Abstract
The pathogenesis of NEC in term infants with critical congenital heart defects (CHD) is mainly associated with hypoxic-ischemic events that initiate an exaggerated systemic inflammatory response. Herein, we investigated the cumulative impact of the cytokine landscape and gut microbiota on the pathobiology leading to NEC onset in term newborns with CHD. This study involved 36 newborns who underwent surgical correction of CHD during the first two weeks of life; eight of them developed NEC within one week after cardiac surgery. Blood and fecal samples were collected at two time points: before and after surgery. Newborns without NEC exhibited significant changes in the levels of 22 cytokines, whereas newborns with NEC had changes in only 4 cytokines during the perioperative period. A panel including IL-1RA, IL-5, IL-18, and MCP-1 showed impressive test performance characteristics for diagnosing NEC at the preclinical stage with an AUC of 0.938, a sensitivity of 100.0%, and a specificity of 85.7%. Fifteen bacterial taxa were differentially abundant between feces samples of newborn groups. The pathobionts Collinsella and Mediterraneibacter gnavus group, known to be associated with increased intestinal permeability, were enriched in NEC newborns’ feces before cardiac surgery. Our study demonstrated that the gut microbiota mediates the equilibrium of cytokine network dynamics under a broad spectrum of “friend or foe” conditions, effectively suppressing excessive inflammatory responses during early postnatal adaptation. In contrast, under conditions of low microbial diversity, a strong imbalanced cytokine feedback loop formed, resulting in deviations from normal immune response maturation. These findings offer new insights into understanding the fine-tuning of gut microbiota-immune system interactions in the first days of life.
Similar content being viewed by others
Introduction
Necrotizing enterocolitis (NEC) is a severe neonatal multifactorial gastrointestinal disease characterized by bowel inflammation and necrosis that afflicts 5–12% of preterm newborns1,2. Another risk group for the development of NEC in the neonatal period is term infants with congenital heart defects (CHD), among whom the incidence rate reaches 2–6%3,4,5,6.
The etiology and specific molecular mechanisms of NEC pathogenesis are still unclear. In preterm infants, the development of NEC is mainly associated with the composition of the microbial community colonizing the functionally immature intestine, low secretion of mucus, decreased intracellular junction integrity, and uncoordinated peristalsis2,7,8. Many studies report the dysbiotic microbiota (low bacterial diversity, predominance of Pseudomonadota (formerly Proteobacteria), presence of pathogens) in preterm infants who developed NEC8. In term newborns with CHD, triggers of NEC can include circulatory hypoxia leading to bowel hypoperfusion and ischemia, which initiate an exaggerated systemic inflammatory response; abnormal colonization of the mature intestine by microorganisms; and aberrant reactions of the innate immune system caused by the adaptation process in the first weeks of life2,3,5. Common risk factors are formula feeding, perinatal asphyxia, prolonged antibiotic administration, sepsis, and an Apgar score < 7 at 5 min9,10.
Bacterial colonization of the intestine in the early neonatal period serves as the driving force for the activation and training of the immune system in a new environment. This process is guided by the “friend or foe” principle, where the immune system learns to tolerate commensal bacteria while remaining active against pathogens, which results in fine-tuning of the immune system functioning.
Both processes, bacterial colonization and intestinal ischemia, have been found to mediate activation of toll-like receptor 4 (TLR4), a pathogen recognition receptor for lipopolysaccharides found in Gram-negative bacteria that is expressed by immune cells and intestinal epithelial cells (IECs)11,12,13. TLR4 signaling through the MyD88 (myeloid differentiation factor 88)-dependent pathway results in NF-κB (nuclear factor kappa B) activation and production of pro-inflammatory cytokines (such as IL-1β, IL-2, IL-6, IL-12p40, IL-18, IL-23, and TNFα), while signaling through the TRIF (Toll/IL-1R domain-containing adapter-inducing IFNβ)-dependent pathway induces the transcription of type I interferons (IFNα and IFNβ)12,14,15,16. Cytokines are a broad group of small secreted proteins that drive various intercellular signaling and communication processes and regulate growth, differentiation, and activation of the immune cells, including the dynamic regulation of innate lymphoid cell (ILC) development, T cell ‘polarization’, and chemotaxis17. The cytokines form complex networks that differ in the degree of redundancy and activated pathways. The unique cytokine fingerprints govern the pattern of immune response and, subsequently, the disease outcome18.
Although it is known that alterations in immune-microbiota interaction can lead to aberrant immune responses in adults19, there is still a gap in understanding the complex interplay between the immune system and gut microbiota during the postnatal adaptation period that may explain why some newborns have increased susceptibility to pathogenic invasion and therefore infectious and inflammatory diseases, such as NEC. Herein, we employed multiplex analysis of 47 plasma cytokines/chemokines/growth factors and 16S rRNA gene sequencing of fecal samples, both collected before and after cardiac surgery, to investigate the cumulative impact of the cytokine landscape and gut microbiome on the pathobiology leading to NEC onset in term neonates with critical CHD.
Results
Clinical characteristics of newborns
All 36 enrolled newborns underwent surgical correction of CHD on median day of life (DOL) 8 (interquartile range: 6–10). Eight of them developed NEC (Bell’s stage IIA) within one week after cardiac surgery (hereafter referred to as NEC newborns, NEC_BS (before surgery), or NEC_AS (after surgery). Twenty-eight newborns, who did not develop NEC postoperatively, were considered a control group (hereafter referred to as no-NEC newborns, no-NEC_BS (before surgery), or no-NEC_AS (after surgery).
No differences in sex, gestational age, birth weight, mode of delivery, Apgar score, type of CHD, or laboratory parameters were observed between NEC and no-NEC newborns both before and after cardiac surgery (Supplementary Tables 1, 2). There was no statistical difference (p = 0.69) in the administration of antibacterial therapy between the no-NEC and NEC newborns, neither before nor after surgery. However, levels of hemoglobin, platelets, and lymphocytes decreased significantly in no-NEC newborns postoperatively, whereas the levels of segmented neutrophils and CRP increased (Supplementary Table 2). In NEC newborns, none of the studied routine laboratory parameters changed significantly after cardiac surgery.
Cytokine profile predicts NEC development
Four (IL-1β, IL-3, IL-22, and GM-CSF) out of 47 studied plasma cytokines had values below the detectable range in more than 80% of plasma samples, and, therefore, they were excluded from follow-up analysis.
No-NEC newborns had significant changes in the levels of 22 signaling molecules during the perioperative period (Table 1). The concentrations of 21 cytokines were decreased, while the IL-27 level increased. The largest decrease was observed for M-CSF, EGF, IL-12p40, IL-1α, sCD40L, VEGF-A, and IL-18. Contrariwise, NEC newborns had a significant decrease in the levels of only 4 signaling molecules: IL-12p40, TNFα, IP-10, and MDC.
The two groups of newborns significantly differed in the levels of four cytokines: IL-5, IL-18, IL-1RA, and MCP-1 preoperatively (Fig. 1A). No-NEC newborns had higher levels of IL-18 and IL-1RA and lower levels of IL-5 and MCP-1 compared to NEC newborns. Furthermore, the IL-5 and MCP-1 levels remained unchanged in no-NEC newborns in the postoperative period, but they increased in NEC newborns; the opposite pattern was observed for the IL-18 levels. Both groups had a similar dynamic of changes in IL-1RA levels. Postoperatively, no-NEC newborns showed a 15-fold decrease in the M-CSF level compared to NEC newborns, in whom it remained unchanged (Fig. 1A).
