Abstract
This study aimed to investigate the association between dietary vitamin K intake and all-cause mortality in individuals with non-alcoholic fatty liver disease (NAFLD). We analyzed data from 7857 NAFLD participants in the National Health and Nutrition Examination Survey (NHANES 2005–2018) linked to mortality outcomes from the National Death Index (NDI). Dietary vitamin K intake was log-transformed (ln[VK]) for analysis. Multivariable Cox proportional hazards models and restricted cubic splines were used to evaluate dose-response relationships. Sensitivity analyses, subgroup analyses, and receiver operating characteristic (ROC) curves were performed to validate findings. Over 180 months of follow-up, 842 deaths occurred. Higher ln[VK] was associated with reduced mortality risk, demonstrating an adjusted hazard ratio (HR) of 0.81 per 1-unit increasement (95% confidence interval CI 0.67–0.98, P-trend = 0.028). Restricted cubic splines revealed a U-shaped relationship (P-nonlinear = 0.0009), with an optimal threshold at 121 µg/day (ln[VK] = 4.71). Below this threshold, each unit increase in ln[VK] corresponded to a 33% lower mortality risk (HR = 0.67; 95% CI 0.55–0.81), whereas no significant association was observed above it (HR 1.07; 95% CI 0.67–1.71). The fully adjusted prediction model showed robust discriminative ability, with an area under the curve (AUC) of 0.832. Results remained consistent across sensitivity analyses. Moderate dietary vitamin K intake (up to 121 µg/day) is associated with reduced all-cause mortality in NAFLD patients, with a threshold effect informing tailored dietary recommendations. This study provides novel evidence for optimizing vitamin K intake in NAFLD management.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a growing global public health concern, characterized by an excessive accumulation of fat in the liver. Its prevalence has risen significantly, from 25.3% between 1990–2006 to 38.0% in 2016–20191,2. The pathogenesis of NAFLD involves multiple factors such as diet, insulin resistance, oxidative stress, and mitochondrial dysfunction3,4,5. Recent studies suggest a potential link between vitamin deficiencies and NAFLD6,7,8, with particular attention to vitamin K due to its antioxidant, anti-inflammatory, and lipid metabolism regulatory properties9,10.
Vitamin K, a fat-soluble vitamin, has been shown to reduce oxidative stress and mitigate liver cell damage caused by reactive oxygen species (ROS)11. It may also regulate lipid metabolism by influencing fatty acid synthesis and breakdown, potentially reducing hepatic fat accumulation12. Preclinical studies, particularly in high-fat diet mouse models, suggest that vitamin K2 may protect against NAFLD progression by modulating key enzymes such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) and reducing body weight and fat accumulation13.
However, the association between vitamin K and long-term outcomes, particularly all-cause mortality in NAFLD patients, remains unclear. To address this gap, this study aims to investigate the relationship between dietary vitamin K intake and long-term all-cause mortality in NAFLD patients, utilizing data from the National Health and Nutrition Examination Survey (NHANES) and the National Death Index (NDI).
Materials and methods
Study design
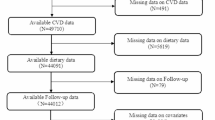
The NHANES data were used to assess the nutritional and health status of the non- institutionalized U.S. population14. The study protocol was approved by the Institutional Review Board of the National Center for Health Statistics, and relevant data are freely available for download from the NHANES website. Interview data were collected from participants between 2005 and 2018. These data were linked with the National Death Index (NDI) to establish a longitudinal follow-up cohort for determining participant survival status15. The National Center for Health Statistics (NCHS) Ethics Review Board approved this study, and all participants provided informed consent forms. No additional informed consent or ethical review was required for this analysis. Of the 60,041 participants from 2005 to 2018, 12,727 with U.S. Multi-Ethnic Fatty Liver Index (US-FLI) ≥ 30 were selected for further analysis (Fig. 1). The final study population included 7857 participants. The patient selection process is illustrated in Fig. 1.
Flowchart of the non-alcoholic fatty liver disease (NAFLD) study population from NHANES (2005–2018). Note: Some exclusion criteria may overlap or not be listed separately, causing discrepancies in the total excluded count; Participants with cancer were excluded during initial screening due to high missing values of cancer history data across survey cycles.
