Abstract
Extracorporeal membrane oxygenation (ECMO) is increasingly being used in intensive care units. However, it can alter drug pharmacokinetics and lead to underexposure associated with treatment failure. The aim of the present study was to determine the influence of ECMO on the pharmacokinetics of midazolam (MDZ). An in vivo rat model was developed using a bolus injection of MDZ, with concentration monitoring for 180 min. Rats that underwent ECMO were compared with a control group. Pharmacokinetic analysis of in vivo data (noncompartmental analysis and nonlinear mixed effects modeling) was performed to determine the influence of ECMO. The activity of the enzyme CYP3A, which catalyzes MDZ metabolism was examined in the livers of ECMO-treated rats. In vivo pharmacokinetic models were developed, showing an increase in the volume of distribution and the area under the curve, but a decreased the clearance, which was associated with decreased CYP3A activity. These results suggest that ECMO may affect the efficacy or increase the risk of side effects associated with MDZ.
Similar content being viewed by others
Introduction
Critically ill patients can undergo pathophysiological changes, including septic shock, multiple organ dysfunction, and acute respiratory distress syndrome. Extracorporeal membrane oxygenation (ECMO) is a form of extracorporeal life support that provides adequate oxygen, gas exchange, or blood supply to sustain life. ECMO provides prolonged cardiac and respiratory support to patients whose hearts and lungs have reduced capacity1.
Compared with other benzodiazepines, midazolam (MDZ) works faster, with a rapid onset and offset of sedation. MDZ is metabolized into inert metabolites primarily through glucuronic acid conjugation and hydroxylation2. The sedative effect of MDZ is dependent on its plasma concentration3. Patient characteristics and pharmacokinetic (PK) variability during ECMO may contribute to inconsistent clinical responses to MDZ4.
Adsorption to ECMO circuits has been considered for various drugs and is strongly affected by the lipophilic and protein-binding characteristics of the drug4,5,6. The adsorption of drugs to ECMO circuits changes the PK in of patients and may affect the efficacy or produce side effects. MDZ and albumin, which are associated with protein-binding, are extracted by the oxygenator in the ECMO circuit5,6,7,8. Despite its clinical relevance, limited data are available on the effects of ECMO on the PK of MDZ.
ECMO-related physiological changes can affect the properties and pharmacological effects of drugs. ECMO alters PK by increasing the volume of distribution (Vd) through mechanisms such as drug adsorption to ECMO circuit components, hemodilution, and impaired drug elimination and sequestration in the ECMO circuit9. Hemodilution caused by the priming solution at the beginning of ECMO, ongoing blood product transfusions, isolation of the drug in the circuit, and administration of fluid to maintain the flow of the circuit notably affect the Vd of drugs whose distribution is limited to the plasma compartment. This is particularly relevant for hydrophilic medicines10,11. ECMO is also associated with reduced drug excretion and clearance, resulting from factors such as renal impairment in patients, decreased drug metabolism, and decreased local hepatic blood flow, especially for drugs with high liver extraction rates11. A lack of response or toxicity, resulting from changes in the PK of important drugs, during and after extracorporeal circulation, can endanger the patient’s life and prolong the recovery process. Changes in PK during ECMO have been studied; however, data on the metabolism of drugs administered to patients receiving ECMO are limited.
Therefore, this study aimed to examine, for the first time, the effects of ECMO on the PK of MDZ and the activity of cytochrome P450 3A (CYP3A), the enzyme responsible for MDZ metabolism, using an in vivo ECMO rat model.
Methods
Ethics
All experiments were approved by the Animal Care and Use Committee of Niigata University of Health and Welfare (Ethical Code 22,003) and conducted in accordance with the guidelines of the National Institute of Health for Laboratory Animal Welfare. This study is reported in accordance with the ARRIVE guidelines.
Experimental design
Figure 1a shows a schematic of the experimental design. The experimental procedure was conducted as described by Fujii et al.12. For the in vivo experiments, a rat model was selected, and the human adult ECMO device was reduced to 1/50–1/100. Briefly, Sprague–Dawley rats (males, 14–16 weeks old, 400–450 g, n = 12) (CLEA Japan, Inc., Tokyo, Japan) were anesthetized with 4.5–5.0% isoflurane, mixed with oxygen-enriched air delivered via a vaporizer. Six rats were divided into two groups: a normal MDZ-treated ECMO group (ECMO-treated rats, n = 3) and an MDZ-treated non-ECMO group (sham rats, n = 3). A blood pressure measurement line was secured in the femoral artery, the blood vessel was inserted from the left common carotid artery and the prolapse was inserted from the right external jugular vein. In the ECMO-treated rats, 400 μg/kg of MDZ was rapidly administered via the jugular vein 2 min before the start of ECMO, ECMO was started at an auxiliary flow rate of 60 ~ 70 ml/kg/min, and blood samples were collected at eight defined time points: 2 min after treatment with MDZ before ECMO initiation and 5–180 min after the ECMO initiation. After collection, the samples were pipetted into cryovials, which were immediately stored at − 80 °C. During the experiment, blood pressure and heart rate were continuously monitored, and blood gas analysis was performed as necessary to ensure biological management. After the study, the rats were euthanized by an overdose of pentobarbital.