Dynamics of plasma cytokines of term newborns with critical congenital heart defects with or without necrotizing enterocolitis (NEC) across the perioperative period. (A) Violin plots of plasma cytokine levels statistically deferent between two groups of newborns. (B) ROC analysis statistics for the plasma cytokines. (C) Discriminant analysis scatterplot showing four clusters formed by the samples obtained from the two groups of newborns (Wilks’ lambda = 0.00807, approx. F (105.102) = 3,9134, p < 0.0000). Each dot represents one sample. No-NEC_BS and No-NEC_AS represent no-NEC newborns before and after cardiac surgery (n = 28); NEC_BS and NEC_AS represent NEC newborns before and after cardiac surgery (n = 8), respectively. AUC: Area under the curve, Se: Sensitivity, Sp: Specificity. Data represented as median ± IQR.
The receiver-operating characteristic (ROC) analysis revealed that IL-5 was the most promising cytokine for diagnosing NEC at the preclinical stage (Fig. 1B). A logistic regression model including IL-1RA, IL-5, IL-18, and MCP-1 significantly improved prognostic accuracy (Fig. 1B). In contrast, M-CSF demonstrated potential for NEC detection in the postoperative period (Fig. 1B).
The discriminant analysis established a high degree of discrimination for 35 out of 43 studied cytokines between NEC and no-NEC newborns in the perioperative period (Supplementary Table 3). All samples formed four completely separate clusters depending on the group and the operative period, which was supported by 100% predictive accuracy according to a classification matrix (Fig. 1C).
Correlation analysis revealed different cytokine profiles in two infant groups (Fig. 2). A complex network of cytokine interactions of moderate strength was observed in no-NEC newborns in the preoperative period, which expanded in the postoperative period, mainly due to the IL-17 family, MCP-3, MIP-1α, MIP-1β, PDGF-AA, PDGF-AB/BB, TGFα, VEGF-A, G-CSF, and IP-10 (Fig. 2). Only strong positive cytokine correlations were found in NEC newborns in the preoperative period, which involved a smaller number of cytokines, including IL-17 A, IL-17E/IL-25, IL-17 F, FGF-2, IL-1RA, and IL-12p70, compared to no-NEC newborns (Fig. 2). Moreover, the interaction between cytokines dramatically declined postoperatively in this group of infants (Fig. 2).
Correlation heat map between the cytokine plasma levels of term newborns with critical congenital heart defects with or without necrotizing enterocolitis (NEC) across the perioperative period. Color scale bars show a range of nonparametric Spearman correlation (r). The red color corresponds to a strong positive correlation, the purple color - a strong negative correlation. Only significant correlations are presented (p < 0.01). No-NEC_BS and No-NEC_AS represent no-NEC newborns before and after cardiac surgery (n = 28); NEC_BS and NEC_AS represent NEC newborns before and after cardiac surgery (n = 8), respectively.
No-NEC newborns exhibited a negative correlation between the platelet level and M-CSF, MDC, IL-15, IL-9, and FLT3L and a positive correlation between the hemoglobin level and IL-7, IL-12p70, MIP-1β, and PDGF-AA in the preoperative period (Supplementary Fig. 1). The correlation trend remained predominantly negative in the postoperative period. In contrast, NEC newborns had a strong positive correlation between the leukocyte level and IL-4, IL-7, TNFβ, and VEGF-A in the preoperative period. Postoperatively, correlations were restricted to MCP-3 with hemoglobin, IL-27 with platelets, and IL-1α with lymphocytes.
Finally, we examined the balance between cytokines associated with type 1, type 2, and type 3 immune responses in the two newborn groups. Type 1 responses are linked predominantly to the activity of T helper 1 (Th1) cells and ILC1, type 2 to Th2 and ILC2 cells, and type 3 to Th17 and ILC3 cells20,21. This analysis revealed pronounced differences between newborn groups in the ratio of type 2-response cytokines and IL-18 (Fig. 3). The impact of type 2-associated cytokines almost doubled in no-NEC_AS, whereas the influence of IL-18 greatly decreased (6-fold). The opposite dynamics of the same cytokines were observed in NEC newborns. Remarkably, a significant decrease in the IL-12p40 level, which is a key cytokine promoting type 1 response, in both groups of infants after cardiac surgery was observed. Meanwhile, the proportion of type 1-associated cytokines did not alter. Furthermore, changes in the ratio of chemokines and growth factors in both infant groups were similar, except for the M-CSF, which increased in NEC newborns during the perioperative period and did not alter in no-NEC newborns (Supplementary Figs. 2–3).
Ratio of mean plasma cytokine levels of term newborns with critical congenital heart defects with or without necrotizing enterocolitis (NEC) across the perioperative period. No-NEC_BS and No-NEC_AS represent no-NEC newborns before and after cardiac surgery (n = 28); NEC_BS and NEC_AS represent NEC newborns before and after cardiac surgery (n = 8), respectively.
Gut microbiota signatures during the perioperative period
For 16S rRNA gene sequencing, 37 feces samples from 20 newborns (12 no-NEC and 8 NEC) were available according to the study design. Taxonomic assignment of 503 ASVs revealed 2 domains, 12 phyla, 18 classes, 49 orders, 90 families, 163 genera, and 231 species. The cumulative microbiota profile was mainly composed of Bacillota (formerly Firmicutes), Pseudomonadota (formerly Proteobacteria), Actinomycetota (formerly Actinobacteriota), and Bacteroidota (formerly Bacteroidetes) (Supplementary Fig. 4A, B).
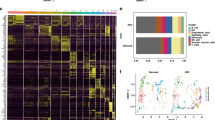
No-NEC and NEC newborns had equal mean relative abundance of Bacillota and Pseudomonadota but differed in Bacteroidota (10% and 2%, respectively) and Actinobacteriota (2% and 11%, respectively) preoperatively (Fig. 4A). Escherichia-Shigella Staphylococcus, Enterococcus, and Bacteroides predominated in no-NEC newborns, whereas Streptococcus, Enterococcus, Corynebacterium, and other members of the family Enterobacteriaceae were the most prominent in NEC newborns (Fig. 4B).
Taxonomic composition of gut microbiome communities of term newborns with critical congenital heart defects with or without necrotizing enterocolitis (NEC) across the perioperative period. (A) Mean relative abundance at the phylum level in each studied cohort group. (B) Mean relative abundance at the genus level in each studied cohort group. (C) Alpha-diversity based on Faith’s phylogenetic diversity, Pielou evenness diversity, Shannon diversity, and Observed species metrics between studied groups. Data is shown in box plots with median and 25–75th percentiles (boxes) and 10–90th percentiles (whiskers). Each dot represents one sample. (D) The two-dimensional UMAP with Jaccard metric visualization of the beta diversity between infant groups at the phylum (up) and genus (down) levels. Each dot represents one sample. (E) Venn diagram representing distribution of all identified 231 taxa (left) and the top 40 most abundant taxa (right) between cohort groups. (F) Mean relative abundance at the species level in each cohort group. No-NEC_BS (n = 11) and No-NEC_AS (n = 12) represent no-NEC newborns before and after cardiac surgery; NEC_BS (n = 8) and NEC_AS (n = 6) represent NEC newborns before and after cardiac surgery, respectively.
