Abstract
Alzheimer’s disease (AD) diagnosis relies on cerebrospinal fluid (CSF) biomarkers or amyloid PET. Alternatives for AD diagnosis from blood samples are needed to develop a fully-automated early-diagnosis approach, potentially implemented in a cognitive disorder unit. Plasma p-Tau217 was determined in patients diagnosed with AD (n = 134) or non-AD (n = 132), from CSF biomarkers (Aβ42/Aβ40). A logistic regression model was developed. The predictive performance was assessed using a training set (70% of data) and internally validated with a test set (30% of data) and 1000 iterations. A nomogram and a double cut-off strategy were proposed to visualize the model results, and stratify patients (AD, non-AD, uncertain), respectively. The model (plasma p-Tau217, ApoE, age) showed satisfactory performance (AUC 0.94, sensitivity 0.85, specificity 0.89); so, together with the corresponding nomogram, it could be applied in specialized clinical contexts. The model including only plasma p-Tau217 (AUC 0.93, sensitivity 0.72, specificity 0.96) would be a useful approach in less specialized clinics. The corresponding two-cut-off strategy for the first model were as AD probability (< 0.41 non-AD, > 0.57 AD). This study provided clinical tools (nomogram, double cut-off) for identifying Aβ positivity at a cognitive disorder unit, which would lead to reduce the CSF analysis.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) has a high incidence in the population, especially in those over 65 years of age1. Currently, specific CSF biomarkers [amyloid β42 (Aβ42), Aβ42/Aβ40, phosphorylated Tau181 (pTau181)] and PET imaging (amyloid, tau, FDG) are commonly used for studying AD pathology2. However, CSF sampling is an invasive procedure that presents some contraindications and side effects, and it must be performed by specialists. At the same time, PET generates a high expenditure of resources (in terms of time and equipment maintenance), which are not always available3,4. This limits access to testing and, consequently to early diagnosis of AD, which is crucial for the initiation of treatment5,6. Therefore, there is a need to develop new alternatives for AD diagnosis using minimally invasive samples7. Different plasma biomarkers have been evaluated as potential AD biomarkers, such as biomarkers of astrogliosis (glial fibrillary acidic protein, GFAP) associated with the onset of Aβ deposition8, neurodegeneration (NfL), amyloid beta (Aβ42, Aβ40) and p-Tau isoforms (p-Tau181, p-Tau217, p-Tau231)9. Among them, plasma p-Tau biomarkers have been postulated as the most promising plasma biomarkers for early diagnosis of AD10. In this regard, p-Tau at different phosphorylation sites has been correlated to amyloid positivity since preclinical stages, amyloid plaques and cognitive stage11,12. Specifically, plasma p-Tau217 has been described as a predictor of Aβ deposition and brain structural changes from early AD stages13, and its levels change before Tau abnormalities are detected14. Moreover, p-Tau217 has been included in the new National Institute on Aging and the Alzheimer’s Association (NIA-AA) 2024 criteria for AD diagnosis15, to be used in an investigation context. However, studies with clinical application across different demographic populations are still needed. In addition, age and ApoE genotype, which are the main risk factors for AD, showed correlation with plasma Tau biomarkers16.
The previous development of different technologies capable of quantifying low levels of peptides in fluids (e.g. mass spectrometry (MS), single molecule array (Simoa), lumipulse, meso scale discovery (MSD))17, together with the new assays’ development, have enabled the determination of plasma p-Tau levels as biomarkers. The accuracy of these biomarkers and technologies has been evaluated, showing generally high and consistent accuracy for p-Tau immunoassays in AD detection17. Recently, automated immunoassays developed for plasma p-Tau217, which are simple and do not require highly qualified personnel, have shown high sensitivity to detect low p-Tau217 plasma levels. Results from well-characterized cohorts are valuable contributions to the body of evidence needed to refine and harmonize cut-off points across different clinical setting18.
The aim of this work is to develop an early AD diagnosis approach, as a predictive model from plasma p-Tau217 levels, using an automated technique available in clinics. From this, some clinical tools (double cut-offs, nomogram) were proposed for their implementation in a cognitive disorder unit; and potential relationships between plasma p-Tau217 and CSF biomarkers, neuropsychological scores and plasma GFAP were assessed.
Results
Participants description
Table 1 describes the clinical and demographic data for the participant groups (AD, non-AD). As expected, CSF biomarkers and neuropsychological scores showed in general differences between groups. In addition, age was different between participant groups. Also, some medications and comorbidities such as cerebrovascular disease showed statistically significant differences.
Regarding the ApoE genotype, statistically significant differences were observed in the number of ε4-allele carriers between the AD (52%) and non-AD (19.5%) groups. Also, 7% of AD subjects were homozygous ε4-allele carrier vs 1.5% of non-AD subjects. In general, the distribution obtained for the ApoE genotype is similar to the European distribution, in which the ε3 allele has the highest penetrance19; the ε4 allele prevalence in northern Europe is approximately 25%, and in the Mediterranean area is less than 10%20. On the other hand, ε4 alleles are over-represented in AD population, approximately 60%–80% of subjects with AD21,22.
Plasma p-Tau217 levels
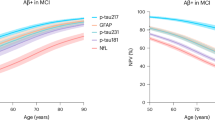
The plasma p-Tau217 levels (median, inter-quartile range (IQR)) for each participants group (AD (0.5 (0.3–0.7) pg mL−1), non-AD (0.1 (0.1, 0.2) pg mL−1)) showed statistically significant differences between groups (p < 0.0001). The median concentration of p-Tau217 was 5 times higher in AD than in non-AD participants (see Fig. 1).
