Abstract
Lynch syndrome is rarely associated with rectal cancer (RC) and thus, metachronous RC has been scarcely investigated. This study aimed to analyze the mucosal immune microenvironment in sporadic and metachronous RC. We analyzed the mucosal immune microenvironment in the 25 metachronous RCs present in the IMMUNOREACT 1 and 2 multicentre observational studies (624 patients). A panel of immune markers was retrospectively investigated at immunohistochemistry: CD3, CD4, CD8, CD8b, Tbet, FoxP3, PD-L1, MSH6, and PMS2 and CD80. Single-cell suspensions were subjected to flow-cytometry to determine the proportion of epithelial cells (pan-cytokeratin) acting as antigen-presenting cells (expressing CD80, CD86, HLA-ABC) and the proportion of activated CD8 + T cells (CD8 + positive for CD28, CD38), inhibitory T cells (CD3 + CTLA-4+) of activated CD4 + T helper cells (CD4 + CD25+) and activated T regulatory cells (CD4 + CD25 + FoxP3+). No mismatch repair gene deficiencies were observed in the patients. The previous history of colorectal adenoma was significantly more frequent in metachronous RC. In healthy epithelial cells, HLA-ABC expression was significantly higher in patients with metachronous RC. In therapy-naïve metachronous RC patients, a significantly lower level of circulating lymphocytes and CD3 + T-cell infiltration in the healthy mucosa surrounding the RC was observed compared to patients with non-metachronous cancer. Our study supports the hypothesis that metachronous RC can occur in a cancerization field in patients with weak systemic and local immune systems. The peculiar site of RC makes the mismatch-repair genes deficiency in metachronous cancer onset less relevant.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most frequent cancer and the second leading cause of cancer-related deaths globally1,2. Metachronous CRC is defined as a second primary CRC that is not a recurrence or a metastatic deposit of the primary one3,4. The risk of developing is around 2–12% within five years after surgery for initial CRC, and the cumulative incidence is 1.1% at 3 years, 2.0% at 6 years, and 3.1% at 10 years5,6. The development of metachronous CRC has been recognized since 19327, while there was a common belief about a first colorectal tumor conferring immunity against a second tumor3. Metachronous CRC is predominantly detected during the first three years after the first CRC diagnosis and gradually declines thereafter to a risk comparable to the general population. Patients with previous CRC have a higher risk of developing CRC later than the general population, and this risk varies between 0.6% and 9%. Moreover, several studies showed that the presence of synchronous adenomas, besides synchronous CRC, increased the risk of development of metachronous CRC8.
Several hypotheses tried to explain the development of metachronous CRC, including an alternative pathway of neoplastic development different from the adenoma-carcinoma pathway or inadequate colonoscopies that missed synchronous lesions4,8,9. Risk factors associated with the occurrence of metachronous CRC are proximally located primary CRC, the presence of mucinous histology, younger age at the time of primary tumor, and the presence of synchronous adenomas or CRC at first diagnosis10. Moreover, previous studies observed signs of local immune impairment in metachronous CRC and metachronous oesophageal squamous cell carcinoma, as also reported in the metastatic process4,11,12. Finally, metachronous CRCs are usually associated with microsatellite instability (MSI) and mismatch repair gene deficiency (genes MLH1, MSH2, MSH6, PMS2), related to Lynch syndrome, the most common hereditary cancer of the colon and rectum11. However, not all metachronous CRC have microsatellite instability3 and the incidence of mismatch repair gene deficiency is rare in RC13. A recent systematic review stated that, in RC, the incidence of MSI was low, at 7% of cases. Due to the relatively low incidence of MSI in rectal cancer, limited evidence regarding its prognostic and predictive value exists but the authors showed that there is no effect of MSI on overall survival or disease-free survival14.