Distinct trajectories of changes in microbiota profiles were observed in two groups of infants postoperatively. In no-NEC newborns, the mean relative abundance of Bacillota and Actinomycetota increased, while Pseudomonadota and Bacteroidota decreased. Conversely, in NEC newborns, the mean relative abundance of Pseudomonadota and Bacteroidota increased, but Bacillota and Actinomycetota decreased. However, no significant differences between infant groups at the phylum level were observed. At the genus level, the proportion of Escherichia-Shigella and Streptococcus decreased (23% vs. 11% and 8% vs. 1%, respectively), while the proportion of Enterococcus, Bifidobacterium, and Lactobacillus increased (11% vs. 26%, 0.8% vs. 5%, and 0.1% vs. 5%, respectively) in no-NEC newborns. The mean relative abundance of Staphylococcus dramatically increased from 4 to 25%, whereas Streptococcus decreased from 24 to 5% in NEC newborns. Both groups of infants differed by the proportion of Streptococcus (p = 0.03) and Senegalimassilia (p = 0.01). Following pairwise comparisons revealed differences in Streptococcus between no-NEC_AS vs. NEC_BS (p = 0.03) and in Senegalimassilia between no-NEC_AS vs. NEC_AS (p = 0.02), no-NEC_BS vs. NEC_AS (p = 0.03), and NEC_BS vs. NEC_AS (p = 0.04).
Three alpha-diversity metrics indicated similar dynamic changes in the microbiota profile of no-NEC newborns during the perioperative period, while the Pielou’s evenness index showed opposite dynamics (Fig. 4C). However, no significant differences between any studied sample groups were found by any index, and no samples showed clustering based on beta-diversity (Fig. 4D).
Based on a Venn diagram, the gut microbiota of NEC newborns comprised a noticeably lower number of species compared to those of no-NEC newborns during the perioperative period (Fig. 4E). Twenty out of the top 40 species with an abundance of more than 1% in feces samples were common for all sample groups (Fig. 4E, Supplementary Table 4). Seven species, namely Lacticaseibacillus rhamnosus (formerly Lactobacillus rhamnosus), Phocaeicola massiliensis (formerly Bacteroides massiliensis), Paenibacillus cookii, Parabacteroides merdae, g. [Clostridium] innocuum group, g. Stenotrophomonas, and f. Sporolactobacillaceae (g. uncultured), were present in feces of no-NEC newborns throughout the entire period but were absent in samples from NEC newborns (Supplementary Tables 4, 5). Meanwhile, the microbiota structures of NEC newborns differed from those of no-NEC newborns by the presence of Ruminococcus lactaris, Burkholderia-Caballeronia-Paraburkholderia in the preoperative period, and Blautia throughout the entire period. Of note, in all studied groups, the relative abundance of identified taxa varied widely across individuals.
DESeq2 analysis revealed a differential abundance of nine bacterial genera and six species between feces samples of no-NEC and NEC newborns (Fig. 5A). No-NEC newborns had a higher abundance of Parabacteroides, Enhydrobacter, Bacteroides, Clostridioides, Actinomyces, and Escherichia-Shigella in the preoperative period and a lower abundance of f. Enterobacteriaceae in the postoperative period compared to NEC newborns. Surprisingly, at the species level, no-NEC newborns had a higher abundance of Clostridioides difficile and a lower abundance of f. Enterobacteriaceae in the preoperative period compared to NEC newborns (Figs. 4F and 5A). Moreover, feces samples of no-NEC_AS were enriched for Lactobacillus, Bacteroides, and Staphylococcus compared to NEC newborns, whereas NEC newborn samples varied in the number of Collinsella in the perioperative period (Fig. 5A).
Series analysis reveals distinct gut microbiome landscapes of term newborns with critical congenital heart defects, comparing those with and without necrotizing enterocolitis (NEC), across the perioperative period. (A) Differentially abundant bacterial taxa, identified by DESeq2 analysis between cohort groups. The threshold for statistical significance was a false detection ratio (FDR) < 0.05 (adjusted p < 0.05). A positive LFC (Log2FoldChange) value means that the corresponding bacterial taxa are higher represented in group 1 vs. group 2. A negative LFC value means that the corresponding bacterial taxa are lower represented in group 1 vs. group 2. (B-C) Differentially abundant taxa identified by linear discriminant analysis of effect size (LefSe) between cohort groups before (left) and after (right) cardiac surgery. (B) Distribution histogram based on linear discriminant analysis (LDA) with a threshold value > 2.0 and p < 0.05. (C) Taxonomic cladogram. Red and green colors represent bacteria with increased relative abundance during the perioperative period. No-NEC_BS (n = 11) and No-NEC_AS (n = 12) represent no-NEC newborns before and after cardiac surgery; NEC_BS (n = 8) and NEC_AS (n = 6) represent NEC newborns before and after cardiac surgery, respectively.
Additionally, the LEfSe analysis revealed that feces samples of no-NEC_BS newborns had a higher abundance of Peptostreptococcus-tissierellales, Anaerococcus, and Anaerococcus (unidentified sp.), whereas feces samples of NEC_BS newborns had a higher prevalence of Streptococcaceae, Mediterraneibacter gnavus (basonym: Ruminococcus gnavus) group, and Mediterraneibacter gnavus group (unidentified sp.) (Fig. 5B, C). Besides, feces samples from NEC_AS newborns were significantly enriched for Eggerthellaceae, uncultured Senegalimassilia, uncultured Senegalimassilia (unidentified sp.), and Lactococcus compared to no-NEC_AS newborns (Fig. 5B, C).
Aberrant cytokines-gut microbiota interactions impact the development of NEC
Finally, a correlation analysis between plasma cytokine levels and the 23 most abundant bacterial genera was performed (Fig. 6). A predominantly moderate positive relationship between bacterial taxa and plasma cytokine levels was revealed in no-NEC newborns preoperatively but predominantly negative postoperatively. Of note, multiple correlation patterns were observed for Staphylococcus, Stenotrophomonas, Lachnoclostridium, and Cutibacterium in the preoperative period. Meanwhile, many correlations were noticed in the postoperative period for Lactobacillus, Veillonella, Clostridium_sensu_stricto_1, Lachnoclostridium, and Flavonifractor (Fig. 6). Additionally, positive interactions within the microbiota community became more complex and stronger in the postoperative period compared to those in the preoperative period. Correlations between the microbiota and plasma cytokine levels were almost absent in NEC newborns during the observation period (Fig. 6). Moreover, there were scarcely any correlations within the microbiota community. Collectively, these results emphasize the impact of the immune-gut microbiota axis on the risk of NEC development.
Correlation heat map between the cytokine plasma levels, laboratory parameters and the top 23 most abundant genera in gut microbiota of term newborns with critical congenital heart defects with or without necrotizing enterocolitis (NEC) across the perioperative period. Color scale bars show a range of nonparametric Spearman correlation (r). The red color corresponds to a strong positive correlation, the purple color - a strong negative correlation. Only significant correlations are presented (p < 0.03). No-NEC_BS and No-NEC_AS represent no-NEC newborns before and after cardiac surgery, NEC_BS and NEC_AS represent NEC newborns before and after cardiac surgery, respectively.
