Abstract
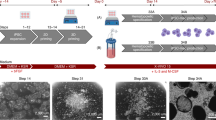
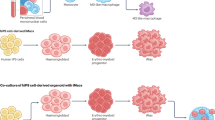
Macrophages are crucial in immune responses, tissue repair and homeostasis, making them prime candidates for translational applications. Induced pluripotent stem cell (iPS cell)-derived macrophages hold considerable promise for regenerative medicine, cancer therapy, inflammatory disease treatment and in vitro bioassays. However, cost-effective, standardized intermediate-scale bioreactor systems tailored for early-stage research and drug discovery in academia remain limited. Here, we present an extension of our previously published protocol that is feeder free, semi-defined and user friendly, enabling the standardized production of iPS cell-derived macrophages in an intermediate (10–50 mL)-scale benchtop bioreactor. This Protocol can be implemented by users with basic iPS cell culture experience without requiring advanced bioprocessing expertise. This method consists of two primary endpoints: the generation of mesoderm-primed aggregates with hematopoietic potential, termed hemanoids, and the standardized production of iPS cell-derived macrophages that are ready for downstream applications. This Protocol enables continuous macrophage generation in long-term cultures, with a minimum of five consecutive collections, yielding an average of 2–3 × 107 cells per collection per vessel. Four vessels operate independently, each with a maximum culture volume of up to 50 mL, while critical process parameters (CO2, temperature and pH) are monitored. This semi-automated platform and in-process monitoring improve process control, leading to higher yields, reproducibility and cell quality compared with other systems. The simplified process spans 24 d, starting from single-cell iPS cells to ready-to-use macrophages. By bridging the gap between small- and large-scale systems, this approach provides scalable, standardized manufacturing of iPS cell-derived macrophages, making it a valuable tool for academics focused on human immune cells such as macrophages.
Key points
-
This Protocol Extension details the standardized production of iPS cell-derived macrophages in an intermediate-scale benchtop bioreactor. This method is divided into two stages: the generation of mesoderm-primed aggregates with hematopoietic potential, termed hemanoids, and the standardized production of iPS cell-derived macrophages that are ready for downstream applications.
-
The use of standardized intermediate-scale bioreactor systems is tailored for early-stage research and drug discovery in academic and industrial settings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
We declare that the data supporting the findings of this study are available within the supporting Protocol13 and its Supplementary information. The scRNA sequencing datasets are deposited in the NCBI GEO repository under accession number GSE268458. Should any raw data files be needed in another format, they are available from the corresponding author upon reasonable request.
References
Ng, E. S. et al. Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells. Nat. Biotechnol. 43, 1274–1287 (2024).
Frenz-Wiessner, S. et al. Generation of complex bone marrow organoids from human induced pluripotent stem cells. Nat. Methods 21, 868–881 (2024).
Leung, C. M. et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2, 33 (2022).
Rowe, R. G. & Daley, G. Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 20, 377–388 (2019).
Kyrysyuk, O. & Wucherpfennig, K. W. Designing cancer immunotherapies that engage T cells and NK cells. Annu. Rev. Immunol. 41, 17–38 (2023).
Park, M. D., Silvin, A., Ginhoux, F. & Merad, M. Macrophages in health and disease. Cell 185, 4259–4279 (2022).
Alsinet, C. et al. Robust temporal map of human in vitro myelopoiesis using single-cell genomics. Nat. Commun. 13, 2885 (2022).
Lee, B. et al. Cell culture process scale-up challenges for commercial-scale manufacturing of allogeneic pluripotent stem cell products. Bioengineering 9, 92 (2022).
Godoy, P. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87, 1315–1530 (2013).
Ackermann, M. et al. Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Nat. Commun. 9, 5088 (2018).
Ackermann, M. et al. Continuous human iPSC-macrophage mass production by suspension culture in stirred tank bioreactors. Nat. Protoc. 17, 513–539 (2022).
Abdin, S. M. et al. Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia. J. Immunother. Cancer 11, e007705 (2023).
Ackermann, M. et al. Standardized generation of human iPSC-derived hematopoietic organoids and macrophages utilizing a benchtop bioreactor platform under fully defined conditions. Stem Cell Res. Ther. 15, 171 (2024).