Current guidelines propose endoscopic surveillance with colonoscopy for follow-up after surgery for CRC, with the first colonoscopy one year after surgery and the following every 3–5 years in the absence of adenomas15. Hence, defining the risk factors for the development of metachronous CRCs may be useful for the identification of patients at risk and the personalization of the endoscopic surveillance scheme3. Given the molecular and epidemiological peculiarity of RC (rarer MSI occurrence, higher frequency in male and in younger patients, less advanced T stage at presentation, and better survival16), this study aimed to analyze the mucosal immune microenvironment in these sporadic and metachronous cancers.
Methods
Study design
This study was a sub-analysis of data from two multicentric observational cohort studies, IMMUNOREACT 1 (clinicaltrials.gov NCT04915326) and 2 (clinicaltrials.gov NCT04917263), that included all patients with early and locally advanced RC, respectively. The IMMUNOREACT project was based on the analysis of the immune infiltration within the healthy mucosa surrounding RC, to evaluate the quality of the constitutive immunosurveillance mechanisms and the field of cancerization. The rationale of the project is that the local immune activation may involve the “healthy” rectal mucosa surrounding the cancer and the sampling of this mucosa may provide useful information about rectal cancer behavior. In fact, a combination of different factors, including molecular signaling networks within a different cell population, the presence of soluble chemical factors, and the quantity/quality of immune cells infiltrate will decide rectal cancer behavior and its response to neoadjuvant chemoradiation. Moreover, tumor cells actively interact with the microenvironment secreting and degrading extracellular matrix components, and the release of soluble molecules can significantly influence the inflammatory and immune responses of the “healthy” rectal mucosa surrounding the cancer16,17,18,19,20. In the present study, IMMUNOREACT 9, immune markers within the healthy rectal mucosa were compared between patients with metachronous RC and those with a sporadic one. Metachronous RC was defined as a second primary RC occurring more than six months after the first CRC which was not local recurrence or metastatic deposit of the primary tumor.
The IMMUNOREACT protocol was approved by the Ethical Committee of the coordinating center (Padova University Hospital Ethical Committee code 4448/AO/20) and each of the collaborating centers. Patients gave their written informed consent to be enrolled in the study. The study was conducted according to Helsinki’s declaration principles, and it received funding from the Associazione Italiana per la Ricerca sul Cancro (AIRC) under IG 2019 - ID. 23,381 project to the principal investigator (M.S.).
Patients
Inclusion criteria for the metachronous RC group were the presence of RC regardless of neoadjuvant therapy, previous surgery for a first CRC that could have any localization in the colorectal area, and a time interval between the two tumors of six months or more, to define the second cancer as a metachronous. The eligible patients were identified by reviewing the anamnestic and clinical data that were previously collected for the IMMUNOREACT project. Data were collected anonymously using the REDCap (Research Electronic Data Capture) web platform21. Data collection included demographic data, past medical history, family history, and details of RC relating to the site of the neoplasm (rectum < 15 cm or rectosigmoid junction), cancer stage, associated diseases, including inflammatory bowel diseases, hereditary syndromes or history of previous neoplasms or previous colorectal adenomas. All data were reviewed and updated.
Tissue sampling
Samples of healthy mucosa were taken 3 to 15 cm proximal to RC at the time of surgery (either anterior rectal resection, Miles abdominoperineal amputation, or transanal excision) according to the concept of field cancerization. Field cancerization is a biological process in which large areas of cells at a tissue surface or within an organ are affected by carcinogenic alterations22. Field cancerization characterizes various epithelial cancers, including CRC, implying that the molecular events in tumorigenesis still occur in normal adjacent tissues23. An internal assessment showed the homogeneity of expression of immunological markers at this distance (Supplementary Table 1).
