Abstract
Pain and inflammation are the severe problems of the world. Essential oils (EOs) have been considered for treating pain and inflammation. As EOs are unstable and insoluble in water, nanoemulsions can improve bioavailability and efficacy. This study formulated Pelargonium graveolens essential oil nanoemulsion (PGEO-NE) using Tween 80 as a carrier. The activity of PGEO-NE (50 and 100 mg/kg) and the involvement of the opioid pathway were investigated in different models of nociception and inflammation in mice. The droplets in PGEO-NE are spherical, with a particle size of around 553 nm and a polydispersity index of 0.113. GC/MS analysis of PGEO revealed citronellol (34.9%), geraniol (16.1%), citronellyl formate (12.3%), and linalool (6.8%) as the main constituents. PGEO-NE significantly reduced the duration of formalin-induced paw licking/biting in both phases and the number of acetic acid-induced writhings, and increased the hot plate latency, compared to vehicle and PGEO (10 mg/kg), equivalent to the PGEO-NE dose of 100 mg/kg. The anti-nociceptive potential of PGEO-NE (100 mg/kg) in the hot plate and acetic acid tests was reversed by the opioid antagonist. After formalin and carrageenan injections, PGEO-NE significantly inhibited paw edema, but PGEO (10 mg/kg) had no effect. These results confirm that PGEO-NE inhibits pain and inflammation via the opioid pathway.
Similar content being viewed by others
Introduction
Pain and inflammation are serious problems with a high prevalence worldwide, adversely affecting animal and human health1,2. In many cases, pain and inflammation accompany each other. Pain is one of the symptoms of inflammation and also modulates the immune system’s inflammatory response. The inflammatory mediators, such as bradykinin3and prostaglandins, and pro-inflammatory mediators such as cytokines and chemokines (tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β)4, stimulate the nociceptive sensory neurons and initiate the pain response5. On the other hand, the immune system is modulated by neuropeptides and neurotransmitters released from nociceptors5. Although pain and inflammation are vital defense systems against pathological situations, they can disturb the body’s function. Also, they are consequences of many acute and chronic diseases. Therefore, the prevention and treatment of pain and inflammation are necessary. Despite the large number of antinociceptive and anti-inflammatory drugs available, they have some limitations, such as the high cost, side effects, and low efficacy. Thus, it is necessary to find alternative strategies to achieve more effective and safe treatment. For thousands of years, herbal medicines have been valued for their ability to treat pain6and inflammation7 with low side effects and multi-target efficacy. The bioactive components in medicinal plants can have diverse therapeutic effects and may even enhance each other’s effectiveness.
Essential oils (EOs) are one of the most important natural products of medicinal plants; some of their bioactive constituents show anti-inflammatory8and antinociceptive9effects. However, EOs consist of volatile and hydrophobic components, making them highly unstable and prone to rapid degradation by external factors such as light, humidity, oxygen, and temperature. Nanoencapsulation is a well-known method to overcome these limitations by coating bioactive substances. In this context, nanoemulsion is the biphasic drug delivery system and forms the small droplet emulsion of oil and water with diameters of approximately 50 to 1000 nm10. Nanoemulsion can improve the bioactivity and solubility of hydrophobic-based particles with low side effects by reducing the effective dose.
Pelargonium graveolens is an aromatic medicinal plant belonging to the Geraniaceae family, which originated in South Africa and is distributed worldwide. In folk medicine, P. graveolensand its bioactive constituents have been reported to have several biological and therapeutic effects, such as antioxidant11,12,13, antifungal11, antimicrobial11, antiviral11, antidiabetic11, anxiolytic, antidepressant12, and anti-inflammatory13. Several reports indicate the anti-nociceptive and anti-inflammatory properties of geraniol14,15,16, linalool17,18, and citronellol19,20, the main components of Pelargonium graveolens essential oil (PGEO).
Despite the beneficial effects of P. graveolens in animal models and traditional medicine, the poor water solubility of its essential oil remains a major challenge in optimizing the bio-efficacy of its constituents. Therefore, this study aimed to formulate Pelargonium graveolens essential oil nanoemulsion (PGEO-NE) and evaluate its anti-nociceptive and anti-inflammatory activities using experimental nociception and inflammation models in mice. Additionally, the role of the opioid pathway in the mechanism of action of PGEO-NE was investigated.
Results
Chemical analysis of essential oil
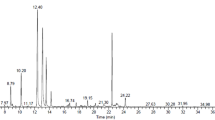
The chemical composition of PGEO was analyzed using Gas chromatography-mass spectrometry (GC-MS), with its chromatogram shown in Fig. 1. GC-MS analysis identified 31 compounds in the PGEO profile, listed in Table 1, in order of their retention indices. As presented in Table 1, the major constituents of PGEO were citronellol (34.9%), geraniol (16.1%), citronellyl formate (12.3%), and linalool (6.8%). Additionally, neryl formate (4.6%), 1-epi-γ-eudesmol (4%), menthone (3.9%), and iso-menthone (3%) were detected at significant levels. The PGEO is rich in oxygenated monoterpenes (90.1%), a characteristic of the Pelargoniumspecies21,22,23which contribute to its potent biological activities, particularly antimicrobial, anti-inflammatory, and anti-nociceptive properties14,15,16,17,18,19,20. The high concentrations of citronellol, citronellyl formate, geraniol, linalool, menthone, and iso-menthone compounds known for their potent anti-inflammatory effects-suggest that PGEO has significant potential for use in natural anti-inflammatory formulations23.