Lachmann, N. et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 4, 282–296 (2015).
Karlsson, K. R. et al. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp. Hematol. 36, 1167–1175 (2008).
Borys, B. S. et al. Optimized serial expansion of human induced pluripotent stem cells using low-density inoculation to generate clinically relevant quantities in vertical-wheel bioreactors. Stem Cells Transl. Med. 9, 1036–1052 (2020).
Cuesta-Gomez, N. et al. Suspension culture improves iPSC expansion and pluripotency phenotype. Stem Cell Res. Ther. 14, 154 (2023).
Kwok, C. K. et al. Scalable expansion of iPSC and their derivatives across multiple lineages. Reprod. Toxicol. 112, 23–35 (2022).
Abdin, S. M. et al. Sensor macrophages derived from human induced pluripotent stem cells to assess pyrogenic contaminations in parenteral drugs. Biofabrication 16, 035017 (2024).
Pouyanfard, S. et al. Human induced pluripotent stem cell-derived macrophages ameliorate liver fibrosis. Stem Cells 39, 1701–1717 (2021).
Nenasheva, T. et al. Macrophages derived from human induced pluripotent stem cells are low-activated “naive-like” cells capable of restricting mycobacteria growth. Front. Immunol. 11, 1016 (2020).
Schinke, M. Polarization of human iPSC-derived macrophages directs their immunological response to secondary pro-inflammatory stimuli. J. Immunol. Regen. Med. 17, 100061 (2022).
Cao, X. et al. Differentiation and functional comparison of monocytes and macrophages from hiPSCs with peripheral blood derivatives. Stem Cell Rep. 12, 1282–1297 (2019).
Monkley, S. et al. Optimised generation of iPSC-derived macrophages and dendritic cells that are functionally and transcriptionally similar to their primary counterparts. PLoS One 15, e0243807 (2020).
Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol. 23, 563–579 (2023).
Kulle, A., Thanabalasuriar, A., Cohen, T. S. & Szydlowska, M. Resident macrophages of the lung and liver: the guardians of our tissues. Front. Immunol. 13, 1029085 (2022).
Gomez Perdiguero, E. et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518, 547–551 (2015).
Williamson, A. E. et al. Discovery of an embryonically derived bipotent population of endothelial-macrophage progenitor cells in postnatal aorta. Nat. Commun. 15, 7097 (2024).
Hoeffel, G. et al. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity 42, 665–678 (2015).
Kent, G. M. et al. Human liver sinusoidal endothelial cells support the development of functional human pluripotent stem cell-derived Kupffer cells. Cell Rep. 43, 114629 (2024).
Buchrieser, J., James, W. & Moore, M. D. Human induced pluripotent stem cell-derived macrophages share ontogeny with MYB-independent tissue-resident macrophages. Stem Cell Rep. 8, 334–345 (2017).
Lock, R. I. et al. Macrophages enhance contractile force in iPSC-derived human engineered cardiac tissue. Cell Rep. 43, 114302 (2024).
Landau, S. et al. Primitive macrophages enable long-term vascularization of human heart-on-a-chip platforms. Cell Stem Cell 31, 1222–1238.e1210 (2024).
Long, C. et al. Effects of macrophages on the proliferation and cardiac differentiation of human induced pluripotent stem cells. Cell Commun. Signal. 20, 108 (2022).
Qi, L. et al. Human iPSC-derived proinflammatory macrophages cause insulin resistance in an isogenic white adipose tissue microphysiological system. Small 19, e2203725 (2023).
Park, D. S. et al. iPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature 623, 397–405 (2023).
Zhang, W. et al. Microglia-containing human brain organoids for the study of brain development and pathology. Mol. Psychiatry 28, 96–107 (2023).
Schafer, S. T. et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell 186, 2111–2126.e2120 (2023).
Maxwell, K. G. & Millman, J. R. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med. 2, 100238 (2021).
Cheng, W., Zeng, Y. & Wang, D. Stem cell-based therapy for pulmonary fibrosis. Stem Cell Res. Ther. 13, 492 (2022).
