Abstract
Although various joint injuries result in post-traumatic osteoarthritis (PTOA), differences in chondrocyte response to specific injuries, such as blunt compression or fracture, are unclear. Furthermore, the role of underlying joint inflammation, or synovitis, is often not considered. We investigated how injury mechanisms and underlying synovitis affect chondrocyte gene expression using osteochondral injury models with synovial co-culture. We hypothesized that the state of synovitis as well as the mechanism of biomechanical cartilage injury differentially alter the gene expression of chondrocytes and that these responses are regulated by the pro-inflammatory cytokine interleukin 1 (IL-1). The mechanism of injury and level of synovial inflammation both significantly regulated chondrocyte gene expression and associated pathways, uncovering distinct characteristics of fracture and compression injury mechanisms. Targeting IL-1 following injury reduced the inflammatory response and could have clinical implications. The results from this study show that crosstalk between biomechanics and inflammation in the context of synovitis and cartilage injury mechanism is an important consideration for PTOA.
Similar content being viewed by others
Introduction
Post-traumatic osteoarthritis (PTOA) is an important contributor to the overall burden of osteoarthritis (OA)1. Two aspects of PTOA make study of this condition particularly compelling: (1) PTOA is an important cause of joint disability, accounting for approximately 3.2 million symptomatic cases of arthritis in the US2, and (2) trauma is often a known event that heralds the onset of PTOA, which provides an opportunity for investigation of the onset of disease. The injury mechanism leading to PTOA can vary widely between patients, ranging from a blunt compression of the articular surface with an underlying bone bruise, to frank intra-articular fracture extending through the articular cartilage and underlying bone. However, the progression of PTOA following these two common injury mechanisms, blunt compression versus articular fracture, on articular cartilage and the joint as a whole is not fully elucidated.
Blunt compression of the cartilage, as can occur with an ACL injury, results in bone bruises without articular surface disruption3,4,5, changes in synovial pro-inflammatory cytokines6,7,8,9,10, and measurable changes in articular cartilage using MRI as early as 12 months following injury11,12,13,14,15. Patients with ACL injuries are at increased risk of developing advanced OA within 10 years of injury16,17,18,19,20,21,22,23. On the other hand, patients with displaced intra-articular fractures can present with end-stage OA within 1–2 years of injury24. This rapid presentation of end-stage disease could reflect a more severe articular cartilage injury due to intra-articular fracture. Similar to ACL injury, an acute increase in pro-inflammatory cytokines, including IL-1, in the synovial fluid has been reported following intra-articular fracture25,26,27,28,29,30. The inflammatory pathways activated in articular cartilage following either compression or fracture injury are likely influenced by other joint tissues, and this interaction may drive PTOA development. Importantly, the inhibition of IL-1 in this context has been shown to significantly reduce the severity of PTOA in a preclinical model31,32. However, the respective roles of different cell types in the joint, and their interactions following joint injury, are not fully understood. As the response to joint injury is thought of as an organ system response that has the potential to involve all tissues in the intra-articular environment, including articular chondrocytes, synoviocytes and immune cells in the synovium, bone, and bioactive mediators released by these cells into the synovial fluid. The development of PTOA from a local injury to whole joint arthritis as an organ system response is supported by animal models and patient data33,34,35. Importantly, differences in the acute inflammatory response of articular cartilage to different injuries is not fully elucidated.
To examine specific mechanisms of crosstalk between synovium and articular cartilage, we used explant injury models to allow for the direct study of the acute effects of specific injury types on articular cartilage, while excluding variability that may occur in vivo with animal models36. We previously characterized porcine osteochondral core loading models with 2-distinct injury mechanisms: (1) uniform compression without fracture that results in apoptosis throughout the articular surface, and (2) point loading with osteochondral fracture that predominantly results in cell necrosis along the fracture37. After each injury type, culture media from osteochondral cores stimulated activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, with fracture media having the greatest stimulatory effect37. These findings suggest that, similar to humans, both compression and fracture injury mechanisms activate pro-inflammatory pathways that are involved in disease progression37,38. These prior experiments were performed without co-culture with synovial tissue. However, both human and animal data indicate that synovitis develops in the injured joint following injury28,39. Combining articular injury of osteochondral cores with synovial cells in co-culture may better represent the articular environment following an articular injury40,41,42. In normal joints, healthy articular cartilage is surrounded by synovial tissue, which can become inflamed following different types of joint injury resulting in synovitis. The interaction of underlying synovial inflammation on the cartilage response to different articular injuries has not been explored.
We hypothesized that high synovitis co-culture and articular injury will increase inflammatory chondrocyte gene expression and that inhibiting IL-1 will modulate this response. To test this hypothesis, articular injury was induced in osteochondral cores generating either blunt compression injury of the articular surface or articular fracture. To simulate the joint organ system, cores were then co-cultured with cells from normal (low synovitis) synovium versus cells from inflamed (high synovitis) synovium with and without IL-1 receptor antagonist (IL-1RA) to determine the potential role of IL-1 in this response, as implicated by previous in vivo studies of PTOA31 (Fig. 1). The objectives of this study were to determine if the type of articular injury with co-culture of low synovitis cells versus high synovitis cells differentially regulate chondrocyte inflammatory gene expression and, secondarily, if inhibition of IL-1 signaling alters the cross-talk in the co-culture injury models.
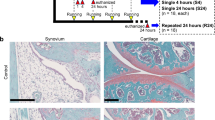
Study design (a) Experimental conditions for co-culture studies included osteochondral injury mode (no injury, compression, fracture), co-culture with synovial cells (no cells, low synovitis, high synovitis) and IL-1 inhibition with IL-1RA (0 ng/ml, 1000 ng/ml). (b) Study outcomes with assays performed.
Materials and methods
Synovitis assessment
Fresh intact porcine knee joints were obtained from 2–3 y.o. skeletally mature female pigs sacrificed at a local abattoir within 12 h of slaughter. Using sterile technique, porcine joint capsules were opened the same day, and gross images of the anterior region of the synovium overlying the infra-patellar fat pad region were obtained (Fig. 2a). Additionally, three synovial punch biopsies from each synovium (Supplemental Fig. S1) were obtained, formalin fixed, paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E). The level of underlying synovitis in the synovium was determined by three blinded graders using both a gross synovitis score of images of the entire region of the synovium to be harvested43 and the Krenn histopathologic synovitis score44 of H&E sections from the three biopsied regions in each synovium. The gross and histologic synovitis scores were summed for a combined score, with a maximum of 12, indicating a high level of synovitis, and a minimum score of 0, indicating little to no synovitis.
Synovial cell co-culture with osteochondral injury models. (a) Synovial cell superlots of normal (low synovitis) and inflamed (high synovitis) cells were isolated and cultured on transwells for 4 days. (b) Osteochondral cores from lateral condyles were subjected to fracture (Fx), injurious compression, or no injury, and (c) co-cultured for 3 days.
Synovial cell superlots
Synovial cells were isolated by mechanical disruption and then enzymatic digestion from the remaining synovial tissue of each knee after the biopsy punches were obtained. Minced synovial tissue was first digested with 0.5% pronase (Calbiochem 53,702) in Isocove’s modified dubelco’s medium (IMDM, Corning 10-016-CV) with 37.5 µg/mL L-ascorbic acid 2-phosphate (Sigma #A8960-5G), 0.9 mM sodium pyruvate (Gibco #11,360,070), and 10X antibiotic–antimycotic solution (pen/ptrep/fungiezone, HyClone #SV30079.01) for 1 h and then 0.2% collagenase type I (Worthington LS004196) in the same medium above plus 10% fetal bovine serum (Hyclone SH30071.02) overnight. Cells were then sequentially filtered (200 µm CellMicroSieve, BioDesign Inc of New York #N200S, and 70 µm cell strainer, Falcon #352,350), followed by a red blood cell lysis using ACK lysing buffer (Lonza #10-549E). The cells isolated from synovial tissue of each knee joint were cryopreserved in IMDM with 10% FBS and 10% DMSO (Sigma #D2650). Based on synovitis grading (described above), synovial cells from the 4 joints with the least synovial inflammation were thawed and combined to create a low synovitis superlot and synovial cells from 4 knees with the highest evidence of synovial inflammation were combined to create a high synovitis superlot (Fig. 3). The superlots were cryopreserved in IMDM with 10% FBS and 10% DMSO (Sigma #D2650) for future use.