Relationship between plasma p-Tau217 and CSF biomarkers
The correlations between plasma p-Tau217 and CSF biomarkers in the AD group showed statistically significant relationships between plasma p-Tau217 and CSF p-Tau181 (rho = 0.554, p < 0.001), CSF t-Tau (rho = 0.503, p < 0.001), CSF t-Tau/Aβ42 (rho = 0.493, p < 0.001), and CSF NfL (rho = 0.302, p < 0.001). In the non-AD group, plasma p-Tau217 showed statistically significant correlation with CSF p-Tau181 (rho = 0.37, p < 0.001), CSF t-Tau (rho = 0.337, p < 0.001), CSF t-Tau/Aβ42 (rho = 0.391, p < 0.001), CSF NfL (rho = 0.335, p < 0.001), and CSF Aβ42/Aβ40 (rho = − 0.224, p = 0.01) (see Table 2).
Relationship between plasma p-Tau217 and plasma GFAP
The correlation between plasma p-Tau217 and GFAP was analyzed. The correlation between both plasma biomarkers was statistically significant in the AD group (rho = 0.433, p < 0.001), as well as in the non-AD group (rho = 0.346, p < 0.001).
Relationship between plasma p-Tau217 and cognitive status
The correlations between plasma p-Tau217 levels and neurophysiological tests scores are shown in Table 3. In the AD group, p-Tau217 showed statistically significant correlations with MMSE (rho = − 0.37, p < 0.001), RBANS_IM (rho = − 0.422, p < 0.001), RBANS_L (rho = − 0.275, p < 0.001), RBANS_A (rho = − 0.187, p < 0.04), RBANS_DM (rho = − 0.318, p < 0.001), and GDS (rho = − 0.242, p = 0.006). In the non-AD group, plasma p-Tau217 showed significant correlations with MMSE (rho = − 0.26, p = 0.003), RBANS_V/C (rho = − 0.201, p < 0.03), RBANS_L (rho = − 0.174, p < 0.05), RBANS_DM (rho = − 0.225, p = 0.01), FAQ (rho = 0.23, p = 0.008), and ADCS_ADL_MCI (rho = − 0.279, p = 0.001).
Predictive models from plasma p-Tau217 biomarker
Different predictive models were developed to detect AD patients, using different predictor variables. Table 4 shows the different values obtained from each model (A, B). Model A (using plasma p-Tau217 alone as a predictor showed an AUC-ROC of 0.93 (CI 95% 0.88–0.97), and model B (using plasma p-Tau217, age, and ApoE genotype) showed an AUC-ROC of 0.94 (CI 95% 0.90–0.98). Also, it was tested the combination of plasma p-Tau217 and plasma GFAP, but the AUC-ROC was 0.93, not observing any improvement. For model B, performance showed higher sensitivity, lower specificity, and higher NPV and accuracy than model A. Therefore, model B would be selected to be applied in specialized clinical contexts. However, the model A (with only p-Tau217) would be a very useful and reliable approach in less specialized clinics. Figure 2a represents the median and 95% CI curve ROC obtained from the 1000 ROC curves performed for model B, and Fig. 2b represents the median and 95% CI curve ROC obtained from the 1000 ROC curves performed for model A.
The equation obtained for the model B (including p-Tau217, ApoE, and age) is:
being the coefficients: β0 = − 4.01, β1 = 1.71, β2 = 0.74, β3 = 0.47, β4 = 0.05.
The output of this model is AD probability (score between 0 and 1).
Figure 3 represents the obtained nomogram for the optimum model (B). In this nomogram, each predictor is assigned a specific point value, and the sum of these values corresponds to a predicted probability of AD on the bottom axis. For the ApoE genotype, Ɛ4 allele non-carriers (e.g. those with Ɛ3/Ɛ3 genotype) correspond to zero on both “Heterozygous ApoE4” and “Homozygous ApoE4” scales, yet each of these baseline positions still confers a certain number of points due to the underlying model parameterization. As it can be seen, p-Tau217 is the variable with greater weight in the model followed by age and ApoE4 homozygous genotype. This nomogram visually represents the developed model and constitutes a promising clinical tool.
Double-cut-off strategy development
The cut-offs for the developed model A (plasma p-Tau217), model B (plasma p-Tau217 + ApoE + age), and for plasma p-Tau217 levels, are shown in Table 5.
Model including p-Tau217, age and ApoE genotype
First, a threshold criterion based on 95% Sensitivity and 95% Specificity (95% Sens/Spec) was used, obtaining two different probability cut-offs, one with a sensitivity > 95% and the other with the specificity > 95%. The lower (sensitivity 95.5%) cut-off was 0.41 (AD probability), and the higher (specificity 95.5%) cut-off was 0.57 (AD probability) (see Table 5). As shown in Fig. 4a, a red area was defined as positive for AD (including 36.8% of participants); a blue area was defined as negative for AD (37.6% of participants); and a grey area of uncertainty in between was defined, including 25.6% of total cases. Overall, 25.6% of patients could not be classified (uncertain area) and 74.4% were classified as AD or non-AD using these cut-offs. Of the latter, 6% were false positives or false negatives.
Density diagram representing the cut-offs optimization for the model (plasma p-Tau217, age, ApoE genotype) (AD probability): (a) one cut-off established with a sensitivity > 95% and the other with a specificity > 95%; (b) one cut-off established with a PPV > 95% and the other with a NPV > 95%. Density diagram representing the cut-offs optimization for only p-Tau217 (plasma concentration): (c) one cut-off established with a sensitivity > 95% and the other with a specificity > 95%; (d) one cut-off established with a PPV > 95% and the other with a NPV > 95%.
