Abstract
Southeast Asia includes several global biodiversity hotspots and bats account for nearly one-third of mammal species currently known in the region. While acoustic methods have become widespread in bat research, basic information is often lacking on the echolocation calls produced by Asian bat species. Since such information can aid a wide variety of research and conservation initiatives, descriptions of the calls emitted by Asian bats are fundamental. The aim of our study was to provide a standardized analysis and description of the Vietnamese bat echolocation calls. We analyzed call recordings of 87 species arranged in eight families. This constitutes 74% of the echolocating bats presently known in Vietnam and includes the first call descriptions for five taxa. Our use of an open-source software and the deposition of recordings in the ChiroVox repository will facilitate comparative studies in Asia and the information we provide represents one of the most comprehensive bioacoustic databases for Asian bats to date.
Similar content being viewed by others
Introduction
Exploring the wildlife of tropical areas, heavily affected by the biodiversity crisis, is one of the most urgent and important research fields today. The rainforests of Southeast Asia encompass four of the 25 biodiversity hotspots globally and are among the most endangered habitats worldwide1,2,3. Bats are an important component of this diversity, accounting for nearly one-third of mammal species in Southeast Asia4,5,6, but are also among the least known vertebrates in the region4. Unfortunately, it has been estimated that ≈40% of regional bat species could become extinct by the end of the twenty-first century if current rates of deforestation continue4,7,8. In response to this, more comprehensive conservation studies are urgently needed to improve knowledge regarding patterns of diversity and distribution as well as the natural history of bats in Southeast Asia4,5,9.
Bats are elusive animals, being primarily active during the night and often roosting in hidden places. Traditional capture methods (mist-nets & harp-traps) are usually suitable for catching bats up to a few meters above ground level, with the result that species flying within or above the forest canopy are typically under-represented in ground-based surveys. However, with the exception of most of the Old World fruit bats (Pteropodidae), bats use an echolocation system which allows us to study their sounds without trapping and stressing them unnecessarily. These acoustic signals play an important role in the life of bats, are used to orient and hunt in the dark10,11 and are adapted to different environments and resources, as their primary goal is to provide adequate sensitivity at a given foraging site11,12,13,14,15,16. The use of bat detectors is widespread and has a long history in temperate areas17 and is gradually increasing in Asian countries18,19,20,21,22,23,24,25,26,27.
The main advantage of acoustic surveys over traditional capture methods is that a lot of data can be collected during a short period of time without disturbing the bats28. Passive acoustic monitoring can be used to sample multiple locations simultaneously, to reveal trends in population size and to examine their behavior and habitat preferences29. As a consequence, the description of bat echolocation calls is of great importance for acoustic research and certain ecological studies14,30,31. However, acoustic sampling methods also have limitations. The directionality of high frequency calls and the intensity of calls may differ between species, hence certain taxa (e.g., “whispering” bats) can be underrepresented in surveys. In many cases, bats can only be determined at the species-group level, and accurate information about the sex, age, reproductive status and other aspects of individuals cannot be obtained. The echolocation signals emitted by bats can vary greatly depending on geographical location, habitat structure, flight altitude, and other environmental factors12,14,15,32. Moreover, differences in body size, sex and age can also cause interspecific variation in calls and individual variation can occur as bats traverse different habitats33,34. These variations may hinder exact species identification and must be considered in analyses. They underline the necessity for open-access call repositories with multiple recordings of the same taxon in different conditions.
With 129 bat species currently known (unpublished data of the authors), the bat fauna of Vietnam is highly diverse35. Nearly 40 species were described as new for science or new for the country in recent decades (e.g., Kerivoula titania, Murina eleryi, Mu. beelzebub, Mu. walstoni, Mu. annamitica, Myotis ancricola, My. annamitica, My. annatessae, My. indochinensis, My. phanluongi, Hipposideros griffini)36,37,38,39,40,41. The first publication containing information on Vietnamese bat echolocation was Borissenko and Kruskop (2003)42, whereas the first research was published by Furey et al. (2009)19, who described the calls of 31 bat species occurring in Kim Hy Nature Reserve, northern Vietnam and concluded that acoustic identification of local bat species was feasible. Since then, several studies in other locations in Vietnam have provided information on the call characteristics of additional Vietnamese bat species22,26,43,44,45,46,47,48,49,50.
Acoustic monitoring has appeared as a reliable and standardized method for rapid surveys, but it has to be based on localized (country or province level) acoustic libraries. Therefore, there is an urgent need for bat acoustic studies from many regions of the world. Although several papers have included echolocation parameters of Southeast Asian bat species, comprehensive studies are scarce. Elusive or rare species are often missing from these studies and the call analyses are rarely repeatable as the recordings are not available for further studies. The goal of our study was to describe and characterize echolocation calls of Vietnamese bats to facilitate research and conservation activities in the wider Southeast Asian region. We ensure repeatability by using open-source software and providing all recordings in the ChiroVox sound database51, so as to allow comparative analyses and identification of anonymous bat calls.
