Abstract
This study asked if combining different motor learning mechanisms—adaptation and reinforcement—could produce immediate improvements in over ground walking after stroke. Fifteen adults with stroke engaged in three conditions: (1) reinforcement following adaptation, (2) reinforcement alone, and (3) adaptation alone. Adaptation involved split-belt treadmill walking to produce after-effects that reduce step asymmetry. Reinforcement involved the use of real-time auditory feedback about step length asymmetry. Auditory feedback was binary, signaling whether steps were asymmetric or equal, but not whether to shorten or lengthen either step. Change in step length asymmetry was the outcome assessed during over ground walking. Reinforcement following adaptation led to reductions in step length asymmetry that persisted into an immediate retention period. Importantly, it led to the desired pattern of lengthening the shorter step in a majority of participants. Reinforcement alone led to no significant change in step length asymmetry, and sometimes produced a non-optimal pattern of shortening the longer step. Our control condition of adaptation alone led to more transient reductions in step length asymmetry. These findings reveal the potential for utilizing serial delivery of adaptation and reinforcement to influence a complex movement in the real-world context of over ground walking, in people with stroke.
Similar content being viewed by others
Introduction
Locomotor learning is not a unitary process, but rather, refers to an array of different mechanisms with distinctive features and neural substrates1. These include: instructive learning (e.g. “take a bigger step!”), error-based adaptation (e.g. split-belt treadmill walking), and binary reinforcement (e.g., “correct” vs. “incorrect”)2,3,4. In clinical practice, teaching and learning a new gait pattern relies heavily on instructive learning5. However, there is significant potential to improve gait rehabilitation outcomes by moving beyond purely instructive learning and directly implementing approaches that leverage other learning mechanisms in isolation or in combination.
Reinforcement is an important mechanism for learning new movement patterns. This learning is driven by reward prediction and may engage basal ganglia and primary motor cortex circuits6,7,8. During reinforcement, feedback about movement success or failure drives exploration9. With practice, a person learns which movement produces reward and repeats it, eventually acquiring a new movement pattern. While acquisition of new movements via reinforcement may occur slowly, once learned, movements can be well-retained10,11. Because retention of learned movements is a primary objective of clinical rehabilitation interventions, reinforcement-based rehabilitation interventions warrant further investigation.
Reinforcement-based protocols have been shown to promote learning and retention of novel reaching movements in neurologically intact adults12 as well as in adults with stroke13. The ability of people with stroke to modulate their over ground walking patterns via reinforcement signaling has not yet been established. One potential concern is that people with motor control deficits (such as those present after stroke) may be unable to explore the walking pattern that elicits reinforcement feedback. For instance, if the rehabilitation objective is to reduce step length asymmetry, participants would need some capacity to modulate their step lengths. Without the ability to generate movements that fall within the reinforcement window, learning through this modality may be limited.
Another form of motor learning, locomotor adaptation, may represent a method of addressing this concern by shifting the movements that can be generated by people with stroke toward the desired pattern. Adaptation is driven by delivering a predictable perturbation that causes errors to a given motor pattern. In split-belt walking adaptation, people normally learn to account for the perturbation over hundreds of steps and alter their movement pattern to reduce or eliminate errors. In walking, this does not require the person to voluntarily attempt to correct the pattern. There are several reasons that adaptation may be useful for improving gait in persons with supratentorial strokes: (1) relevant subcortical neural substrates—e.g., cerebellum14—are typically intact, (2) adaptation can be paired with instructive learning without interference15, and (3) several clinical populations have shown the ability to learn new gait patterns via adaptation16,17,18.
Moreover, our lab has shown that people with chronic stroke can restore step length symmetry following locomotor adaptation via split-belt treadmill walking2. In split-belt treadmill adaptation, the feet walk on two independent belts that move at different speeds. The split-belt treadmill perturbation exaggerates a person’s baseline step length asymmetry, leading to sensory prediction errors (i.e., the belts and their legs move at different speeds than the person expects) and abrupt postural instability. With continued split-belt treadmill walking, the person experiences locomotor adaptation—their nervous system learns to predict the environment and produce an appropriate motor pattern to restore postural stability and walk in the split-belt treadmill environment19,20. Importantly, this new pattern is stored and does not immediately return to normal when the perturbation is removed. Consequently, adaptation allows the participant to temporarily walk with more symmetrical step lengths on a treadmill2 and when walking over ground21, even if they normally have difficulty doing so volitionally. Long-term split-belt treadmill training can lead to improvements in step symmetry when walking over ground but these effects are incomplete and not all people with stroke show benefits22. Thus, the clinical utility of adaptation alone may be limited by its relative transience and incomplete transfer from the treadmill to the real-world context of walking over ground.
While pre-adapting the walking pattern before engaging in a reinforcement-based learning task has not yet been tested in stroke, there is evidence that pairing adaptation and reinforcement enhances retention of a reaching task in healthy adults23. A similar approach of leveraging adaptation and reinforcement mechanisms sequentially may also result in rapidly acquired and longer-lasting improvements in post-stroke gait.
The purpose of this study was to determine whether pre-adapting the walking pattern before engaging in binary reinforcement learning-based training yielded greater learning and immediate retention of an over ground gait pattern compared with reinforcement learning without pre-adaptation. Specifically, we strove to elicit and maintain improvements in step length difference (SLD) during over ground walking in people with stroke. We targeted SLD because it is common after stroke24 and is associated with instability, slow walking speeds, and elevated energy cost25,26,27. Reinforcement feedback was signaled when a subject successfully produced symmetric steps during walking. Importantly, reinforcement could drive an individual to explore different patterns: they could lengthen the shorter step, shorten the longer step or change both steps to achieve symmetry. We hypothesized that pre-adapting step lengths via split-belt treadmill walking before over ground training with reinforcement signaling would lead to greater improvements in SLD in people with stroke, relative to training with reinforcement signaling alone. We also expected that participants who pre-adapted would be better able to maintain that pattern when the reinforcement signal was withdrawn.