Immunohistochemistry
Immunohistochemical (IHC) analyses were performed using standard procedures. A tissue microarray instrument was used and tissue microarrays were constructed and cut into 5-µm sections for IHC. TMA area was chosen avoiding germinative follicula and other irregularities. Immunocomplexes were detected using an avidin-biotin-peroxidase conjugate and 3–3’ diaminobenzidine tetrahydrochloride chromogen as a substrate (Dako, Glostrup, Denmark) in formalin-fixed paraffin-embedded sections. The resulting sections were evaluated by a single pathologist in a blinded fashion. The first 50 patients were evaluated by two pathologists (M.F. and V.P.) with a high agreement coefficient (Cohen K > 0.80). IHC staining was performed using monoclonal antibodies. A panel of immune markers was retrospectively investigated at immunohistochemistry: CD3, CD4, CD8, CD8β, Tbet, FoxP3, PD-L1, MSH6, and PMS2 and CD80. Although a two-antibody approach for the detection of mismatch repair gene deficiency (PMS2, MSH6) can miss some cases it has been demonstrated to be feasible and in great agreement with a four antibodies approach24,25. The absolute number of positive cells was obtained by considering the mean number of positive cells observed under a 5-high power field (40x). The panel of immunohistochemical markers of immune surveillance was tested on healthy rectal mucosa according to the concept of field cancerization. Immune markers within the healthy rectal mucosa were compared between patients with metachronous RC and those with a sporadic one. Antibodies characteristics are shown in Supplementary Table 2.
Flow cytometry
Prospectively collected rectal mucosa samples were minced into 3 to 4 mm pieces and incubated in HBSS supplemented with 1 mM DTT and 0.5 mM EDTA with shaking at 37 °C for 20 min. After washing, tissue pieces were treated with 1 U/ml Dispase (Stemcell Technologies, Vancouver, Canada) in HBSS at 37 °C for 30 min with gentle stirring and then filtered through a sterile stainless-steel mesh (pore size 80 μm, Sigma Aldrich) in order to obtain a single-cell suspension. Freshly isolated cells (105) were stained in PBS/2% FBS with appropriate combinations of FITC-, PE-, PE-Cy7-, and APC-conjugated antibodies (Supplementary Table 3). Single-cell suspensions were subjected to flow cytometry to determine the proportion of epithelial cells (pan-Cytokeratin, Ck+) acting as antigen-presenting cells (expressing CD80, CD86, HLA ABC) and the proportion of activated CD8 + T cells (CD8 + positive for CD28, CD38), inhibitory T cells (CD3 + CTLA-4+) of activated CD4 + Th cell (CD4 + CD25+) and T regulatory cells (CD4 + CD25 + FoxP3+). Flow cytometric analysis was performed using a FACSCalibur based on CellQuest software (BD-Becton Dickinson, Franklin Lakes, USA).
Gene expression profiling analysis
Total RNA was extracted from snap-frozen rectal mucosa using the RNeasy Plus mini kit (Qiagen); RNA quantification was performed with a Nanodrop 1000 spectrophotometer (ThermoFisher Scientific), and the RNA integrity and quality were evaluated with the Agilent 2100 Bioanalyzer System. Gene expression profiling was performed using the PanCancer IO 360™ panel (NanoString Technologies), which measures the expression of 770 genes involved in the complex interplay between the tumor, microenvironment, and immune response in cancer. Samples were processed according to the manufacturer’s instructions provided by NanoString Technologies. The nCounter Advanced Analysis module V.2.0.134 software (NanoString Technologies) was used for differential expression analysis, and to obtain scores for cell types profiling and pathways analysis, based on the expression of predefined genes.
Statistical analysis
Data were summarized as mean and interquartile range (IQR) (continuous variables), or absolute and relative frequency (categorical variables). Data were compared among groups using the Mann-Whitney test, Chi-square test, and Fisher’s exact test, as appropriate. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) were used to explore the relationship between metachronous RC, the cancerization field, and the immune response. All tests were 2-sided and a p-value < 0.05 was considered statistically significant. Comparisons were corrected for multiple tests according to Benjamini and Hochberg False Discovery Rate method. Statistical analysis was conducted using the R 4.3 version for non-parametric analysis (R Foundation for Statistical Computing, Vienna, Austria)26.