Results
We analyzed a total of 3,438 echolocation pulses in 1,042 recordings manually (in some cases, multiple harmonics were measured). These recordings stemmed from 87 species caught and identified in the field or later through DNA analyses and two species/species groups for whom identification was uncertain (e.g., Hipposideros grandis/poutensis and Kerivoula cf. hardwickii). The bats belong to 28 genera arranged in eight families: Pteropodidae, Megadermatidae, Emballonuridae, Hipposideridae, Rhinolophidae, Molossidae, Miniopteridae and Vespertilionidae (Table S1) and account for 74% of echolocating bat species in Vietnam. We provide an overview for each family, including the basic characteristics of the calls and species analyzed.
Pteropodidae
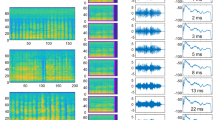
Within the family Pteropodidae we analyzed the calls of one species from the genus Rousettus. Members of this genus are well known for producing echolocation sounds by making clicks with their tongue. We were able to measure echolocation clicks of R. leschenaultii. These were atonal calls with single dominant, low frequency FM structure (Fig. 1, Table 1).
Echolocation call characteristics of bats belonging to the Pteropodidae, Megadermatidae and Emballonuridae families. Multiple harmonics are shown for the megadermatids and emballonurids. Abbreviations: R. les.–Rousettus leschenaultii, L. lyr.–Lyroderma lyra, M. spa.–Megaderma spasma, T. the.—Taphozous theobaldi, T. mel.–T. melanopogon, T. lon.–T. longimanus.
Megadermatidae
Recordings of two species in two genera were analyzed from the Megadermatidae: Lyroderma lyra and Megaderma spasma. Both species emitted short duration narrowband FM calls with multi-harmonic signals (Fig. 1, Table 1). Among the multi-harmonic signals, the second and the third harmonics were measured in most cases.
Emballonuridae
We were able to study three species within the Emballonuridae, all belonging to the genus Taphozous: T. longimanus, T. melanopogon and T. theobaldi. All three species emit long multi-harmonic QCF or FM signals, out of which the second harmonic is the most dominant, although the other harmonics were also relatively strong (Fig. 1, Table 1). We consequently measured the second harmonic in all cases and the third and fourth harmonics in many cases.
Hipposideridae
The calls of 15 species arranged in three hipposiderid genera were measured (Fig. 2, Table 2). All but one (Coelops frithii) of these emitted calls with a typical CF-FM structure. The second harmonic of each call was measured because it contained the maximum energy. Coelops frithii emitted calls with a special CF-FM structure; these usually contained an initial and very short narrowband component, but the dominant energy was always contained in the FM sweep.
Echolocation call characteristics of Hipposideridae (only the second harmonics shown). Abbreviations: H. swi.–Hipposideros swinhoei, H. scu.–H. scutinares, H. arm.–H. armiger, H. lyl.–H. lylei, H. gri.–H. griffini, H. a. sun.–H. alongensis sungi, H. a. alo.–H. alongensis alongensis, H. pou.–H. poutensis, H. kha.–H. khaokhouayensis, H. gra.–H. grandis, H. gal.–H. galeritus, H. gen.–H. gentilis, A. don.–Aselliscus dongbacanus, A. sto.–A. stoliczkanus, H. cin.–H. cineraceus, C. fri.–Coelops frithii.
Rhinolophidae
Seventeen species were included in our dataset for the Rhinolophidae: Rhinolophus acuminatus, R. affinis, R. chaseni, R. episcopus, R. lepidus, R. perniger, R. malayanus, R. marshalli, R. microglobosus, R. pearsonii, R. pusillus, R. rex, R. shameli, R. siamensis, R. sinicus, R. stheno and R. thomasi. All of these species emitted the characteristic FM-CF-FM calls of horseshoe bats with the maximum energy in the second harmonic (Fig. 3, Table 3).
Echolocation call characteristics of Rhinolophidae (only the second harmonics shown). Abbreviations: R. rex.–Rhinolophus rex, R. per.–R. perniger, R. mar.–R. marshalli, R. pea.–R. pearsonii, R. epi.–R. episcopus, R. sha.–R. shameli, R. aff.–R. affinis, R. sia.–R. siamensis, R. sin.–R. sinicus, R. cha.–R. chaseni, R. tho.–R. thomasi, R. mal.–R. malayanus, R. sth.–R. stheno, R. mic.–R. microglobosus, R. acu.–R. acuminatus, R. lep.–R. lepidus, R. pus.–R. pusillus.
Molossidae
Recordings for one molossid species were measured: Mops plicatus. This species emitted multi-harmonic narrowband FM calls with relatively low peak frequencies from which the first harmonic was measured (Fig. 4, Table 4). A partial echo of the first harmonic is included in the exemplar signal provided in Fig. 4, which includes a small hook at the beginning of the call.
Echolocation call characteristics of Molossidae, Miniopteridae and Pipistrellini (Vespertilionidae: Vespertilioninae) . Abbreviations: M. pli.–Mops plicatus, M. mag.–Miniopterus magnater, M. ful.–M. fuliginosus, M. pus.–M. pusillus, N. pla.–Nyctalus plancyi, G. buc.–Glischropus bucephalus, P. cor.–Pipistrellus coromandra, P. jav.–P. javanicus, P. abr.–P. abramus, P. ten.–P. tenuis.
