Abstract
The present study aimed to identify nutrients (UPLC-PDA-ESI-MS/MS, HPLC-RI method) and biological activities (antioxidant activity to reduce Fe3+ and ABTS·+, pancreatic lipase inhibitory effect, α-amylase, and α-glucosidase, anti-bacterial) of 14 highbush blueberries (Vaccinium corymbosum L.) cultivars (Northern type) as well as a principal component analysis (PCA) to assess the variation of these properties in the context of biodiversity. Most of the cultivars in this research have been first presented in this paper. Phytochemical profiling of the tested highbush blueberry fruit revealed 75 bioactive compounds, including 5 macroelements, 7 microelements, 7 monophosphate nucleotides, 15 anthocyanins, 1 phenolic acid, 14 flavonols, 11 essential amino acids, 8 non-essential amino acids, 2 sugars, 7 organic acids. The PCA showed that the profile and contents of the analyzed compounds as well as their anti-bacterial, antioxidant, anti-diabetic, and anti-obesity potentials depended significantly on the tested cultivars. Thus, the study provides comprehensive data on cultivar-specific biodiversity and correlations that can be used to design novel extracts rich in polyphenolic, amino acids, and/or minerals extracts from the selected cultivars of highbush blueberry as natural and alternative sources to fulfill the growing industry demand for supplements, pharmaceuticals, and nutraceutical products.
Similar content being viewed by others
Introduction
Highbush blueberries (Vaccinium corymbosum L.) are widely cultivated in many countries around the world. It is a species of perennial plants from the Ericaceae family, whose bushes are up to 2.5 m high and characterized by large leaves and berries in bunches with a diameter of 1.5–2.5 cm, flat in shape, aromatic, and covered with a light blue coating. It is estimated that these fresh fruits are available all year round as they are cultivated commercially in many countries1,2.
Fruits are an excellent source of polyphenolic compounds, mainly of polymeric procyanidins and anthocyanins which account for approx. 68 and 25% of all polyphenolic compounds2,3. Anthocyanins are natural pigments responsible for the blue color of fruit especially delphinidin and malvidin, which account for approx. 75% of their total anthocyanin content3. Apart from anthocyanins, blueberries contain significant amounts of flavonols (kaempferol, quercetin, myricetin), phenolic acids (mainly hydroxycinnamic acids), and stilbene derivatives. Quercetin is the major and dietarily significant flavonol, that shows as high as 80% bioavailability during digestion3. In addition, highbush blueberries are rich in ascorbic acid (approx. 20–100 mg/100 g), minerals such as magnesium, potassium, calcium, iron, zinc, organic acids, vitamins (A, E, B1, B2, B3, B6, and B9), as well as dietary fibers, carotenoids, and tocopherols3,4,5.
These phytochemical profiles significantly modulate their high biological activity, as they are potent too, e.g. prevent osteoporosis and diseases of the urinary system, exhibit antioxidative effects by eliminating the excessive amount of reactive oxygen species, as well as elicit anti-inflammatory, anti-carcinogenic, anti-diabetic, anti-bacterial, and anti-obesity effects6. In addition, they are also characterized by an interesting, balanced, and desirable sweet–sour sensory profile developed by the above-mentioned compounds. However, numerous studies on the phytochemical composition of blueberries3,7 have shown that the chemical diversity and abundance of their compounds strictly depend on the genetic variability of cultivars, plant maturity stage, and agrotechnical or environmental conditions. Analyses of the phytochemical and biological biodiversity of various fruit cultivars are of key importance, not only to the cultivation programs aimed at modifying and improving their nutritional value but also to the development of dietetic functional food with targeted health properties. On the other hand, a comprehensive analytical approach to phytochemicals is essential to determine their biological value and to indicate the relationship between the components. Existing literature sources have shown chemical composition of blueberries to be different for different cultivars9,10,11, depending on the cultivar3 and/or growing/maturity stage8. Our research presents a holistic approach to the cultivar-dependent assessment of blueberry fruits to identify varietal biodiversity as well as cultivar-specific phytochemical benefits. In addition, some of the phytochemicals and/or some cultivars presented in this report are investigated for the first time.
Therefore, this study aimed to assess the nutritional biodiversity and biological activity of 14 old and new highbush blueberry cultivars using the UPLC-PDA-ESI-MS/MS technique. Statistical multivariate analysis such as agglomerative hierarchical cluster analysis (HCA), principal component analysis (PCA), and Pearson correlations were deployed to illustrate cultivar-specific differences in terms of phytochemical composition and health-promoting value in vitro as well as the correlations between many variables tested.
Results and discussion
Basic physical and chemical parameters
The color parameters of the blueberry fruits were measured in the CIE L*a*b* system with the photo colorimetric method (Table 1). The lightness parameter (parameter L*) was significantly different and depended on the cultivar (cv.). The parameter L* values ranged from 37.20 (‘Liberty’) to 55.25 (‘Bonus’). The parameter a* values also depended on the cultivar and were between 28.64 (‘Lateblue’) and 48.42 (‘Aurora’), and were correlated with the NAI index suggesting that the anthocyanin pigments affected fruit color. Similar values of the parameter L* were noted for the cv. ‘Brigitta Blue’12, in turn, the values of the a* and b* parameters were different. In our study, the parameter a* value was approximately 2 times higher, while the parameter b* value indicated the presence of a yellow pigment, not a blue one.
Fruit dry matter content ranged from 10.09 to 17.08% for cvs. ‘Brigitta Blue’ and ‘Lateblue’, respectively (Table 1). The analyzed fruits of tested highbush blueberry cultivars were also characterized by low levels of harmful nitrates III and V, which, according to the applicable standards, were within their permissible range. The permissible NO3 content intended for consumption by infants and children should not exceed 200 mg in vegetables, and 500 mg in leafy vegetables; whereas the daily intake of nitrates should not exceed 0.07 mg/kg of body weight/day. This suggests that the analyzed blueberry fruits were safe and suitable for consumption. Similar results were obtained for the cvs. ‘Brigitta Blue’ and ‘Duke’12,13. In our studies, other cvs. have not been analyzed in this respect so far. In turn, the content of soluble solids in these fruits of tested cvs. varied significantly and ranged from 10.30 °Brix (‘Bonus’) to 15.70 °Brix. (‘Spartan’). It was similar to the result for the cv. ‘Kimcheon’ lowbush and ‘Pyungtaek’ highbush blueberries5. In the berries of cv. ‘Duke’, it was 13.2 °Brix, which was in line with the results obtained by Ochmian13, while it reached 62.67 and 62.58 °Brix, respectively in Korean and American blueberries10.
Sugars and organic acids are mainly responsible for the sensory characteristics of fruits, and acids additionally affect their health values7. In fruits of our tested highbush blueberry cvs., the main sugars were glucose accounted for 57% of the total sugar content on average, and fructose for 43%, which was also confirmed by Song et al.5, Li et al.7, Moon et al.10 (Fig. 1a). Additionally, Moon et al.10 reported the content of sucrose (average 1.4%) in the composition of the blueberry fruits in South Korea, while it was not present in blueberries in North America. In our studies, the highest contents of glucose and fructose were recorded for cvs. ‘Duke’ and ‘Nelson’, which may suggest that these fruits were relatively sweet. Whereas, the sugar profile has not been previously analyzed in the fruits of cvs. ‘Bonus’ and ‘Bonifacy’. Fruits of tested highbush blueberry cvs. contained additionally 7 organic acids (Fig. 1b), of which citric acid was the major acid and accounted for 58% on average. Similar observations were reported by Li et al.7, pointing to citric acid as the main organic one, followed by quinic acid evaluated in fruits of cv. O’Neal’s (highbush blueberry, Southern type). The highest content of organic acids was found in the fruits of cv. ‘Liberty’ and it was about 5 times higher than in the cv. ‘Bluegold’, which had the lowest organic acid content. Although organic acids play important roles in the proper functioning of the body, it should be emphasized that their excessive intake may diminish the bioavailability of many macronutrients and micronutrients. Phytic acid and oxalic acid are particularly responsible for reducing the absorption of elements. Moreover, excessive oxalic acid salts can generate sparingly soluble14 calcium oxalate crystals in various organs of the body.
Mineral compounds
The appropriate daily intake of macroelements and microelements is essential to the proper development and functioning of the human body14. The total ash content and contents of individual mineral components in analyzed fruits were significantly (p ≤ 0.05) different depending on the cvs. (Fig. 1c, d). The ash content in the fruit varied between 0.13% for the cv. ‘Brigitta Blue’ up to 0.28% for the cv. ‘Lateblue’. On the other hand, the ash content in blueberry fruits in South Korea and North America was 1.43% and 1.41%, respectively10, but in the fruits of cv. ‘Kimcheon’ lowbush blueberry it was 0.14%, and in the cv. ‘Pyungtaek’ highbush blueberry it was 0.17%5.
