Abstract
Fritillaria cirrhosa, an endangered medicinal plant in the Qinghai-Tibet Plateau, is facing resource scarcity. Artificial cultivation has been employed to address this issue, but problems related to continuous cultivation hinder successful transplantation. Imbalanced microbial communities are considered a potential cause, yet the overall changes in the microbial community under continuous cropping systems remain poorly understood. Here, we investigated the effects of varying durations of continuous cropping on the bacterial and fungal communities, as well as enzymatic activities, in the rhizospheric soil of F. cirrhosa. Our findings revealed that continuous cropping of F. cirrhosa resulted in soil acidification, nutrient imbalances, and increased enzyme activity. Specifically, after 10 years of continuous cropping, there was a notable shift in the abundance and diversity (e.g., Chao1 index) of soil bacteria and fungi. Moreover, microbial composition analyses revealed a significant accumulation of harmful microorganisms associated with soil-borne diseases (e.g., Luteimonas, Parastagonospora, Pseudogymnoascus) in successively cropped soils, in contrast to the significant reduction of beneficial microorganisms (e.g., Sphingomonas, Lysobacter, Cladosporium) that promote plant growth and development and protect against diseases such as Fusarium sp.These changes led to decreased connectivity and stability within the soil microbial community. Structural equation modeling and redundancy analysis revealed that alkaline hydrolytic nitrogen and available phosphorus directly influenced soil pH, which was identified as the primary driver of soil microbial community changes and subsequently contributed to soil health deterioration. Overall, our results highlight that soil acidification and imbalanced rhizosphere microbial communities are the primary challenges associated with continuous cropping of F. cirrhosa. These findings establish a theoretical foundation for standardized cultivation practices of F. cirrhosa and the bioremediation of continuously cultivated soils.
Similar content being viewed by others
Fritillaria cirrhosa, bulbous medicinal plant from the Liliaceae family, flourishes in the high-altitude meadows, thickets, and grasslands of the Himalayan region, ranging from 3200 to 4600 m above sea level1. The dried bulbs of F. cirrhosa hold a rich tradition of use as a valuable expectorant, antitussive, and asthma-relieving herb in China and Southeast Asian countries, owing to their notable therapeutic efficacy, low toxicity, and minimal adverse effects2,3,4. However, due to its specific growth requirements, extended growth cycle, low reproductive rate, and overexploitation, F. cirrhosa has become an endangered species, leading to its elevation from a Grade III to a Grade II protected herb in China5,6,7. The scarcity of genuine wild F. cirrhosa has resulted in a significant price surge, escalating from US$60 per kg in 2002 to US$540 per kg in 2022, thus posing a substantial challenge to its clinical utilization8, 9. The very high economic value and high demand of F. cirrhosa has created a strong incentive for continuous cropping, especially with its limited suitable cultivation areas9,10,11. However, the yield and quality of F. cirrhosa from continuous field cropping gradually declined, especially when it was planted continuously for more than 5 years, its yield was significantly reduced by more than 80%. Therefore, understanding the underlying mechanisms responsible for continuous cropping is crucial for developing effective strategies to optimize cultivation practices and ensure the sustainable production of high-quality F. cirrhosa.
Soil microorganisms are crucial factors in soil fertility, soil quality, and productivity, which play an important role in the ecosystem10,11,12. Several studies have demonstrated that the variations in rhizospheric soil microorganisms will impact the uptake and transformation of soil minerals, which in consequence affects the growth, reproduction, accumulation of hypometabolism and physiological status of medicinal plants13,14,15. In particular, continuous monoculture cultivation has been proven to alter the rhizospheric fungal composition and diversity of in the soil16, 17. For instance, Yasir et al. observed that continuous crop cultivation in tea plantations has not only reduced some beneficial fungal species, such as Mortierella elongatula and Mortierella alpina, but also enhanced potentially pathogenic fungal species over time, such as Fusarium oxysporum, Fusarium solani and Microidum phyllanthi18. Li et al. found that long-term continuous croppin peanut altered soil chemical properties and raised the relative abundance of the potential pathogenic fungal genera, thus increasing the underlying risk of soil-borne diseases and diminishing its yield and quality19. In addition, multiple studies have indicated that the improper usage of chemical fertilizers leads to changes in the physicochemical properties of the soil and thus affects the composition of the fungal community20,21,22. To date, it remains unknown how long-term continuous cropping affects the rhizospheric soil fungal and bacterial community (diversity and structure) under the F. cirrhosa system.
A microbial community consists not only of the numbers and composition of its members but also of the ecological relationships among them23. To study complex microbial communities, co-occurrence network analysis presents a new and innovative approach24,25,26. This type of analysis allows for the prediction of potential interactions between microbial species, including competition, facilitation, and inhibition26. Moreover, it enables the examination of how different taxonomic groups respond to agricultural practices and the identification of keystone species that play a crucial role in agroecosystem functioning. The complexity of the microbial network serves as an important indicator of ecosystem stability and function. However, it remains unclear how the connectivity of the soil bacterial community, as represented by the network, changes when continuously cultivating the F. cirrhosa system.
This study aimed to investigate the changes in the number, diversity, composition, and network complexity of rhizospheric bacteria and fungi in response to continuous cropping of F. cirrhosa. Additionally, we explored the potential connections between alterations in the bacterial and fungal communities and soil properties. In accordance with the hypotheses formulated for this study, it was postulated that (1) continuous cropping would exert a deleterious effect on soil microbial populations, diversity, and microbial network complexity, and (2) continuous cropping would also result in alterations to soil physicochemical properties and soil enzyme activities. Furthermore, we aimed to compare the number, diversity, and co-occurrence patterns of bacteria and fungi in soils with different durations of continuous cropping and examine the correlations among soil properties, bacterial communities, and soil quality. The findings of this study may offer valuable agricultural strategies to mitigate the detrimental impacts of long-term monoculture.
Materials and methods
Experimental site and soil sampling
The research site is situated in Tu Autonomous County of Huzhu (36°59′E, 101°59′N; at an elevation of 3050 m above sea level), which is part of Haidong City in the Qinghai Province of China. The climate type is continental cold temperate zone, characterized by a long cold season and a short warm period, with an annual average temperature of 5 ℃and an annual average rainfall of 520.3 mm. Soil samples were collected from crop fields of F. cirrhosa plants with a cultivation history of more than 10 years. Each treatment group had three biological replicates to ensure data reliability and reproducibility. The initial characterisation of the soil is as follows: organic carbon (30.39 g/kg), total nitrogen (2.88 g/kg), effective phosphorus (83.11 mg/kg), total phosphorus (1.38 g/kg), total potassium (21.66 g/kg), alkaline nitrogen decomposition (171.75 mg/kg), quick-acting potassium (234.00 mg/kg), and moisture content (28.78%). The climate is cold and continental with an average annual temperature of 5.8 ℃. There are on average 2581.7 annual sunshine hours. The frost-free period lasts for 114 days, and the mean annual precipitation is 477.4 mm, the annual evaporation 1198.3 mm, and the annual relative air humidity 63%. The base fertiliser is mostly farmyard manure, 1500 kg of compost, stable manure, 50 kg of calcium superphosphate, 100 kg of oil cake or 4000 kg of rotted stable manure per acre, 4000 kg of rotted stable manure per acre, and the compost is spread evenly on the beds after it has rotted. The herbal plants were cultivated using seeds, and mature bulbs were harvested after at least four years. Soil samples were collected on April 25, 2021, when the soil had completely thawed after the long winter. The soil samples included three-year-old (Y3), five-year-old (Y5), seven-year-old (Y7), and ten-year-old (Y10) soils, as well as a blank soil (CK) that had never been cultivated with F. cirrhosa. Soil samples were collected from each treatment F. cirrhosa planting field. The planting fields were not more than 100 m apart and were collected at a depth of 0–20 cm. Three replicate soil samples were collected from each treatment group and the three replicate soil samples from each treatment group were thoroughly mixed to form a composite sample, which was then transported back to the laboratory within three hours. Subsequently, the soil composite samples from each treatment group were divided into two portions. To evaluate its physicochemical characteristics, one part was allowed to air dry, and the other was kept at -80 °C to compare the microbial populations in the soil.
