Abstract
The mitochondrial translocase of the outer membrane (TOM) and translocase of the inner membrane 23 (TIM23) complexes are coupled to control protein import across the outer and inner membranes, respectively. However, the mechanisms of protein recognition and sorting in the TOM–TIM23 pathway remain unclear. Here we report cryo-electron microscopy structures of a translocating polypeptide substrate captured in the active TOM–TIM23 supercomplex from Saccharomyces cerevisiae. In the TOM complex, the polypeptide substrate adopts multiple conformations stabilized by hydrophilic residues from distinct regions of the Tom40 channel. In the TIM23 complex, the Tim17 and Mgr2 subunits create the translocation pathway, with a central restriction formed by four highly conserved hydrophobic residues. The substrate primarily interacts with hydrophobic residues along the Tim17–Mgr2 pathway. Substrate hydrophobicity modulates the association of Mgr2 with Tim17, enabling dynamic regulation of protein sorting toward either the matrix or membrane. These findings reveal a sophisticated translocation mechanism of the TOM–TIM23 supercomplex that ensures the efficient import of diverse mitochondrial proteins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The cryo-EM maps of the substrate-engaged TOM complex and the substrate-engaged TIM23 complex were deposited to the EM Data Bank under accession numbers EMD-61256 and EMD-61257. The atomic structure coordinates of the substrate-engaged TOM complex the substrate-engaged TIM23 complex were deposited to the PDB under accession numbers 9J99 and 9J9B. MD simulation data are available on figshare (https://doi.org/10.6084/m9.figshare.27802086 (ref. 64)). Data and materials can be obtained from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The code for the distribution landscape of TIM relative to TOM is available from GitHub (https://github.com/GuoQLabPKU/TIMTOM-distribution). The code for residue orientation analysis in MD is also available from GitHub (https://github.com/ComputBiophys/TIMOrientationAnalysis).
References
Busch, J. D., Fielden, L. F., Pfanner, N. & Wiedemann, N. Mitochondrial protein transport: versatility of translocases and mechanisms. Mol. Cell 83, 890–910 (2023).
Makki, A. & Rehling, P. Protein transport along the presequence pathway. Biol. Chem. 404, 807–812 (2023).
Bykov, Y. S., Rapaport, D., Herrmann, J. M. & Schuldiner, M. Cytosolic events in the biogenesis of mitochondrial proteins. Trends Biochem. Sci. 45, 650–667 (2020).
Callegari, S., Cruz-Zaragoza, L. D. & Rehling, P. From TOM to the TIM23 complex—handing over of a precursor. Biol. Chem. 401, 709–721 (2020).
Mokranjac, D. How to get to the other side of the mitochondrial inner membrane—the protein import motor. Biol. Chem. 401, 723–736 (2020).
Araiso, Y., Imai, K. & Endo, T. Role of the TOM complex in protein import into mitochondria: structural views. Annu. Rev. Biochem. 91, 679–703 (2022).
Shiota, T. et al. Molecular architecture of the active mitochondrial protein gate. Science 349, 1544–1548 (2015).
Araiso, Y. et al. Structure of the mitochondrial import gate reveals distinct preprotein paths. Nature 575, 395–401 (2019).
Tucker, K. & Park, E. Cryo-EM structure of the mitochondrial protein-import channel TOM complex at near-atomic resolution. Nat. Struct. Mol. Biol. 26, 1158–1166 (2019).
Guan, Z. Y. et al. Structural insights into assembly of human mitochondrial translocase TOM complex. Cell Discov. 7, 22 (2021).
Zarsky, V. & Dolezal, P. Evolution of the Tim17 protein family. Biol. Direct 11, 54 (2016).
Fielden, L. F. et al. Central role of Tim17 in mitochondrial presequence protein translocation. Nature 621, 627–634 (2023).
Sim, S. I., Chen, Y., Lynch, D. L., Gumbart, J. C. & Park, E. Structural basis of mitochondrial protein import by the TIM23 complex. Nature 621, 620–626 (2023).