Measurement of dietary vitamin K intake
Dietary data were derived from two 24-h recall interviews, one conducted at the Mobile Examination Center (MEC) and the other via telephone 3 to 10 days later. All eligible participants completed both interviews, during which they reported the types and quantities of food consumed in the 24 h preceding each interview. The dietary data were analyzed using the Food and Nutrient Database for Dietary Studies (FNDDS) and the standardized Automated Multiple-Pass Method (AMPM) to ensure accuracy. Finally, the daily dietary vitamin K intake was calculated as the average of the two dietary recalls; if only one recall was available, that value was used. Based on NHANES data, primary dietary sources of vitamin K in our cohort included leafy greens (spinach, kale), vegetable oils).
Definition of fatty liver based on the U.S. multi-ethnic fatty liver index
NAFLD was defined using the U.S. Multi-Ethnic Fatty Liver Index (US-FLI), The US-FLI is calculated using the following validated formula16: \(x = (e^{{ - 0.8073*non - Hispanic\;black + 0.3458*}}\)\(^{{Mexican\;American + 0.0093*age + 0.6151*\log _{e} \left( {GGT} \right)}}\)\(^{ + 0.0249*waist\;circumference + 1.1792*\log _{e} \left( {insulin} \right) + 0.8242*}\)\(~^{{\log _{e} \left( {glucose} \right) - 14.7812}} /(1 + e^{{ - 0.8073*non - Hispanic\;black}}\)\(^{{ + 0.3458*Mexican\;American + 0.0093*age + 0.6151*}}\) \(^{{\log _{e} \left( {GGT} \right) + 0.0249*waist\;circumference + 1.1792*\log _{e} \left( {insulin} \right) + 0.8242*\log _{e} \left( {glu\cos e} \right) - 14.7812}} )*100\)
Which incorporates demographic factors (age, race/ethnicity) and biochemical markers (insulin, glucose, γ-glutamyltransferase, and waist circumference). Participants classified as "Non Hispanic Black " or “Mexican American” are assigned a value of 1 for the corresponding variable; otherwise, the value is set to 0. A US-FLI score greater than 30 indicates the presence of fatty liver. This definition has been validated with an area under the receiver operating characteristic (ROC) curve of 0.80 (95% CI 0.77–0.83) for predicting NAFLD, as confirmed by ultrasound.
Survival status
The National Center for Health Statistics (NCHS) established the NDI in collaboration with states, utilizing a series of identifiers including social security numbers and birth dates. In this study, the NCHS linked NHANES data with the NDI to determine the survival status of participants. The follow-up for the participants ended on December 31, 2019. All-cause mortality is defined as death resulting from any cause.
Covariates
Age was categorized into three groups (20–40, 40–60, and over 60 years), and race/ethnicity into four groups (Mexican American, Non-Hispanic Black, Non-Hispanic White, and Other). Educational level was classified into three categories: below high school, high school, and above high school. Smoking status was divided into three groups: never smokers (those who have smoked fewer than 100 cigarettes in their lifetime), former smokers (those who have smoked more than 100 cigarettes and have since quit), and current smokers (those who have smoked more than 100 cigarettes and currently smoke daily or occasionally). Body mass index (BMI) was calculated as weight divided by height squared and classified into three categories: normal weight (BMI < 25 kg/m2), overweight (BMI 25–30 kg/m2), and obese (BMI > 30 kg/m2). Diabetes was defined as meeting any of the following criteria: (1) HbA1c ≥ 6.5% or fasting blood glucose > 7.0 mmol/L; (2) self-reported diabetes diagnosis or current insulin use. Hypertension and total cholesterol intake were both estimated based on self-reported data from the 24-h recall interviews. Heavy drinking was defined as an average daily alcohol intake exceeding 20 g for men or 10 g for women. Cardiovascular disease history was defined as self-reported diagnosis of congestive heart failure, coronary artery disease, angina pectoris, or myocardial infarction. Individuals affirming ≥ 1 condition were categorized as positive. The Healthy Eating Index 2020 (HEI-2020), based on the 2020–2025 Dietary Guidelines for Americans (DGA), assesses diet quality across 13 food components, with scores ranging from 0 to 10017. Higher scores indicate better diet quality and greater adherence to the DGA. Cancer history (self-reported via MCQ220) was not included as a covariate due to extensive missing data (90.4%) alongside potential misclassification bias in self-reports.