The main macroelements in the fruits of highbush blueberry cvs. were as follows: phosphorus (P; accounting for 56% of all macroelements) > nitrogen (N; 32%) > potassium (K; 6%) > calcium (Ca; 4%) > magnesium (Mg; 3%), while the main microelements were iron (Fe; accounting for 69% of all microelements) > manganese (Mn; 28%) > zinc (Zn; 2%) > copper (Cu), nickel (Ni), cadmium (Cd), and selenium (Se) (below 1%). Similar results were reported by Moon et al.10 and Song et al.5. Zhang et al.15 did not detect N in blueberry fruits, but instead found Ca to be the dominant macroelement. However, it should be mentioned that the contents of individual elements in that study differed significantly depending on the cvs.
Nitrogen was found to be highest in the fruit of the ‘Duke’ cultivar (11.6 mg/g) and lowest in the ‘Lateblue’ cultivar (4.87 mg/g). Nitrogen and magnesium are often considered synergistic elements. However, our results showed that cvs. with higher N content had little Mg in the fruit—e.g. ‘Duke’, ‘Aurora’, ‘Toro’. In contrast, the highest Mg content was in ‘Lateblue’ fruit, which had low N.
Levels of micronutrients (Fe, Mn, Zn, Cu) and the known heavy metals Ni and Cd were below 1%. Micronutrients also become toxic to humans after a certain threshold. Cadmium and nickel are not very toxic to plants and do not cause a decrease in yield even in highly contaminated soils, but they are dangerous to humans.
The content of both macronutrients and micronutrients was at similar levels as in the studies of Ochmian et al.16 and Zhang et al.15. The macronutrient content of blueberry fruit grown in South Korea were also comparable, with the exception of Ca which was more than 2 times higher in their fruit10. According to literature data, differences in mineral content may be influenced by, among others, soil properties, insecticides and fungicides, fertilization applied, plant physiology, genotype, and environmental conditions15. In our studies fruits of cvs. ‘Bonus’ and ‘Lateblue’ were not analyzed in this report.
Consumption of blueberry fruits can enrich an everyday diet with selected minerals, especially K, Fe, and Mn. It is worth noting that the absorption of K can be enhanced by the presence of Mg in the diet, the absorption of Fe—by the presence of Co, Cu, and Mn in the diet, and additionally by the presence of vitamins B6, B9 and B12; whereas that of Mn—by the presence of Zn in the diet, and vitamins, such as B1, E, K, C17. Therefore, it is important to analyze the chemical composition of the fruit, which allows the selection of appropriate cvs. with fruits for direct consumption. In addition, the chemical composition of blueberries, including in terms of macroelements and micronutrients, was compared with their recommended daily intake (RDI) with the diet17. Comparing the obtained results with the RDN for macroelements and microelements, it was found that 100 g d.m. of fruit covered on average 5% of the demand for Ca, 11% for P, 12 and 9% for Mg, respectively, 15% for N at 50 kg of body weight, and 5% for Se. However, in the case of K, Fe, and Mn, the contents were strongly dependent on the cultivar, and 100 g d.m. of ‘Lateblue’ fruit could provide Fe to 119% of the RDN for men and 66% of the RDN for women. In the case of K—the analyzed ‘Bluecrop’ fruits provided as much as 38% of the RDN, whereas in the case of Mn to 189% (Liberty) of the RDN for men and to 241% of the RDN for women.
Free amino acids
The results of the determinations of free amino acids (FAA) are summarized in Table S1. From the nutritional perspective, amino acids are functional and construction units that can be divided into two main groups—those synthesized in the body (as non-essential amino acids; NEFAA) and those not synthesized in the body to meet its demands (as essential amino acids; EFAA), which makes that they need to be supplied to the body with food18. Therefore, the presence of 19 FAA was determined in the analyzed fruits of highbush blueberry cvs. grown in Poland, 11 of which belonged to EAA and 8 to NEAA in an average ratio of 3:1. In turn, the total FAA content was significantly dependent on the cultivar (p ≤ 0.05) and ranged from 78.10 (‘Toro’) to 801.93 mg/100 g dry weight (dw) (‘Aurora’). However, the FAA content in fruits has been determined for the first time for the following highbush blueberry cvs. (‘Aurora’, ‘Bonifacy’, ‘Lateblue’, and ‘Nelson’). In addition, one more group could be distinguished in the division of nutritional amino acids, i.e. conditionally essential amino acids (CEAA), such as arginine (synthesized from glutamate/aspartate), cysteine (from methionine and/or serine), glutamine (from glutamic acid), proline (from glutamine), tyrosine (from phenylalanine)18. Thus, the share of the main FAA groups in the examined fruits ranged from 63% (‘Spartan’) to 85% (‘Draper’) for EFAA, including 53% of CEAA on average, as well as from 15% (‘Draper’) to 37% (‘Spartan’) for NEFAA, including 1% of CEAA on average. These results indicated that the higher the EFAAs content was in the fruit of individual cultivars, the relatively lower their content of non-essential amino acids. In turn, Zhang et al.15 reported the presence of only 12 FAA (187.5 mg/g), which suggested that the amount of FAA identified was influenced by dilution and extraction methods. Thus, 12 FAA (390.7 mg/100 g) were detected in the blueberry fruit in South Korea, including 4 EFAA (which accounted for 68% of total FAA) and 9 NEFAA; in North America—9 FAA, including 2 EFAA (which accounted for 49% of total FAA) and 7 NEFAA10; in the blueberries in Argentina—22 FAA19; which may indicate that the quality, and thus the quantity of blueberry fruits, may additionally be affected by, among others, place of cultivation, growing and environmental conditions or genotype. The dominant content of arginine was also confirmed in other studies, where it was also noted that the content of individual amino acids depended on the tested cultivar5,10,15,20. The content of arginine in our studies significantly depended on the cultivar and ranged from 21.79 (‘Toro’) to 598.25 mg/100 g dw (‘Duke’). Arginine is an essential amino acid that is used in some biological processes, including in the immune response, similar to monophosphate nucleotides20, in protein biosynthesis, nitric oxide production, to the umami taste or the urea cycle21. It is also worth mentioning the role of glutamic and aspartic acid salts, which, by connecting with the taste receptors T1R1, T1R3, and mGluR4, contribute to the umami taste development. In turn, the interaction with monophosphate nucleotides affects the intensification of taste sensations8. Hence, the presence of FAA in highbush blueberries may affect both the human body and the palatability of fruits and processed fruit products.
Monophosphate nucleotides
Results of the quantitative and qualitative assessment of monophosphate nucleotides (MNs) are presented in Table S2. According to the available data from the literature, this assessment has been carried out for highbush blueberries for the first time ever. The legitimacy of the analysis of these compounds is related to their important role in human energy metabolism, fat metabolism, and beneficial involvement in iron absorption or immune response20. In fruits of tested highbush blueberry cvs., seven compounds were detected and their total content ranged from 3.57 (‘Brigitta Blue’) to 13.89 mg/100 g dw (‘Duke’). However, the highest content of these compounds was determined in xanthine-5ʹ-monophosphate (XPM), which accounted for 33% of total MNs on average, followed by inosine 5ʹ-monophosphate (IMP; 23%) > uridine 5ʹ-monophosphate (UMP; 16%) > guanosine 5ʹ-monophosphate (GMP; 9%) > thymidin 5ʹ-monophosphate (TMP) = adenosine 5ʹ-monophosphate (AMP; 8% each) > cytidine 5ʹ-monophosphate (CMP; 4%). These compounds, in synergy with free amino acids, i.e. l-glutamic acid and l-aspartic acid, are responsible for enhancing the palatability of some foods and the feeling of satisfaction, i.e. for intensifying the umami taste20. MNs enhance the taste sensation in the order of GMP followed by IMP > XMP, and finally AMP by combining with taste receptors T1R1 + T1R38,21. As in the fruit of the Saskatoon berry, syn. serviceberry (Amelanchier alnifolia Nutt.), XMP was the compound with the highest content noted and constituted 30% of total MNs on average5. Thus, in the highbush blueberry fruits, XMP was mainly responsible for palatability intensification, followed by IMP. In addition, it is worth noting that the fruits of cv. ‘Duke’. were the richest in CMP, GMP, XMP, and TMP. For comparison, the AMP content of banana fruit was around 1 µg/g fresh weight (fw)22, while the AMP content of litchi fruit was 7.9 µg/g fw of AMP22. However, the cAMP and cGMP contents in fresh jujube (Ziziphus jujuba Mill.) fruit were 130.9 and 150.2 µg/g dw, respectively, and the drying process contributed to their loss23. According to the information provided by Yang et al.24, the content of other flavour MNs was up to 100 mg/100 g, which was deemed low. The low content of flavour 5′-nucleotides was also noted in mushrooms: Morchella crassipes, Morchella deliciosa, Enteloma saunders, Panus tigrinus20. Thus, the obtained results indicated that the taste sensations of highbush blueberry fruits can be due to the presence of monophosphate nucleotides, which also occurred in mushrooms8.
