Abstract
Exposure to pulmonary tuberculosis (PTB) culminates in heterogeneous outcomes, including variation in Mtb antigen-specific interferon-gamma (IFN-γ) T-cell responses. IFN-γ-independent cytokines, including tumor necrosis factor (TNF) and interleukin (IL-2), offer potential diagnostic improvements and insights into pathogenesis. We hypothesized that ESAT6/CFP10 TNF and IL-2 responses improve Mtb infection detection among exposed household contacts (HHCs) and are associated with index case Mtb aerosolization (i.e., cough aerosol culture positive for Mtb growth, CAC+) and HIV co-infection. We enrolled individuals with PTB and their HHCs in a longitudinal study in Nairobi, Kenya. We measured TNF and IL-2 in HHCs from QuantiFERON-TB Plus TB1 tube supernatants. An additional 9.2% (25) HHCs beyond the 58.6% (129) with an IFN-γ response demonstrated an antigen-specific increase in IL-2 and TNF. HHCs of CAC + participants were more likely to have positive IL-2 (84.6% vs. 53.8%, p = 0.02) and IFN-γ (88.0% vs. 54.9%, p = 0.01), but not TNF responses, compared to CAC-negative individuals. While HIV co-infection in the index was negatively associated with IFN-γ responses in HHCs (35.7% vs. 62.3%, p = 0.03), IL-2 and TNF responses did not differ. Antigen-specific ESAT6/CFP10 IL-2 and TNF may increase rates of Mtb infection detection and provide insights into Mtb transmission and pathogenesis.
Similar content being viewed by others
Introduction
Mycobacterium tuberculosis (Mtb) is the leading infectious disease cause of death1. Most tuberculosis (TB) cases are preceded by asymptomatic Mtb infection. Interferon-gamma (IFN-γ), a critical pro-inflammatory cytokine and the endpoint of the interferon-gamma release assays (IGRAs) is pivotal in Mtb infection ascertainment2. In individuals heavily exposed to pulmonary TB, there is significant variation in Mtb infection rates, with some remaining IGRA-negative despite heavy exposure3,4,5,6,7. Alternative correlates of immunity in these individuals have been proposed3,7,8,9,10,11,12. Understanding these alternative correlates of immunity may aid in developing new tools for preventing, diagnosing, and treating Mtb.
Interleukin (IL)-2 and tumor necrosis factor (TNF) are T helper (Th) 1 pro-inflammatory cytokines promoting cell-mediated immunity, demonstrated to have a protective and immunodiagnostic role in TB13,14,15,16,17,18,19,20. Both cytokines have been detected in “resisters”10,11,21 and show promise in improving sensitivity in diagnosing Mtb infection22. Although these data suggest that Mtb-specific TNF and IL-2 responses are alternative endpoints, further research is needed to support their utility in enhancing the detection of Mtb infection. Additionally, differences in IFN-γ, TNF, and IL-2 responses following exposure to Mtb are not fully understood.
While previous studies have been conducted on cohorts with strong epidemiologic evidence of exposure to Mtb10,11, the effect of differential levels of exposure on immunologic responses has not been explored. Differential exposure to Mtb may activate a distinct T-helper pathway with varying immunologic responses23. As Mtb is transmitted through aerosolization, bioaerosol sampling to estimate differential exposure may provide further insights23. The cough aerosol sampling system (CASS) is a cough bioaerosol sampler validated to estimate Mtb infectiousness24,25,26. Cough-generated aerosol cultures (CAC) are shown to predict Mtb infection measured by IFN-γ better than other measures of infectiousness, including sputum smear microscopy or culture26,27,28. The magnitude and directionality of the effect of the aerosolized Mtb on antigen-specific IFN-γ-independent cytokine responses following exposure to Mtb is unknown.
Further, although persons living with HIV (PLWH) are more likely to develop TB1, the likelihood of Mtb transmission to contacts is thought to be lower than that of persons without HIV29,30,31. One reason is the lower bacillary load associated with the altered pathogenesis30,31. However, results on infectiousness of PLWH with TB have varied30,32. In addition, previous studies evaluating the infectiousness of PLWH with TB have used conventional tests assessing sensitization to Mtb antigens, including the tuberculin skin test and IGRAs, to indicate Mtb transmission to contacts29,30,31,32. It has not been determined before, to our knowledge, how Mtb antigen-specific non-IFN-γ cytokine responses in contacts vary with HIV coinfection in the index. Non-IFN-γ responses, including IL-2 and TNF, might provide additional information on transmission dynamics beyond IFN-γ.
To address these gaps in knowledge, we determined Mtb-specific TNF and IL-2 responses among household contacts (HHCs) of TB participants to assess the utility in improving Mtb infection detection and the effect of aerosolized Mtb and HIV on the cytokine responses.
Methods
Study setting, design, and population
This study used enrolment data from participants in an ongoing longitudinal cohort study designed to assess TB aerobiology, immunology, and transmission (TBAIT) in Nairobi, Kenya33. Adults newly diagnosed with pulmonary TB by GeneXpert and/or liquid culture for Mtb were enrolled from outpatient clinics and through a door-to-door prevalence survey (index TB participants). HHCs of any age were enrolled if they resided in the same household for at least 60 days at the time of the index patient TB diagnosis.