Discussion
The main factor in intestinal homeostasis is mucosal immunity, which comprises innate and adaptive immune cells, intestinal epithelial and connective tissue cells, as well as commensal bacteria22,23. To our knowledge, no investigations of the immune-microbiota axis during the development of NEC in term neonates with CHD in the early neonatal period have been previously performed. Here, we found that a skewed post-birth cytokine landscape, characterized by a decrease in inflammasome-associated cytokine IL-18 and an increase in type1/type2-associated cytokines, is one of the triggers contributing to the later onset of NEC in term infants with CHD. A plasma cytokine signature, consisting of 35 cytokines, enabled the differentiation of newborns with CHD at high risk of NEC development. A panel including IL-1RA, IL-5, IL-18, and MCP-1 showed impressive test performance characteristics for identifying NEC at the preclinical stage, while M-CSF demonstrated potential for diagnosing NEC at the clinical stage. The microbiota community mediated the balanced cytokine network dynamics under a wide spectrum of “friend or foe” conditions, effectively dampening excessive inflammatory responses during early postnatal adaptation. However, under low microbial diversity, a strong imbalanced cytokine feedback loop formed, resulting in deviations from normal immune response maturation and, consequently, an aberrant immune response in the early postoperative period.
In the first weeks of life, cytokines play a pivotal role in the adaptation of the immune system to a new environment, the development of lymphoid progenitors, and the formation of immune tolerance to commensal bacteria, but unbalanced release of pro-inflammatory cytokines can lead to inflammation and increased intestinal epithelial permeability24,25,26. In healthy term newborns during the first week of life, plasma levels of type 1 immune response-associated cytokines, such as IFNγ, CXCL10 (IP10), GM-CSF, and plasma levels of IL-5, which contributes to type 2 response and eosinophil activation, significantly increased, while IL-1RA, IL-6, IL-10, CCL4 (MIP-1β), CXCL8 (IL-8), G-CSF, TGFα, and IL-12p40 decreased, altogether reflecting the resolution of the birthing process25. In our study, during the first week of life, the ratio of type 1/type 2 immune response-associated cytokines appeared to be different in the two newborn groups, primarily driven by elevated levels of IL-12p40 and IL-17E/IL-25 in NEC newborns, and it seems to reflect an aberrant shift in immune adaptation to the new environment in this group of infants. This observation was further supported by the variety of multiple type 1, type 2, and type 3 immune response-associated cytokine correlations in the two infant groups.
Intriguingly, the proinflammatory cytokine IL-18, produced by the inflammasome and stimulating both innate and adaptive immunity, facilitating a type 1 immune response in the presence of IL-12 or activating Th2 cytokine production in the presence of IL-227,28, had the highest impact on the difference in the proportions of plasma cytokines between the two groups of neonates. A variety of cells are known to produce IL-18, including IECs, in response to LPS binding to TLR427. Epithelial-derived IL-18 participates in intestinal homeostasis by regulating intestinal CD4 + T cell subsets, particularly by limiting Th17 cell differentiation and promoting Foxp3 + Treg cells that prevent systemic and tissue-specific autoimmunity and play a key role in immune tolerance29,30. IL-18 enhances host defense against bacterial infections improving bacterial clearance27. Another IEC-derived cytokine involved in intestinal immune homeostasis is IL-17E/IL-25, activating Th2 cytokine production by ILC2s that contributes to epithelial barrier function and tissue repair31,32,33. Although the IL-17E/IL-25 level did not differ between no-NEC and NEC newborns, it had a high impact on the difference in the proportions of plasma cytokines, along with IL-18, in no-NEC newborns during the perioperative period. After surgery, the proportion of IL-18 dramatically decreased, while the proportion of IL-17E/IL-25 doubled. Thus, the effective activation followed by down-regulation of the NLRP3 inflammasome during the first week of life may be one of the crucial steps in the development of the balanced immune response towards type1/type2/type3, which may explain why newborns with NEC have an increased susceptibility.
Many studies revealed that elevated plasma levels of IL-8 in preterm infants are associated with NEC pathogenesis, which is more pronounced in the surgical stage of NEC, and directly correlate with mortality34,35,36,37. IL-8 in combination with IL-24 and CCL20 was found to give the best prediction value for NEC and control, NEC and sepsis, as well as distinguish the severity of NEC in preterm infants36. Also, intestinal villi damage in NEC rat models was found to be associated with high levels of pro-inflammatory cytokines, including IL-838. In our study, IL-8 levels significantly decreased in no-NEC newborns after surgery but remained unchanged in NEC newborns. Given that IL-8 stimulates neutrophil migration to the site of inflammation39, our data may reflect its potential for continued neutrophil recruitment to local tissue inflammation sites and neutrophil extracellular traps (NETs) formation in NEC pathogenesis. Recently, it has been supposed that reducing NET formation would be beneficial in preventing hyperinflammatory damage, at least after the start of antibiotic therapy at NEC onset40.
Both groups had equal M-CSF levels preoperatively, whereas they dramatically decreased in no-NEC_AS newborns. In contrast, M-CSF levels in NEC newborns remained unchanged and showed no correlations. Increased M-CSF levels have been reported in inflammatory bowel disease (IBD) and many inflammatory/autoimmune diseases41,42. Given that M-CSF, produced by macrophages and endothelial cells, stimulates the differentiation and maturation of villi42, the obtained results suggest that after surgery, low M-CSF levels may serve as a limiting factor, potentially hindering the involvement of a greater intestinal surface in the inflammatory process.
Newborns with CHD often receive antibiotic therapy both before and after surgical intervention to manage suspected infections, and in some cases, probiotic administration may also be considered. These interventions are known to influence gut microbiota colonization, skew immune response polarization, disrupt the establishment of tolerance to commensal microorganisms, and ultimately alter cytokine production, potentially leading to imbalances in plasma cytokine levels. Although the impact of antibiotics on cytokine production may vary across different conditions, the similar antibiotic administration between the two newborn groups in our study suggests a consistent effect of antibiotics on plasma cytokine profiles and indicates that other factors may influence cytokine levels.
During the early neonatal period, aberrant and depleted bacterial colonization of the small intestine may influence an imbalance of cytokines released in response to a new environment, leading to systemic and local inflammation, intestinal barrier injury, impaired tolerance development to the commensal microbiota, increased intestinal permeability, and subsequent NEC development. Indeed, the symbiotic microbiota, both directly and indirectly, through generated metabolites, participates in constitutive cytokine secretion and Th cell ‘polarization’ toward Treg cells27,43,44.
The impact of abnormal gut microbiota on the risk of developing NEC has been previously confirmed45. Several studies revealed that low diversity of intestinal microbiota, with the prevalence of Pseudomonadota (class Gammaproteobacteria, family Enterobacteriaceae) and a decrease in Bacillota species, is typical for preterm infants who develop NEC46,47,48,49. Two different gut microbiome compositions in the first few weeks of life were found to be associated with NEC development in preterm infants: one with predominant Bacillota (> 98%), particularly Staphylococcus and Enterococcus, and the second with dominant Gram-negative Pseudomonadota, specifically the family Enterobacteriaceae, genera Enterobacter, and Escherichia50. Also, term neonates with CHD had a depleted microbiome composition, characterized by a decrease in Actinomycetota, Bacteroidota, and Enterobacteriaceae and an increase in Bacillota compared to healthy neonates51.