Polyphenolic compounds
UPLC-PDA-ESI–MS/MS analysis enabled the detection of 15 anthocyanin compounds, 1 phenolic acid, 14 flavonols and flavan-3-ols (procyanidins group). Anthocyanins type and quantities were significantly dependent on analyzed cultivars (Table 2). Derivatives of delphinidin ([M–H]+ at m/z = 303), petunidin ([M–H]+ at m/z = 317), peonidin ([M–H]+ at m/z = 301), malvidin ([M-H]+ at m/z = 331), cyanidin ([M–H]+ at m/z = 287) were confirmed among the identified compounds, with loss of hexoside and pentoside moiety25. In addition to the monoglucosides identified in blueberry fruit, there were also 6 acetylated compounds (loss of 42 units (u)) in combination with acetic acid. Among the tested cvs., all anthocyanins (TAC) were identified only in fruits of 5 cvs. i.e. ‘Bluecrop’, ‘Bonifacy’, ‘Bonus’, ‘Nelson’ and ‘Spartan’. However, no anthocyanins have been reported for cvs. ‘Lateblue’ and ‘Bonus’ so far. It was also shown that only the acetylated forms of anthocyanins significantly depended on the cultivar. They accounted for 8% (‘Boniface’) to 24% (‘Bluecrop’ and ‘Bonus’) of TAC. The high proportion of acetylated forms was due to the high content of delphinidin 3-O-(6’’-methyl-glucoside) and cyanidin 3-O-(6’’-acetyl-glucoside), which were present only in the above-mentioned cvs. In addition, petunidin 3-O-(6’’-acetyl-galactoside) was additionally identified in the fruit of cv. ‘Toro’, and compounds 11, 14, and 15 were also detected for the cvs. ‘Bluegold’ and ‘Toro’ (Table 2). The lack of acetylation with ferulic acid, p-coumaric acid, and caffeic acid indicates a higher activity of acetyltransferase, the production of which, according to observations, depends on the genome of the tested cvs. In addition, anthocyanin acetylation affects co-pigmentation through intramolecular reactions and strong stability of pigment molecules, which is desired in food, as well as in pharmaceutical and/or cosmetic applications26. In turn, glycosidic derivatives occurred in fruits of all tested highbush blueberry cvs. The content of anthocyanins in the fruit depended significantly on the cultivar and ranged from 466.6 mg/100 g dw for the cv. ‘Spartan’ up to 1353.51 mg/100 g dw for the cv. ‘Bonifacy’, with the average content being 741.35 mg/100 g dw. Malvidin derivatives accounted for 44%, followed by delphinidin—31%, petunidin—18%, peonidin—6.7%, and cyaniding—0.3%. This order was probably due to the biosynthetic pathway of individual anthocyanins, as similar patterns of the accumulation of petunidin with those of delphinidins were found, and it was explained in the example of grapes that petunidin was obtained from delphinidin and then was converted to malvidin by methyltransferase induction26,27. The major compound was malvidin3-O-glucoside which accounted for 17–45% of TAC in the fruits of cvs. ‘Spartan’ and ‘Duke’, respectively. This was also confirmed by Prior et al.25 in the analyzed highbush blueberry fruits. In turn, in the cv. ‘Kimcheon’ lowbush and in the cv. ‘Pyungtaek’ highbush blueberry, the content of anthocyanins in their fruits was 22 and 18.1 mg/100 g, respectively5.
One cryptochlorogenic acid ([M–H]– at m/z = 191) and 14 flavonols were also identified in the fruits of tested highbush blueberry cvs., including 2 myricetin ([M–H]– at m/z = 317), 10 quercetin ([M–H]– at m/z = 301), and 3 isorhamnetin ([M–H]– at m/z = 315)3. Monoglucosides (12 compounds) were identified during main ion fragmentation which lost pentoside (132 u), rhamnoside (146 u), and hexoside (162 u), as well as deoxyhexoside group (146 u + 162 u = 308 u). Thus, they enabled the identification of arabinose or xylose, glucose or galactose, and rhamnose, respectively. In addition to the monoglucosides identified in blueberry fruit, there were also 2 acetylated compounds (the loss of an acetyl moiety –42 u and malonyl moiety –86 u) in combination with acetic and malonic acid, and one aglycone (isorhamnetin). However, the number of identified compounds significantly (p ≤ 0.05) depended on the analyzed cultivar, with quercetin 3-O-galactoside and 3-O-rabinobioside being most affected (absent in the fruit of cvs. ‘Aurora’ and ‘Bluegols’), quercetin 3-O-rhamnoside (missing in the cvs, ‘Bliegold’, ‘Duke’ and ‘Liberty’); isorhamnetin 3-O-rutinoside (missing in the cvs. ‘Chandler’, ‘Bluegold’ and ‘Duke’); and isorhamnetin 3-O-pentoside (missing in the cv. ‘Bluecrop’). In turn, no flavonols have been detected in the fruits of cvs. ‘Bonifacy’, ‘Bonus’ and ‘Lateblue’. The content of flavanols (TFL) significantly (p ≤ 0.05) depended on the tested cultivar and ranged from 76.69 (‘Spartan’) to 268.82 mg/100 g dw (‘Aurora’). Among the identified groups of compounds, the largest share was noted for quercetin, which accounted for 65–83% of TFL in the fruits of cvs. ‘Bonus’ and ‘Nelson’, respectively, followed by myricetin (1–30% for cvs. ‘Duke’ and ‘Liberty’) and isorhamnetin (3–28% for cvs. ‘Aurora’ and ‘Chandler’). This contribution is probably due to the flavonol biosynthesis pathway, as O-methylation at the 3ʹ position of quercetin by flavonoid 3′-hydroxylase converts it to isorhamnetin, while hydroxylation at the 5ʹ position of quercetin leads to the formation of flavonol myricetin27. In turn, the major compound in the highbush blueberry fruit turned out to be quercetin 3-O-glucoside (34%), which was also confirmed by other authors3. Quercetin is the most widely studied phenolic compound in blueberries; it is characterized by high bioavailability (about 80%), and elicits positive effects on health, including among others, on the circulatory and respiratory systems, and inhibits the activity of certain forms of cancer3. The content of 3-O-caffeoylquinic acid also depended on the cultivar and it ranged from 135.20 (‘Bonus’) to 329.14 mg/100 g dw (‘Aurora’), accounting for 6% of total all polyphenols on average.
Flavan-3-ols as polymeric procyanidins (PP) have been quantified in highbush blueberry (V. corymbosum L.) fruits for the first time ever. It was found that they belonged to the subclasses of proanthocyanidins. These compounds are known for their valuable biological activities, e.g. antioxidative, anti-atherosclerotic, anti-carcinogenic, hypolipidemic, hypotensive, hypoglycemic, and anti-inflammatory activities28. The content of PP in the fruits differed significantly (p ≤ 0.05) among tested cvs., with the highest content recorded for the cv. ‘Bluegold’ (4243.36 mg/100 g dw) and the lowest one for the cv. ‘Duke’ (2019.19 mg/100 g dw). Compared to chokeberry (Aronia melanocarpa Eliot.) fruit, these values were respectively 1.2-fold and 2.6-fold lower29. In turn, the average content of PP in highbush blueberries was twofold higher compared to the Saskatoon berry (Amelanchier alnifolia Nutt.) cv. ‘Thiessen’ fruits11. In addition, condensed tannins are composed primarily of repeating monomeric units, which affects their uniqueness, and the varying degree of polymerization (DP; the number of flavanol units) indicates their biological activity30. In this study, there was no correlation between PP content in the analyzed blueberry fruits and the DP, while significant (p ≤ 0.05) differences were noted depending on the cultivar tested. The DP expressed by having up to 10 monomer units in the polymerization process, noted for the following cvs. ‘Bluecrop’, Bluegold’, ‘Bonus’, ‘Nelson’, ‘Toro’, ‘Chandler’ and ‘Duke’ indicated the presence of oligomers, while higher DP values determined in the remaining cvs. indicated the presence of polymers28. In addition, the low DP value determined the low degree of astringency and bitterness in fruits6. It indicated that the fruits of cvs. ‘Spartan’, ‘Lateblue’ and ‘Brigitta Blue’ may have the highest astringency compared to other tested highbush blueberry cvs. A similarly low DP value (4.8–6.6) was recorded in the fruit of the Saskatoon berry, which also indicated the presence of oligomers with imperceptible bitterness11. In the case of the cultivars characterized by the presence of polymers, a higher DP was noted in lowbush blueberry, i.e. from 19.9 to 114.131, which also may indicate a more astringent taste of these fruits. A higher DP was also noted in the fruit (23) and pomace (35) of Aronia melanocarpa Eliot.29 compared to the highbush blueberry (V. corymbosum L.) cv. ‘Spartan’ , i.e. by approx. 1.3 and 2 times, respectively.