Ethical approvals
The ongoing study was conducted per the principles of the Declaration of Helsinki. It was approved by the Kenya Medical Research Institute Scientific and Ethics Review Unit (048/3988), the University of Washington Institutional Review Board (STUDY00009209), and Kenyatta National Hospital-University of Nairobi Ethics and Research Committee (KNH-ERC/A/221). All participants provided written informed consent. All data were obtained and stored in compliance with the associated Institutional Ethics Review Unit regulations.
Study interventions and clinical protocol
Members of the study team administered a questionnaire to obtain clinical and demographic information, including age, sex, HIV status, alcohol use, smoking status, information on HHC sharing a bedroom with index, and anthropometric measures, including mid-upper arm circumference (MUAC), blood pressure (BP), weight, and height; Body mass index (BMI) was calculated from weight and height measurements.
Among index TB participants, sputum was collected for acid-fast bacilli (AFB) smear, culture, and GeneXpert testing. HIV testing was performed using an opt-out approach, and CD4 lymphocyte count testing was performed where applicable. Chest X-rays (CXR) were obtained, and cavitation was documented. Phlebotomy was done to measure white cell count (WBC) and C-reactive protein (CRP). Infectious aerosols were collected using a cough aerosol sampling system (CASS), as we described previously33. Briefly, CASS consists of a six-stage Andersen Cascade Impactor (Thermo Fischer Scientific, IL) within a larger stainless-steel chamber attached to the mouthpiece tubing from the participant and with a vacuum pump, creating an airflow through the system24,25,33. Each of the six stages holds a Middlebrook plate with 7H10 or 7H11 solid agar media on which aerosolized particles settle based on size, with the smallest particles settling on the lowest plate24,25,33. Participants coughed into the mouthpiece for 5 min, after which the plates were incubated at 37 °C and observed weekly for growth for a maximum of 8 weeks33.
HHCs underwent symptom screening (ongoing cough, hemoptysis, weight loss, chest pain, fevers, chills, or night sweats), and CXR was obtained to rule out active TB. HHCs with symptoms suggestive of TB and/or abnormal CXR underwent further TB evaluations. HHCs with microbiological confirmation of TB were enrolled as TB cases and referred for treatment. Phlebotomy for HIV testing (using an opt-out approach) and QuantiFERON-TB Plus (QFT-Plus, IGRA) testing were performed.
Laboratory assays
Index TB participants
GeneXpert MTB/RIF (Xpert MTB/RIF) or GeneXpert Ultra (Xpert Ultra) (Cepheid, Sunnyvale, CA) were performed on raw sputum samples. Results were recorded as a cycle threshold (Ct) value and semi-quantitative grade, including “Trace” for Xpert Ultra. AFB smears and culture were performed on concentrated sputum. AFB smears were examined using fluorescence microscopy (FM), and the WHO AFB scale was used for grading. Sputum cultures were performed in liquid (MGIT Becton-Dickinson, Franklin Lakes, NJ, supplemented with BD BBLTM MGITTM PANTA) using an automated BACTEC MGIT 960 machine for incubation. Positive cultures were confirmed using Ziehl-Neelsen smear microscopy, and the MGIT TBc Identification Test (Becton-Dickinson Diagnostic Instrument Systems, Sparks, MD) was used to detect MTBc-specific MPT64-antigen. Time to detect Mtb growth (TTD of Mtb) was recorded. For CAC, agar plates with infectious aerosols were incubated at 37 °C for 8 weeks and observed weekly for growth. Colony-forming units (CFU) were counted where growth was identified as Mtb. Otherwise, agar plates were discarded as contaminated. The time to detect Mtb and the plates where Mtb was identified were documented.
Household contacts
The QFT-Plus test was performed at KEMRI/CRDR following the manufacturer’s instructions (Qiagen)34. Briefly, whole blood was collected into lithium heparin vacutainers, and 1 mL was transferred into each of the four QFT-Plus blood collection tubes within 4 h of collection. The tubes were incubated at 37 °C for 16 h and centrifuged for 15 min at 3000 relative centrifugal force (RCF), after which plasma was collected and assessed using the standard ELISA. Results were recorded using standard QFT-Plus interpretations from the manufacturer. The remaining supernatants from the nil, mitogen, and TB1 tubes with ESAT6/ CFP10 antigens were frozen at -800c. Frozen QFT-Plus supernatants were shipped to Seattle, WA, and tested for TNF and IL-2 using sandwich ELISA according to the manufacturer’s instructions (R&D Systems Inc. Minneapolis, USA.) Briefly, the supernatants were transferred from the vials to the master plate and then spun at 500 g for 5 min at 4oc. Pelleted debris from fibrin was lifted from the bottom, and the plates were spun again at 1200 rpm for 10 min. Assays were performed in duplicate for validation unless in cases where volumes were low, and the average value was generated. The lower limit of detection (LLD) was 5 pg/ml for IL2 and 15 pg/ml for TNF.
