Abstract
The Rhizobium rhizogene-transformed root culture from Physalis peruviana L. (P. peruviana) may be a promising and novel source of valuable phenolics, including caffeoylputrescine (CP), which is known for antioxidant, antidiabetic, insect-resistant, disease-resistant, and neuroprotective properties. In this study, to improve the production efficiency of phytochemical components in P. peruviana hairy root cultures, we optimized various culture conditions, including the inoculum size, liquid volume, culture media type, carbon source, sucrose concentration, initial pH, and application of elicitors, to enhance the total phenolic content and CP yield in these hairy root cultures. The findings indicate that the use of sucrose as carbon source resulted in the highest biomass (13.28 g DW/L), total phenolic content (6.26 mg/g), and CP yield (2.40 mg/L). The White medium excelled in enhancing the total phenolic content (9.35 mg/g), whereas the B5 medium was most effective for the biomass (13.38 g DW/L) and CP yield (6.30 mg/L). A sucrose concentration of 5% was best for the biomass (18.40 g DW/L), whereas a sucrose concentration of 4% was ideal for the CP yield. Optimal culture conditions were as follows: an inoculum size of 0.5 g/100 mL, a liquid volume of 100 mL in a 250-mL flask, B5 medium, 4% sucrose, and a pH of 5.5. Among the tested elicitors, methyl jasmonate (MeJA) at 100 µM significantly increased the biomass (21.3 g/L), total phenolic content (23.34 mg/g), and CP yield (141.10 mg/L), which represent 0.96-, 2.12-, and 13.04-fold increases, respectively, over the control after 8 days. The optimized HR culture of P. peruviana provides a promising system to enhance the production of CP for pharmaceutical applications.
Similar content being viewed by others
Introduction
Plants are valuable sources of secondary metabolites, and they play crucial roles in nutrition and pharmaceuticals1. However, plants can be negatively impacted by various biotic and abiotic stresses in their natural environment, which reduces the productivity and affects the production of secondary metabolites2. To address this issue, in vitro culture technology can be effectively utilized for the mass propagation, conservation, and extraction of secondary metabolites from these plants3. Hairy root (HR) cultures of plants are established by infecting explants with a T-DNA, which has been integrated into the genomic DNA of the target plant through the natural genetic engineering process of Agrobacterium rhizogenes at the site of plant injury4. The T-DNA region contains genes that are responsible for auxin and cytokinin synthesis in the roots and genes that produce opines (unusual amino acids)5. The resulting HR tissues are genetically stable and have a high growth rate in a hormone-free medium6. Compared to their parent plants, HR cultures can often better produce and increase secondary metabolites7. In vitro culture enables the application of diverse strategies to enhance the accumulation of secondary metabolites.
Previous studies have shown that factors such as the type and concentration of the carbon source, inoculum size, liquid volume in the flask, mediums, and initial pH of the medium can impact the growth and secondary metabolism of HR cultures8,9. Therefore, optimizing these parameters can improve the growth and secondary metabolite biosynthesis in this culture system.
In addition, elicitation is a valuable biotechnological tool to alter the metabolic pathways of medicinal plants by stimulating the plant defense and stress-induced responses10. Elicitors, which are classified as biotic or abiotic based on their origin, can enhance the secondary metabolism in plants11. Methyl jasmonate (MeJA), salicylic acid (SA), and sodium nitroprusside (SNP) are powerful elicitors for the secondary metabolite production in medicinal plants. Jasmonic acid and its methyl esters are crucial in the signal transduction process that regulates the defense mechanisms in plants12,13. MeJA has been successfully used to increase the production of 3,5-dicaffeoylquinic acid in Cichorium intybus L14. SA, which is a phenolic phytohormone, triggers various plant defense responses and promotes the synthesis of secondary metabolites15. SA treatment can enhance the yield of phenolic acids in Salvia miltiorrhiza16. SNP, which acts as a donor of nitric oxide (NO), plays a role in plant signaling, defense mechanisms, and processes such as plant growth and development17. SNP has been effective as an elicitor in increasing flavonoids, including rutin, in Haplophyllum virgatum18.
Phenolic compounds represent a group of secondary metabolites that plants produce19. These compounds are subdivided into different groups, including phenolic acids, flavonoids, stilbenes, lignans, and polymeric lignans20. There are two main groups of phenolic acids: hydroxybenzoic and hydroxycinnamic21. Phenolic compounds have been linked to various beneficial properties, including antioxidant, antidiabetic, anticancer, and anti-inflammatory effects20,22. Caffeoylputrescine (CP) is a hydroxycinnamic acid amide compound that results from the conjugation of caffeic acid and putrescine23. The molecular architecture of CP features a conjugated double bond, which bestows upon it the potential to exist as two geometric isomers: (E)-N-caffeoylputrescine and (Z)-N-caffeoylputrescine. It was initially discovered in the callus tissue culture of Nicotiana tabacum24 and subsequently found in various plants of the Solanaceae family such as Scopolia tangutica25, Solanum lycopersicum13, and Capsicum annuum26. Our preliminary research also detected CP in Physalis peruviana L. (P. peruviana). In Nicotiana attenuata, CP is believed to have defensive and developmental functions. It functions acts as a direct defense mechanism by inhibiting the growth of Manduca sexta caterpillars27. In Capsicum annuum L., CP compounds act as the phytoalexin to defend against the Colletotrichum gloeosporioide pathogen28. CP demonstrates moderate antimicrobial activity against various bacterial strains and exhibits anti-inflammatory properties in tomato leaves29. Additionally, the CP compound in pepper leaves may enhance antidiabetic effects and provide a natural approach to improve the human glucose metabolism30. Kim et al.31 have reported that CP and anthocyanin from Capsicum annuum exhibit the greatest antioxidant activity, with potent radical-scavenging potential. Furthermore, CP enhances the analgesic and sedative properties of Anisodus tanguticus (Maxim) Pascher, bolstering its role as an auxiliary in managing neurodegenerative diseases32.
Although numerous studies investigated the optimal culture conditions and elicitors of HR and their impact on secondary metabolites in various plants, no research has specifically evaluated the production of phytochemical components (e.g., polyphenols and CP) in P. peruviana HRs. The production of these phytochemical components must be optimized to enhance the industrial potential of P. peruviana HRs. This study aimed to establish the optimal culture conditions to improve the growth, total phenolic content, and CP content of P. peruviana HRs. Additionally, P. peruviana. HRs were treated with different elicitors (MeJA, SA, and SNP), and the most effective elicitor was selected for further treatment. The findings of this study are expected to provide a reference for the cultivation of P. peruviana HRs and synthesis and regulation of secondary metabolites.