Results
Patients characteristics
The analysis included 624 patients (25 with metachronous RC and 599 without metachronous RC). Patient characteristics are summarized in Table 1. No statistically significant difference between the two groups was found in terms of age, sex, cancer site and stage, and neoadjuvant therapy. Patients’ characteristics are shown in Table 1.
Metachronous RC and the cancerization field (IMMUNOREACT 9: whole cohort)
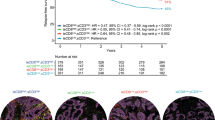
MLH1, MSH2, MSH6, and PMS2 were analyzed by immunohistochemistry (Fig. 1A). Although no mismatch-repair gene deficiency was observed in the two groups in the cancerization field or in the cancer tissue, the previous history of colorectal adenoma was more frequent in metachronous RC patients (p < 0.001; Fig. 1B). As shown in Fig. 1C and 4 metachronous RC patients (16%) had a previous right colon cancer, 4 had a previous RC (16%) and 14 (68%) had a previous left or sigmoid colon cancer (for 3 patients the localization of the previous colorectal cancer was unknown). Flow cytometry analysis revealed that in epithelial cells the expression of HLAabc in the cancerization field was significantly higher in patients with metachronous RC (Fig. 1D), suggesting a higher presence of antigens presented in the healthy tissue surrounding RC (p = 0.031). No other differences were observed in terms of costimulatory molecules (CD80 and CD86) expression on epithelial cells or lymphocyte subpopulation within the normal rectal mucosa between the two groups (Table 2).
Metachronous RC and patients’ characteristics(IMMUNOREACT 9: whole cohort). (A) Representative immunohistochemical analysis of PMS2, MLH1, MSH2, and MSH6, (20x bar = 0.050 mm) in the cancer tissue. (B) Frequency of a previous history of colorectal adenoma (CRA) in metachronous and non-metachronous RC patients. (C) Illustration of primary cancer site in the metachronous cancers group. (D) Flow cytometric analysis of HLAabc + epithelial (cytokeratin+, CK+) cells within the cancerization field. Representative images of flow cytometric analysis of CK + HLAabc + cells are shown. ROC curve showing the accuracy of CK + HLAabc + mean fluorescence intensity (MFI) in predicting metachronous RC.
Potential differences in gene expression in the cancerization field of metachronous RC patients and non-metachronous RC patients were investigated. Due to the limited tissues available, only 3 metachronous RC and 30 non-metachronous RC were considered for the analysis. In the cancerization field of metachronous RC patients, 20 genes (CD40, CD45RO, CLEC7A, CXCL10, CXCL11, CXCL9, CYBB, GBP1, GBP4, HCK, IFIH1, IFIT3, IL15, ITGA4, ITGB2, LAIR1, MB21D1, S100A9, STAT1, TYMP) resulted significantly up-regulated compared to non metachronous RC patients (Fig. 2A). Based on the expression of cell-type and signature-associated predefined genes present in the panel, a trend for a higher expression of genes associated with immune cells was observed in metachronous compared to non-metachronous RC patients; in particular, metachronous RC patients showed a higher expression of genes associated with B-cells, T-cells and neutrophils (Fig. 2B). Furthermore, the cancerization field of metachronous RC patients showed a higher expression of gene signatures associated with the lymphoid and myeloid compartment, as well as with cytokines and chemokines signaling and immune cell adhesion and migration pathways as compared to non-metachronous RC patients (Fig. 2C).
Metachronous RC and differential gene expression in the cancerization field ((IMMUNOREACT 9: whole cohort). (A) Volcano plot of differential gene expression in the cancerization field of metachronous RC patients (n = 3) vs. non-metachronous RC patients (n = 30). The 20 statistically significant genes are labeled in the plot. (B) Differential expression of gene-based cell types abundance scores in the cancerization field of metachronous RC patients vs. non-metachronous RC patients. (C) Differential expression of gene signatures pathways scores in the cancerization field of metachronous RC patients vs. non-metachronous RC patients.