Second, a threshold criterion based on 95% NPV and 95% PPV (95% NPV/PPV) was used, obtaining two probability cut-offs, one with a NPV > 95% and the other with a PPV > 95%. The lower (NPV 95.1%) cut-off was 0.38 (AD probability), and the higher (PPV of 96%) cut-off was 0.58 (AD probability) (see Table 5). As shown in Fig. 4b, these cut-off values reduced the number of patients classified as positive (red area, 35.3%) or negative (blue area, 30.8%), reducing false positives and false negatives (4.5%), and increasing the number of patients, who could not be classified (33.9% of participants in the uncertain area).
Third, a threshold criterion based on 90% Sensitivity and Specificity (90% Sens/Spec) was applied for comparison with previous results, since these criteria are considered acceptable according to global CEOi guidelines on plasma test performance23. From this, it was observed an increase in false positives and false negatives, along with a reduction in uncertain cases within the grey area (see Table 5).
Model including plasma p-Tau217
First, a threshold criterion based on 95% Sens/Spec was used, obtaining two different probability cut-offs. The lower cut-off was 0.37 (AD probability), and the higher cut-off was 0.49 (AD probability) (see Table 5).
Second, a threshold criterion based on 95% NPV/PPV was used, obtaining two probability cut-offs. The lower cut-off was 0.36 (AD probability), and the higher cut-off was 0.49 (AD probability) (see Table 5).
Plasma p-Tau217 levels
First, a threshold criterion based on 95% Sens/Spec was used, obtaining two different concentration cut-offs, one with a sensitivity > 95% and the other with a specificity > 95%. The lower (sensitivity 95.5%) cut-off was 0.12 pg mL−1 and the higher (specificity 96.2%) cut-off was 0.29 pg mL−1 (see Table 5). As shown in Fig. 4c, a red area included 39.1% of participants; a blue area included 32.7% of participants; and a grey area, defined between both cut-offs, included 28.2% of total cases. In general, with these cut-offs, 28.2% of patients could not be classified and 71.8% were classified as AD or non-AD. Of the latter, 6% would be false positives or false negatives.
Second, a threshold criterion based on 95% NPV/PPV was used, obtaining two different concentration cut-offs. The lower (NPV 95.5%) cut-off was 0.10 pg mL−1, and the higher cut-off (PPV 95.2%) was 0.29 pg mL−1 (see Table 5). As shown in Fig. 4d, these cut-off values reduced the number of patients classified as negative (blue area, 24.8%), reducing false positives and false negatives (4.7%), and increasing the number of patients, who could not be classified (36.1% of participants in the uncertain area).
Discussion
Recently, some anti-amyloid drugs have been approved, and because of that, changes in the AD patient’s detection would be expected24,25,26. In this scenario, screening, accessible, and easily interpretable biomarkers are of particular importance for detecting early stages of disease and for implementation in primary care. For this, studies on the accuracy of plasma biomarkers are needed. Various plasma biomarkers have been measured to detect early AD27,28. In this context, p-Tau217 has shown high accuracy and is considered a promising candidate for this purpose. The present study evaluated the ability of plasma p-Tau217, measured using an automated technology, to differentiate between AD and non-AD patients (classified from CSF Aβ42/Aβ40) in a cohort of individuals with cognitive impairment. From this, a predictive model and a double-cut-off approach were developed as the first screening step for clinical implementation. Then, other AD risk factors (age and apoE genotype) were included to develop another predictive model, which improved previous model diagnostic accuracy. From this optimum model, a nomogram and a double-cut-off approach were developed as promising tools for clinicians. Finally, significant relationships were observed for plasma p-Tau217 vs CSF biomarkers (Aβ42, t-Tau, p-Tau181, Aβ42/Aβ40, NfL, and Aβ42/t-Tau), vs cognitive tests (CDR sum of boxes, MMSE, RBANS-DM, ADCS-ADL-MCI), and vs plasma GFAP.
In the present work, plasma p-Tau217 levels were increased in AD patients (0.5 pg mL−1) compared to non-AD patients (0.1 pg mL−1). Similar results were found in recent studies using the same technology (Lumipulse) as the present work, in which the median levels were 0.15 pg mL−1 for cognitively unimpaired patients, 0.34 pg mL-1 for MCI-AD, and 0.59 pg mL−1 for dementia patients, respectively29. Other studies have reported levels between 0.94 and 1.43 pg mL−1 in the amyloid-positive group and between 0.28 and 0.38 pg mL−1 in the amyloid-negative group, using the immunoassay ALZpath30. Additionally, using the meso scale discovery31, the found levels were 0.25 pg mL−1 for cognitively unimpaired, 0.5 pg mL−1 for MCI, and 0.7 pg mL−1 for dementia patients.