Statistical analysis
Analyses of NHANES incorporated sample weights, stratification, and primary sampling units. Continuous variables were presented as medians with interquartile ranges, while categorical variables were reported as counts and percentages. The three groups were compared using either ANOVA or the Kruskal–Wallis test for continuous variables, and the χ2 test for categorical variables. To reduce skewness in dietary vitamin K intake and approximate a normal distribution, a log transformation was applied. The characteristics of the study population were stratified based on the tertiles of the natural logarithm of dietary vitamin K intake.
A weighted multivariate Cox proportional hazards regression model was applied to assess the association between dietary vitamin K intake and all-cause mortality. The proportional hazards assumption was validated through residual analysis using Schoenfeld residuals, and no violations were detected. Three regression models were constructed. The non-adjusted Model included only the dietary vitamin K intake. The model I adjusted for age, gender, race, and educational level, while model II was fully adjusted for all covariates, including lifestyle factors and comorbidities. To avoid multicollinearity, variables with a variance inflation factor (VIF) greater than 10 were excluded (Tale A1 in the Appendix). Additional adjustments were made for characteristics that, when added to the model, altered the hazard ratio by at least 10%18. We used a weighted multivariate Cox regression model with restricted cubic splines to detect non-linearity and identify inflection points. Based on these points, we developed segmented Cox proportional hazards models, along with Kaplan–Meier analyses and Log-Rank tests, to identify significant predictors of survival. Model performance was evaluated using Receiver operating characteristic curves (ROC) and AUC, with differences in AUCs compared using the z-test.
Stratified analyses were conducted for significant covariates, taking into account potential effect modifiers including age, gender, race, education level, history of hypertension, alcohol consumption, dietary supplement usage, HEI-2020 score, diabetes history, triglycerides, hypertension history, total energy intake, cardiovascular disease, smoking status and cholesterol levels. Sensitivity analyses included: (1) excluding participants who died within 2 years of follow-up to minimize reverse causality bias; (2) excluding participants with only one dietary recall; (3) examining the predictive value of serum vitamin K for mortality using FLI (Fatty Liver Index) ≥ 60 as the diagnostic criterion for NAFLD19. The E-value was calculated to assess the impact of unmeasured confounders on the relationship between dietary vitamin K intake and all-cause mortality20,21, with higher E-values indicating greater robustness of the observed association. (4) Sensitivity analyses evaluated potential confounding by vitamin E, including interaction tests and multicollinearity diagnostics. Full details of sensitivity analyses are provided in Supplementary Material. Data analysis was performed using R software (version 4.3.1). A P-value < 0.05 was considered statistically significant.
Results
General characteristics of participants
Based on the tertiles of dietary vitamin K intake, baseline characteristics of 7857 participants are summarized in Table 1. Compared with those in the lowest tertile, participants in the higher tertiles (T2 and T3) were more likely to be men, aged 40–60 years, and to have education above high school. They generally have significantly higher HEI-2020 scores, and dietary supplement use. No significant differences were found among groups for hypertension history, diabetes history, triglyceride levels, total cholesterol, BMI categories, cardiovascular disease, or moderate-intensity exercise duration.
Univariate cox regression of factors predicting all-cause mortality in NAFLD
Univariate analysis identified several factors significantly associated with all-cause mortality in NAFLD. These factors included demographic and clinical characteristics (age, sex, race, educational level, BMI category, diabetes history, hypertension history, cardiovascular disease status), lifestyle factors (alcohol consumption history, smoking status, dietary supplement use), and dietary factors (total energy intake, total cholesterol intake, HEI-2020 score, and dietary vitamin K intake). All variables were statistically significant (P < 0.05) (Table A2 in the Appendix).
