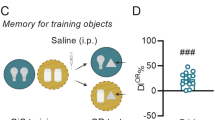
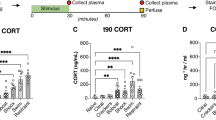
Abstract
Noradrenergic activation of the basolateral amygdala (BLA) promotes strong and lasting memories of emotionally arousing experiences. However, in our lives, we often encounter similar events that may be confused and result in emotional strengthening of incorrect associations. Here we provide evidence, in rats, that noradrenergic activation of the BLA promotes the formation of discrete memories of similar events that were experienced close in time, via a miR-134-regulated consolidation process within the dentate gyrus of the hippocampus. Targeted downregulation of miR-134 in the hippocampus was sufficient to induce memory specificity, without affecting the strength of the memory. Notably, noradrenergic activation of the BLA did not recruit this hippocampal miR-134-mediated mechanism in enhancing memory of a single event. These findings indicate that the BLA engages a qualitatively different neural mechanism on an ‘as-needed’ basis to facilitate the separation of similar memory representations, enabling the selective strengthening of correct associations into long-term memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data are available via Zenodo at https://doi.org/10.5281/zenodo.15496898 (ref. 71) or in this paper and Supplementary Information.
References
Roozendaal, B. & McGaugh, J. L. Memory modulation. Behav. Neurosci. 125, 797–824 (2011).
McGaugh, J. L. Memory—a century of consolidation. Science 287, 248–251 (2000).
McIntyre, C. K., Hatfield, T. & McGaugh, J. L. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur. J. Neurosci. 16, 1223–1226 (2002).
Phelps, E. A. & LeDoux, J. E. Contributions of the amygdala to emotional processing: from animal models to human behavior. Neuron 48, 175–187 (2005).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).
Liu, Y., Xu, M. & Li, W. G. Segregating memories: targeting microenvironment of neuronal ensembles. Sig. Transduct. Target Ther. 7, 363 (2022).
Silva, A. J., Zhou, Y., Rogerson, T., Shobe, J. & Balaji, J. Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395 (2009).
Rogerson, T. et al. Synaptic tagging during memory allocation. Nat. Rev. Neurosci. 14, 157–169 (2014).
Roozendaal, B. & Mirone, G. Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology 114, 104588 (2020).
Bahtiyar, S., Gulmez Karaca, K., Henckens, M. J. A. G. & Roozendaal, B. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol. Cell. Neurosci. 108, 103537 (2020).
Dunsmoor, J. E. & Paz, R. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry 78, 336–343 (2015).
Atucha, E. & Roozendaal, B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front. Behav. Neurosci. 9, 60 (2015).
Atucha, E. et al. Noradrenergic activation of the basolateral amygdala maintains hippocampus-dependent accuracy of remote memory. Proc. Natl Acad. Sci. USA 114, 9176–9181 (2017).
LaLumiere, R. T., Buen, T.-V. & McGaugh, J. L. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J. Neurosci. 23, 6754–6758 (2003).
Roozendaal, B., Castello, N. A., Vedana, G., Barsegyan, A. & McGaugh, J. L. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol. Learn. Mem. 90, 576–579 (2008).
Clewett, D. & Murty, V. P. Echoes of emotions past: how neuromodulators determine what we recollect. eNeuro 6, ENEURO.0108-18.2019 (2019).
Clewett, D., Gasser, C. & Davachi, L. Pupil-linked arousal signals track the temporal organization of events in memory. Nat. Commun. 11, 4007 (2020).
Dunsmoor, J. E. et al. Event segmentation protects emotional memories from competing experiences encoded close in time. Nat. Hum. Behav. 2, 291–299 (2018).
Marr, D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971).
Leutgeb, J. K., Leutgeb, S., Moser, M. B. & Moser, E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966 (2007).
Yassa, M. A. & Stark, C. E. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525 (2011).
Kandel, E. R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001).
He, L. & Hannon, G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5, 522–531 (2004).
Schratt, G. microRNAs at the synapse. Nat. Rev. Neurosci. 10, 842–849 (2009).