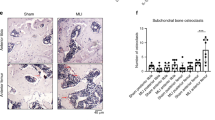
Synovial pathology and immune cell phenotypes in porcine knee joint capsule. Representative images of (a) Anterior joint capsule with synovium overlying the infra-patellar fat pad from porcine knees with normal synovial tissue (low synovitis) on left and inflamed synovial tissue (high synovitis) on right. (b) Representative images of histology sections stained with hematoxylin and eosin showing cellular infiltrate in porcine synovium with low and high synovitis. Scale bar = 1 mm. (c) Combined synovitis score summed from gross scoring of the synovium and histologic scoring of synovitis. Data presented as mean + SEM (n = 4 per group) with statistical analysis performed using unpaired t-test (*p = 0.04). (d) Representative image of immune cell phenotype of normal (low synovitis) and inflamed (high synovitis) porcine synovial cell superlots. High synovitis CD14+ monocytes expressed higher frequencies of SLA-DR+ CD163– and SLA-DR– CD163+ subsets compared to low synovitis superlots. Data reported as percent of parent gate.
Phenotyping of cells in the low and high synovitis superlots
Antibodies The following antibodies were selected for specificity and reactivity with porcine according to the manufacturer’s specifications and used for flow cytometry: Alex Fluor 700-conjugated CD14 (clone TUK4), Alexa Fluor 647-conjugated CD21 (clone CC51), PE-conjugated CD163 (clone 2A10/11), and FITC-conjugated SLA-DR (clone 2E9/13) from Bio Rad (Hercules, CA); and PerCp-Cy5.5-conjugated CD3e (clone BB23-8E6-8C8) and BUV395-conjugated CD90 (Thy-1) (clone 5E10) from BD Pharmingen (San Jose, CA). After thawing cryopreserved low and high synovitis synovial superlot cells and washing twice with RPMI medium (Gibco 21870-containing 10% FBS (R10), cell number and viability were calculated. A total of 1 × 106 viable cells per sample were plated in a 96-well round bottom plate in R10 media. After centrifugation and removal of media, cells were stained with Zombie viability dye (Biolegend; San Diego, CA) for 15 min at room temperature. Cells were washed with PBS, then stained for 30 min at 4 °C with an antibody cocktail for surface proteins listed above. Following cell surface staining, cells were washed twice with PBS + 2% FBS, acquired on a LSR Fortessa flow cytometer (BD Biosciences; San Jose, CA), and data were analyzed using Flowjo software (BD Biosciences).
IL-1-stimulated NF-κB activity in Low and High Synovitis Cells
As IL-1-induced NF-κB activation is known to play a role in synovial inflammation associated with arthritis development45,46,47, the level of NF-κB transcriptional activity was assessed in the low and high synovitis populations. Cells from each superlot were transduced with a lentivirus reporter produced as previously described48. The lentiviral construct contains a negative regulatory element (NRE), tandem NF-κB response elements (NF-κB RE), and a TATA box from the minimal CMV promoter to drive expression of the firefly luciferase (Luc) transgene in response to IL-1-induced NF-κB transcriptional activity (Fig. 4a). To transduce cells with lentiviral vectors containing this reporter, synovial cells were incubated overnight with 1:1 virus and base media plus 4 μg/ml polybrene. Base media contained IMDM (Corning 10–016-CV) with 10% fetal bovine serum (FBS, Hyclone SH30071.02), 37.5 µg/mL L-ascorbic acid-2-phosphate (Sigma A8960), and 1X antibiotic–antimycotic solution (pen/strep/fungizone, HyClone #SV30079.01). After transduction, cells were plated on collagen-coated plates (Corning 354,249) and then treated with IL-1α (R&D Systems 680-PI-010) at concentrations of 0, 0.1, 1, or 10 ng/ml (n = 6 per group). Cells were lysed and assayed at 0, 8, or 12 h for luminescence using the One-Glo luminescence assay kit (Promega E6110), according to the manufacturer’s instructions. Luminescence was normalized to background levels in untreated cells and reported as fold change in NF-κB activity. In a second experiment to assess the ability of IL-1RA (anakinra, Sobi) to modulate IL-1-stimulated NF-κB activity, low and high synovitis cells were transduced and then treated with 1 ng/mL IL-1α in combination with 0, 100, or 1000 ng/mL IL-1RA for 8 h. Luminescence data were analyzed at each time point using a two-way ANOVA and Tukey’s post-hoc test with synovitis level and either IL-1 or IL-1RA dose as the second factor.
IL-1-stimulated NF-κB activity in Low and High Synovitis cell superlots. (a) NF-κB activity assay—schematic showing viral construct driving IL-1 mediated luciferase (Luc) expression under control of NF-κB response elements. RE: regulatory element; NRE: negative regulatory element (b) NF-κB activity due to IL-1 dosing in low and high synovitis superlot cells expressed as fold-change of luminescence units relative to control at 4 h (left), 8 h (middle), and 12 h (right). (c) IL-1RA modulation of IL-1-mediated NF-κB response in low and high synovitis superlot cells expressed in fold-change of luminescence units relative to control at 8 h with IL-1RA. Data presented as mean + SEM (n = 6 per group) with statistical analysis performed using two-way ANOVA with Tukey's post-hoc, *p = 0.0187, **p ≤ 0.0079, ***p ≤ 0.006, ****p < 0.0001.
Injury and co-culture experiments
Co-culture transwell inserts (Nunc 141,002) were coated with 500 μL of type 1 collagen (Corning 354,249) at 40 µg/ml in PBS (Gibco #10,010–023). Previously cryopreserved synovial superlot cells were then thawed and plated at a cell density of 4.7 × 105 cells per transwell and cultured for 4 days prior to co-culture with osteochondral cores (Fig. 2a). Base media, as detailed above, was used for all subsequent co-culture experiments (IMDM with 10% FBS, 37.5 µg/mL L-ascorbic acid-2-phosphate, and 1X antibiotic–antimycotic solution). Media were changed on day 3 (one day prior to co-culture).
On day 4, porcine osteochondral cores were harvested from freshly obtained porcine joints without gross evidence of arthritis (no fibrillation or loss of cartilage) or inflamed synovium and prepared in the manner indicated previously37. Briefly, three osteochondral cores with a diameter of 6.35 mm and a bone thickness of 1 mm were obtained from the lateral femoral condyle of 36 different joints using a sterile coring bit and drill press. Cores from the anterior, middle and posterior regions of the condyle (Fig. 2b) were distributed to the various loading conditions outlined below. Prior to loading, all cores were washed with sterile PBS followed by sterile base media with 10 × antibiotic–antimycotic solution for 1 h.
Two osteochondral loading models, compression or fracture, were used to represent articular injury using previously published methods37. Briefly, all cores were loaded at 100%/s strain rate using a uniaxial load frame (Endura TEC SmartTest). For samples assigned to compression injury (blunt loading without fracture), the load was applied using a flat surface to the cartilage side of the osteochondral core at 70% cartilage strain (Supplemental Methods – video S1). For fracture injury, the load was applied with a spherical surface to the bone side of the osteochondral core at 90% strain (Supplemental Methods – video S1). Samples assigned to “no injury” underwent no loading, as outlined in Fig. 2b.
Next, osteochondral cores were co-cultured with or without the previously prepared cells from the low synovitis or high synovitis superlots (Fig. 2c). Synovial cell isolates did not come in direct contact with the osteochondral cores but were instead separated by the transwell system (Nunc 141002) permitting only small molecule transfer with a pore size of 0.4 μm. One group of osteochondral cores without synovial cell isolates was kept as control. Samples were co-cultured for a total of 3 days. At the end of three days, media was collected and stored at − 80 °C for further analysis. Cartilage from the osteochondral cores was harvested and stored in RNALater (Thermo Fisher) at 4ºC.
IL-1 inhibition
The above injury and co-culture experimental conditions were then repeated using freshly harvested osteochondral cores, and for these experiments, IL-1RA (1000 ng/ml, anakinra, Sobi) was added to base media at the onset of co-culture to investigate the effect of inhibiting IL-1 on chondrocyte gene expression.
Cytokine multiplex assay
Inflammatory cytokines were quantified in media collected at day 3 of co-culture without the addition of IL-1RA. The Luminex MAGPIX® system (Luminex Corp, Austin, TX) was used with the Milliplex porcine cytokine 13-plex panel (Cat# PCYTMG-23 K-13PX, Millipore Sigma, Burlington, MA) which quantified GM-CSF, IFNγ, IL-1α, IL-1β, IL-1RA, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-18, and TNF-α. All samples (n = 5 per group) were analyzed as recommended by the manufacturer, using 25 μL of undiluted cell culture supernatant. Statistical analysis was performed using two-way ANOVA with Tukey’s post-hoc test with synovial cells (no cells, low synovitis, high synovitis) as one factor and osteochondral core state (no core, uninjured core, compression injury, fracture) as the second factor.