Variables and definitions
Cough aerosol culture status
Cough aerosol culture-positive status (CAC +) was defined by culture growth on any CASS plates. Participants were considered cough aerosol culture-negative (CAC-) if no CASS plates were positive for Mtb growth and no more than two of the six plates were discarded as contaminated with fungal or bacterial overgrowth.
IGRA measures and positivity
IGRA positivity was determined using standard QFT-Plus interpretations from the manufacturer with responses dichotomized as positive (> 0.35 IU/mL, and ≥ 25% of Nil value) or negative (< 0.35 IU/mL, or ≥ 0.35 IU/mL and < 25% of Nil value, with a response to the mitogen control ≥ 0.5 IU/mL) after excluding indeterminate responses34.
Defining positivity by TNF and IL-2 responses
We simulated the IGRA criteria to define positivity by TNF and IL-2 responses. TNF and IL-2 responses were considered positive if all of the following criteria were met: (1) TB1 ≥ LLD (5 pg/ml for IL2 and 15 pg/ml for TNF); (2) TB1-Nil ≥ LLD; (3) TB1-Nil ≥ 25% of sample Nil. TNF and IL-2 responses were considered negative if the patient did not meet the abovementioned criterion with a response to the mitogen control that was ≥ LLD.
Mtb infection endpoints
We categorized participants as having Mtb infection (latent TB infection, LTBI) (inferred based on evidence of Mtb-specific CD4 T-cell responses) in four ways based on the above criteria of defining positivity: IFN-γ status based on the standard IGRA criteria, IL2 status, TNF status, and finally, using a composite endpoint of any ESAT6/CFP10 response among IFN-γ, IL2, and TNF, “composite measure.” Using the IGRA criteria, we excluded 6 HHC with indeterminate and 4 HHC missing QFT-Plus test results in categorizing participants as having Mtb infection. However, these participants were assessed for IL2 and TNF responses.
Statistical analysis
Index and household contact characteristics analyses
We compared baseline characteristics of HHC by IGRA status using Pearson’s chi-square or Fisher’s exact tests and the t-test or Wilcoxon rank-sum as applicable. We used bivariate logistic regression with random intercepts, without adjustment, to account for clustering for index participant characteristics.
IFN-γ, TNF, and IL-2 responses in HHC
IFN-γ, TNF, and IL-2 concentrations in the TB1 and mitogen tubes were background-corrected before analysis by subtracting the concentration from the negative control (nil) sample. Correlation plots were created for the TB1 minus nil (TB1-Nil) responses to assess potential associations between cytokines (Supplementary Figure S1).
Associations with cytokine responses and positivity in household contacts
Our primary outcome of interest was cytokine responses and positivity in household contacts, and our primary predictor was the cough aerosol culture status of the index participant. Using the defined endpoints, cytokine responses were dichotomized as positive or negative (excluding indeterminate and unclassified responses). Wilcoxon rank sum test with continuity correction was used to assess the association of cytokine concentrations (pg/ml) with cough aerosol culture status, cavitary disease, and other categorical variables. Spearman’s correlation was used to examine the association of cytokine responses with continuous and ordinal variables. We further assessed associations between predictors and outcomes using bivariate and multivariable logistic regression models with random intercepts to account for clustering by index participants. Multivariable models included covariates with statistically significant results based on p-values < 0.10 in bivariate analyses after assessing multicollinearity between our independent variables using variance inflation factors. We removed any explanatory variables with evidence of multicollinearity from the model. We introduced an interaction term between cavitary disease and CAC status to test the difference between these associations by the presence of cavitary disease. Significance was set at a α = 0.05. Missing data were addressed by pairwise deletion to reduce the chance of biased estimates. Analyses were performed using STATA V.17 (StataCorp, College Station, TX) and RStudio: Integrated Development Environment for R version 4.4.0 (2024-04-24).
Results
Participant characteristics and Mtb-specific TNF and IL-2 responses in HHC
Among 230 HHCs, 58.7% (n = 135) were female, the median age was 14.9 years (interquartile range [IQR] 6.5–32.0), the median BMI was 20 kg/m2 (IQR 16.6–25.4), and the median MUAC was 23 cm (IQR 16.5–27.5). Only 3.5% (n = 8) were PLWH, 9.0% (n = 19) reported alcohol use, and 3.3% reported tobacco use. Mtb infection (IGRA positive) was detected in 58.6% of participants (129/220, excluding 6 with indeterminate and 4 with missing results). 65% of households had two or more HHCs for each index participant (Table 1), and 29 of 92 (13%) had both IGRA positive and negative HHCs. Compared to HHCs with a negative IGRA, HHCs with a positive IGRA were older (median 17.4 vs. 12.8 years, p = 0.02) and had larger MUAC (median 23.0 vs. 22.0 cm, p = 0.03). We observed no difference in IGRA positivity rates as the number of contacts increased in households. (Supplementary Table 1). Characteristics of the index participants have been described previously33.