Soil analysis of physicochemical properties
The soil chemical properties were determined following the standard protocols outlined in the reference book “Soil and Agricultural Chemistry Analysis” and the “National Technical Specification for Soil Testing and Formula Fertilization.” Each treatment was conducted with three biological replicates to ensure the accuracy and repeatability of the results. In particular, Alkaline Hazardous Nitrogen (AHN) levels were determined using the alkaline hydrolysis diffusion method. Available phosphorus (AP) and available potassium (AK) were extracted with 1 mol/L HNO3 and 2% (m/V) citric acid solution, respectively, and subsequently quantified using flame photometry (model 410 photometer; Corning, Halstead, UK) and the colorimetric method. Total nitrogen (TN) was determined using an AA3 continuous flow analyzer following Kjeldahl digestion. Total phosphorus (TP) was analyzed using a spectrophotometer based on the NaOH melting method–molybdenum antimony resistance colorimetry. Total potassium (TK) was measured using an FP6410 multi-element flame photometer. Organic matter (OM) was assayed through potassium dichromate oxidation-outer heating. Lastly, soil pH was measured using a pH analyzer.
Enzymatic activity within different groups was assessed using specific assay kits. The researchers followed the instructions provided with the assay kits to analyze the sucrase activities (SA), urease activity (UA), catalase activity (CA), acid phosphatase activity (ACA), and alkaline phosphatase activity (ALA). The assay kits used in the study were obtained from Suzhou Grace Biotechnology Co., Ltd (Suzhou, China). The soil health index was calculated with reference to Lu’s methodology27.
DNA extraction and PCR amplification
Total DNA was extracted from a 0.5 g soil sample using the FastDNA® Spin Kit for Soil (MP Biomedicals, Inc., Santa Ana, CA, USA). The primers ITS1F (5-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS4-2409R (5′-TCCTCCGCTTATTGATATGC-3′) were used to amplify ITS2 sequences of fungi. The 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGACTTAACCCCAATCGC-3′) primers were used to target the V3-V4 region of the 16 S rRNA gene of bacteria. PCR was performed with an initial denaturation of 5 min at 94 °C, followed by 28 cycles of denaturation at 94 ℃ for 30 s, annealing at 55 ℃ for 30 s, and extension at 72 ℃ for 60 s. The amplicons were sequenced using the Illumina NovaSeq6000 platform (Illumina, USA). The raw sequences have been submitted to the Sequence Read Archive of the National Center for Biotechnology Information.
Co-occurrence network construction
In order to study the symbiotic networks of fungi and bacteria in the successional soils, we analysed them using the R package “ggClusterNet” and calculated their topological properties, including node degree, clustering coefficients and median values28. These results were then visualised using Gephi (0.10) software to illustrate the interrelationships between microbial communities in the successional soils. Soil quality was calculated based on the previous method29.
Bioinformatics analysis
In this study, the original OTU sequences were quality filtered by QIIME software to obtain representative sequences of operational classification units (OTUs). First, low-quality and ambiguous OTU sequences were removed, and then non-repetitive sequences (excluding single sequences) were clustered at 97% similarity. During clustering, chimeras are eliminated. Next, the optimized sequences were mapped to the OTU representative sequences and selected for matching with sequences with more than 97% similarity. To assess the diversity and richness among samples, we employed the Mothur software (v. 1.30.1) to analyze alpha diversity, including the Chao1 index. Additionally, we utilized the R package “ggplot2” to visualize the diversity indices. Furthermore, we conducted Mantel’s test and correlation analysis using the R package “ggcor” to explore the correlation between the samples. Furthermore, we conducted a comprehensive analysis of the relationships between microbial communities, soil enzymes, and bioactive compounds using several R packages. This included analysis of variance (ANOVA) using the R package “vegan” to examine the interactions between microbial communities and soil enzymes and bioactive compounds. Finally, in order to investigate the effects of continuous cropping on microbial diversity, soil physicochemical properties, and soil health, we employed the R package “lavaan” for structural equation modeling (SEM) analysis. SEM analysis facilitated the determination of causal relationships among the variables30, thereby enhancing our comprehension of the effects of continuous cropping on soil ecosystems.
Statistical analysis
In this study, data were processed, analyzed, and plotted using Excel 2017, IBM SPSS v.20.0, and GraphPad Prism 9 software. Excel 2017 was employed to organize and initially analyze the data in order to better understand the trends and distribution of the data. Next, IBM SPSS v.20.0 software was utilized for more in-depth analysis and processing of the data. In this case, one-way ANOVA was employed to identify significant differences (p < 0.05) between samples, thereby determining the impact of the distinct treatments on the outcomes of the experiment. The analytical mapping of the soil microbiome was conducted via a cloud-based platform (https://www.omicshare.com). An online microbiome analysis tool was employed for the rapid and accurate analysis of microbial community structure in soil samples. Principal Coordinates Analysis (PCoA) was utilized to classify the differences in microbial structure between various successional soil samples. Redundancy Analysis (RDA) was employed to determine the degree of correlation between soil physicochemical properties and microbial communities.
Results
Physicochemical properties of soil
The results demonstrated that, over the course of increasing cultivation years, there was a consistent upward trend in TN content. This was particularly evident after 10 years of continuous cultivation, where the TN content saw an 82.5% increase compared to the control soil (CK). In contrast, the TP content remained relatively stable across the five-, seven-, and ten-year-old soils, showing no significant differences (P > 0.05, Table 1). The TK content exhibited a similar stable trend, maintaining approximately 19 g/kg across soils of different cultivation years. However, the AN content began to show a substantial increase after five years of continuous cultivation. It is noteworthy that in the 10-year-old soil, the AN content reached a remarkable 144.52 ± 1.05 mg/kg, which was nearly double the 73.86 ± 1.43 mg/kg found in the CK soil. Furthermore, significant changes were observed in the levels of AP and AK. In comparison to the CK soil, the AP content demonstrated a two- to three-fold increase, with the highest concentration observed in the three-year-old soil at 122.83 ± 1.89 mg/kg. Similarly, the AK content displayed a marked increase, reaching up to 578.67 ± 8.33 mg/kg in soils after ten years of continuous cultivation, representing a 250.8% increase compared to the CK soil. The organic matter content in soils across varied tillage years also demonstrated a notable upward trend. However, the pH level of the soil exhibited a significant downward trend.