Materials and methods
Plant material and hairy root culture establishment
All plant experiments complied with relevant institutional standards. P. peruviana seeds were purchased from China National Seed Group Co., Ltd., Beijing, China, and stored at the Jiangxi Key Laboratory of Natural Products and Functional Food, in Nanchang, Jiangxi, China. The seeds were identified by Professor Lu Zhang of the College of Forestry at Jiangxi Agricultural University. HRs were induced using the method described by Fu et al.33. These pre-cultured explants were immersed in a culture medium of Agrobacterium rhizogenes for 10 min; then, the surface bacteria and moisture were removed using sterile filter paper. The infected explants were placed on the surface of a 1/2 MS solid medium and cultured at 28 °C in the dark. After 2 d of co-culture, the explants were transferred to a 1/2 MS solid medium supplemented with cefotaxime sodium (Cef) to eliminate any remaining bacteria. To confirm the plant transformation, a DNA polymerase chain reaction (PCR) analysis was performed. To obtain the optimal harvest time for the biomass of P. peruviana HRs in liquid culture, liquid cultivation was first conducted following the method of Hajati et al.34, and a growth curve was plotted. Then, 0.5 g fresh weight (FW) of HR was inoculated in 250-mL flasks containing 100 mL of 1/2 MS liquid medium supplemented with 30 g/L of sources. Samples were collected every 4 days until day 36. The FW and dry weight (DW) were recorded.
Optimization of culture conditions
To establish the optimal growth conditions to produce phytochemical components, the effects of different carbon sources, sucrose concentrations, inoculum sizes, liquid volumes in the flask, and media were evaluated on in vitro HR cultures of P. peruviana. In total, 500 mg of HRs was inoculated in 250-mL Erlenmeyer’s flasks that contained 100 mL of 1/2 MS medium supplemented with 30 g/L of different carbon sources (maltose, glucose, sucrose, or fructose). Different inoculum sizes (0.1, 0.3, 0.5, 0.7, or 0.9 g/100 mL), liquid volumes in the flask (60, 80, 100, 120, or 140 mL), media (MS, 1/2 MS, B5, WPM, or White), sucrose concentrations (1%, 2%, 3%, 4%, 5%, or 6%), and initial pH values of the medium (4.5, 5.0, 5.5, 6.0, or 6.5) were applied depending on the objective of the experiment. The increases in total biomass, total phenolic content, and secondary metabolite content were evaluated after 24 days of subculture.
Elicitor treatments of the hairy root culture
To improve the HR biomass and phytochemical component production, different elicitor treatments were studied to assess their influence on the amount of phytochemical components. The study used elicitors such as SA, SNP, and MeJA at concentrations of 50, 100, 200, and 400 µM. These elicitors were added to the B5 medium and individually applied to 20-day-old liquid HR cultures. The control group was treated without using any elicitors. The HRs were harvested at 2, 4, 6, and 8 days after being treated with the elicitors.
Determination of root biomass
To calculate the FW of the collected HRs, they were washed twice with distilled water, delicately pressed onto filter papers to eliminate excess water, and weighed. The DW was determined by dehydrating the fresh HRs in an oven set at 60 °C until a stable weight was reached.
Extraction of phenolic
The ultrasound-assisted extraction was performed as reported by Alcántara et al.35 Then, 200 mg of the dried and grinded HR sample was extracted using 2 mL of 70% methanol through sonication at 80 °C for 1 h, followed by centrifugation at 3000 rpm for 15 min. The supernatant was collected, and the solution was filtered through a 0.22-mm-pore-size filter to determine the total phenolic content (TPC) and for the high-performance liquid chromatography (HPLC) analysis. The ultrasonic plant material extraction procedure was repeated three times.
Total phenolic content determination and quantitative analysis of caffeoylputrescine
The TPC of transgenic HR and non-transformed roots was determined using the Folin-Ciocalteau method, as described by Singleton and Rossi36 and Cheng et al.37 with modifications. A mixture was prepared with 0.1 mL of sample solution, 0.5 mL of Folin-Ciocalteu reagent, 7.9 mL of water, and 1.5 mL of 10% sodium carbonate (w/v). After 1 h, absorbance was measured at 760 nm using a MicroplateReader (SpectraMax M2; Molecular Devices, Shanghai, China). The TPC was determined in milligrams of gallic acid equivalents using an equation from the gallic acid calibration curve.
CP in the HR was identified via HPLC-TOF-MS (6430 HPLC-MS system; Agilent Technologies, Santa Clara, CA, USA) and quantified via HPLC (1260 HPLC system; Agilent Technologies, Santa Clara, CA, USA). The HPLC conditions were as follows: the chromatographic column was a Waters Symmetry C18 column (4.6 mm × 250 mm, 5 μm; MA, USA). The mobile phase consisted of 0.3% formic acid (A) and acetonitrile (C) using the following gradient program: 0–10 min, 97% (A); 10–18 min, 97–85% (A); 18–28 min, 87–75% (A); 28–30 min, 75–5% (A); 30–40 min, 5% (A); 40–41 min, 5–97% (A); 41–45 min, 97% (A). The column temperature was maintained at 40 °C with a flow rate of 1 mL/min and an injection volume of 3 µL. The target compound was quantified using specific calibration curves and reported as mg/g with respect to the DW of the roots.
Data statistics and analysis
Each experiment was repeated thrice, and the results are presented as mean values ± standard deviation. Data were analyzed using the GraphPad Prism 8.0.2 (https://www.graphpad-prism.cn/) and SPSS Statistics 17 software (https://www.ibm.com/cn-zh/spss). Statistical analysis was performed using an analysis of variance followed by Duncan’s test (at P ≤ 0.05).
Results
Establishment of a suspension culture system for P. peruviana hairy roots.