Physicochemical properties of PGEO-NE
Fourier transform infrared (FTIR) spectra of PGEO and PGEO-NE are shown in Fig. 2a. In the FTIR spectrum of PGEO, the broad absorption band at 3375 cm−1 is related to the O‒H stretching vibration of the alcohol. The peaks at 2964 and 2926 cm−1 are attributed to aliphatic C‒H stretching, and the peak at 2850 cm−1 is associated with aldehyde C‒H (O = C‒H) stretching. Besides, the peaks at 1730 cm−1 and 1672 cm−1 are related to C = O stretching in carbonyl compounds and C = C stretching frequency, respectively. In addition, the absorption bands observed at 1058, 1047 and 1008 cm−1 are assigned to C‒O or C‒O‒C stretching vibrations. The recorded peaks at 1454 cm−1 and 1378 cm−1 are due to CH3 and CH2 bending vibrations. Weak absorption peaks in the range of 400–1000 cm−1are related to C-H out-of-plane bending vibrations. Also, similar absorption bands appeared in FTIR spectrum of PGEO-NE, with a slight shift in the frequency, indicating the interaction of PGEO compounds with the emulsifier Tween 80 in the formulated nanoemulsion21,22,23.
Scanning electron microscopy (SEM) was used to determine the morphology of the nanoemulsion droplets. in the SEM image (Fig. 2b), the droplets are spherical, either free or clustered. In addition, dynamic light scattering (DLS) technique revealed a particle size distribution of 553 nm (Fig. 2c), a polydispersity index (PDI) of 0.11, and a zeta potential of + 28 mV for PGEO-NE. The DLS and PDI results indicate that the formulation is nano-sized and the particles are monodispersed10.
Nociception
Formalin-induced paw-licking test
Compared to the vehicle-treated group, both doses of PGEO-NE reduced the licking time in both phases of formalin-induced nociception 1 (P < 0.001) and 2 (P < 0.01). This effect was comparable to that of the standard antinociceptive drugs; morphine and diclofenac sodium (P < 0.001). PGEO (10 mg/kg), an equivalent dose to the PGEO-NE (100 mg/kg), did not significantly diminish licking time in both phases of nociception compared to the vehicle-treated group. The anti-nociceptive effects of morphine were abolished by naloxone in both phases 1 and 2 (P < 0.05) (Fig. 3). However, naloxone could not prevent the anti-nociceptive activity of PGEO-NE in phases 1 and 2 (Fig. 3).
Effects of PGEO-NE on formalin-induced nociception behavior in mice. The graph shows the duration of biting/licking after the formalin injection in phase-1 (neurogenic, 0–10 min) and phase-2 (inflammatory, 11–45 min) and the involvement of opioid receptor antagonist, Naloxone, on PGEO-induced anti-nociception in the formalin-induced nociception. DIC: diclofenac sodium, PGEO-NE: Pelargonium graveolens essential oil nanoemulsion, NLX: Naloxone. The data are presented as means ± SEM. **P < 0.01, ***P < 0.001 versus the vehicle-treated group, ## P < 0.01, ### P < 0.001 versus the naloxone group, and §§§ P < 0.001 versus the morphine group in ANOVA test.
Acetic acid-induced writhing test
PGEO-NE (50 and 100 mg/kg) significantly inhibited the number of writings induced by acetic acid (P < 0.001) as well as standard drugs (Fig. 4), but PGEO (10 mg/kg) had no significant effect. However, compared to vehicle, the co-administration of naloxone and PGEO-NE exerts anti-nociceptive effects (P < 0.01). However, compared with the PGEO-NE (100 mg/kg) group, pretreatment of naloxone was able to abolish the anti-nociceptive effects of PGEO-NE in a significant manner (P < 0.01) (Fig. 4).
Effects of PGEO-NE on acetic acid induced nociception in mice. The graph shows total number of abdominal writhings in 30 min after acetic acid injection and the involvement of opioid receptor antagonist, Naloxone, in the acetic acid test. DIC: diclofenac sodium, PGEO-NE: Pelargonium graveolens essential oil nanoemulsion, NLX; Naloxone. The data are presented as means ± SEM. *P < 0.05, **P < 0.01, *** P < 0.001 versus the vehicle-treated group, ## P < 0.01 versus the PGEO-NE (100 mg/kg) group, and §§§ P < 0.001 versus the morphine group, in ANOVA test.
Hot plate test
PGEO-NE significantly increased the latency of the physiological nociception threshold in response to a hot stimulus compared to the vehicle (P < 0.001) and morphine (Fig. 5). PGEO (10 mg/kg) did not exhibit a significant anti-nociceptive effect. Notably, the anti-nociceptive effect of PGEO-NE was comparable to that of morphine and was reversed by naloxone (P < 0.001) (Fig. 5).