Soil enzymatic activity
Enzyme activity in the soil increased as the number of years F. cirrhosa was planted increased. For example, the activities of major soil enzymes such as sucrase, urease, catalase, acid phosphatase and alkaline phosphatase were significantly higher in the Y10 treatment group compared to CK by 148.01, 93.95, 22.77, 339.86 and 64.08%, respectively(Fig S1). However, it is noteworthy that catalase activity in the soil of Y5 treatment group instead decreased by 9.74% compared to CK group. And there was no significant difference in urease activity in soil of Y3 and Y5 treatment groups compared to CK group.
Community and diversity composition of soil bacterial and fungal
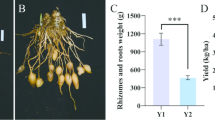
Figure 1a illustrates healthy F. cirrhosa plants. We employed the Chao 1 index to evaluate the microbial communities’ alpha diversity. Years of consistent F. cirrhosa planting led to a progressive increase in soil bacterial numbers and alpha diversity (Fig. 1b). Compared with the control CK, the Y10 treatment increased the Chao 1 index by 12.35%. Likewise, fungal diversity displayed an increasing trend over the same period (Fig. 1c), with the Y10 treatment showing a 21.97% increase in the Chao 1 index compared to the CK. Additionally, the beta-diversity analysis based unweighted unifrac Principal Coordinates Analysis (PCoA) showed identified distinct differences in microbial community composition and structures between the CK and continuous cropping soil samples. The first two principal coordinates, which accounted for 52.3 and 40.85% of the variance in bacterial and fungal communities, respectively, during continuous cropping, are visualized in Fig. 1d and e. Significant differences in bacterial and fungal communities were observed throughout the continuous cropping process. The non-parametric multivariate analysis of variance test further confirmed the significant separation of bacterial and fungal communities induced by continuous cropping (R2 = 0.84, P = 0.001; R2 = 0.90815, P = 0.001). These findings suggest significant alterations in microbial diversity and community composition resulting from continuous cropping.
Schematic diagram of F. cirrhosa (a) Chao1 index of bacteria, showing the diversity of bacterial communities across different years of continuous cropping(b). Chao1 index of fungi, showing the diversity of fungal communities across different years of continuous cropping (c). Principal Coordinate Analysis (PCoA) of bacterial communities based on weighted UniFrac distances, illustrating the differences in bacterial community composition among various continuous cropping years (d). Principal Coordinate Analysis (PCoA) of fungal communities based on weighted UniFrac distances, illustrating the differences in fungal community composition among various continuous cropping years (e).
Relative abundance of bacterial and fungal taxa
To determine the relative abundance of bacterial and fungal taxa, soil samples underwent high-throughput sequencing, resulting in operational taxonomic unit (OTU) sequences. These sequences were then clustered at a 97% similarity threshold, and chimeras were carefully removed. This process yielded 7649 representative bacterial OTU sequences and 3098 representative fungal OTU sequences. Specifically, the Y3, Y5, Y7, and Y10 treatments produced 1541, 1636, 1668, and 1675 bacterial OTUs, respectively. These bacterial OTUs were assigned to 69 phyla, 156 classes, 331 orders, 475 families, and 817 genera. The dominant phyla across all samples were Proteobacteria (26.63–41.08%), Bacteroidota (5.40–25.12%), and Acidobacteriota (4.06–16.55%). Moreover, regarding fungi, a total of 444, 720, 789, and 1200 fungal OTUs were obtained for Y3, Y5, Y7, and Y10, respectively. These fungal OTUs were assigned to 15 phyla, 60 classes, 137 orders, 292 families, and 570 genera. The dominant phyla across all samples were Ascomycota (40.65–68.97%), Basidiomycota (1.84–10.36%), and Mortierellomycota (2.07–12.53%).
At the Bacteria phylum level, the relative abundance of Proteobacteria showed a noticeable increase in all continuous soil samples, particularly in Y5, where its abundance increased by 51.9% (P ≤ 0.05) compared to the CK (Fig. 2a). On the contrary, the relative abundance of Bacteroidota in the continuous soil appeared to be dramatically reduced when compared with CK, especially in Y5 whose relative abundance was found to be as low as 5.12% (P ≤ 0.05). At the genus level, we observed a significant decrease of 59.8% (P ≤ 0.05) and 34.0% (P ≤ 0.05) in the relative abundance of Lysobacter and Sphingomonas, respectively, and a significant increase of 74.7% (P ≤ 0.05) in the relative abundance of Luteimonas(Fig. 2b).
In addition, both Ascomycota and Mortierellomycota showed a significant increase in relative abundance of 69.7% (P ≤ 0.05) and 180.5% (P ≤ 0.05) respectively, whereas Basidiomycota showed a significant decrease in relative abundance of 77.5% (P ≤ 0.05) in the Y10 treatment group compared to CK (Fig. 2c). At the genus level, the relative abundance of Mortierella and Parastagonospora increased significantly by 164.8% (P ≤ 0.05) and 106.7% (P ≤ 0.05) respectively, whereas the relative abundance of Plectosphaerella decreased significantly by 99.45% (P ≤ 0.05) (Fig. 2d).
Distinct microbial taxa and associated functional potential during continuous cropping
LDA effect size analysis enables the identification of microbial taxa that significantly differ in abundance between different continuous cropping years, allowing for the discovery of potential biomarkers associated with specific cropping practices. (Figs S2, S3) shows the community composition difference of bacteria. The phylum Acidobacteriota together with its orders Vicinamibacterales and Pyrinomonadales were dominant in the Y3 treatment, while the the phylum Bacteroidota together with its order Sphingobacteriales was dominant in the Y10 treatment. The phylum Ascomycota together with its orders Helotiales, Thelebolales, Pezizales, Glomerellales and Hypocreales were dominant in the Y3 treatment, while the the phylum Ascomycota together with its orders Pleosporales, Sordariales, and Mortierellales were dominant in the Y10 treatment.
Bacterial and fungal network complexity and functions
As continuous cultivation had a significant impact on microbial community composition, we constructed further networks to explore the effect of continuous cultivation on microbial interactions (Fig. 3a and c). The main topological properties are illustrated in Fig. 3b and d. The number of nodes (n) and edges (L) in Y10 was lower than that in CK, indicating that continuous cropping decreased the fungal co-occurrence network complexity. The relative modularity index, which indicates the tendency of networks to naturally divide into clusters with similar characteristics (e.g., biological functions), exhibited a similar trend. After continuous cropping, the topological properties of networks in Y10 and Y5 were lower than those in CK, with fewer nodes and edges, a lower average degree as well as a lower relative modularity. Conversely, networks exhibiting greater topological properties are indicative of more complex and stable network structures. Conversely, the number of positive edges, which represent mutualistic relationships among microorganisms, was significantly reduced in Y5, Y7 and Y10.