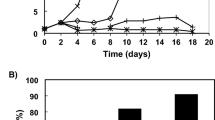
Figure 1A–D show the time course of P. peruviana HR growth in the 1/2 MS liquid medium. The entire growth cycle can be divided into four stages: adaptive phase (0–4th day), exponential phase (4th − 24th day), stationary phase (24th − 28th day), and decline phase (after the 28th day). The maximal biomass concentration of 18.6 g DW/L was achieved on the 24th day of cultivation, and it represented a significant increase compared to the initial inoculation. Subsequently, the biomass concentration of HRs decreased, and the liquid culture medium gradually became yellow after the 28th day.
P. peruviana HRs were analyzed using HPLC-TOF-MS and HPLC to identify the extracts. The results showed that a compound identified as CP had pseudo-molecular ions at m/z 251 [M + H] +, which matched those reported by Baumert et al.38. We confirmed this compound by comparing the retention times of authentic standards and extracts from the treated HR cultures (Fig. 1E,F). The standard curve was generated using a series of CP solutions of known concentrations of 1–1000 µg/mL, which were prepared with methanol. The solutions were analyzed using an HPLC method, and the peak area was plotted against the concentration to obtain the standard curve equation: \(\:y=9.0559x-8.7285\) with a high correlation coefficient (R2 = 0.9999), which indicates excellent linearity.
(A) Fresh weight and dry weight of hairy root of Physalis peruviana L. (P. peruviana) on different days after a sucrose treatment. (B) Identified hairy roots. Growth of hairy roots in a liquid 1/2MS medium: (C) 4 days - adaptive phase; (D) 24 days - stationary phase. Mass spectra of the identified caffeoylputrescine (CP) in the positive ion mode. (E) HPLC chromatograms of standard CP and methanolic extracts of P. peruviana HRs. (F) Secondary mass spectrometry of CP.
Optimizing the culture condition effects
Effect of the inoculum size on the TPC and CP synthesis of P. Peruviana HRs
This study identified that an optimal HR inoculum size of 0.5 g FW/100 mL resulted in the highest accumulation of biomass (16.57 g DW/L), TPC (10.39 mg/g DW), CP (0.53 mg/g DW), and CP yield (8.03 mg/L) after 24 days of culture (Fig. 2 [1(a), 1(b)]). A higher or lower inoculum size was not advantageous because they decreased the biomass and phytochemical component accumulation.
Effect of the liquid volume on the TPC and CP synthesis of P. Peruviana HRs
In this experiment, 250-mL triangular bottles were used with varying liquid volumes (60, 80, 100, 120, and 140 mL). The results in Fig. 2(2(a) and 2(b)) illustrate the effects of these different liquid volumes on the biomass and production of secondary metabolites. Among the tested volumes, HRs at 140 mL exhibited the highest biomass accumulation (27.97 g DW/L) after 24 days of incubation. Additionally, the highest total phenolics accumulation (8.90 mg/g DW) was observed at 60 mL, whereas the highest accumulation of CP content (0.27 mg/g DW) and CP yield (5.23 mg/L) occurred at 100 mL.
Effect of the carbon sources on the TPC and CP synthesis of P. Peruviana HRs
This study assessed the effects of four carbon sources on the HR biomass and production of secondary metabolites (Fig. 2[3(a) and 3(b)]). Among these sources, sucrose was the most optimal for the biomass (13.28 g DW/L) and TPC (6.83 mg/g DW) accumulation in the HRs, and glucose was more favorable for CP (0.27 mg/g DW) accumulation. Interestingly, there was no significant difference in CP production between sucrose (2.40 mg/L) and glucose (2.50 mg/L). As a result, sucrose was selected for this study due to its commonality and cost-effectiveness.
Optimization of culture conditions to enhance the biomass, total phenolic content (TPC), and caffeoylputrescine production from hairy roots. The effects of the inoculum size (1(a) and 1(b)), liquid volume (2(a) and 2(b)), and carbon source sucrose (3(a) and 3(b)) were investigated. The presented values are the mean ± standard error of three replicates; the data recorded were after 24 days of culture.
Effect of the media on the TPC and CP synthesis of P. Peruviana HRs
To determine the most suitable medium for HR growth, five different media were evaluated: MS, 1/2 MS, WPM, White, and B5. The results in Fig. 3(1(a) and 1(b)) show significant variations in growth capacity and CP production among different media, and B5 was superior. The B5 medium exhibited the highest biomass accumulation (13.38 g DW/L), the maximum CP content (0.47 mg/g DW), and the highest CP yield (6.30 mg/L). Although the White medium showed the highest total phenol accumulation, its CP yield (0.78 mg/L) was only 0.12 times higher than that of the B5 medium. Therefore, B5 was identified as the most effective medium for CP production.
Effect of the concentration of sucrose on TPC and CP synthesis of P. Peruviana HRs
The increasing trend was notably apparent with sucrose levels of 1–4% but inversely proportional at concentrations exceeding 4% which affected the CP content and yield in HRs. Optimal results were observed with 4% sucrose: the CP content and yield increased by 1.56 and 1.92 times, respectively, compared to the 3% sucrose levels (Fig. 3[2(a) and 2(b)]). However, there was no significant difference in the production levels of the growth capacity and TPC at higher sucrose concentrations.
Effect of the initial pH on the TPC and CP synthesis of P. Peruviana HRs
The study evaluated the effect of the initial pH (4.5–6.5) on the HR growth and secondary metabolite production on the 24th day of culture (Fig. 3[3(a) and 3(b)]). The pH levels of 4.5–5.5 showed no significant effect on the biomass and CP productivities, whereas pH levels above 5.5 had an inverse relationship with these factors and affected the biomass, TPC, CP content, and yield in HRs. Notably, HRs grown at pH 5.5 exhibited the highest TPC (10.31 mg/g DW), CP content (2.25 mg/g DW), and CP yield (28.49 mg/L).
Optimization of culture conditions to enhance the biomass, total phenolic content (TPC), and caffeoylputrescine production of HRs. The effects of the media (1(a) and 1(b)), sucrose concentration (2(a) and 2(b)), and initial pH (3(a) and 3(b)) were investigated. The presented values are the mean ± standard error of three replicates; the data were recorded after 24 days of culture.
