Abstract
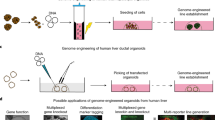
Hepatocytes are one of the most important cell types in the liver, carrying out key functions. They are essential for hepatocyte-based therapy, disease modeling and drug development. However, the availability of primary human hepatocytes (PHHs) is limited by a shortage of donors. It is therefore of great value to expand PHHs in large quantities. Here we provide a detailed protocol for the large-scale expansion of PHHs (proliferating human hepatocytes, ProliHHs) derived from healthy donors and patients with inherited liver diseases, which can be rematured in a three-dimensional culture system. Moreover, we provide a protocol for the genetic manipulation of ProliHHs, including lentivirus transduction and CRISPR–Cas9-mediated knockout and knock-in. The protocol described here will help to realize the full potential of ProliHH-based therapy, organoid-based liver disease modeling and drug screening. The protocol to expand PHHs takes ~1–2 months, the protocol to establish the 3D-cultured ProliHHs takes ~8 d and the protocol to perform gene editing takes ~3 d. Personnel with basic scientific training can conduct these protocols.
Key points
-
This Protocol is for the large-scale expansion of proliferating human hepatocytes derived from human donor livers. Proliferating human hepatocytes can be rematured in a three-dimensional culture system and are amenable to genetic manipulation.
-
This overcomes limitations in using primary human hepatocytes, for which there is a shortage of donors and which display a low efficiency of gene manipulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in either this paper or in our original research study15,18,19,20,21. The RNA-seq data were reanalyzed from the public repository Gene Expression Omnibus database: GSE112866 (related to Extended Data Fig. 1f and Supplementary Code 1). All raw data are provided as source data files. Any additional data required for research purposes are available from the corresponding authors upon request. Source data are provided with this paper.
References
Sun, Z. et al. Hepatocyte transplantation: the progress and the challenges. Hepatol. Commun. https://doi.org/10.1097/HC9.0000000000000266 (2023).
Hu, W. & Lazar, M. A. Modelling metabolic diseases and drug response using stem cells and organoids. Nat. Rev. Endocrinol. 18, 744–759 (2022).
Grossman, M. et al. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat. Genet. 6, 335–341 (1994).
Hickey, R. D. et al. Curative ex vivo liver-directed gene therapy in a pig model of hereditary tyrosinemia type 1. Sci. Transl. Med. 8, 349ra399 (2016).
VanLith, C. J. et al. Ex vivo hepatocyte reprograming promotes homology-directed DNA repair to correct metabolic disease in mice after transplantation. Hepatol. Commun. 3, 558–573 (2019).
Artegiani, B. et al. Fast and efficient generation of knock-in human organoids using homology-independent CRISPR–Cas9 precision genome editing. Nat., Cell. Biol. 22, 321–331 (2020).
Kim, T. W. et al. Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell 28, 343–355 e345 (2021).
Touboul, T. et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51, 1754–1765 (2010).
Baxter, M. et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 62, 581–589 (2015).
Huang, P. et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14, 370–384 (2014).
Grandy, R., Tomaz, R. A. & Vallier, L. Modeling disease with human inducible pluripotent stem cells. Annu. Rev. Pathol. 14, 449–468 (2019).
Xiang, C. et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science 364, 399–402 (2019).
Fu, G. B. et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 29, 8–22 (2019).
Hu, H. et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175, 1591–1606 e1519 (2018).
Zhang, K. et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell 23, 806–819 e804 (2018).
Katsuda, T. et al. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. eLife https://doi.org/10.7554/eLife.47313 (2019).
Kim, Y. et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J. Hepatol. 70, 97–107 (2019).
Wang, C. et al. Dedifferentiation-associated inflammatory factors of long-term expanded human hepatocytes exacerbate their elimination by macrophages during liver engraftment. Hepatology 76, 1690–1705 (2022).
Zhang, K. et al. Ex vivo factor VIII-modified proliferating human hepatocytes therapy for haemophilia A. Cell Prolif. 56, e13467 (2023).
Yuan, X. et al. Preclinical efficacy and safety of encapsulated proliferating human hepatocyte organoids in treating liver failure. Cell Stem Cell 31, 484–498 e485 (2024).
Zhang, K. et al. Efficient expansion and CRISPR–Cas9-mediated gene correction of patient-derived hepatocytes for treatment of inherited liver diseases. Cell Stem Cell https://doi.org/10.1016/j.stem.2024.04.022 (2024).
Nicolas, C. T. et al. Ex vivo cell therapy by ectopic hepatocyte transplantation treats the porcine tyrosinemia model of acute liver failure. Mol. Ther. Methods Clin. Dev. 18, 738–750 (2020).
Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250 (2013).
Huch, M. et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160, 299–312 (2015).