Metachronous RC and immunological response (IMMUNOREACT 9: therapy naïve patients)
In therapy-naïve patients, low circulating lymphocyte levels were more frequent in metachronous RC patients (p = 0.031) (Fig. 3A). In addition, low levels of CD3 + T-cell infiltration in the healthy mucosa surrounding the RC were more frequent in metachronous RC patients (p = 0.017) (Fig. 3B). Finally, no difference was observed in terms of lymphocyte subpopulation infiltration within the rectal mucosa between the two groups (Fig. 4) either in terms of CD80 + ve and PDL1 + ve cells infiltration (Fig. 5A) or MSH6 and PMS2 (Fig. 5B).
Metachronous RC and immunological response (IMMUNOREACT 9: therapy naïve patients). (A) Association between metachronous RC and circulating lymphocytes. ROC curve showing the accuracy of circulating lymphocytes in predicting metachronous RC.RC (B) Association between metachronous RC and CD3 + T-cell infiltration in the RCcancerization field. Representative immunohistochemical analysis of CD3 is shown (20x bar = 0.050 mm). ROC curve showing the accuracy of CD3 + cells in predicting metachronous RC.
Metachronous RC and immunological response (IMMUNOREACT 9: therapy naïve patients). Representative immunohistochemical analysis of CD80, and PDL-1 + leukocytes within the cancerization field, (20x bar = 0.050 mm). Representative immunohistochemical analysis of PMS2, and MSH6 within the cancerization field, (20x bar = 0.050 mm).
Discussion
Colonoscopy plays a fundamental role in the prevention of metachronous CRC, and may also impact overall survival15,27. Our study aimed to analyze the healthy mucosa immune microenvironment in sporadic and metachronous RC to identify risk factors for metachronous RC occurrence. While risk factors for metachronous RCs have not been fully evaluated so far28, we believe that such information may help to identify high-risk patients and implement personalized surveillance.
In our series, no mismatch-repair gene deficiency was observed in the healthy rectal mucosa surrounding the RC. Usually, metachronous CRC is associated with microsatellite instability and mismatch repair gene deficiency, which is found in 90% of CRC in the context of Lynch syndrome but also in 15% of sporadic cancers29. We may speculate that the distal cancer site may explain the absence of mismatch repair gene deficiency in both sporadic and metachronous cancers. Lynch syndrome-related CRC prevalence decreases along the large bowel length by up to 2.5% in the rectum30.
Nevertheless, most of the metachronous RC patients had previous left or sigmoid colon cancer and a frequent previous history of colorectal adenoma. Several studies have shown that CRC patients with synchronous adenomas or neoplasms have an increased risk of developing metachronous adenomas or tumors after the first surgery31. Moreover, recent studies observed a greater occurrence of developing metachronous adenomas in patients who underwent previous surgery for left colon cancer18, supporting the hypothesis that metachronous RC may frequently occur in the same site as primary ones because of the presence of a cancerization field32,33.
In our series, epithelial cells acting as antigen-presenting cells (HLAabc + ve) in the healthy mucosa, surrounding RC were significantly higher in patients with metachronous RC. A group of genetically altered cells that are not in a histologically identifiable state of malignancy but are at a higher risk of transforming into one, is defined as a field of cancerization35. A higher expression of HLAabc suggests a higher presence of antigens to be presented in the healthy tissue surrounding RC. HLAabcs correspond to MHC class I (A, B, and C), all of which are the HLA Class 1 group, and present peptides from inside the damaged cell36,37. Thus, we can conclude that metachronous RC occurs in a cancerization field.
In our series, therapy-naïve metachronous RC patients had more frequently lower levels of circulating lymphocytes and CD3 + T-cell infiltration in the healthy mucosa surrounding RC than patients with non-metachronous RC. Previous studies have shown not only that immune infiltration is a good predictor of patient survival, but also that the adaptive immune response may likely influence the behavior of tumors38. Weak activation of cytotoxic T cells was associated with the progression of low-grade dysplasia in the context of inflammatory colorectal carcinogenesis39, and patients with metachronous CRC and oesophageal squamous cell carcinoma had a higher frequency of absent infiltration of lymphomononuclear cells within the tumor3,4. All these data suggest a constitutive failure of the immune surveillance of the colorectal mucosa favoring the environment for the development of metachronous RCs.