Effect of different elicitors on the TPC and CP synthesis of P. peruviana HRs
Figure 4I–L show the production of HRs after treatment with elicitors. The MeJA-induced HRs (Fig. 4J) exhibited a yellow-brown color, which was darker than that of the control group (Fig. 4I). In contrast, the HRs induced with SA (Fig. 4K) and SNP (Fig. 4L) showed an orange-yellow color, which was lighter than that of the control group. The SA-induced HRs exhibited a finer root system than MeJA-induced HRs. Furthermore, the results in Fig. 4A–H clearly demonstrate that different types of elicitors have varying effects on the accumulation of phytochemicals in P. peruviana HRs. Compared to the non-treated control, significant changes in CP content (14.52-, 0.47-, and 0.82-fold increases) and yield (13.54-, 0.58-, and 0.83-fold increases) were obtained when MeJA, SA, and SNP (100 µM) were induced in P. peruviana HRs for 8 days respectively (Fig. 4C,D). The TPC significantly increased by 2.13-, 0.91-, and 0.96-fold when P. peruviana HRs were subjected to MeJA, SA, and SNP (100 µM) for 8 days, respectively (Fig. 4B). Thus, the best MeJA concentration to further enhance CP in P. peruviana HRs was 100 µM, which yielded the highest level of CP (7.26 mg/g).
The P. peruviana HRs exhibited varying levels of TPC and CP when treated with SA, MeJA, and SNP at different time points (2, 4, 6, and 8 days). The CP content was 0.42–0.82 mg/g, whereas TPC was 10.42–11.31 mg/g in the non-treated control group. The CP content significantly increased to 7.26 mg/g after 8 days of MeJA treatment (Fig. 4G). Notably, similar yields of CP were observed at 2, 6, and 8 days of treatment with values of 140.13, 141.11, and 141.10 mg/L, respectively (Fig. 4H). In contrast, both TPC and biomass were lower after 8 days of MeJA treatment than after 2 days of treatment, with TPC levels at 23.34 mg/g and biomass at 19.43 g/L after 8 days of treatment. With SA treatment, the TPC level also maximized (10.45 mg/g) at 4 days. Concurrently, the maximum content and yield of CP were observed at 2 days, with values of 0.46 mg/g and 8.58 mg/L, respectively. In comparison, SNP significantly increased the TPC (13.05 mg/g), CP (0.86 mg/g), and CP yield (15.43 mg/L) after 2 days of treatment. No significant differences were noted between treated samples and controls at 2, 4, 6, and 8 days.
Overall, MeJA was more effective at enhancing the accumulation of TPC and CP in P. peruviana HRs than SA and SNP. MeJA showed no significant impact on the biomass, whereas SA slightly increased the biomass production (Fig. 4A,E).
Elicitor effects on the HR parameters of P. peruviana HR. (A–D) Impact of MeJA, SA, and SNP at concentrations of 50, 100, 200, and 400 µM on the (A) biomass, (B) total phenolic content (TPC), (C) caffeoylputrescine (CP) content, and (D) CP yield in 20-day-old P. peruviana HRs after an 8-day exposure. (E–H) Impact of the same elicitors at a fixed concentration of 100 µM on the biomass (E), TPC (F), CP (G), and CP yield (H) under different exposure durations (2, 4, 6, and 8 days). (I–L) Morphological effects of various elicitors on P. peruviana HRs: (I) non-treated control roots, (J) MeJA (100 µM) treated, (K) SA (100 µM) treated, and (L) SNP (100 µM) treated. CK denotes the untreated control group. The presented values are the mean ± standard error of three replicates. Bars = 1 cm.
Discussion
This study aimed to optimize the culture conditions for HRs of P. peruviana and to screen for the most effective elicitors to increase the production of phenolic compounds, especially CP. Our results comprehensively optimized the culture parameters, specifically the inoculum size, liquid volume, carbon source, medium, sucrose concentration, and initial pH, to effectively cultivate P. peruviana HRs.
The inoculum size and liquid volume play a crucial role in the efficiency of tissue culture to produce secondary metabolites39,40, which directly impacts the nutrient availability, oxygen exchange, and overall growth kinetics41. Our results indicate that inoculum sizes of 0.1–0.9 g/100 mL had a minimal effect on the biomass of HRs. The optimal inoculum size of 0.5 g/100 mL provided a balanced starting point for growth without overcrowding the culture vessel, which can lead to localized nutrient depletion or uneven growth distribution. Meanwhile, the liquid volume significantly affected the biomass and metabolite accumulation. The ideal liquid volume of 100 mL in a 250-mL flask well balanced the nutrient and oxygen availability and promoted efficient growth and metabolic activity. Interestingly, we noted a decrease in CP accumulation at high inoculum sizes (0.7–0.9 g/100 mL) and liquid volumes (120–140 mL), possibly due to nutrient limitations, oxygen limitations, and flask volume constraints42.
A suitable culture medium plays a crucial role in regulating the plant cell growth and secondary metabolite accumulation43. This study revealed that the highest biomass production in P. peruviana HRs occurred when they were cultured on B5 and 1/2 MS media, whereas WPM and White media were considered unsuitable. The greatest accumulation of CP was observed in HRs grown on the B5 medium, followed by those grown in MS, 1/2 MS, White, and WPM media. The high salt levels and nitrogen content in the MS medium may impact the growth and secondary metabolite accumulation of HRs44. The 1/2 MS medium, which has lower nutrient concentrations than MS, was more effective for the biomass accumulation in P. peruviana HRs, although it resulted in a lower CP content than the B5 medium. Thus, a low inorganic nitrogen source (1/2 MS) and a high organic nitrogen source (B5 vitamin) were more favorable for the HR growth and secondary metabolite accumulation45. The WPM, which is commonly used for woody plant tissue culture, was not conducive to the growth and CP production of P. peruviana HRs, possibly due to its low total ion concentration46. Similarly, the White medium significantly decreased the biomass for P. peruviana HRs, which was also observed in Anisodus acutangulus HRs due to the suboptimal total nitrogen content in the White medium47. Notably, the distinctive feature of the B5 medium was its high thiamine concentration, a crucial element for cell biosynthesis and metabolism. Thiamine is essential for the continuous growth of isolated root cultures in vitro48. Among the five evaluated media compositions (MS, 1/2 MS, B5, WPM, and White), the B5 medium emerged as the most suitable option. Similar results were observed in Artemisia vulgaris49 and Picrorhiza kurroa2.
This research also enhanced the biomass and secondary metabolite yields by optimizing the medium pH before autoclaving. Specifically, an initial pH of 5.5 was found to be conducive for the biomass accumulation and CP production. Similar results were observed in the HR cultures of Plumbago indica, where the initial pH of 5.5 was favorable for the production of plumbagin production50. In Isatis tinctoria HRs, an initial pH of 5.8 favored the biomass accumulation and bioactive alkaloid production51.