Regarding the developed models, the diagnostic indexes obtained for plasma p-Tau217 model (AUC 0.93), were similar to those reported in previous studies using SIMOA (AUC 0.92–0.96)30, meso scale diagnostics (AUC 0.89–0.97)17,31 and Janssen R&D (AUC 0.86)17. The differences found could be related to the specific characteristics of the cohort and the reference measure (e.g. CSF Aβ42/Aβ40, amyloid PET)33. In fact, the present cohort is quite heterogeneous, containing patients with AD at different stages, as well as those with subjective cognitive impairment, MCI, or dementia not due to AD (e.g. LBD, FTD, vascular dementia). In this regard, the ability of p-Tau217 to discriminate between patients with AD and those with other pathologies exhibiting similar clinical manifestations (FTD, vascular dementia, LBD, psychiatric conditions) was enhanced. Similarly, in previous work, p-Tau217 was considered specific for detecting early AD pathology among other degenerative pathologies (e.g. FTD)34. It could be useful for selecting prodromic AD patients in clinical trials, or even for designing new clinical trials for non-AD pathologies. Also, the advantage of using a plasma biomarker is the easy confirmation of AD in patients who may be eligible for anti-amyloid therapies. Moreover, the present study showed that p-Tau217 could be a promising biomarker of Aβ pathology in preclinical AD, like previous studies35. In general, p-Tau217 emerges as a robust AD-specific biomarker with a large fold-change in Aβ positive patients, outperforming other p-Tau biomarkers in previous studies13,16,36,37. Another promising plasma biomarker to detect AD is GFAP. In the present work, plasma GFAP also showed good accuracy as a biomarker (AUC 0.76), but adding GFAP to the p-Tau217 model did not lead to any improvement (AUC 0.93). Therefore, only plasma p-Tau217 was selected because it provided better performance as a potential screening biomarker.
In this work, pTau217 was assessed as a first-line screening biomarker. To achieve this, a double cut-off approach was proposed32. A 95% sensitivity and 95% specificity were the main criteria used to achieve a balance between AD detection and solid exclusion of AD, given that AD is a condition with significant implications. In this regard, a good balance was achieved in reducing both false positives and false negatives, which could improve diagnostic accuracy. In general, the cut-off value may need to be adjusted based on population prevalence. In fact, under conditions of high prevalence, reducing sensitivity to enhance specificity could be beneficial for to refine the AD diagnosis. Additionally, based on the experience with plasma biomarkers, sensitivity and specificity could be lowered to 90% for clinical use, in accordance with global CEOi guidelines on plasma test performance23. However, in this study, a more stringent approach was chosen (95% Sensitivity and Specificity); increasing the uncertain cases and reducing the false positives and false negatives. Although potential false-positive and false-negative cases could still occur (as well as with CSF or PET biomarkers), the interpretation of the results must always be done within the clinical context and considering the symptoms. In cases where suspicion remains, additional diagnostic procedures should be performed to complete the diagnosis.
Additionally, the inclusion of ApoE genotype and age as AD risk factors in an AD diagnosis model improved accuracy38. Also, from this model, a double cut-off for AD probability and a nomogram were developed as useful clinical tools. In fact, this model could be applied in specialized clinical contexts; while the model including only plasma p-Tau217 would be a very useful and reliable approach in less specialized clinics. Previous studies described the association between p-Tau217, age, and ApoE genotype39. However, Clifford40 showed that incorporating age, sex and ApoE genotype improved the accuracy of plasma biomarker models, but in the present study sex variable did not improve the model performance. In general, the proposed models (p-Tau217 alone or combined with ApoE and age) and the double cut-off strategies would significantly reduce the application of current diagnosis methods (PET amyloid, CSF biomarkers), and so the number of lumbar punctures. Only patients with uncertain results—falling between both cut-offs (grey area)—would require the procedure. Specifically, using the p-Tau217-only model, approximately 32–39% of cases, depending on the chosen criteria (95% Sens/Spec or 95% PPV/NPV), would require a lumbar puncture. With the complete model (p-Tau217, ApoE, and age), this percentage decreases to approximately 25–34%. As a result, between 61 and 75% of lumbar punctures could be avoided. A previous study reported the utility of pTau217 to screen for amyloid pathology in a memory clinic cohort41, and the present study confirms these findings and provides relevant information from another memory clinic cohort using a different automated assay.
Regarding relationships, plasma p-Tau217 correlated with clinical CSF biomarkers. Specifically, strong correlations were found with CSF p-Tau181, t-Tau, tau/β42, and NfL, similar to previous works29,33. Regarding the cognitive status, plasma p-Tau217 showed correlations with MMSE and some RBANS domains. Also, a semi-automated method using venous-dried plasma spots reported a weak association of p-Tau217 levels with CDR42. In addition, Mendes31 found an association between plasma p-Tau217 and MMSE. However, they found significant differences in plasma p-Tau217 between cognitively unimpaired and MCI, and between cognitively unimpaired and dementia patients, but not between MCI and dementia groups. Therefore, p-Tau217 could be an early disease stage biomarker (differentiating between non-AD and AD individuals), but when amyloid is present in the brain, it could not differentiate between stages. However, the cause of these correlations (cognitive status or pathology) should be more extensively studied. Finally, statistically significant correlation was observed with plasma GFAP, so it could be also a promising plasma AD biomarker.
Regarding the blood analytical conditions, plasma p-Tau217 determinations showed higher robustness over the pre-analytical factors (e.g. the collection tube) than the CSF biomarkers43. Specifically, the CSF biomarkers require the use of polypropylene tubes rather than glass or polystyrene44,45. Also, diurnal variability in amyloid biomarkers levels has been studied46, while p-Tau217 levels are quite stable47,48. Moreover, the ApoE genotype determination in blood is simple and reliable, showing the ε4 allele as the most consistent genetic risk factor for sporadic AD49,50, mainly for homozygous Apoe ε4-carriers51. One relevant factor that could impact the accuracy of plasma biomarkers is kidney function, since it plays an important role in the clearance of blood substances (proteins…); so, impaired kidney function (reflected as a low estimated glomerular filtration rate) may affect the concentration of these biomarkers. Regarding p-Tau217, recent studies have shown that renal function does not have a clinically relevant impact (excluding severe renal function52), while other biomarkers, such as plasma GFAP, could be altered53. In addition, the participants included in the present study did not show kidney dysfunction.