Our results revealed that the gut microbiota of NEC newborns before NEC onset exhibited a tendency toward lower alpha-diversity in terms of phylogeny, evenness, richness, and abundance and was characterized by increased Actinomycetota and decreased Bacteroidota, which is generally consistent with previous studies. As expected, Enterobacteriaceae, previously associated with NEC, were found to be more abundant in NEC newborns at NEC onset. However, no specific, unique pathogen has been implicated in NEC development. Two potentially proinflammatory taxa, Burkholderia-Caballeronia-Paraburkholderia and Ruminococcus lactaris, were present only in the gut microbiota of a few newborns before NEC onset. Enrichment in Burkholderia-Caballeronia-Paraburkholderia was found in patients with sepsis-induced cholestasis and in mice with induced colitis46,52. Ruminococcus lactaris and its metabolites (succinate, formate, and ethanol) were found to stimulate the production of inflammatory signaling molecules and are implicated in oxidative stress53,54. Interestingly, a dramatic difference between the presence of Collinsella before and after surgery was found in NEC newborns. Although Collinsella can produce ursodeoxycholate, which suppresses proinflammatory cytokines and has antiapoptotic and antioxidant effects55,56, this genus is generally considered a pathobiont. Collinsella increases intestinal permeability57 and its relative abundance is elevated in pediatric patients with penetrating Crohn’s disease58. Besides, the pathobiont Mediterraneibacter gnavus (basonym: Ruminococcus gnavus) group, found to be abundant in feces of NEC newborns prior to cardiac surgery, has been linked to increased intestinal paracellular permeability, IBD, and diarrhea-predominant irritable bowel syndrome59,60,61. Moreover, Mediterraneibacter gnavus exopolysaccharides influence host immune response by inducing the production of cytokines and chemokines involving the TLR4 signaling pathway62.
In contrast, the gut microbiota of no-NEC newborns was more diverse during the first week of life, with six genera (Parabacteroides, Enhydrobacter, Bacteroides, Actinomyces, Escherichia-Shigella, and Clostridioides) being differentially abundant compared to NEC newborns, although the relative abundance of these genera varied widely across newborns. Among those listed above, unexpectedly, Clostridioides difficile, a potential pathobiont63, was differentially abundant in no-NEC newborns compared to NEC newborns prior to surgery.
In addition, potentially protective taxa Phocaeicola massiliensis and Parabacteroides merdae, found only in no-NEC_BS, have been associated with the production of short-chain fatty acids (SCFAs)64,65. Production of SCFAs by gut commensal bacteria was found to regulate Treg/Th17 balance and protect against epithelial injury66. Considering a wide correlation network between plasma cytokines and the microbiota consortium observed only in no-NEC newborns, the results suggest that the diversity of microorganisms, each with potentially unique properties essential for the immune system adaptation and fine-tuning in the first days of life, could be a major factor in protecting against NEC. This is supported by the lack of the mono-strain probiotic effect on the fecal microbiota of neonates with CHD compared to healthy ones51, as well as the positive effect of a multi-strain probiotic on the incidence and severity of NEC in preterm neonates67.
The main limitations of our study are the lack of a standardized uniform nutrition protocol for all newborns enrolled in this study and the fact that fecal samples were obtained within a 72-hour window around the time point of blood collection. Moreover, the sample size was relatively small, and larger-scale studies are necessary to validate the findings.
In conclusion, a disrupted balance in the immune-microbiota axis in NEC newborns with CHD throughout the entire perioperative period had a crucial impact on NEC onset. A panel including IL-1RA, IL-5, IL-18, and MCP-1 shows promise for diagnosing NEC at the preclinical stage in newborns with CHD and could greatly improve outcomes for this high-risk group. Taking together, our findings further encourage focusing on factors in the antenatal and intrapartum periods that influence the post-birth cytokine landscape and gut microbiota diversity, as well as whether the inflammatory mediators we found in our research are specific for NEC or are common to other inflammatory disease processes in the early neonatal period.
Methods
Study design
Thirty-six term neonates with a gestational age of more than 37 weeks and a birth weight of more than 2500 g with critical CHD were enrolled in this study (Supplementary Fig. 5). The spectrum of CHD included: transposition of the great arteries, coarctation of the aorta, pulmonary atresia/stenosis, aortic valve stenosis, hypoplastic left heart syndrome, double-inlet left ventricle, unbalanced (RV dominant) atrioventricular septal defect, tricuspid atresia, double outlet right (single) ventricle, and double outlet right ventricle (Supplementary Table 6). All participants were born at the Almazov National Medical Research Centre (Saint-Petersburg, Russia). The exclusion criteria were the development of NEC before cardiac surgery, congenital malformations of the gastrointestinal tract, death during the first two weeks of life, ECMO, lack of material at two time points (before and after cardiac surgery), a discrepancy in the timing of feces and blood collection by more than 72 h, and unwillingness to participate in the study for any personal reason.
NEC was diagnosed based on both abnormal plain abdominal radiography and clinical signs; the severity of the disease was classified according to the modified Bell staging criteria68. The median infant age at NEC diagnosis was 12 days (interquartile range: 6–16).
This study was approved by the Ethics Committee of Almazov National Medical Research Center (protocol no. 1702-21, February 15, 2021) and complied with the Helsinki Declaration. Written informed consent was obtained from the parents of all participating neonates.
Routine management of newborns
CHD was suspected prenatally in all newborns, and it was confirmed by postnatal echocardiography and computed tomography. Following birth, newborns were admitted to the neonatal intensive care unit and administered an intravenous prostaglandin E1 infusion before cardiac surgery due to duct-dependent circulation. Three no-NEC and three NEC newborns required Rashkind / stenting procedure on the first day of life (p = 0.109).
In the preoperative period, newborns were fed according to the internal protocol, which involved initiating enteral nutrition with preterm formula (80 Kcal/100mL), followed by mixed feeding or feeding with the mother’s own expressed milk. The types of feeding substrates before cardiac surgery are presented in Supplementary Table 1. The volume of enteral feeding the day before cardiac surgery was 61.5 (35.5–85.5) mL/kg/day in no-NEC neonates and 72.30 (43.5–92.3) mL/kg/day in NEC newborns (p = 0.394). In the early neonatal period, 13 (46%) no-NEC newborns and 5 (62%) NEC newborns required antibiotic therapy. Five no-NEC newborns suffered congenital pneumonia. Other indications for antibacterial therapy in no-NEC group, due to suspected infection, included increasing CRP levels (8 newborns). In NEC newborns, antibacterial therapy was initiated in four neonates due to maternal chorioamnionitis (1 neonate), increasing leukocytosis (1 newborn), increasing neutrophilia (1 newborn), and increasing CRP levels (2 newborns). The initial antibiotic therapy was ampicillin/sulbactam.