Co-occurrence network analysis of (a) bacterial community and (b) main topo-logical properties in the F. cirrhosa soils with varying continuous cropping years. Co-occurrence network analysis of (c) fungal community and (d) main topological proper-ties in the F. cirrhosa soils with varying continuous cropping years. (Spearman’s |r| > 0.6 and P < 0.05).
With the increase in the number of years that F. cirrhosa has been planted, the microbial functional community in the soil has undergone a gradual change. In the predictive analysis of bacterial functions, membrane translocation emerged as a critical function in bacterial ecosystems. This function is crucial for soil nutrient cycling and transportation, as it involves the movement of molecules across bacterial cell membranes, facilitating essential processes such as nutrient uptake and waste removal. It was found that the membrane translocation function in the Y10 treatment group showed higher activity and efficiency compared with CK. In the predictive analysis of fungal functions, the saprotroph function is the first to be identified. This function plays a crucial role in the decomposition of soil organic matter and the cycling of nutrients. The results of the study indicate that the saprotroph function of the Y10 treatment group was significantly more effective than that of CK. This suggests that the number of microorganisms with nutrient conversion and metabolism functions in the soil increased as a consequence of the planting year of F. cirrhosa, thereby enhancing the decomposition rate of soil organic matter and the effectiveness of nutrients. Using PICRUSt2 for functional prediction analysis, we identified significant changes in functional groups that impact plant health and growth. The analysis revealed alterations in pathways related to nitrogen cycling, phosphorus solubilization, and plant hormone synthesis. Specifically, there was an increase in the abundance of genes related to nitrogen fixation and phosphorus solubilization in soils with longer durations of continuous cropping. This shift suggests a potential adaptation of the microbial community to the changing soil environment, which could have significant implications for plant nutrient uptake and overall soil health(Fig. 4).
Correlation between soil enzyme activity and microbial population
A Random Forest Analysis was conducted to identify the main environmental variables affecting soil microorganisms. The results showed that OM, TN, TP, AK contributed the most to explain the variation in bacterial abundance across years, with Chao1 recording the highest (92.69%), followed by UA (90.51%). TN, OM, AK are positively correlated with observed_species (significance coefficients 10.90714694, 6.184066921, 10.23795348, respectively). TN, AK significantly affected Chao1 (significance coefficients of 11.97892077, 11.04688709, respectively). Interestingly in fungi, OM has almost no effect on observed_species, but has a large and positive effect on ACE (Fig. 5a, b).
The contributions of environmental factors to bacterial (a) and fungal (b) richness were determined using a correlation analysis and random forest modeling. The circle sizes represent the variables’ importances, which were calculated as percentages of the increase in mean square error using a random forest model. The correlations among microbial communities were also evaluated. The Mantel test was used to determine the correlations between physicochemical properties and the bacterial and fungal communities (c). The following significance levels were used in this analysis: *P < 0.05, **P < 0.01, ***P < 0.001 sucrase activities (SA), urease activity (UA), catalase activity (CA), acid phosphatase activity (ACA), and alkaline phosphatase activity (ALA).
To evaluate the relationships between the physicochemical parameter matrices and the distance matrices of the bacterial and fungal communities, the Mantel test was utilized.The positive correlation of TN (r = 0.233, P = 0.031), AK (r = 0.224, P = 0.029), CA (r = 0.239, P = 0.038) with bacterial communities was more significant. CA (r = 0.4636, P = 0.001), AK (r = 0.4764, P = 0.001), AN (r = 0.4737, P = 0.001) were more closely and significantly positively correlated with fungal communities (Fig. 5c).
The influence of soil environmental factors on the structure of microbial communities and their metabolites is crucial for understanding ecosystem processes. RDA was conducted to determine the contributions of selected environmental variables to soil bacterial and fungal community structure. The findings demonstrate that these environmental factors explained 61.51% of the variation in the bacterial community and 47.06% in the fungal community. Specifically, soil pH (r^2 = 0.992, P = 0.001) and urease activity (UA) (r^2 = 0.892, P = 0.001) significantly influenced bacterial community composition, while pH (r^2 = 0.653, P = 0.002) and AP (r^2 = 0.731, P = 0.001) were significantly correlated with fungal community composition (Fig. 6a and b).
Ecological linkages between soil chemical properties and microbial diversity
SEM was utilized to elucidate the relationships among environmental factors and the diversity and structure of bacterial and fungal communities. It was found that OM was positively correlated with bacterial community diversity (r = 0.51, P ≤ 0.001) and fungal community diversity (r = 0.68, P ≤ 0.001). Soil AHN values were positively correlated with soil OM values (r = 1.04, P ≤ 0.001) and soil pH values (r = 0.70, P ≤ 0.001). Soil bacterial community diversity was negatively correlated with soil health (r = 0.67, P ≤ 0.001), while fungal community structure was also negatively correlated with soil health (r = 0.27, P ≤ 0.01). Interestingly, there was a negative correlation between soil microbial diversity and soil health with increasing years of cultivation (Fig. 7).
SEM describes the relationship between continuous crop barriers, soil physicochemical properties, and soil health, and the alpha and beta diversity of soil microbial communities. In addition, blue and red arrows indicate positive and negative effects, respectively. Significance of each predictor: *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01; ***, P ≤ 0.001.
Discussion
The increasing popularity of traditional Chinese medicine has garnered greater attention for F. cirrhosa. However, its unique growth requirements and extensive excavation have led to its classification as a second-class protected plant, underscoring the urgency of its cultivation. Furthermore, the absence of an established histoculture system has resulted in a continued reliance on traditional seed propagation in artificial cultivation. This necessitates the use of large seed quantities for new F. cirrhosa seedlings in practical production. Regrettably, F. cirrhosa requires a minimum of five years for seed maturation, and its mortality rate escalates annually after five years of cultivation, posing a significant challenge to its artificial conservation. Additionally, extended cultivation periods have been shown to markedly reduce its yield. Soil microbial communities are pivotal in this context. On one hand, inter-root microbes have the capacity to aid host plants in nutrient uptake and enhance their resilience to biotic and abiotic stresses31. On the other hand, specific harmful inter-root microorganisms can detrimentally affect plants, leading to decreased resistance and yield32, 33. Consequently, inter-root microorganisms play a critical role in the growth and development of host plants33. This study integrates soil health index indicators with microbiomics to explore the impact of varied cultivation periods on inter-root microorganisms. The ensuing findings are elaborated in the subsequent sections.
Effect of continuous tillage on soil physicochemical properties
Previous studies have demonstrated that continuous cropping can affect rhizosphere soil nutrients, leading to decreased nutrient uptake by plant roots34,35,36. In our observations, we consistently observed an increase in TN, AN, AP, AK, and organic matter content as the number of cultivation years increased, particularly noticeable after 10 years of continuous cultivation. These findings suggest that continuous cropping enhances the total nitrogen, phosphorus, and potassium content in the soil. Similar results have been reported in studies on maize, potato, and wheat37,38,39,40. The results of RDA and SEM analyses also indicate that nutrient enrichment in continuously cropped soils directly influences the composition of inter-root fungi and bacteria.Therefore, we propose that the imbalance in soil nutrient content, particularly the decrease in organic matter and available nitrogen, may represent a significant factor contributing to the challenges of continuous cultivation of F. cirrhosa. This imbalance can lead to reduced soil fertility and adverse effects on plant health, making continuous cultivation more difficult over time.