Zhou, X. et al. Molecular pathway of mitochondrial preprotein import through the TOM–TIM23 supercomplex. Nat. Struct. Mol. Biol. 30, 1996–2008 (2023).
Gebert, M. et al. Mgr2 promotes coupling of the mitochondrial presequence translocase to partner complexes. J. Cell Biol. 197, 595–604 (2012).
Ieva, R. et al. Mgr2 functions as lateral gatekeeper for preprotein sorting in the mitochondrial inner membrane. Mol. Cell 56, 641–652 (2014).
Rassow, J. et al. Mitochondrial protein import: biochemical and genetic evidence for interaction of matrix Hsp70 and the inner membrane protein Mim44. J. Cell Biol. 127, 1547–1556 (1994).
Schneider, H. C. et al. Mitochondrial Hsp70/Mim44 complex facilitates protein import. Nature 371, 768–774 (1994).
D’Silva, P., Liu, Q., Walter, W. & Craig, E. A. Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat. Struct. Mol. Biol. 11, 1084–1091 (2004).
Ting, S. Y., Yan, N. L., Schilke, B. A. & Craig, E. A.Dual interaction of scaffold protein Tim44 of mitochondrial import motor with channel-forming translocase subunit Tim23. eLife 6, e23609 (2017).
Kang, P. J. et al. Requirement for Hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348, 137–143 (1990).
Liu, Q., D’Silva, P., Walter, W., Marszalek, J. & Craig, E. A. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 300, 139–141 (2003).
Matouschek, A., Pfanner, N. & Voos, W. Protein unfolding by mitochondria. The Hsp70 import motor. EMBO Rep. 1, 404–410 (2000).
Vogtle, F. N. et al. Global analysis of the mitochondrial N-proteome identifies a processing peptidase critical for protein stability. Cell 139, 428–439 (2009).
Mossmann, D., Meisinger, C. & Vogtle, F. N. Processing of mitochondrial presequences. Biochim. Biophys. Acta 1819, 1098–1106 (2012).
Truscott, K. N. et al. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 8, 1074–1082 (2001).
Alder, N. N., Jensen, R. E. & Johnson, A. E. Fluorescence mapping of mitochondrial TIM23 complex reveals a water-facing, substrate-interacting helix surface. Cell 134, 439–450 (2008).
Rapoport, T. A., Li, L. & Park, E. Structural and mechanistic insights into protein translocation. Annu. Rev. Cell Dev. Biol. 33, 369–390 (2017).
van den Berg, B. et al. X-ray structure of a protein-conducting channel. Nature 427, 36–44 (2004).
Ma, C. et al. Structure of the substrate-engaged SecA–SecY protein translocation machine. Nat. Commun. 10, 2872 (2019).
Hegde, R. S. & Keenan, R. J. The mechanisms of integral membrane protein biogenesis. Nat. Rev. Mol. Cell Biol. 23, 107–124 (2022).
Dekker, P. J. et al. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70–Tim44. EMBO J. 16, 5408–5419 (1997).
Botte, M. et al. Cryo-EM structures of a LptDE transporter in complex with Pro-macrobodies offer insight into lipopolysaccharide translocation. Nat. Commun. 13, 1826 (2022).
Malhotra, K., Sathappa, M., Landin, J. S., Johnson, A. E. & Alder, N. N.Structural changes in the mitochondrial Tim23 channel are coupled to the proton-motive force. Nat. Struct. Mol. Biol. 20, 965–972 (2013).
Hessa, T. et al. Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450, 1026–1030 (2007).
Herrmann, J. M., Neupert, W. & Stuart, R. A. Insertion into the mitochondrial inner membrane of a polytopic protein, the nuclear-encoded Oxa1p. EMBO J. 16, 2217–2226 (1997).
Bohnert, M. et al. Cooperation of stop-transfer and conservative sorting mechanisms in mitochondrial protein transport. Curr. Biol. 20, 1227–1232 (2010).
