Abstract
Following prolonged exposure to hypoxic conditions, for example, due to ascent to high altitude, aging or stroke, cognitive deficits can develop. The exact nature and genesis of hypoxia-induced cognitive deficits remain unresolved. Curcumin has been reported to stimulate neurogenesis and reduce neuronal degeneration. This study aimed to investigate the effect of curcumin on cognitive deficits in hypoxic-brain injury mice and its potential mechanism. Eight-week-old male C57BL/6J mice were exposure to normobaric-hypoxia (13%O2) 14 days to establish hypoxic-brain injury models. Morris water maze and novel object recognition were used to detect the cognitive function of each mouse. Immunofluorescence assays, including Fluoro-Jade C (FJC) and bromodeoxyuridine (BrdU), were used to detect neuronal degeneration and neurogenesis. Thy1-YFP transgenic mice were used to detect synapse plasticity. Our results showed that curcumin administration rescued the impaired cognition of mice, shown as enhanced BrdU+ and dendritic spine in hippocampus. At the molecular level, curcumin was found to promote the expression of brain-derived neurotrophic factor (BDNF) and postsynaptic density protein 95 (PSD95). The results of primary hippocampal neuron detection showed that curcumin could promote dendritic growth. In conclusion, our study indicates that curcumin, increased BDNF and PSD95 expression and contacted with interneurons, salvaged of interneurons may normalize ambient neuroplasticity, resulting in the preservation of neurogenesis processes as well as contributing to improve cognitive performance.
Similar content being viewed by others
Introduction
Oxygen deprivation can be detrimental. Hypoxia contributes to several leading causes of mortality in the world - stroke, myocardial infarction, and respiratory failure1. Cellular hypoxia is the common final pathway of brain injury that occurs after high altitude, aging or stroke. Inability to adjust to hypoxia may lead to dementia, cardiovascular and cerebrovascular dysfunction, and even death2,3. Accumulating evidence has shown that hypoxia can lead to cognitive impairment and dementia4. Additionally, our preliminary research has confirmed that chronic hypoxic-induced cognitive impairment is irreversible5. Unfortunately, there has been no effective therapies to treat hypoxic-brain injury, of which the pathogenesis is disturbingly complex6. Therefore, there is an urgent need to develop novel strategies for the treatment.
Neurogenesis and neuroplasticity are crucial for the maintenance of hippocampal function which alterations related to memory deficits7. Neurogenesis and synaptic plasticity are negatively regulated by age and stress, and increased by exercise, drugs, and physiological activation8,9. Neurogenesis involves a balance between neural stem cells proliferation, migration, differentiation to neurons, and regulation of hippocampus-dependent learning and memory processes10. To achieve stable integration and uniquely contribute to hippocampal function, immature neurons are endowed with a critical period of heightened synaptic plasticity11. Recent evidences suggest reduced neurogenesis in neurodegenerative disorders such as Parkinson’s disease and Alzheimer’s disease12. Induction of neurogenesis and synaptic plasticity through pharmacological may reduce neurodegeneration and slow disease progression in demented disease13. These data suggest that promoting neurogenesis and synaptic plasticity is beneficial to ameliorate cognitive deficits may help in the development of regenerative medicines for the hypoxic brain injury.
Neurotrophic factor of BDNF play pivotal roles in the pathogenesis of hypoxic-brain injury. Increasing BDNF expression could preservation of adult neurogenesis processes as well as contributing to improving cognitive function14. HOMER protein homolog 1 (Homer1) is a well-established regulator of synaptic plasticity and neuronal excitability, which has been linked to a plethora of nervous system disease15. Postsynaptic density protein 95 (PSD95) is pivotally involved in formation, elimination and pruning of synapses and modulation of their strength and plasticity16. M Llorens-Martín et al. reported that injecting PSD95-GFP-expressing retroviruses can determine that hippocampal causes dramatic alterations in both dendritic tree morphology and post-synaptic densities in newborn neurons17. Synaptic proteins, including PSD95 and Homer1, are involved in synaptic transmission and in maintaining synaptic structure and stability, played a neuroprotective effect by promoting neuroplasticity18. In accordance with above roles, we studied the proteins PSD95, Homer1, and BDNF. Therefore, up-regulating BDNF and PSD95 most likely results in activation of neurogenesis and synaptic plasticity.
Curcumin is a polyphenolic substance extracted from the rhizomes of plants in the genus Curcuma. It has various pharmacological effects such as anti-inflammatory, antioxidant, and anti-apoptotic properties19. Due to its relatively low or null cytotoxicity, some scholars begin to investigate its biological activity in nervous system disease and found that it has good neuroprotective ability8. Studies have shown that curcumin improves the spatial learning and memory abilities of the Alzheimer’s disease model mice20 and reduces cell apoptosis and stimulates neurogenesis by downregulating caspase-3 mRNA21. In addition, the significant biological function and neuroprotective effects of curcumin in Parkinson’s disease occur via the activation of BDNF22. In the pathological process of normobaric-hypoxic brain injury mice, whether curcumin can improve nerve regeneration and its related mechanisms is still unclear. We assume that curcumin has a fundamental role in neurogenesis and neuroplasticity via the targeting of the proteins of BDNF, PSD95 and Homer1.