Schratt, G. M. et al. A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 (2006).
Bicker, S., Lackinger, M., Weiß, K. & Schratt, G. MicroRNA-132, -134, and -138: a microRNA troika rules in neuronal dendrites. Cell. Mol. Life Sci. 71, 3987–4005 (2014).
Gao, J. et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466, 1105–1109 (2010).
Im, H. I. & Kenny, P. J. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 35, 325–334 (2012).
Silva, A. J., Kogan, J. H., Frankland, P. W. & Kida, S. CREB and memory. Annu. Rev. Neurosci. 21, 127–148 (1998).
Mizuno, M., Yamada, K., Olariu, A., Nawa, H. & Nabeshima, T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J. Neurosci. 20, 7116–7121 (2000).
Bekinschtein, P. et al. BDNF in the dentate gyrus is required for consolidation of “pattern-separated” memories. Cell Rep. 5, 759–768 (2013).
Bekinschtein, P. et al. Brain-derived neurotrophic factor interacts with adult-born immature cells in the dentate gyrus during consolidation of overlapping memories. Hippocampus 24, 905–911 (2014).
Jimenez-Mateos, E. M. et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 18, 1087–1094 (2012).
Campbell, A. et al. AntimiR targeting of microRNA-134 reduces seizures in a mouse model of Angelman syndrome. Mol. Ther. Nucleic Acids 28, 514–529 (2022).
Eyileten, C. et al. The relation of the brain-derived neurotrophic factor with microRNAs in neurodegenerative diseases and ischemic stroke. Mol. Neurobiol. 58, 329–347 (2021).
Wang, G. et al. Antidepressant-like effect of ginsenoside Rb1 on potentiating synaptic plasticity via the miR-134–mediated BDNF signaling pathway in a mouse model of chronic stress-induced depression. J. Ginseng Res. 46, 376–386 (2022).
Baby, N., Alagappan, N., Dheen, S. T. & Sajikumar, S. MicroRNA‐134‐5p inhibition rescues long‐term plasticity and synaptic tagging/capture in an Aβ(1–42)-induced model of Alzheimer’s disease. Aging Cell 19, e13046 (2020).
Li, Y. et al. MicroRNA134 of ventral hippocampus is involved in cocaine extinction-induced anxiety-like and depression-like behaviors in mice. Mol. Ther. Nucleic Acids 19, 937–950 (2020).
Kandel, E. R. The molecular biology of memory: cAMP, PKA, CRE, CREB‐1, CREB‐2, and CPEB. Mol. Brain 5, 14 (2012).
Tao, X., Finkbeiner, S., Arnold, D. B., Shaywitz, A. J. & Greenberg, M. E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20, 709–726 (1998).
Joilin, G. et al. Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front. Mol. Neurosci. 7, 98 (2014).
Krol, J. et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141, 618–631 (2010).
Chatterjee, S. & Grosshans, H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature 461, 546–549 (2009).
Lee, D., Park, D., Park, J. H., Kim, J. H. & Shin, C. Poly(A)-specific ribonuclease sculpts the 3ʹ-ends of microRNAs. RNA 25, 388–405 (2019).
Boele, J. et al. PAPD5-mediated 3′ adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proc. Natl Acad. Sci. USA 111, 11467–11472 (2014).
Burroughs, A. M. et al. A comprehensive survey of 3′ animal miRNA modification events and a possible role for 3′ adenylation in modulating miRNA targeting effectiveness. Genome Res. 20, 1398–1410 (2010).
Kai, Z. S. & Pasquinelli, A. E. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat. Struct. Mol. Biol. 17, 5–10 (2010).
Katoh, T. et al. Selective stabilization of mammalian microRNAs by 3′ adenylation mediated by the cytoplasmic poly(A) polymerase GLD-2. Genes. Dev. 23, 433–438 (2009).
Sharot, T., Delgado, M. R. & Phelps, E. A. How emotion enhances the feeling of remembering. Nat. Neurosci. 7, 1376–1380 (2004).