Association of dietary vitamin K intake with all-cause mortality in NAFLD
Weighted Cox proportional hazards regression model was conducted to analyze the relationship between In (dietary vitamin K intake) and all-cause mortality risk in participants with NAFLD. Over a total follow-up period of 180 months, 842 all-cause deaths were recorded. Table 2 presents the results from three Weighted Cox regression model analyses. Non-adjusted Model and Adjust Model I indicate downward trends between In (dietary vitamin K intake) and all-cause mortality in participants with NAFLD (P for trend < 0.05).After adjusting for age, sex, race, educational level, BMI category, alcohol consumption status, HEI-2020 score, dietary supplement use, total cholesterol, triglycerides, hypertension history, diabetes history, total energy intake, cardiovascular disease and smoking status in the Adjusted Model II, the HRs and 95% confidence intervals (CIs) were 1.00 (reference), 0.76(0.62,0.93), 0.76(0.54,1.08), for the T1,T2, and T3 groups, respectively, for all-cause mortality (P for trend = 0.028).The continuous models indicate that every one-unit increase in the In(dietary vitamin K intake) was associated with an reduced risk of 0.81(0.67,0.98) for all-cause mortality after adjusting for confounding factors (Table 2).
Non-linear relationship between dietary vitamin K intake and all-cause mortality
Utilizing Cox proportional hazards regression models with restricted cubic splines, the association between the In (dietary vitamin K intake) and all-cause mortality was examined. A non-linear relationship was observed between the In (dietary vitamin K intake) and mortality incidence (Fig. 2). Additionally, inflection points for all-cause mortality were identified as 4.71 (Table 3).
Nonlinear relationship between In(Dietary Vitamin K Intake) and All-Cause Mortality in NAFLD Adjust for: Age, sex, race, education level, BMI group, alcohol consumption status, HEI-2020 score, dietary supplement use, total cholesterol, triglycerides, hypertension history, diabetes history, total energy intake, cardiovascular disease and smoking status. HR: Hazard Ratio. The red line represents HR, and the blue shaded area represents the 95% confidence interval.
Impact of dietary vitamin K intake on all-cause mortality risk: threshold analysis
As shown in Table 3, after adjusting for various factors, the risk of all-cause mortality decreased by 33% (HR: 0.67, 95% CI 0.55, 0.81) for each unit increase in In(dietary vitamin K intake) below the threshold value (P < 0.001). However, the In (dietary vitamin K intake) above the threshold value did not significantly associate with the risk of all-cause mortality (P > 0.05).
Survival analysis of dietary vitamin K intake: stratified mortality rates by intake levels
We grouped the study population based on In (dietary vitamin K intake) (< 4.71, ≥ 4.71) and divided these two groups into three quantiles to generate survival curves. Interestingly, In (dietary vitamin K intake) stratified survival curves demonstrated that significant differences in mortality rates among groups persisted in the population with In (dietary vitamin K intake) < 4.71 (All-cause mortality: P for log-rank test = 0.0037, Fig. 3B). However, the differences in mortality among patients with In (dietary vitamin K intake) ≥ 4.71 did not reach statistical significance (All-cause mortality: P for log-rank test = 0.20, Fig. 3A).
Kaplan–Meier survival curves based on Ln (dietary vitamin K intake). A: In (dietary vitamin K intake) > 4.71, B: In (dietary vitamin K intake) < 4.71. T: tertiles of dietary vitamin K intake (T1: lowest tertile, T2: middle tertile,T3: highest tertile). Note: The cutoff point of 4.71 was identified based on a segmented Cox regression analysis, corresponding to a dietary vitamin K intake of 121 μg/day.
E-value
The E-value of 1.88 suggests that an unmeasured confounder would need a relative risk of at least 1.88 for both dietary vitamin K intake and NAFLD mortality to explain the observed association, indicating the relationship is robust against confounding.
Subgroup analysis and model evaluation
Table 4 presents the results of a stratified subgroup analysis by age, sex, education level, hypertension history, alcohol consumption history, dietary supplement use, HEI-2020 score, diabetes history, and cholesterol levels. No significant interactions were observed among the subgroups. The ROC curve in Fig. 4 illustrates that when using In (dietary vitamin K intake) to assess survival status, the fully adjusted model demonstrated superior discriminatory power and accuracy than the univariate Cox regression model. The AUC value of the fully adjusted model was 0.832, which is significantly higher than that of the unadjusted model (P < 0.001).
Comparison of ROC curves for fully adjusted and univariate cox regression models in asessing survival status using In (Dietary Vitamin K intake). The ROC curves compare the performance of the Multivariate Cox Model (Adjusted II model, adjusted for Age, sex, race, educational level, BMI category, alcohol consumption status, HEI-2020 score, dietary supplement use, total cholesterol, triglycerides, hypertension history, diabetes history, total energy intake, cardiovascular disease and smoking status.) and the Univariate Cox Model in predicting survival based on natural log-transformed dietary vitamin K intake without additional covariates.