Methods
Following publication of this manuscript, the data that support the findings of this study will be made available on Github or from the corresponding author upon reasonable request.
Participants
Fifteen adults with stroke ≥ 4 months prior (8 male; 55.5 ± 8.3 years old) participated in this study. Inclusion criteria were: (1) diagnosis of ischemic or hemorrhagic stroke confirmed by a neurologist and/or magnetic resonance imaging, (2) ability to ambulate with or without an assistive device, (3) score of < 34 on the lower extremity subscale of the Fugl-Meyer, (4) baseline step length difference (|short step length—long step length|) of ≥ 2.0 cm, and (5) gait speed of > 0.40 m/s. Participants were excluded if they had neurological injury other than stroke, cerebellar signs or evidence of cerebellar involvement as confirmed by a neurologist or MRI, pregnancy, orthopedic or other medical condition that could compromise walking performance or introduce a step-length asymmetry, or unilateral spatial neglect with a Star Cancellation Test score of < 44/55. We did not exclude participants with aphasia or imperfect MoCA scores. The study protocol was approved by the Johns Hopkins Medicine Institutional Review Board and was conducted in compliance with the Declaration of Helsinki. Participants provided written informed consent prior to beginning the study and were compensated for their time. Because the objective of this study was to test a protocol intended to normalize step length asymmetry, we did not test neurologically-intact individuals who do not display this gait deficit.
Clinical assessments
Participants underwent clinical examination during a pretest session. We administered the Montreal Cognitive Assessment (MoCA)28 to assess cognitive function, The Star Cancellation Test29 to screen for unilateral spatial neglect, the lower extremity subscale of the Fugl-Meyer test (FM-LE)30 to quantify motor impairment. For proprioception testing, participants were supine with their eyes closed. The examiner stabilized the proximal joint segment and passively moved the distal segment to a position above or below the neutral starting position (neutral position was midway through the joint’s range of motion). The participant reported whether the position of the joint was above or below the starting position. Paretic hip, knee, and ankle joints were each tested at six different positions (18 total probes). We measured self-selected and fast over ground walking speeds by having participants walk two passes at each speed across a six-meter electronic walkway (Zeno Walkway, ProtoKinetics, Havertown, PA). SLD, used to determine study eligibility, was measured by the electronic walkway during the self-selected walking speed assessment. Participants who customarily wore an ankle–foot orthosis or walked with a cane, continued using these items throughout the study.
Experimental design
Participants completed a pretest session during which they signed consent forms, underwent eligibility screening and clinical examination, and performed: (1) 2 min of baseline over ground walking, (2) 2 min of ‘voluntary correction’ over ground walking, (3) 2 min of baseline treadmill walking, and (4) 2 min of ‘voluntary correction’ treadmill walking. For voluntary correction blocks, participants were instructed to “try to take equal length steps”, but were not told which was their shorter or longer stepping leg. These periods of walking with instruction allowed us to determine whether or not participants were able to voluntarily correct step length difference with just simple instructions (rather than requiring our protocol of reinforcement with or without pre-adaptation).
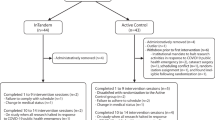
Eligible participants then engaged in three, randomly ordered sessions (2 experimental, 1 control), each involving a different learning condition (Fig. 1A). Sessions were separated by at least 3 days (mean: 9 days; range: 3–33 days) During the Reinforcement + pre-adaptation condition (AR), participants performed split-belt treadmill walking followed by over ground walking with reinforcement signaling. During the Reinforcement Alone condition (R), tied-belt treadmill walking was followed by over ground walking with reinforcement signaling. Tied belt-walking was not expected to elicit learning or improvements in the walking pattern but was included in order to match the total amount of time spent walking between conditions and control for potential fatigue effects. During the control condition of Adaptation Alone (A), participants were exposed to split-belt treadmill walking followed by over ground walking without reinforcement signaling. We included this control condition so that if an advantage of AR over R were detected, we would be able to determine whether improvements resulted from the pairing of pre-adaptation with reinforcement or were derived entirely from the adaptation process.
(A) Experimental paradigm. Participants engaged in three, randomly ordered experimental sessions, each involving a different learning condition. Experimental sessions included Reinforcement with pre-adaptation (purple), Reinforcement Alone (magenta), and the control condition of Adaptation Alone (green). Experimental blocks shown in white took place over ground and blocks shown in grey took place on the treadmill. All conditions involved: 2 min of baseline walking over ground, 2 min of baseline walking on the treadmill, 10 min of treadmill walking, 10 min of over ground training, and 10 min of over ground retention. During the Reinforcement with pre-adaptation condition, participants walked on a split-belt treadmill before engaging in over ground training that included reinforcement feedback. During the Reinforcement Alone condition, participants walked on a treadmill with tied-belts before over ground training that included reinforcement feedback. During the Adaptation Alone condition, participants walked on a split-belt treadmill and then walked over ground without reinforcement feedback. No reinforcement signaling/auditory feedback was provided during over ground retention for any of the conditions. (B) Illustration depicting the calculation of example step lengths. Step length was measured during periods of double support and was defined as the vector between right and left heel positions projected onto the direction of travel. In this example, the shorter step (blue) was taken by the right leg and the longer step (orange) was taken by the left leg. The metric of step length difference is agnostic to whether the right or left step is longer and is merely the difference between the step lengths of a pair of steps (SLD = short step length—long step length).