Porcine inflammatory cytokines and receptors PCR array
RNA isolation from articular cartilage was performed using a two-step TRIzol protocol. Briefly, cartilage was separated from the subchondral bone and immediately pulverized in TRIzol (Life Technologies, 15,596,026) in a liquid nitrogen-cooled multi-sample biopulverizer (BioSpec 59012MS), frozen at − 80 °C overnight and then homogenized using ceramic beads (Omni International 19-627) in the BeadRuptor 12 (Omni International) for 6 cycles of 30 s on high. Following separation with chloroform, RNA was then purified using the RNEasy universal mini kit (Qiagen 1,062,832) following the manufacturer’s protocol. RNA was pooled from two cores (n = 3 pooled samples per group, run in duplicate when sufficient RNA was available). The commercially available Pig Inflammatory Cytokines and Receptors RT2 Profiler PCR array (Qiagen 330,231, Supplemental Table S1) was used to evaluate 84 inflammatory genes. The array was chosen based on published data indicating that genes for cytokines, chemokines and their receptors are upregulated following injurious loading and exposure to catabolic factors, like cartilage matrix components49,50. Using 1 µg of RNA, relative mRNA expression of each gene of interest was normalized to the housekeeping gene, RPL13A, and then compared to the mRNA expression of the control group using the 2−ΔΔCT method51.
Gene expression statistical analysis
Statistical analysis was performed on the gene expression data using Matlab (Mathworks, Natick, MA) via multifactor ANOVA with a Bonferroni correction for multiple comparisons for the various experimental conditions with a significance level of p < 0.05 for the adjusted p-values52. Custom MATLAB code was utilized to format and analyze the PCR array data (see Supplemental Methods). To achieve normal distributions, the relative fold change in gene expression was log transformed. Statistical analysis was performed independently for each set of experiments.
For the experiment evaluating the effect of injury mode and synovial co-culture, relative chondrocyte gene expression was compared to the control group representing a “normal joint” (low synovitis and no injury). A second analysis was performed with relative gene expression determined from direct comparisons of injury modes with synovial co-culture conditions. Statistical significance was determined using a two-way ANOVA with injury mode as one factor (no injury, compression, fracture), and synovial co-culture condition (no cells, low synovitis, high synovitis) as the second factor.
For the experiment evaluating the effect of IL-1 inhibition, relative chondrocyte gene expression with IL-1RA was compared to no IL-1RA for each individual injury mode and synovial co-culture condition. Statistical significance was again determined using a two-way ANOVA with injury mode as one factor (no injury, compression, fracture) and IL-1RA (0 ng/ml, 1000 ng/ml) as the second factor.
Ingenuity pathway analysis (IPA)
The default core analysis of Ingenuity Pathway Analysis (IPA) software (version 01-20-04, Qiagen) was used to identify enriched canonical pathways and molecular networks from differentially expressed genes53. This software compared the gene expression for the porcine genes with human analogs of its Ingenuity® Knowledge base. For all genes in the array, gene IDs, expression data, and adjusted p-values from multiple ANOVA comparisons (including all experimental groups assessing chondrocyte gene expression by osteochondral injury mode and synovial co-culture condition) were uploaded together to the IPA core analysis applying a Benjamini–Hochberg (B–H) correction for false discovery rate (FDR) errors. This allowed for all gene expression data to be compared to find the pathways significantly enriched by the varying experimental states. Analysis was performed using standard settings, and results with B–H multiple-testing corrected p-values (q-values) < 0.05 were considered significant. Top canonical pathways enriched with osteochondral injury and synovial cell co-culture were identified based on the resulting activation z-score.
IL-1 was identified as an upstream regulator for these injury states. For this reason, the above experimental conditions were repeated with IL-1 inhibited using IL-1RA (1000 ng/ml). IPA analysis was again run to identify top canonical pathways related to osteochondral injury and synovial co-culture that were altered by inhibition of IL-1.
Results
Characterizing synovial cell superlots
Synovial pathology
Synovial cells from porcine knees with little or no gross synovial pathology (Fig. 3a) were pooled to generate a low synovitis cell superlot (N = 4). Synovium from these low synovitis joints showed minimal cellular infiltrate histologically (Fig. 3b). Joints with grossly inflamed synovium were used to generate a high synovitis cell superlot (Fig. 3a, N = 4) and showed increased cellular infiltrate histologically (Fig. 3b). The combined synovitis score (Fig. 3c) for the high synovitis superlot, 10.3 ± 3.1, was significantly greater than that of the low synovitis superlot, 6.0 ± 2.1 (p = 0.04).
Cellular subsets in low and high synovitis superlots
Flow cytometry analyses revealed similar populations of cells in the high and low synovitis populations, with very low (< 0.01%) or undetectable percentages of T cells and B cells (Supplemental Table S2). The fraction of cells expressing CD90 + , a synovial fibroblast marker54,55,56,57, was lower in the high synovitis population compared to low synovitis (Supplemental Table S2). Within the CD14 + monocyte population, high synovitis cells expressed higher frequencies of both activated SLA-DR + CD163- monocytes, 21.4%, and SLA-DR + CD163 + mature macrophages, 21.4%, compared to low synovitis cells comprised of 9.3% activated SLA-DR + monocytes and 9.4% activated SLA-DR + macrophages (Fig. 3d).
IL-1-stimulated NF-κB activity in low and high synovitis cell superlots
To identify functional differences between the superlots of synovial cells from joints with low and high synovitis, a reporter assay was used to assess IL-1 mediated NF-κB activity (Fig. 4a). Following transduction of cells from each superlot with the reporter construct, cells were challenged with three different doses of IL-1α for up to 12 h (Fig. 4b). Low synovitis cells showed a lower fold change in NF-κB activity compared to high synovitis for a given dose of IL-1 at 4 and 8 h (p ≤ 0.046). At 12 h, the fold change in NF-κB activity for Low synovitis cells was statistically lower than High Synovitis cells at the higher doses of 1 and 10 ng/ml (p ≤ 0.00001), but not the lowest dose of 0.1 ng/ml (p = 0.9998). Low synovitis cells also differed from the high synovitis superlot cells in terms of the dose response (Fig. 4b). In particular, high synovitis superlot cells showed increased NF-κB activity with increasing IL-1 concentrations up to 1 ng/mL with a plateau at 10 ng/mL. On the other hand, low synovitis superlot cell NF-κB activity showed an increase from low to mid doses but then a decrease from mid to high doses. To see if NF-κB activity could be mitigated, cells were then incubated with IL-1 at 1 ng/ml for 8 h with the addition of IL-1RA at various concentrations and fold change in NF-κB activity was assessed. Again, high synovitis superlot cells had greater fold increases in NF-κB activity in the presence of IL-1. However, IL-1RA significantly reduced IL-1-mediated NF-κB activity to baseline levels in both high and low synovitis superlot cells (Fig. 4c).
Effect of injury mode and synovial co-culture conditions
Cytokine release from osteochondral injury models with synovial cell co-culture
Two cytokines, IL-6 and IFN-γ, changed significantly in osteochondral—synovial co-cultures; IL-6 responded to injury, while IFN-γ responded to level of synovitis. IL-6 release into media was significantly greater in all conditions with osteochondral cores compared to media from synovial cells alone (Fig. 5a). Synovial cells alone, either low or high synovitis, released minimal levels of IL-6. Compared to no injury, compression yielded greater IL-6 release regardless of synovial cell condition (Tukey’s post-hoc, p = 0.018). IL-6 release with fracture was also greater than no injury with no effect of synovial co-culture condition, but was not statistically significant (p = 0.08), indicating that the release of IL-6 is related to the presence of the osteochondral core and either cartilage injury mechanism increases the level of IL-6.
Cytokine release from osteochondral injury models with synovial cell co-culture. (a) IL-6 concentrations in media from osteochondral injury models (no injury, compression, fracture) with synovial co-culture conditions (no cells, low synovitis, high synovitis). The osteochondral injury state resulted in statistical differences in IL-6 (p = 0.008), with no significant interaction from underlying synovitis (ANOVA, p = 0.671). Compared to no injury, IL-6 release was greater with compression injury (Tukey’s post-hoc, **p = 0.018) and was greater with fracture, although not statistically significant (post-hoc, ***p = 0.080) regardless of synovial cell condition. (b) IFNγ release was significantly greater for all samples with high synovitis cells compared to samples with low synovitis cells (post-hoc, *p = 0.016) or osteochondral cores cultured without synovial cells (post-hoc, **p = 0.007). All media collected after 3 days of co-culture. Data presented as mean ± SD (n = 5 per group) with statistical analysis performed using two-way ANOVA with Tukey’s post-hoc test.