Park, K., Jung, S. J., Kim, H. & Kim, H. Mode of membrane insertion of individual transmembrane segments in Mdl1 and Mdl2, multi-spanning mitochondrial ABC transporters. FEBS Lett. 588, 3445–3453 (2014).
Richter, F. et al. ROMO1 is a constituent of the human presequence translocase required for YME1L protease import. J. Cell Biol. 218, 598–614 (2019).
Nouwen, N., Berrelkamp, G. & Driessen, A. J. Charged amino acids in a preprotein inhibit SecA-dependent protein translocation. J. Mol. Biol. 386, 1000–1010 (2009).
Gakh, O., Cavadini, P. & Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 1592, 63–77 (2002).
Ford, H. C. et al. Towards a molecular mechanism underlying mitochondrial protein import through the TOM and TIM23 complexes. eLife 11, e75426 (2022).
Ralser, M. et al. The Saccharomyces cerevisiae W303-K6001 cross-platform genome sequence: insights into ancestry and physiology of a laboratory mutt. Open Biol. 2, 120093 (2012).
Matsuda, T. & Cepko, C. L. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl Acad. Sci. USA 101, 16–22 (2004).
Kirchhofer, A. et al. Modulation of protein properties in living cells using nanobodies. Nat. Struct. Mol. Biol. 17, 133–138 (2010).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Kimanius, D., Dong, L., Sharov, G., Nakane, T. & Scheres, S. H. W. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem. J. 478, 4169–4185 (2021).
Zhang, K. Gctf: teal-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370–383 (2021).
Jiang, W. et al. A transformation clustering algorithm and its application in polyribosomes structural profiling. Nucleic Acids Res. 50, 9001–9011 (2022).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Wittig, I., Braun, H. P. & Schagger, H. Blue native PAGE. Nat. Protoc. 1, 418–428 (2006).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Huang, J. et al. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Parrinello, M. & Rahman, A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 52, 7182–7190 (1981).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Hess, B., Bekker, H., Berendsen, H. J. C. & Fraaije, J. G. E. M. LINCS: a linear constraint solver for molecular simulations. J. Comput. Chem. 18, 1463–1472 (1997).
Michaud-Agrawal, N., Denning, E. J., Woolf, T. B. & Beckstein, O. MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J. Comput. Chem. 32, 2319–2327 (2011).
Yang, Y. et al. Dynamic TOM-TIM23 supercomplex directs mitochondrial protein translocation and sorting. figshare https://doi.org/10.6084/m9.figshare.27802086 (2025).
Acknowledgements
We thank X. Zhou for helping with sample preparation, N. Gao, X. Zhang and N. Li for helping with cryo-EM calculation and the National Center for Protein Sciences at Peking University for assistance with protein purification and mass spectrometry. The cryo-EM data were collected at the cryo-EM platform of Peking University. The computation was supported by the High-Performance Computing Platform of Peking University. This work was supported by the National Natural Science Foundation of China (32271269 to L.L. and T2321001 to C.S.), Qidong-SLS Innovation Fund (to L.L.) and National Key R&D Program of China (2024YFA0916800 to L.L. and C.S.). C.S. was supported in part by the Frontier Innovation Fund of Peking University Chengdu Academy for Advanced Interdisciplinary Biotechnologies.
Author information
Authors and Affiliations
Contributions
Y.Y. and Y.L. prepared the protein samples for structural studies. Y.Y., S.W. and Y.L. performed the functional assays. Y.Y. and G.W. collected the cryo-EM data and determined the structures. L.X. performed the MD simulations. W.J. and Q.G. analyzed the relative orientation between TOM and TIM23. L.L. and C.S. supervised the project. All authors were involved in paper preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Paul Schanda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Katarzyna Ciazynska, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
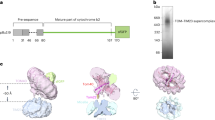
Extended Data Fig. 1 Purification and structure determination of the TOM-TIM23 supercomplex.