Miniopteridae
Three miniopterid species were analyzed: Miniopterus fuliginosus, M. magnater and M. pusillus (Fig. 4, Table 4). These species all emitted FM-QCF signals (FM calls with narrowband tails) and the first harmonic was measured in all cases since it contained the maximum energy. In some cases, a second harmonic was apparent in higher intensity signals.
Vespertilionidae
Recordings from 46 species belonging to 18 vespertilionid genera were analyzed (Figs. 4, 5, 6, 7) (Tables 4, 5, 6). These revealed the remarkable diversity of signal types within the family, with three major types identified: FM, FM-QCF and cDFM (checked downward FM).
Of the Pipistrellini tribe we measured the calls of six species from three genera: Nyctalus plancyi, Glischropus bucephalus, Pipistrellus coromandra, P. javanicus, P. abramus and P. tenuis. Half of the species emitted both FM and FM-QCF calls, but in the case of P. abramus we found only FM-QCF type calls and in the case of P. coromandra and P. tenuis we only found FM type calls (Fig. 4, Table 4).
Echolocation call characteristics of Plecotini (Vespertilioninae) and Myotinae. Abbreviations: P. hom.–Plecotus homochrous, M. chi.–Myotis chinensis, M. pil.–M. pilosus, M. sic.–M. sicarius, M. lan.–M. laniger, M. hor.–M. horsfieldii, M. for.–M. formosus, M. ann.–M. annectans, M. alta.–M. altarium, M. mon.–M. montivagus, M. mur.–M. muricola, M. ate.–M. ater, M. alti.–M. alticraniatus, E. den.–Eudiscopus denticulus.
Echolocation call characteristics of Murininae and Kerivoulinae species. Abbreviations: H. har.–Harpiocephalus harpia, M. fio.–Murina fionae, M. bee.–M. beelzebub, M. har.–M. harrisoni, M. hut.–M. huttoni, M. ann.–M. annamitica, M. cyc.–M. cyclotis, M. fea.–M. feae, M. ele.–M. eleryi, P. jag.–P. jagorii, K. kac.–Kerivoula kachinensis, K. tit.–K. titania, K. dep.–K. depressa, K. don.–K. dongduongana, K. fur.–K. furva, K. cf. har.–K. cf. hardwickii.
Echolocation call characteristics of Vespertilioninae (excl. Pipistrellini and Plecotini) bats. Abbreviations: M. jof.–Mirostrellus joffrei, H. cad.–Hypsugo cadornae, H. pul.–H. pulveratus, T. ful.–Tylonycteris fulvida, S. orn.–Scotomanes ornatus, T. aur.–Thainycteris aureocollaris, A. cir.–Arielulus circumdatus, S. hea.–Scotophilus heathii, S. kuh.–S. kuhlii.
Within the subfamily Myotinae we measured the calls of 12 Myotis species: M. altarium, M. alticraniatus, M. annectans, M. ater, M. chinensis, M. formosus, M. horsfieldii, M. laniger, M. montivagus, M. muricola, M. pilosus, M. sicarius and Eudiscopus denticulus (Fig. 5). Aside from M. alticraniatus and M. muricola, Myotis species emitted only short-duration, broadband FM calls; whereas Eudiscopus denticulus uses FM-QCF signals, Myotis alticraniatus and M. muricola also emitted FM-QCF as well as cDFM-QCF calls. In the case of E. denticulus, we also observed the “unique” call type described by Zsebők et al. (2010)52. In contrast to the above species, Plecotus homochrous (Vespertilioninae: Plecotini) emitted short, low intensity, narrowband FM type signals with multiple harmonics.
Within the Murininae subfamily, we studied the calls of nine species from two genera: Harpiocephalus harpia and Murina annamitica, M. beelzebub, M. cyclotis, M. eleryi, M. feae, M. fionae, M. harrisoni and M. huttoni. Almost all of these species emitted calls of typical cDFM structure (Fig. 6), which begins with an upward modulated component followed by a downward phase. Harpiocephalus harpia also emitted FM and FM-QCF calls, while Murina species FM type beside cDFM. From M. beelzebub we could only examine FM type sounds, although this may be due to the fact that, in many cases, the initial stage of this type of call had only a low decibel value, so in these cases only the values of the FM structure could be measured accurately.
Of the Kerivoulinae subfamily, we were able to study the calls of seven species: Phoniscus jagorii, Kerivoula depressa, K. dongduongana, K. furva, K. cf. hardwickii, K. kachinensis and K. titania. All of these species emitted broadband FM calls with relatively low intensities and short durations (Fig. 6) and we found also cDFM types in case of Kerivoula kachinensis and K. titania.
The subfamily Vespertilioninae (with the exception of Pipistrellini and Plecotini shown above) is represented by the following 10 species: Arielulus circumdatus with FM signals, Hypsugo cadornae and H. pulveratus with both FM and FM-QCF signals, Ia io with only FM signals, Mirostrellus joffrei with both FM and FM-QCF signals, Scotophilus heathii and S. kuhlii with both FM and FM-QCF signals, Scotomanes ornatus with only FM signals, Thainycteris aureocollaris with only FM signals, and Tylonycteris fulvida with both FM and FM-QCF signals (Figs. 5 and 7).