Materials and methods
Animals
All experiments were performed according to a protocol approved by the Institute of Basic Theory for Traditional Chinese Medicine, China Academy of Chinese Medical Sciences. All animal experiments fully complied with the related laboratory animal regulations (No.2023EC-KY-009), and conducted in accordance with ethical requirements and ARRIVE guidelines (PLoS Bio 8(6), e1000412,2010).
Eight-week-old male C57BL/6J mice obtained from SPF Biotechnology (Beijing, China). Eight-week-old male Thy1-YFP transgenic mice with C57BL/6 background were included as described above23. All mice were housed in groups of five and were maintained under standard laboratory conditions (22 ± 2℃, 12/12-hour light/dark cycle, free access to food and water).
Normobaric-hypoxic treatment
All animals were randomly assigned to each group. Normobaric-hypoxic-induced brain injury mice were administered hypoxic stimulation in a closed hypoxic chamber (China Innovation Instrument Co., Ltd, Ningbo, Zhejiang, China), which accurately set the desired hypoxic concentration and pattern. Mice were treated continuously with 13% O2 (simulates oxygen concentrations at an altitude of 3,700 m, a common altitude around the world) for 14 days5,24. The hypoxic chamber was opened briefly for food and water additions every 3 days. Control animals were left uninterrupted, except for regular cage cleaning. The curcumin groups were given curcumin on time according to the designed dose.
Drugs administration
Curcumin (Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd., 458-37-7) was dissolved in 0.1% dimethyl sulfoxide (DMSO) as a stock solution with a concentration of 30 mg/ml. Then, the stock solution was diluted with physiological saline to obtain concentrations of 10 and 20 mg/ml. The solutions were stored in a refrigerator at 4 °C. Curcumin was gavaging for 7 days.
Brdu (Sigma-Aldrich, B5002) was dissolved into 10 mg/ml in 0.9% NaCl to be injected intraperitoneally into the animal at a dose of 50 mg/kg body weight 3 times per day in proliferation assay.
Open field test (OFT)
Open field test was performed in a quiet environment. Each mouse was placed in the center of the bottom of the box (length, width, height were 40, 40 and 40 cm). The inner wall and bottom of the box were thoroughly cleaned by 75% medical alcohol before the next test. The activity of mice was monitored for 5 min in the smart 3.0 behavior analysis system.
Novel object recognition (NOR)
For the novel object recognition tests, the experimental device was a rectangular box with a length, width, and height of 40 cm. Three stages: adaptation, familiarity, and testing. Objects A and B were performed for different shapes and colors. Adaptive phase: no object in the box, give mice 10 min to adapt to the environment; Familiarity phase: two identical objects A in the box, give mice 5 min to explore the object A; Testing phase: different object A and B in the box, give mice 5 min to explore the object A and B. Recognition Index (RI) was calculated with the formula RI = N / (N + F), where N represented the total time of mice exploring the object B, and F represented the total time of mice exploring object A.
Morris water maze (MWM)
The device was a circular tank 120 cm in diameter with a platform, filled with water at a temperature of 24 ± 1 ℃. There were four quadrants and a small platform in the tank. Four different shape and color pictures were posted along the curtains, which served as spatial reference cues. During the training stage (5 days), the platform was submerged 1–2 cm below the water surface, and the mice were placed into one of quadrants to search the platform for 60 s. If mice failed to find the platform, they would be guided to the platform and kept on the platform for 5 s. On testing stage (the 6th day), removed the platform, mice were put into the water at one quadrant swimming 60 s, during which, the mice’ first-time finding of the location as well as the number of crossing the platform were recorded.
Immunofluorescence
Mice were anesthetized with 2% sodium pentobarbital (i.p.), followed by perfusion with 4% paraformaldehyde (PFA). Brains were cut in the coronal plane in 20 μm thick sections by a freezing microtome (Thermo, USA) and then processed for immunofluorescence. Mice brain sections were boiled in citric acid buffer (pH = 6.0) for 10 min for antigen repair; After 0.1 M PBS washing (10 min per time, 3 times), sections were placed in 1% PBST and incubated at 25℃ for 30 min; Sections placed in 5% BSA and sealed for 2 h at 25℃; Sections were incubated overnight at 4 °C in primary antibody: Brdu (Sigma, YA818), MAP2 (Abcam, ab32454); Sections were incubated 2 h at 25 °C in secondary antibody: Alexa Fluor 488 (Invitrogen, A11029); DAPI was used for nuclei staining (Sigma, D9542).