Effects of PGEO-NE on hot plate induced nociception in mice. The graph shows the latency time to lick the paw or jump on 55 °C Hot Plate and the involvement of opioid receptor antagonist, naloxone, in the hot plate test. DIC: diclofenac sodium, PGEO-NE: Pelargonium graveolens essential oil nanoemulsion, NLX; Naloxone. The data are presented as means ± SEM. *P < 0.05, **P < 0.01, *** P < 0.001 versus the vehicle-treated group, ## P < 0.01 versus the PGEO-NE (100 mg/kg) group, and §§§ P < 0.001 versus the morphine group, in ANOVA test.
Inflammation
Formalin-induced paw edema
PGEO-NE at the higher dose (100 mg/kg) decreased the paw edema starting 1 h after formalin injection and continuing until 24 h compared to vehicle (P < 0.05). The inhibitory effect of PGEO-NE 50 mg/kg on the induction of paw edema started 4 h after formalin injection and continued until 24 h (P < 0.05) (Table 2). PGEO (10 mg/kg) had no anti-inflammatory effect against formalin paw injection (Table 2).
Carrageenan-induced paw edema test
Doses of PGEO-NE 50 and 100 mg/kg exhibited significant inhibition of paw edema from 1 h to 5 h after carrageenan injection (p < 0.05) compared to vehicle (Table 3), but PGEO (10 mg/kg) had no significant anti-inflammatory effect.
Discussion
In the present study, we have shown for the first time that PGEO-NE exerts significant central and peripheral anti-nociceptive effects in several models of nociception. Furthermore, PGEO-NE exhibited potent anti-inflammatory activity in the early and second phases of chemically induced inflammation. These results clearly showed that PGEO-NE was more effective than pure PGEO at the same dose as PGEO-NE against nociception and inflammation in animal models.
The EOs of medicinal plants are a mixture of natural volatile molecules with various biological activities. The nanoencapsulation technique is recommended to improve the solubility of EOs in the aqueous environment and to increase their stability10. So, this study investigated the possible anti-nociceptive effect of PGEO-NE on central and peripheral pain pathways in three different nociception models, including the formalin-induced biphasic pain test, the acetic acid-induced abdominal writhing test, and the hot plate test.
The formalin test assesses two types of pain: neurogenic pain (the first phase) indicates centrally mediated pain, and inflammatory pain (the second phase) reflects peripherally mediated pain24. Neurogenic pain arises from the direct action of formalin on pain receptors and inflammatory pain induced by the release of inflammatory mediators, such as histamine, prostaglandins, and bradykinins25,26. The results revealed that PGEO-NE significantly reduced the duration of licking behavior during both phase 1 and 2 of the formalin assessment. Moreover, PGEO-NE demonstrated superior inhibition of acute pain in both phases compared to PGEO at the same dose.
To further explain the antinociceptive effects of PGEO-NE, an acetic acid test was performed.
Acetic Acid directly and indirectly stimulates the central and peripheral nociceptors, causing the release of pain mediators27,28and endogenous pro-inflammatory mediators, which collectively stimulate nociceptors29. In this study, PGEO-NE decreased abdominal writhing, while the same dose of PGEO had no significant effect. Besides, the hot plate model evokes the central nociceptors, and substances that increase the latency time to jump or lick in this test can reduce pain via a supraspinal mechanism30. PGEO-NE significantly reduced the latency time in the hot plate test, whereas PGEO, at the same dose as the PGEO-NE, did not produce significant changes compared to the vehicle. Therefore, these results suggest that PGEO-NE may reduce the release of pain mediators and nociceptor activation.
To our knowledge, no studies have investigated the anti-nociceptive impact of PGEO-NE in the nociceptive models used in this research. However, some studies have demonstrated the antinociceptive activity of the bioactive constituents of PGEO. In this context, geraniol14, linalool17,18, and citronellol20, the main constituents of PGEO, exerted significant antinociceptive activity in these animal models of nociception. Geraniol dose-dependently inhibited the paw-licking time of the formalin test only in the second phase14. Pretreatment with citronellol resulted in anti-nociceptive effects in the first and second phases of the formalin-induced nociceptive behavior19. Linalool decreased the licking time in both phases of nociception induced by paw injection of formalin17,18. Linalool has been shown to reduce writhing and licking time in acetic acid and hot plate tests31. Additionally, citronellol20and linalool18 prolonged the latency of response when mice were exposed to a hot plate.
It is generally accepted that centrally acting antinociceptive agents, including opioids, inhibit both the non-inflammatory (early) and inflammatory (late) phases of nociception32. Opioid receptors are found on primary afferent neurons33,34and reduce pain by lowering nociceptor excitability and reducing pain mediators like substance P33. In the present study, naloxone (a non-selective opioid receptor antagonist) effectively inhibited the anti-nociceptive effects of morphine.
Pretreatment with naloxone reversed the anti-nociceptive effects of PGEO-NE in the hot plate and acetic acid tests, but not in the formalin test. This shows that the antinociceptive action of PGEO-NE is mainly related to the central opioid system, with some involvement of peripheral opioid receptors. The central antinociceptive effect of citronellol20and linalool17was blocked by naloxone in the hot plate and formalin tests, respectively. In the acetic acid test, the inhibitory effect of linalool was reversed by both naloxone (opioid receptor antagonist) and atropine (muscarinic receptor antagonist)31. Additionally, linalool odor exerted anti-nociceptive activity through the TRPV1 pathway18. However, the antinociceptive effect of geraniol was not altered by naloxone pretreatment in the acetic acid test17. It can be suggested that the linalool and citronellol in PGEO-NE bind to the opioid receptors on the peripheral terminals of primary afferent (sensory) neurons, contributing to the anti-nociceptive effect. In addition, the result of the formalin test suggests that PGEO-NE may induce anti-nociception by a mechanism other than the opioid system.