Carbon sources were recently recognized as compounds that act as energy sources and can serve as signaling molecules that affect the growth, development, and metabolism of cultured cells52. Sucrose was identified as the optimal carbon source for biomass accumulation and CP yield, followed by glucose and maltose. Conversely, the lowest accumulation of biomass and CP content was observed in the fructose-supplemented medium. Similar to our results, Fu et al.33 demonstrated that sucrose significantly enhanced the biomass accumulation in Stevia rebaudiana and resulted in higher levels of TP-CADs than other carbon sources. Praveen and Murthy53 found that sucrose was the optimal carbon source for the biomass accumulation and withanolide A production in Withania somnifera HR. Sucrose is commonly preferred as a carbohydrate source in cell and tissue culture media due to its abundance in the phloem sap of many plants, effective absorption through the cell membrane, economic feasibility, and easy accessibility54. Previous studies showed that P. peruviana HRs and seedlings exhibited poor growth when glucose and fructose were used as carbon sources, but certain plant species can thrive on alternative carbohydrates aside from sucrose55,56. The level of sucrose can affect the productivity of secondary metabolites in plant tissue cultures, particularly the growth of transformed roots57. In the present study, lower sucrose concentrations (1–3%) negatively impacted the biomass production and CP accumulation in P. peruviana HRs. In contrast, higher sucrose concentrations of 4.0–6.0% had a significant effect on the CP accumulation but minimal impact on the biomass yield. Optimal CP accumulation was observed in cultures supplemented with approximately 4% sucrose, and this concentration was also suitable for biomass production. Similar findings were noted in the HR cultures of Picrorhiza kurroa, where a 4% sucrose concentration favored biomass accumulation and picroliv production58. Generally, sucrose serves a dual role as a carbon source and an osmotic agent in plant cell and organ culture media59. Increased sucrose consumption was associated with faster root growth, but higher sucrose concentrations led to elevated osmolality in the culture media, as reported by Jiao et al.39. This behavior may reduce the cell viability due to the dehydration and enhanced diffusion of metabolites from tissues into the media, which ultimately decreases the CP accumulation in P. peruviana HRs.
The optimal culture conditions for HRs were determined to be an inoculum size of 0.5 g/100 mL, a liquid volume of 100 mL in a 250-mL flask, sucrose as a carbon source, B5 medium, a sucrose concentration of 4%, and a pH of 5.5. Compared to P. peruviana HRs before optimization, the TPC, CP content, and CP yield significantly increased (by 1.26-, 8.24-, and 5.45-fold, respectively) after the optimization of the cultivation conditions.
Furthermore, the production levels can be enhanced by the application of elicitors in HR culture systems. The secondary metabolism is activated by intricate interactions between the plant tissue and the elicitor, and the response is contingent on various factors, including the elicitor specificity and concentration, duration of elicitation, and specific developmental stage of the plant tissue60. Various elicitors have distinct impacts on the accumulation of secondary metabolites in plants. For example, MeJA has been applied to enhance the production of scopolamine in Duboisia genus61 and glucoraphanin and sulforaphane in Brassica oleracea var. italica62. The SA treatment increased the yield of friedelin and epifriedelanol in HRs of Cannabis sativa L63. SNP acted as a nitric oxide donor and enhanced the growth of Artemisia annua HRs64 and the accumulation of four tanshinone compounds in Salvia miltiorrhiza HRs65. Our study indicate that compared to the elicitors SA and SNP, MeJA is more effective at enhancing CP accumulation in P. peruviana HRs. Based on the results obtained from this experiment, adding MeJA to the culture media increased the CP to be 14.52-fold higher than that in the non-treated control. Recently, several studies have been reported to enhance secondary metabolites of respective abiotic elicitors in various Solanaceous. Piñeros-Castro et al.66 reported that 10 mM SA significantly increased the production of 4β-Hydroxywithanolide E in Physalis peruviana L. HR. Halder et al.67 also demonstrated that both SA and MeJA substantially stimulated the accumulation of withaferin A in Physalis minima L. HRs, with SA and MeJA inducing 21.40-fold and 20.39-fold increases, respectively, compared to non-treated control. Similarly, Halder et al.68 showed that SA and MeJA significantly boosted withanolides production in Withania somnifera (L.) Dunal HRs, with SA (4.14 mg/L) and MeJA (6.75 mg/L) inducing 11.49-fold and 11.00-fold increases, respectively, compared to the non-treated control. These findings underscore the potential of SA and MeJA as potent elicitors for enhancing the production of bioactive compounds within the Solanaceae family.
MeJA is a well-known elicitor, and it has been reported as a signal transduction elicitor for plant defense response and production of plant secondary metabolites. It plays an important role in plants from morphological to molecular functions. Therefore, the jasmonate (JA) signaling pathway is generally considered an integral signal to biosynthesize many secondary plant products69. Chen et al.13 demonstrated that the production of CP in tomato plants was tightly regulated by the JA signaling pathway, and exogenous MeJA elicited a massive accumulation of CP in leaves. The general timing and high level of induction of this response appear to be similar to those in N. attenuata70. Pieterse et al.71 demonstrated that SA suppressed JA accumulation. S-nitrosoglutathione reductase (GSNOR) is associated with NO levels. Furthermore, GSNOR is required for the MeJA-induced accumulation of defense-related CP72. Sodium nitroprusside has been used as an NO donor in plant physiological studies to increase the NO level and CP accumulation. Notably, the biochemical mechanisms by which MeJA alterations activate CP biosynthesis in P. peruviana HRs remain unknown. A more comprehensive understanding of this issue will provide invaluable insight into the mechanisms of jasmonate homeostasis, which may inform the development of novel approaches to manipulate the plant secondary metabolism. Furthermore, the combined application of different elicitors in bioreactors may be considered a useful strategy to increase the yield of effective compounds.