Yanger, K. et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 27, 719–724 (2013).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Tanimizu, N., Nishikawa, Y., Ichinohe, N., Akiyama, H. & Mitaka, T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM−) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J. Biol. Chem. 289, 7589–7598 (2014).
Yuan, X., Sun, Z., Wu, J., Hui, L. & Zhang, L. Cell therapy for liver diseases: from hepatocyte transplantation to bioartificial livers. Curr. Opin. Biomed. Eng. 30, 100530 (2024).
Labanieh, L. et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763 e1722 (2022).
Peng, W. C. et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 175, 1607–1619 e1615 (2018).
Guo, R. et al. IL6 supports long-term expansion of hepatocytes in vitro. Nat. Commun. 13, 7345 (2022).
Cox, D. B., Platt, R. J. & Zhang, F. Therapeutic genome editing: prospects and challenges. Nat. Med. 21, 121–131 (2015).
Dunbar, C. E. et al. Gene therapy comes of age. Science https://doi.org/10.1126/science.aan4672 (2018).
Ma, C. et al. CD47 and PD-L1 overexpression in proliferating human hepatocytes attenuated immune responses and ameliorated acute liver injury in mice. Am. J. Transplant. 23, 1832–1844 (2023).
Gao, Y. et al. Distinct gene expression and epigenetic signatures in hepatocyte-like cells produced by different strategies from the same donor. Stem Cell Rep. 9, 1813–1824 (2017).
Akbari, S. et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Rep., 13, 627–641 (2019).
Feng, S. et al. Large-scale generation of functional and transplantable hepatocytes and cholangiocytes from human endoderm stem cells. Cell Rep. 33, 108455 (2020).
Ma, C. et al. Single cell Raman spectroscopy to identify different stages of proliferating human hepatocytes for cell therapy. Stem Cell Res. Ther. 12, 555 (2021).
Qiao, S. et al. Functional proliferating human hepatocytes: in vitro hepatocyte model for drug metabolism, excretion, and toxicity. Drug Metab. Dispos. 49, 305–313 (2021).
Fennema, E., Rivron, N., Rouwkema, J., van Blitterswijk, C. & de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 31, 108–115 (2013).
Marsee, A. et al. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832 (2021).
Yamaguchi, T. et al. Generation of functional human hepatocytes in vitro: current status and future prospects. Inflamm. Regen. 39, 13 (2019).
Meng, X., Liu, A., Phangthavong, O. & Sun, Y. A novel strategy for treating acute liver failure: encapsulated proliferating human hepatocyte organoids. Biomater. Transl. 5, 444–446 (2024).
Yu, L. et al. Manganese is a potent inducer of lysosomal activity that inhibits de novo HBV infection. PLoS Pathog. 21, e1012800 (2025).
Lee, S. M., Schelcher, C., Demmel, M., Hauner, M. & Thasler, W. E. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. J. Vis. Exp. https://doi.org/10.3791/50615 (2013).
Knobeloch, D. et al. Human hepatocytes: isolation, culture, and quality procedures. Methods Mol. Biol. 806, 99–120 (2012).
Broutier, L. et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 11, 1724–1743 (2016).
Sun, L. et al. Modelling liver cancer initiation with organoids derived from directly reprogrammed human hepatocytes. Nat. Cell. Biol. 21, 1015–1026 (2019).
Ogawa, S. et al. Three-dimensional culture and cAMP signaling promote the maturation of human pluripotent stem cell-derived hepatocytes. Development 140, 3285–3296 (2013).
Azuma, H. et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat. Biotechnol. 25, 903–910 (2007).
Acknowledgements
We thank the core facilities for molecular biology, cell biology and animal care at the Center for Excellence in Molecular Cell Science (CEMCS) (Shanghai Institute of Biochemistry and Cell Biology, China). This project was supported by the National Natural Science Foundation of China (NSFC) (grant nos. 92168202, 32370793 and 32221002), the Shanghai Science and Technology Committee (grant no. 22JC1403001), the Shanghai Municipal Science and Technology Major Project and the National Center of Technology Innovation for Biopharmaceuticals (grant no. NCTIB2023XB01019). The schematic view was adapted from BioRender.com.
Author information
Authors and Affiliations
Contributions
The data in the paper are derived from independent experiments conducted by multiple individuals (K.Z., X.Y., S.L., Y.S. and C.W.). K.Z. performed most of the experiments; X.Y. and C.W. performed PHHs and 3D-cultured ProliHHs experiments; S.L. and Y.S. assisted with the cell culture and the manuscript preparation; J.C. performed the HTx; K.Z., X.Y. and L.H. analyzed the data and wrote the manuscript and L.H. supervised and coordinated the project.
Corresponding authors
Ethics declarations
Competing interests
L.H., L.Z. and K.Z. declare financial interests via patents filed by Center for Excellence in Molecular Cell Science on the production of ProliHHs.