Therefore, the two main results of this study, epithelial cells with high HLA-ABC expression associated with a low T cell infiltration, suggest a situation of high presentation of damaged peptide unbalanced by a weak immune presence. This disequilibrium can potentially open the way to metachronous rectal cancer through an insufficient generation of CD45RO + T cells, memory T cells, that are crucial to respond again to antigens that had already been reckoned40.
The main limitations of this study are the small number of patients with metachronous RC, which suggests caution in the interpretation of the findings, and the study design that does not allow drawing any causal associations between the relevant variables in the analysis nor making any inference on effect on survival. Moreover, in the present study, no mismatch repair gene sequencing of the 25 cases could be done and this information could have completed the whole picture. In fact, in TMAs some cases with heterogenous MMR expression41 and/or rare staining patterns could have been missed42. Furthermore, we did have not any information about the histologic subtype of the first cancer and we used simple manual cell counting at IHC. Finally, the results of the HLA expression on epithelial cells would have been more clinically meaningful if obtained also by immunohistochemistry.
In conclusion, our study supports the hypothesis that metachronous RC can occur in a cancerization field in patients with weak systemic and local immune systems. The peculiar site of RC makes the mismatch-repair genes deficiency in metachronous cancer onset less relevant. These results support the hypothesis that CRC with rectal mucosa with high HLAabc expression associated with low CD3 + T cell infiltration may benefit from a more intensive postoperative endoscopic surveillance program. Further studies should focus on the design of a dedicated surveillance protocol in patients with low CD3 + infiltration and, possibly, high HLA-abc expression.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at University of Padova (https://www.unipd-ubep.it).
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 136, E359–E386 (2015).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. https://doi.org/10.3322/caac.21834 (2024).
Park, I. J. et al. Metachronous colorectal cancer. Colorectal Dis. 8, 323–327 (2006).
Angriman, I. et al. Metachronous colorectal cancer have a similar microsatellite instability frequency but a lower infiltration of lymphomononuclear cells than primary lesions. Surgery 171, 1605–1611 (2022).
Bulow, S., Svendsen, L. B. & Mellemgaard, A. Metachronous colorectal carcinoma. Br. J. Surg. 77, 502–505 (1990).
Bouvier, A. M. et al. The lifelong risk of metachronous colorectal cancer justifies long-term colonoscopic follow-up. Eur. J. Cancer. 44, 522–527 (2008).
Warren, S. & Gates, O. Multiple primary malignant tumors: a survey of the literature and statistical study. Am. J. Cancer. 16, 1358–1344 (1932).
Mulder, S. A. et al. The incidence and risk factors of Metachronous Colorectal Cancer: an indication for follow-up. Dis. Colon rectum. 55, 522–531 (2012).
Le Clercq, C. M. et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointes Endosc. 82, 325–333 (2015).
Win, A. K. et al. Risk of metachronous Colon Cancer following surgery for rectal Cancer in Mismatch Repair Gene Mutation Carriers. Ann. Surg. Oncol. 20 (6), 1829–1836 (2013).
Mlecnik, B. et al. The tumor microenvironment and immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl Med. 8, 327ra26 (2016).
Businello, G. et al. Esophageal squamous cell carcinoma metachronous to head and neck cancers. Pathol. Res. Pract. 219, 153346. https://doi.org/10.1016/j.prp.2021.153346 (2021).
Iseas, S. et al. Prognostic impact of an Integrative Landscape of Clinical, Immune, and molecular features in non-metastatic rectal Cancer. Front. Oncol. 11, 801880. https://doi.org/10.3389/fonc.2021.801880 (2022).