Biological activity
Antimicrobial potency
Highbush blueberry fruits are rich in phytochemical components used as natural antioxidants, which may, depending on the bioactive substance, elicit a health-promoting effect. Antimicrobial properties were assessed against Gram-negative and Gram-positive bacteria (Table S3). The results showed that the fruits had an inhibitory effect on B. cereus, except for the cvs. ‘Bluegold’, ‘Boniface’, ‘Brigitta Blue’, ‘Liberty’ and ‘Spartan’; against P. aeruginosa except for the cvs ‘Liberty’ and ‘Spartan’; and against V. harveyi except for the cv. ‘Boniface’. On the other hand, fruits of cvs. ‘Duke’ and ‘Bonus’ did not show any ability to inhibit the activity of the analyzed microorganisms. In turn, the highest ability to inhibit the growth and development of B. cereus, P. aeruginosa, and V. harvei strains was shown by the fruits of cvs. ‘Aurora’ and ‘Nelson’ It is well known that P. aeruginosa causes nosocomial infections, as well as infections with a high risk of mortality, mainly for people with compromised immunity, e.g. after surgery or chemotherapy and with HIV infection. It is worth mentioning that the bacterium is highly resistant to antibiotics. On the other hand, V. harveyi, is common to wild and farmed fish. In turn, B. cereus is known to be relatively pathogenic, causing food poisoning, but also pneumonia, inflammatory endocardial infections, meningitis, and eyeball inflammation. The blueberry leaves showed to exhibit strong inhibitory activity against the growth and development of P. aeruginosa, E. coli, A .baumannii, E. faecalis, K. pneumonia, S. typhimurium and S. aureus)2,32 due to their polyphenolic compounds. The blueberry fruits and skins of the cv. ‘Toro’ showed inhibitory activity against the growth of S. aureus, E. faecalis, L. monocytogenes, B. subtilis, E. coli, P. aeruginosa, S. typhimurium and C. freundii33. Other cvs. were not analyzed in this respect. On the other hand, the inability to inhibit the growth and development of F. prausnitzii, B. longum and B.subtilis is a good feature, because they are well known to be also beneficial microorganisms with probiotic properties that can produce IgA antibodies in the intestines and upper respiratory tract and to exhibit anti-inflammatory properties (especially F. prausnitzii), or inhibitory abilities against ailments associated with allergies, celiac disease and irritable bowel syndrome (IBS). This may suggest the influence of blueberry bioactive compounds, especially polyphenolic compounds (which also act as prebiotics), on the development of these microorganisms2. However, these observations need to be confirmed in subsequent scientific works.
Enzyme inhibition potency
The anti-diabetic and anti-obesity properties were evaluated as the ability to inhibit activities of digestive enzymes, like α-amylase, α-glucosidase, and pancreatic lipase, and expressed as IC50 (Table 3). The obtained results for the highbush blueberry fruits differed significantly (p ≤ 0.05) depending on the tested cultivar. The inhibitory effect against α-amylase and α-glucosidase refers to limiting the breakdown of polysaccharides into simple sugars while inhibiting or preventing increasing levels of glucose in the blood. The inhibitory effect against pancreatic lipase reduces the breakdown of triglycerides into fatty acids and glycerol34. The highest inhibitory activity against α-amylase and α-glucosidase was found in fruits of cvs. ‘Nelson’ and ‘Lateblue’ (≤ IC50 = 16.3 mg/mL and 18.8 mg/mL), respectively. In addition, a 1.3-fold higher ability to inhibit α-glucosidase than α-amylase was noted in the fruit of the cvs. ‘Nelson’, ‘Lateblue’. Similar results of anti-diabetic activity were reported for the cv. ‘Brigitta Blue’12. The other cvs. were not analyzed for their inhibitory capacity against α-amylase and α-glucosidase. Moreover, as reported by Johnson et al.35 and Ochmian et al.12, the ability to inhibit the activity of digestive enzymes was strongly correlated with procyanidin polymers, the content of which was the highest in blueberry extracts with the lowest IC50 value for α-glucosidase35. In turn, the highest anti-obesity properties were noted for the cvs. ‘Bluegold’, ‘Draper’, and ‘Lateblue’ (≤ IC50 = 66 mg/mL). Anthocyanins in particular are responsible for the high ability to inhibit pancreatic lipase activity36,37. In turn, inoculation of the blueberry pomace with the probiotic Lactobacillus casei increased significantly its ability to inhibit pancreatic lipase, reducing its value to IC50 = 1.28 mg/mL, comparable to that of orlistat38.
Antioxidant activity
The antioxidative properties of highbush blueberries were assessed using antiradical scavenging activity against the ABTS·+ radical and reducing power from Fe3+ to Fe2+ in the FRAP tests, the results of which are presented in Table 3. The antioxidant activity (AA) ability of plasma differed significantly depending on the cultivar tested and the type of bioactive compounds. The mean ABTS·+ and FRAP values were 22.69 and 11.87 mmol Trolox/100 g dw, respectively. The highest ability to scavenge excess free radicals was noted for the fruits of cvs. ‘Lateblue’, ‘Duke’, ‘Chandler’, and ‘Bluegold’ (≤ 26 mmol Trolox/100 g dw), while fruits of cvs. ‘Bluegold’, ‘Lateblue’, and ‘Aurora’ had the highest ferrous ion reducing potential (≤ 15 mmol Trolox/100 g dw). In turn, the antioxidant potential has not yet been analyzed in ‘Bonus’. Similar values of antioxidant activity against ABTS and FRAP radicals were previously reported for fruits of cv. ‘Brigitta Blue’ of highbush blueberry12. Other authors in their research suggest that both TPC and other bioactive compounds were responsible for AA12,32. In addition, in this study, a linear correlation was observed between the ferric-reducing ability of plasma and the content of TPC, which was also confirmed by Ochmian et al.12. The contribution of flavonols within flavonoids to the development of antioxidant power was due to the unique pattern of free hydroxyl groups substituted in the flavonoid skeleton39.
Statistical analysis
To explain the relationship between bioactive compounds and biological activity, Pearson’s correlation analysis was performed between the studied factors (Fig. S1). Irrespective of the cultivar tested, significant (p ≤ 0.05) correlations were shown by determining the correlation coefficient (r). As already mentioned, the AA to reduce Fe3+ and ABTS+ radicals in vitro strongly correlated with the content of PP (r = 0.62, r = 0.82) and the total content of anthocyanins (r = 0.82, r = 0.77), especially with malvidin glycosides (r = 0.87, r = 0.79), and peonidin (r = 0.77, r = 0.79). On the other hand, an average positive correlation was found with petunidin and delphinidin glycosides (0.04 < r < 0.70), while a negative average correlation was observed with cyanidin. Similar observations of high correlations were found when analyzing the effect of anthocyanins on antioxidant activity3. High correlations between AA and total TAC of highbush blueberry fruits were also noted by Moyer et al.40 and Castrejon et al.41. In turn, the coefficient of correlation with FL (rABTS = 0.33, rFRAP = 0.40) and PA (rABTS = 0.46, rFRAP = 0.58) was moderate, while similarly to cyanidin, a negative moderate correlation was noted with myricetin. The high dependence of AA on the contents of TPC was based primarily on the capture of free radicals by donating electrons or hydrogen atoms32. In addition to polyphenolic compounds, the AA was also strongly positively influenced by TMP (rABTS = 0.83), AMP (rABTS = 0.74, rFRAP = 0.72), and a total of MP (rABTS = 0.74), while moderate correlations were confirmed with UMP, CMP, GMP, XMP (0.04 < r < 0.70).