Experimental paradigm
Experimental sessions consisted of (1) Over ground baseline: 2 min of over ground walking (no reinforcement). (2) Treadmill baseline: 2 min of treadmill walking with tied-belts. (3) Treadmill walking: 10 min of walking on the treadmill with either split-belts (Reinforcement with Pre-adaptation and Adaptation Alone conditions) or tied-belts (Reinforcement Alone condition). (4) Over ground training: 10 min of over ground walking with either reinforcement signaling on (Reinforcement with Pre-adaptation and Reinforcement Alone conditions) or no reinforcement signaling (Adaptation Alone condition). During the over ground training portion of all conditions, participants were instructed to try to take equal length steps. (5) Over ground retention: 10 min of over ground walking with no reinforcement signaling. During over ground retention for all conditions, participants were told that auditory feedback was ‘off’ and were encouraged to continue trying to take equal length steps. As described above, the term retention here refers to the period of over ground walking during which participants attempted to continue producing equal length steps in the absence of the reinforcement signaling. It is critical that the behavioral changes elicited by any gait training protocol outlast the feedback used during training. Therefore, measuring SLD during retention was essential for us to identify whether any changes in step length difference persisted in the absence of feedback. To mitigate fatigue, participants had 2-min sitting rest breaks every 5 min during treadmill walking and every 2.5 min during over ground walking.
To gauge subjective experience with the different conditions, participants completed a four-item questionnaire after each session. Participants scored the following questions on a visual analog scale ranging from 0 to 10: (1) How symmetric were your steps during the over ground walking task? (0: never symmetric—10: always symmetric), (2) How fatigued were you after completing the task? (0: not fatigued—10 extremely fatigued), (3) How much attention did you pay to the task? (0: never paid attention—10 always paid attention), (4) What effect did the [reinforcement signaling] ‘tone’ have on your walking performance? (0: very harmful—10 very helpful).
Locomotor adaptation via split-belt treadmill walking
Treadmill walking portions of the experiment took place on a custom split-belt treadmill (Woodway USA, Waukesha, WI) with separate treadmill belts under each leg, driven by independent motors. In the treadmill walking period of Reinforcement + pre-adaptation and Adaptation Alone conditions, participants walked with “split-belts”, where right and left belts moved at different speeds. All other treadmill walking periods occurred with “tied-belts”, where both belts moved at the same speed. The treadmill was controlled with custom-written MATLAB (R2017a, The MathWorks, Inc., Natick, Massachusetts) software. During treadmill walking, participants were instructed to look straight ahead as they walked (not down at their feet or at the treadmill belts) and to notify the experimenter if they experienced discomfort. Participants were not given specific instructions about their walking pattern and were not given information about the relative treadmill belt speeds at any point in the experiment. Participants wore a safety harness and held onto a front handrail during treadmill walking.
Treadmill belt speeds were based on each individual’s over-ground walking speed during pretest and were held constant throughout the experimental conditions. During tied-belt walking, both belts moved at the individual’s self-selected speed. During split-belt walking, the fast belt speed equaled the participant’s fast over ground walking speed and the slow belt was set to half of that speed. The leg that took the shorter step during over-ground walking was placed on the fast belt during split-belt walking (paretic leg n = 6; non-paretic leg n = 9). Previous studies have shown that this protocol reliably generates locomotor adaptation of step lengths and results in a motor after-effect of more equal step lengths in people with stroke2,4. In this experiment, split-belt exposure was used as a tool to elicit the desired motor after-effect of more equal step lengths to be exploited during over ground walking. Feedback was not delivered at any point during split-belt or tied-belt treadmill walking.
Binary reinforcement signaling
Reinforcement signaling consisted of real-time binary auditory feedback about SLD during over ground walking. The method of reinforcement delivery was designed to be simple and not overly prescriptive or complex in order to enable participants with varying levels of attention and cognition to explore and solve the motor problem. Custom MATLAB (R2017a, The MathWorks, Inc., Natick, Massachusetts) software was synchronized with Vicon software (Nexus 2.7.1, Vicon Motion Systems Ltd, Oxford, UK) to give a tone (frequency: 1000 Hz; duration: 0.02 s) at heel strike if the participant achieved a step length that fell within their reinforcement window (otherwise there was no tone). Step length was calculated as the vector between right and left heel position, projected onto the direction of travel (Fig. 1B). When reinforcement signaling was on (over ground training epochs for AR and R conditions), participants were told that they would hear a tone if the length of a step was equal to the length of the previous step and were encouraged to try to earn as many ‘tones’ as possible. Equal length steps could be achieved by (1) shortening the longer step, (2) lengthening the shorter step, or (3) concurrently shortening the longer step and lengthening the shorter step; no guidance nor constraints were placed on which strategy a participant used. While the global objective of taking equal length steps was known, participants were not told how to do this. Consistent with classic reinforcement learning paradigms, participants had to explore different movements and discover which combination of muscle activations generated a movement that elicited the auditory reward. When reinforcement signaling was off during over ground retention (all conditions), participants were told that they would not hear tones or get feedback but that they should keep trying to take equal length steps.
The binary reinforcement window was centered around equal length steps (SLD = 0) and its width was customized such that during the first minute of over ground training, steps that fell + /− 0.8 standard deviations (SD) of the individual’s pretest SLD were reinforced. Immediately after the first minute of over ground training, we applied a one-time adjustment of the reinforcement window width based on the number of steps that were reinforced during the first minute of over ground training:
We did this to ensure that participants operated within a range where the reinforcement signaling was neither absent nor constant and could be used to shape behavior.
Motion analysis
During over ground walking, we collected kinematic data at 330 Hz using eight Vicon Vero 2.2 cameras (Vicon Motion Systems Ltd., Oxford, UK) positioned around a rectangular capture space (5.5 × 2.7 m). Participants continuously walked within this space. The majority of steps were taken on the long straight sides of the rectangular capture space; participants walked in the direction that placed their short-stepping leg on the outside during the brief turns at the short ends of the rectangular capture space (e.g., participant with a shorter left step walked clockwise). Eight passive reflective markers were placed bilaterally on the foot (second metatarsal head), heel (midpoint of calcaneus), ankle (lateral malleolus), mid-shank, knee (lateral joint space), mid-thigh, and pelvis (anterior superior iliac crest and posterior superior iliac crest).