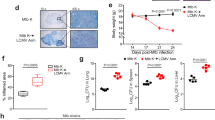
We analyzed levels of TNF and IL-2 in QFT-Plus supernatants among HHCs of TB index cases. TNF responses demonstrated moderate background (median 34.4 pg/mL, IQR 12.5–99.5), strong mitogen responses (mitogen-nil 187.5 pg/mL, IQR 38.4-709.2), with a small margin between TB1 and nil levels (median 3.9 pg/mL), and no precise bimodal distribution (TB1-nil range 0–845.3, IQR 0–45.9), with 57% participants having TB1-nil > 0 (Fig. 1A and Supplementary Table 2), and 47% (71/151) having TB1-nil > 25% of the Nil. IL2 responses had low background (median nil 6.2 pg/mL, IQR 0-50.5), strong mitogen responses (mitogen-nil 305.1 pg/mL, IQR 59.2-851.9), with a wide margin between TB1 and nil levels (median 35.4 pg/mL), and no precise bimodal distribution for TB1-nil (TB1-nil range 0–2795.6 pg/mL, IQR 0-195.0) with 63.8% participants having TB1-nil > 0 (Fig. 1A and Supplementary Table 2), and 59% (124/210) participants having TB1-nil > 25% of the Nil. For the TB1-Nil responses, we found a weak correlation between IL-2 and TNF (r = 0.165, p = 0.04) and between QFT-Plus IFN-γ and TNF (r = 0.179, p = 0.03). QFT-Plus IFN-γ and IL-2 had moderate correlation (r = 0.459, p < 0.001) (Supplementary Figure S1). In a secondary analysis, we found that IL-2 concentrations (TB1-nil) were significantly higher among IGRA positive HHC compared to IGRA negative (150.6 pg/mL IQR 64.33–393.8 vs. 0 pg/mL IQR 0–0; p < 0.0001) (Supplementary Table 3). In contrast, TNF concentrations (TB1-nil) were not different (4.47 pg/mL IQR 0-33.09 vs. 2.25 pg/mL IQR 0-64.5, p = 0.52) (Supplementary Table 3). Together, these data show that IL-2 and TNF responses among HHC of TB participants lack a bimodal distribution and are not highly correlated with each other or with QFT-Plus IFN-γ.
Household contact IL2 and TNF cytokines measured from the QFT-Plus supernatants. (A) IL2 and TNF concentrations (pg/mL) measured from the QFT-Plus supernatant of the nil, mitogen, and TB1 (ESAT6/CFP10 stimulated) tubes. We observed no precise bimodal distribution for ESAT6/CFP10 IL-2 and TNF responses (B) Positivity results by cytokine. 129 of 220 (58.6%) HHC were positive by QFT-Plus IFN-γ interpretation, 121 of 210 (57.6%) HHC were regarded positive by IL-2, and 55/151(36.4%) HHC were regarded positive by TNF. Using a combined endpoint of any response among IFN-γ + IL-2 + TNF+, we identified a total of 154 of 227 (67.8%) with an antigen-specific response, a 9.2% increase from using QFT-Plus IFN-γ interpretation alone.
Positivity by Mtb-specific TNF and IL-2 responses in HHC
Modeling the positive/negative criteria of QFT-Plus, we defined positivity thresholds for TNF and IL-2 responses in HHC. The median concentrations (IQR) among participants regarded positive by IL-2 and TNF were 152.0 (69.5-412.8) and 71.1 (38.0-153.3), respectively. Compared with 58.6% (129 of 220) HHC considered positive by QFT-Plus interpretation, 57.6% (121 of 210) HHC were regarded positive by IL-2 (eight being negative and one having an indeterminate result by QFT-Plus IFN-γ) and 36.4% (55/151) HHC were considered positive by TNF (19 being negative by QFT-Plus IFN-γ) (Fig. 1B). Eight (8) HHCs considered positive by QFT-Plus IFN-γ were regarded negative by both IL-2 and TNF, and 34 of 147 (23%) HHCs with complete data for the three cytokines were positive by all three criteria (IFN-γ + IL-2 + TNF+). Using a combined endpoint of any response among IFN-γ, IL-2, and TNF, we identified 25 additional HHC with evidence of an ESAT6/CFP10-specific response (6 by IL-2 alone, 17 by TNF alone, and 2 by IL-2 and TNF). This brought the total classified as having Mtb infection to 154 of 227 (67.8%), a 9.2% increase from using QFT-Plus IFN-γ interpretation alone (Fig. 1B).
We compared HHCs with a positive QFT-Plus result (IGRA+, N = 129) with the 25 HHCs who had negative QFT-Plus (IGRA-) but had positive IL-2 or TNF (IGRA-&IL-2 +/TNF+). IGRA-&IL-2+/TNF + HHCs were younger (median 11.5 IQR 5.3–23.4 vs. 17.4 IQR 9.1–35.3 years, p = 0.04); other characteristics did not differ. Additionally, we compared HHCs that were positive by all three criteria (IFN-γ + IL-2 + TNF) with HHCs that were positive by one or two criteria and found no differences in the characteristics (Supplementary Table 4). Together, these data suggest that IL-2 and TNF endpoints improve the detection of Mtb infection in HHC of TB participants compared with an IFN-γ endpoint alone.