a, Size exclusion chromatography of the TOM-TIM23 supercomplex. The grey-shaded elution fractions are analyzed by BN-PAGE as shown on the right. b, Representative raw image of the supercomplex particles. c, Representative 2D classes of the supercomplex. Individual components of the supercomplex, including sfGFP, TOM, TIM23, and Fab, are visible in some classes. d, Flow chart of data processing for structure determination of the TOM complex. Details of data processing are provided in the Methods section. e, Focused 3D classification of the substrate in the Tom40 channel. f, Eulerian angle distribution of the particles used for TOM reconstruction, generated from RELION. g, Gold standard Fourier shell correlation (FSC) curve. The estimated resolution of the final map at 0.143 is generated from RELION.
Extended Data Fig. 2 Structure determination of TIM23 in the supercomplex.
a, Flow chart of data processing for structure determination of the TIM23 complex. The starting particles are derived from the TOM calculation (Extended Data Fig. 1d). b, Representative 2D classes of the TIM23 complex. c, Gold standard Fourier shell correlation (FSC) curve. The estimated resolution of the final map at 0.143 is generated from CryoSPARC. d, Eulerian angle distribution of the particles used for TIM23 reconstruction, generated from CryoSPARC. e, 3D classification of the TOM-TIM23 supercomplex using the selected particles from focused TOM and TIM23 classifications and refinements. f, Distribution landscape of TIM23 relative to TOM. The dots indicate the spatial positions of TIM23 relative to TOM. The dots are color-coded by particle density, with red representing the areas of highest concentration.
Extended Data Fig. 3 Examples of the fit of the models into density maps.
a, Density maps (meshes) and models of the selected peptide segments from the TOM complex. b, Density maps (meshes) and models of the selected peptide segments from the TIM23 complex. c, Density maps (meshes) and models of the selected peptide segments from and the substrate in the TIM23 complex are shown. Residues at the beginning and end of each segment are labeled. The residue side chains are shown as sticks.
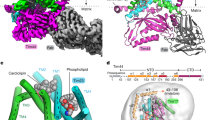
Extended Data Fig. 4 Structures of the TOM complex and its substrate in the supercomplex.
a, Structural comparison of Tom7 in the idle structure (Mandy’s pink, PDB ID: 6UCU) and the active structure (forest green). The two copies of the subunit in a dimeric TOM complex are both shown in a side view and a top view. The phospholipid clamped by Tom7 and Tom40 in the active structure is shown as yellow balls and sticks with transparent density. b, Structural model of an anti-GFP Macrobody. MBP and the anti-GFP nanobody are colored khaki and plum, respectively. c, Purification of the supercomplex complexed with the Macrobody. The elution fractions (grey shaded) from size exclusion chromatography (left) are analyzed by BN-PAGE (top right) and SDS-PAGE (bottom right). BN-PAGE is stained with Coomassie blue. SDS-PAGE is subjected to immunoblotting with an anti-His tag antibody for detection of the Macrobody and Tom22. d, Cryo-EM analysis of the supercomplex with the Macrobody. The raw image (left), 2D classification (middle), and 3D reconstruction (right) are shown. The GFP density in the 2D classes is marked by green arrows. The resolution estimations are from the outputs of 3D classification using RELION. e, Focused 3D classification of the substrate in the Tom40 channel. The six classes are the calculation results from Extended Data Fig. 1e. The three representative classes are also shown in Fig. 1d–f.