During treadmill walking, kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Waterloo, ON, Canada). Bilateral infrared-emitting markers were placed over the fifth metatarsal head, lateral malleolus, lateral femoral epicondyle, greater trochanter, iliac crest, and acromion process.
Data analysis
SLD was defined as the difference between the short step length and the long step length. SLD of 0 indicates equal length steps, the desired gait pattern in the experimental sessions. For each participant, legs were assigned as “short” and “long” based on the over ground walking pattern demonstrated during the pretest session; this designation was maintained throughout the experiment. In other words, all participants initially had negative SLDs; a positive SLD in would indicate that the leg that initially took the shorter step was now taking the longer step.
We characterized over ground walking by averaging values in five epochs: baseline (mean of over ground baseline), early training (first fifteen pairs of steps of over ground training), late training (last fifteen pairs of steps of over ground training), early retention (first fifteen pairs of steps of over ground retention), and late retention (last fifteen pairs of steps of over ground retention).
To better understand how changes in SLD were achieved we also analyzed kinematics during over ground walking. We looked at whether the shorter step was lengthening (or vice versa) and measured the range of motion of hip and knee joints.
Statistics
Statistical analyses were completed using SPSS Statistics 24 (IBM, Armonk, NY). The criterion for statistical significance was set at α ≤ 0.05. If there were multiple comparisons, we used a more conservative level of α ≤ 0.0125 (0.05/4). Further analyses were performed on baseline-subtracted values in order to account for the wide range of starting values found in this heterogeneous group of people with stroke. To best visualize the results in figures, time series data were smoothed by a moving average of three steps; statistics were performed on the unsmoothed data.
We first performed a number of preparatory analyses to contextualize our results. To determine whether or not participants were able to voluntarily correct step length difference with instruction alone, we used a paired samples t-test to compare SLD during a pretest period of ‘voluntary correction’ with SLD during pretest baseline walking. To confirm that there were no differences in baseline SLD values between conditions, and that the available range for improvement in SLD was the same for each condition, we conducted a one-way ANOVA. Lastly, we wanted to assess whether the order of conditions experienced by a participant influenced task performance (e.g., assess the possibility that greater improvements in SLD occurred during the 3rd session, regardless of condition type). Thus, we conducted a repeated measures ANOVA with SLD data classified by the session order (1,2,3) instead of by condition.
We tested our hypothesis that the Reinforcement with pre-adaptation condition would produce greater or longer lasting improvements in SLD compared to Reinforcement Alone or the control condition of Adaptation Alone, by performing a 4-by-3, epoch-by-condition, repeated measures ANOVA to compare changes in SLD from baseline across epoch, between conditions. If the assumption of sphericity were violated (Mauchly’s Test p < 0.05), a Greenhouse–Geisser correction was applied. If applicable, post-hoc analysis was performed using the Bonferroni correction.
We then performed planned post-hoc tests to analyze each learning condition separately in order to better understand how the learning developed over the course of a given condition. This consisted of using one-sample t-tests (test value = 0) to determine if a SLD in an epoch was different from SLD at baseline. This was done for all three conditions.
To better understand how changes in step length difference were achieved during over ground walking for a given condition, we performed a series of secondary exploratory analyses. Using repeated-measures ANOVAs for each condition separately, we assessed: (1) change in short step length, (2) change in long step length, (3) change in hip kinematics (flexion, extension, range of motion), (4) change in knee kinematics (flexion, extension, range of motion), across epochs. If the assumption of sphericity were violated (Mauchly’s Test p < 0.05), Greenhouse–Geisser corrections were applied. We used pair-wise comparisons to determine which epochs differed, as well as one-sample t-tests (test value = 0) to determine if the value for an epoch was different from baseline. To account for multiple comparisons, we used a level of α ≤ 0.0125 (0.05/4). To better understand how individual participants adjusted their step lengths, we dichotomized participants as either ‘lengthening’ their short step length during training (change ≥ 2 cm) compared with their baseline versus no change (< 2 cm). Note that we chose this threshold based on the inclusion criterion requiring a minimal step length difference 2 cm.
Finally, we were interested in understanding whether there was a relationship between baseline characteristics/training parameters and the magnitude of retention. Therefore, we conducted a set of bivariate correlations (either Spearman or Pearson, depending on data type) between step length difference in the early retention epoch and each of the following: age, gender, time post-stroke, lower extremity Fugl-Meyer impairment score, MoCA cognitive score, baseline SLD, walking speed, number of steps taken during training, percentage of steps reinforced during training). These correlations were conducted for each condition. Assumptions of linearity, significant outliers, and normality were assessed.
An a priori power analysis for sample size estimation was conducted using G*Power31. The analysis used data from a published study Savin et al. 2014 in which gait adaptation was followed by over ground walking in people with and without stroke32. The effect size of post-adaptation step asymmetry after effects in this study was large, d = 1.07. With an alpha = 0.05 and power = 0.80, and a more conservative effect size of 0.80, the projected sample size needed for this repeated measure, between condition design was approximately n = 15.
Results
Demographics
We screened 25 individuals and enrolled 15 participants with stroke and mild-to-moderate lower extremity motor deficits (Table 1) who met inclusion/exclusion criteria. All participants provided informed consent. All participants reported intact hearing; none wore hearing aids. Baseline measures, adaptation factors, reinforcement signaling factors (e.g., reinforcement window widths), and questionnaire responses were similar across conditions (Table 2). Step length difference during a pretest period of voluntary correction was not different from pretest baseline (t(14) = − 1.208, p = 0.248), confirming that voluntary correction alone was insufficient to improve SLD during over ground walking (Fig. S1). Moreover, repeated measures ANOVAs applied to data classified by session order demonstrated that there was no order effect (all p > 0.05).