Swets, M. et al. Microsatellite instability in rectal cancer: what does it mean? A study of two randomized trials and a systematic review of the literature. Histopathology 81 (3), 352–362. https://doi.org/10.1111/his.14710 (2022).
Kahi, C. J. et al. Colonoscopy Surveillance after Colorectal Cancer Resection: recommendations of the US Multi-society Task Force on Colorectal Cancer. Gastroenterology 150 (3), 758–768e11. https://doi.org/10.1053/j.gastro.2016.01.001 (2016).
van der Sijp, M. P. et al. Differences between colon and rectal cancer in complications, short-term survival, and recurrences. Int. J. Colorectal Dis. 31 (10), 1683–1691. https://doi.org/10.1007/s00384-016-2633-3 (2016).
Simoni, O. et al. IMMUNOREACT Study Group. IMMUNOREACT 7: regular aspirin use is associated with immune surveillance activation in colorectal cancer. Cancer 130 (13), 2272–2286. https://doi.org/10.1002/cncr.35297 (2024).
Stepanyan, A. et al. IMMUNOREACT 0: biopsy-based immune biomarkers as predictors of response to neoadjuvant therapy for rectal cancer-A systematic review and meta-analysis. Cancer Med. 12 (17), 17878–17890. https://doi.org/10.1002/cam4.6423 (2023).
Spolverato, G. et al. IMMUNOREACT Study Group. IMMUNOREACT 6: weak immune surveillance characterizes early-onset rectal cancer. Br. J. Surg. 110 (11), 1490–1501. https://doi.org/10.1093/bjs/znad219 (2023).
Spolverato, G. et al. IMMUNOREACT 5: female patients with rectal cancer have better immune editing mechanisms than male patients - a cohort study. Int. J. Surg. 109 (3), 323–332. https://doi.org/10.1097/JS9.0000000000000214 (2023).
Harris, P. A. et al. The REDCap consortium: building an international community of software partners. J. Biomed. Inf. 9. https://doi.org/10.1016/j.jbi.2019.103208] (2019).
Rubin, H. Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture. Bioessays 33 (3), 224–231. https://doi.org/10.1002/bies.201000067 (2011).
18 et al. Integrated transcriptomic analysis of distance-related field cancerization in rectal cancer patients. Oncotarget 8 (37), 61107–61117. https://doi.org/10.18632/oncotarget.17864 (2017).
Hall, G., et al. Immunohistochemistry for PMS2 and MSH6 alone can replace a four-antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology. 42(5), 409–413. https://doi.org/10.3109/00313025.2010.493871 (2010)
Wong, S., Hui, P. & Buza, N. Frequent loss of mutation-specific mismatch repair protein expression in nonneoplastic endometrium of Lynch syndrome patients. Mod. Pathol. 33 (6), 1172–1181. https://doi.org/10.1038/s41379-020-0455-x (2020).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2023). https://www.R-project.org.
Green, R. J. et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann. Intern. Med. 136 (4), 261–269. https://doi.org/10.7326/0003-4819-136-4-200202190-00005 (2002).
Nam, K. & Shin, J. E. Risk factors of advanced metachronous neoplasms in surveillance after colon cancer resection. Korean J. Intern. Med. 36, 305–312 (2021).
Koshiji, M. et al. Genetic alterations in normal epithelium of colorectal cancer patients may be a useful indicator for subsequent metachronous tumor development. Ann. Surg. Oncol. 9 (6), 580–586. https://doi.org/10.1007/BF02573895 (2002).
Farchoukh, L. F. et al. DNA mismatch repair-deficient rectal Cancer is frequently Associated with Lynch Syndrome and with poor response to Neoadjuvant Therapy. Am. J. Surg. Pathol. 46 (9), 1260–1268. https://doi.org/10.1097/PAS.0000000000001918 (2022).