New call descriptions
The echolocation calls of the following species are described in detail as their characteristics become available for the first time.
Glischropus bucephalus
Based on recordings of three males flying in a flight tent, this species emits broadband FM calls without strong harmonics (Figs. 4 and 8, Table 4). The maximum start frequency of these calls was 112.6 kHz and the minimum end frequency was 48.3 kHz. The peak energy occurred at an average frequency of 56.7 ± 1.8 (mean ± SD) kHz. The mean call duration was 2.6 ± 0.5 ms with the longest call lasting 3.0 ms. Regarding interpulse intervals, most calls followed each other at intervals between 23 and 68 ms (44.9 ± 18.2 ms).
Kerivoula depressa
Based on signals emitted by a male and a female released within a flight tent, this species emits characteristic broadband FM type calls (Figs. 6 and 8, Table 6). The maximum start frequency measured was 215.4 kHz and the minimum end frequency was 77.3 kHz. Peak energy was at a frequency of 129.0 ± 15.8 kHz. The mean call duration was 2.3 ± 0.7 ms and the longest call was 3.6 ms. Most calls followed each other at intervals between 12 and 23 ms (15.5 ± 3.8 ms).
Kerivoula dongduongana
Based on four males and one female flying in a flight tent, this species emits a characteristic broadband FM call (Figs. 6 and 8, Table 6). The maximum start frequency was at 237.5 kHz and the minimum end frequency was 72.6 kHz. The peak energy was at 127.8 ± 13.1 kHz, whereas the mean call duration was 2.9 ± 0.8 ms and the longest call was 5 ms. Regarding interpulse intervals, most calls followed each other at intervals between 11 and 69 ms (21.3 ± 15.3 ms).
Myotis laniger
Based on one female flying in a flight tent, this species emits typical broadband FM calls (Figs. 5 and 8, Table 7). The maximum start frequency was found to be 96.0 kHz and the minimum end frequency was 30.3 kHz. The peak energy was at 54.7 ± 2.6 kHz on average. The mean call duration was around 3.4 ± 0.5 ms and the longest call was 4.3 ms. As for the interpulse interval, most of the calls followed each other at intervals between 40 and 55 ms (46 ± 5.3 ms).
Myotis sicarius
The calls of M. sicarius were characterized by Csorba et al. (1999) as relatively high intensity FM echolocation calls, but their frequency range was not determined53. Based on one male flying in a flight tent (the first record of this species from Vietnam54), M. sicarius emits FM type calls with strong upper harmonics (Figs. 5 and 8, Table 7). The maximum start frequency was 80.2 kHz and the minimum end frequency was 36.0 kHz. The peak energy was at 36.6 ± 0.6 kHz on average. The mean call duration was 2 ms. Most of the calls followed each other at interpulse intervals between 117 and 165 ms (137.3 ± 24.8 kHz).
Discussion
Acoustic identification of bats typically begins with compilation of a library of reference calls from known species within a region, considering potential intraspecific geographic variation. Although some baseline acoustic data are available for Vietnam (e.g., Furey et al. 2009), a comprehensive call library covering such a large number of distinct (morphologically and/or genetically) species, as included in this study, did not exist for any single country in mainland Asia.
We analyzed reference echolocation calls produced by 88 of the 118 echolocating bat species presently known to occur in Vietnam, making this study one of the most comprehensive bioacoustic studies involving Asian bats to date. For several species, we were also able to analyze large datasets of echolocation calls, some of which were very poorly known e.g., Aselliscus dongbacanus, Hypsugo cadornae, Kerivoula titania, Murina feae, Myotis alticraniatus, Myotis ater, however in some cases (e.g., Hipposideros pratti, Miniopterus magnater, Miniopterus pusillus, Myotis altarium etc.), the number of investigated calls was limited and the variation in calls recorded for these species may not be sufficiently representative and should be interpreted with caution. We also provide the first description of echolocation calls produced by five species. Every sound recording analyzed can be found in the ChiroVox public sound database51, hence the calls can be reanalyzed and new parameters can be measured or even studied with a novel identification method.
Descriptions of bat echolocation calls are very useful for faunistic surveys, ecological studies, taxonomic research and also in the planning of developments affecting natural habitats19,55,56. For example, an increasing number of wind farms are being established in Southeast Asia, and according to the good practices for environmental impact assessment during the construction and operation phases of these projects, it is recommended to carry out surveys to gather baseline information of local bat populations57,58. As wind farms are often sited in open areas where the effectiveness of capture-based methods are inherently limited, acoustic surveys can play an important role in ensuring accurate baselines to be created for subsequent monitoring the health of local bat assemblages59.
In recent years, several software applications, e.g., SonoChiro, Kaleidoscope, and BatClassify, have been developed to automatically detect and identify bats from recordings based on large datasets on bat echolocation calls. Although such tools greatly facilitate the processing of bat acoustic data, users have to be cautious when interpreting the results made by the identifiers even in the temperate zone60, while the tools may be even more imprecise in identifying bat species in regions with high biodiversity. The information presented in our study can aid efforts to overcome this shortcoming and also help to validate the outputs of such software.