Changes in step length difference during over ground walking
Comparison between conditions
Figure 2 shows SLDs for Reinforcement with pre-adaptation (Fig. 2A), Reinforcement Alone (Fig. 2B), and Adaptation Alone (Fig. 2C) conditions during the over ground walking portions of the experiment. Note that the gold shaded regions in Fig. 2A–C indicate treadmill walking periods (described below).
Step length difference results. Group mean time series for step length difference during over ground walking in the (A) Reinforcement with pre-adaptation condition (purple), (B) Reinforcement Alone condition (magenta), (C) Adaptation Alone condition (green). Time series data are smoothed by a running average of 3 steps; shaded areas around each line represent SE; gold bars indicate the point within the experiment where subjects walked on the treadmill; horizontal dashed line is the group’s average over ground baseline step length difference for that session; BL = over ground baseline; SLD = step length difference; symmetry = step length difference of 0. D) Group average change in step length difference during early training (first 15 pairs of steps of training), late training (last 15 pairs of steps of training), early retention (first 15 pairs of steps of retention) and late retention (last 15 pairs of steps of retention) epochs. Data shown are means ± SE. Asterisks highlight epochs in which step length difference was significantly different (improved) compared with that condition’s over ground baseline step length difference. E) Time series of treadmill walking portions of the experiment for all conditions. Data are smoothed with a running average of 3 steps; shaded areas around each line represent SE; BLTread = treadmill baseline; Walking = treadmill walking; dashed lines indicate the mean over ground baseline step length difference for each condition (Purple = Reinforcement with pre-adaptation; Magenta = Reinforcement Alone; Green = Adaptation Alone).
Over ground baseline SLDs were similar across conditions (F(2,42) = 0.202, p = 0.818). A 3-by-4 repeated measures ANOVA comparing changes in SLD between conditions, across epoch of over ground walking showed a robust main effect of epoch (F(3, 126) = 5.452, p = 0.001) as individuals were able to change their behavior during training and retention. We also saw a marked condition-by-epoch interaction (F(6,126) = 2.994, p = 0.009) because the pattern of learning was different in different conditions. Post-hoc comparisons showed differences in the early training epoch between Reinforcement Alone and Reinforcement with pre-adaptation (p = 0.032) and between Reinforcement Alone and Adaptation Alone conditions (p < 0.001). The late training epoch was different between Reinforcement with pre-adaptation and Adaptation Alone conditions (p = 0.049). All other post-hoc comparisons were not significant (all p > 0.05). We did not find a main effect of condition (F(2,42) = 2.008, p = 0.137), likely due to the similarity in improvements seen during the early training epoch in Reinforcement with pre-adaptation and Adaptation Alone conditions.
How did step length difference change within each learning condition?
We conducted planned post-hoc tests for each learning condition separately to better understand how the learning developed over the course of a given condition (Fig. 2D). In the Reinforcement with pre-adaptation condition (Fig. 2A), exposure to split-belt treadmill adaptation before reinforcement-based training led to improvements in SLD during over ground walking. Specifically, Reinforcement with pre-adaptation led to large improvements in SLD in early (4.996 ± 5.93 cm, p = 0.006) and late over ground training (3.609 ± 3.19 cm, p = 0.001). A significant portion of these improvements lasted into early retention (4.369 ± 5.41 cm, p = 0.007). SLD was not different from baseline at late retention: (2.959 ± 7.14 cm, p = 0.131). The Reinforcement Alone condition (Fig. 2B) produced only minimal, non-significant changes in SLD from baseline (early training: 1.055 ± 3.22 cm; late training: 1.374 ± 3.83 cm; early retention: 2.067 ± 3.43 cm; late retention: 0.967 ± 4.25 cm, all p > 0.0125), despite the reduction in SLD observed during tied-belt walking (Fig. 2E magenta trace). This suggests that the systematic change in SLD during tied-belt treadmill exposure was likely due to the mechanical advantage of treadmill walking rather than a learning phenomenon. The control condition of Adaptation Alone (Fig. 2C) led to marked improvements in SLD during over ground walking immediately after treadmill exposure (early training 6.470 ± 4.27 cm, p < 0.001), an effect that gradually decayed in the absence of reinforcement signaling (late training: 1.352 ± 2.80 cm, p = 0.082; early retention: 2.313 ± 4.25 cm, p = 0.053; late retention: 1.923 ± 1.76 cm, p = 0.100).
Figure 2E shows the group time series during treadmill portions of the experiment. SLD was similar during the split-belt treadmill periods in Reinforcement with pre-adaptation and Adaptation Alone conditions. Interestingly, tied belt treadmill exposure during the Reinforcement Alone condition partially reduced SLD (not statistically significant), a mechanical phenomenon that has been previously described33,34.
Secondary analyses
How were changes in step length difference achieved?
To understand whether the improvements in SLD resulted from adjustments to the short step length, the long step length, or both, we calculated and plotted change in step length from baseline for the short and long steps, during training and retention (Fig. 3). At the group level, this exploratory analysis suggests that changes in SLD may be generated differently depending on the condition. In the Reinforcement with pre-adaptation condition, the group demonstrated a pattern of primarily adjusting step length difference by lengthening their short step (Fig. 3A filled purple bar). This pattern persisted into retention (Fig. 3D filled purple bar). In the Reinforcement Alone condition, the group data indicates that change from baseline to training may be most driven by shortening the long step length (Fig. 3B unfilled magenta bar), an effect which diminished in retention (Fig. 3E). In the control condition of Adaptation Alone the short step length was increased during training and retention (Fig. 3C,F, filled green bars), though to a lesser extent than in the Reinforcement with Pre-adaptation condition.