Discussion
This nationwide study revealed a nonlinear association between dietary vitamin K intake and all-cause mortality in U.S. adults with NAFLD, characterized by a threshold at 121 μg/day using restricted cubic spline analysis (P-nonlinearity < 0.001). These findings suggest that maintaining dietary vitamin K intake near the identified threshold (121 μg /day)-equivalent to 100 g of cooked spinach or 150 g of broccoli daily-may optimize survival benefits in NAFLD, avoiding both insufficiency and pharmacologically redundant high intake.
The observed non-linear relationship between dietary vitamin K intake and mortality in NAFLD patients-marked by a maximal protective effect at 121 μg/day—aligns with biological saturation kinetics. Vitamin K serves as an obligate cofactor for the γ-carboxylation of glutamic acid residues in hepatic proteins, most notably matrix Gla protein (MGP) and osteocalcin22,23,24. In cardiovascular health, carboxylated MGP (cMGP) directly inhibits vascular calcification by sequestering calcium phosphate crystals25, a process critical for NAFLD patients who exhibit amplified calcification risks due to chronic inflammation and insulin resistance26. Our threshold of 121 μg/day likely represents the intake required to achieve near-maximal carboxylation of these proteins; beyond this point, excess vitamin K no longer enhances activity, as evidenced by the plateau in mortality reduction (HR 1.07 beyond 121 μg/day).
Significant thresholds were identified in previous studies. For example, In the Danish cohort (n = 56,048), mortality risk decreased by 24% (HR 0.76) when vitamin K1 intake increased from the lowest quintile (median 57 μg/day) to the highest (192 μg/day), with benefits plateauing at ≥ 87 μg/day27. In U.S. adults (n = 37,037), achieving sex-specific Adequate Intake (AI) levels (90 μg/day for women; 120 μg/day for men) via diet reduced mortality by 21% (RR = 0.79)28. High-risk cohorts further illustrate threshold variability. Chronic kidney disease (CKD) patients meeting AI levels (men = 120 μg/day; women = 90 μg/day) exhibited a 15% mortality reduction (HR 0.85), though only 28% achieved this intake29, while Danish atherosclerotic cardiovascular disease (ASCVD) cohorts required higher doses (192 μg/day) for maximal protection (HR 0.76, highest quintile [192 ug/day] vs. lowest quintile [57ug/day])27. This variability extends to populations with baseline intakes exceeding physiological demands. In the EPIC-NL cohort (median = 170 μg/day)30, no incremental benefit was observed, mirroring our findings of a plateau above 121 μg/day in NAFLD patients. The threshold in our NAFLD cohort (121 μg/day) approximates the U.S. male AI (120 μg/day)31, yet exceeds the median national intake range (85–120 μg/day) and general population thresholds (87–90 μg/day). This divergence likely reflects disease-specific metabolic demands, such as upregulated hepatic MGP carboxylation in steatosis to counteract inflammation and calcification26. In NAFLD patients, vitamin K intake below 121ug/day was associated with a 33% reduction in all-cause mortality (adjusted HR 0.67), significantly greater than the reductions observed in CKD (15%28 and general populations (21–24%)25,26. This discrepancy underscores the necessity of higher vitamin K intake for optimal protection in NAFLD. Notably, our identified threshold (120 μg/day) aligns closely with the USDA Dietary Guidelines’ Adequate Intake (AI) for men (120 μg/day) but exceeds the recommended level for women (90 μg/day)29.While the median U.S. vitamin K intake (85–120 μg/day) recorded in the Food and Nutrient Database for Dietary Studies (FNDDS) meets nutritional adequacy standards, it remains below the optimal threshold (121 μg/day) associated with maximal mortality reduction in NAFLD patients. This disparity suggests that NAFLD imposes unique physiological demands-likely through increased hepatic requirements for vitamin K-dependent processes (e.g., MGP activation to counteract steatosis-related calcification)-rather than reflecting simple population-wide deficiency26. These findings underscore that vitamin K thresholds are not fixed; instead, they dynamically adapt to three key factors: baseline intake levels, disease-driven metabolic priorities (e.g., MGP activation in NAFLD vs. vascular protection in CKD), and clinical endpoints (all-cause vs. disease-specific mortality).