Western blotting
Hippocampus and neuron were homogenized in lysis buffer (RIPA plus protease inhibitor cocktail) on ice for 0.5 h, and subsequently centrifuged at 12,000 rpm for 10 min at 4℃. Extract the supernatant, and use BCA method to determin the protein quantification. 30 µg protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and, subsequently immunoblotted onto polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% non-fat milk at room temperature for 1 h. After TBST washing (10 min per time, 3 times), the membrane was incubated with indicated primary antibodies at 4℃ with shaking overnight. Primary antibodies: PSD95 (CST, 2507 S), Homer1 (CST, 8231 S), BDNF (CST, 16696 S), β-actin (Sigma, A5316), After which, the membrane was incubated with secondary antibodies at 25℃ for 2 h.
Primary neurons culture and treatments
24 h new-born C57BL/7 mice was disinfected and its head was cut off immediately. Hippocampus were dissected and incubated for 30 min in trypsin at 37 ℃ and subsequently, mechanically dissociated. The cell suspension was passed through a 40 μm filter and plated at a density of 9 × 104 cells/cm2 on poly-L-lysine-coated plates and kept in neurobasal medium supplemented with 1% B27 and 1% PS. Cells were incubated for 7 days at 37 ℃ and 5% CO2 and 99% humidity. Half of the medium was changed every 3 days after cell adhesion.
Cells were treated continuously with 0.1% O2 for 4 h to establish hypoxia injury model in vitro5, and then treated with 1、5、10、20、25 µM curcumin for 24 h. MAP2 staining and WB were performed after cell collection.
Cell viability detection
Cell viability was detected by the MTT assay kit (Sigma, M2128). 5 mg MTT dissolved in 1 ml PBS buffer, kept at − 20 °C and protected from light. Discarded the cell supernatant, 90 µl culture solution and 10 µl MTT solution were added to the pre-designed 96-well plate, and then incubated 4 h at 37 °C with 5% CO2. The supernatant was discarded again and 100 µl DMSO was added and mixed for 10 min while avoiding light. OD values at 490 nm of each well were detected by enzyme-labeling apparatus.
Cytotoxicity detection
Cytotoxicity was detected by the LDH assay (Roche, 4744926001). The supernatant was used to detect LDH. The reaction solution was added to each well for 100 µl, incubated at room temperature for 30 min away from light, then 50 µl termination solution was added to each well for 10 min. OD values at 490 nm of each well were detected by enzyme-labeling apparatus.
Statistical analysis
The experimental data were displayed as the mean ± standard error (mean ± SEM) and its analysis was carried out using excel and GraphPad Prism 9.0 software. Statistical significance was assessed by performing Student’s t-test, one-way analysis of variance (ANOVA), and two-way ANOVA. The p values of < 0.05 was deemed significant.
Results
Effects of different dosages of curcumin on mice body weight
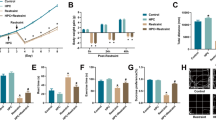
To investigate the potential effects of curcumin on normobaric-hypoxic-induced cognitive deficits, different dosages of curcumin were administrated to hypoxic brain injury mice. According to previous reports25,26, three doses of curcumin of 100, 200, 300 mg/kg/day were selected. As shown in Fig. 1A, mice were subjected to continued hypoxia (13%O2) for 14 days, and thereafter intragastrically administered curcumin at a dosage of 100, 200, or 300 mg/kg/day for 7 days. We first evaluated the changes in the body weight of mice in different groups, there was little difference in initial weight among all groups (Fig. 1B). Compared with the control (Con) group, the body weight in mice treated continuously with 13% O2 for 14 days (H14d) group experienced a perceptibly slow increase (P<0.05, P<0.01), where the administration of curcumin did not have evident impact on the body weight gain of hypoxic brain injury mice. However, a curcumin dose as high as 300 mg/kg was found to have a significant impact on the body weight gain (P<0.05, P<0.01) (Fig. 1C).
Experimental design and effects of curcumin on body weight of mice. A Schematic representation of the experimental design. Mice were adapted for 3 days before the onset of hypoxia (13%O2) stress. Subsequently, mice were subjected to a 2-weeks hypoxia stress followed by behavioral tests. B Initial body weight. There was no significant difference in initial body weight among each group mice. C Effects of curcumin on mice weight. Mice subjected to hypoxia stress exhibited reduced body weight compared with the control group, and 300 mg curcumin had negative effect on mice body weight. Con = control group; H14d = mice treated with 13% O2 for 14 days group; cur-1/2/3 = curcumin (100, 200, and 300 mg/kg) groups; H14d + Cur-1/2/3 = H14d + curcumin (100, 200, and 300 mg/kg) groups. All data are expressed as mean ± SEM, n = 6, *p < 0.05, **p < 0.01 versus Con group, #p < 0.05, ##p < 0.01 versus H14d group.
Different dosages of curcumin had no significant effect on spontaneous activity of mice
Open field test (OFT) was conducted to observe the effects of long-term mild hypoxia stimulation on spontaneous activity of mice and curcumin administration on mice. As shown in Fig. 2A-D, each group mice showed no significant difference in spontaneous activity. We noticed that the spontaneous activity of mice in 300 mg curcumin group showed a decreasing trend compared with other groups.