Some studies suggest that several neurotransmitters play a vital role in processing pain behaviors during the first and second phases of the formalin-induced nociceptive response35. The administration of 5-HT36and serotonin reuptake inhibitors (SSRIs)37 reduced pain behaviors in the formalin model. The EO of Pelargonium roseumhas been shown to exert anxiolytic effects via the serotoninergic system in the elevated plus maze test12. Moreover, the glutamatergic38and gabaergic39systems are also involved in formalin-induced nociception40. The antagonists of glutamatergic receptors attenuated the formalin-induced nociceptive-like behavior38. Furthermore, gamma-aminobutyric acid (GABA) receptor agonists exert antinociceptive effects via central and peripheral pathways in the formalin test39,41.
Previous studies have reported that PGEO has potent anti-inflammatory activity in experimental models13,42. PGEO attenuated the edema induced by carrageenan and croton oil42and suppressed the inflammatory cell infiltration in the colon of rats with acetic acid-induced colitis13. In addition, several data from the literature describe the anti-inflammatory properties of the main components of PGEO15,16,19,20,42. PGEO and PGEO-NE inhibited the expression level of inflammatory mediators like cytokines in macrophage cells against Candida albicans23.
PGEO-NE reduced the paw edema formalin over 24 and 5 h after local injection of formalin and carrageenan, respectively. But PGEO at the same nanoemulsion dose had no anti-inflammatory effect. The anti-inflammatory effect of PGEO-NE on the paw edema induced by formalin corroborates the peripheral anti-nociceptive effect of PGEO-NE. The paw edema response to carrageenan is biphasic; the first phase (0–2 h) is partly related to the injection-induced tissue damage response, including neurotransmitter (serotonin and histamine) release. The late phase (2–5 h) is associated with prostaglandin and cytokines activation43. Thus, these results indicate the possible involvement of central or peripheral pathways in the anti-inflammatory effect of PGEO-NE.
The present study indicated that the inhibitory effect of PGEO-NE on the paw edema induced by formalin and carrageenan corroborates the central and peripheral anti-nociceptive effects. In this line, citronellol, the main component of Pelargonium graveolens, downregulated the inflammatory markers such as IL-6, and TNFα, while upregulated the anti-inflammatory marker, IL-1019. Citronellol also abolished the leukocyte migration and high levels of TNFα induced by carrageenan in the peritoneal cavity20. Treatment with geraniol diminished the release of inflammatory markers such as IL-6, IL-1β, and TNFα15,16. Linalool reduced the inflammatory cell infiltration and secretion of inflammatory markers including Cytokines44,45, Cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS)45.
Conclusion
It can be concluded that Pelargonium graveolens essential oil nanoemulsion is an effective antinociceptive and anti-inflammatory formulation in experimental models of pain and inflammation. In addition, our results suggest that the specific components of PGEO, citronellol, geraniol and linalool, as well as the synergistic interaction between the different components, may be responsible for the beneficial effects on pain and inflammation. It is also worth mentioning that the anti-nociceptive action of PGEO-NE interacts with the opioidergic pathway. Further investigations are needed to comprehend the precise mechanisms of action of PGEO-NE in nociception and inflammation.
Materials and methods
Animals
256 adult male NMRI mice weighing 25–35 g were acquired from the Pasteur Institute of Iran, Amol. Animals were maintained at a regulated temperature (22 ± 2 °C), humidity (50–55%), a 12-hour light/dark cycle, and ad libitumaccess to water and food. All experiments were conducted with the ethical approval of the Animal Ethics Committee of Amol University of Special Modern Technologies (authorization code IR.ASMT.REC.1403.003) to reduce the number of animals utilized and minimize their distress. All procedures were performed according to the relevant guidelines and regulations, and in accordance with the ARRIVE (Animal Research: Reporting of in Vivo Experiments) guidelines for the care and use of laboratory animals. Mice were randomly assigned into groups (8 in each group). Finally, animals were euthanized by i.p. ketamine/xylazine combination (10:1, doses of 100 mg/kg ketamine and 10 mg/kg xylazine). The calculation of the animal sample size was performed according to Arifin and Zahiruddin46. Following the acceptable range for the degrees of freedom (DF), with a minimum of 10 and a maximum of 20, the minimum and maximum quantities of animals per group are defined as minimum n = 10/k + 1 and maximum n = 20/k + 1, where k and n represent the number of groups and the number of subjects allocated to each group, respectively. In this study, the effects of PGEO-NE were statistically analyzed in two steps in different models of antinociception and inflammation induction, as follows:
-
1.
Evaluation of the antinociceptive and anti-inflammatory effects of PGEO-NE (by comparing the results of the groups: vehicle, two standard drugs, PGEO (10 mg/kg), PGEO-NE (50 mg/kg), and PGEO-NE (100 mg/kg).
-
2.
Evaluation of the role of the opioid pathway in the mechanism of action of PGEO-NE (by comparing the results of the groups: vehicle, Morphine, PGEO-NE (100 mg/kg), NLX, NLX + Morphine, NLX + PGEO-NE (100 mg/kg).