Conclusions
In this study, a culture system of P. peruviana HRs was successfully established. The optimal culture conditions for HRs were as follows: an inoculum size of 0.5 g/100 mL, a liquid volume of 100 mL in a 250-mL flask, sucrose as a carbon source, B5 medium, a sucrose concentration of 4%, and a pH of 5.5. The effects of MeJA, SA, and SNP on enhancing the biomass accumulation and CP production in P. peruviana HR cultures were investigated. Compared to the non-treated control, the CP yield significantly increased by 13.54-, 0.58-, and 0.83-fold when SA, MeJA, and SNP (100 µM) were induced in P. peruviana HRs for 8 days, respectively. This established protocol will serve as a valuable guide for the large-scale production of P. peruviana biomass accumulation and CP production.
Data availability
All data generated or analysed during this study are included in this published article.
References
Hussain, M. S. et al. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied Sci. 4, 10–20. https://doi.org/10.4103/0975-7406.92725 (2012).
Verma, N. & Shukla, S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. AROMA 2, 105–113. https://doi.org/10.1016/j.jarmap.2015.09.002 (2015).
Isah, T. et al. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue and Organ Culture PCTOC 132, 239–265. https://doi.org/10.1007/s11240-017-1332-2 (2018).
Veena, V. & Taylor, C. G. Agrobacterium Rhizogenes : Recent developments and promising applications. In Vitro Cell. Dev. Biol. Plant 43, 383–403. https://doi.org/10.1007/s11627-007-9096-8 (2007).
Rawat, J. M., Bhandari, A., Raturi, M. & Rawat, B. Agrobacterium Rhizogenes Mediated Hairy Root Cultures: A Promising Approach for Production of Useful Metabolites, in New and Future Developments in Microbial Biotechnology and Bioengineering, 103–118 (Elsevier, 2019).
Hu, Z. B. & Du, M. hairy root and its application in plant genetic engineering. J. Integr. Plant Biol. 48, 121–127. https://doi.org/10.1111/j.1744-7909.2006.00121.x (2006).
Banerjee, S., Singh, S. & Rahman, L. U. Biotransformation studies using hairy root cultures—A review. Biotechnol. Adv. 30, 461–468. https://doi.org/10.1016/j.biotechadv.2011.08.010 (2012).
Guo, Y. et al. Effects of different carbon sources on growth and active component contents in Salvia Miltiorrhiza and S. Castanea F. Tomentosa hairy roots. China J. Chin. Mater. Med. 45, 2509–2514. https://doi.org/10.19540/j.cnki.cjcmm.20200329.112 (2020).
Rahmat, E. & Kang, Y. Adventitious root culture for secondary metabolite production in medicinal plants: A review. J. Plant Biotechnol. 46, 143–157. https://doi.org/10.5010/JPB.2019.46.3.143 (2019).
Gorelick, J. & Bernstein, N. Elicitation: An underutilized tool in the development of medicinal plants as a source of therapeutic secondary metabolites. Adv. Agron. 124, 201–230. https://doi.org/10.1016/B978-0-12-800138-7.00005-X (2014).
Andrea, V. & Ricardo, B. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 172, 861–875. https://doi.org/10.1016/j.plantsci.2007.01.006 (2007).
Walker, T. S., Bais, H. P. & Vivanco, J. M. Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s Wort). Phytochemistry 60, 289–293. https://doi.org/10.1016/S0031-9422(02)00074-2 (2002).
Chen, H., Jones, A. D. & Howe, G. A. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 580, 2540–2546. https://doi.org/10.1016/j.febslet.2006.03.070 (2006).
Bernard, G. et al. MeJA elicitation of chicory hairy roots promotes efficient increase of 3, 5-DiCQA accumulation, a potent antioxidant and antibacterial molecule. Antibiotics 9, 659. https://doi.org/10.3390/antibiotics9100659 (2020).
Ahmad, F., Singh, A. & Kamal, A. Salicylic Acid-Mediated Defense Mechanisms to Abiotic Stress Tolerance 355–369 (Elsevier, 2019).
Li, X. et al. Salicylic acid-induced cytosolic acidification increases the accumulation of phenolic acids in Salvia Miltiorrhiza Cells. Plant Cell Tissue Organ Cult. (PCTOC) 126, 333–341. https://doi.org/10.1007/s11240-016-1001-x (2016).
Morot-Gaudry-Talarmain, Y. et al. Nitrite accumulation and nitric oxide emission in relation to cellular signaling in nitrite reductase antisense tobacco. Planta 215, 708–715. https://doi.org/10.1007/s00425-002-0816-3 (2002).
Abedi, M., Karimi, F., Saboora, A. & Razavi, K. Elicitation of flavonoid biosynthesis in cell suspension cultures of Haplophyllum Virgatum by sodium nitroprusside. Plant Biosyst. 157, 992–1002. https://doi.org/10.1080/11263504.2023.2234906 (2023).
Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 11, 153–177. https://doi.org/10.1007/s11101-012-9242-8 (2012).
Tatipamula, V. B. & Kukavica, B. Phenolic compounds as antidiabetic, anti-inflammatory, and anticancer agents and improvement of their bioavailability by liposomes. Cell Biochem. Funct. 39, 926–944. https://doi.org/10.1002/cbf.3667 (2021).
Han, X., Shen, T. & Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 8, 950–988. https://doi.org/10.3390/i8090950 (2007).
Dias, M. et al. Recent technological advances in phenolic compounds recovery and applications: Source of nutraceuticals for the management of diabetes. Appl. Sci. 12, 9271. https://doi.org/10.3390/app12189271 (2022).
Leubner-Metzger, G. & Amrhein, N. The distribution of hydroxycinnamoylputrescines in different organs of Solanum Tuberosum and other solanaceous species. Phytochemistry 32, 551–556. https://doi.org/10.1016/S0031-9422(00)95135-5 (1993).
Mizusa, S., Tanabe, Y. & Noguchi, M. A new aromatic amide, caffeoylputrescine from callus tissue culture of Nicotiana Tabacum. Agric. Biol. Chem. 34, 972–973. https://doi.org/10.1080/00021369.1970.10859716 (1970).
Long, Z. et al. Amide alkaloids from Scopolia tangutica. Planta Med. 80, 1124–1130. https://doi.org/10.1055/s-0034-1382961 (2014).
Tebayashi, S. et al. Induction of resistance against the leafminer, Liriomyza trifolii, by jasmonic acid in sweet pepper. Biosci. Biotechnol. Biochem. 71, 1521–1526. https://doi.org/10.1271/bbb.70033 (2007).