Soil pH plays a critical role in influencing the composition and structure of microbial communities and bacterial diversity41, 42. The findings of this study indicate a significant decrease in soil pH as the number of cultivation years increases. Numerous studies have shown that long-term management of fertilizers is associated with an accelerated rate of nitrification, leading to increased soil acidification43,44,45. Previous research has demonstrated that the extended use of inorganic fertilizers in field conditions is linked to soil acidification, which can contribute to the proliferation and spread of soil-borne pathogens46, 47. Additionally, with each subsequent planting, the capacity of rhizobial microorganisms to utilize potassium and phosphorus diminishes, ultimately resulting in soil acidification. These findings align with previous studies that have explored the limitations of continuous cultivation for medicinal plants like Salvia miltiorrhiza Bunge and Panax ginseng47, 48. It is worth noting that the soil samples used in this study were obtained from sites where F. cirrhosa had been cultivated under long-term fertilization practices. The incidence of F. cirrhosa disease in continuously planted soils was found to be as high as 90%. Therefore, prolonged field fertilization has been observed to enhance nitrification activity, leading to the release of protons and exacerbation of soil acidity. This may play a crucial role in the rapid spread of soil-borne diseases. There seems to be a close correlation between soil nitrification, soil acidification, and disease outbreaks in F. cirrhosa growing areas. Based on these findings, we hypothesize that soil acidification is likely to be an important factor influencing the growth of F. cirrhosa, leading to a decline in yield and quality after successive plantings.
The findings of this study suggest that as the duration of F. cirrhosa cultivation increases, there is a gradual rise in the activities of soil invertase, urease, catalase, acid phosphatase, and alkaline phosphatase. Specifically, when the plants have been cultivated for 10 years, these soil enzymes exhibit the highest levels of activity. These enzymes play a crucial role in the growth process of plants as they participate in urea degradation, organic phosphorus and carbohydrate conversion, and other processes. These conversion processes are essential for the nutrient cycle in the soil, ensuring that plants efficiently acquire the necessary nutrients for growth. It is hypothesized that the observed increase in soil enzyme activity is associated with the application of fertilizers and organic fertilizers during the annual planting and management of F. cirrhosa. Long-term use of fertilizers and organic fertilizers may enhance soil fertility, thereby promoting the elevation of soil enzyme activity. This hypothesis aligns with similar studies, such as the research conducted by Barbara Symanowicz and colleagues, who observed increased activities of soil urease, catalase, acid phosphatase, and alkaline phosphatase in the soil of Medicago sativa L. as a result of long-term fertilization49. Therefore, in the future, it would be beneficial to investigate the specific fertilizer requirements of F. cirrhosa and develop appropriate strategies for fertilizer application. This approach would be an effective means to overcome the challenges associated with continuous cultivation at the root level.
Impact of continuous cropping on bacterial and fungal abundance and diversity in F. cirrhosa
Numerous studies have highlighted the influence of continuous cropping on the structure of microbial communities in the rhizosphere, exacerbating the issue of soil health deterioration associated with continuous cropping38, 50. To comprehensively understand the inter-root soil bacterial community structure of F. cirrhosa over different years of continuous cultivation, an Illumina NovaSeq approach was employed. The results revealed that the diversity of bacteria and fungi increased as the years of continuous cultivation progressed. This phenomenon can be attributed to changes in the proportion of dominant flora during different cultivation years, consequently impacting the structural homogeneity of fungi and bacteria. The alterations in abundance and uniformity ultimately led to changes in the microbial diversity of F. cirrhosa in the inter-root region. These findings differ from previous studies on sweet potato, rice, cotton, wolfberry, and peanut, where microbial diversity either increased or decreased with the duration of continuous cultivation51, 52. In addition, these results are consistent with previous research findings, which demonstrated that continuous planting of sugarcane at different times significantly affected the microbial community. Similar observations were also made in the continuous cropping of groundnut, and alfalfa52, 53. Overall, continuous cropping had an impact on the composition of inter-root soil bacterial communities. The variations in fungal and bacterial abundance and diversity observed in different studies may be attributed to differences in the duration of continuous cropping, cropping patterns, soil environmental conditions, plant types, and other factors.
Microorganisms can be classified into beneficial growth-promoting bacteria and mycorrhizal fungi, biocontrol bacteria, and harmful pathogens based on their roles53, 54. Beneficial microorganisms stimulate the growth of medicinal plants, facilitate the production of medicinal compounds, and improve the resistance of medicinal plants55. These microorganisms enhance plant disease resistance through four mechanisms: competition, parasitism, antimicrobial activity, and induction of systemic resistance .
In our study, a significant decrease in the abundance of Lysobacter, a beneficial microorganism known for its resistance to bacterial wilt, was observed56. This decrease of 59.8% (P ≤ 0.05) in Y10 soil aligns with the high incidence of wilt disease in Trichoderma reesei planted successively in these soils during actual production. These findings are consistent with previous research conducted by She et al., who reported a significant reduction in the abundance of tobacco, which was negatively correlated with bacterial wilt disease57. Furthermore, our study revealed that the abundance of Sphingomonas, a microorganism known for its disease resistance and plant growth promotion properties, was significantly higher in the Y10 soil compared to the other groups. Sphingomonas has the ability to survive under nutrient-limited conditions and plays a crucial role in the breakdown of complex organic matter58. This microorganism exhibits great potential for ecological remediation and is likely to become a focal point in microbial environmental remediation in the future. Previous reports have also shown that Sphingomonas is involved in phosphorus solubilisation and positively correlated with phosphatase activity59. In our study, we observed a significant increase in AP content by 132.82% in the Y10 soil. This suggests that the higher relative abundance of Sphingomonas in successional soils may contribute to an increased supply of AP to plants and fungi.
Moreover, the study indicated a notable reduction in the prevalence of Bacillus, a bacterium renowned for its capacity to liberate potassium from soil and to stimulate the growth of rice seedlings, ultimately enhancing the yield. In this study, the abundance of Bacillus was found to have declined by approximately 60% (P ≤ 0.05). Additionally, a notable increase of 74.66% (P ≤ 0.05) in the abundance of Luteimonas, a harmful microorganism associated with soil-borne pathogens60, was observed in continuously ploughed F. cirrhosa soil.