A strong effect of Cd (rABTS = 0.76) and a moderate effect of P, Fe, methionine, lysine, serine, tyrosine, and asparagine on the antioxidant activity were also noted based on Pearson’s correlation analysis. It is worth mentioning that methionine has protective properties against abiotic stress in plants and has a protective and antioxidant effect against oxidative damage to cells39. A study by Castrejón et al.39 indicated a strong effect of anthocyanins on anti-diabetic and anti-obesity activity; however, the type of anthocyanins identified in blueberry fruits did not show a significant effect (p ≤ 0.05). On the contrary, a negative weak correlation was noted in the case of malvidin and peonidin, whereas cyanidin and myricetin showed a moderate correlation with anti-obesity activity and α-glucosidase activity (r = 0.45, r = 0.21). Cyanidin compounds, especially cyanidin 3-glucoside, alleviate obesity by causing the activation of brown adipose tissue and the formation of beige cells in the white adipose tissue of the groin of rats38. In the case of inhibiting the growth and development of V. harveyi, asparagine may exert a particularly significant effect, whereas NEAA, such as valine, lysine, methionine, proline, PP, and microelements like Se may have a moderately positive effect. In turn, the activity of P. aeruginosa may be inhibited by lysine, arginine, and PP, whereas that of B. cereus by macroelements, like Mg and K. Previous reports showed that FAA or OA in complexes with macroelements and microelements had positive antibacterial properties, especially against Gram-positive bacteria41. Stănilă et al.41 reported that cobalt and copper complexes, especially with lysine, valine, methionine and phenylalanine, leucine, and histidine, featured antibacterial activity. In turn, Tripatchi et al.32 in their research on blueberry fruit extracts also noted a strong antibacterial effect ascribed, in particular, to polyphenolic compounds. Interesting relationships were noted for FAA, especially tyrosine and methionine from PP, where a strong positive correlation was found with antibacterial activity; while the CMP, TMP, and AMP correlation coefficient was moderately positive. Tyrosine was reported to be a precursor to polyphenolic compounds, including PP8, while tryptophan was a precursor to serotonin, auxin, niacin, and methionine responsible for nutritional and hormonal function in the body8. In turn, the identified organic acids had a medium effect on the DP. This may have a slight effect on the taste of the fruit. There was also a strong correlation between AMP and Cd and the TAC, and malvidin (r = 0.71, r = 0.68). In turn, UMP, AMP, and Cd correlated with delphinidin, and peonidin glycosides, which might be related to their effect on amino acid biosynthesis. Interdependencies also seem to be worth mentioning, where cyanidin showed a negative correlation with other anthocyanin derivatives, while malvidin, petunidin, and delphinidin showed strong interdependencies. In the case of flavonols, there was a strong correlation between quercetin and isorhamnetin (r = 0.76), while negative correlation was observed with myricetin (r = − 0.42). An interesting relationship was found in the group of anthocyanins, including especially a positive correlation between cyanidin and myricetin, and a negative one between these two compounds and other flavonols. However, quercetin and isorhamnetin were positively correlated with the other anthocyanins. As mentioned earlier, that was probably due to the biosynthesis pathway for the occurrence of individual anthocyanins26 and a similar biosynthesis pathway of anthocyanins to that of flavonols27, which significantly depended on the cultivar. Both mentioned pathways of biosynthesis, closely related to each other, used two identical enzymes–flavonoid 3′-hydroxylase and flavanoid 3′,5′-hydroxylase–based on delphinidin and cyanidin and myricetin and quercetin. The authors suggested that the 5’ hydroxylation of cyanidin and quercetin led to the formation of delphinidin and myricetin in grapes (Vitis vinifera L.), but the reported negative correlation of these compounds in highbush blueberry (V. corymbosum L.) did not indicate such a pathway. On the other hand, O-methylation of the 3ʹ position of quercetin and delphinidin to form isorhamnetin, peonidin, petunidin and additionally 3ʹ and 5ʹ of delphinidin to form petunidin and malvidin occurred27. The presence of flavonols in blueberries, in addition to their nutritional properties, may enhance the color of berries and stabilize them in the co-pigmentation reaction with anthocyanins27. In the case of NEFAA and EFAA, there was a strong positive correlation, especially between Cu and NEFAA (including arginine –r = 0.76, histidine –r = 0.77), asparagine and quercetin (r = 0.80) and between Se and tryptophan. The moderate dependence of asparagine and glutamic acid on nitrogen proves the participation of these amino acids in the transport and storage of nitrogen, playing an important role in nitrogen and carbon metabolism8. For MP, a strong influence of cadmium was observed on all MP except IMP; that of iron on CMP, GMP, XMP, TMP, and that of fumaric, malic, and tartaric acids on CMP. This was probably related to the taste of ripe fruit, the key drivers of which were organic acids and MPs8. There was also a strong influence of TMP on other MPs except for IMP and the interdependence of CMP, GMP, and XMP. As previously mentioned, e.g. IMP, AMP affect the intensification of taste sensations in synergy with the amino acids (aspartic acid and glutamic acid)8, which was confirmed by the correlation coefficients r = 0.51, r = 0.48, respectively. The correlations between the identified organic acids and Mg, between K and Mg (r = 0.76), and between Mn and selected OA seemed also important. These correlations, especially between Mg and K, were responsible for metabolism and significantly improved the absorption of these elements8. Mg absorption may also be affected by the supply of Ca and P. In addition, macroelements and micronutrients were negatively and positively correlated, which may be influenced by synergism and antagonism between them43, where, for example, high P content may have an antagonistic effect on micronutrients. However, our study showed the synergism of P with Zn and Cu. Synergism was also noted for Cu with N and P, Zn with P, Ca with Fe, and Se with Mn.
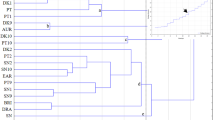
The use of HCA as exploratory tool to assess their heterogeneity allowed description of 4 areas of interactions between the level of chemical and physical components in the 14 highbush blueberry cultivars (Fig. 2). The first area was the cv. ‘Lateblue’, which contained the highest amounts of dry weight, ash, OA, PA, TAC, PP, TPC, Fe, MP, and FAA and the lowest amount of sugar, and parameter a*, and b*; the second area consisted of cvs. ‘Brigitta Blue’, ‘Draper’, ‘Spartan’, and ‘Toro’, which had the highest Mn, Zn, and the lowest TAC, PP, TPC, FAA, parameter L*; the third area concerned cvs. ‘Blue gold’, ‘Bonus’, ‘Duke’, ‘Nelson’, distinguished by the highest amount of colour parameter L*, b*, and sugar, and the lowest content of OA, PA, TFL, Fe, and the last area included cvs. ‘Aurora’, ‘Bluecrop’, ‘Bonifacy’, ‘Chandler’, and ‘Liberty’, which were characterized by a particularly high TFL, and parameter a*, and low Mn, Zn, MP, and parameter L*.
To better diversify the abundance of the analyzed phytochemical compounds and biological activity assessed by in vitro tests, PCA analysis was performed, which allowed for a clear cultivar-specific differentiation (Fig. 3). The PCA results (Fig. 3a) explained 45.4% of the total variance, of which PC1 explained 25.9% and PC2—19.5% of the variance between the main components and the primary variables. Thus, the diversity of the first component variants was most affected by FAA, malvidin, and peonidin compounds and acylated forms of anthocyanins, PP, and AA, while the second component variants were defined by antibacterial activity and micronutrient content. Cultivars located close to each other were similar in terms of the analyzed data3. The vectors presented in Fig. 3b expressed the analyzed variables, while their direction and length testified to the influence on the main components. It was noted that most of the analyzed variables were located close to the circle, which might mean that the PCA could carry most of the information contained in them. In turn, vectors placed close to each other might indicate a strong correlation, while those placed perpendicularly—no correlation, and opposite to each other – had a negative correlation between the factors. Similar observations were noted by Ochmian et al.43, where anti-diabetic activity was negatively correlated with blueberry polyphenolic compounds in the fruit, while a positive correlation was shown for AA with these components. The PCA–biplot (Fig. 3c) illustrated the 4 identified groups: the first was represented by cvs. Bonifacy’; ‘Bonus’, ‘Chandler’ with a high content of myricetin, and anti-diabetic and anti-obesity activity; the second group contained cvs. ‘Bluecrop’, ‘Brigitta Blue’, ‘Nelson’, ‘Spartan’, ‘Toro’ with a high content of total sugar content (TSC) and cyanidin; the third group consisted of cvs. ‘Aurora’, ‘Draper’ being the richest in PP, TFL, TPA, NEFAA, EFAA, SFAA, TMiC, quercetin, isorhamnetin, having high DP and showing high antibacterial activity against VH, BC, PA; and group 4 comprised cvs. ‘Bluegold’, ‘Duke’, ‘Lateblue’, ‘Liberty’ characterized by an extremely high amount of TAC, glycosidic derivatives of peonidins, malvidins, delphinidins, petunidins, TMC, total organic acids, sum of monophosphate nucleotides, and high AA. Summing up, the PCAs’ results showed differences in the quantitative sum of monophosphate nucleotides and qualitative composition between the tested highbush blueberry cvs. Similar observations were reported by Li et al.9, they analyzed fruits of blueberry cvs. from different regions of China in terms of the profile and variability of anthocyanin content. They noted that the cultivars and climatic factors could have a significant impact on the content of individual color compounds, especially malvidin and delphinidin. In turn, Manninen et al.8, in their PCA analysis of immature and ripened fruits of blueberry cultivars, showed that the content of individually analyzed FAAs was significantly dependent on the cultivars, which was also noted in our studies. Thus, the results provide comprehensive reports focusing on the indication of biodiversity and the relationship between the analyzed components and biological value.