Extended Data Fig. 5 Structures of the core translocase subunits in TIM23 complex.
a, Superimposition of the idle (PDB ID: 8SCX) and active structure of Tim17-Tim23-Tim44. The subunits in the idle structure are all colored grey. Tim17, Tim23, and Tim44 in the active structure are colored the same as in Fig. 2. b, Superimposition of Tim17 in the idle (grey) and active (brownish pink) structures. c, Superimposition of Tim44-CTD in the idle (grey) and active (dark sea green) structures. d, Fitting of the idle Tim23 and Tim44 structures into the density of the active state. The density map is low pass filtered to 6 Å to emphasize the overall fitting the model. Views are shown from the Tim23 side (left) and top (right). Idle Tim23 and Tim44 are colored cyan and magenta, respectively. Tim44 in the active state is colored lime. TM3-4 of the Tim23 idle structure is outside the density map of the active state. The side chains of the Tim44 α2-3 helices in the active state are shown as sticks, with hydrophobic and hydrophilic residues labeled in black and lime, respectively. The shift of the Tim44 α2 and α3 helices is indicated by an arrow. e, Comparison of cryo-EM densities of the active and inactive TIM23 complexes (EMD-27825). Side views illustrate the thinned membrane at the Tim23 side of the active structure. The density map is low-pass filtered to 15 Å, with estimated membrane thicknesses labeled. f, Alignments of Tim17 sequences from representative eukaryotic species. The sequences are from Saccharomyces cerevisiae (S. cerevisiae, Uniprot P39515), Neurospora crassa (N. crassa, Uniprot P59670), Schizosaccharomyces pombe (S. pombe, Uniprot P87130), Kluyveromyces lactis (K. lactis, Uniport Q6CXS9), Candida glabrata (C. glabrata, Uniprot A0A0W0CKS7), Caenorhabditis elegans (C. elegans, Uniprot O44477), Drosophila melanogaster (D. melanogaster, Uniprot Q9VGA2), Mus musculus (M. musculus, Uniprot Q545U2), Homo sapiens (H. sapiens, Uniprot Q99595). g, Alignments of Mgr2 sequences from representative eukaryotic species. The sequences are from Saccharomyces cerevisiae (S. cerevisiae, Uniprot Q02889), Neurospora crassa (N. crassa, Uniprot Q7SDL7), Schizosaccharomyces pombe (S. pombe, Uniprot P79082), Caenorhabditis elegan (C. elegans, Uniprot Q93511), Drosophila melanogaster (D. melanogaster, Uniprot Q9VUM2), Mus musculus (M. musculus, Uniprot P60603), Homo sapiens (H. sapiens, Uniprot P60602).
Extended Data Fig. 6 Structural comparison of the Tim17-Mgr2 complex and the Sec translocon.
a, Surface presentations of Tim17-Mgr2 in a side view (left) and a cutaway side view (right). The lateral opening between Tim17 and Mgr2 in the lower half of the complex is outlined by black lines. The translocation pathway inside the complex is outlined by green lines. The restriction residues are colored green. b, Hydrophobicity distribution along the Tim17-Mgr2 translocation pathway. c, Charge distribution along the Tim17-Mgr2 translocation pathway. d, Surface presentations of the Sec translocon (PDB ID: 8RJC), shown in a side view (left) and a cutaway side view (right). The restriction (pore ring) residues are colored green. e, Hydrophobicity distribution in the Sec channel. f, Charge distribution in the Sec channel. g, Superimposition of the restriction residues in the Tim17-Mgr2 pathway and the Sec channel. Tim17, Mgr2, and Sec (PDB ID: 6ITC) are colored brownish pink, gold, and grey, respectively. The restriction residues of Tim17-Mgr2 and Sec are shown as balls and sticks, and colored forest green and grey, respectively. h, The substrate exit site of Tim17-Mgr2. The translocation direction toward the β sheet of Tim44 is indicated by a black arrow. i, same as h, except showing the hydrophobicity distribution of the exit site. The polypeptide substrate is represented as a curved line.