Materials and methods
Study sites and sound recordings
Bat calls were recorded between 2006 and 2023 from nearly all regions of Vietnam (specifically from 30 provinces and three municipalities) (Fig. 9). Bats were captured using mist-nets or harp-traps and kept in cloth bags until further processing. The data providers tried to ensure that–if the circumstances allowed–netting was carried out also at canopy height. They were provisionally identified based on morphology in the field based on recent literature. In cases where species identity could not be confirmed, DNA barcoding and/or morphological investigation of voucher specimens were employed. Sex and age were determined, and forearm length and weight were measured using a digital caliper and a scale, respectively. All experimental protocols and samplings were approved by local authorities and the Forest Protection Department of the Ministry of Agriculture and Rural Development, Hanoi, Vietnam (permit numbers: 1206/TCLN-BTTN, 2064/TCLN-BTTN, 326/TCLN-BTTN, 2833 /UBND-NC, 299/CV-BQL, 1508/UBND-NC, 7281/VPUBND-THNV, 317/UBND-NV, 631/UBND-NV). All methods were carried out in accordance with the guidelines of Sikes and the Animal Care and Use Committee of the American Society of Mammalogists61. The study is reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Whenever possible, free-flying recordings or flying sequences inside a flight tent were used for call descriptions. However, hand-held, resting, and hand-release sequences were also included in statistical measures when only those calls were available. For flight tent recordings (tent dimensions were at least 3 m long, 2 m wide and 2 m high), we distinguished between calls emitted when the bats were resting and calls produced during flight. Resting sequences were identified when a bat landed on the tent, whereas flying sequences were registered when it was flying in the tent. A small number of our older recordings were made with a D240x (Pettersson Elektronik AB) bat detector in time expansion mode and also with an Echo Meter Touch 1 (Wildlife Acoustics) with a 256 kHz sampling frequency (capable of recording calls up to 128 kHz); hence recordings taken by these devices from bats emitting high frequency calls (e.g., Kerivoulinae) were not used in our analysis. However, most of the sounds were recorded with D1000x, D980, M500 (Pettersson Elektronik AB), SM4BAT-FS (Wildlife Acoustics) detectors and the PCTape System (University of Tübingen). The sampling frequencies were set to the highest available for each specific recording device or system (up to 500 kHz). In some cases, multiple recordings were gathered from the same individual to achieve the best quality.
Sound analysis
The free, open-source Sonic Visualiser software (version 4.5.2)62 was used to measure various call parameters. Standardization was important for our analysis, so we worked with well-defined methods to ensure the data obtained was comparable. Spectrograms were generated using the following settings: FFT (Fast Fourier Transformation) size at 512, 93.75% overlapping, and Hann window. Three consecutive pulses were measured from each individual recording. These “triplets” were selected from the section with the highest signal-to-noise ratio (at least 20 dB over background noise). The parameters we measured were chosen from those most frequently used in the literature and suited for species identification12,20,22,23,31,63,64,65,66,67,68. Thus in general, eight parameters were manually measured from selected pulses depending on their call structure: frequency of maximum energy (FmaxE, the frequency containing the maximum energy on a power spectrum, kHz), start frequency (SF, frequency value at the start of the call, kHz), end frequency (EF, frequency value at the end of the call, kHz), highest frequency (HF, the highest frequency value of the call, kHz), lowest frequency (LF, the lowest frequency value of the call, kHz), bandwidth (BW, the difference between the highest and the lowest frequency value, kHz), call duration (D, duration of a single call, ms) and inter-pulse interval (IPI, time from the start of one call to the start of the next call, ms). SF and EF values were determined visually. The time parameters (D and IPI) were obtained from oscillograms, SF, EF, HF and LF from sonograms, and BW and FmaxE from power spectra.
Depending on the different structures of the calls, some characteristic parameters were also measured. For example, in the case of the FM-CF-FM (frequency modulated-constant frequency-frequency modulated) and FM-QCF (frequency modulated-quasi-constant frequency) calls, we measured the durations (ms) of the CF and QCF components (DCF & DQCF, respectively) and their frequency values (kHz) (FCF & FQCF, respectively). The number of harmonics and the measured harmonic(s) were also recorded. Where multiple call harmonics exhibited sufficient amplitude for measurement, we assessed the same parameters for these additional harmonics alongside the dominant or fundamental (first) harmonic, with the exception of rhinolophid, hipposiderid, emballonurid, and megadermatid bats, where the second harmonic always contained the maximum energy. For bats producing broadband calls (such as Myotis species), the cursor was positioned at the middle of the spectrum when FmaxE was not clearly defined. We calculated mean values and standard deviations of each parameter for each species as well as the specific parameters (in the case of FM-CF-FM and FM-QCF bats) mentioned. Descriptive statistics were calculated in Microsoft Excel. Spectrograms of typical call types emitted by each species were exported from BatSound Pro 4.7 (Pettersson Elektronik AB, Uppsala). All of the recordings and associated metadata (collection locality, date, type of recording device etc.) are available via ChiroVox (www.chirovox.org) (Table 8). Further details are provided in Supplementary Table S1.