The main strength of the present study is the optimization of plasma p-Tau217 cut-offs using analytical measures with an automated method. There are several analytical techniques capable of determining plasma biomarkers54,55,56, but those that are more automated and have the capacity to analyze many samples simultaneously would be preferred for screening purposes. In addition, it allowed for the differentiation of AD from other clinically similar pathologies, which also course with cognitive impairment. However, site-specific cut-offs are not yet generalizable, so for the clinical use of plasma p-Tau217, the use of site-specific cut-offs is recommended. In general, further research (standardizing cut-offs among multiple sites, using samples from the general population, external validation, follow-up, comorbidities…) is required to address the clinical implementation of the present approach.
Regarding limitations, the present cohort does not fully represent the general population at risk for AD. Also, this study has not been validated in an external cohort. The external validation is important for assessing generalizability across different population. While our findings are supported by strong internal validation, and the models were obtained after 1000 iterations to ensure robust estimates, future studies should include independent cohorts to confirm the applicability of our results in broader clinical settings. Further validation efforts will be crucial to ensure the robustness of the proposed workflow across diverse demographic and clinical characteristics. Moreover, this is a cross-sectional study that evaluates the ability to diagnose AD patients, without considering the stage of the disease. Nevertheless, patients at different AD stages were included, so the relationship between cognitive stage and plasma p-Tau217 levels was evaluated. Additionally, kidney dysfunction was not considered, so plasma p-Tau217 concentrations could be affected. However, patients with this comorbidity were not included in this study. Also, although the predictive model shows high accuracy (95% sensitivity and specificity), false positives and false negatives may occur. For this reason, it is important to correlate the analytical levels with the clinical suspicion.
Conclusion
Plasma p-Tau217 levels measured by an automatic method provided a high accuracy (AUC 0.93) for early AD diagnosis in a cognitive disorder cohort. The inclusion of age and ApoE genotype as predictive variables together with plasma p-Tau217 improved the accuracy (AUC 0.94), obtaining a promising predictive model, which could constitute a useful screening approach for early AD detection in a cognitive disorder unit. In general, this approach could reduce the number of lumbar punctures more than 70%, with a relevant impact on the public health system. For this, two clinical tools (a double cut-off, and nomogram) were developed, and further studies are required to validate them in external cohorts and address their clinical implementation.
Methods
Study design and participants
This is a cross-sectional study carried out in the Cognitive Disorder Unit from the Neurology Service in the University and Polytechnic Hospital La Fe (Valencia, Spain), in which the patients were recruited consecutively. The participants are patients between 50 and 80 years old (n = 259). They were classified in AD (preclinical-AD (n = 9), mild cognitive impairment (MCI) due to AD (n = 98), mild dementia-AD (n = 20)), and non-AD (healthy controls (n = 57), fronto-temporal dementia (FTD) (n = 15), vascular dementia (n = 8), Lewy bodies dementia (LBD) (n = 2), MCI due to other causes (n = 50)), as described in a previous study57. Their diagnosis was based on the National Institute on Aging and the Alzheimer’s Association (NIA-AA) criteria of 202415.
The Aβ positivity classification was carried out attending to CSF biomarkers levels; specifically, patients were classified as AD if CSF Aβ42/Aβ40 levels were impaired (< 0.069)15. The CSF sampling was carried on with a 20G traumatic lumbar needle, and the sample was collected between 9 and 11 am using a polypropylene tube. The CSF levels of Aβ42, t-Tau, p-Tau, and Aβ40 were determined by chemiluminescence (CLIA) immunoassay (Lumipulse® G, Fujirebio, Japan)57 and CSF NfL levels were determined by Enzyme Immunoassay (NF-light® ELISA de Uman Diagnostics AB, Sweden). The cut-off values considered for each individual CSF biomarker were (reference level) > 830 pg mL−1 for Aβ42, < 380 pg mL−1 for t-Tau, < 60 pg mL−1 for p-Tau181, > 0.069 Aβ42/Aβ40, < 0.41 t-Tau/Aβ42, and < 810 pg mL−1 for NfL58.
In the non-AD group, all patients showed normal levels for CSF Aβ42/Aβ40 (> 0.069). This heterogeneous group was composed of patients considered healthy controls (normal neuropsychological tests results according to age and educational level); patients diagnosed with FTD, who met the International Behavioural Variant FTD Criteria Consortium (FTDC)59 criteria or the 2011 international expert consensus for the diagnosis of Primary Progressive Aphasia60; vascular dementia patients defined by the International Society of Vascular Behavioural and Cognitive Disorders (VAS-COG) criteria61; patients with LBD, who met the criteria developed by the DLB Consortium62 (without AD co-pathology); and patients classified as MCI due to other causes. This last subgroup consisted of patients with altered neuropsychological tests results, but without evidence of neurodegenerative primary pathologies (reasonably excluded by CSF biomarkers and other evaluations), and it included patients with anxious depressive syndrome, pluripathology, history of having received chemotherapy and/or radiotherapy, history of drug use, etc.