In contrast, IFN-γ concentrations (Fig. 5b) in the media were statistically elevated in all conditions cultured with high synovitis superlot cells compared to both low synovitis superlot co-culture (p = 0.016) or osteochondral cores cultured without synovial cells (p = 0.007). However, there was no significant effect on IFN-γ release in the presence of osteochondral cores or injury mode (p = 0.494). The increase in IFN-γ levels from low to high synovitis state, regardless of injury, suggests that synovial cells are the source of IFN-γ.
For all other measured cytokines, no statistically significant differences were found in concentrations of IL-4, IL-8, IL-18 and IL-1RA, and the concentrations of the remaining cytokines were below the level of detection for the assay (Supplemental Table S3).
Chondrocyte gene expression of inflammatory cytokines and receptors
Establishment of low synovitis with uninjured core as control for comparison Data presentation of gene expression requires a control group for comparison across differing injury and co-culture conditions. Uninjured cores co-cultured with low synovitis superlot cells (normal synovium) were selected as the control group for relative comparisons in gene expression. This group was chosen as it represented conditions in a “normal joint”. The low synovitis cells were obtained from joints with a normal, white appearance of the synovium, as indicated by significantly lower synovitis scores. Comparing this group to uninjured cores alone, only one gene, CSF3, was significantly upregulated compared to normal synovitis control (Fig. 6a), indicating that cells from normal synovium had a minimal effect on chondrocyte gene expression of uninjured cores.
Chondrocyte gene expression and Ingenuity Pathway Analysis differentially regulated by osteochondral injury mode and synovial cell co-culture. Relative gene expression for (a) Injury mode (no injury, compression, fracture) and co-culture with synovial cells (No Cells, Low synovitis, High synovitis) compared to Low synovitis cells and No injury, representing “normal joint” control group and (b) direct comparisons of injury modes, compression (C) and fracture (Fx), co-cultured with Low or High synovitis cells. Gene expression data (n = 3 per group) presented as fold change (FC) with statistical analysis performed using two-way ANOVA with Bonferroni’s correction for multiple comparisons (adjusted p-values reported). Genes highlighted in orange are significantly upregulated, and genes highlighted in blue are significantly downregulated. Significant p-values shown in red. Ingenuity pathway analysis activation z-scores with B–H corrected p-values for top canonical pathways enriched by (c) injury mode (no injury, compression, fracture) and co-culture with synovial cells (no cells, low synovitis, high synovitis) compared to low synovitis cells and no injury, representing “normal joint” control group; and (d) Direct comparisons of compression (C) and fracture (Fx) injury modes co-cultured with low or high synovitis cells.
Effect of injury with low synovitis cells Compression injury with low synovitis co-culture significantly upregulated 9 genes (CCL20, CCL5, CCL8, CCR1, CXCR4, LIF, IL33, TNFRSF11B, LOC396594) and downregulated 0 to compared to control (Fig. 6a). Fracture injury with low synovitis co-culture only upregulated 5 genes (CCL20, CCL4, CCL5, IL5, TNFRSF11B) and downregulated 0 compared to control. Both injury modes upregulated CCL20, CCL5 and TNFRSF11B.
Effect of injury with high synovitis cells In contrast to co-culture with low synovitis cells, compression injury with high synovitis cells significantly upregulated only 2 genes (CCL5, TNFRSF11B) and downregulated 1 gene (CCL1) compared to control (Fig. 6a). However, fracture injury with high synovitis cells significantly altered the same genes as compression injury (upregulated CCL5 and TNFRSF11B and downregulated CCL1) but also upregulated an additional 4 genes (CCL20, IL1RN, IL4R, NAMPT) compared to control.
Direct comparison of injury modes A secondary analysis directly compared relative chondrocyte gene expression of fracture to compression injury with the co-culture conditions of low or high synovitis superlot cells (Fig. 6b). In low synovitis co-cultures, fracture resulted in 2 genes with significantly higher relative expression (FLT3LG, TNFSF10) and 4 genes with significantly lower relative expression (CCR1, CXCL8, IL33, LOC100621682) compared to compression injury. With high synovitis co-culture, fracture injury resulted in 1 gene with significantly higher relative expression (IL1RN) and no genes with significantly lower relative expression compared to compression injury.
Canonical pathways altered by injury mode and synovial co-culture
Ingenuity pathway analysis (IPA) analyzed gene expression patterns among all groups to identify significantly enriched pathways. NF-κB was the top canonical pathway activated by both compression and fracture injury co-cultured with low or high synovitis superlot cells (Fig. 6c). Fracture compared to injurious compression had lower pro-inflammatory pathway activation with low synovitis superlot cells but greater activation with high synovitis superlot cells (Fig. 6c). Direct comparisons between compression injury and fracture in low synovitis conditions (Fig. 6d) highlighted that the NF-κB pathway, along with many others, was less activated with fracture compared to compression injury. However, with high synovitis cells, the opposite was true in that fracture resulted in greater NF-κB pathway activation. This trend of activation showed that in a low synovitis environment, compression activated more pro-inflammatory pathways than fracture. However, in a high synovitis environment, fracture activated more pro-inflammatory pathways than compression. Additional pathways of interest to PTOA demonstrated the same differential patterns of activation between the experimental groups, including the wound healing pathway, osteoarthritis pathway, HMGB1 signaling, and toll-like receptor signaling (Fig. 6d). Other cytokines and pathways differentially activated by synovitis level and injury included IL-17, IL-6, acute phase response signaling, and adrenomedullin signaling, which are associated with the response to trauma or injury58,59,60. These data support our hypothesis that co-culture of injured osteochondral cores with high synovitis cells upregulated chondrocyte gene expression of pro-inflammatory pathways. The greater activation of pro-inflammatory pathways with high synovitis co-culture of injured osteochondral cores supports our hypothesis that chondrocyte gene expression is upregulated in injured osteochondral cores when co-cultured with high synovitis cells.
Differential chondrocyte gene expression with IL-1RA in osteochondral injury models with synovial co-culture
To identify IL-1-mediated inflammatory cytokines and receptor genes regulated by osteochondral injury and synovial co-culture, relative chondrocyte gene expression with the addition of IL-1RA was directly compared to co-culture without IL-1RA for each experimental group.
Effect of IL-1RA with no cells The addition of IL-1RA to osteochondral cores without synovial cells resulted in downregulation of various inflammation-related genes. Without injury, 14 genes were significantly downregulated with the addition of IL-1RA (Fig. 7a). With compression injury, only 2 genes were downregulated with IL-1RA (CCL4, CSF1), and with fracture, only CCR10 was significantly downregulated.
Effect of inhibition of IL-1 on chondrocyte gene expression and associated pathways in osteochondral injury models with synovial co-culture. (a) Relative gene expression for all experimental groups in the presence of IL-1RA compared to the same group without IL-1RA. Data (n = 3 per group) presented as fold change (FC) with statistical analysis performed using two-way ANOVA with Bonferroni’s correction for multiple comparisons (adjusted p-values reported). Genes highlighted in orange are significantly upregulated, and genes highlighted in blue are significantly downregulated. Significant p-values shown in red. (b) Ingenuity pathway Analysis activation z-scores with B–H corrected p-values for top canonical pathways differentially altered with IL-1RA by injury mode (no injury, compression, fracture) and co-culture with synovial cells (no cells, low synovitis, high synovitis).
Effect of IL-1RA with low synovitis superlot cells With low synovitis co-culture, IL-1RA downregulated 1 gene (CXCL8) with no injury. IL-1RA added to low synovitis co-culture downregulated 1 gene (CCL20) with compression and 7 genes (CCL4, CCL5, CCR3, CXCL10, CXCL12, IFNG, IL5RA) with fracture (Fig. 7a). These data suggest that IL-1RA was more effective at downregulating inflammatory genes in cartilage injured by fracture than compression in the presence of cells from normal synovium (low synovitis).
Effect of IL-1RA with high synovitis superlot cells With high synovitis co-culture and no injury, interestingly four genes were significantly upregulated (CCL22, CCR3, IL1B, IL5RA) with the addition of IL-1RA, and no genes were downregulated. With osteochondral injuries and high synovitis co-culture, the introduction of IL-1RA significantly downregulated 7 genes with compression (CCL22, CCR3, IL1B, IL5RA, IL6R, OSM, TNFSF10), but upregulated 5 genes with fracture (CCL17, CCL22, CCR3, IL1B, IL5RA) relative to the same injury state without IL-1RA (Fig. 7a). With compression injury, IL-1RA was effective at mitigating the chondrocyte inflammatory response with co-culture of cells from high synovitis superlot. However, IL-1RA was unable to modulate chondrocyte inflammatory gene expression with high synovitis superlot cells and fracture.