Data availability
Data is provided within the manuscript or supplementary information files. The analyzed calls can be freely accessed through the ChiroVox bat call repository: https://www.chirovox.org.
References
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Sodhi, N. S., Koh, L. P., Brook, B. W. & Ng, P. K. L. Southeast Asian biodiversity: An impending disaster. Trends Ecol. Evol. 19, 654–660 (2004).
Furey, N. M., Mackie, I. J. & Racey, P. A. Bat diversity in Vietnamese limestone karst areas and the implications of forest degradation. Biodivers. Conserv. 19, 1821–1838 (2010).
Kingston, T. Research priorities for bat conservation in Southeast Asia: A consensus approach. Biodivers. Conserv. 19, 471–484 (2010).
Kingston, T. Response of bat diversity to forest disturbance in Southeast Asia: Insights from long-term research in Malaysia. In Bat Evolution, Ecology, and Conservation (eds Adams, R. A. & Pedersen, S. C.) 169–185 (Springer, New York, 2013).
Simmons, N. B. & Cirranello, A. L. Bat Species of the world: A taxonomic and geographic database. Version 1.5. Accessed on 03/28/2024. (2024).
Lane, D. J. W., Kingston, T. & Lee, B. P. Y. H. Dramatic decline in bat species richness in Singapore, with implications for Southeast Asia. Biol. Conserv. 131, 584–593 (2006).
Tuan, L. Q. et al. Potential individual and interactive effects of climate and land-cover changes on bats and implications for conservation planning: A case study in Vietnam. Biodivers. Conserv. 32, 4481–4508 (2023).
Francis, C. M. et al. The role of DNA barcodes in understanding and conservation of mammal diversity in Southeast Asia. PLoS ONE 5, e12575 (2010).
Jones, G. & Teeling, E. C. The evolution of echolocation in bats. Trends Ecol. Evol. 21, 149–156 (2006).
Jones, G. & Holderied, M. W. Bat echolocation calls: Adaptation and convergent evolution. Proc. R. Soc. B Biol. Sci. 274, 905–912 (2007).
Jones, G., Vaughan, N. & Parsons, S. Acoustic identification of bats from directly sampled and time expanded recordings of vocalizations. Acta Chiropterologica 2, 155–170 (2000).
Parsons, S. & Jones, G. Acoustic identification of twelve species of echolocating bat by discriminant function analysis and artificial neural networks. J. Exp. Biol. 203, 2641–2656 (2000).
Russo, D. & Jones, G. Identification of twenty-two bat species (Mammalia: Chiroptera) from Italy by analysis of time-expanded recordings of echolocation calls. J. Zool. 258, 91–103 (2002).
Schnitzler, H.-U., Moss, C. F. & Denzinger, A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 18, 386–394 (2003).
Pfalzer, G. & Kusch, J. Structure and variability of bat social calls: Implications for specificity and individual recognition. J. Zool. 261, 21–33 (2003).
Brigham, R. M., Kalko, E. K. V., Jones, G., Parsons, S. & Limpens, H. J. G. A. Bat Echolocation Research: Tools, Techniques and Analysis. (Bat Conservation International, Austin, Texas, 2004).
Chakravarty, R., Ruedi, M. & Ishtiaq, F. A recent survey of bats with descriptions of echolocation calls and new records from the western Himalayan region of Uttarakhand India. Acta Chiropterologica 22, 197–224 (2020).
Furey, N. M., Mackie, I. J. & Racey, P. A. The role of ultrasonic bat detectors in improving inventory and monitoring surveys in Vietnamese karst bat assemblages. Curr. Zool. 55, 327–341 (2009).
López-Bosch, D. et al. Bat echolocation in continental China: A systematic review and first acoustic identification key for the country. Mammal Res. 66, 405–416 (2021).
McArthur, E. & Ali Anwarali Khan, F. Towards a regional call library: Classifying calls of a species-rich bat assemblage in a Bornean karst rainforest. Barbastella 14, 95–117 (2021).
Pham, L. K., Tran, B. V., Le, Q. T., Nguyen, T. T. & Voigt, C. C. Description of echolocation call parameters for urban bats in Vietnam as a step towards a more integrated acoustic monitoring of urban wildlife in Southeast Asia. Diversity 13, 18 (2021).
Phauk, S., Sarith, P. & Furey, N. M. Cambodian bat echolocation: A first description of assemblage call parameters and assessment of their utility for species identification. Cambodian J. Nat. Hist. 2013, 16–26 (2013).
Rai, V., Thapa, S., Chalise, P. & Shah, K. B. Record of bats and their echolocation calls from southern Dolakha, central Nepal. Mammalia 85, 557–567 (2021).
Raman, S. & Hughes, A. C. Echobank for the bats of Western Ghats biodiversity hotspot. Acta Chiropterologica 22, 349–364 (2021).
Thong, V. D., Limbert, H. & Limbert, D. First records of bats (Mammalia: Chiroptera) from the world’s largest cave in Vietnam. Diversity 14, 534 (2022).