Kaur, H., Heinzel, N., Schöttner, M., Baldwin, I. T. & Gális, I. R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol. 152, 1731–1747. https://doi.org/10.1104/pp.109.151738 (2010).
Park, S. et al. Determination of polyphenol levels variation in Capsicum annuum L. Cv. chelsea (Yellow Bell Pepper) infected by anthracnose (Colletotrichum Gloeosporioides) using liquid chromatography-tandem mass spectrometry. Food Chem. 130, 981–985. https://doi.org/10.1016/j.foodchem.2011.08.026 (2012).
Roumani, M. et al. Characterization of biological properties of individual phenolamides and phenolamide-enriched leaf tomato extracts. Molecules 28, 1552. https://doi.org/10.3390/molecules28041552 (2023).
Assefa, S. T. et al. Identification of Α-glucosidase inhibitors from leaf extract of pepper (Capsicum Spp.) through metabolomic analysis. Metabolites 11, 649. https://doi.org/10.3390/metabo11100649 (2021).
Kim, W. et al. Antioxidant activity of phenolics in leaves of three red pepper (Capsicum annuum) cultivars. J. Agri. Food Chem. 62, 850–859. https://doi.org/10.1021/jf403006c (2014).
Jiang, Y. et al. Spectrum-effect relationships between high performance liquid chromatography fingerprint and analgesic property of Anisodus tanguticus (Maxim) pascher (Solanaceae) roots. Trop. J. Pharm. Res. 16, 379–386. https://doi.org/10.4314/tjpr.v16i2.17 (2017).
Fu, X. et al. Production of chlorogenic acid and its derivatives in hairy root cultures of Stevia rebaudiana. J. Agri. Food Chem. 63, 262–268. https://doi.org/10.1021/jf504176r (2014).
Hajati, R. J., Payamnoor, V. & Chashmi, N. A. Effect of biotic and abiotic elicitors on production of betulin and betulinic acid in the hairy root culture of Betula pendula roth. Prep. Biochem. Biotech. 49, 1010–1019. https://doi.org/10.1080/10826068.2019.1650372 (2019).
MotoliniaAlcántara, E. A. et al. Phenolic compounds from wild plant and in vitro cultures of Ageratina pichichensis and evaluation of their antioxidant activity. Plants-Basel 12, 1107. https://doi.org/10.3390/PLANTS12051107 (2023).
Singleton, V. & Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J. Enol. Viticult. 16, 144–158. https://doi.org/10.5344/ajev.1965.16.3.144e (1965).
Cheng, K., Wu, J., Lin, J. & Liu, W. Enhancements of isoflavone aglycones, total phenolic content, and antioxidant activity of black soybean by solid-state fermentation with Rhizopus Spp.. Eur. Food Res. Technol. 236, 1107–1113. https://doi.org/10.1007/s00217-013-1936-7 (2013).
Baumert, A. et al. Patterns of phenylpropanoids in non-inoculated and potato virus Y-inoculated leaves of transgenic tobacco plants expressing yeast-derived invertase. Phytochemistry 56, 535–541. https://doi.org/10.1016/S0031-9422(00)00422-2 (2001).
Jiao, J. et al. Optimization of Astragalus membranaceus hairy roots induction and culture conditions for augmentation production of astragalosides. Plant Cell Tissue Organ Culture (PCTOC) 120, 1117–1130. https://doi.org/10.1007/s11240-014-0668-0 (2015).
Srivastava, S. & Srivastava, A. K. Statistical medium optimization for enhanced Azadirachtin production in hairy root culture of Azadirachta indica. In Vitro Cell Dev-Plant. 48, 73–84. https://doi.org/10.1007/s11627-011-9395-y (2012).
Amaral, P. F. F. et al. Beneficial effects of enhanced aeration using perfluorodecalin in Yarrowia lipolytica cultures for lipase production. World J. Microbiol. Biotechnol. 23, 339–344. https://doi.org/10.1007/s11274-006-9229-y (2007).
Jeong, C. S., Murthy, H. N., Hahn, E. J., Lee, H. L. & Paek, K. Y. Inoculum size and auxin concentration influence the growth of adventitious roots and accumulation of ginsenosides in suspension cultures of Ginseng (Panax Ginseng CA Meyer). Acta Physiol. Plant 31, 219–222. https://doi.org/10.1007/s11738-008-0206-y (2009).
Murakami, Y., Shimomura, K., Yoshihira, K. & Ishimaru, K. Polyacetylenes in hairy root cultures of Trachelium caeruleum L.. J. Plant Physiol. 152, 574–576. https://doi.org/10.1016/S0176-1617(98)80279-4 (1998).
Ooi, C. T., Syahida, A., Stanslas, J. & Maziah, M. Efficiency of different Agrobacterium rhizogenes strains on hairy roots induction in Solanum mammosum. World J. Microbiol. Biotechnol. 29, 421–430. https://doi.org/10.1007/s11274-012-1194-z (2013).
Ashraf, M. F. et al. Establishment of Persicaria minor hairy roots and analysis of secreted Β-caryophyllene in medium broth. Plant Cell Tissue Organ Cult. (PCTOC) 121, 11–20. https://doi.org/10.1007/s11240-014-0674-2 (2015).
Carlín, A. P., Tafoya, F., Alpuche Solís, A. G. & Pérez-Molphe-Balch, E. Effects of different culture media and conditions on biomass production of hairy root cultures in six Mexican cactus species. In Vitro Cell. Dev. Biol. Plant 51, 332–339. https://doi.org/10.1007/s11627-015-9681-1 (2015).
Li, L. et al. Optimization of induction and culture conditions and tropane alkaloid production in hairy roots of Anisodus acutangulus. Biotechnol. Bioproc. E 13, 606–612. https://doi.org/10.1007/s12257-008-0035-2 (2008).
Jacob, A. & Malpathak, N. Manipulation of MS and B5 components for enhancement of growth and solasodine production in hairy root cultures of Solanum khasianum clarke. Plant Cell Tissue Organ Cult. 80, 247–257. https://doi.org/10.1007/s11240-004-0740-2 (2005).
Sujatha, G., Zdravković-Korać, S., Ćalić, D., Flamini, G. & Ranjitha Kumari, B. D. High-efficiency agrobacterium rhizogenes-mediated genetic transformation in Artemisia vulgaris: Hairy root production and essential oil analysis. Ind. Crop. Prod. 44, 643–652. https://doi.org/10.1016/j.indcrop.2012.09.007 (2013).