Effect of IL-1RA on canonical pathways altered by injury and synovial co-culture
Pathway analysis of post-injury gene activation was used to assess if IL-1 inhibition could regulate pathways that may be implicated with PTOA development. IPA analysis identified that the addition of IL-1RA with no cells was effective at downregulating the top pathways altered by both compression and fracture injury (Fig. 7b). In the context of low synovitis, IL-1RA downregulated almost all top canonical pathways in the absence and presence of injury (compression and fracture) (Fig. 7b). Interestingly, in the context of low synovitis, IL-1RA was most effective at downregulating pathways activated by fracture injury, including IL-17 signaling, FAK signaling, would healing signaling pathway, Th17 activation, and HMGB1 signaling. In the context of high synovitis, IL-1RA downregulated almost all top canonical pathways induced by compression, similar to the effect of with low synovitis cells (Fig. 7b). In the context of high synovitis, IL-1RA upregulated canonical pathways induced by fracture, including the following relevant pro-inflammatory pathways: IL-17 signaling, FAK signaling, Th17 activation, chemokine signaling, HIF1α signaling, ID1 signaling pathway, and IL-23 signaling (Fig. 7b). These data indicate that the addition of IL-1RA modulated the effects of co-culture with high synovitis cells with injurious compression, but not so with articular fracture. This pathway analysis identifies important differences in chondrocyte pro-inflammatory gene expression resulting from these two injury mechanisms.
Discussion
This study found that chondrocyte gene expression following two distinct articular injuries was highly reliant on the level of inflammation in the synovial environment. Differential chondrocyte gene expression resulting from compression or fracture of osteochondral cores is enhanced by co-culture with normal and high synovitis cells. Under normal, or low inflammatory synovial conditions, injurious compression of the cartilage activates more inflammatory pathways than fracture. However, in an inflamed synovial environment the opposite is observed, and fracture activated more inflammatory pathways than injurious compression (Fig. 8). Inhibition of IL-1 effectively decreased chondrocyte inflammatory gene expression and associated pathway activation in a low synovitis environment following injurious compression or fracture. However, in a high synovitis environment, IL-1 inhibition downregulated chondrocyte pro-inflammatory pathways following injurious compression but not following fracture. Characterization of the high synovitis superlot in the absence of osteochondral cores showed that IL-1-mediated NF-κB activity could be reduced to baseline with IL-RA. However, with the interaction of fracture and a high synovitis environment, chondrocyte inflammatory pathways could not be downregulated by IL-1RA. Therefore, this study shows that both the mechanism of injury to articular cartilage and the state of inflammation in the synovium significantly regulate chondrocyte gene expression and associated pathways.
Importance of underlying synovial inflammation in the response of cartilage to different articular injuries. (a) Synovial cells from inflamed synovium (high synovitis superlot) had greater IL-1-mediated NF-κB activity, released more IFN-γ, and had greater percentages of activated (SLA-DR+) monocytes (CD14+) and macrophages (CD163+) compared to synovial cells from normal synovium (Low Synovitis superlot). (b) Injury and synovial co-culture models with the most robust chondrocyte inflammatory responses: (1) With low synovitis cells, compression injury activated more inflammatory genes and pathways compared to fracture, which could be downregulated with IL-1RA. (2) In contrast, with high synovitis cells, fracture activated more inflammatory genes and pathways than compression injury, but IL-1RA was unable to mitigate this response.
The mechanism by which inflamed synovium affects chondrocyte gene expression and pathways is complex40,41,42,54,61,62,63,64,65, and its role following injurious loading of cartilage may provide new targets for intervening with PTOA progression. In our model, high synovitis cells from porcine synovium had increased percentages of SLA-DR + , CD14 + monocytes and CD163 + tissue macrophages compared to low synovitis cells (Fig. 8a). Increased surface expression of SLA-DR on porcine monocytes and macrophages is likely indicative of an activated phenotype, as reported for the human analog, HLA-DR with human monocytes and macrophages66. HLA-DR expression is generally thought to be induced by cytokine stimulation. HLA-DR on macrophages is regulated by GM-CSF, TNF-α, and IFN-γ67,68. In our porcine co-culture model, GM-CSF and TNF-α were not detectable. However, IFN-γ was significantly elevated in media cultured with high synovitis cells. These findings support the notion that the underlying level of synovitis in an injured joint may synergistically drive inflammatory pathways that lead to PTOA development.
Pathway analysis was used to gain mechanistic insight into the genes differentially regulated by both the type of cartilage injury and synovial inflammatory state. We identified NF-κB as the top canonical pathway, along with other activated pathways with potential relevance to PTOA including, osteoarthritis (OA), HMGB1, and toll-like receptor signaling (Fig. 8b). NF-κB was the most significantly activated pathway across all injury types and level of synovitis. Prior work has shown that the NF-κB signaling pathway is highly associated with the presence of OA69. Under a physical stress like injury, HMGB1 can induce inflammation via NF-κB activation with signaling triggered through toll-like receptors70. Many other pathways identified in this study showed similar trends, including IL-17, wound healing, and the FAK signaling pathway. Each of these pathways has been identified to play a role in OA69,71,72,73 and are shown in this study to be activated acutely following articular injury and additionally regulated by the underlying synovial inflammatory state.
As IL-1 was identified as an upstream regulator for the pathways enhanced by these injury states, the effect of IL-1 inhibition was also investigated. In clinical trials, IL-1RA administered intra-articularly did not show improvements in symptoms in patients with established OA74,75. However, in our model of acute osteochondral injury, IL-1RA was effective at reducing inflammatory gene expression for compression within a normal joint environment, or with low synovitis superlot cells, and an inflamed environment, or high synovitis superlot cells. These results are supported by clinical data in which IL-1RA administered intra-articularly within one month of ACL tear, which may cause a compression type injury, reduced acute knee pain and improved function76. However, with fracture, we found that IL-1RA was effective at modulating inflammatory gene expression and downregulating pathways in a normal environment (low synovitis), but not in an inflamed environment (high synovitis). Although not directly assessed in this study, the mechanism of cell death may influence the IL-1 pathway and activation of other pathways. Our previous study showed that the compression injury used in this study with 70% strain loading of osteochondral cores resulted in predominantly apoptosis, whereas fracture resulted in predominantly necrosis only along the fracture line with little or no change in viability on the articular surface37. Interestingly, IL-1 inhibition in an inflamed environment (high synovitis) with no injury showed similar patterns of pathway regulation as fracture (Fig. 7b). However, we previously showed that fracture significantly increased release of sulfated glycosaminoglycans, double stranded DNA, and factors that stimulated NF-κB activity in a reporter cell line compared to the no injury control37. The complex interaction of the synovial environment (low versus high synovitis cells) on chondrocyte gene expression following chondral injury and the associated pathways regulated needs further investigation.
Inhibiting IL-1 in different injury models provides insight on differentially regulated pathways that may aid in identifying new targets for interventional strategies to mitigate post-injury inflammation associated with PTOA development. With fracture in an inflamed environment, several pathways remained upregulated with IL-1RA, including Th17 activation, IL-17 signaling, HIF1α signaling, IL-23 signaling, and chemokine signaling. In our functional phenotyping of the synovial cell superlots for this investigation, IL-1RA was able to reduce IL-1-mediated NF-κB activity back to baseline and the NF-κB signaling pathway was downregulated by IL-1RA for both compression and fracture with both low and high synovitis states, suggesting that targeting IL-1 following joint injury may have potential clinical implications77. However, some post-injury pathways did not appear to be mediated by IL-1.
In this study, IL-6 concentrations were elevated in media from osteochondral cores with compression and fracture compared to uninjured cores and was undetectable with synovial cells alone. These data suggest that cartilage is the likely source of IL-6 as reported in the literature78,79,80,81. IL-6 is upregulated by injury and loading in cartilage7,9,25,29,49,79,81,82 and has been implicated in cartilage degenerative changes associated with OA78,80,83,84. IL-6 and its role in the pathways upregulated with fracture may be a potential target for future investigations.