Cardiac surgery was performed at the Almazov National Medical Research Centre. The groups were comparable by the incidence of cardiopulmonary bypass during cardiac surgery (Supplementary Table 1). During surgery, some newborns underwent thymectomy to provide access, depending on the type of CHD and surgical intervention. The groups of newborns were comparable in terms of the frequency of thymectomy during cardiac surgery (Supplementary Table 1).
All newborns received prophylaxis with intravenous cefuroxime, administered at a dose of 50 mg/kg 30 min before surgery and then at a dose of 50 mg/kg every 8 h (three times) after surgery. Early postoperative period in no-NEC newborns was complicated by the development of pneumonia (6 newborns), an inflammatory process with increasing CRP levels that required a change in antibiotic therapy (2), a urinary tract infection (1), a positive blood culture with Streptococcus epidermidis (1), and severe hemodynamic disturbances requiring extracorporeal membrane oxygenation (1).
Enteral feeding (with a hydrolyzed formula at a rate of 2 mL/h) was initiated during the early postoperative period once hemodynamics had stabilized, contingent upon the absence of NEC symptoms as determined by ultrasound examination.
At NEC onset, newborns were kept on nil per os, total parenteral nutrition, and antibiotic therapy. The management and treatment of newborns with NEC were provided according to national guidelines69.
Sample collection
Blood and fecal samples were collected at two time points: before (median DOL 5, interquartile range: 4–7) and after (median DOL 14, interquartile range: 12–15) surgical intervention. Blood samples were obtained into EDTA tubes as part of the pre- and postoperative diagnostic workup. To avoid excessive blood loss and pain in newborns, only leftover blood after routine laboratory tests was collected; plasma was separated within 8 h after collection and stored at -40 °C until use. In this study, only fecal samples obtained within a 72-hour window around the time point of blood collection were included. Besides, the majority of fecal samples (89%) were collected within 24 h of blood sampling, and 11% of samples were collected within 72 h. Fecal samples were collected from diapers, placed into sterile microcentrifuge tubes, and stored at -40 °C until DNA extraction.
Cytokine and chemokine quantification
Plasma cytokine, chemokine, and growth factor concentrations were assessed by multiplex analysis using the MILLIPLEX® MAP Human Cytokine/Chemokine/Growth Factor Panel A (HCYTA-60 K-PX48, MilliporeSigma, Burlington, MA, USA) according to the manufacturer’s instructions. Overnight incubation at 4 °C and a handheld magnet were utilized.
The following 47 factors were evaluated: soluble CD40L (sCD40L), epidermal growth factor (EGF), eotaxin, fibroblast growth factor-2 (FGF-2), FMS-like tyrosine kinase 3 ligand (FLT3L), fractalkine, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), growth-regulated oncogene (GROα), interferons (IFNα2, IFNγ), interleukins (IL-1α, IL-1β, IL-1RA, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17 A, IL-17E/IL-25, IL-17 F, IL-18, IL-22, IL-27), interferon γ-induced protein 10 (IP-10), monocyte chemoattractant proteins (MCP-1, MCP-3), macrophage colony-stimulating factor (M-CSF), macrophage-derived chemokine (MDC), monokine induced by interferon-gamma (MIG), macrophage inflammatory proteins (MIP-1α, MIP-1β), platelet-derived growth factors (PDGF-AA, PDGF-AB/BB), transforming growth factor (TGFα), tumor necrosis factors (TNFα, TNFβ), vascular endothelial growth factor (VEGF-A).
The plates were analyzed on a Luminex MAGPIX System (Luminex Corporation, Austin, TX, USA) and the data were generated with xPONENT software version 4.3. The results were expressed in picograms per milliliter (pg/mL).
16S rRNA gut microbiota profiling
The gut microbiota profiles were determined by high-throughput sequencing of the V4 region of the bacterial 16S rRNA gene. Total DNA was extracted from 250 mg of fecal samples using the QIAamp PowerFecal Pro DNA Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The quality and quantity of extracted DNA were assessed using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The DNA was stored at -20 °C until use. Amplicons were generated using NEXTflex 16 S V4 Amplicon-Seq Kit 2.0 (PerkinElmer, Inc., Waltham, MA, United States), according to the manufacturer’s instructions. All amplification steps were performed on a Veriti Thermal Cycler (Applied Biosystems, Waltham, MA, United States). Amplicons were purified with AMPure XP Reagent (Beckman Coulter, Inc., Brea, CA, United States) and quantified with a Qubit 2.0 Fluorometer (Invitrogen, Waltham, MA, United States). Sequencing was performed on the MiSeq platform using MiSeq Reagent Kit v2 (250 bp × 2) (Illumina Inc., San Diego, CA, United States).
Bioinformatic analysis
The raw sequences were processed using the QIIME 2 pipeline version 2023.570. Adapters and primers in forward and reverse sequences were trimmed with the q2-cutadapt plugin, and the reads were denoised and merged using the DADA2 (q2-dada2 denoise-paired) plugin71. The average sequence depth was 161.888 (with a range of 124.539-230.167) reads per sample, while denoising 5.989.845 raw reads were processed to 5.602.536 merged reads. Finally, a total of 5187 amplicon sequence variants (ASVs) were obtained. Taxonomic classification was performed with a pre-trained Naive Bayes classifier on the SILVA v.138 NR 99 database for the V4 region72. ASVs with an abundance of less than 0.01% in each sample and undefined taxa or taxonomic assignment as mitochondria or chloroplasts were filtered and removed. As a result, 503 ASVs, accounting for 5.050.918 quality clean reads, remained for subsequent analysis.
Alpha-diversity metrics, Shannon’s diversity index, Faith’s phylogenetic diversity (Faith’s PD), Pielou’s evenness, and observed species were assessed with the q2-diversity plugin in QIIME 270. Differences in alpha diversity metrics between sample groups were calculated using Kruskal-Walli’s test (p < 0.05) in QIIME 2. Beta-diversity (Bray-Curtis and Jaccard) statistics were calculated with the Scipy package in Python73, and dimensionality reduction was performed with the Uniform Manifold Approximation and Projection (UMAP)74.
The differential abundance of taxa was determined by pairwise comparison between infant groups using DESeq2 (v. 1.38.3)75 in R (v. 4.2.3), with fitType = local and a threshold of an adjusted p-value < 0.05. Linear discriminant analysis (LDA) Effect Size (LEfSe) was performed on logarithmically transformed data using the ‘microbiomeMarker’ package (Bioconductor version 3.20)76 in R (v. 4.2.3), with a logarithmic LDA score threshold of > 2.0 and a p-value < 0.05. The Venn diagram was created with the Pyvenn package in Python.
Statistical analysis
Continuous variables are expressed as medians and interquartile ranges, and categorical variables as numbers and percentages. For continuous variables, the Mann-Whitney U test was used to assess differences between no-NEC and NEC newborns. The effect of surgery on cytokine levels was determined with the Wilcoxon Matched Pairs Test. For categorical variables, differences between groups were detected using the two-tailed Fisher’s exact test and the Kruskal-Wallis ANOVA test. ROC analysis was performed to assess the diagnostic accuracy of cytokines for NEC prediction. The Youden index was employed to determine the optimal cutoff value. A predictive model was developed using logistic regression. Correlation analysis of plasma cytokine levels was performed using a nonparametric Spearman rank test. The significance was set at p < 0.01. A forward stepwise discriminant analysis was performed to determine cytokines, chemokines, and growth factors able to distinguish infant groups. A discrimination level was evaluated by Wilks’ lambda. The interpretation of the results was based on scatterplots of canonical values, a classification matrix, and Mahalanobis squared distance. All statistical analyses were performed using Statistica 10.0 (StatSoft, USA) and Prism 9 (GraphPad, USA); p < 0.05 was considered statistically significant.