Change in long and short step lengths during: Training (top row) and Retention (bottom row) for each condition. Conditions included Reinforcement with pre-adaptation condition (A, D), Reinforcement Alone condition (B, E), and Adaptation Alone (C, F). Group data (purple, magenta, green bars) are the means ± SE of the change in step length. Individual data are shown in multi-colored inset plots; each color represents a different participant. Unfilled bars: long step length; Filled bars: short step length; Values below zero indicate a reduction of step length compared with baseline and values above zero indicate an increase in step length.
We then determined how many individuals responded by lengthening their short step in the different conditions. These data are illustrated in the inset bar plots shown in Fig. 3. We dichotomized participants as either ‘lengthening’ their short step length during training (change ≥ 2 cm) compared with their baseline versus no change (< 2 cm). In the Reinforcement with pre-adaptation condition, 10 out of 15 participants lengthened their short step, whereas only 5 of 15 did so in the Reinforcement Alone condition. In our Adaptation Alone condition, 8 of 15 individuals increased their short step. These trends in this secondary analysis suggest that learning conditions may have distinct effects on the way SLD is modulated by individual participants with stroke.
Hip and knee joint angles, particularly sagittal plane flexion and extension, influence foot placement and therefore step lengths during walking. While our learning conditions targeted SLD rather than particular joint kinematics, we were interested in exploring whether there were systematic changes in joint kinematics based on condition. For instance, conditions that involved split-belt treadmill walking could have resulted in increased hip range of motion of the short step due simply to the mechanics of that leg being placed on the fast treadmill belt. Figure S2 shows data for hip and knee range of motion for short and long steps during each epoch for all three learning conditions. We found that there were no systematic differences in hip or knee kinematics (flexion, extension, range of motion) across epoch, during any of the three conditions (all p > 0.0125). This finding suggests that participants modulated their step lengths within their existing range of motion by changing the relative timing of kinematics between their legs, rather than by adjusting their joint ranges of motion.
Which baseline characteristics or training parameters influenced retention of an improved walking pattern?
We were primarily interested in determining if there were improvements in SLD during the early retention epoch. We targeted this epoch for cross-condition comparisons because the protocol is identical among conditions at this point in the experiment and because retention of improvements is a critical objective of gait training protocols for people with stroke. We found that the amount of retention that resulted from any of the conditions was not correlated with age, gender, time post-stroke, lower extremity Fugl-Meyer impairment score, MoCA cognitive score, baseline SLD, walking speed, number of steps taken during training, nor the percentage of steps reinforced during training (all p > 0.05). The lack of correlation did not result from a truncated range of scores (i.e. Fugl-Meyer range 19–33; MoCA range 20–30; walking speed range 0.41–1.18 m/s) which suggests that the training protocol described in this study might be effective for a broad range of individuals with stroke.
Discussion
In this study, we investigated whether split-belt treadmill adaptation before binary reinforcement-based training would improve step length asymmetry during over ground walking in people with stroke. As hypothesized, we found that exposing participants to locomotor adaptation before initiating a reinforcement-based training protocol (pre-adapting the reinforcement training) resulted in improvements in SLD that persisted into an immediate retention period. Moreover, in Reinforcement with pre-adaptation and Adaptation Alone conditions, improvements in SLD were primarily achieved by increasing the length of the short step. This was not the case in the Reinforcement Alone condition. Thus, adapting before over ground reinforcement training helped people with stroke produce a more typical gait pattern.
One of the first laboratory studies combining reinforcement with adaptation showed that this combination could improve retention of motor after-effects during reaching within a robotic device23. That study of healthy controls showed proof-of-principle that these mechanisms could be leveraged sequentially. More recent work in reaching showed that people with stroke could also benefit from reinforcement of an adapted reaching pattern within a robotic setting13. Our study takes an important translational step by showing that serial exposure to treadmill adaptation and reinforcement training can improve post-stroke gait in an unconstrained, over ground environment. In other words, these effects are not specific to training on a treadmill.
The walking pattern induced by the split-belt training prior to over ground walking is key—when verbally instructed to take equal steps during the pretest session, people with stroke were not able produce the desired outcome (Fig. S1). With reinforcement alone, most participants appear to use a compensatory strategy of shortening their longer step. Treadmill adaptation deployed before over ground reinforcement allowed the majority of participants to achieve symmetry by lengthening their shorter step, thus moving out of their normal range of step size and promoting a more clinically desirable pattern.
An important feature of this work is that improvements in step length differences were observed during over ground walking. There is accumulating evidence that people with stroke can improve many features of their walking. But the majority of these reported improvements are achieved and measured on the treadmill2,4,22. The context in which improvements are observed is critical because, relative to over ground walking, treadmill walking induces more predictable inter-stride time dynamics, constrains fluctuations in stride times, equalizes stride lengths, and reduces vertical ground reaction forces33,34. Aligned with these findings, we showed that tied-belt treadmill walking (Reinforcement Alone condition) reduced SLD measured on the treadmill, but improvements did not transfer to the over ground environment. This lack of transfer suggests that tied-belt treadmill walking provided mechanical assistance rather than eliciting a transferrable learning phenomenon.
Our lab has previously found that people with stroke have the capacity to improve step length symmetry during treadmill walking35. Given this capacity, can people with stroke just voluntarily correct SLD during over ground walking? Here, we found that participants were not able to significantly improve SLD during over ground walking with voluntary correction. Instead, meaningful changes in SLD during over ground walking required the ‘boost’ of the adaptation after-effect to shift their steps near symmetry before they were able to generate more equal length steps. The finding that voluntary correction alone is insufficient to address SLD has direct clinical implications. In current clinical practice, teaching and learning relies heavily on explicit instructions for voluntary correction and on feedback from the clinician5. Our results suggest that uncovering greater step length symmetry with pre-adaptation before using reinforcement-based feedback to maintain these gains may be more effective than delivering reinforcement-based feedback alone.