Expression of inflammatory cytokines, chemokines, and receptors at low levels over long periods of time can promote various pathological conditions. We profiled the acute expression of inflammatory cytokine, chemokine, and receptor genes in chondrocytes in order to determine the state of and the mechanisms behind inflammation in osteochondral injury models. This study showed that many chemokine genes were differentially regulated in articular cartilage with osteochondral injury and synovial co-culture. Chemokines are small in size and may easily facilitate crosstalk between cartilage and synovium. Notably, articular fracture versus injurious compression produced altered expression patterns of inflammatory genes that were dependent upon the underlying level of synovitis present at the time of injury. Other in vitro studies have reported upregulation of chemokines in articular cartilage49,50. Additionally, normal cartilage and cartilage from end-stage OA are reported to have moderate to high expression of chemokine receptors85. These reports, in combination with our results identifying that the mode of injury and underlying level of synovitis affect inflammatory gene expression, suggest that regulation of chemokines and the pathways identified here likely play an important role in joint homeostasis and the cartilage response to injury.
There were several limitations of this study. While this study used co-culture to replicate the joint organ response to injury, not all tissues or bioactive components within the intra-articular environment were included. Although human samples were not used due to limited accessibility of fresh tissue and cells, porcine models have been shown to recapitulate human disease compared to other species used for studying joint injuries and arthritis86,87. The homology between the porcine and human genome is conserved to a greater degree than with mouse or other rodents88. Additionally, the porcine model has recently been identified as an alternative model of spontaneous OA and an excellent source of tissue for in vitro and ex vivo studies89,90. The degree of gross synovitis and intra-articular inflammation in normal and spontaneously osteoarthritic commercial porcine knees has been previously reported43. Much like the human condition, the pathophysiology of the synovitis observed is not fully elucidated. However, McNulty et al. reported elevated concentrations of both IL-1α and IL-1β in synovial fluid from porcine knees with evidence of synovial inflammation (McNulty gross synovitis score) and moderate OA of the articular cartilage. The physiologic levels measured were sufficient to promote tissue degradation of the cartilage. Additionally, the histomorphology of the synovitis observed in the pig joints for this study was similar to human synovium, and the histologic assessment of the synovitis was made using a system developed for human synovial biopsies (Krenn histopathology synovitis score)44. The homology between pigs and humans suggest that spontaneous synovial inflammation may have similar etiology, but there may be differences that this study did not attempt to identify. Explant studies have an inherent difference to an intact joint in that the harvesting process introduces a potential effect. However, other co-culture explant studies, which also aimed to better replicate the articular environment, have previously identified anabolic and catabolic changes with the addition of synovial tissue to cartilage40,41,42,54,63,64,65,81,91,92,93. However, in this study, the acute response of cartilage to different types of injuries were determined, and importantly, the contribution and interaction with varying states of synovial cell inflammation were assessed. The finding that IL-1RA did not downregulate pro-inflammatory genes and associated pathways in the co-culture of high synovitis and fracture may reflect an insufficient dose of IL-1RA, although this dose was able to reverse changes in the fracture core alone, as well as with high synovitis cells alone. Overall, our results suggest that modulating inflammation may be more challenging with fracture in a high synovitis environment than other articular injuries.
We have demonstrated that both injury mechanism and synovitis state are independent contributors to the cartilage response to injury. With regard to the clinical problem, the injury type is established when patients present for care. Our findings suggest that developing treatments to acutely reduce inflammation in the injured joint from a high to a low synovitis state may protect against increased pro-inflammatory gene expression potentially leading to enhanced articular cartilage damage following articular cartilage injury. Compression injury, as may be found with an ACL tear, and osteochondral disruption as occurs in an articular fracture, result in differential gene expression that is dependent upon the local synovitis environment. The osteochondral injury and co-culture models allow us the opportunity to study different types of cartilage injuries and improve our understanding of how to modulate the intra-articular environment to reduce irreversible damage to the joint.
Conclusions
This study demonstrates that the clinically relevant injury mechanisms of articular fracture and articular surface compression drive distinct responses in chondrocyte gene expression that are differentially enhanced by co-culture with cells from low and high synovitis states. Specifically, IL-1 inhibition reduces pro-inflammatory gene expression following articular surface compression in co-cultures with low or high synovitis cells. Furthermore, IL-1 inhibition is effective in reducing pro-inflammatory gene expression after articular fracture in co-culture with low synovitis cells; however, it is not as effective with high synovitis cells. These data suggest that urgent treatment to reduce intra-articular inflammation after injury is important, and for articular fracture the underlying inflammatory state may play a larger role. This may be particularly important from a clinical perspective for patients presenting with articular fracture, as our study demonstrated that IL-1RA was ineffective at downregulating inflammatory genes and pathways in the setting of a fracture in an established high synovitis environment. More work is needed to confirm that these clinical injury mechanisms require different approaches to prevent PTOA development following an acute injury. There is a spectrum of injuries that may result in PTOA. Translational research should investigate the specific joint injuries and biologic cross-talk with in a joint organ system that may influence the development of PTOA and inform therapeutic development to halt the progression of PTOA following these injuries.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Post-Traumatic Arthritis. (eds Olson, S. A. & Guilak, F) chap. 28, 360 (Springer, New York, NY, 2015).
Brown, T. D., Johnston, R. C., Saltzman, C. L., Marsh, J. L. & Buckwalter, J. A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20, 739–744. https://doi.org/10.1097/01.bot.0000246468.80635.ef (2006).
Bretlau, T. et al. Bone bruise in the acutely injured knee. Knee Surg. Sports Traumatol. Arthrosc. 10, 96–101. https://doi.org/10.1007/s00167-001-0272-9 (2002).
Owusu-Akyaw, K. A. et al. Determination of the position of the knee at the time of an anterior cruciate ligament rupture for male versus female patients by an analysis of bone bruises. Am. J. Sports Med. 46, 1559–1565. https://doi.org/10.1177/0363546518764681 (2018).
Pezeshki, S., Vogl, T. J., Pezeshki, M. Z., Daghighi, M. H. & Pourisa, M. Association of the type of trauma, occurrence of bone bruise, fracture and joint effusion with the injury to the menisci and ligaments in MRI of knee trauma. Muscles Ligaments Tendons J 6, 161–166. https://doi.org/10.11138/mltj/2016.6.1.161 (2016).
Amano, K. et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am. J. Sports Med. 46, 890–899. https://doi.org/10.1177/0363546517749834 (2018).
Bigoni, M. et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 31, 315–321. https://doi.org/10.1002/jor.22208 (2013).
Catterall, J. B., Stabler, T. V., Flannery, C. R. & Kraus, V. B. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Therapy 12, R229–R229. https://doi.org/10.1186/ar3216 (2010).
Irie, K., Uchiyama, E. & Iwaso, H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10, 93–96 (2003).
King, J. D. et al. Joint fluid proteome after anterior cruciate ligament rupture reflects an acute posttraumatic inflammatory and chondrodegenerative state. Cartilage https://doi.org/10.1177/1947603518790009 (2018).
Crook, B. S. et al. Effect of walking on in vivo tibiofemoral cartilage strain in ACL-deficient versus intact knees. J. Biomech. 116, 110210. https://doi.org/10.1016/j.jbiomech.2020.110210 (2021).
DeFrate, L. E. Effects of ACL graft placement on in vivo knee function and cartilage thickness distributions. J. Orthop. Res. 35, 1160–1170. https://doi.org/10.1002/jor.23541 (2017).
Okafor, E. C. et al. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J. Biomech. 47, 96–101. https://doi.org/10.1016/j.jbiomech.2013.10.003 (2014).
Sutter, E. G. et al. Effects of anterior cruciate ligament deficiency on tibiofemoral cartilage thickness and strains in response to hopping. Am. J. Sports Med. 47, 96–103. https://doi.org/10.1177/0363546518802225 (2019).
Theologis, A. A. et al. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg. Sports Traumatol. Arthrosc. 22, 298–307. https://doi.org/10.1007/s00167-013-2397-z (2014).
Englund, M. & Lohmander, L. S. Risk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomy. Arthritis Rheumatol. 50, 2811–2819 (2004).
Fithian, D. C., Paxton, L. W. & Goltz, D. H. Fate of the anterior cruciate ligament-injured knee. Orthop. Clin. North Am. 33, 621–636 (2002).
Grossman, M. G., ElAttrache, N. S., Shields, C. L. & Glousman, R. E. Revision anterior cruciate ligament reconstruction: Three- to nine-year follow-up. Arthroscopy 21, 418–423 (2005).
Meunier, A., Odensten, M. & Good, L. Long-term results after primary repair or non-surgical treatment of anterior cruciate ligament rupture: A randomized study with a 15-year follow-up. Scand. J. Med. Sci. Sports 17, 230–237. https://doi.org/10.1111/j.1600-0838.2006.00547.x (2007).