Furthermore, Mortierella, a pathogenic fungus in medicinal plant production61, exhibited a striking increase in abundance by 164.82% (P ≤ 0.05) over ten years of continuous sitting in our study. Similarly, the abundance of Parastagonospora, a necrotrophic fungus causing Septoria nodorum spot disease in wheat62, exhibited an sharp increase of 106.69% (P ≤ 0.05). This increase in pathogenic fungi may be a significant factor contributing to the reduced yield and increased disease frequency of F. cirrhosa under continuous cultivation. Furthermore, the abundance of the harmful microorganism Pseudogymnoascus increased by 232.34% (P ≤ 0.05) in the Y10 soil. In contrast to its classification as a beneficial fungus that antagonises root rot disease caused by Fusarium sp.5, 63, our study observed a decrease in the abundance of Epicoccum by 60.74%. Similarly, we found a increase in the abundance of the pathogenic genus Cladosporium, which is commonly associated with plant diseases64. Furthermore, our study observed a decrease in the abundance of beneficial microorganisms. The abundance of Mortierella, which is known to promote plant growth65, 66, decreased by 68.14%, while that of Plectosphaerella, another beneficial microorganism that promotes plant growth, decreased significantly by 99.45% (P ≤ 0.05). These findings provide valuable insights into the changes in microbial abundance associated with continuous cultivation, highlighting the decrease in beneficial microorganisms and the increase in harmful microorganisms. These findings contribute to our understanding of the complex dynamics within the soil microbiome and their implications for plant health and productivity.
We found that the rhizospheric soil bacterial and fungal community associated with F. cirrhosa increased with continuous cropping, likely due to changes in soil properties influenced by the duration of continuous cropping. The increase of pathogenic bacteria in the rhizosphere, where they compete with beneficial bacteria for nutrients, may be partially responsible for this loss. In our study, soil quality was adversely affected by continuous long-term F. cirrhosa farming. Additionally, different durations of continuous cropping resulted in variations in the abundance of specific fungal genera. For instance, the phylum Ascomycota, including its associated orders, showed dominance in different treatments. These shifts may represent a self-regulation mechanism of the bacterial community to cope with increased pathogen pressure during continuous cropping. Co-occurrence network analyses expanded our understanding of symbiotic patterns among microbes in various terrestrial ecosystems. Notably, the number of links in the bacterial and fungal community network was significantly less in the Y10 group than in the CK group, suggesting that continuous planting reduced the size of the microbial community network and the number of taxa involved in microbial interactions (Fig. 3). These findings further support the idea that continuous cultivation of F. cirrhosa for 10 years leads to a more simplistic bacterial and fungal community structure. Thus, when the soil environment is altered by continuous cultivation of F. cirrhosa, the soil microbial community structure is disrupted, leading to a decrease in the inter-compactness of the soil microbial community structure, and a subsequent decrease in the ability of the soil microbial community to interact with each other, resulting in a decrease in the efficiency of soil nutrient conversion, which has an impact on the growth and development of plants.
According to the results of RDA, soil pH was identified as the primary factor influencing bacterial and fungal communities (Fig. 5). This finding highlights the crucial role of soil pH in regulating soil microbial communities, which is essential for comprehending the functioning and sustainability of the F. cirrhosa soil ecosystem. The improper use of fertilizers in F. cirrhosa cultivation can result in their entry into the soil, where a portion is absorbed and utilized by the plant to support its growth, while a significant amount remains in the soil. These residual fertilizers can impact the soil environment by altering its pH. High concentrations of fertilizers can lead to soil acidification or alkalization, thereby affecting the survival and activity of microorganisms such as bacteria and fungi67.
Our results have demonstrated that changes in soil pH can induce variations in the composition of bacterial and fungal communities within the soil. For instance, some microorganisms that thrive in acidic conditions may flourish, while those adapted to alkaline environments may be suppressed. Such alterations in community composition can influence the diversity and function of soil microbial communities, ultimately affecting soil health and plant growth. The improper use of pesticides can also contribute to changes in soil microbial communities. Pesticide residues can directly poison microorganisms or modify the soil environment, thereby impacting microbial growth and activity29. Collectively, these factors can lead to an imbalance in the structure and reduced diversity of soil microbial communities.
To ensure the sustainability of F. cirrhosa cultivation and soil health, it is imperative to adopt more scientifically sound strategies for fertilizer and pesticide use. For instance, adjusting the application rate of fertilizers based on soil pH can help maintain soil pH stability66. Additionally, measures such as precision fertilizer application and reduced pesticide use can minimize the residues of fertilizers and pesticides in the soil. The implementation of these measures will preserve the balance and diversity of soil microbial communities, thereby promoting soil health and plant growth. In this study, soil health showed a decreasing trend with the number of years of cultivation.
Furthermore, conducting further research on the relationship between soil pH and microbial communities can provide a scientific foundation for the development of precise fertilizer and pesticide management strategies. Moreover, it is crucial to consider the impacts of various environmental factors, including temperature, humidity, and soil texture, on soil microbial communities. This attention will contribute to a better comprehension of soil ecosystem functioning and the promotion of sustainable development. These studies will strongly support the healthy development of F. cirrhosa cultivation in the future.
The results of SEM analyses indicated that soil organic matter content was the primary driver influencing the diversity of soil microbial communities. Soil organic matter encompasses various plant and animal residues, microorganisms, and their decomposition products within the soil. An increase in soil organic matter enhances soil fertility, providing more habitat and nutrient sources for microorganisms, thereby promoting the diversity of soil microbial communities. Additionally, it was observed that soil pH had a significant negative effect on the diversity of soil microbial communities. pH serves as a critical indicator of soil acidity and alkalinity, and pH fluctuations affect the solubility and effectiveness of various soil elements, subsequently influencing the survival and activities of microorganisms. When soil pH deviates from the appropriate range, certain microorganisms may struggle to adapt, resulting in a decrease in soil microbial community diversity.
This study also revealed a significant correlation between soil alkaline dissolved nitrogen and soil organic matter content and pH. Alkaline dissolved nitrogen represents an important form of soil nitrogen, and its content is closely tied to soil fertility. Experimental findings demonstrated a significant positive correlation between soil alkaline dissolved nitrogen and soil organic matter content and pH. This suggests that optimizing the diversity of soil microbial communities can be achieved to a certain extent by appropriately regulating soil alkaline dissolved nitrogen content while ensuring soil fertility. It is important to note that the microbial community structure in continuously cropped soil is a major factor contributing to soil health degradation. Continuous cropping refers to the repetitive cultivation of the same crop on the same land, which tends to disrupt the balance of soil microbial communities, thereby impacting soil health. This is due to the potential increase in pathogenic bacteria and decrease in beneficial microorganisms, ultimately resulting in reduced diversity of the soil microbial community. Taking these findings into account, it can be concluded that the unscientific application of excessive amounts of chemical fertilizers is a significant factor contributing to soil health deterioration. Excessive chemical fertilizer use can disrupt the balance of soil organic matter and pH, leading to a decline in soil microbial community diversity. Therefore, in order to maintain soil health and promote sustainable agricultural development, it is crucial to implement scientific and rational fertilizer application strategies that ensure the equilibrium of soil organic matter and pH, thereby preserving soil microbial community diversity. Additionally, soil health issues caused by continuous cropping can be effectively addressed through practices such as crop rotation, intercropping, or the use of green organic fertilizers as substitutes for chemical fertilizers.