Gangopadhyay, M., Sircar, D., Mitra, A. & Bhattacharya, S. Hairy root culture of Plumbago indica as a potential source for plumbagin. Biol. Plant. 52, 533–537. https://doi.org/10.1007/s10535-008-0104-6 (2008).
Gai, Q. et al. Establishment of hairy root cultures by Agrobacterium rhizogenes mediated transformation of Isatis tinctoria L. for the efficient production of flavonoids and evaluation of antioxidant activities. PLOS ONE 10, e0119022. https://doi.org/10.1371/journal.pone.0119022 (2015).
Yaseen, M., Ahmad, T., Sablok, G., Standardi, A. & Hafiz, I. A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 40, 2837–2849. https://doi.org/10.1007/s11033-012-2299-z (2013).
Praveen, N. & Murthy, H. N. Synthesis of withanolide a depends on carbon source and medium PH in hairy root cultures of Withania somnifera. Ind. Crops Prod. 35, 241–243. https://doi.org/10.1016/j.indcrop.2011.07.009 (2012).
Fuentes, S. R., Calheiros, M. B., Manetti-Filho, J. & Vieira, L. G. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult. 60, 5–13. https://doi.org/10.1023/A:1006474324652 (2000).
Wang, Y. & Weathers, P. J. Sugars proportionately affect artemisinin production. Plant Cell Rep. 26, 1073–1081. https://doi.org/10.1007/s00299-006-0295-2 (2007).
Park, C. H. et al. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. https://doi.org/10.1177/1934578X1601100625 (2016).
Rao, S. R. & Ravishankar, G. A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 20, 101–153. https://doi.org/10.1016/S0734-9750(02)00007-1 (2002).
Verma, P. C. et al. Yield enhancement strategies for the production of picroliv from hairy root culture of Picrorhiza kurroa royle ex benth. Plant Signal Behav. 10, e1023976. https://doi.org/10.1080/15592324.2015.1023976 (2015).
Mukherjee, S. K. I. A., Rathinasabapathi, B. & Gupta, N. Low sugar and osmotic requirements for shoot regeneration from leaf pieces of Solanum melongena L.. Plant Cell Tissue Organ Cult. 25, 13–16. https://doi.org/10.1007/bf00033906 (1991).
Narayani, M. & Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 16, 1227–1252. https://doi.org/10.1007/s11101-017-9534-0 (2017).
Singh, P. et al. Silencing of quinolinic acid phosphoribosyl transferase (QPT) gene for enhanced production of scopolamine in hairy root culture of Duboisia leichhardtii. Sci. Rep.-UK https://doi.org/10.1038/s41598-018-32396-0 (2018).
Bao, J. et al. Transcriptome analysis of genes related to glucoraphanin and sulforaphane synthesis in methyl jasmonate treated broccoli (Brassica oleracea Var. Italica) hairy roots. J. Plant Res. 135, 757–770. https://doi.org/10.1007/s10265-022-01407-7 (2022).
Mahendran, G. & Vimolmangkang, S. Effect of carbon source and elicitors on biomass production and accumulation of friedelin and epifriedelanol in hairy roots of hemp (Cannabis Sativa L.). Plant Cell Tissue Organ Cult. 156, 61. https://doi.org/10.1007/s11240-023-02675-4 (2024).
Zheng, L. P., Guo, Y. T., Wang, J. W. & Tan, R. X. Nitric oxide potentiates oligosaccharide-induced artemisinin production in Artemisia annua hairy roots. J. Integr. Plant Biol. 50, 49–55. https://doi.org/10.1111/j.1744-7909.2007.00589.x (2008).
Du, X. et al. Nitric oxide plays a central role in water stress-induced tanshinone production in Salvia miltiorrhiza hairy roots. Molecules 20, 7574–7585. https://doi.org/10.3390/molecules20057574 (2015).
Piñeros-Castro, Y., Otálvaro-álvarez, Á. & Velásquez-Lozano, M. Efecto de la aplicación de elicitores sobre la producción de 4B-hidroxiwithanólido E, en raíces transformadas de Physalis peruviana L.. Universitas Scientiarum 14, 23–28 (2009).
Tarun, H. & Biswajit, G. Hairy root cultures of Physalis minima L.—An alternative source of withaferin a production. Plant Cell Tiss. Org. 152, 31–44. https://doi.org/10.1007/s11240-022-02386-2 (2023).
Halder, T. & Ghosh, B. Withania somnifera (L.) dunal: Enhanced production of withanolides and phenolic acids from hairy root culture after application of elicitors. J. Biotechnol. 388, 59–71. https://doi.org/10.1016/j.jbiotec.2024.04.010 (2024).
Mishra, A. K., Sharma, K. & Misra, R. S. Elicitor recognition, signal transduction and induced resistance in plants. J. Plant Interact. 7, 95–120. https://doi.org/10.1080/17429145.2011.597517 (2012).
Keinänen, M., Oldham, N. J. & Baldwin, I. T. Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glycosides in Nicotiana attenuata. J. Agr. Food Chem. 49, 3553–3558. https://doi.org/10.1021/jf010200+ (2001).
Pieterse, C. M. J., Leon-Reyes, A., Van der Ent, S. & Van Wees, S. C. M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316. https://doi.org/10.1038/nchembio.164 (2009).
Wünsche, H., Baldwin, I. T. & Wu, J. S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata. J. Exp. Bot. 62, 4605–4616. https://doi.org/10.1093/jxb/err171 (2011).
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
The authors gratefully acknowledge the support of the National Natural Science Foundation of China [32460638].
Author information
Authors and Affiliations
Contributions
Y.J.Z: Methodology, Validation, Investigation, Data curation, Writing–original draft, Writing–review, and editing. S.F.W: Conceptualization, Methodology. L.Z: Investigation, Validation, Formal analysis. Z.P.Y: Conceptualization, Methodology, Supervision, Funding Acquisition. Y.H.X: Data curation. J.G.C: Conceptualization, Writing - review and editing, Visualization, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhong, Yj., Wu, Sf., Zhang, L. et al. In vitro strategy to enhance the production of bioactive polyphenols and caffeoylputrescine in the hairy roots of Physalis peruviana L.. Sci Rep 14, 27600 (2024). https://doi.org/10.1038/s41598-024-77698-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77698-8