Data availability
The 16S rRNA data were deposited at the NCBI sequence read archive under the BioProject PRJNA1087717. All other data that support the findings of this study are available in the article or from the corresponding author upon reasonable request.
References
Neu, J., Mshvildadze, M. & Mai, V. A roadmap for Understanding and preventing necrotizing Enterocolitis. Curr. Gastroenterol. Rep. 10, 450–457 (2008).
Wertheimer, F., Arcinue, R. & Niklas, V. Necrotizing Enterocolitis: Enhancing awareness for the general practitioner. Pediatr. Rev. 40, 517–527 (2019).
Kashif, H., Abuelgasim, E., Hussain, N., Luyt, J. & Harky, A. Necrotizing Enterocolitis and congenital heart disease. Ann. Pediatr. Cardiol. 14, 507 (2021).
Bubberman, J. M. et al. Necrotizing Enterocolitis associated with congenital heart disease: A different entity?? J. Pediatr. Surg. 54, 1755–1760 (2019).
van der Heide, M. et al. Hypoxic/ischemic hits predispose to necrotizing Enterocolitis in (near) term infants with congenital heart disease: A case control study. BMC Pediatr. 20, 553 (2020).
Velazco, C. S. et al. Morbidity and mortality among big babies who develop necrotizing Enterocolitis: A prospective multicenter cohort analysis. J. Pediatr. Surg. 53, 108–112 (2018).
Ou, J., Courtney, C. M., Steinberger, A. E., Tecos, M. E. & Warner, B. W. Nutrition in necrotizing Enterocolitis and following intestinal resection. Nutrients 12, 520 (2020).
Duess, J. W. et al. Necrotizing Enterocolitis, gut microbes, and sepsis. Gut Microbes 15, (2023).
Gephart, S. M., McGrath, J. M., Effken, J. A. & Halpern, M. D. Necrotizing Enterocolitis risk. Adv. Neonatal. Care. 12, 77–87 (2012).
Aydoğan, S. et al. Lactobacillus rhamnosus sepsis associated with probiotic therapy in a term infant with congenital heart disease. Fetal Pediatr. Pathol. 41, 823–827 (2022).
Molteni, M., Gemma, S. & Rossetti, C. The role of toll-like receptor 4 in infectious and noninfectious inflammation. Mediat. Inflamm. 2016, 1–9 (2016).
Cho, S. X., Berger, P. J., Nold-Petry, C. A. & Nold, M. F. The immunological landscape in necrotising Enterocolitis. Expert Rev. Mol. Med. 18, e12 (2016).
Jang, J. H. et al. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediat. Inflamm. 2015, 1–12 (2015).
Lu, P., Sodhi, C. P. & Hackam, D. J. Toll-like receptor regulation of intestinal development and inflammation in the pathogenesis of necrotizing Enterocolitis. Pathophysiology 21, 81–93 (2014).
Werts, A. D. et al. A novel role for necroptosis in the pathogenesis of necrotizing Enterocolitis. Cell. Mol. Gastroenterol. Hepatol. 9, 403–423 (2020).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2, 17023 (2017).
Tisoncik, J. R. et al. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32 (2012).
Cui, A. et al. Dictionary of immune responses to cytokines at single-cell resolution. Nature 625, 377–384 (2024).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell. Res. 30, 492–506 (2020).
Annunziato, F., Romagnani, C. & Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 135, 626–635 (2015).
Zhu, X. & Zhu, J. CD4 T helper cell subsets and related human immunological disorders. Int. J. Mol. Sci. 21, 8011 (2020).
Stras, S. F. et al. Maturation of the human intestinal immune system occurs early in fetal development. Dev. Cell. 51, 357–373e5 (2019).
Hodzic, Z., Bolock, A. M. & Good, M. The role of mucosal immunity in the pathogenesis of necrotizing Enterocolitis. Front. Pediatr. 5 (2017).
Burge, K. Y. et al. Clinical characteristics and potential pathogenesis of cardiac necrotizing Enterocolitis in neonates with congenital heart disease: A narrative review. J. Clin. Med. 11, 3987 (2022).
Smolen, K. K. et al. Ontogeny of plasma cytokine and chemokine concentrations across the first week of human life. Cytokine 148, 155704 (2021).
Bennike, T. B. et al. Preparing for life: Plasma proteome changes and immune system development during the first week of human life. Front. Immunol. 11, (2020).
Yasuda, K., Nakanishi, K. & Tsutsui, H. Interleukin-18 in health and disease. Int. J. Mol. Sci. 20, 649 (2019).
Vecchié, A. et al. IL-18 and infections: Is there a role for targeted therapies? J. Cell. Physiol. 236, 1638–1657 (2021).
Harrison, O. J. et al. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3 + Treg cell function in the intestine. Mucosal Immunol. 8, 1226–1236 (2015).
Lei-Leston, A. C., Murphy, A. G. & Maloy, K. J. Epithelial cell inflammasomes in intestinal immunity and inflammation. Front. Immunol. 8, (2017).
Amatya, N., Garg, A. V. & Gaffen, S. L. IL-17 signaling: The Yin and the Yang. Trends Immunol. 38, 310–322 (2017).
Ham, J., Shin, J. W., Ko, B. C. & Kim, H. Y. Targeting the Epithelium-Derived innate cytokines: From bench to bedside. Immune Netw. 22, (2022).
Sunaga, S., Tsunoda, J., Teratani, T., Mikami, Y. & Kanai, T. Heterogeneity of ILC2s in the intestine; homeostasis and pathology. Front. Immunol. 13, (2022).
Benkoe, T. et al. Interleukin-8 predicts 60-day mortality in premature infants with necrotizing Enterocolitis. J. Pediatr. Surg. 49, 385–389 (2014).
Bhatia, A., Stoll, B., Cismowski, M. & Hamrick, S. Cytokine levels in the preterm infant with neonatal intestinal injury. Am. J. Perinatol. 31, 489–496 (2013).
Dong, H. et al. Screening inflammatory protein biomarkers on premature infants with necrotizing Enterocolitis. Inflamm. Res. 72, 757–768 (2023).
Nanthakumar, N. et al. The mechanism of excessive intestinal inflammation in necrotizing Enterocolitis: An immature innate immune response. PLoS One. 6, e17776 (2011).
Zhu, H., Lin, Y. & Liu, Y. miR-34a increases inflammation and oxidative stress levels in patients with necrotizing Enterocolitis by downregulating SIRT1 expression. Mol. Med. Rep. 24, 664 (2021).
Matsushima, K., Yang, D. & Oppenheim, J. J. Interleukin-8: An evolving chemokine. Cytokine 153, 155828 (2022).
Klinke, M., Chaaban, H. & Boettcher, M. The role of neutrophil extracellular traps in necrotizing Enterocolitis. Front. Pediatr. 11, (2023).
Ushach, I. & Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 100, 481–489 (2016).