Analysis of main components: (A) distribution of 14 cultivars of highbush blueberry fruits; (B) distribution of the analyzed parameters; (C) PCA-biplot. Explanations: TSC, sum of sugar; TOA, sum of organic acid; SMP, sum of monophosphate nucleotides; SFAA, sum of free amino acids; NEFAA, sum of non-essential amino acids; EFAA, sum of essential amino acids; TMC, sum of macroelements; TMiC, sum of microelements; TAC, sum of anthocyanins; TPA, sum of phenolic acid; TFL, sum of flavonols; PP, sum of polymeric procyanidins; DP, degree of polymerization; VH, V. harvey; PA, P.aeruginosa; BC, B. cereus; 1, ‘Aurora’; 2, ‘Bluecrop’; 3, ‘Bluegold’; 4, ‘Bonifacy’; 5, ‘Bonus’; 6, ‘Brigitta Blue’; 7, ‘Chandler’; 8, ‘Draper’; 9, ‘Duke’; 10, ‘Lateblue’; 11, ‘Liberty’; 12, ‘Nelson’; 13, ‘Spartan’; 14, ‘Toro’.
Material and methods
Plant materials
The 14 old and new cvs. of highbush blueberry (V. corymbosum L,) Northern type were used in this research. The study materials were the fruits of following cultivars (cvs.): ‘Bluecrop’, ‘Bluegold’, ‘Bonifacy’, ‘Bonus’, ‘Brigitta Blue’, ‘Chandler’, ‘Duke’, ‘Lateblue’, ‘Nelson’, ‘Spartan’ (old cvs.) and ‘Aurora’, ‘Draper’, ‘Liberty’, and ‘Toro’ (new cvs.). Most of these cultivars have commonly been cultivated commercially in Poland and other countries worldwide. The highbush blueberry fruits were collected, with permission, manually from the bushes grown in the cultivar trial (experiment) established in the field of the Pomological Orchard in Dąbrowice (51° 55′ 24″ N, 020° 5′ 58″ E), and all specimens were deposited for future access to the National Institute of Horticultural Research (InHort) in Skierniewice, central Poland. The material was identified by Prof. Dr. Stanisław Pluta, a geneticist and breeder of the highbush blueberry working at the Department of Horticultural Crop Breeding of the InHort. The fruits were harvested at full ripeness, determined on the basis of color and the strength of the fruit’s adhesion to the peduncle. The highbush blueberry fruits were frozen at − 25 °C, freeze-dried (24 h; FreeZone; Labconco Corporation, USA), and crushed (IKA A.11, Germany). The powders were kept in a refrigerator (− 80 °C) until analysis. All experiments in the manuscript were performed with relevant International Union for Conservation of Nature guidelines.
Color analysis
The color of the tested highbush blueberry fruits was analyzed in CIE L*a*b* system, as described by Ochmian et al.43, Lachowicz et al.6. The a* value showed the place of appearing in the color gamut, in the range from green to red on the surface of dried fruits of analyzed genotypes. The b* parameter described the color in the range from yellow to blue on the surface of dried fruits of tested genotypes. The value of L* parameter ranges from 0 to 100, black to white, respectively. Relative changes in anthocyanins, as a normalized anthocyanin index—NAI = (I780–I570)/(I780 + I570) with a disposition of both parameters normalized to between –1 (redness) and + 1 (red) was measured6, where Ix is the remittance spectra intensity at a given wavelength x.
Total soluble solids, sugars and organic acids
The total soluble solids of tested material was measured using a laboratory refractometer PCE-DR (PCE Instruments Polska Sp. z o. o., Sosnowiec, Poland) at 20 °C and expressed as °Brix. The organic acids and sugar contents were tested by the HPLC method with refractive index detection42. The samples fruits (4–5 g) were diluted with redistilled water (50 mL). The extraction was performed by incubation for 15 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland) and with occasional shaking and then incubation at 90 °C for 30 min. Next, the slurry was centrifuged at 19,000×g for 10 min, and the supernatant was filtered through Sep-Pak C-18 Cartridges (Waters Millipore) and through a Hydrophilic PTFE 0.20-mm membrane (Millex Samplicity Filter, Merck) and used for analysis. The chromatographic equipment SYKAM (Eresing, Germany) consisting of sample injector S5250, pump system S1125, column oven S4120 and RI detector S3590 was used. Separation was carried out using Polymer IEX H column (6 µm, 250 × 8 mm; SETREX). The mobile phase was used with an acetonitrile water mixture (75:25) for isocratic elution; the flow rate was 0.5 mL/min at column temperature set at 30 °C and injection volume of 10 mL. The volume of injected sample was 20 µL and 30 min was needed to complete the analysis. The results are given as mg/100 g dw.
Ash, microelements and macroelements
The dry matter and total ash were determined according to Polish Standards. The results were expressed at.%. The content of elements in fruits of 14 highbush blueberry cvs. was assessed after mineralisation: Ca, P, K, and N were tested after wet mineralisation in H2SO4 (96%) and HClO4 (70%); Cu, Zn, Mn, Fe, Se were measured after mineralisation in HNO3 (65%) and HClO4 (70%) in a ratio 3:1. The total nitrogen concentration in plants was determined by the Kjeldahl distillation method using a Vapodest 30 (Gerhardt GmbH, Germany). The concentration of K was tested by atomic emission spectrometry; Mg, Ca, Cu, Zn, Mn, Fe concentration was tested by the flame atomic absorption spectroscopy using iCE 3000 Series (Thermo Fisher Scientific, UK), the content of P was measured by the colourimetric method on a Specol 221 apparatus (Carl Zeiss, Germany). Results for Ash, microelements and macroelements were expressed as mg per g dw and ug per g dw, respectively.
Chemical extraction
For bioactive compounds: all samples (1 g) were extracted with 10 mL of mixture containing UPLC-grade methanol (30%), and acetic acid (1% of reagent). The extraction was performed twice by incubation for 20 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland) and with occasional shaking. Next, the slurry was centrifuged at 19,000g for 10 min, and the supernatant was filtered through a hydrophilic PTFE 0.20 μm membrane (Millex Samplicity Filter, Merck, Darmstadt, Germany) and used for analysis.
Monophosphates nucleotides
The quantification and identification of monophosphate nucleotides were performed as reported by Lachowicz et al.6 by UPLC-PDA-ESI–MS/MS (Waters Corporation, Milford, USA). LC–MS was performed under the following conditions: instrument, Waters UPLC™ and system with an electrospray ionization (ESI) interface and a quadrupole mass detection system (Waters, Milford, MA, USA); software, MassLynxTM; column, ACQUITY UPLC™ Column HSS T3 (2.1 mm ID and 100 mm length, 1.8-μm particle size) (Waters, Milford, MA, USA). The ESI source was operated at 120 °C with a desolvation temperature of 450 °C, 800 L/h desolvation gas flow rate, and a capillary voltage set at 3.5 kV. The cone voltage was 30 V and collision energies 30 eV. Integration and quantitation were performed using the Waters MassLynxTM software. The results are given as mg/100 g of dw.
Free amino acids
The analysis of free amino acids was performed aptly to the protocol described by Lachowicz et al.6 by UPLC-PDA-ESI–MS/MS (Waters Corporation, Milford, USA). UPLC-PDA-ESI-MS/MS analysis was carried out on a Waters ACQUITY (Waters Corporation, Milford, U.S.A.). The ESI source was operated at 120 °C with a desolvation temperature of 450 °C, 800 L/h desolvation gas flow rate, and a capillary voltage set at 3.5 kV. The cone voltage varied from 20 to 35 V and collision energies within 10–40 eV, depending on the free amino acid investigated. Integration and quantitation were performed using the Waters MassLynxTM software. Detection of these compounds was performed in the positive ionization. Amino acid standards (certified reference material) was purchased from Sigma–Aldrich (Wrocław, Poland). The results are given as mg/100 g dw.
Polyphenolic compounds
The determination of phenolic compounds was performed aptly to the protocol described by Kapusta et al.42 by UPLC-PDA-ESI-MS/MS (Waters Corporation, Milford, USA). The separation was carried out using BEH C18 column (100 mm × 2.1 mm i.d., 1.7 µm, Waters) kept at 50 °C. For the anthocyanins investigation the following solvent system: mobile phase A (2% formic acid in water v/v) and mobile phase B (2% formic acid in 40% ACN in water v/v) were applied. For other polyphenolic compounds, lower concentration of formic acid was used (0.1% v/v). The gradient program was set as follows: 0 min 5% B, from 0 to 8 min linear to 100% B, and from 8 to 9.5 min for washing and back to initial conditions. The injection volume of the samples was 5 µl (partial loop with needle overfill) and the flow rate was 0.35 mL/min. The following parameters were used for TQD: capillary voltage 3.5 kV; con voltage 30 V in positive and negative mode; the source was kept at 250 °C and desolvation temperature was 350 °C; con gas flow 100 L/h; and desolvation gas flow 800 L/h. Argon was used as collision gas at a flow rate of 0.3 mL/min. The characteristic UV-spectra were collected at the following wavelengths: λ = 520 nm, anthocyanins; λ = 320, phenolic acids; λ = 360, flavonols; and λ = 280, flavan-3-ols.Quantification was achieved by injection of solutions of known concentrations ranging from 0.05 to 5 mg/mL (R 2 ≤ 0.9998) of phenolic compounds as standards. Waters MassLynx software v.4.1 was used for data acquisition and processing. The results are given as mg/100 g dw.