The cognitive status was characterized by neuropsychological evaluation (clinical dementia rating (CDR), composed of a scale compromising global score and the sum of boxes; Mini-Mental State Examination (MMSE); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS and its domains IM, V/C, A, L, DM); Functional Activities Questionnaire (FAQ); Alzheimer’s Disease Cooperative Study—Activities of Daily Living (ADCS-ADL); Geriatric Depression Scale (GDS)). According to the neuropsychological performance, the patients were classified as cognitively unimpaired, mild cognitive impairment (MCI), and mild dementia. The preclinical AD group includes patients with positive CSF AD biomarkers and cognitively unimpaired; the MCI-AD group includes patients with positive CSF AD biomarkers and impairments in at least one cognitive domain, without an impairment in daily function; the mild dementia AD group includes patients with positive CSF AD biomarkers and cognitive impairment in one or more cognitive domains that precludes activities of daily living.
The study was conducted in accordance with the Declaration of Helsinki, and it was approved by the Ethics Committee (CEIC) from Health Research Institute La Fe (Valencia, Spain) (reference number: 2022-990-1; date: 8 February 2023). All participants signed informed consent prior to their recruitment.
Blood and plasma samples collection and analysis
Blood samples were obtained from all the participants, using tubes containing EDTA. A whole blood sample was used for Apolipoprotein E (ApoE) genotyping. It was determined following the manufacturer protocol by PCR using Light-Mix® Kit ApoE C112R R158C from Roche Diagnostics57,63. A specific ApoE genotype informed consent was obtained for each patient.
The other blood samples were centrifuged (1160g, 15 min, 25 °C), to separate the plasma fraction into a new tube. The plasma samples were stored at −80 °C until analysis.
For the analysis, plasma samples were thawed on ice and centrifuged. After that, plasma p-Tau217 was determined by means of Lumipulse® technology (G600II automated platform, Fujirebio Diagnostics, Malvern, USA), following the manufacturer recommendations.
Statistical analysis
Differences between groups (AD, non-AD) were analysed using a Wilcoxon rank sum test or Kruskal Wallis or Mann Whitney tests for continuous variables, and Pearson’s Chi squared test or Fisher’s exact test for categorical variables. Correlations between numerical variables (plasma p-Tau217, cognitive tests (CDR, MMSE, RBANS, FAQ, ADCS-ADL, GDS), CSF biomarker levels (Aβ42, Aβ42/Aβ40, p-Tau181, t-Tau, NfL, Aβ42/t-Tau)) were evaluated using Spearman’s rank correlation coefficient (rho). For all the analyses, statistical significance was established as p-value < 0.05.
Discriminatory capacity of plasma p-Tau217 levels for AD vs non-AD patients was evaluated using Ridge penalized logistic regression to develop a predictive model. A repeated holdout approach was used (1000 iterations), randomly splitting the data into a 70:30 ratio (70% training set, 30% testing set). The proportion of AD and non-AD cases was maintained in all iterations. Two different models based on different variable selection were developed: (i) only plasma p-Tau217, and (ii) plasma p-Tau217, age and ApoE genotype). These models were adjusted using the observations of the training set and validated using the testing set. In order to obtain a more robust and representative estimate for each model, the process was replicated 1000 times, varying in each case the observations selected for the training set and testing set.
The diagnosis indexes (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, area under curve Receiver Operating Characteristics (AUC-ROC)) were calculated. A confidence interval (CI, 95%) was estimated for the performance metrics of the models. Then, the optimal cut-off points were stablished using different threshold criteria (sensitivity and specificity > 95%, or PPV and NPV > 95%).
The R software (version 4.2.3.) was used in all the analysis64, and the different packages were glmnet (version 4.1.7.)65 caret (version 6.0.94)66, cutpoint (version 1.1.2)67 and ggplot2 (version 3.5.1)68.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AD:
-
Alzheimer disease
- ADCS-ADL:
-
Alzheimer’s disease cooperative study-activities of daily living
- AUC:
-
Area under the curve
- AUC-ROC:
-
Area under curve receiver operating characteristics
- Aβ42:
-
Amyloid β42
- Aβ40:
-
Amyloid β40
- CSF:
-
Cerebrospinal fluid
- CDR:
-
Clinical dementia rating
- CI:
-
Confidence interval
- CU:
-
Cognitively unimpaired
- FAQ:
-
Functional Activities Questionnaire
- GDS:
-
Geriatric Depression Scale
- GFAP:
-
Glial fibrillary acidic protein
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini-Mental State Examination
- NPV:
-
Negative predictive value
- RBANS:
-
Repeatable battery for the assessment of neuropsychological status
- PPV:
-
Positive predictive value
- p-Tau181:
-
Phosphorylated Tau181
- p-Tau217:
-
Phosphorylated Tau217
- T-tau:
-
Total tau
References
Alzheimer’s disease facts and figures. Alzheimer’s Dement. 19, 1598–1695 (2023).
Jack, C. R. et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562. https://doi.org/10.1016/j.jalz.2018.02.018 (2018).
Engelborghs, S. et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 8, 111–126 (2017).
Villa, C., Lavitrano, M., Salvatore, E. & Combi, R. Molecular and imaging biomarkers in Alzheimer’s disease: A focus on recent insights. J. Pers. Med. 10, 61 (2020).
Mahaman, Y. A. R. et al. Biomarkers used in Alzheimer’s disease diagnosis, treatment, and prevention. Ageing Res. Rev. 74, 101544 (2022).
Liu, E., Zhang, Y. & Wang, J.-Z. Updates in Alzheimer’s disease: from basic research to diagnosis and therapies. Transl. Neurodegener. 13, 45 (2024).