Association of index case CAC status with Mtb-specific IFN-γ, TNF, and IL-2 responses in HHC
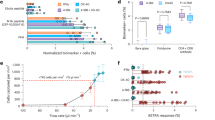
Next, we examined the impact of index case aerosolized Mtb on HHC TNF and IL-2 compared with QFT-Plus IFN-γ responses. Median IL-2 concentrations (TB1-nil) were significantly higher among HHC of CAC + compared to those of CAC- index participants (142.06 pg/mL IQR 32.58-381.88 vs. 21.21 pg/mL IQR 0-158.56; p = 0.01). The same trend was observed with QFT-Plus IFN-γ concentrations (TB1-nil) comparing HHC of CAC + and CAC- index participants (3.42 IU/mL IQR 0.81–4.68 vs. 0.49 IU/mL IQR 0-3.50; p = 0.001) (Table 2; Fig. 2A). In contrast, TNF concentrations were not different (3.96 pg/mL IQR 0-45.87 vs. 1.76 pg/mL IQR 0-28.53, p = 0.99) (Table 2). We also assessed the association of cavitary lung disease and other measures of bacillary burden with IFN-γ, IL-2, and TNF responses in HHCs. IL-2 (p = 0.02) and IFN-γ (p = 0.001), but not TNF (p = 0.79), were positively associated with cavitary lung disease regardless of CAC status (Table 3; Fig. 2B). We observed no association between TNF, IL-2, and IFN-γ concentrations and bacillary burden measured by GeneXpert cycle threshold (p-values 0.50, 0.79, and 0.11), Xpert Grade (p-values 0.06, 0.40, and 0.15), sputum smear grade (p-values 0.05, 0.19, and 0.05) and TTD of Mtb (p-values 0.66, 0.44, and 0.05), respectively.
Distribution of household contact cytokine concentrations stratified by index case Cough Aerosol Culture (CAC) and chest x-ray lung cavitation statuses. Scatter points represent individual household contact cytokine measures, and the colors represent the index case CAC status (black, CAC negative, and red, CAC positive). (A) Household contact cytokine concentrations stratified by index case CAC status. IL-2 and QFT-Plus IFN-γ concentrations were significantly higher among HHC of CAC-positive than those of CAC-negative index participants. (B) Household contact cytokine concentrations stratified by index case chest x-ray lung cavitation status. IL-2 and QFT-Plus IFN-γ concentrations were significantly higher among the HHC of index participants with, compared to those without, lung cavitary disease.
We then examined whether aerosolized Mtb was associated with Mtb infection, as defined by various positivity endpoints using ESAT6/CFP10 responses for TNF, IL-2, and QFT-Plus IFN-γ (IGRA). We used mixed-effects logistic regression models to account for clustering by index participants. Comparing HHC of CAC + and CAC- index participants, exposure to a CAC + index was associated with Mtb infection when using IL2 (84.6% vs. 53.8%, odds ratio [OR] 6.98, 95% confidence interval [CI] 1.33–36.75, p = 0.02) and IGRA (88.0% vs. 54.9%, OR 11.5, CI 1.68–78.7, p = 0.01), but not TNF (35.3% vs. 36.6%, OR 0.96, CI 0.30–3.09, p = 0.94) endpoints independently (Table 4). Using any positive ESAT6/CFP10-specific cytokine response (composite criteria including IL-2, TNF or IFN-γ), exposure to a CAC + index was still associated with Mtb infection (88.5% vs. 66.2% comparing CAC + and CAC-, respectively, OR 5.37, CI 1.07-27.0, p = 0.04). This also held when the endpoint was narrowed to include any positive of QFT-Plus or either of the two non-IFN-γ endpoints: using IL2/ QFT-Plus (OR 5.70, CI 1.10-29.56, p = 0.04) and TNF/QFT-Plus (OR 6.48, CI 1.27–33.11, p = 0.03) (Table 4). In multivariable models, the observed associations with CAC status were maintained, and we observed that chest X-ray cavitation modified the effect of CAC-positive status on all Mtb infection endpoints (Table 4). Together, these data suggest that IFN-γ and IL-2 endpoints are associated with aerosolized Mtb independently or as part of a measure comprising any positive ESAT6/CFP10-specific cytokine response, including IL-2, TNF, or IFN-γ.
Association of index case HIV co-infection with Mtb-specific IFN-γ, TNF, and IL-2 responses in HHC
We also examined the impact of index case HIV co-infection on Mtb infection as defined by various positivity endpoints using ESAT6/CFP10 responses for TNF, IL-2, and QFT-Plus IFN-γ. Mtb infection status differed by index case HIV co-infection status for the QFT-Plus IFN-γ endpoint (35.7% of PLWH vs. 62.3% of persons without HIV with Mtb infection by a positive QFT-Plus, OR 0.17, CI 0.04–0.82, p = 0.03), but not TNF or IL-2 endpoints (Table 5). Together, this data suggests that HIV co-infection may be associated with significantly lower odds of Mtb infection in HHC when the QFT-Plus IFN-γ endpoint is used alone.