Procyanidins
The freeze-dried samples were weighed in an amount of 5 mg into 2-mL Eppendorf vials. Subsequently, 0.8 mL of the methanolic solution of phloroglucinol (75 g/L) and ascorbic acid (15 g/L) were added to samples. After addition of 0.4 mL of methanolic HCl (0.3 M), the vials were incubated for 30 min at 50 °C with continuous vortexing in a thermo shaker (TS-100, BioSan, Riga, Latvia). The reaction was terminated by placing the vials in an ice bath, drawing 0.6 mL of the reaction medium and diluting with 1.0 mL of sodium acetate buffer (0.2 M). The samples were centrifuged immediately at 20,000×g for 10 min at 4 °C and stored at 4 °C before reverse-phase HPLC (RP-HPLC) analysis.
The determination of phloroglucinolysis was performed aptly to the protocol described by Lachowicz et al.11. Phloroglucinolysis products were separated on a Cadenza CD C18 (75 mm × 4.6 mm, 3 μm) column (Imtakt, Japan). Analysis was carried out using a Waters (Milford, MA) system equipped with Waters 474 diode array and scanning fluorescence detectors and Waters 717 plus autosampler. The mobile solvents were 0.25% aqueous acetic acid (A) and acetonitrile (B). The fluorescence detection was monitored at 278 nm and 360 nm. The calibration curves were established using ( +)-catechin and ( −)-epicatechin-phloroglucinol adducts standards. The results are given as mg/100 g dw.
Anti-microbiological potency
The anti-microbiological activity of the freeze-dried highbush blueberry fruit was checked on the following strains of microorganisms: (1) Gram-negative bacteria: Salmonella spp. (ATCC 29890), Escherichia coli (ATCC 10536), Pseudomonas aeruginosa (ATCC 15442), Vibrio harveyi (ATCC 12126); (2) Gram-positive bacteria: Bacillus subtilis (ATCC 13640), Staphylococcus aureus (ATCC 9538), Enterococcus faecalis (ATCC 29212), Enterococcus hirae (ATCC 10542), Faecalibacterium prausnitzii (ATCC 27768), Bifidobacterium longum (ATCC 15707), Bacillus cereus(ATCC 6633). All microorganisms were bought from Merck (Darmstadt, Germany). Ready-made sterile Petri dishes (Ø 90 mm) were used. The cell suspensions (100 µL) were evenly distributed on the Petri dishes. Gentamycin was used as a control for bacteria. The antimicrobial activity was evaluated by measuring the diameter of the circular inhibition zones around the well. The bacteria were grown in nutrient broth medium at 37 °C, except B. subtilis (ATCC 6633) which was grown at 30 °C. The yeast was grown in Yeast Extract Peptone Glucose (YPD) medium at 30 °C. The agar was added to the medium at a concentration of 2% when it was needed6.
Enzyme inhibition potency
Anti-diabetic activity such as α-amylase and α-glucosidase inhibitory, and anti-obesity activity such as lipase inhibitory effect of the samples were protocols described already by Ochmian et al.43. The extraction of mixed parts of fruits was done with 70% acetone (or water) at room temperature for 60 min with constant stirring. After centrifuging at 4000 rpm for 10 min, and filtration, the supernatants were concentrated at 40 °C (vacuum evaporator) to remove the acetone and the aqueous phase was diluted with water. For further analytical and biological activity assays, a gradient of concentrations was prepared via serial dilution of the fruit extracts in pure water. The results of enzyme inhibition activity are given as IC50 value (mg of powder material per 1 mL of the reaction mixture under assay conditions).
Antioxidant activity
Samples (1 g) was mixed with 80% of methanol and water (10 mL) + 1% hydrochloric acid, and incubated for 20 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland). Next, the slurry was centrifuged at 19,000×g for 10 min, and the supernatant was filtered through a hydrophilic PTFE 0.20 μm membrane (Merck, Darmstadt, Germany) and used for analysis.
The antiradical cations activity (ABTS), and the ferric reducing power (FRAP) methods were used in our studies as reported by Re et al.44 and Benzie and Strain45. Briefly, 10 µL of the supernatant was mixed with 990 µL of ABTS or FRAP. After 10 min of reaction, absorbance was measured at 734 nm for ABTS and 593 nm for FRAP. Determinations by ABTS and FRAP methods were performed using a UV-2401 PC spectrophotometer (Shimadzu, Kyoto, Japan). The results of antioxidant activity are given as mmol Trolox/100 g dw.
Statistical analysis
All experimental results were mean ± SD of two parallel experiments (N = 18 for bioactive compounds, non-enzymatic and enzymatic activities; N = 20 for colour parameters). Extractions were repeated three times for all analyzed samples. Statistica 12.5 (StatSoft, Kraków, Poland) was used for statistical analyses, including one-way ANOVA and Tukey’s HSD test to assess significant differences (p ≤ 0.05) between cvs. The multivariate analysis was fulfilled by applying principal component analysis (PCA) and agglomerative hierarchical cluster analysis (HCA) by the Ward method (Euclidean distance).
Conclusions
To recapitulate, the assessed phytochemical profile indicated the presence of 75 compounds, including 30 polyphenolic compounds such as 15 anthocyanins (derivatives of delphinidin and petunidin were predominate), 14 flavonols (the dominant fraction was derivative of quercetin) and hydroxybenzoic acid. PP content significantly depended on the cultivar, averaging 2840 mg/100 g dw. DP indicated that the ‘Aurora’, ‘Boniface’, ‘Brigitta Blue’, ‘Draper’, ‘Lateblue’, ‘Liberty’, and ‘Spartan’ cvs. contained oligomers resulting from the polymerization process and the remaining 7 cultivars contained polymers. 19 free amino acids were identified, of which 8 were essential amino acids along with branched-chain amino acids, and the most abundant was arginine. Mineral analysis revealed 5 macroelements and 7 microelements, of which N, K were the most abundant. However, on average, fruit consumption may enrich the diet with K, Fe, and Mn, which can cover 64–241% of the daily requirement. MP profiling indicated 7 compounds, of which XMP and IMP dominated, and the cultivar ‘Duke’ was the most abundant in these compounds. In addition, the analysis of sugars and organic acids indicated fructose and glucose, respectively, and 7 acids arranged in decreasing order of amount: citric acid > quinic acid > tartaric acid > succinic acid > malic acid > fumaric > oxalic. Higher inhibitory activity against α-amylase than α-glucosidase was also noted, including a positive correlation indicated with myricetin, while inhibition of pancreatic lipase activity indicated a positive correlation with cyanidin. The analyzed cultivars showed high inhibitory activity against V. harveyi, P. aeruginosa, and B. cereus. Antioxidant activity averaged 22.69 and 11.87 mmol Trolox/100 g dw (ABTS, FRAP assay, respectively), and strongly correlated with PP, TAC, especially with malvidin glycosides, and peonidin, TMP, AMP, TMP, and Cd. Thus, the study provides comprehensive data on cultivar-specific biodiversity and interdependence that pharmaceutical industries can use to design novel products with a high perspective to develop in the food industry tailored to individual needs and diet-related disorders. Rich polyphenolic, amino acids, and/or mineral extracts of selected cultivars of blueberry can be good natural and alternative sources in the face of the growing market and pharmacy industry demand for supplements, pharmaceuticals, and nutraceuticals products.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. Any further information is available from the corresponding author on reasonable request.
Abbreviations
- AA:
-
Antioxidant activity
- ABTS:
-
The antiradical cations activity
- AMP:
-
Adenosine 5ʹ-monophosphate
- CEAA:
-
Conditionally essential amino acids
- CMP:
-
Cytidine 5ʹ-monophosphate
- DP:
-
Degree of polymerization
- EFAA:
-
Essential amino acids
- FRAP:
-
The ferric reducing power
- FAA:
-
Free amino acids
- GMP:
-
Guanosine 5ʹ-monophosphate
- InHort:
-
The National Institute of Horticultural Research
- IBS:
-
Irritable bowel syndrom
- IMP:
-
Inosine 5ʹ-monophosphate
- MNs:
-
Monophosphate nucleotides
- NEFAA:
-
Non-essential amino acids
- NAI:
-
Normalized anthocyanin index
- PCA:
-
Principal component analysis
- PP:
-
Polymeric procyanidins
- RDI:
-
Recommended daily intake
- SMP:
-
Sum of monophosphate nucleotides
- SFAA:
-
Sum of free amino acids
- TFL:
-
Total of flavanols
- TAC:
-
Total anthocyanins
- TPC:
-
Total phenolic compounds
- TPA:
-
Total phenolic acids
- TMC:
-
Total macroelements compounds
- TMP:
-
Thymidin 5ʹ-monophosphate
- TOA:
-
Total organic acids
- TMiC:
-
Total microelements compounds
- UMP:
-
Uridine 5ʹ-monophosphate
References
Cvetković, M. et al. When is the right moment to pick blueberries? Variation in agronomic and chemical properties of blueberry (Vaccinium corymbosum) cultivars at different harvest times. Metabolites 12(9), 798 (2022).