Salmon, L. J., Pinczewski, L. A., Russell, V. J. & Refshauge, K. Revision anterior cruciate ligament reconstruction with hamstring tendon autograft: 5- to 9-year follow-up. Am. J. Sports Med. 34, 1604–1614. https://doi.org/10.1177/0363546506288015 (2006).
Salmon, L. J. et al. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: Minimum 13-year review. Am. J. Sports Med. 34, 721–732. https://doi.org/10.1177/0363546505282626 (2006).
Segawa, H., Omori, G. & Koga, Y. Long-term results of non-operative treatment of anterior cruciate ligament injury. Knee 8, 5–11 (2001).
von Porat, A., Roos, E. M. & Roos, H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: A study of radiographic and patient relevant outcomes. Ann. Rheum. Dis. 63, 269–273 (2004).
Dirschl, D. R. et al. Articular fractures. J. Am. Acad. Orthop. Surg 12, 416–423. https://doi.org/10.5435/00124635-200411000-00006 (2004).
Adams, S. B. Jr. et al. Inflammatory cytokines and cellular metabolites as synovial fluid biomarkers of posttraumatic ankle arthritis. Foot. Ankle Int. 35, 1241–1249. https://doi.org/10.1177/1071100714550652 (2014).
Adams, S. B., Reilly, R. M., Huebner, J. L., Kraus, V. B. & Nettles, D. L. Time-Dependent effects on synovial fluid composition during the acute phase of human intra-articular ankle fracture. Foot. Ankle Int. 38, 1055–1063. https://doi.org/10.1177/1071100717728234 (2017).
Adams, S. B. et al. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot. Ankle Int. https://doi.org/10.1177/1071100715611176 (2015).
Furman, B. D. et al. Brief report: Articular ankle fracture results in increased synovitis, synovial macrophage infiltration, and synovial fluid concentrations of inflammatory cytokines and chemokines. Arthritis Rheumatol. 67, 1234–1239. https://doi.org/10.1002/art.39064 (2015).
Haller, J. M., McFadden, M., Kubiak, E. N. & Higgins, T. F. Inflammatory cytokine response following acute tibial plateau fracture. J. Bone Joint Surg. Am. 97, 478–483. https://doi.org/10.2106/JBJS.N.00200 (2015).
Papathanasiou, I. et al. Molecular changes indicative of cartilage degeneration and osteoarthritis development in patients with anterior cruciate ligament injury. BMC Musculoskelet Disord. 17, 21. https://doi.org/10.1186/s12891-016-0871-8 (2016).
Furman, B. D. et al. Targeting pro-inflammatory cytokines following joint injury: Acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res. Ther. 16, R134. https://doi.org/10.1186/ar4591 (2014).
Kimmerling, K. A. et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur. Cells Mater. 29, 124–139; discussion 139–140, https://doi.org/10.22203/ecm.v029a10 (2015).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheumatol. 64, 1697–1707. https://doi.org/10.1002/art.34453 (2012).
Scanzello, C. R. & Goldring, S. R. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257. https://doi.org/10.1016/j.bone.2012.02.012 (2012).
Mathiessen, A. & Conaghan, P. G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 19, 18. https://doi.org/10.1186/s13075-017-1229-9 (2017).
Nickien, M., Heuijerjans, A., Ito, K. & van Donkelaar, C. C. Comparison between in vitro and in vivo cartilage overloading studies based on a systematic literature review. J. Orthop. Res. https://doi.org/10.1002/jor.23910 (2018).
Stolberg-Stolberg, J. A. et al. Effects of cartilage impact with and without fracture on chondrocyte viability and the release of inflammatory markers. J. Orthop. Res. 31, 1283–1292. https://doi.org/10.1002/jor.22348 (2013).
Yan, H. et al. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl. Acad. Sci. U S A 113, E6199–E6208. https://doi.org/10.1073/pnas.1608245113 (2016).
Lewis, J. S. et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 19, 864–873. https://doi.org/10.1016/j.joca.2011.04.011 (2011).
Haltmayer, E. et al. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS One 14, e0214709. https://doi.org/10.1371/journal.pone.0214709 (2019).
Lee, C. M., Kisiday, J. D., McIlwraith, C. W., Grodzinsky, A. J. & Frisbie, D. D. Synoviocytes protect cartilage from the effects of injury in vitro. BMC Musculoskelet. Disord. 14, 54. https://doi.org/10.1186/1471-2474-14-54 (2013).
Sward, P. et al. Coculture of bovine cartilage with synovium and fibrous joint capsule increases aggrecanase and matrix metalloproteinase activity. Arthr. Res. Ther. 19, 157. https://doi.org/10.1186/s13075-017-1318-9 (2017).
McNulty, A. L., Rothfusz, N. E., Leddy, H. A. & Guilak, F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J. Orthop. Res. 31, 1039–1045. https://doi.org/10.1002/jor.22334 (2013).
Krenn, V. et al. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology 49, 358–364. https://doi.org/10.1111/j.1365-2559.2006.02508.x (2006).
Roman-Blas, J. A. & Jimenez, S. A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 14, 839–848. https://doi.org/10.1016/j.joca.2006.04.008 (2006).
Ahmed, A. S. et al. Activation of NF-kappaB in synovium versus cartilage from patients with advanced knee osteoarthritis: A potential contributor to inflammatory aspects of disease progression. J. Immunol. 201, 1918–1927. https://doi.org/10.4049/jimmunol.1800486 (2018).
Berke, I. M. et al. NF-kappaB-mediated effects on behavior and cartilage pathology in a non-invasive loading model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 29, 248–256. https://doi.org/10.1016/j.joca.2020.10.008 (2021).
Brunger, J. M., Zutshi, A., Willard, V. P., Gersbach, C. A. & Guilak, F. Genome engineering of stem cells for autonomously regulated, closed-loop delivery of biologic drugs. Stem Cell Rep. 8, 1202–1213. https://doi.org/10.1016/j.stemcr.2017.03.022 (2017).
Pulai, J. I. et al. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J. Immunol. 174, 5781–5788. https://doi.org/10.4049/jimmunol.174.9.5781 (2005).
Sandell, L. J. et al. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthr. Cartil. 16, 1560–1571. https://doi.org/10.1016/j.joca.2008.04.027 (2008).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Brinster, R. et al. Control procedures and estimators of the false discovery rate and their application in low-dimensional settings: An empirical investigation. BMC Bioinf. 19, 78. https://doi.org/10.1186/s12859-018-2081-x (2018).
Kramer, A., Green, J., Pollard, J. Jr. & Tugendreich, S. Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30, 523–530. https://doi.org/10.1093/bioinformatics/btt703 (2014).
Pretzel, D., Pohlers, D., Weinert, S. & Kinne, R. W. In vitro model for the analysis of synovial fibroblast-mediated degradation of intact cartilage. Arthritis Res. Ther. 11, R25. https://doi.org/10.1186/ar2618 (2009).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791. https://doi.org/10.1038/s41467-017-02659-x (2018).
Huang, W. et al. Parallel comparison of fibroblast-like synoviocytes from the surgically removed hyperplastic synovial tissues of rheumatoid arthritis and osteoarthritis patients. BMC Musculoskelet. Disord. 20, 591. https://doi.org/10.1186/s12891-019-2977-2 (2019).
Onuora, S. Rheumatoid arthritis: RA synovium harbours distinct fibroblast subsets. Nat. Rev. Rheumatol. 14, 250. https://doi.org/10.1038/nrrheum.2018.47 (2018).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340, 448–454. https://doi.org/10.1056/NEJM199902113400607 (1999).
Eto, T. A review of the biological properties and clinical implications of adrenomedullin and proadrenomedullin N-terminal 20 peptide (PAMP), hypotensive and vasodilating peptides. Peptides 22, 1693–1711. https://doi.org/10.1016/s0196-9781(01)00513-7 (2001).
Giannoudis, P. V. et al. Correlation between IL-6 levels and the systemic inflammatory response score: Can an IL-6 cutoff predict a SIRS state?. J. Trauma 65, 646–652. https://doi.org/10.1097/TA.0b013e3181820d48 (2008).
Chou, C. H. et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 10, 10868. https://doi.org/10.1038/s41598-020-67730-y (2020).
Dwivedi, G. et al. Inflammatory cytokines and mechanical injury induce post-traumatic osteoarthritis-like changes in a human cartilage-bone-synovium microphysiological system. Arthritis Res. Ther. 24, 198. https://doi.org/10.1186/s13075-022-02881-z (2022).