Voigt, C. C. & Kingston T. (eds.) Bats in the Anthropocene: Conservation of Bats in a Changing World. 1-606 (Springer, Cham, 2016).
Flaquer, C., Torre, I. & Arrizabalaga, A. Comparison of sampling methods for inventory of bat communities. J. Mammal. 88, 526–533 (2007).
Russo, D. & Voigt, C. C. The use of automated identification of bat echolocation calls in acoustic monitoring: A cautionary note for a sound analysis. Ecol. Indic. 66, 598–602 (2016).
Russo, D. & Jones, G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 26, 197–209 (2003).
Hughes, A. C. et al. Using echolocation calls to identify Thai bat species: Vespertilionidae, Emballonuridae Nycteridae and Megadermatidae. Acta Chiropterologica 13, 447–455 (2011).
O’Farrell, M. J., Corben, C. & Gannon, W. Geographic variation in the echolocation calls of the hoary bat (Lasiurus cinereus). Acta Chiropterologica 2, 185–196 (2000).
Barclay, R. M., Fullard, J. H. & Jacobs, D. S. Variation in the echolocation calls of the hoary bat (Lasiurus cinereus): Influence of body size, habitat structure, and geographic location. Can. J. Zool. 77, 530–534 (1999).
Sun, K. et al. Geographic variation in the acoustic traits of greater horseshoe bats: Testing the importance of drift and ecological selection in evolutionary processes. PLoS ONE 8, e70368 (2013).
Kruskop, S. V. Bats of Vietnam (Russian Academy of Sciences, 2013).
Csorba, G. A new species of Glischropus from the Indochinese subregion (Mammalia: Chiroptera: Vespertilionidae). Zootaxa 2925, 41–48 (2011).
Csorba, G., Thong, V. D., Bates, P. J. J. & Furey, N. M. Description of a new species of Murina from Vietnam (Chiroptera: Vespertilionidae: Murininae). Occas. Pap. Mus. Tex. Tech Univ. 268, 1–10 (2007).
Francis, C. M. & Eger, J. L. A review of tube-nosed bats (Murina) from Laos with a description of two new species. Acta Chiropterologica 14, 15–38 (2012).
Furey, N. M., Thong, V. D., Bates, P. J. J. & Csorba, G. Description of a new species belonging to the Murina ‘suilla -group’ (Chiroptera: Vespertilionidae: Murininae) from North Vietnam. Acta Chiropterologica 11, 225–236 (2009).
Thong, V. D. et al. A new species of Hipposideros (Chiroptera: Hipposideridae) from Vietnam. J. Mammal. 93, 1–11 (2012).
Tu, V. T., Hassanin, A., Furey, N. M., Son, N. T. & Csorba, G. Four species in one: multigene analyses reveal phylogenetic patterns within Hardwicke’s woolly bat, Kerivoula hardwickii-complex (Chiroptera, Vespertilionidae) in Asia. Hystrix Ital. J. Mammal. 29, 111–121 (2018).
Borissenko, A. V. & Kruskop, S. V. Bats of Vietnam and Adjacent Territories: An Identification Manual. 1-300 (Russian Vietnamese Science and Technological Tropical Centre, and the Zoological Museum of Moscow, M.V. Lomonosov State University, 2003).
Fukui, D. et al. First record of the genus Plecotus from Southeast Asia with notes on the taxonomy, karyology and echolocation call of P. homochrous from Vietnam. Acta Chiropterologica 22, 57–74 (2020).
Son, N. T. et al. Bats (Chiroptera) of Bidoup Nui Ba National Park, Dalat Plateau Vietnam. Mammal Study 46, 53–68 (2021).
Thong, V. D. Remarks on the diversity and echolocation calls of hipposiderid bats (Chirroptera: Hipposideridae) in Cuc Phuong National Park, northeastern Vietnam. Acad. J. Biol. 45, 1–9 (2023).
Thong, V. D. New data on distribution, morphology and echolocation of Hipposideros khaokhouayensis Guillén-Servent & Francis, 2006 (Chiroptera: Hipposideridae). Acta Zool. Bulg. 75, 469–476 (2023).
Thong, V. D. New records of Hipposideros griffini from lava caves and the threats to its conservation in Vietnam. Tap Chi Sinh Hoc J. Biol. 41, 31 (2019).
Thong, V. D. et al. Importance of mangroves for bat research and conservation: A case study from Vietnam with notes on echolocation of Myotis hasselti. Diversity 14, 258 (2022).
Thong, V. D. et al. Echolocation calls of Myotis alticraniatus (Chiroptera: Vespertilionidae) in Vietnam. J. Sci. Nat. Sci. 67, 133–140 (2022).
Thong, V. D. et al. Further records of Murina tiensa from Vietnam with first information on its echolocation calls. Hystrix 22, 129–138 (2011).
Görföl, T. et al. ChiroVox: A public library of bat calls. PeerJ 10, e12445 (2022).
Zsebők, S., Son, N. T. & Csorba, G. Acoustic characteristics of the echolocation call of the disc-footed bat, Eudiscopus denticulus (Osgood, 1932) (Chiroptera, Vespertilionidae). Acta Acust. United Acust. 100, 767–771 (2014).