Effects of different doses of curcumin on spontaneous activity of mice. A–D. Open field test. Each group mice showed no significant difference in spontaneous activity. 300 mg curcumin group showed a decreasing spontaneous activity trend compared with other groups. (A) Distance in edge zone of mice in the OFT. (B) Total distance of mice in the OFT. (C) Edge/total distance of mice in the OFT. (D) Track map of mice in the OFT. All data are expressed as mean ± SEM, n = 6, *p < 0.05, **p < 0.01 versus Con group, #p < 0.05, ##p < 0.01 versus H14d group.
Curcumin improved normobaric hypoxic-induced impairments of learning and memory
Morris water maze (MWM) and novel object recognition (NOR) were conducted to examined changes in learning and spatial memory of each group mice. As shown in Fig. 3A, during the 5 days of training stage, average escape latency of H14d group was increased compared with the Con group (P<0.05, P<0.01). However, 100 mg and 200 mg curcumin treatment were found to significantly reduce the time of escape latency under long-term mild hypoxia stimulation (P<0.05). As shown in Fig. 3B-D, in the testing stage, H14d group showed obvious spatial memory impairments, as demonstrated by fewer crossings and shorter latency compared with the Con group (P<0.05). But now all of these parameters were reversed by 200 mg curcumin treatment (P<0.05). Similarly, NOR results showed that H14d group had significantly shorter novel object exploration times compared with the Con group (P<0.01); and this discrepancy was nevertheless reversed by 100 mg and 200 mg curcumin treatment (P<0.01) (Fig. 3E). The result suggests that curcumin effectively improved normobaric hypoxic-induced learning and memory impairment. Curcumin at 200 mg produced the best effect, we decided to use a dose of 200 mg for the following studies.
Hypoxic-induced impairments of learning and memory were reversed by curcumin. A Escape latency to find the hidden platform during the training period of mice in each group. B The mean latency in first-time passing the location of the original platform during the probe test of mice in each group. C The mean number of platform crossing during the probe test of mice in each group. D Track map of mice in the MWM. E Discrimination index of NOR was evaluated. All data are expressed as mean ± SEM, n = 6, *p < 0.05, **p < 0.01 versus Con group, #p < 0.05, ##p < 0.01 versus H14d group.
Curcumin improved hypoxic-induced hippocampal neurodegeneration
Increasing evidence suggests that hypoxia may cause neurodegenerative changes. Fluoro-Jade C (FJC) is a fluorescent tracer derived from fluorescein and has been widely used for histochemical labeling of degenerating neurons27. As shown in Fig. 4A-C, Compared with the Con group, the numbers of FJC-positive cells in hippocampal cornu ammonis1 (CA1) field and dentate gyrus (DG) regions of the H14d group were significantly increased (P<0.01). However, the numbers of FJC-positive cells in hippocampal CA1 and DG regions of H14d group were significantly decreased after curcumin treatment (P<0.01), suggesting that curcumin has the potential to improve normobaric hypoxic-induced hippocampal neurodegeneration.
Curcumin improved hypoxic-induced hippocampal neurodegeneration. FJC staining was used to evaluate the neurodegeneration of the CA1 and DG regions in the mice hippocampus of each group. (B–C) Histograms showing the number of FJC-positive cells as shown in (A). Data are expressed as mean ± SEM, n = 3, ***p < 0.001 versus Con group, ##p < 0.01, ###p < 0.01 versus H14d group.
Curcumin rescued decreased of hippocampal neurogenesis and synaptic deficits induced by long-term normobaric hypoxia
Previous studies confirmed that cognitive impairment is associated with decreased neurogenesis and synaptic deficits28. To evaluate this effect, BrdU was administered at the experimental endpoint to verify the influence of curcumin on survival of newborn neurons in hippocampus. As shown in Fig. 5A-B, a modulate number of BrdU-positive cells were observed in the Con group. In contrast, a significant decrease in the number of BrdU-positive cells was observed in H14d group (P<0.01). Whereas H14d group treated with curcumin displayed an increase in the number of BrdU-positive cells (P<0.05), indicating that curcumin alleviated the negative effect of long-term mild hypoxic stimulation on neurogenesis.
Curcumin improved hippocampal neurogenesis and synaptic plasticity in mice subjected to hypoxia stress. A Fluorogram of BrdU-positive (newborn) cells in the subgranular zone-granule cell layer of the hippocampus in mice. B Histograms showing the number of BrdU-positive cells as shown in BrdU staining. C, D Representative dendritic segments of CA1 pyramidal neurons (AO = apical oblique dendrite) in each group of Thy-l-YFP mice. E Dendritic spine density expressed as the number of spines normalized to 15 μm of dendritic length. F, G Western blots of PSD95, Homer1 and BDNF in the hippocampus (the original blots/gels are presented in Supplementary Fig. 1). Histograms on the right show relative protein band density. All data are expressed as mean ± SEM, n = 3, *p < 0.05, **p < 0.01 versus Con group, #p < 0.05 versus H14d group.