Discussion
We assessed the utility of Mtb-specific TNF and IL-2 (from supernatants of the QFT Plus TB1 antigen tube) in improving Mtb infection detection in HHCs of TB participants and the effect of index case aerosolized Mtb and HIV co-infection on cytokine responses. We had several primary findings, including that 9.2% of HHCs had an antigen-specific TNF and IL-2 response, yet no response by QFT-Plus IFN-γ; aerosolized Mtb exposure was associated with IL-2 and IFN-γ, but not TNF, in HHCs; and HIV co-infection in the index was associated with IFN-γ responses, but not IL-2 or TNF responses in HHCs.
Our findings of weak to moderate correlation of the three cytokines suggest that each may have complementary roles in Mtb infection detection. Like QFT-Plus IFN-γ, ESAT6/CFP10 responses for IL-2 and TNF lacked a bimodal distribution, which can be used to define positivity thresholds. Modeling the positive/negative criteria of QFT-Plus34, we identified 9.2% of HHCs that were Mtb uninfected by traditional criteria (lacked evidence of sensitization to Mtb antigens based on in vitro release of IFN-γ) yet had evidence of sensitization to Mtb-specific TNF and IL-2. We did not test the criteria for defining positivity thresholds in individuals from a non/low-endemic area. However, we observed that for IL-2 concentration, the lower quartile among participants inferred to have Mtb infection (69.5 pg/mL) approximated the threshold (66 pg/mL) identified for discriminating TB-infected from uninfected persons in a low-endemic area in a previous study9. Our findings extend past evidence where we and others highlight that IFN-γ independent T cell responses may identify Mtb exposure not reflected by the current IFN-γ based diagnostic tests10,11,12,21,22.
We also found that the additional HHCs identified were more likely to be younger. Recently, we found that Mtb-specific non-IFN-γ cytokine responses have improved sensitivity in detecting Mtb infection in HIV-exposed infants compared with IGRA22. Our new data in Mtb-exposed HHCs suggest that IL-2 and TNF may have a role in detecting Mtb exposure in younger persons, such as children, who have reduced sensitivity to IGRA35,36. Our findings and past evidence have implications for developing innovative tests with potential use in TB diagnostics in line with The End TB Strategy37.
The role of IFN-γ, IL-2, and TNF in TB pathogenesis has been studied extensively. Distinct profiles of Mtb-specific IFN-γ, IL-2, and TNF CD4 + T-cells are thought to be associated with different Mtb infection states and the related bacterial loads within the host38,39,40. Mtb-specific IL-2 and TNF have also been detected in individuals heavily exposed to Mtb without evidence of an immunologic response based on traditional in vitro release of IFN-γ10,11,21. CAC is an important measure of TB infectiousness26,27,28, given it is a direct measure of Mtb aerosolization supported by existing evidence of the crucial role of aerosols in infectivity and immunopathology23,41,42. Data on the differential cytokine expression by levels of exposure is sparse and limited to the IFN-γ response26,27,43. In keeping with this past evidence, we recently found that the CAC status of the index predicted QFT Plus positivity in HHCs33. Our new data extends these findings to show that IL-2 and IFN-γ, but not TNF, independently or as part of a measure comprising any positive ESAT6/CFP10-specific cytokine response, are associated with aerosolized Mtb (CAC status).
Consistent with previous findings suggesting a lower likelihood of Mtb transmission from PLWH compared to persons without HIV29,30,31,44, we recently found that HIV coinfection in the index predicted a negative QFT Plus in HHCs33. In the current study, we extend these findings to assess the association between HIV coinfection in the index and HHC Mtb infection defined by various positivity endpoints using ESAT6/CFP10 responses for TNF, IL-2, and QFT-Plus IFN-γ. As reported previously33, there was a negative association between HIV co-infection in the index and Mtb infection in HHC using the QFT-Plus IFN-γ endpoint independently. Although PLWH had lower odds of having HHCs with Mtb infection using the IL-2 endpoint or the composite measure comprising any positive ESAT6/CFP10-specific cytokine response, there was no evidence of a significant association. There have been contradictory reports in the past, with other studies finding no evidence for an association between HIV coinfection and Mtb transmission30,32. Together, these data suggest that additional studies using different methodologies, including exploring the role of non-IFN-γ responses in confirming Mtb infection and using Mtb-specific antigens beyond ESAT-6/CFP10, may provide further insights into Mtb transmission dynamics among PLWH.
Our study has several limitations. TNF and IL-2 lacked a bimodal distribution, limiting the ability to define appropriate cutoffs. As this was like QFT-Plus IFN-γ, we mimicked the QFT-Plus criteria in defining positivity. Given that the IGRAs cutoff is also suggested to have an uncertainty zone and that there is a high likelihood of conversion within a year of exposure45,46,47, these cutoffs may need optimization in future research. We had a small number of index participants who were CAC positive and living with HIV, limiting the ability to study associations comprehensively. In this analysis, we do not report follow-up data to show evidence of protection from TB or risk of TB progression based on the various ESAT6/CFP10-specific cytokine responses and CAC status. We had some missing data points, which we addressed by pairwise deletion to reduce the chance of biased estimates. Despite these limitations, our study has strengths, including using multiple diagnostic approaches to maximize test sensitivity in diagnosing possible Mtb infection in HHCs and determining, for the first time to our knowledge, associations with Mtb aerosolization and HIV coinfection.