Extended Data Fig. 7 Substrate and its interaction residues in the Tim17-Mgr2 pathway.
a, Mapping of the substrate in the TIM23 complex. The substrate residues around the α1 and α2 regions of the B2 segments are mutated to Bpa. The supercomplexes are assembled in the yeast strain yLS420 transformed with the plasmids pLSP2-x that encode the photoactive substrates. The cells are subjected to UV irradiation, followed by Mgr2 pulldown. The substrate, Mgr2, and their crosslinking products are detected by immunoblotting against the StrepII tag (Mgr2) and Flag tag (substrate). Position 155 of the substrate outside the TIM23 complex is included as a negative control. b, Comparison of the substrate passing through the restrictions of Tim17-Mgr2 (left) and the Sec channel (right, PDB ID: 6ITC). Tim17 and Mgr2 are colored brownish pink and gold, respectively. The N-half and C-half of the Sec channel are colored sky blue and plum, respectively. The restriction residues are shown as balls and sticks with those from Tim17-Mgr2 in crystal blue and those from Sec in red. The substrate density is colored green. The distances between the restriction residues and the centers of the substrate density are labeled. c, Spot assays to examine the growth of yeast strains with point mutations of the substrate-interacting residues of Tim17. d, Same as c except with additional Mgr2 knockout. e, Tim17 mutant selection on 5-FOA plates. The yeast strains, yLS270, yLS280, and yLS290, are transformed with Tim17 mutation plasmid pLSA4-X and selected on 5-FOA plates. The mutants that could not grow during selection are shown. f, Import of the metabolite carrier GGC1 via the TIM22 pathway into mitochondria with mutations of the restriction residues in Tim17. The imported and correctly folded GGC1 is detected as a ~ 100 kDa band in BN-PAGE 78.
Extended Data Fig. 8 MD simulations of protein translocation in TIM23.
a, Side chain orientations of all substrate residues from α2 of the modeled substrate during simulated translocation through the Tim17 groove in the presence (left) or absence (right) of Mgr2. This panel extends the analysis from Fig. 4c to include all substrate residues, providing a comprehensive view of their orientations. b, Initial model used for the MD simulations. The side chains of the substrate are labeled and shown as balls and sticks. c, MD simulations of the substrate-engaged TIM23 complex, demonstrating that the complex remains impermeable to water diffusion, irrespective of Mgr2’s presence. Water molecules are depicted as red and white spheres. The orange spheres are the phosphorous atoms of lipids.
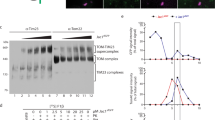
Extended Data Fig. 9 Double crosslinking sites of the substrate to detect TIM23 subunits.
a, Scheme of the supercomplex assembly, showing two adjacent positions 135 and 139 of the substrate located in the TIM23 complex. Both positions are mutated to an amber codon for Bpa incorporation. b, Photo-crosslinking of the substrate with double Bpa sites. In wild type mitochondria, the double crosslinking product containing the substrate, Tim17, and Mgr2 could be detected. When Mgr2 is knocked out, no higher crosslinking bands above the “substrate x Tim17” band are observed.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Supplementary Video 1
MD simulations trajectories illustrating the translocation of a polypeptide substrate through the Tim17–Mgr2 pathway. The substrate was steered into the matrix (downward) by a pulling force in MD simulations. The polypeptide is depicted in green with side chains highlighted as balls and sticks. Mgr2 is shown in transparent yellow. Key hydrophobic residues of Tim17 and Mgr2 that interact with the substrate are shown as color-coded balls and sticks: orange, F65 of Tim17; lime, W68 of Tim17; purple, L12 of Tim17; deep pink, F67 of Mgr2; silver, F70 of Mgr2. Notably, the applied force was not constant and could be accumulated in certain polypeptide-trapped locations during simulations. This could result in uneven translocation rates of the polypeptide substrate.
Supplementary Video 2
Same as Supplementary Video 1, except that the substrate was pulled toward the IMS.
Supplementary Video 3
Same as Supplementary Video 1, except that the simulation was conducted without Mgr2.
Supplementary Video 4
Same as Supplementary Video 2, except that the simulation was conducted without Mgr2.
Source data
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed western blots and gels.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Wang, S., Wang, G. et al. Dynamic TOM–TIM23 supercomplex directs mitochondrial protein translocation and sorting. Nat Struct Mol Biol 32, 2231–2241 (2025). https://doi.org/10.1038/s41594-025-01662-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41594-025-01662-x