Thy1-YFP transgenic mice, which express the fluorescent yellow fluorescent protein (YFP) in neurons, were subjected to the 13%O2 for 14 days, including treatment with or without 200 mg/kg/day curcumin. As shown in Fig. 5C–E, the results of brain slice examination in H14d group showed a lower density of dendritic spines was present in hippocampal CA1 pyramidal cells compared with the Con group (P<0.01). Interestingly, curcumin treatment was found to obviously rescue the decreased of density of dendritic spines induced by long-term normobaric hypoxia (P<0.05), indicating that curcumin could promote synaptic plasticity.
To explore potential molecular mechanisms underlying the beneficial effect of curcumin, we first examined changes of synaptic protein PSD95 and Homer1, and brain-derived neurotrophic factor (BDNF) in vivo. As shown in Fig. 5F, G, curcumin rescued decreased protein levels of PSD95 and Homer1 in H14d model animals, indicating that PSD95 and Homer1 could be target proteins for the effect of curcumin on synaptic plasticity (P<0.05). In various groups of mice, there is not much change in BDNF protein, but its change trend is consistent with PSD95 and Homer1 protein.
Curcumin promoted synaptic growth in primary hippocampal neurons in vitro
Primary neurons from mouse hippocampus were cultured for 7 days and then treated with 1% O2 for 4 h to to establish hypoxia injury model in vitro5. To investigate the potential effects of curcumin on degenerated neuron, different dosages of curcumin were administrated to degenerated neurons. According to previous reports29,30, five doses of curcumin of 1, 5, 10, 20, 25 µM were selected. As shown in Fig. 6A and B, we first evaluated the changes of neurons’ cell viability and cytotoxicity of in each group by 3-(4,5)-dimethylthiahiazo-(z-y1)-3,5-di-phenytetrazoliumromide (MTT) and lactate dehydrogenase (LDH) assays. The results showed that 5, 10, 20, 25 25 µM curcumin could ameliorate hypoxic-induced decreased cell viability, and 20 µM curcumin significantly reduced cytotoxicity induced by hypoxia. Balancing the results of MTT and LDH, the dose of 20 µM for the following studies (P<0.05, P<0.01).
Curcumin promoted dendritic growth of primary hippocampal neurons subjected to hypoxia stress in vitro. A, B Effects of curcumin on cell viability and cytotoxicity of primary neurons subjected to hypoxia stress. C Representative images of MAP2 staining. D, E Quantification of total cultured neuron numbers and total dendritic length. F, G Western blot of PSD95 and BDNF in the hippocampus neurons (the original blots/gels are presented in Supplementary Fig. 2). The histogram below shows the relative protein band density. Con = control group; H4h = neurons treated with 0-0.1% O2 for 4 h group; cur-1/2/3/4/5 = curcumin (1, 5, 10, 20, 25 µM) groups; H14d + Cur = H14d + curcumin group. All data are expressed as mean ± SEM, n = 3, *p < 0.05, **p < 0.01 versus Con group, #p < 0.05, ##p < 0.01 versus H4h group.
Microtubule-associated protein 2 (MAP2) staining was conducted to ascertain whether curcumin promoted dendritic growth in vitro. As shown in Fig. 6C–E, the dendritic length of hippocampal neurons in H4h group were significantly shorter than Con group (P<0.01). However, after curcumin treatment, the dendritic length of hippocampal neurons was noticeably increased compared with H4h group neurons (P<0.01). In addition, curcumin increased protein levels of PSD95 and BDNF (P<0.05, P<0.01) (Fig. 6F, G). These results arguably offer proof that curcumin promotes synaptic growth both in vivo and in vitro.
Discussion
In this study, we found that curcumin exhibits neuroprotective properties in a normobaric-hypoxic brain injury mice model. The results of the behavioral tests and FJC staining showed that curcumin not only improved the memory impairment but also reversed the neuronal degeneration caused by long-term hypoxia. At the molecular level, curcumin was proved to promote neurogenesis and synaptic plasticity through up-regulating postsynaptic structural proteins PSD95 and Homer1, and neurotrophic factors protein BDNF. Taken together, we believe these findings collectively demonstrate that curcumin is a promising and effective agent for treatment of hypoxic-induced neurological disorders.
Long-term hypoxia is common in life and includes environmental hypoxia, such as space exploration, altitude exposure, marine aviation and hypoxia of the body or organs during aging and disease31. The brain is highly sensitive to hypoxia due to its high metabolic characteristics. Our experimental protocol was successful in inducing normobaric-hypoxic brain injury mice5. Our previous research found that mice exposed to 13%O2 (simulates oxygen concentrations at an altitude of 3,700 m, a common altitude around the world) for 7 and 14 days exhibited cognitive impairments. Furthermore, the cognitive function of mice exposed for 14 days was more severely than those exposed for 7 days. Even after restoring O2 supply, their cognitive function did not recover, indicating that long-term mild hypoxic-induced neural damage is irreversible5,24. Therefore, the mice treated continuously with 13% O2 for 14 days (H14d) were selected as hypoxic brain injury models in this study.