The number of groups in each test of each step, except for the hot plate test, was 6, and in the hot plate test, it was 5.
Additionally, with an anticipated attrition rate of 10% (the possibility of death), the adjusted range of the sample size per group was determined to be 4 to 6. Furthermore, it is essential to acknowledge that the biological variability present in the animal subjects utilized in experimental investigations, particularly in behavioral assessments, arises from genotypic and phenotypic variations, as well as environmental factors. Therefore, to gather sufficient experimental data and ensure the robustness of the results, consistent with preceding studies47, the sample size for each group was established to be 8. Mice were randomly assigned to groups. Finally, animals were euthanized by i.p. ketamine/xylazine combination (10:1, doses of 100 mg/kg ketamine and 10 mg/kg xylazine).
Drugs and chemicals
The chemicals used in the study were acquired from various suppliers: PGEO (Barij Essence Pharmaceutical Company); Tween 80 (Sigma-Aldrich, product number: 655207), diclofenac sodium (DIC, Exir Pharmaceutical Company, Iran); morphine (Daroupakhsh Pharmaceutical Company, Iran); dexamethasone (DEX, Zahravi Pharmaceutical Company, Iran), naloxone (NLX, Caspian Tamin Pharmaceutical Company, Iran); formaldehyde solution (Sigma-Aldrich, product no. 252549); acetic acid (Sigma-Aldrich, product number: 695092), carrageenan formaldehyde solution (Sigma-Aldrich, product code: 22048).
PGEO-NE and all standard drugs (morphine, DEX, and DIC) were diluted with normal saline as a vehicle and administered intraperitoneally (i.p.). The control group received the vehicle. Naloxone was used as an opioid receptor antagonist. The selected concentrations of PGEO-NE (50 and 100 mg/kg) were based on the EO used in the previous study42. As the nanoemulsion was prepared at a concentration of 10%, 10 mg/kg of PGEO, the equivalent dose of PGEO-NE 100 mg/kg, was also tested in all models. Since dose 10 mg/kg of PGEO did not show anti-nociceptive and anti-inflammatory effects in this study, dose 5 mg/kg of PGEO, the equivalent dose of PGEO-NE 50 mg/kg, was not tested based on the recommendation of the Animal Ethics Committee to reduce the number of animals used.
The control groups received Tween 80 (10%) as vehicle42. Carrageenan and formalin were injected into the right hind paw of the mice and acetic acid was administered i.p.
GC-MS analysis and identification of EO components
The analysis of EO was performed on an Agilent 7890 A gas chromatograph connected to an Agilent 5975 C quadrupole mass spectrometer (Agilent Technologies, USA) equipped with a DB-5 column (length 30 m, internal diameter 0.25 mm and stationary phase thickness 0.25 μm). The column temperature program included an initial temperature increase from 60 °C to 220 °C at a rate of 3 °C/min, followed by an increase to 260 °C at a rate of 20 °C/min, and a final hold at 260 °C for 3 min. The injector and transfer line temperatures were set at 260 °C and 280 °C, respectively. Helium was used as the carrier gas at a flow rate of 30.6 cm/s. The mass spectrometer operated in electron ionization mode at 70 eV, with a scan range of m/z 30–400 at 1 s scan48,49,50.
The EO was diluted 1:10 with dichloromethane and 1 µL of the diluted sample was injected into the GC-MS. The components of the EO were characterized by comparing their mass spectra with those stored in the GC-MS library (Adams, 2017)40or with authentic compounds, and then confirmed by comparing their retention indices (RI) with published data in the literature and by the co-injection of authentic standards available in our laboratory. The relative amount of each component in EO was determined based on the peak area without an internal standard or considering response factor48,49.
Formulation of P. graveolens essential oil nanoemulsion (PGEO-NE)
The process for formulating PGEO-NE was executed according to the method documented by Hein et al., (2021)51. In summary, a mixture comprising 10 g of PGEO and 20 g of Tween 80 as surfactant was stirred at 800 rpm for 15 min. Subsequently, the mixture of oil and surfactant was slowly added dropwise to a volume of 70 mL of distilled water. Following the titration, the system was stirred continuously on a magnetic stirrer for 30 min. The resultant sample was then subjected to fixation at −15 °C for 24 h, followed by complete thawing at ambient temperature. Finally, PGEO-NE was stored at room temperature and prepared for evaluation of anti-nociceptive effects.
Characterization of the PGEO-NE
The morphology of the prepared PGEO-NE was determined by scanning electron microscopy (SEM, TESCAN model Vega3, Netherlands). Dynamic light scattering (DLS) was used to measure the droplet size distribution, zeta potential, and polydispersity index (PDI) of the nanoemulsion using a nanoparticle size analyzer (Microtrac Nanotrac Wave II, USA). To avoid multiple scattering effects, the PGEO-NE was diluted to 1:20 with deionized (DI) water before evaluation.