Beyond cardiovascular benefits, vitamin K may mitigate NAFLD progression by suppressing inflammatory drivers. Chronic inflammation promotes steatosis-to-NASH transition via NF-kB-mediated cytokine release (e.g., TNF-α, IL-6)26,32. Vitamin K attenuates this process by inhibiting NF-kB nuclear translocation, potentially reducing fibrosis and hepatocellular carcinoma risk. Vitamin K has been shown to improve insulin sensitivity by enhancing insulin receptor substrate (IRS) phosphorylation, which promotes insulin signaling. This is particularly relevant for NAFLD patients, as insulin resistance is a major driver of hepatic fat accumulation and subsequent liver injury.
The null association above 121 μg/day may also reflect nutrient interactions. High-dose vitamin K and E compete for hepatic metabolism via CYP4F2-mediated ω-hydroxylation33 and rodent studies show α-tocopherol (vitamin E) supplementation reduces plasma phylloquinone (vitamin K1) levels34. This aligns with our sensitivity analysis showing attenuation of the vitamin K-mortality association after vitamin E adjustment (HR: 0.81 → 0.85, ΔHR = + 0.04). This effect may primarily reflect dietary collinearity (e.g., shared food sources like spinach and broccoli) rather than direct biological antagonism, as the threshold effect persisted in fully adjusted models. Finally, the observed threshold (121 μg/day) likely represents synergistic benefits of nutrient-dense diets (e.g., combined effects of vitamin K, polyphenols, and fiber) rather than isolated vitamin K effects. While we adjusted for the Healthy Eating Index-2020, residual confounding by unmeasured dietary components cannot be excluded. Future studies should integrate biomarkers (e.g., plasma phylloquinone, α-tocopherol) and metabolomics to disentangle these relationships.
Our study demonstrated that dietary vitamin K intake is a significant predictor of all-cause mortality in NAFLD patients, as indicated by the ROC curve with an AUC value of 0.832. This suggests that dietary vitamin K intake can effectively discriminate between patients at higher and lower risk of mortality. In clinical practice, this finding could help physicians assess patient risk and develop personalized interventions. For example, NAFLD patients with insufficient vitamin K intake may benefit from dietary modifications or supplementation to reduce the mortality risk. However, despite the promising predictive power of vitamin K intake, further randomized controlled trials are needed to establish a causal relationship and confirm the applicability of these findings in broader populations.
This study provides important insights into the role of dietary vitamin K in reducing mortality among NAFLD participants. The identification of a threshold effect at 121 μg/day has significant clinical implications, suggesting that increasing vitamin K intake in patients with low baseline levels may confer significant survival benefits. From a public health policy perspective, these findings support the advocacy of vitamin K-rich diets, particularly in populations at high risk for NAFLD. Achieving the vitamin K threshold of 121 μg/day can be practically accomplished through common dietary sources. For example, 25 g raw spinach (~ 120 μg) or 65 g steamed broccoli (~ 100 μg) alone meet the target. Alternatively, combining smaller portions: 15 g spinach (72 μg) with 50 g romaine lettuce (48 μg) plus 1 tsp olive oil provides sufficient intake (total 128 μg). These strategies align with Mediterranean dietary patterns by emphasizing plant-based fats and vegetables.
Strengths
This study addresses a gap in the existing literature by investigating the long-term relationship between dietary vitamin K intake and all-cause mortality in NAFLD participants. We identified both linear and non-linear associations, calculated cutoff points, and demonstrated the predictive value of vitamin K intake. This study has several notable strengths. We utilized data from the NHANES, a nationally representative cohort with rigorous data collection protocols, ensuring high generalizability to the U.S. population. Our analysis incorporated a comprehensive adjustment for potential confounders, including demographic, lifestyle, and clinical variables, enhancing the robustness of the observed associations. The use of restricted cubic splines allowed us to identify a non-linear relationship and a clinically relevant threshold (121 μg/day), which may guide dietary recommendations. Additionally, sensitivity analyses and subgroup assessments demonstrated consistent results, further supporting the reliability of our findings. Finally, the application of the E-value method strengthened our conclusion by quantifying the potential impact of unmeasured confounding.