Pais, M. V., Forlenza, O. V. & Diniz, B. S. Plasma biomarkers of Alzheimer’s disease: A review of available assays, recent developments, and implications for clinical practice. J. Alzheimers Dis. Rep. 7, 355–380 (2023).
Pereira, J. B. et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain 144, 3505–3516 (2021).
Mielke, M. M. et al. Comparison of plasma phosphorylated tau species with amyloid and tau positron emission tomography, neurodegeneration, vascular pathology, and cognitive outcomes. JAMA Neurol. 78, 1108 (2021).
Therriault, J. et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 80, 188 (2023).
Chatterjee, P. et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s Dement. 18, 1141–1154 (2022).
Ding, X. et al. Ultrasensitive assays for detection of plasma tau and phosphorylated tau 181 in Alzheimer’s disease: a systematic review and meta-analysis. Transl. Neurodegener. 10, 10 (2021).
Telser, J., Risch, L., Saely, C. H., Grossmann, K. & Werner, P. P-tau217 in Alzheimer’s disease. Clin. Chim. Acta 531, 100–111 (2022).
Salvadó, G. et al. Specific associations between plasma biomarkers and postmortem amyloid plaque and tau tangle loads. EMBO Mol. Med. 15, (2023).
Jack, C. R. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 20, 5143–5169 (2024).
Snellman, A. et al. Head-to-head comparison of plasma p-tau181, p-tau231 and glial fibrillary acidic protein in clinically unimpaired elderly with three levels of APOE4-related risk for Alzheimer’s disease. Neurobiol. Dis. 183, 106175 (2023).
Janelidze, S. et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer’s disease. Brain 146, 1592–1601 (2023).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21, 726–734 (2022).
Troutwine, B. R., Hamid, L., Lysaker, C. R., Strope, T. A. & Wilkins, H. M. Apolipoprotein E and Alzheimer’s disease. Acta Pharm. Sin. B 12, 496–510 (2022).
Huebbe, P. & Rimbach, G. Evolution of human apolipoprotein E (APOE) isoforms: Gene structure, protein function and interaction with dietary factors. Ageing Res. Rev. 37, 146–161 (2017).
Farrer, L. A. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA 278, 1349 (1997).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1979(261), 921–923 (1993).
Schindler, S. E. et al. Acceptable performance of blood biomarker tests of amyloid pathology—recommendations from the Global CEO Initiative on Alzheimer’s disease. Nat. Rev. Neurol. 20, 426–439 (2024).
Kang, C. Donanemab: First approval. Drugs 84, 1313–1318 (2024).
Hoy, S. M. Lecanemab: First approval. Drugs 83, 359–365 (2023).
Li, W., Sun, L., Yue, L. & Xiao, S. Diagnostic and predictive power of plasma proteins in Alzheimer’s disease: a cross-sectional and longitudinal study in China. Sci. Rep. 14, 17557 (2024).
Torres Robledillo, S. et al. Validation of plasma biomarkers in Alzheimer disease. Clin. Chim. Acta 558, 118996 (2024).
Arranz, J. et al. Diagnostic performance of plasma pTau217, pTau181, Aβ1-42 and Aβ1-40 in the LUMIPULSE automated platform for the detection of Alzheimer disease. Alzheimers Res. Ther. 16, 139 (2024).
Ashton, N. J. et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 81, 255 (2024).
Mendes, A. J. et al. Head-to-head study of diagnostic accuracy of plasma and cerebrospinal fluid p-tau217 versus p-tau181 and p-tau231 in a memory clinic cohort. J. Neurol. 271, 2053–2066 (2024).
Brum, W. S. et al. A three‐range approach enhances the prognostic utility of CSF biomarkers in Alzheimer’s disease. Alzheimer’s & Dement. Transl. Res. Clin. Interv. 8, (2022).
Gonzalez-Ortiz, F. et al. A novel ultrasensitive assay for plasma p-tau217: Performance in individuals with subjective cognitive decline and early Alzheimer’s disease. Alzheimer’s Dement. 20, 1239–1249 (2024).
Thijssen, E. H. et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 20, 739–752 (2021).
Ashton, N. J. et al. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 28, 2555–2562 (2022).
Ashton, N. J. et al. Plasma and CSF biomarkers in a memory clinic: Head-to-head comparison of phosphorylated tau immunoassays. Alzheimer’s Dement. 19, 1913–1924 (2023).
Montoliu-Gaya, L. et al. Mass spectrometric simultaneous quantification of tau species in plasma shows differential associations with amyloid and tau pathologies. Nat. Aging 3, 661–669 (2023).
Armstrong, A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 57, 87–105 (2019).
Cogswell, P. M. et al. Modeling the temporal evolution of plasma p-tau in relation to amyloid beta and tau PET. Alzheimer’s Dement. 20, 1225–1238 (2024).
Jack, C. R. et al. Predicting amyloid PET and tau PET stages with plasma biomarkers. Brain 146, 2029–2044 (2023).
Brum, W. S. et al. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nat. Aging 3, 1079–1090 (2023).
Huber, H. et al. Biomarkers of Alzheimer’s disease and neurodegeneration in dried blood spots—A new collection method for remote settings. Alzheimer’s Dement. 20, 2340–2352 (2024).
Fourier, A. et al. Pre-analytical and analytical factors influencing Alzheimer’s disease cerebrospinal fluid biomarker variability. Clin. Chim. Acta 449, 9–15 (2015).
Bjerke, M. et al. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J. Alzheimers Dis. 2010, 1–11 (2010).