Bech, J. M. et al. Proteomic profiling of colorectal Adenomas identifies a predictive risk signature for development of Metachronous Advanced Colorectal Neoplasia. Gastroenterology 165 (1), 121–132e5. https://doi.org/10.1053/j.gastro.2023.03.208 (2023).
Lam, Y. F. et al. Rates of metachronous adenoma after curative resection for left-sided or right-sided colon cancer. Intest Res. 16 (4), 619–627 (2018).
Fuccio, L. et al. The higher adenoma recurrence rate after left- versus right-sided colectomy for colon cancer. Endoscopy Gastrointest. 82 (2), 337–343 (2015).
Rugge, M. et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinicopathological follow-up study. Aliment. Pharmacol. Ther. 31 (10), 1104–1111. https://doi.org/10.1111/j.1365-2036.2010.04277.x (2010).
Gee Young Yun, G. Y. et al. Left-sided colectomy: one of the important risk factors of Metachronous colorectal adenoma after colectomy for Colon cancer. Dig. Dis. Sci. 63, 1052–1061 (2018).
Ahmed, S. et al. Expressional variations of Kaiso: an association with pathological characteristics and field cancerization of OSCC. BMC Cancer. 22 (1), 990. https://doi.org/10.1186/s12885-022-10014-7 (2022).
Matsumura, M., Fremont, D. H., Peterson, P. A. & Wilson, I. A. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science 257 (5072), 927–934. https://doi.org/10.1126/science.1323878 (1992).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313 (5795), 1960–1964 (2006).
Kotsafti, A. et al. Weak cytotoxic T cells activation predicts low-Grade Dysplasia persistence in Ulcerative Colitis. Clin. Translational Gastroenterol. 10, e–00061 (2019).
Hu, G. & Wang, S. Tumor-infiltrating CD45RO + memory T lymphocytes predict favorable clinical outcome in solid tumors. Sci. Rep. 7 (1), 10376. https://doi.org/10.1038/s41598-017-11122-2 (2017).
Li, X. et al. Heterogeneous expression of mismatch repair proteins and interpretation of immunohistochemical results in colorectal cancer and endometrial cancer. Pathol. Res. Pract. 248, 154647. https://doi.org/10.1016/j.prp.2023.154647 (2023).
Reitsam, N. G. et al. Concurrent loss of MLH1, PMS2 and MSH6 immunoexpression in digestive system cancers indicating a widespread dysregulation in DNA repair processes. Front. Oncol. 12, 1019798. https://doi.org/10.3389/fonc.2022.1019798 (2022).
Funding
The project is funded by AIRC IG 2019 Id.23381.
Author information
Authors and Affiliations
Contributions
B.S., Me.S., In.C., S.P., M.F. and Ma.S. conceived the study and wrote the main manuscript; V.P., A.S., R.S., A.K., S.B., G.B., L.L., V.G., L.D.S., C.C., A.P.D.T., Iv.C., and A.S. preformed immunohistochemical and flow cytometry analysis; F.S., D.G. V.C., G.T., B.D.C., and F.C. performed the statistical analysis; G.R., O.D.S., G.B., S.N., C.V., G.S., C.R., I.A., F.B., I.M., M.Z., F.M., L.F., M.M., A.P., T.S., V.Z., P.P., B.F., G.P., M.G., G.B., G.P., A.R., I.M., C.D.L., R.M., D.V., L.S., S.G., A.P., G.N., R.C., G.P., C.C., M.Z., S.C., L.G., F.R., M.O., M.G., G.T., M.T., U.T., A.P., M.A., Q.R.B., and R.B collected patients’ consent, mucosal samples for the analysis and patients’ history. All authors participated in the discussion for the design of the study and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salmaso, B., Scarpa, M., Pellegrini, V. et al. IMMUNOREACT 9 metachronous rectal cancers have high HLA-ABC expression on healthy epithelium but a lower infiltration of CD3+ T cells than primary lesions. Sci Rep 14, 29821 (2024). https://doi.org/10.1038/s41598-024-80299-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80299-0