Limitations
Despite these strengths, our study has several limitations. hypertension status was self-reported, which may introduce misclassification bias; dietary vitamin K intake was assessed via two non-consecutive 24-h recalls, while this method reduces day-to-day variability compared to single-day records, it may not fully capture habitual intake, and results could be influenced by recall bias or seasonal variations in food consumption (e.g., higher leafy greens intake in summer);the observational design precludes causal inference, necessitating future randomized trials to confirm our findings; NAFLD diagnosis relied on the US-FLI rather than gold-standard methods (e.g., liver biopsy or imaging), potentially misclassifying cases; residual confounding may persist despite extensive adjustments. For instance, we lacked data on physical activity intensity (a potential modifier of NAFLD progression) and socioeconomic factors (e.g., income, healthcare access) that influence dietary patterns; cancer history was not included as a covariate due to extensive missing data (90.4% missingness) and potential misclassification from self-reports (MCQ220). Failure to adjust for this variable may have introduced residual confounding, as cancer survivors may exhibit distinct dietary behaviors (e.g., higher consumption of vitamin K-rich vegetables) and elevated mortality risk unrelated to NAFLD mechanisms. Future studies should integrate longitudinal dietary assessments, such as food frequency questionnaires (FFQs) -with plasma phylloquinone biomarkers to improve validity.
Conclusion
Our study identified dietary vitamin K as a reliable predictor of all-cause mortality in NAFLD participants. Specifically, Higher dietary vitamin K intake is associated with reduced all-cause mortality in NAFLD participants, with a threshold effect observed at 121 μg/day. Dietary vitamin K intake may serve as a useful predictor of survival in this population. These findings have important implications for dietary guidelines and public health approaches targeting the reduction of mortality risk in NAFLD participants.
Data availability
The data used in this study can be accessed from the National Health and Nutrition Examination Survey (NHANES) at the following link: https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- Anova:
-
Analysis of variance
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- DGA:
-
Dietary guidelines for Americans
- EFSA:
-
European Food Safety Authority
- FLI:
-
Fatty liver index
- HEI:
-
Healthy eating index
- HbA1c:
-
Hemoglobin A1c
- NCHS:
-
National Center for Health Statistics
- NDI:
-
National death index
- ROC:
-
Receiver operating characteristic
- HEI-2020:
-
Healthy Eating Index 2020
- HR:
-
Hazard ratio
- CKD:
-
Chronic kidney disease
- ASCVD:
-
Atherosclerotic cardiovascular disease
- MGP:
-
Matrix Gla protein
- FNDDS:
-
Food and Nutrient Database for Dietary Studies
- AI:
-
Adequate intake
References
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 77, 1335–1347 (2023).
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861 (2022).
Lee, K. C., Wu, P. S. & Lin, H. C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 29, 77–98 (2023).
Karkucinska-Wieckowska, A. et al. Mitochondria, oxidative stress and nonalcoholic fatty liver disease: A complex relationship. Eur. J. Clin. Invest. 52, e13622 (2021).
Hernández-Alvarez, M. I. et al. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell 177, 881–895e17 (2019).
Scorletti, E. et al. Dietary vitamin E intake is associated with a reduced risk of developing digestive diseases and nonalcoholic fatty liver disease. Am. J. Gastroenterol. 117, 927–930 (2022).
Vogli, S., Naska, A., Marinos, G. & Kasdagli, M. I. The effect of vitamin E supplementation on serum aminotransferases in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis. Nutrients 15, 3733 (2023).
Tripathi, M. et al. Vitamin B12 and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J. Hepatol. 77, 1246–1255 (2022).
Shea, M. K. & Booth, S. L. Vitamin K. Adv. Nutr. 13, 350–351 (2022).
Mishima, E., Wahida, A., Seibt, T. & Conrad, M. Diverse biological functions of vitamin K: From coagulation to ferroptosis. Nat. Metab. 5, 924 (2023).
Welsh, J., Bak, M. J. & Narvaez, C. J. New insights into vitamin K biology with relevance to cancer. Trends Mol. Med. 28, 864–881 (2022).
Silva, N. G., Preto, M., Vasconcelos, V. & Urbatzka, R. Reduction of neutral lipid reservoirs, bioconversion and untargeted metabolomics reveal distinct roles for vitamin K isoforms on lipid metabolism. Food Funct. 15, 2170–2180 (2024).
Zhao, P. et al. Vitamin K2 protects mice against non-alcoholic fatty liver disease induced by high-fat diet. Sci. Rep. 14, 3075 (2024).