Douglass, T. G. et al. Macrophage colony stimulating factor: Not just for macrophages anymore! A gateway into complex biologies. Int. Immunopharmacol. 8, 1354–1376 (2008).
Opipari, A. & Franchi, L. Role of inflammasomes in intestinal inflammation and Crohnʼs disease. Inflamm. Bowel Dis. 21, 173–181 (2015).
Colliou, N. et al. Commensal Propionibacterium strain UF1 mitigates intestinal inflammation via Th17 cell regulation. J. Clin. Investig. 127, 3970–3986 (2017).
Kaplina, A. et al. Necrotizing Enterocolitis: The role of hypoxia, gut microbiome, and microbial metabolites. Int. J. Mol. Sci. 24, 2471 (2023).
Wang, Y. J., Li, Q. M., Zha, X. Q. & Luo, J. P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 13, 143–160 (2022).
Mai, V. et al. Fecal microbiota in premature infants prior to necrotizing Enterocolitis. PLoS One. 6, e20647 (2011).
Lindberg, T. P. et al. Preterm infant gut microbial patterns related to the development of necrotizing Enterocolitis. J. Maternal-Fetal Neonatal Med. 33, 349–358 (2020).
Kelleher, S. T., McMahon, C. J. & James, A. Necrotizing Enterocolitis in children with congenital heart disease: A literature review. Pediatr. Cardiol. 42, 1688–1699 (2021).
Morrow, A. L. et al. Early microbial and metabolomic signatures predict later onset of necrotizing Enterocolitis in preterm infants. Microbiome 1, 13 (2013).
Ellis, C. L. et al. Probiotic administration in congenital heart disease: A pilot study. J. Perinatol. 33, 691–697 (2013).
Zhang, B. et al. Acute Gastrointestinal injury and altered gut microbiota are related to sepsis-induced cholestasis in patients with intra-abdominal infection: A retrospective and prospective observational study. Front. Med. (Lausanne) 10 (2023).
Geng, J., Liu, C., Xu, J., Wang, X. & Li, X. Potential relationship between tourette syndrome and gut Microbiome. J. Pediatr. (Rio J). 99, 11–16 (2023).
Togo, A. H. et al. Description of Mediterraneibacter Massiliensis, gen. Nov., Sp. Nov., a new genus isolated from the gut microbiota of an obese patient And reclassification of Ruminococcus faecis, Ruminococcus lactaris, Ruminococcus torques, Ruminococcus gnavus And Clostridium glycyrrhizinilyticum as Mediterraneibacter faecis comb. Nov., Mediterraneibacter lactaris comb. Nov., Mediterraneibacter torques comb. Nov., Mediterraneibacter gnavus comb. Nov. And Mediterraneibacter Glycyrrhizinilyticus comb. Nov. Antonie Van Leeuwenhoek. 111, 2107–2128 (2018).
Hirayama, M. et al. Intestinal Collinsella May mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS One. 16, e0260451 (2021).
Li, X. et al. Design, synthesis and evaluation of ursodeoxycholic acid-cinnamic acid hybrids as potential anti-inflammatory agents by inhibiting Akt/NF-κB and MAPK signaling pathways. Eur. J. Med. Chem. 260, 115785 (2023).
Mena-Vázquez, N. et al. Expansion of rare and harmful lineages is associated with established rheumatoid arthritis. J. Clin. Med. 9, 1044 (2020).
Kugathasan, S. et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: A multicentre inception cohort study. Lancet 389, 1710–1718 (2017).
Kaczmarczyk, M. et al. The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life. J. Transl. Med. 19, 177 (2021).
Vestergaard, M. V. et al. Gut microbiota signatures in inflammatory bowel disease. United Eur. Gastroenterol. J. 12, 22–33 (2024).
Han, L. et al. Altered metabolome and Microbiome features provide clues in Understanding irritable bowel syndrome and depression comorbidity. ISME J. 16, 983–996 (2022).
Laplanche, V. et al. The human gut symbiont Ruminococcus gnavus displays strain-specific exopolysaccharides modulating the host immune response. Carbohydr. Polym. 347, 122754 (2025).
Schönherr-Hellec, S. & Aires, J. Clostridia and necrotizing Enterocolitis in preterm neonates. Anaerobe 58, 6–12 (2019).
Bajic, D. et al. HMOs exert marked bifidogenic effects on children’s gut microbiota ex vivo, due to Age-Related Bifidobacterium species composition. Nutrients 15, 1701 (2023).
Qiao, S. et al. Gut Parabacteroides merdae protects against cardiovascular damage by enhancing branched-chain amino acid catabolism. Nat. Metab. 4, 1271–1286 (2022).
Liu, Y. J. et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10, 5225–5241 (2020).
Sowden, M. et al. Effect of a Multi-Strain probiotic on the incidence and severity of necrotizing Enterocolitis and feeding intolerances in preterm neonates. Nutrients 14, 3305 (2022).
Walsh, M. C. & Kliegman, R. M. Necrotizing Enterocolitis: Treatment based on staging criteria. Pediatr. Clin. N. Am. 33, 179–201 (1986).
Dorofeyeva, E. I. et al. Diagnosis and conservative treatment of necrotizing enterocolitis in newborn (project of clinical practice guidelines). Neonatol. News Views Educ. 2, 84–92 (2014).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible Microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: High-resolution sample inference from illumina amplicon data. Nat. Methods. 13, 581–583 (2016).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2012).
Virtanen, P. et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods. 17, 261–272 (2020).
McInnes, L., Healy, J. & Melville, J. U. M. A. P. Uniform Manifold Approximation and Projection for Dimension Reduction (2018).
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Cao, Y. et al. MicrobiomeMarker: an R/Bioconductor package for Microbiome marker identification and visualization. Bioinformatics 38, 4027–4029 (2022).
Acknowledgements
We would like to thank the doctors of the Perinatal Centre of Almazov National Medical Research Centre who treated and cared for newborns: Nikiforov V, Latypov A, Volkova Yu, Alkhova T, Kim M, Shemyakina O, Vagina E, Podgurskaya T, Zaretskaya Yu, Khavkina M, Poskrebysheva S, Klimenko A, Kiseleva N, Guryanova N, Islamova K, Treskina N, and neonatal nurses for their help in the investigation.
Funding
This work was supported by the Russian science Foundation (project No. 19-75-20076, https://rscf.ru/project/19-75-20076/).
Author information
Authors and Affiliations
Contributions
E.K., A.K., N.P., A.G., and O.K. conceptualized the study. E.K. performed experiments. A.K., N.P. and T.P. coordinated and provided patient samples. D.B. analyzed sequencing data. E.K., S.S., I.K., A.G., and O.K. analyzed and interpreted the data. A.K., and T.P. supervised the research. E.K., A.K., S.S., I.K., A.G., and O.K. wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Ethics Committee of Almazov National Medical Research Center (protocol no. 1702-21, February 15, 2021) and complied with the Helsinki Declaration. Written informed consent was obtained from the parents of all participating neonates.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zaikova, E., Kaplina, A., Belozertsev, D. et al. An early post-birth immune-microbiota landscape predicts the development of necrotizing enterocolitis in term newborns with congenital heart defects. Sci Rep 15, 33811 (2025). https://doi.org/10.1038/s41598-025-02542-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-02542-6