Thilakarathna, W. W., Langille, M. G. & Rupasinghe, H. V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr. Opin. Food Sci. 20, 51–57 (2018).
Li, D. et al. Polyphenols, anthocyanins, and flavonoids contents and the antioxidant capacity of various cultivars of highbush and half-high blueberries. J. Food Compos. Anal. 62, 84–93 (2017).
Rupasova, Z. et al. Genotypic distinctions of variability of biochemical composition of fruits of Vacciniaceae species under conditions of Belarus. Eur. Chem. Bull. 6(1), 5–12 (2017).
Song, H. N., Park, M. S., Youn, H. S., Park, S. J. & Hogstrand, C. Nutritional compositions and antioxidative activities of two blueberry varieties cultivated in South Korea. Korean J. Food Preserv. 21(6), 790–798 (2014).
Lachowicz, S., Wiśniewski, R., Ochmian, I., Drzymała, K. & Pluta, S. Anti-microbiological, anti-hyperglycemic and anti-obesity potency of natural antioxidants in fruit fractions of Saskatoon berry. Antioxidants 8(9), 397 (2019).
Li, X., Li, C., Sun, J. & Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 309, 125617 (2020).
Manninen, H., Rotola-Pukkila, M., Aisala, H., Hopia, A. & Laaksonen, T. Free amino acids and 5′-nucleotides in Finnish forest mushrooms. Food Chem. 247, 23–28 (2018).
Li, D., Meng, X. & Li, B. Profiling of anthocyanins from blueberries produced in China using HPLC-DAD-MS and exploratory analysis by principal component analysis. J. Food Compos. Anal. 47, 1–7 (2016).
Moon, H. K., Lee, S. W. & Kim, J. K. Physicochemical and quality characteristics of the Korean and American blueberries. Korean J. Food Preserv. 20(4), 524–531 (2013).
Lachowicz, S., Seliga, Ł & Pluta, S. Distribution of phytochemicals and antioxidative potency in fruit peel, flesh, and seeds of Saskatoon berry. Food Chem. 305, 125430 (2020).
Ochmian, I., Błaszak, M., Lachowicz, S. & Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 10(1), 1–14 (2020).
Ochmian, I. D. The impact of foliar application of calcium fertilizers on the quality of highbush blueberry fruits belonging to the ‘Duke’ cultivar. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 40(2), 163–169 (2012).
Khan, N. et al. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 52(2), 355–363 (2020).
Zhang, H. et al. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an amino acid analyzer and ICP-MS with micro-wave digestion. Food Chem. 147, 189–194 (2014).
Ochmian, I. et al. The feasibility of growing highbush blueberry (V. corymbosum L.) on loamy calcic soil with the use of organic substrates. Sci. Hortic. 257, 108690 (2019).
Jarosz, M., Rychlik, E., Stoś, K., & Charzewska, J. Normy żywienia dla populacji Polski i ich zastosowanie (pp. 68–437). Warsaw, Poland: Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny (2020).
Alagawany, M. et al. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health–a comprehensive review. Vet. Q. 41(1), 1–29 (2021).
Montecchiarini, M. L. et al. Metabolic and physiologic profile during the fruit ripening of three blueberries highbush (Vaccinium corymbosum) cultivars. J. Berry Res. 8(3), 177–192 (2018).
Ranogajec, A., Beluhan, S. & Šmit, Z. Analiza nukleozydów i nukleotydów monofosforanowych z grzybów metodą HPLC z odwróconymi fazami. J. Sep. Sci. 33(8), 1024–1033 (2010).
Li, D., Limwachiranon, J., Li, L., Du, R. & Luo, Z. Involvement of energy metabolism to chilling tolerance induced by hydrogen sulfide in cold-stored banana fruit. Food Chem. 208, 272–278 (2016).
Liu, H., Jiang, Y., Luo, Y. & Jiang, W. A simple and rapid determination of ATP, ADP and AMP concentrations in pericarp tissue of litchi fruit by high-performance liquid chromatography. Food Technol. Biotechnol. 44(4), 531–534 (2006).
Wang, R. et al. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci. Biotechnol. 25, 137–143 (2016).
Yang, J. H., Hsiu-Ching, L. & Jeng-Leun, M. Non-volatile taste components of several commercial mushrooms. Food Chem. 72(4), 465–471 (2001).
Prior, R. L., Lazarus, S. A., Cao, G., Muccitelli, H. & Hammerstone, J. F. Identification of procyanidins and anthocyanins in blueberries and cranberries (Vaccinium spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 49(3), 1270–1276 (2001).
Kapusta, I., Cebulak, T. & Oszmiański, J. The anthocyanins profile of red grape cultivars growing in south-east Poland (Subcarpathia region). J. Food Meas. Charact. 11, 1863–1873 (2017).
Figueiredo, M. et al. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 130(1), 9–19 (2012).
Yang, H. et al. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 111, 114–127 (2021).
Oszmiański, J. & Wojdylo, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 221(6), 809–813 (2005).
Bitzer, Z. T. et al. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J. Nutr. Biochem. 26(8), 827–831 (2015).
Gu, L. et al. Fractionation of polymeric procyanidins from lowbush blueberry and quantification of procyanidins in selected foods with an optimized normal-phase HPLC−MS fluorescent detection method. J. Agric. Food Chem. 50(17), 4852–4860 (2002).
Tripathi, S. et al. Evaluation of anti microbial and antioxidants potential of blueberry extracts. Int. J. Aguat. Sci. 12(2), 5478–5492 (2021).
Burdulis, D. et al. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Poloniae Pharm. 66(4), 399–408 (2009).
Podsedek, A., Majewska, I., Redzynia, M., Sosnowska, D. & Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 62(20), 4610–4617 (2014).
Johnson, M. H., Lucius, A., Meyer, T. & Gonzalez de Mejia, E. Cultivar evaluation and effect of fermentation on antioxidant capacity and in vitro inhibition of α-amylase and α-glucosidase by highbush blueberry (Vaccinium corymbose). J. Agric. Food Chem. 59(16), 8923–8930 (2011).
Fabroni, S., Ballistreri, G., Amenta, M., Romeo, F. V. & Rapisarda, P. Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J. Sci. Food Agric. 96(14), 4713–4723 (2016).
You, Y. et al. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods 41, 62–71 (2018).
Dermengiu, N. E. et al. A dark purple multifunctional ingredient from blueberry pomace enhanced with lactic acid bacteria for various applications. J. Food Sci. 87(10), 4725–4737 (2022).
Castrejón, A. D. R., Eichholz, I., Rohn, S., Kroh, L. W. & Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 109(3), 564–572 (2008).
Moyer, R. A., Hummer, K. E., Finn, C. E., Frei, B. & Wrolstad, R. E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 50(3), 519–525 (2002).
Stănilă, A., Braicu, C. & Stănilă, S. Antibacterial activity of copper and cobalt amino acids complexes. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 39(2), 124–129 (2011).
Kapusta, I., Cebulak, T. & Oszmiański, J. Characterization of polish wines produced from the interspecific hybrid grapes grown in southeast Poland. Eur. Food Res. Technol. 244(3), 441–455 (2018).
Ochmian, I., Oszmiański, J., Lachowicz, S. & Krupa-Małkiewicz, M. Rootstock effect on physicochemical properties and content of bioactive compounds of four cultivars Cornelian cherry fruits. Sci. Hortic. 256, 108588 (2019).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med. 26(9–10), 1231–1237 (1999).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 239(1), 70–76 (1996).
Acknowledgements
Authors acknowledge the financial support provided by the A. S. Dekaban Foundation for supporting the stay of Sabina Lachowicz-Wiśniewska as Visiting Assistant Professor at the UBC Food Process Engineering Laboratory, Vancouver, Canada (01.2020-05.2020).
Funding
This research was partially supported by the National Science and Engineering Research Council of Canada (NSERC) Discovery grant (grant No. RGPIN-2018-04735).
Author information
Authors and Affiliations
Contributions
S.L.-W.: conceptualization, methodology, software, investigation, formal analysis, writing—original draft, writing—review & editing, funding acquisition, project administration. A.P.-S.: conceptualization, investigation, writing—original draft, writing—review & editing, funding acquisition, I.O.: methodology, formal analysis, writing—original draft. I.K.: methodology, formal analysis, writing—review & editing, S.P.: resources, writing—review & editing. A.K.: software, writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lachowicz-Wiśniewska, S., Pratap-Singh, A., Ochmian, I. et al. Biodiversity in nutrients and biological activities of 14 highbush blueberry (Vaccinium corymbosum L.) cultivars. Sci Rep 14, 22063 (2024). https://doi.org/10.1038/s41598-024-71114-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71114-x