In summary, we show that IL-2 and TNF, in addition to QFT outcomes, may provide additional benefits in Mtb infection diagnosis and phenotyping, help predict Mtb transmission events, and provide further insights into Mtb transmission dynamics among PLWH. Our findings have implications for developing innovative tests with potential use in TB diagnostics in line with The End TB Strategy for moving forward to the 2035 targets. We recommend further studies to optimize the diagnosis of Mtb infection using non-IFN-γ cytokines and study associations with CAC status and HIV coinfection.
Data availability
The data supporting this study’s findings are not publicly available due to privacy policy but will be available from the corresponding author upon reasonable request.
Change history
27 February 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41598-025-90994-1
References
Global tuberculosis report & World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (Accessed 10 Feb 2024). (2023).
World Health Organisation. Consolidated guidelines on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. https://www.who.int/publications/i/item/9789240001503 (Accessed 13 July 2022). (World Health Organization, 2020).
Simmons, J. D. et al. Immunological mechanisms of human resistance to persistent Mycobacterium tuberculosis infection. Nat. Rev. Immunol. 18(9), 575–589 (2018).
Stein, C. M. et al. Long-term stability of resistance to latent Mycobacterium tuberculosis infection in highly exposed Tuberculosis household contacts in Kampala, Uganda. Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am. 68(10), 1705–1712 (2019).
Li, H. et al. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 114(19), 5023–5028 (2017).
Chihota, V. N. et al. Resistance to Mycobacterium tuberculosis infection among highly TB exposed South African gold miners. PLoS ONE. 17(3), e0265036.
Kroon, E. E. et al. An observational study identifying highly tuberculosis-exposed, HIV-1-positive but persistently TB, tuberculin and IGRA negative persons with M. tuberculosis specific antibodies in Cape Town, South Africa. EBioMedicine. 2020, 61. (2022).
Kim, S. Y. et al. The responses of multiple cytokines following incubation of whole blood from TB patients, latently infected individuals and controls with the TB antigens ESAT-6, CFP-10 and TB7.7. Scand. J. Immunol. 76(6), 580–586 (2012).
Rubbo, P. A. et al. Multicytokine detection improves latent tuberculosis diagnosis in health care workers. J. Clin. Microbiol. 50(5), 1711–1717 (2012).
Lu, L. L. et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat. Med. 25(6), 977–987 (2019).
Davies, L. R. L. et al. IFN-γ independent markers of Mycobacterium tuberculosis exposure among male South African gold miners. EBioMedicine 93, 104678 (2023).
Sun, M. et al. Author correction: specific CD4(+) T cell phenotypes associate with bacterial control in people who ‘resist’ infection with Mycobacterium tuberculosis. Nat. Immunol. 25(10), 1976 (2024).
Segueni, N. et al. Innate myeloid cell TNFR1 mediates first line defence against primary Mycobacterium tuberculosis infection. Sci. Rep. 6(1), 22454 (2016).
Flynn, J. L. et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2(6), 561–572 (1995).
Keane, J. et al. Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent. N. Engl. J. Med. 345(15), 1098–1104 (2001).
Bean, A. G. et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162(6), 3504–3511 (1999).
Belardelli, F. Role of interferons and other cytokines in the regulation of the immune response. Apmis 103(3), 161–179 (1995).
Qiu, X. et al. Is interleukin-2 an optimal marker for diagnosing tuberculosis infection? A systematic review and meta-analysis. Ann. Med. 52(7), 376–385 (2020).
Balcells, M. E., Ruiz-Tagle, C., Tiznado, C., García, P. & Naves, R. Diagnostic performance of GM-CSF and IL-2 in response to long-term specific-antigen cell stimulation in patients with active and latent tuberculosis infection. Tuberculosis 112, 110–119 (2018).
Chegou, N. N., Heyckendorf, J., Walzl, G., Lange, C. & Ruhwald, M. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur. Respir J. 43(5), 1472–1486 (2014).
Shey, M. S. et al. Mycobacterial-specific secretion of cytokines and chemokines in healthcare workers with apparent resistance to infection with Mycobacterium tuberculosis. Front. Immunol. 14, 1176615 (2023).
Anterasian, C. et al. Non-IFNγ whole blood cytokine responses to Mycobacterium tuberculosis antigens in HIV-exposed infants. Pediatr. Infect. Dis. J. 40(10), 922–929 (2021).
Fennelly, K. P. & Jones-López, E. C. Quantity and quality of inhaled dose predicts immunopathology in tuberculosis. Front. Immunol. 6, 313 (2015).
Fennelly, K. P. et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir Crit. Care Med. 169(5), 604–609 (2004).
Fennelly, K. P. et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am. J. Respir Crit. Care Med. 186(5), 450–457 (2012).
Acuña-Villaorduña, C. et al. Cough-aerosol cultures of Mycobacterium tuberculosis in the prediction of outcomes after exposure. A household contact study in Brazil. PloS One. 13(10), e0206384 (2018).
Jones-López, E. C. et al. Cough aerosols of Mycobacterium tuberculosis predict new infection: a household contact study. Am. J. Respir Crit. Care Med. 187(9), 1007–1015 (2013).