Conclusions
In conclusion, the alterations in soil physicochemical properties, bacterial and fungal abundance, and diversity were observed to be significantly different in soils where F. cirrhosa was continuously planted compared to control (blank) soils. Moreover, continuous cropping of F. cirrhosa resulted in a significant decrease in potentially beneficial bacterial and fungal populations, while the abundance of pathogenic fungi significantly increased when compared to the CK. These findings suggest that long-term continuous cultivation of F. cirrhosa transformed the microbial community into an unhealthy system. Furthermore, the complexity of co-occurrence networks between bacteria and fungi was significantly reduced due to continuous cultivation. These results indicate that changes in soil nutrient content, soil acidification, microbial diversity, and notable alterations in the abundance of specific taxa in F. cirrhosa may serve as important factors contributing to the challenges associated with continuous cultivation.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Li, X., Dai, Y. & Chen, S. Growth and physiological characteristics of Fritillaria cirrhosa in response to high irradiance and shade in age-related growth phases. Environ. Exp. Bot. 67, 77–83 (2009).
Wang, D. et al. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 139, 189–193 (2012).
Xu, Y., Ming, T. W., Gaun, T. K. W., Wang, S. & Ye, B. A comparative assessment of acute oral toxicity and traditional pharmacological activities between extracts of Fritillaria cirrhosae bulbus and Fritillaria pallidiflora bulbus. J. Ethnopharmacol. 238, 111853 (2019).
Wang, D., Yang, J., Du, Q., Li, H. & Wang, S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 193, 150–158 (2016).
Wang, Y., Xie, H., Yang, T., Gao, D. & Li, X. Primary investigation of phenotypic plasticity in Fritillaria cirrhosa based on metabolome and transcriptome analyses. Cells 11, 3844 (2022).
Wang, Y., Yang, Z., Gao, D. & Li, X. Integrated microbiology and target metabolomics analysis reveal the interaction of plant-microbe-soil interactions leading to the phenotypic plasticity of Fritillaria cirrhosa. Ind. Crops Prod. 219, 118999 (2024).
Jiang, W. et al. Integrated microbiology and metabolomics analysis reveal patterns and mechanisms for improving the yield and alkaloid content of Fritillaria cirrhosa by nitrogen fertilization. Ind. Crops Prod. 218, 119000 (2024).
Wang, Y. et al. Natural drug sources for respiratory diseases from Fritillaria: Chemical and biological analyses. Chin. Med. 16, 40 (2021).
Li, X. W. & Chen, S. L. Diurnal changes in gas exchange and chlorophyll fluorescence parameters of Fritillaria cirrhosa and F. delavayi under field conditions. Photosynthetica 47, 191–198 (2009).
Luo, X. et al. Microbial communities play Important roles in modulating paddy soil fertility. Sci. Rep. 6, 20326 (2016).
Zhang, W. W., Chong, W., Rui, X. & Wang, L. -j. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 18, 1360–1368 (2019).
Schloter, M., Dilly, O. & Munch, J. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 98, 255–262 (2003).
Mudhoo, A. & Garg, V. Sorption, transport and transformation of atrazine in Soils, minerals and composts: A review. Pedosphere 21, 11–25 (2011).
Cui, S., Qi, Y., Zhu, Q., Wang, C. & Sun, H. A review of the influence of soil minerals and organic matter on the migration and transformation of sulfonamides. Sci. Total Environ. 861, 160584 (2023).
Jacoby, R., Peukert, M., Succurro, A., Koprivova, A. & Kopriva, S. The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front. Plant Sci. 8, 292271 (2017).
Lei, H. et al. Diversity patterns of soil microbial communities in the sophora flavescens rhizosphere in response to continuous monocropping. BMC Microbiol. 20, 1–12 (2020).
Town, J. R. et al. Diverse crop rotations influence the bacterial and fungal communities in root, rhizosphere and soil and impact soil microbial processes. Appl. Soil. Ecol. 169, 104241 (2022).
Arafat, Y. et al. Long-term monoculture negatively regulates fungal community composition and abundance of tea orchards. Agronomy 9, 466 (2019).
Li, H. et al. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 12, 2758 (2022).
Ren, J. et al. Rhizosphere soil properties, microbial community, and enzyme activities: Short-term responses to partial substitution of chemical fertilizer with organic manure. J. Environ. Manage. 299, 113650 (2021).
Sun, R. et al. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 18, 5137–5150 (2016).
Yao, Q. et al. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of Northeast China. Soil Biol. Biochem. 110, 56–67 (2017).
Fuhrman, J. A., Cram, J. A. & Needham, D. M. Marine microbial community dynamics and their ecological interpretation. Nat. Rev. Microbiol. 13, 133–146 (2015).
Barberán, A., Bates, S. T., Casamayor, E. O. & Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 6, 343–351 (2012).
Zhu, P. et al. Shifts in soil microbial community composition, function, and co-occurrence network of phragmites Australis in the yellow river delta. Front. Microbiol. 13, 858125 (2022).
Zhan, P. et al. Plant litter decomposition in wetlands is closely associated with phyllospheric fungi as revealed by microbial community dynamics and co-occurrence network. Sci. Total Environ. 753, 142194 (2021).
Lu, H. et al. Soil health assessment under different soil and irrigation types in the agro-pastoral ecotone of Northern China. Catena 235, 107655 (2024).
Li, Y., Li, T., Zhao, D., Wang, Z. & Liao, Y. Different tillage practices change assembly, composition, and co-occurrence patterns of wheat rhizosphere diazotrophs. Sci. Total Environ. 767, 144252 (2021).
Wang, Y. et al. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 725, 138527 (2020).
Wu, H. et al. Biotic and abiotic factors interplay in structuring the dynamics of microbial co-occurrence patterns in tropical mountainsides. Environ. Res. 250, 118517 (2024).
Benitez-Alfonso, Y. et al. Enhancing climate change resilience in agricultural crops. Curr. Biol. 33, R1246–R1261 (2023).
Abdelaal, K. et al. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 10, 520 (2021).
Singh, A. et al. New and Future Developments in Microbial Biotechnology and Bioengineering 1–15 (Elsevier, 2020).
Zhang, Y. et al. Fertilization shapes bacterial community structure by alteration of soil pH. Front. Microbiol. 8, 1325 (2017).
Kang, E. et al. Soil pH and nutrients shape the vertical distribution of microbial communities in an Alpine wetland. Sci. Total Environ. 774, 145780 (2021).
Wang, Z., Kelly, J. & Kovar, J. Depletion of macro-nutrients from rhizosphere soil solution by juvenile corn, cottonwood, and switchgrass Plants. Plant. Soil. 270, 213–221 (2007).
Hontoria, C., García-González, I., Quemada, M., Roldán, A. & Alguacil, M. The cover crop determines the AMF community composition in soil and in roots of maize after a ten-year continuous crop rotation. Sci. Total Environ. 660, 913–922 (2019).
Liu, Z. et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage. Res. 197, 104503 (2020).
Zhao, J. et al. Dissecting the effect of continuous cropping of potato on soil bacterial communities as revealed by high-throughput sequencing. PLoS ONE 15, e0233356 (2020).
Alami, M. M. et al. Continuous cropping changes the composition and diversity of bacterial communities: A meta-analysis in nine different fields with different plant cultivation. Agriculture 11, 1224 (2021).