Nociception tests
Formalin-induced paw-licking test
Mice were habituated to the laboratory conditions in the observation chamber for 30 min. Then, 20 µl of 2.5% formalin solution was injected subcutaneously into the plantar surface of the left paw of the mice24. After the formalin injection, the animals were randomly divided into 6 groups (n= 8 in each): vehicle, PGEO-NE (50 and 100 mg/kg), PGEO (10 mg/kg), morphine (10 mg/kg)47, and diclofenac sodium (20 mg/kg)47. The vehicle, PGEO-NE, and morphine were injected 15 min, and diclofenac sodium was administered 30 min before the formalin injection. The mice were then individually placed in an observation chamber, and the time that the animals spent biting/licking the injected paw was recorded and considered as an indication of nociception for both the early neurogenic phase (0–5 min) and the late inflammatory phase (10–45 min) of this model24,47.
Acetic acid-induced writhing test
This test was performed in 6 groups (n= 8 in each) which received vehicle, PGEO-NE (50 and 100 mg/kg), PGEO (10 mg/kg) or morphine (1 mg/kg) 15 min and diclofenac sodium (10 mg/kg) 30 min before 1% acetic acid injection (10 ml/kg). Each mouse was then individually placed in an observation chamber and the number of writhes was counted every 5 min for 30 min after acetic acid administration47.
Hot plate test
In the hot plate test, mice were randomly divided into 5 groups (n= 8 in each), including PGEO-NE (50 and 100 mg/kg), PGEO (10 mg/kg), morphine (10 mg/kg)52, and vehicle (0.9% saline). The hot plate temperature was set at 55 °C. The latency to lick a hind paw or jump out of the apparatus was recorded 30 min after administration of the test drugs or vehicles.
To verify the possible involvement of the opioid system, we evaluated the effects of naloxone, a non-selective opioid receptor antagonist, on the effects of PGEO-NE in all nociception tests. This was performed in three groups (n = 8 in each); NLX, NLX + morphine, and NLX + PGEO-NE. Naloxone (i.p.) at a dose of 2 mg/kg was employed 15 min before the administration of morphine or PGEO-NE (100 mg/kg).
Inflammation test
Formalin-induced paw edema test
The peripheral anti-inflammatory property of PGEO-NE was assessed by measuring the formalin-induced paw edema following the mentioned method24. Paw edema was induced by subcutaneous injection formalin into the right hind paw, resulting in edema at the injection site. The vehicle, PGEO-NE, and morphine were injected 15 min, and diclofenac sodium was administered 30 min before the formalin injection. The thickness of the paw was measured using a plethysmometer to assess the extent of edema before 1, 2, 3, 4, and 24 h after the formalin injection. The inhibition of edema was determined according to the following equation.
v1: The paw thickness before formalin injection, v2: The paw thickness after formalin injection.
Carrageenan-induced paw edema test
Animals were divided into six groups (n= 8 in each). They were pretreated with vehicle, PGEO-NE (50, 100 mg/kg), PGEO (10 mg/kg), diclofenac (10 mg/kg)53, or dexamethasone (5 mg/kg)3130 min before carrageenan (3.0% ƛ-carrageenan) injection at a volume of 40 µL/animal. As previously outlined, the volume of the mice’s paws up to the ankle joint was also measured at time points 0, 1, 2, 3, 4, and 5 h after carrageenan administration. The degree of inhibition of paw edema was calculated using the following formula: edema = (volume of the foot at the time of measurement - volume of the foot at the initial time)/volume of the foot at the initial time47.
Statistical analysis
The data obtained were analyzed using SPSS for Windows software version 22.0 and expressed as the mean ± standard error of the mean (SEM). Statistical analysis was done using one-way analysis of variance (ANOVA) followed by Tukey’s test. In all experiments, P< 0.05 was considered statistically significant. All graphs were generated using Graph Pad Prism version 10 for Windows.
Data availability
All the data generated or analyzed during this study are included in this published article.
Abbreviations
- TNF-α:
-
Tumor necrosis factor-α
- IL:
-
Interleukin
- EOs:
-
Essential oils
- PGEO-NE:
-
Pelargonium graveolens essential oil nanoemulsion
- PGEO:
-
Pelargonium graveolens essential oil
- GC-MS:
-
Gas chromatography-mass spectrometry
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning electron microscopy
- DLS:
-
Dynamic light scattering
- PDI:
-
Polydispersity index
- DIC:
-
Diclofenac sodium
- NLX:
-
Naloxone
- DEX:
-
Dexamethasone
- RI:
-
Retention indices
- COX-2:
-
Cyclooxygenase-2
- iNOS:
-
Inducible nitric oxide synthase
- IFN-γ:
-
Interferon-gamma
- GABA:
-
Gamma-aminobutyric acid
- SSRIs:
-
Serotonin reuptake inhibitors
References
Kapos, F. P. et al. Social determinants and consequences of pain: toward multilevel, intersectional, and life course perspectives. J. Pain 25, 104608 (2024).
Varela, M. L., Mogildea, M., Moreno, I. & Lopes, A. Acute inflammation and metabolism. Inflammation 41 (4), 1115–1127 (2018).
Wang, S. et al. Phospholipase C and protein kinase A mediate Bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 131, 1241–1251 (2008).
Cunha, T. M. et al. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. U. S. A. 102 (5), 1755–1760 (2005).
Pinho-Ribeiro, F. A., Verri, W. A. & Chiu, I. M. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 38 (1), 5–19 (2017).