Peer review
Peer review information
Nature Protocols thanks Dongho Choi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key references
Zhang, K. et al. Cell Stem Cell 23, 806–819 e804 (2018): https://doi.org/10.1016/j.stem.2018.10.018
Wang, C. et al. Hepatology 76, 1690–1705 (2022): https://doi.org/10.1002/hep.32436
Zhang, K. et al. Cell Prolif. 56, e13467, (2023): https://doi.org/10.1111/cpr.13467
Yuan, X. et al. Cell Stem Cell 31, 484–498 e485 (2024): https://doi.org/10.1016/j.stem.2024.02.005
Zhang, K. et al. Cell Stem Cell 31, 1187–1202 e8 (2024): https://doi.org/10.1016/j.stem.2024.04.022
Extended data
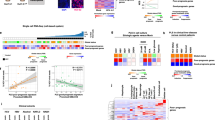
Extended Data Fig. 1 ProliHHs culture in modified HM.
a. Time-lapse images of PHHs (healthy donor: MRW) cultivated in HM and modified HM. b. Relative cell proliferation (fold change) of ProliHHs (P1) in HM and modified HM (n = 3). The data are shown as mean ± SD. ****p < 0.0001; Student’s t test. c. Expression of hepatocyte genes and progenitor-associated genes determined by qPCR in HM and modified HM. Data are normalized to PHH (n = 3). The data were shown as the mean ± SD. d. Representative cell images of cultured hepatocytes (donor: MRW). e. Growth curves of cultured PHHs from healthy donor (LTV and MRW) were analyzed at indicated passages. f. Pearson correlation coefficient-based heat map analysis was performed to compare global gene expression profiles in ProliHHs (P2, P4, P6 and P8 under hypoxia), PHH and hiPSC-derived liver progenitor-like cells (LPCs). The RNA-seq data have been published previously and the accession number of the RNA-seq data in the GEO database is GSE112866. g. Representative images of fibroblast-like ProliHHs.
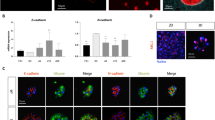
Extended Data Fig. 2 Functional identification of Liver Organoid.
a. Expression of hepatocyte genes was determined by qPCR after ProliHHs maturation induction. Data are normalized to freshly thawed PHH (n =5). The data were shown as the mean ± SD. b. Co-immunofluorescent staining of CYP1A2 and CYP3A4 in ProliHH-derived liver organoids. Scale bars, 100 mm. c. Co-immunofluorescent staining of E-Cadherin and multidrug resistance-associated protein 2 in ProliHH-derived liver organoids. Scale bars, 100 mm. d. Immunofluorescent staining of Ki67 in ProliHH-derived liver organoids. Scale bars, 100 mm. e. Expression of progenitor-associated genes was determined by qPCR after ProliHHs maturation induction (n = 5). Data are normalized to freshly thawed PHH and the data were shown as the mean ± SD. f. Immunofluorescent staining of EPCAM in ProliHH-derived liver organoids. Scale bars, 100 mm. g. Expression of AFP was determined by qPCR after ProliHHs maturation induction (n = 3), the data were shown as the mean ± SD. Data provide the amplification cycles of the AFP gene, using PHH and HepG2 cells as negative and positive controls for AFP expression, respectively. h. Representative time-lapse images of ProliHH-derived liver organoids from a healthy donor demonstrating the uptake of pHrodo™ Green-LDL.
Extended Data Fig. 3 ProliHH cryopreservation and resuscitation.
a. Representative images of Trypan Blue staining and live/dead staining of resuscitated ProliHH after cryopreservation b. The percentage of live cells of resuscitated ProliHH as determined by Trypan Blue exclusion assay (n = 3), the data were shown as the mean ± SD. c. Time-lapse images of resuscitated ProliHH cultivation. d. Relative cell proliferation (fold change) of ProliHHs in normal (n = 3) and resuscitated ProliHH (n = 5), the data were shown as the mean ± SD. e. Expression of hepatocyte genes and progenitor-associated genes in normal and resuscitated ProliHH cells was determined by qPCR (n = 4), the data were shown as the mean ± SD.
Supplementary information
Supplementary Code 1
The code for the Pearson correlation coefficient.
Source data
Source Data Fig. 3
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 5
Statistical source data
Source Data Extended Data Fig. 1
Statistical source data
Source Data Extended Data Fig. 2
Statistical source data
Source Data Extended Data Fig. 3
Statistical source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, K., Yuan, X., Lu, S. et al. Expansion of human hepatocytes and their application in three-dimensional culture and genetic manipulation. Nat Protoc (2025). https://doi.org/10.1038/s41596-025-01211-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41596-025-01211-2