Ren, B. et al. Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of Northeastern China. Sci. Rep. 8, 5619 (2018).
Zeng, M. et al. Model-based analysis of the long-term effects of fertilization management on cropland soil acidification. Environ. Sci. Technol. 51, 3843–3851 (2017).
Xu, R. & Coventry, D. Soil pH changes associated with lupin and wheat plant materials incorporated in a red–brown earth soil. Plant. Soil. 250, 113–119 (2003).
HUANG, P. et al. Proton accumulation accelerated by heavy chemical nitrogen fertilization and its long-term Impact on acidifying rate in a typical arable soil in the Huang-Huai-Hai plain. J. Integr. Agric. 14, 148–157 (2015).
Shi, R. et al. Mechanisms for increasing soil resistance to acidification by long-term manure application. Soil Tillage. Res. 185, 77–84 (2019).
Chen, D. et al. Persistent organic fertilization reinforces soil-borne disease suppressiveness of rhizosphere bacterial community. Plant. Soil. 452, 313–328 (2020).
Liu, S. et al. Traditional Chinese medicine residues promote the growth and quality of salvia miltiorrhiza bunge by improving soil health under continuous monoculture. Front. Plant Sci. 14, 1112382 (2023).
Liao, J. & Xia, P. Continuous cropping obstacles of medicinal plants: Focus on the plant-soil-microbe Interaction system in the rhizosphere. Sci. Hort. 328, 112927 (2024).
Symanowicz, B., Skorupka, W., Becher, M., Jaremko, D. & Krasuski, S. The effect of alfalfa mineral fertilization and times of soil sampling on enzymatic activity. Agronomy 11, 1335 (2021).
Chen, Y. et al. Evolutions and managements of soil microbial community structure drove by continuous cropping. Front. Microbiol. 13, 839494 (2022).
Gao, Z. et al. Effects of continuous cropping of sweet potato on the fungal community structure in rhizospheric soil. Front. Microbiol. 10, 2269 (2019).
Wang, P. et al. Effect of soil management systems on the rhizosphere bacterial community structure of tobacco: Continuous cropping vs. paddy-upland rotation. Front. Plant Sci. 13, 996858 (2022).
Xu, Y. et al. Continuous cropping of alfalfa (Medicago sativa L.) reduces bacterial diversity and simplifies cooccurrence networks in aeolian sandy soil. Soil. Ecol. Lett. 1–13 (2021).
Soni, J., Sinha, S. & Pandey, R. Understanding bacterial pathogenicity: A closer look at the journey of harmful microbes. Front. Microbiol. 15, 1370818 (2024).
Wang, G., Ren, Y., Bai, X., Su, Y. & Han, J. Contributions of beneficial microorganisms in soil remediation and quality improvement of medicinal plants. Plants 11, 3200 (2022).
Han, G. et al. Response of pine rhizosphere microbiota to foliar treatment with resistance-inducing bacteria against pine wilt disease. Microorganisms 9, 688 (2021).
She, S. et al. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 199, 267–275 (2017).
Leys, N. M., Bastiaens, L., Verstraete, W. & Springael, D. Influence of the carbon/nitrogen/phosphorus ratio on polycyclic aromatic hydrocarbon degradation by mycobacterium and sphingomonas in soil. Appl. Microbiol. Biotechnol. 66, 726–736 (2005).
Xu, H., Lv, J. & Yu, C. Combined phosphate-solubilizing microorganisms jointly promote Pinus massoniana growth by modulating rhizosphere environment and key biological pathways in seedlings. Ind. Crops Prod. 191, 116005 (2023).
Erlacher, A., Cardinale, M., Grosch, R., Grube, M. & Berg, G. The Impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 5, 74035 (2014).
Ozimek, E. & Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 11, 7 (2020).
Duba, A., Goriewa-Duba, K. & Wachowska, U. A Review of the interactions between wheat and wheat pathogens: Zymoseptoria Tritici, fusarium spp. and Parastagonospora nodorum. Int. J. Mol. Sci. 19, 1138 (2018).
Hamzah, T. N. T. et al. Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and Identification of potential antagonists against the soil-borne fungus, Fusarium solani. Front. Microbiol. 9, 1707 (2018).
Wang, X. et al. Antifungal activity against plant pathogens of metabolites from the endophytic fungus Cladosporium cladosporioides. J. Agric. Food Chem. 61, 4551–4555 (2013).
Li, F. et al. Rare fungus, Mortierella capitata, promotes crop growth by stimulating primary metabolisms related genes and reshaping rhizosphere bacterial community. Soil Biol. Biochem. 151, 108017 (2020).
Yang, Z. et al. Physiological, cytological and multi-omics analysis revealed the molecular response of Fritillaria cirrhosa to cd toxicity in Qinghai-Tibet Plateau. J. Hazard. Mater. 472, 134611 (2024).
Yang, Z. et al. Transcriptomics combined with physiology reveals the effect of different light qualities on the development of Fritillaria cirrhosa in the Qinghai Tibet Plateau. Environ. Exp. Bot. 224, 105814 (2024).
Acknowledgements
We appreciate two anonymous reviewers for their insightful comments on this paper. This study was financially supported by the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZXKT22061 and ZZ16-XRZ-067); Bijie Technology Innovation Platform and Talent Team (Bikehe [2023] No.66-BJZDSYS 2024-10).
Author information
Authors and Affiliations
Contributions
Author Contributions: D.G.: Conceptualization, Methodology, Validation, Program modification, Formal analysis, Data curation, Writing—original draft preparation, Writing—review and editing, Visualization. X.S.G.: Methodology, Investigation, Writing—review and editing. Y.H.W.: Methodology, Investigation, Writing—review and editing. H.M.H.: Validation, Resources, Project administration. Y.W.: Meth-odology, Investigation, Writing—review and editing. X.Y.Y.: Investigation, Project administration. F.F.L.: Investigation, Project administration. Z.M.Y.: Investigation, Project administration. H.B.Z.: Investigation, Conducting a research. X.W.L: Validation, Resources, Supervision, Project administration, Formal analysis, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, D., Gao, X., Wang, Y. et al. Effects of long-term continuous cultivation on the structure and function of soil bacterial and fungal communities of Fritillaria Cirrhosa on the Qinghai-Tibetan Plateau. Sci Rep 14, 21291 (2024). https://doi.org/10.1038/s41598-024-70625-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-70625-x
Keywords
This article is cited by
-
Influence of salt-tolerant medicinal herbs on soil geochemistry, nutrient cycling, and microbial communities in saline–alkali ecosystems of inner Mongolia
Environmental Geochemistry and Health (2026)
-
Rhizospheric and endophytic bacterial community response to continuous Atractylodes macrocephala cultivation
BMC Plant Biology (2025)
-
Genomic and metabonomic insights into the lignin-degrading potential of a novel halophilic bacterial strain Salinicoccus sp. HZC-1
BMC Genomics (2025)
-
Analysis of Inter-Root Soil Habitat and Physiological Traits in Healthy and Root Rot-Infested Custard Apple (Annona Squamosa L.) Plants
Journal of Soil Science and Plant Nutrition (2025)