In a comprehensive series of studies that hypoxia may affect many pathological aspects of nervous system disease, including neurogenesis, synaptic plasticity, mitochondrial dysfunction, which may collectively result in neurodegeneration32. Chronic exposure or severe forms of stress (as mimicked in the hypoxic brain injury model) can induce long-lasting reductions in neurogenesis, in particular, within the hippocampus33. Indeed, hippocampal neurogenesis and synaptic plasticity are crucial in regulating memory formation and potentially enhancing the resilience to neuronal degeneration related conditions34,35. Hence, stimulation of neurogenesis and neuroplasticity is regarded as a promising strategy for identifying new neuroprotection targets. Consistent with previous reports33,36, long-term hypoxia resulted in reduced neurogenesis and dendrite spine in our study, as evidenced by decreased of BrdU staining in the hippocampus and dendrite spine density detection in Thy1-YFP transgenic mice. Whereas this reduction was reversed by curcumin, suggesting that curcumin plays an important role in neurogenesis and synaptic plasticity. BDNF play a pivotal role in the pathogenesis of neurodegenerative diseases37, and Diniz et al. found that neuroplasticity are require neurotrophic actions of BDNF38. We speculated that the recovery of cognitive function by curcumin is probably attributable to its induction of neuroplasticity and BDNF.
In addition to regulating BDNF, which plays critical roles in neurogenesis and synaptic plasticity, curcumin exerted a similar influence on expression of PSD95 and Homer1, two synaptic structural proteins18,39, indicating that curcumin has probable synaptic protection benefits. PSD95, served as an indicator of synaptic plasticity, played a vital role in the development, functioning, and plasticity of the nervous system16. Notably, PSD95 interacts with various binding partners, such as associated synaptic proteins, cytoskeletal element, and sion channels40, to form a complex network that stabilizes and regulates synaptic function, transmission and formation and maintenance of dendritic spines, and synaptic signaling and plasticity mechanisms41,42. Loss of PSD95 results in severe memory deficit due to synaptic disruption and neuronal loss43. Homer1, a protein that regulate neuronal excitability and synaptic plasticity and linked to nervous system disease, is reduced in the stem cell derived neuronal cultures of schizophrenia patients39. Interestingly, Homer1 knockout mice displayed different behavioral deficits including cognitive deficit44. These results suggest that curcumin may enhance synaptic plasticity by promoting BDNF, PSD95, and Homer1 expression in neuronal cells of normobaric-hypoxic brain injury model mice. Considering that curcumin promoted dendritic growth in primary neurons, it may be involved in dendritic outgrowth by regulating the preservation of synaptic proteins and neurotrophic factor, such as PSD95 and BDNF.
Interestingly, one report indicated that one report indicated that chronic hypoxia promotes hippocampal neurogenesis involving Notch1 signaling in epileptic rats45. However, our research found that long-term hypoxia caused a reduction in neurogenesis and spine density of hippocampal CA1 pyramidal neurons, which was accompanied by a decrease in the level of PSD95. Consistent with previous reports indicated that chronic intermittent hypoxia has been shown to have deleterious and damaging effects on central neurons and to impair synaptic plasticity in the CA1 region of hippocampus46, and chronic intermittent hypoxia also exacerbated memory and synaptic plasticity deficits in P301S mice47.
Curcumin has been widely confirmed to have a beneficial effect on cognitive functions in neurodegenerative diseases29,48. The antioxidant, anti-inflammatory, and neuroprotective properties of curcumin, found in turmeric, have demonstrated promise49. What’s more, scholars have found that curcumin in concentrating on hemorrhagic lesions by promoting neurogenesis, and improving motor functions50. And, Rat offspring who are exposed to an amorphous formula of curcumin from the embryonic stage have anti-anxiety-like behaviors, enhanced fear extinction learning, and increased synaptic plasticity in the hippocampal DG51. In our study, we found that curcumin promotes synaptic plasticity and improves cognitive dysfunction in normobaric-hypoxic brain injury model mice by significantly upregulating PSD95 and Homer1. From this, we hypothesize that curcumin is more effective as a compensation mechanism, but the further mechanism needs to be studied. However, whether it exerts neuroprotective effects in hypoxic-induced brain injury has not been reported yet. Currently, the treatment methods of hypoxic-induced brain injury are very limited due to the complexity of the pathogenesis of hypoxic-induced brain injury, thus there is an urgent need to explore new effective treatment strategies.
In summary, our study demonstrated that curcumin effectively reverses memory impairment by virtue of its facilitating role in neurogenesis and synaptic plasticity. The observed beneficial effects are probably attributable to a novel mechanism for the neuroprotective action of curcumin mediated by a up-regulation of synaptic proteins, such as PSD95 and Homer1, that leads to an increase in interneuron survival following hypoxic-induced neurodegeneration, possibly resulting from increased BDNF expression and contact with interneurons. Moreover, previous authorization of curcumin for clinical application is expected to pave the way for its therapeutic use in the treatment of hypoxic-related diseases.
Data availability
Data availability statementsData is provided within the manuscript or supplementary information files.
References
Midha, A. D. et al. Organ-specific fuel rewiring in acute and chronic hypoxia redistributes glucose and fatty acid metabolism. Cell. Metab. 35(3), 504–516e5 (2023).