Vankemmelbeke, M. N., Ilic, M. Z., Handley, C. J., Knight, C. G. & Buttle, D. J. Coincubation of bovine synovial or capsular tissue with cartilage generates a soluble “Aggrecanase” activity. Biochem. Biophys. Res. Commun. 255, 686–691. https://doi.org/10.1006/bbrc.1999.0266 (1999).
Lai-Zhao, Y., Pitchers, K. K. & Appleton, C. T. Transient anabolic effects of synovium in early post-traumatic osteoarthritis: A novel ex vivo joint tissue co-culture system for investigating synovium-chondrocyte interactions. Osteoarthr. Cartil. 29, 1060–1070. https://doi.org/10.1016/j.joca.2021.03.010 (2021).
Mehta, S., Akhtar, S., Porter, R. M., Önnerfjord, P. & Bajpayee, A. G. Interleukin-1 receptor antagonist (IL-1Ra) is more effective in suppressing cytokine-induced catabolism in cartilage-synovium co-culture than in cartilage monoculture. Arthritis Res. Ther. 21, 238–238. https://doi.org/10.1186/s13075-019-2003-y (2019).
Firestein, G. S. & Zvaifler, N. J. Down regulation of human monocyte differentiation antigens by interferon gamma. Cell Immunol. 104, 343–354. https://doi.org/10.1016/0008-8749(87)90036-0 (1987).
Alvaro-Gracia, J. M., Zvaifler, N. J. & Firestein, G. S. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: A possible role in rheumatoid arthritis. J. Exp. Med. 170, 865–875. https://doi.org/10.1084/jem.170.3.865 (1989).
Zvaifler, N. J. & Firestein, G. S. Cytokines in chronic inflammatory synovitis. Scand. J. Rheumatol. Suppl. 76, 203–210. https://doi.org/10.3109/03009748809102970 (1988).
Liu, Y. X., Wang, G. D., Wang, X., Zhang, Y. L. & Zhang, T. L. Effects of TLR-2/NF-kappaB signaling pathway on the occurrence of degenerative knee osteoarthritis: An in vivo and in vitro study. Oncotarget 8, 38602–38617. https://doi.org/10.18632/oncotarget.16199 (2017).
Pisetsky, D. S., Erlandsson-Harris, H. & Andersson, U. High-mobility group box protein 1 (HMGB1): An alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res. Ther. 10, 209. https://doi.org/10.1186/ar2440 (2008).
Shahrara, S., Castro-Rueda, H. P., Haines, G. K. & Koch, A. E. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res. Ther. 9, R112. https://doi.org/10.1186/ar2318 (2007).
Zhou, Y., Wang, T., Hamilton, J. L. & Chen, D. Wnt/beta-catenin signaling in osteoarthritis and in other forms of arthritis. Curr. Rheumatol. Rep. 19, 53. https://doi.org/10.1007/s11926-017-0679-z (2017).
Na, H. S. et al. Interleukin-1-interleukin-17 signaling axis induces cartilage destruction and promotes experimental osteoarthritis. Front. Immunol. 11, 730. https://doi.org/10.3389/fimmu.2020.00730 (2020).
Chevalier, X. et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: A multicenter study. J Rheumatol 32, 1317–1323 (2005).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 61, 344–352. https://doi.org/10.1002/art.24096 (2009).
Kraus, V. B. et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial (NCT00332254). Osteoarthr. Cartil. 20, 271–278. https://doi.org/10.1016/j.joca.2011.12.009 (2012).
Buchanan, M. W., Furman, B. D., McNulty, A. L. & Olson, S. A. Combination of lidocaine and IL-1Ra is effective at reducing degradation of porcine cartilage explants. Am. J. Sports Med. https://doi.org/10.1177/03635465221090611 (2022).
Flannery, C. R. et al. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Boil. J. Int. Soc. Matrix Biol. 19, 549–553 (2000).
Mohtai, M. et al. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J. Orthop. Res. 14, 67–73. https://doi.org/10.1002/jor.1100140112 (1996).
Pearson, M. J. et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 7, 3451. https://doi.org/10.1038/s41598-017-03759-w (2017).
Sui, Y. et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthr. Rheumatol. 60, 2985–2996. https://doi.org/10.1002/art.24857 (2009).
Elsaid, K. A. et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthr. Rheumatol. 58, 1707–1715. https://doi.org/10.1002/art.23495 (2008).
Attur, M. G., Patel, I. R., Patel, R. N., Abramson, S. B. & Amin, A. R. Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc. Assoc. Am. Phys. 110, 65–72 (1998).
Stannus, O. et al. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 18, 1441–1447. https://doi.org/10.1016/j.joca.2010.08.016 (2010).
Silvestri, T. et al. Down-modulation of chemokine receptor cartilage expression in inflammatory arthritis. Rheumatology (Oxford) 42, 14–18. https://doi.org/10.1093/rheumatology/keg020 (2003).
Kaab, M. J., Gwynn, I. A. & Notzli, H. P. Collagen fibre arrangement in the tibial plateau articular cartilage of man and other mammalian species. J. Anat. 193(Pt 1), 23–34. https://doi.org/10.1017/s0021878298003744 (1998).
Aspden, R. M., Yarker, Y. E. & Hukins, D. W. Collagen orientations in the meniscus of the knee joint. J. Anat. 140(Pt 3), 371–380 (1985).
Humphray, S. J. et al. A high utility integrated map of the pig genome. Genome Biol. 8, R139. https://doi.org/10.1186/gb-2007-8-7-r139 (2007).
Macfadyen, M. A. et al. The commercial pig as a model of spontaneously-occurring osteoarthritis. BMC Musculoskelet. Disord. 20, 70. https://doi.org/10.1186/s12891-019-2452-0 (2019).
Cone, S. G., Warren, P. B. & Fisher, M. B. Rise of the pigs: Utilization of the porcine model to study musculoskeletal biomechanics and tissue engineering during skeletal growth. Tissue Eng. Part C Methods 23, 763–780. https://doi.org/10.1089/ten.TEC.2017.0227 (2017).
Gregg, A. J. et al. Assessment of the catabolic effects of interleukin-1beta on proteoglycan metabolism in equine cartilage cocultured with synoviocytes. Am. J. Vet. Res. 67, 957–962. https://doi.org/10.2460/ajvr.67.6.957 (2006).
Beekhuizen, M. et al. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage: Establishment and characterization of a long-term cartilage-synovium coculture. Arthritis Rheumatol. 63, 1918–1927. https://doi.org/10.1002/art.30364 (2011).
Steinhagen, J. et al. Perfusion culture system: Synovial fibroblasts modulate articular chondrocyte matrix synthesis in vitro. Tissue Cell 42, 151–157. https://doi.org/10.1016/j.tice.2010.03.003 (2010).
Acknowledgements
This work is supported in part by Depuy Synthes research grant 13126, NIH Clinical and Translational Science grant TL1TR001116, Piedmont Orthopedic Foundation Grant 2020-5, NIH Grant P30AG028716, NIH Grant AG046927, and DoD Translational Research Partnership Award Grants W81XWH-12-1-0621, W81XWH-12-1-0622, and W81XWH-12-1-0623. We would like to acknowledge the following individuals for their expertise and/or technical assistance, Dr. Adman Goode, Dr. Holly Leddy, Katelyn Steadman, Dr. Kent Weinhold, Janet Huebner, Dr. Hattie Cutcliffe, and Steve Johnson.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript and provided critical feedback. Conceptualization: DJC, BDF, FG, ALM, SAO; Data curation: MLL, DJC, BDF, SAO; Formal analysis: MLL, DJC, BDF, JSY, JMB; Funding acquisition: DJC, JSY, VBK, FG, SAO; Investigation, including performing experiments and data collection: MLL, DJC, BDF, JSY, JMB; Develop and design methodology: MLL, DJC, BDF, JMB, ALM, FG, SAO; Provided resources: JSY, VBK, FG, SAO Supervision: BDF, JSY, FG, ALM, SAO; Writing original draft: MLL, DJC, BDF, JSY, JMB, SAO; and all authors contributed to the visualization and data presentation of the published work.
Corresponding author
Ethics declarations
Competing interests
Dr. Guilak is an employee and shareholder of Cytex Therapeutics, Inc. All other co-authors have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Libke, M.L., Cunningham, D.J., Furman, B.D. et al. Mode of injury and level of synovitis alter inflammatory chondrocyte gene expression and associated pathways. Sci Rep 14, 28917 (2024). https://doi.org/10.1038/s41598-024-71964-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-71964-5
This article is cited by
-
RNF152-mediated, ubiquitin-dependent degradation of HSP27 activates the PI3K/AKT pathway, driving synovial inflammatory cascades in TMJOA
Arthritis Research & Therapy (2025)