Lewczuk, P. et al. Effect of sample collection tubes on cerebrospinal fluid concentrations of tau proteins and amyloid β peptides. Clin. Chem. 52, 332–334 (2006).
Bateman, R. J., Wen, G., Morris, J. C. & Holtzman, D. M. Fluctuations of CSF amyloid-β levels. Neurology 68, 666–669 (2007).
Orduña Dolado, A. et al. Effects of time of the day at sampling on CSF and plasma levels of Alzheimer’ disease biomarkers. Alzheimers Res. Ther. 16, 132 (2024).
Pichet Binette, A. et al. Confounding factors of Alzheimer’s disease plasma biomarkers and their impact on clinical performance. Alzheimer’s Dement. 19, 1403–1414 (2023).
Vasilevskaya, A. et al. Interaction of APOE4 alleles and PET tau imaging in former contact sport athletes. Neuroimage Clin. 26, 102212 (2020).
Huang, Y.-W.A., Zhou, B., Wernig, M. & Südhof, T. C. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell 168, 427-441.e21 (2017).
Agnello, L. et al. Exploring the effect of APOE ε4 on biomarkers of neurodegeneration in Alzheimer’s disease. Clin. Chim. Acta 562, 119876 (2024).
Bornhorst, J. A. et al. Quantitative assessment of the effect of chronic kidney disease on plasma P-Tau217 concentrations. Neurology 104, (2025).
Arslan, B. et al. The impact of kidney function on Alzheimer’s disease blood biomarkers: implications for predicting amyloid-β positivity. Alzheimers Res. Ther. 17, 48 (2025).
Tieu, M.-V., Choi, S. H., Le, H. T. N. & Cho, S. Electrochemical impedance-based biosensor for label-free determination of plasma P-tau181 levels for clinically accurate diagnosis of mild cognitive impairment and Alzheimer’s disease. Anal. Chim. Acta 1273, 341535 (2023).
Verberk, I. M. W. et al. F2‐07‐04: Plasma amyloid beta 1–42 and 1–40 measured by a novel simoa assay as a diagnostic tool for Alzheimer’s disease pathology. Alzheimer’s Dement. 15, (2019).
Gobom, J., Brinkmalm, A., Brinkmalm, G., Blennow, K. & Zetterberg, H. Alzheimer’s disease biomarker analysis using targeted mass spectrometry. Mol. Cell. Proteomics 23, 100721 (2024).
Baquero, M. et al. Insights from a 7-Year Dementia Cohort (VALCODIS): ApoE genotype evaluation. J. Clin. Med. 13, 4735 (2024).
Peña‐Bautista, C. et al. Clinical usefulness of cerebrospinal fluid biomarkers in Alzheimer’s disease. Eur. J. Clin. Investig. 53, (2023).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477 (2011).
Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014 (2011).
Sachdev, P. et al. Diagnostic criteria for vascular cognitive disorders. Alzheimer Dis. Assoc. Disord. 28, 206–218 (2014).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies. Neurology 89, 88–100 (2017).
R Foundation for Statistical Computing, V. Austria. R: A Language and Environment for Statistical Computing. (2023).
Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 33, (2010).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, (2008).
Thiele, C. & Hirschfeld, G. cutpointr : Improved estimation and validation of optimal cutpoints in R. J. Stat. Softw. 98, (2021).
Wickham, H. Ggplot2. https://doi.org/10.1007/978-3-319-24277-4 (Springer International Publishing, 2016).
Acknowledgements
CC-P acknowledges a postdoctoral “Miguel Servet” grant CPII21/00006 and FIS project PI22/00594 from Health Institute Carlos III and co-funded by European Union; and a consolidation project from Ministerio de Ciencia e Innovación (Spain) (ref. CNS2022-135327). CP-B acknowledges a predoctoral “PFIS” grant FI20/00022 from the Health Institute Carlos III. The authors thank Fujirebio Europe NV for kindly providing the necessary p-Tau217 reagents to perform the study; and B.L. and R.P. from clinics laboratory at Hospital Universitari i Politècnic La Fe for their help in using the analytical equipment.
Funding
Grant CNS2022-135327 funded by MICIU/AEI/https://doi.org/10.13039/501100011033 and by the “European Union NextGenerationEU/PRTR”.
Author information
Authors and Affiliations
Contributions
L.A.-S. Methodology, investigation, and wrote the main manuscript. C.P.-B.: Methodology, investigation, and wrote the main manuscript. L.F.-G.: Methodology, investigation. A.B.: Validation, formal analysis, and prepared Figs. 1, 2, 3, 4, 5. J.L.A.: Validation, formal analysis, writing—review & editing. M.B.: Conceptualization, resources, supervision. C.C.-P.: Conceptualization, investigation, resources, writing—review & editing, supervision, funding acquisition. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical statement standard
This study was approved by the Ethics Committee from Instituto de Investigación Sanitaria La Fe (Valencia, Spain) (Reference number: 2022-990-1).
Informed consent
All participants signed informed consent prior to their recruitment.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Álvarez-Sánchez, L., Peña-Bautista, C., Ferré-González, L. et al. Promising clinical tools for specific Alzheimer disease diagnosis from plasma pTau217 and ApoE genotype in a cognitive disorder unit. Sci Rep 15, 16316 (2025). https://doi.org/10.1038/s41598-025-01511-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01511-3
Keywords
This article is cited by
-
Clinical validation of new alzheimer disease diagnosis tools based on plasma p-Tau217
Scientific Reports (2025)
-
Ethical dilemmas and medicolegal implications of nuclear medicine imaging in dementias
Clinical and Translational Imaging (2025)