National Center for Health Statistics. National Health and Nutrition Examination Survey. Accessed 26 August 2023. https://www.cdc.gov/nchs/nhanes/index.htm (2023).
National Center for Health Statistics. NCHS provides timely and accurate health statistics for the United States. Accessed 26 August 2023. https://www.cdc.gov/nchs/index.htm (2023).
Ruhl, C. E. & Everhart, J. E. Fatty liver indices in the multiethnic United States National health and nutrition examination survey. Aliment. Pharmacol. Ther. 41, 65–76 (2014).
Shams-White, M. M. et al. Healthy eating Index-2020: Review and update process to reflect the dietary guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 123, 1280–1288 (2023).
Kernan, W. N. et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl. J. Med. 343, 1826–1832 (2000).
Biciusca, T. et al. The role of the fatty liver index (FLI) in the management of non-alcoholic fatty liver disease: A systematic review. Diagnostics (Basel). 13, 3316 (2023).
Localio, A. R., Stack, C. B. & Griswold, M. E. Sensitivity analysis for unmeasured confounding: E-values for observational studies. Ann. Intern. Med. 167, 285–286 (2017).
Blum, M. R., Tan, Y. J. & Ioannidis, J. P. A. Use of E-values for addressing confounding in observational studies-an empirical assessment of the literature. Int. J. Epidemiol. 49, 1482–1494 (2020).
Popa, D. S., Bigman, G. & Rusu, M. E. The role of vitamin K in humans: Implication in aging and age-associated diseases. Antioxidants 10, 566 (2021).
Schmölz, L., Birringer, M., Lorkowski, S. & Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 7, 14–43 (2016).
Zhang, S., Guo, L. & Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 26, 549–553 (2019).
Theuwissen, E., Smit, E. & Vermeer, C. The role of vitamin K in soft-tissue alcification. Adv. Nutr. 3, 166–173 (2012).
Simes, D. C., Viegas, C. S. B., Araújo, N. & Marreiros, C. Vitamin K as a diet supplement with impact in human health: Current evidence in age-related diseases. Nutrients 12, 138 (2020).
Palmer, C. R. et al. Association between vitamin K1 intake and mortality in the Danish diet, cancer, and health cohort. Eur. J. Epidemiol. 36, 1005–1014 (2021).
Chen, F. et al. Association among dietary supplement use, nutrient intake, and mortality among U.S. Adults: A cohort study. Ann. Intern. Med. 170, 604–613 (2019).
Cheung, C., Sahni, S., Cheung, B. M. Y., Sing, C. & Wong, I. C. K. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin. Nutr. 34, 235–240 (2015).
Zwakenberg, S. R. et al. A. Vitamin K intake and all-cause and cause specific mortality. Clin. Nutr. 36, 1294–1300 (2017).
Department of Agriculture, U. S. & U.S. Department of Health and Human Services. U.S. Government Publishing Office. Dietary Guidelines for Americans, 2020–2025. 9th ed. (2020). https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_ Guidelines_ for_ Americans_2020–2025.pdf.
Zheng, X. et al. Synthetic vitamin K analogs inhibit inflammation by targeting the NLRP3 inflammasome. Cell. Mol. Immunol. 18, 2422–2430 (2020).
Liao, S. et al. Vitamin E and metabolic health: Relevance of interactions with other micronutrients. Antioxidants 11, 1785 (2022).
Hanzawa, F. et al. Excess α-tocopherol decreases extrahepatic phylloquinone in phylloquinone-fed rats but not menaquinone-4 in menaquinone-4-fed rats. Mol. Nutr. Food Res. 58, 1601–1609 (2014).
Funding
This research did not receive any funding from public, commercial, or non-profit organizations.
Author information
Authors and Affiliations
Contributions
Yu Huang designed the study, wrote the R code, and drafted the manuscript. Xianfeng Zhu collected and cleaned the data. Liang Han conducted secondary analysis and correction of the R code, and reanalyzed the data. Hulan Hu reviewed and revised the manuscript.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, Y., Zhu, X., Han, L. et al. Dietary vitamin K intake associates with reduced all-cause mortality in non-alcoholic fatty liver disease patients. Sci Rep 15, 19272 (2025). https://doi.org/10.1038/s41598-025-03258-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03258-3