Gazdhar, A. et al. The secretome of induced pluripotent stem cells reduces lung fibrosis in part by hepatocyte growth factor. Stem Cell Res. Ther. 5, 123 (2014).
Arias, A. A. et al. Tuberculosis in otherwise healthy adults with inherited TNF deficiency. Nature 633, 417–425 (2024).
Neehus, A. L. et al. Human inherited CCR2 deficiency underlies progressive polycystic lung disease. Cell 187, 390–408.e323 (2024).
Rosain, J. et al. Human IRF1 governs macrophagic IFN-gamma immunity to mycobacteria. Cell 186, 621–645.e633 (2023).
Forde, A. J. et al. Metabolic rewiring tunes dermal macrophages in staphylococcal skin infection. Sci. Immunol. 8, eadg3517 (2023).
Hartung, T. Pyrogen testing revisited on occasion of the 25th anniversary of the whole blood monocyte activation test. ALTEX 38, 3–19 (2021).
Senju, S. et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 18, 874–883 (2011).
Kambal, A. et al. Generation of HIV-1 resistant and functional macrophages from hematopoietic stem cell-derived induced pluripotent stem cells. Mol. Ther. 19, 584–593 (2011).
Choi, K. D. et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 27, 559–567 (2009).
Bitzer, S. et al. Application of human iPSC-derived macrophages in a miniaturized high-content-imaging-based efferocytosis assay. SLAS Discov. 28, 149–162 (2023).
van Wilgenburg, B., Browne, C., Vowles, J. & Cowley, S. A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One 8, e71098 (2013).
Gutbier, S. et al. Large-scale production of human iPSC-derived macrophages for drug screening. Int. J. Mol. Sci. 21, 4808 (2020).
Toda, S. et al. Characteristics of macrophage aggregates prepared by rotation culture and their response to polymeric materials. J. Artif. Organs 27, 410–418 (2024).
Fridley, K. M., Kinney, M. A. & McDevitt, T. C. Hydrodynamic modulation of pluripotent stem cells. Stem Cell Res. Ther. 3, 45 (2012).
Klepikova, A. et al. iPSC-derived macrophages: the differentiation protocol affects cell immune characteristics and differentiation trajectories. Int. J. Mol. Sci. 23, 16087 (2022).
Scott, C. L. et al. The transcription factor ZEB2 is required to maintain the tissue-specific identities of macrophages. Immunity 49, 312–325.e35 (2018).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy (EXC 2155; RESIST; project no. 390874280 and DFG support LA 3680/9-1 and 10-1) (N.L.); the European Research Council (ERC) under the European Union (EU)’s Horizon 2020 research and innovation program (grant agreement 852178); and the EU (grant agreements 101100859 and 101158172) (N.L.). Additional funding was provided by the German Center of Lung Research (DZL) and the Federal Ministry of Research, Technology and Space (BMFTR, SMARTibone project). This work was supported by the Fraunhofer Internal Programs under grant no. attract 40-01696. The work also received funding by the SPARK BIH (01BIHTP2521B) funding scheme within the National Strategy for Gene- and Cell-based Therapies and by the program ‘zukunft.niedersachsen’ of Lower Saxony, Germany for the project ‘MacroAB-Delivery’. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the EU or the ERC. Neither the EU nor the granting authority can be held responsible for them. The project was additionally supported by zukunft.niedersachsen (Federal State of Lower Saxony), R2N.Micro-Replace-Systems. The EBiSC Bank acknowledges Bioneer A/S as the source of the human induced pluripotent cell line BIONi010-C, which was generated with support from the EBiSC project. The EBiSC has received support from the Innovative Medicines Initiative (IMI) Joint Undertaking (JU) under grant agreement no. 115582 and from the IMI-2 JU under grant agreement no. 821362, resources of which are composed of financial contributions from the European Union’s Seventh Framework Programme (FP7/2007–2013), the European Union’s Horizon 2020 research and innovation programme, and EFPIA.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.S. and N.L. Methodology: F.S. and N.L. Investigation: F.S., E.V.M., M.K., I.G., E.N., M.S., A.H.H.N., M.J., D.B.Q. and N.L. Writing—original draft: F.S., E.M.V., E.N., M.S., D.B.Q. and N.L. Writing—review and editing: F.S., E.M.V., M.K., I.G., E.N., M.S., A.H.H.N., M.J., D.B.Q. and N.L. Visualization: F.S., A.H.H.N. and N.L. Project administration: F.S. and N.L. Funding acquisition: N.L.