Krenz, V., Sommer, T., Alink, A., Roozendaal, B. & Schwabe, L. Noradrenergic arousal after encoding reverses the course of systems consolidation in humans. Nat. Commun. 12, 6054 (2021).
Hargreaves, E. L., Rao, G., Lee, I. & Knierim, J. J. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308, 1792–1794 (2005).
Luna, V. M. et al. Adult-born hippocampal neurons bidirectionally modulate entorhinal inputs into the dentate gyrus. Science 364, 578–583 (2019).
Myers, C. E. & Scharfman, H. E. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus 19, 321–337 (2009).
GoodSmith, D. et al. Spatial representation of granule cells and mossy cells of the dentate gyrus. Neuron 93, 677–690 (2017).
Senzai, Y. & Buzsáki, G. Physiological properties and behavioral correlates of hippocampal granule cells and mossy cells. Neuron 93, 691–704 (2017).
Ikegaya, Y., Nakanishi, K., Saito, H. & Abe, K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport 8, 3143–3146 (1997).
Vouimba, R. M. & Richter-Levin, G. Different patterns of amygdala priming differentially affect dentate gyrus plasticity and corticosterone, but not CA1 plasticity. Front. Neural Circuits 7, 80 (2013).
McHugh, S. B. et al. Adult-born dentate granule cells promote hippocampal population sparsity. Nat. Neurosci. 25, 1481–1491 (2022).
Bangasser, D. A., Wiersielis, K. R. & Khantsis, S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 1641, 177–188 (2016).
Borodovitsyna, O., Tkaczynski, J. A., Corbett, C. M., Loweth, J. A. & Chandler, D. J. Age- and sex-dependent changes in locus coeruleus physiology and anxiety-like behavior following acute stressor exposure. Front. Behav. Neurosci. 16, 808590 (2022).
Joshi, N. & Chandler, D. Sex and the noradrenergic system. In Handbook of Clinical Neurology, Vol. 175 (eds Lanzenberger, R., et al.) 167–176 (Elsevier, 2020).
Yagi, S., Chow, C., Lieblich, S. E. & Galea, L. A. M. Sex and strategy use matters for pattern separation, adult neurogenesis, and immediate early gene expression in the hippocampus. Hippocampus 26, 87–101 (2016).
Yagi, S., Lee, A., Truter, N. & Galea, L. A. M. Sex differences in contextual pattern, and functional connectivity within the limbic system. Biol. Sex. Differ. 13, 42 (2022).
Edwards, C. M., Dolezel, T. & Rinaman, L. Sex and metabolic state interact to influence expression of passive avoidance memory in rats: potential contribution of A2 noradrenergic neurons. Physiol. Behav. 239, 113511 (2021).
Graham, B. M. & Scott, E. Effects of systemic estradiol on fear extinction in female rats are dependent on interactions between dose, estrous phase, and endogenous estradiol levels. Horm. Behav. 97, 67–74 (2018).
Do Nascimento, E. B. et al. Memory impairment induced by different types of prolonged stress is dependent on the phase of the estrous cycle in female rats. Horm. Behav. 115, 104563 (2019).
Kirry, A. J., Durigan, D. J., Twining, R. C. & Gilmartin, M. R. Estrous cycle stage gates sex differences in prefrontal muscarinic control of fear memory formation. Neurobiol. Learn. Mem. 161, 26–36 (2019).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th edn (Academic, 2007).
Schmittgen, T. D., Jiang, J., Liu, Q. & Yang, L. A high-throughput method to monitor the expression of microRNA precursors. Nucleic Acids Res. 32, e43 (2004).
Schmittgen, T. D. et al. Real-time PCR quantification of precursor and mature microRNA. Methods 44, 31–38 (2008).
Atucha, E. et al. Noradrenergic activation of the basolateral amygdala facilitates memory specificity for similar events experienced close in time-source data. Zenodo https://doi.org/10.5281/zenodo.15496898 (2025).
Acknowledgements
Funding was provided by Radboud University Topfund (to B.R.); Dutch Research Council Open Research Area grant (no. 464.18.110 to B.R.), and European Community’s Seventh Framework Programme (no. FP7/2007–2013) and Horizon 2020 Programme under grant agreement nos. 603016 and 667302 (to J.C.G.).