While the group level effects described here suggest that Reinforcement with pre-adaptation may be the superior condition for addressing SLD, it is unlikely that a ‘one size fits all’ approach will lead to optimal outcomes for gait rehabilitation. Adaptation alone can change the walking pattern and does not require participants to attend to feedback. Accordingly, this strategy might be useful for some participants who have difficulty with attention or cognition. However, in this group of participants, the amount of attention reported for reinforcement signaling conditions was not statistically greater than for the Adaptation Alone condition (Table 2) and cognition (MoCA score) was not correlated with performance under the Reinforcement Alone condition. The influence of speech/language impairments on responsiveness to the different learning conditions is also unclear. In this study we included two people with expressive aphasia, and one responded best to the Reinforcement Alone condition and one responded best to the control condition of Adaptation Alone. Future work should include a sample of participants with more varied cognitive and language status in order to determine if impaired cognition or language represent barriers for reinforcement-based gait training.
We identified a few participants who responded to Reinforcement Alone, but these individuals could not be predicted from the demographic or clinical information that we collected. In lieu of a demographic or clinical predictor, it is possible that a protocol like this that includes brief exposures to training under different learning conditions (e.g. reinforcement with pre-adaptation, reinforcement alone, adaptation alone) could serve as an assay to assess responsiveness to different learning mechanisms. This information could be used to select the optimal gait training protocols for each individual to improve walking outcomes in people with stroke or other neurological conditions.
There are a few limitations to this work. The focus of this study was on leveraging two learning mechanisms sequentially to improve a common gait deficit in people with stroke. We are not able to draw more general conclusions about the use of these learning approaches in neurologically-intact individuals or people with other neurological conditions, but evidence suggests that healthy people can change their walking pattern in response to sensorimotor perturbations and various forms of feedback36,37,38. Another potential limitation of this study is the sample size. Future work should expand upon this proof-of-principle study by testing the hypotheses with a larger sample size. Specifically, future studies should be powered to detect whether there are significant differences in early and late retention epochs between reinforcement with pre-adaptation and adaptation alone conditions, thus clarifying the extent to which reinforcement learning and adaption learning mechanisms were each engaged. Additionally, our methods involved providing binary reinforcement feedback about task success (e.g. reward), rather than about task failure (e.g. punishment). We are unable to determine if or how the directionality of feedback influences task performance and retention. There are several studies in neurologically-intact individuals suggesting that reward leads to better learning and/or retention of upper extremity motor tasks, relative to punishment or no feedback39,40. In people with stroke, a study conducted by Quattrocchi et al. found that providing reward or punishment during a reaching adaptation task led to similar improvements in performance compared to a no-feedback group, but only the rewarded group showed better retention13. Finally, this study sought to engage reinforcement and adaptation-based learning mechanisms and did not incorporate instructive-based learning mechanisms into the protocol. Future studies may investigate whether pairing clinically plausible instruction with reinforcement-based training influences the retention of a new movement pattern.
Summary/conclusions
Our results suggest that pre-adapting a reinforcement-based training protocol can lead to longer-lasting improvements in step length difference during over ground walking in people with stroke. This proof-of-principle study assessed performance during a single session of each condition. However, we expect the benefits of the Reinforcement with pre-adaptation condition to be compounded and more durable following longer training periods. Future work should directly test this hypothesis. This work provides the preliminary evidence that clinical training approaches incorporating serial delivery of adaptation and reinforcement learning mechanisms might be more effective than standard instruction-based training for addressing complex motor deficits in people with stroke.
Data availability
Data available upon reasonable written request to the corresponding author. Controlled access of data required to protect participant privacy.
References
Krakauer, J. W., Hadjiosif, A. M., Xu, J., Wong, A. L. & Haith, A. M. Motor learning. Compr. Physiol.9, 613–663 (2019).
Reisman, D. S., Wityk, R., Silver, K. & Bastian, A. J. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain.130, 1861–1872 (2007).
Roemmich, R. T., Long, A. W. & Bastian, A. J. Seeing the errors you feel enhances locomotor performance but not learning. Curr. Biol.26, 2707–2716 (2016).
Cherry-Allen, K. M., Statton, M. A., Celnik, P. A. & Bastian, A. J. A dual-learning paradigm simultaneously improves multiple features of gait post-stroke. Neurorehabil. Neural Repair.32, 810–820 (2018).
Johnson, L., Burridge, J. H. & Demain, S. H. Internal and external focus of attention during gait re-education: An observational study of physical therapist practice in stroke rehabilitation. Phys. Ther.93, 957–966 (2013).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science.275, 1593–1599 (1997).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol.80, 1–27 (1998).
(PDF) A Primer on Reinforcement Learning in the Brain: Psychological, Computational, and Neural Perspectives [Internet]. ResearchGate. [cited 2021 Oct 28]. Available from: https://www.researchgate.net/publication/269517554_A_Primer_on_Reinforcement_Learning_in_the_Brain_Psychological_Computational_and_Neural_Perspectives.
Lee, D., Seo, H. & Jung, M. W. Neural basis of reinforcement learning and decision making. Annu. Rev. Neurosci.35, 287–308 (2012).
Galea, J. M., Mallia, E., Rothwell, J. & Diedrichsen, J. The dissociable effects of punishment and reward on motor learning. Nat. Neurosci.18, 597–602 (2015).
Therrien, A. S., Wolpert, D. M. & Bastian, A. J. Effective reinforcement learning following cerebellar damage requires a balance between exploration and motor noise. Brain.139, 101–114 (2016).
Therrien, A. S., Wolpert, D. M. & Bastian, A. J. Increasing motor noise impairs reinforcement learning in healthy individuals. eNeuro5, ENEURO.0050-18.2018 (2018).