Biller, A. et al. Exposure to 16 h of normobaric hypoxia induces ionic edema in the healthy brain. Nat. Commun. 12(1), 5987 (2021).
Wilson, M. H., Newman, S. & Imray, C. H. The cerebral effects of ascent to high altitudes. Lancet Neurol. 8(2), 175–191 (2009).
Zhang, X. et al. Ferroptosis pathways: unveiling the neuroprotective power of cistache deserticola phenylethanoid glycosides. J. Ethnopharmacol. 27:118465 (2024).
Li, G. et al. Chronic hypoxia leads to cognitive impairment by promoting HIF-2α-mediated ceramide catabolism and alpha-synuclein hyperphosphorylation. Cell. Death Discov. 8(1), 473 (2022).
Yang, Y. et al. Effects of hypoxia and ischemia on microRNAs in the brain. Curr. Med. Chem. 22(10), 1292–1301 (2015).
Yi, L. X. et al. Reelin links apolipoprotein E4, tau, and Amyloid-β in Alzheimer’s disease. Ageing Res. Rev. 98, 102339 (2024).
Tiwari, S. K. et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 8(1), 76–103 (2014).
Brown, J. C. et al. Critically assessing the unanswered questions of how, where, and when to induce plasticity in the Posttraumatic Stress Disorder Network with Transcranial Magnetic Stimulation. Biol. Psychiatry. 21, S0006 (2024). 3223(24)01390-8.
Zhao, C., Deng, W. & Gage, F. H. Mechanisms and functional implications of adult neurogenesis. Cell 132(4), 645–660 (2008).
Kochan, S. M. V. et al. Enhanced mitochondrial fusion during a critical period of synaptic plasticity in adult-born neurons. Neuron 112(12), 1997–2014e6 (2024).
Winner, B., Kohl, Z. & Gage, F. H. Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 33(6), 1139–1151 (2011).
Cho, S. R. et al. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J. Clin. Invest. 117(10), 2889–2902 (2007).
Tessier, M. et al. Bumetanide induces post-traumatic microglia-interneuron contact to promote neurogenesis and recovery. Brain 146(10), 4247–4261 (2023).
Lominac, K. D. et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J. Neurosci. 25(50), 11586–11594 (2005).
Nazir, F. H. et al. Expression and secretion of synaptic proteins during stem cell differentiation to cortical neurons. Neurochem Int. 121, 38–49 (2018).
Llorens-Martín, M. et al. GSK-3β over expression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Mol. Psychiatry. 18(4), 451–460 (2013).
Shahar, O. et al. Effect of chemically synthesized psilocybin and psychedelic mushroom extract on molecular and metabolic profiles in mouse brain. Mol. Psychiatry Feb 20. (2024).
Armağan, H. H. & Nazıroğlu, M. Curcumin attenuates Hypoxia-Induced oxidative neurotoxicity, apoptosis, calcium, and Zinc Ion influxes in a neuronal cell line: involvement of TRPM2 Channel. Neurotox. Res. 39(3), 618–633 (2021).
Gu, J. et al. Erythrocyte membrane-coated nanocarriers modified by TGN for Alzheimer’s disease. J. Control Release. 366, 448–459 (2024).
Li, J. et al. Curcumin promotes proliferation of adult neural stem cells and the birth of neurons in Alzheimer’s disease mice via notch signaling pathway. Cell. Reprogram. 21(3), 152–161 (2019).
Jin, T. et al. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol. 164, 113091 (2022).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28(1), 41–51 (2000).
Li, G. et al. Intermittent hypoxic conditioning restores neurological dysfunction of mice induced by long-term hypoxia. CNS Neurosci. Ther. 29(1), 202–215 (2023).
Ran, Y. et al. Curcumin ameliorates White Matter Injury after ischemic stroke by inhibiting Microglia/Macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid. Med. Cell. Longev. 2021, 1552127 (2021).
Lou, S. et al. Curcumin improves neurogenesis in Alzheimer’s Disease mice via the Upregulation of Wnt/β-Catenin and BDNF. Int. J. Mol. Sci. 25(10), 5123 (2024).
Gu, Q. et al. Combining tissue clearing and Fluoro-Jade C labeling for neurotoxicity assessments. Exp. Biol. Med. (Maywood). 248(7), 605–611 (2023).
Ward, C. et al. Developmental disruption of Mef2c in Medial Ganglionic Eminence-derived cortical inhibitory interneurons impairs cellular and circuit function. Biol. Psychiatry 5:S0006 -3223(24)01360-X (2024).
Liu, Y. et al. Brain-targeted biomimetic nanodecoys with neuroprotective effects for precise therapy of Parkinson’s disease. ACS Cent. Sci. 8(9), 1336–1349 (2022).
Xu, B., Chen, J. & Liu, Y. Curcumin interacts with α-synuclein condensates to inhibit amyloid aggregation under phase separation. ACS Omega. 7(34), 30281–30290 (2022).
Yeo, E. J. Hypoxia and aging. Exp. Mol. Med. 51(6), 1–15 (2019).