Csorba, G., Kruskop, S. V. & Borissenko, A. V. Recent records of bats (Chiroptera) from Nepal, with remarks on their natural history. Mammalia 63, 61–78 (1999).
Győrössy, D. et al. The grey zone of taxonomy–the case of the Sikkim Myotis (Myotis sicarius) (Chiroptera: Vespertilionidae), first recorded from Southeast Asia. (in prep).
Barlow, K. E. et al. Citizen science reveals trends in bat populations: The national bat monitoring programme in great Britain. Biol. Conserv. 182, 14–26 (2015).
Stathopoulos, V., Zamora-Gutierrez, V., Jones, K. E. & Girolami, M. Bat echolocation call identification for biodiversity monitoring: A probabilistic approach. J. R. Stat. Soc. Ser. C Appl. Stat. 67, 165–183 (2018).
Frick, W. F. et al. Fatalities at wind turbines may threaten population viability of a migratory bat. Biol. Conserv. 209, 172–177 (2017).
Millon, L., Colin, C., Brescia, F. & Kerbiriou, C. Wind turbines impact bat activity, leading to high losses of habitat use in a biodiversity hotspot. Ecol. Eng. 112, 51–54 (2018).
Voigt, C. C. et al. Toward solving the global green–green dilemma between wind energy production and bat conservation. BioScience 74, 240–252 (2024).
Rydell, J., Nyman, S., Eklöf, J., Jones, G. & Russo, D. Testing the performances of automated identification of bat echolocation calls: A request for prudence. Ecol. Indic. 78, 416–420 (2017).
Sikes, R. S. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 97, 663–688 (2016).
Cannam, C., Landone, C. & Sandler, M. Sonic visualiser: An open source application for viewing, analysing, and annotating music audio files. In Proceedings of the 18th ACM international conference on Multimedia 1467–1468 (ACM, Firenze Italy, 2010). https://doi.org/10.1145/1873951.1874248.
Kingston, T., Jones, G., Akbar, Z. & Kunz, T. H. Echolocation signal design in Kerivoulinae and Murininae (Chiroptera: Vespertilionidae) from Malaysia. J. Zool. 249, 359–374 (1999).
Papadatou, E., Butlin, R. K. & Altringham, J. D. Identification of bat species in Greece from their echolocation calls. Acta Chiropterologica 10, 127–143 (2008).
Barataud, M. Acoustic Ecology of European Bats. Species Identification and Studies of Their Habitats and Foraging Behaviour. (Biotope Editions, Mèze; National Museum of Natural History, Paris, 2015).
Hackett, T. D., Holderied, M. W. & Korine, C. Echolocation call description of 15 species of Middle-Eastern desert dwelling insectivorous bats. Bioacoustics 26, 217–235 (2017).
Görföl, T. et al. A new genus of vespertilionid bat: The end of a long journey for Joffre’s Pipistrelle (Chiroptera: Vespertilionidae). J. Mammal. 101, 331–348 (2020).
Zamora-Gutierrez, V., MacSwiney G., M. C., Balvanera, S. M. & Esquivelzeta, E. R. The evolution of acoustic methods for the study of bats. In 50 years of bat research: Foundations and new frontiers 43–59 (Springer International Publishing, Switzerland, 2021). https://doi.org/10.1007/978-3-030-54727-1.
Acknowledgements
The authors would like to express their sincere thanks for all the people who helped in the field. This research received support from the National Research, Development, and Innovation Fund of Hungary (NKFIH FK137778, FK146466, RRF-2.3.1-21-2022-00010), the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (BO/00825/21) to T.G., the Hungarian-Vietnamese bilateral mobility grant (NKM-2021-39) to T.G. and V.T.T., the Vietnam Academy of Science and Technology to V.T.T. (Project No: QTHU01.01/22–23) and to V.D.T. (Projects “UQDTCB.07/23-25” and “KHCBSS.01/24-25”), Academia Sinica Postdoctoral Scholarship to J.C.C.H., Biodiversity Research Center at Academia Sinica to M.N.T. and JSPS KAKENHI (Grant Nos: JP24405045 and T16K00568) to D.F. The work of D.G. and K.L.S. was supported by the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (grant numbers ÚNKP 20-3-1 and ÚNKP-19-2-1, respectively).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.G. and G.C.; methodology, T.G.; validation, T.G. and G.C.; formal analysis, D.G. and K.L.S.; investigation, D.G., T.G. and G.C.; resources, T.G., G.C., P.E., V.T.T., V.D.T., J.C.-C.H., M.N.T., N.M.F., and D.F.; data curation, T.G., J.C.-C.H. and D.G.; writing—original draft preparation, D.G., T.G., G.C., and K.L.S.; writing—review and editing, T.G., G.C., N.M.F., V.D.T. and S.Z.; visualization, D.G. and T.G.; supervision, G.C. and T.G.; project administration, G.C. and T.G.; funding acquisition, T.G. and G.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Győrössy, D., Csorba, G., Szabadi, K.L. et al. The calls of Vietnamese bats: a major step toward the acoustic characterization of Asian bats. Sci Rep 14, 23335 (2024). https://doi.org/10.1038/s41598-024-72436-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-72436-6