Quattrocchi, G., Greenwood, R., Rothwell, J. C., Galea, J. M. & Bestmann, S. Reward and punishment enhance motor adaptation in stroke. J. Neurol. Neurosurg. Psychiatry.88, 730–736 (2017).
Morton, S. M. & Bastian, A. J. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J. Neurosci.26, 9107–9116 (2006).
Long, A. W., Roemmich, R. T. & Bastian, A. J. Blocking trial-by-trial error correction does not interfere with motor learning in human walking. J. Neurophysiol.115, 2341–2348 (2016).
Morton, S. M., Tseng, Y.-W., Zackowski, K. M., Daline, J. R. & Bastian, A. J. Longitudinal tracking of gait and balance impairments in cerebellar disease. Mov. Disord.25, 1944–1952 (2010).
Choi, J. T., Vining, E. P. G., Reisman, D. S. & Bastian, A. J. Walking flexibility after hemispherectomy: Split-belt treadmill adaptation and feedback control. Brain.132, 722–733 (2009).
Reisman, D. S., Bastian, A. J. & Morton, S. M. Neurophysiologic and rehabilitation insights from the split-belt and other locomotor adaptation paradigms. Phys. Ther.90, 187–195 (2010).
Darter, B. J., Labrecque, B. A. & Perera, R. A. Dynamic stability during split-belt walking and the relationship with step length symmetry. Gait Posture.62, 86–91 (2018).
Jacobs, J. V. & Horak, F. B. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp. Brain Res.179, 29–42 (2007).
Reisman, D. S., Wityk, R., Silver, K. & Bastian, A. J. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil. Neural Repair23, 735–744 (2009).
Reisman, D. S., McLean, H., Keller, J., Danks, K. A. & Bastian, A. J. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil. Neural Repair27, 460–468 (2013).
Shmuelof, L. et al. Overcoming motor “forgetting” through reinforcement of learned actions. J. Neurosci.32, 14617–14621 (2012).
Patterson, K. K. et al. Gait asymmetry in community-ambulating stroke survivors. Arch. Phy. Med. Rehabil.89, 304–310 (2008).
Titianova, E. B. & Tarkka, I. M. Asymmetry in walking performance and postural sway in patients with chronic unilateral cerebral infarction. J. Rehabil. Res. Dev.32, 236–244 (1995).
Patterson, K. K., Gage, W. H., Brooks, D., Black, S. E. & McIlroy, W. E. Changes in gait symmetry and velocity after stroke: A cross-sectional study from weeks to years after stroke. Neurorehabil. Neural Repair24, 783–790 (2010).
Awad, L. N., Palmer, J. A., Pohlig, R. T., Binder-Macleod, S. A. & Reisman, D. S. Walking speed and step length asymmetry modify the energy cost of walking after stroke. Neurorehabil. Neural Repair29, 416–423 (2015).
Nasreddine, Z. S. et al. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc.53, 695–699 (2005).
Wilson, B., Cockburn, J. & Halligan, P. Development of a behavioral test of visuospatial neglect. Arch. Phys. Med. Rehabil.68, 98–102 (1987).
Fugl-Meyer, A. R., Jääskö, L., Leyman, I., Olsson, S. & Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med.7, 13–31 (1975).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods39, 175–191 (2007).
Savin, D. N., Morton, S. M. & Whitall, J. Generalization of improved step length symmetry from treadmill to overground walking in persons with stroke and hemiparesis. Clin. Neurophysiol.125, 1012–1020 (2014).
Brouwer, B., Parvataneni, K. & Olney, S. J. A comparison of gait biomechanics and metabolic requirements of overground and treadmill walking in people with stroke. Clin. Biomech. (Bristol, Avon).24, 729–734 (2009).
Hollman, J. H. et al. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture43, 204–209 (2016).
Roemmich, R. T., Leech, K. A., Gonzalez, A. J. & Bastian, A. J. Trading symmetry for energy cost during walking in healthy adults and persons poststroke. Neurorehabil. Neural Repair33, 602–613 (2019).
Malone, L. A. & Bastian, A. J. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. J. Neurophysiol.103, 1954–1962 (2010).
Day, K. A., Cherry-Allen, K. M. & Bastian, A. J. Individualized feedback to change multiple gait deficits in chronic stroke. J. Neuroeng. Rehabil.16, 158 (2019).
French, M. A., Morton, S. M. & Reisman, D. S. Use of explicit processes during a visually guided locomotor learning task predicts 24-h retention after stroke. J. Neurophysiol.125, 211–222 (2021).
Wächter, T., Lungu, O. V., Liu, T., Willingham, D. T. & Ashe, J. Differential effect of reward and punishment on procedural learning. J. Neurosci.29, 436–443 (2009).
Abe, M. et al. Reward improves long-term retention of a motor memory through induction of offline memory gains. Curr. Biol.21, 557–562 (2011).
Acknowledgements
We thank Matthew Statton and Christian Hernandez for their technical contributions in the early stages of the Project.
Funding
This work was supported by NIH 4R37 NS090610-14 to AB, NIH 5R01 HD053793-13 to PC, and NIH F32 HD096775-01 to KMC-A.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.M.C.-A., A.B., P.C.; Data Collection: K.M.C.-A., H.D.H.; Data Analysis: K.M.C.-A., A.B., H.D.H.; Writing—original draft: K.M.C.-A.; Writing—review and editing: K.M.C.-A., A.B., P.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cherry-Allen, K.M., Huang, H.D., Celnik, P.A. et al. Serial engagement of distinct motor learning mechanisms to alter walking after stroke. Sci Rep 14, 22706 (2024). https://doi.org/10.1038/s41598-024-73502-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-73502-9
Keywords
This article is cited by
-
Split-belt treadmill training improves gait symmetry and lower limb function in patients with stroke
Scientific Reports (2025)
-
Error-driven intralimb and interlimb adaptations under asymmetric treadmill and cueing conditions
Scientific Reports (2025)