Author information
Authors and Affiliations
Contributions
Conceptualization: E.A. and B.R. Data acquisition: E.A., M.P., G.R., C.S., P.A., D.R., K.L., G.S. and B.R. Funding acquisition: J.C.G. and B.R. Supervision: J.C.G., A.A. and B.R. Writing—original draft: E.A. and B.R. Writing—review and editing: J.L.M., J.C.G. and A.A. J.C.G. and A.A. contributed equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Michael Fanselow, Ki Goosens, Natalie Tronson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 NE administration into the BLA of home-cage control rats does not affect miR-134, CREB or BDNF levels within the dDG.
NE (1.0 µg in 0.2 µl) was administered unilaterally into one BLA and saline into the other BLA, counterbalanced across animals, of home-cage control rats. NE administration did not affect miR-134, CREB or BDNF levels within the dDG 30 min later. mRNA levels are normalized to the saline-treated hemisphere (mean + s.e.m.) (n = 9, paired Student’s t-test: miR-134: t8 = 0.52, P = 0.62; CREB: t8 = 0.46, P = 0.66; BDNF: t8 = 0.98, P = 0.36).
Extended Data Fig. 2 Ant-134 administration into the dHPC does not alter Arc, Npas4 or c-Fos levels within the dDG.
Ant-134 (10 pmol in 0.5 µl) was administered unilaterally into one dHPC and scrambled control (Scr, 10 pmol in 0.5 µl) into the other dHPC, counterbalanced across animals, immediately after training on the dual-event inhibitory avoidance task. Ant-134 administration did not affect mRNA levels of several plasticity-related genes that are considered non-targets of miR-134 within the dDG 30 min later. mRNA levels are normalized to the scrambled-treated hemisphere (mean + s.e.m.) (n = 7, paired Student’s t-test: activity-regulated cytoskeleton-associated protein (Arc): t6 = 1.73, P = 0.13; neuronal PAS domain protein 4 (Npas4): t6 = 1.86, P = 0.11; c-Fos: t6 = 1.09, P = 0.32).
Extended Data Fig. 3 Noradrenergic activation of the BLA does not increase CREB or pCREB protein levels within the ventral blade of the DG.
NE (1.0 µg in 0.2 µl) was administered unilaterally into one BLA and saline into the other BLA, counterbalanced across animals, immediately after training on the dual-event inhibitory avoidance task. NE administration did not affect CREB or pCREB protein levels within the ventral blade of the DG granule cell layer 3 h later. Protein levels are normalized to the saline-treated hemisphere (mean + s.e.m.) (n = 7, paired Student’s t-test: CREB: t6 = 1.43, P = 0.20; pCREB: t6 = 1.01, P = 0.35).
Extended Data Fig. 4 Overexpression of miR-134 in the dHPC induces an efficient down-regulation of CREB and BDNF mRNA levels within the dDG.
Mimic-134 (0.25 pmol in 0.5 µl) was administered unilaterally into one dHPC and non-target control (NT, 0.25 pmol in 0.5 µl) into the other dHPC, counterbalanced across animals, immediately after training on the dual-event inhibitory avoidance task. Mimic-134 administration resulted in a 6.60 ± 0.91 log2FC (mean ± s.e.m) increase in total-tissue miR-134 levels within the dDG 30 min later (n = 6, paired Student’s t-test: t5 = 7.26, P < 0.01). mRNA levels are normalized to the non-target-treated hemisphere (mean + s.e.m.) (n = 6, paired Student’s t-test: CREB: t5 = 3.22, P < 0.05; BDNF: t5 = 3.28, P < 0.05). *P < 0.05 vs non-target.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atucha, E., Pais, M., Ronzoni, G. et al. Noradrenergic activation of the basolateral amygdala facilitates memory specificity for similar events experienced close in time. Nat Neurosci 28, 1910–1918 (2025). https://doi.org/10.1038/s41593-025-02014-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41593-025-02014-0