Pinky, N. et al. Age-related pathophysiological alterations in molecular stress markers and key modulators of hypoxia. Ageing Res. Rev. 90, 102022 (2023).
Lucassen, P. J. et al. Regulation of adult neurogenesis and plasticity by (early) stress, glucocorticoids, and inflammation. Cold Spring Harb Perspect. Biol. 7(9), a021303 (2015).
Lee, J. et al. Phospholipase C beta 1 in the dentate gyrus gates fear memory formation through regulation of neuronal excitability. Sci. Adv. 10(27), eadj4433 (2024).
Tresky, R. et al. TRMT10A dysfunction perturbs codon translation of initiator methionine and glutamine and impairs brain functions in mice. Nucleic Acids Res. 2:gkae520 (2024).
Wang, F. et al. Enhancing oligodendrocyte myelination rescues synaptic loss and improves functional recovery after chronic hypoxia. Neuron 99(4), 689–701e5 (2018).
Rezaee, D. et al. The role of microRNAs in the pathophysiology of human central nervous system: a focus on neurodegenerative diseases. Ageing Res. Rev. 92, 102090 (2023).
Diniz, C. R. A. F. et al. Fluoxetine and ketamine trigger the p75NTR proteolytic pathway and enhance extinction memory and brain plasticity through p75NTR. Biol. Psychiatry 28:S0006 -3223(24)01425–2 (2024).
Zimmerman, A. J. et al. A psychiatric disease-related circular RNA controls synaptic gene expression and cognition. Mol. Psychiatry. 25(11), 2712–2727 (2020).
Chen, X. et al. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc. Natl. Acad. Sci. U S A. 112(50), E6983–E6992 (2015).
Dore, K., Malinow, R. & Elevated PSD-95 blocks Ion-flux independent LTD: a potential New Role for PSD-95 in synaptic plasticity. Neuroscience 456, 43–49 (2021).
Coley, A. A. & Gao, W. J. PSD95: a synaptic protein implicated in schizophrenia or autism? Prog Neuropsychopharmacol. Biol. Psychiatry. 82, 187–194 (2018).
Han, K. & Kim, E. Synaptic adhesion molecules and PSD-95. Prog Neurobiol. 84 (3), 263–283 (2008).
Klugmann, M. et al. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol. Cell. Neurosci. 28(2), 347–360 (2005).
Sun, C. et al. Chronic mild hypoxia promotes hippocampal neurogenesis involving Notch1 signaling in epileptic rats. Brain Res. 1714, 88–98 (2019).
Wall, A. M. et al. Effects of prolyl-hydroxylase inhibition and chronic intermittent hypoxia on synaptic transmission and plasticity in the rat CA1 and dentate gyrus. Neurobiol. Dis. 62, 8–17 (2014).
Kazim, S. F. et al. Chronic intermittent hypoxia enhances pathological tau seeding, propagation, and Accumulation and exacerbates Alzheimer-like memory and synaptic plasticity deficits and molecular signatures. Biol. Psychiatry. 91(4), 346–358 (2022).
Yang, P. et al. Precise modulation of pericyte dysfunction by a multifunctional nanoprodrug to ameliorate Alzheimer’s disease. ACS Nano. 18(22), 14348–14366 (2024).
Pei, J. et al. Curcumin-loaded polymeric nanomaterials as a novel therapeutic strategy for Alzheimer’s disease: a comprehensive review. Ageing Res. Rev. 99, 102393 (2024).
Xu, X. et al. Platelet membrane-coated Curcumin-PLGA nanoparticles promote astrocyte-neuron transdifferentiation for Intracerebral Hemorrhage Treatment. Small 18:e2311128 (2024).
Tang, Q. et al. DNA methylation-altered genes in the rat hippocampal neurogenic niche after continuous exposure to amorphous curcumin. J. Chem. Neuroanat. 137, 102414 (2024).
Acknowledgements
This research was supported by the grants from China Academy of Chinese Medical Sciences, Special Fund for Young Scientific and Technological Talents (No. ZZ16-YQ-034), the Institute of Basic Theory of Traditional Chinese Medicine Cultivation Fund, China Academy of Chinese Medical Sciences (No. YZX-202233) and The Fundamental Research Funds for the Central Public Welfare Research Institutes (No. YZX-202416).
Author information
Authors and Affiliations
Contributions
Author contributionG.L : writing-original draft, funding acquisition, experimental operation, data curation; Q.W: partial experimental operation; C.W: partial experimental operation; P. D: partial experimental operation; J. L: partial experimental operation; Z.Z: partial experimental operation; Y. L: writing-review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Compliance with ethical standards
All animal experiments fully complied with the related laboratory animal regulations (No.2023EC-KY-009), and conducted in accordance with ethical requirements and ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, G., Wu, Q., Wang, C. et al. Curcumin reverses cognitive deficits through promoting neurogenesis and synapse plasticity via the upregulation of PSD95 and BDNF in mice. Sci Rep 15, 1135 (2025). https://doi.org/10.1038/s41598-024-82571-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82571-9