Quintans, J. S. S., Antoniolli, A. R., Almeida, J. R. G. S., Santana-Filho, V. J. & Quintans-Júnior, L. J. Natural products evaluated in neuropathic pain models—A systematic review. Basic Clinpharmacol. Toxicol. 114 (6), 442–450 (2014).
Nisar, A. et al. Phytochemicals in the treatment of inflammation-associated diseases: the journey from preclinical trials to clinical practice. Front. Pharmacol. 14, 1177050 (2023).
Zhao, Q., Zhu, L., Wang, S., Gao, Y. & Jin, F. Molecular mechanism of the anti-inflammatory effects of plant essential oils: A systematic review. J. Ethnopharmacol. 301, 115829 (2022).
9. Sarmento-Neto, J. F., do Nascimento, L. G., Felipe, C. F. & de Sousa, D. P. Analgesic potential of essential oils. Molecules 21, E20 (2015).
Kumar, A., Kanwar, R. & Mehta, S. K. Nanoemulsion as an effective delivery vehicle for essential oils: properties, formulation methods, destabilizing mechanisms and applications in agri-food sector. Next Nanatechnol. 7, 100096 (2025).
Hamidpour, R., Hamidpour, S., Hamidpour, M., Marshall, V. & Hamidpour, R. Pelargonium graveolens (Rose Geranium)-A novel therapeutic agent for antibacterial, antioxidant, antifungal and diabetics. Arch. Can. Res. 5, 1–5 (2017).
Abouhosseini Tabari, M., Hajizadeh Moghaddam, A., Maggi, F. & Benelli, G. Anxiolytic and antidepressant activities of Pelargonium roseum essential oil on Swiss albino mice: possible involvement of serotonergic transmission. Phytother. Res. 32, 1014–1022 (2018).
Bastani, M., Mousavi, Z., Asgarpanah, J. & Assar, N. Biochemical and histopathological evidence for beneficial effects of Pelargonium graveolens essential oil on the rat model of inflammatory bowel disease. Res. J. Pharmacogn. 6, 77–84 (2019).
La Rocca, V. et al. Geraniol induces antinociceptive effect in mice evaluated in behavioural and electrophysiological models. Basic Clin. Pharmacol. Toxicol. 120, 22–29 (2017).
Younis, N. S., Abduldaium, M. S. & Mohamed, M. E. Protective effect of geraniol on oxidative, inflammatory and apoptotic alterations in isoproterenol-induced cardiotoxicity: role of the Keap1/Nrf2/HO-1 and PI3K/Akt/mTOR pathways. Antioxidants 9, 977 (2020).
Ye, C. J., Li, S. A., Zhang, Y. & Lee, W. H. Geraniol targets KV1.3 ion channel and exhibits anti-inflammatory activity in vitro and in vivo. Fitoterapia 139, 104394 (2019).
Peana, A. T. et al. Profile of spinal and supra-spinal antinociception of (-)-linalool. Eur. J. Pharmacol. 485, 165–174 (2004).
Kashiwadani, H., Higa, Y., Sugimura, M. & Kuwaki, T. Linalool odor-induced analgesia is triggered by TRPA1-independent pathway in mice. Behav. Brain Funct. 17, 3 (2021).
Jayaganesh, R., Pugalendhi, P. & Murali, R. Effect of citronellol on NF-kB inflammatory signaling molecules in chemical carcinogen-induced mammary cancer in the rat model. J. Biochem. Mol. Toxicol. 34, e22441 (2020).
Brito, R. G. et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J. Nat. Med. 66, 637–644 (2012).
Kujur, A., Kumar, A., Yadav, A. & Prakash, B. Antifungal and aflatoxin B1 inhibitory efficacy of nanoencapsulated Pelargonium graveolens L. essential oil and its mode of action. LWT Food Sci. Technol. 130, 109619 (2020).
Santos, F. N. D. et al. Antimicrobial activity of geranium (Pelargonium graveolens) essential oil and its encapsulation in carioca bean starch ultrafine fibers by electrospinning. Int. J. Biol. Macromol. 265, 130953 (2024).
Giongo, J. L. et al. Anti-inflammatory effect of geranium nanoemulsion macrophages induced with soluble protein of Candida albicans. Microb. Pathog. 110, 694–702 (2017).
Hirota, I., Koyama, Y. & Shimada, S. Histochemical analysis of the biphasic properties of formalin pain-induced behavior. Biochem. Biophys. Rep. 34, 101467 (2023).
Spindola, H. M. et al. Derivatives of furanditerpenes from pterodon genus: Pharmacological studies disclose their potential as chronic pain relief in mice. Eur. J. Pharmacol. 804, 68–77 (2017).
Hunskaar, S. & Hole, K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30, 103–114 (1987).
Gurdap, C. O., Markwalter, P. S. Jr., Neddenriep, B., Bagdas, D. & Damaj, M. I. Neuropathic insult increases the responsiveness to acetic acid in mice. Behav. Pharmacol. 30, 534–537 (2019).
Petho, G. & Reeh, P. W. Sensory and signaling mechanisms of Bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol. Rev. 92, 1699–1775 (2012).
Shahraki, F. N. et al. Licofelone attenuates acetic acid-induced colitis in rats through suppression of the inflammatory mediators. Inflammation 46, 1709–1724 (2023).