Corresponding author
Ethics declarations
Competing interests
N.L. is an author of the patent application (European patent application number PCT/EP2018/061574) entitled ‘Stem-cell derived myeloid cells, generation and use thereof’. The priority date of the application is 4 May 2017. N.L. is an author on the patent application (European patent application number PCT/EP2021/083371) entitled ‘Application of stem cell derived monocytes in a monocyte activation test for the assessment of pyrogenicity and inflammatory potential’. The priority date of the application is 29 November 2021. N.L. receives research funding from Novo Nordisk and holds a consultancy agreement with Evotec (scope outside the manuscript). All other authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Megumu Saito and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Key references
Ackermann, M. et al. Stem Cell Res. Ther. 15, 171 (2024): https://doi.org/10.1186/s13287-024-03785-2
Abdin, S. M. et al. J. Immunother. Cancer 11, e007705 (2023): https://doi.org/10.1136/jitc-2023-007705
Ackermann, M. et al. Nat. Protoc. 17, 513–539 (2022): https://doi.org/10.1038/s41596-021-00654-7
Ackermann, M. et al. Nat. Commun. 9, 5088 (2018): https://doi.org/10.1038/s41467-018-07570-7
This Protocol is an extension to Nat. Protoc. 17, 513–539 (2022): https://doi.org/10.1038/s41596-021-00654-7
Extended data
Extended Data Fig. 1 Bioreactor process monitoring and assessment during mesoderm priming and macrophage production.
a) Representative graphs of bioreactor process parameters (CO2, pH, and temperature) during Mesoderm priming. b) Representative graphs of bioreactor process parameters (CO2, pH, and Temperature) during macrophage production. c) IL-6 secretion in naïve macrophages (iPSC lines: 1, 2, and 3). d) Phagocytosis of macrophages across different harvests 1b, 2, 3, 4, 5, 6, 7 (89.7 ± 7.3%, 84.8 ± 6.68%, 80.8 ± 6.03%, 81.2 ± 15.6%, 92.4 ± 7.97%, 95.2 ± 5.09%, SD +/- mean) (iPSC lines 1, 2, 3, 4, and 5, mean ± SD, n=10).
Extended Data Fig. 2 Morphological and cytological characterization of bioreactor generated iPSC-derived macrophages across multiple harvests and lines.
Representative of image of iPSC-derived macrophages produced in the benchtop bioreactor for iPSC 1, 2, 3, 4, and 5 at harvests 1a/1b, 3, 4 and 6. Top: brightfield, (magnification 10x, scale bare 100 µm) bottom: cytospin (magnification 20x) stained with May-Grünwald-Giemsa. Cytospin images were taken using Keyence BZ-X800 (Keyence, Japan) with 20x plan Achromat objective.
Extended Data Fig. 3 Single-cell transcriptomic profiling of iPSC-derived macrophages produced in bioreactors from various iPSC lines.
a) UMAP representation of dataset13 from three independent iMac harvests generated from CERO benchtop bioreactor, the cells are grouped by cell line. (iPSC lines 1, 2, and 3 were used). b) UMAP representation split by cell line and grouped by cluster identity. c) UMAP representation with normalized expression of hematopoietic/myeloid lineage marker genes (PTPRC, ITGAM, CD33, SPI1). d) Dot plot displaying the normalized expression of myeloid progenitor, macrophage, mast cell (MC), granulocyte (Gran), lymphoid lineage and fibroblast marker genes grouped by cell line (iPSC lines: 1-3). Panels a and b adapted from ref. 13, CC BY 4.0.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saleh, F., Valdivia Malqui, E.E., Gensch, I. et al. Harnessing intermediate-scale bioreactors for next-generation macrophage production and application. Nat Protoc (2026). https://doi.org/10.1038/s41596-025-01313-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41596-025-01313-x