Jones-López, E. C. et al. Cough aerosols of Mycobacterium tuberculosis in the prediction of incident tuberculosis disease in household contacts. Clin. Infect. Dis. 63(1), 10–20 (2016).
Espinal, M. A. et al. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet 355(9200), 275–280 (2000).
Martinez, L., Sekandi, J. N., Castellanos, M. E., Zalwango, S. & Whalen, C. C. Infectiousness of HIV-seropositive patients with tuberculosis in a high-burden African setting. Am. J. Respir Crit. Care Med. 194(9), 1152–1163 (2016).
Martinez, L. et al. Transmission dynamics in tuberculosis patients with human immunodeficiency virus: a systematic review and meta-analysis of 32 observational studies. Clin. Infect. Dis. 73(9), e3446–e55 (2021).
Cruciani, M., Malena, M., Bosco, O., Gatti, G. & Serpelloni, G. The impact of human immunodeficiency virus type 1 on infectiousness of tuberculosis: a meta-analysis. Clin. Infect. Dis. 33(11), 1922–1930 (2001).
Nduba, V. et al. Mycobacterium tuberculosis cough aerosol culture status associates with host characteristics and inflammatory profiles. Nat. Commun. 15(1), 7604 (2024).
QuantiFERON-TB Gold Plus (QFT-Plus). ELISA Package Insert 02/2016. http://www.quantiferon.com/wp-content/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf (Accessed Oct 2019).
Mandalakas, A. M., Detjen, A. K., Hesseling, A. C., Benedetti, A. & Menzies, D. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 15(8), 1018–1032 (2011).
Machingaidze, S. et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr. Infect. Dis. J. 30(8), 694–700 (2011).
World Health Organization. The end TB strategy. World Health Organization. https://iris.who.int/handle/10665/331326. (Accessed 26 May 2024) (2015).
Caccamo, N. et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40(8), 2211–2220 (2010).
Biselli, R. et al. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin. Microbiol. Infect. 16(8), 1282–1284 (2010).
Gourgouillon, N. et al. TNF-α/IL-2 ratio discriminates latent from active tuberculosis in immunocompetent children: a pilot study. Pediatr. Res. 72(4), 370–374 (2012).
Sultan, L. et al. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. Am. Rev. Respir Dis. 82, 358–369 (1960).
Riley, R. L. et al. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. 1959. Am. J. Epidemiol. 142(1), 3–14 (1995).
Williams, C. M. et al. Exhaled Mycobacterium tuberculosis predicts incident infection in household contacts. Clin. Infect. Dis. 76(3), e957–e64 (2022).
Windels, E. M. et al. HIV co-infection is associated with reduced Mycobacterium tuberculosis transmissibility in sub-saharan Africa. PLoS Pathog. 20(5), e1011675 (2024).
Nemes, E. et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am. J. Respir Crit. Care Med. 196(5), 638–648 (2017).
van Zyl-Smit, R. N., Zwerling, A., Dheda, K. & Pai, M. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PloS One. 4(12), e8517 (2009).
Andrews, J. R. et al. The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents. Am. J. Respir Crit. Care Med. 191(5), 584–591 (2015).
Acknowledgements
We would like to thank the participation of the individual study participants and their families. We thank Chris Le for his technical assistance. We thank Lenis Njagi, Geoffrey Onchiri, Ruth Munyasya, Isaac Kibet, Stella Nthambi, Kevin Munge, Patrick Isinidu, Joash Omolo, Hastings Koech, Inviolata Sakwa of KEMRI CRDR Nairobi for their support in data collection. LNN acknowledges training support provided by the Health-Professional Partnership Initiative Kenya (NIH R25TW011212), the Consortium for Advanced Research Training in Africa, and Dr. Jared Mecha of the University of Nairobi for supervisory and administrative support during training.
Funding
This research was funded by the National Institutes of Health/NIAID (NIH grant 5R01AI150815 -DJH/TRH and UH2AI152621 - DJH), the National Center for Advancing Translational Sciences of the NIH (UL1 TR002319), the University of Washington/Fred Hutch Center for AIDS Research, (AI027757) and the Firland Foundation (TRH), NIH D43 TW011817-01 (LNN, VN, WBM, JM, DJH, TRH). KF was funded entirely by the NHLBI Division of Intramural Research.
Author information
Authors and Affiliations
Contributions
Funding acquisition VN, KF, DJH, and TRH; conceptualization LNN, VN, DJH, and TRH; laboratory analysis WBM, ZM, KC, and RC; data curation and analysis LNN, VN, JM, DJH, and TRH; writing—original draft LNN; Writing-review and editing LNN, VN, KF, DJH, and TRH; all authors read and approved the final draft for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: In the original version of this Article, the legend of Supplementary Figure 1 was omitted. Full information regarding the correction made can be found in the correction for this Article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Njagi, L.N., Nduba, V., Murithi, W.B. et al. Association of Mycobacterium tuberculosis aerosolization and HIV coinfection in the index case with T cell responses in household contacts. Sci Rep 15, 224 (2025). https://doi.org/10.1038/s41598-024-83965-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83965-5