Verma, S., Mundkinajeddu, D., Agarwal, A., Chatterjee, S. S. & Kumar, V. Effects of turmeric curcuminoids and metformin against central sensitivity to pain in mice. J. Trad Complement. Med. 7, 145–151 (2016).
Peana, A. T. et al. P. (-)-Linalool produces antinociception in two experimental models of pain. Europ. J. Pharmacol. 460, 37–41 (2003).
Zhao, C. S. et al. Role of micro-opioid receptors in formalin-induced pain behavior in mice. Exp. Neurol. 184, 839–845 (2003).
Reeves, K. C., Shah, N., Muñoz, B. & Atwood, B. K. Opioid receptor-mediated regulation of neurotransmission in the brain. Front. Mol. Neurosci. 15, 919773 (2022).
Llorca-Torralba, M., Pilar-Cuéllar, F., da Silva Borges, G., Mico, J. A. & Berrocoso, E. Opioid receptors mRNAs expression and opioids agonist-dependent G-protein activation in the rat brain following neuropathy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 99, 109857 (2020).
Cao, B. et al. Pathology of pain and its implications for therapeutic interventions. Signal. Transduct. Target. Ther. 9 (1), 155 (2024).
Zhang, L. et al. Analgesic effects of duloxetine on formalin-induced hyperalgesia and its underlying mechanisms in the CeA. Front. Pharmacol. 9, 317 (2018).
Zhang, T. T. et al. Evaluation of the analgesic effects of Ammoxetine, a novel potent serotonin and norepinephrine reuptake inhibitor. Acta Pharmacol. Sin. 37, 1154–1165 (2016).
Seo, Y. J. et al. Changes in pain behavior induced by formalin, substance P, glutamate and pro-inflammatory cytokines in immobilization-induced stress mouse model. Brain Res. Bull. 71, 279–286 (2006).
Hassanpour, S., Rezaei, H. & Razavi, S. M. Anti-nociceptive and antioxidant activity of betaine on formalin- and writhing tests induced pain in mice. Behav. Brain Res. 390, 112699 (2020).
Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry 5th edn. (Texensis Publishing, 2017).
Chen, J., Hasanein, P., Komaki, A. & Yari, S. Effects of GABAA receptors in nucleus cuneiformis on the cannabinoid antinociception using the formalin test. Psychopharmacology 238, 1657–1669 (2021).
Boukhatem, M. N., Kameli, A., Ferhat, M. A., Saidi, F. & Mekarnia, M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med. 8, 22520 (2013).
Patil, K. R. et al. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 20, 4367 (2019).
Ma, J. et al. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 29 (2), 708–713 (2015).
Nawaz, S. et al. Monoterpene alcohol effectiveness in chronic synovitis through Lowering Interleukin-17, spleen and thymus indices. Int. Immunopharmacol. 121, 110517 (2023).
Arifin, W. N. & Zahiruddin, W. M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 24, 101–105 (2017).
Yin, Z. Y. et al. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 6, 27129 (2016).
Solouki, A., Mehrjerdi, M. Z., Aliniaeifard, S. & Azimi, R. Postharvest light and temperature elicitors improve chemical composition and level of essential oils in Basil (Ocimum basilicum L.) through boosting antioxidant machinery. Postharvest Biol. Technol. 199, 112279.24 (2023).
Jafari Ghoshchi, M., Abbaszadeh, B., Oraei, M., Azimi, R. & Faramarzi, A. Effects of different drying methods on phytochemical quality and microbial load of Satureja spicigera. J. Essent. Oil Bear. Plants 27, 1347–1361 (2024).
Mahdi Navehsi, F., Abdossi, V., Abbaszadeh, B., Azimi, R. & Dianat, M. Effect of gamma rays on the essential oil and biochemical characteristics of the Satureja mutica fisch & C. A. Mey. Sci. Rep. 14, 7581 (2024).
Hien, L. T. M. & Dao, D. T. A. Black pepper essential oil nanoemulsions formulation using EPI and PIT methods. J. Food Process. Preserv. 45, e15216 (2021).
Yimer, T., Birru, E. M., Adugna, M., Geta, M. & Emiru, Y. K. Evaluation of analgesic and anti-Inflammatory activities of 80% methanol root extract of Echinops kebericho M. (Asteraceae). J. Inflamm. Res. 13, 647–658 (2020).
Shajib, M. S. et al. Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) exert antinociceptive and neuropharmacological effects in mice. Front. Pharmacol. 9, 85 (2018).
Owusu Obeng, A., Hamadeh, I. & Smith, M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy 37, 1105–1121 (2017).
Funding
This study was supported by grant 14/20/20237 from the Iranian Ministry of Science by Amol University of Special Modern Technologies, Iran.
Author information
Authors and Affiliations
Contributions
Study design: HG and MAT; Preparing the nanoemulsion: MAT; Analyzing and characterizing the nanoemulsion structure: RA; Performing the animal experiments: MAM and RSH; Data acquisition and analysis, drafting of figures and manuscript: HG and RA; Interpretation of results: HG and RA. Editing and finalizing manuscript: HG and RA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gavzan, H., Azimi, R., Mashayekhpour, M.A. et al. The anti-nociceptive and anti-inflammatory effects of Pelargonium graveolens essential oil nanoemulsion in mice. Sci Rep 15, 15754 (2025). https://doi.org/10.1038/s41598-025-00722-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00722-y