Analysis of samples
MDZ plasma samples were prepared via protein precipitation and quantified using an Acquity UPLC system coupled with a Xevo-TQS triple quadrupole mass spectrometer equipped with an electrospray ionization source in positive mode (Waters Co., MA, USA)13. Carteorol was used as an internal standard. Data acquisition and instrument control were performed using the MassLynx 4.2 software (Waters Corp., USA). The method was accurate and precise in the linearity range of 10–1000 ng/mL, with intra- and inter-day assay variabilities < 10% for all quality control samples. The analytical validation was treated with full validation of the total densitometry system according to the guidance of the US Food and Drug Administration on bioanalytical method validation14.
PK analysis
PK analysis was performed using two conventional approaches: non-compartmental analysis via moment analysis and compartmental modeling using the NONMEM software, version 7.5.1 (ICON Development Solutions, Gaithersburg, Maryland). NONMEM runs were performed using the Perl-speaks-NONMEM version (University of Uppsala, Uppsala, Sweden). Different approaches, including one- or two-compartment modeling, were tested to describe the MDZ kinetic profile, while proportional modeling was tested to describe the residual variability. Once the null model (i.e., the model without factors explaining interindividual variability) was selected, one documented covariate (ECMO) was tested.
The model was parameterized using the following key PK parameters: Vd in the central compartment (V1), clearance (CL), Vd in the peripheral compartment (V2), and inter-compartmental clearance (Q). Interindividual variability was modeled using an exponential model:
where θi is the estimated individual PK parameter for the ith individual, θ is the median value of the PK parameter for the population, and ηi is the interindividual random effect for the ith individual, assumed to be normally distributed with a mean of 0 and a variance ω2.
An initial combined additive residual error model was tested:
where \({Y}_{o,ii}\) and \({Y}_{p,ij}\) are the observed and predicted \(j\) th drug concentrations for the ith individual, respectively, and where \({\varepsilon }_{pro,ij}\) is the proportional error, with a mean of 0 and a variance of \({\sigma }^{2}\).
The influence of ECMO was tested as a dichotomous covariate of \({\theta }_{i}\):
where \({\theta }_{i}\) is the estimated individual PK parameter for the ith individual, \({\theta }_{pop}\) is the median value of the PK parameter of the population, and dic (ECMO) is the dichotomous covariate (0 or 1) for the ith individual.
Model evaluation
Goodness-of-fit (GOF) plots were used to evaluate model performance. The following plots were generated from the GOF plots: observed vs. individual predicted concentration scatterplots and residual plots (individual weighted residuals vs. time and predicted concentrations). The significant influence of covariates on interindividual variability was determined using the likelihood ratio test. A decrease of > 3.84 in the criterion (p value = 0.01, χ2 distribution, 1 degree of freedom) was considered significant. A bootstrapping method involving the simulation of 500 rats was used to assess the stability of the final model and the precision of parameter estimates, with a priori target of > 90% for the proportion of observations found within the 5th and 95th percentiles deemed acceptable.
Simulation-based analysis
From the PK parameters obtained in this study, Monte Carlo simulations (n = 500) were conducted to calculate the plasma concentration for each dose of MDZ (200, 400, and 800 µg/kg) with and without ECMO.
Measurement of plasma cytokine and liver enzyme levels
The levels of tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 were measured via an enzyme-linked immunosorbent assay (ELISA) kit (Cosmo Bio, Tokyo, Japan). Additionally, the concentrations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in blood plasma samples were measured using an ELISA kit (Abcam, Cambridge, UK). Blood samples for these analyses were collected before the start of ECMO and 180 min after ECMO.
Preparation of liver microsomes
Rat livers were promptly removed 120 min after the start of ECMO and perfused with ice-cold potassium chloride solution (1.15%). (Fig. 1b). The separated liver with ice-cold potassium chloride solution (1.15%) were immediately homogenized in four volumes of potassium chloride solution (1.15%), followed by centrifugation at 9 000 × g for 20 min and ultracentrifugation at 105 000 × g for 60 min. The resulting pellet was suspended in sodium–potassium phosphate buffer (0.1 M, pH 7.4). Protein concentration was determined using the bicinchoninic acid method15.
Evaluation of microsomal CYP3A activity
The CYP3A activity by rat liver microsomes was quantified by measuring testosterone 6β-hydroxylation activity using the van der Hoeven method16. The reaction medium (0.5 mL) consisted of hepatic microsomes (500 μg protein) in phosphate buffer (pH 7.4). The substrate concentration of testosterone was 5 mM. The reaction was initiated by adding NADPH-generating system (1 mM nicotinamide adenine dinucleotide phosphate hydrogen, 66 mM β-glucose-6-phosphate, 40 U/mL glucose-6-phosphate dehydrogenase, 66 mM magnesium chloride). After incubation at 37 °C for 30 min, the reaction was stopped by ice cooling. Betamethasone valerate (500 μL, 10 μmol/L in tert-butyl methyl ether) was added to the reaction medium as an internal standard and extracted. The mixture was subsequently centrifuged at 2 500 × g for 3 min at 4 °C, and the supernatant was evaporated under a nitrogen gas stream at 40 °C. The residue was dissolved in 100 μL of methanol, and a 50-μL aliquot of each sample was injected into the HPLC–UV system and analyzed.
Western blotting analysis of CYP3A protein
Microsomal proteins (2.5 μg) were resolved on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) according to the method described by Laemmli17 and electrophoretically transferred to polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, USA) at 100 mA for 70 min. The membranes were immunolabeled with a polyclonal anti-rat CYP3A2 antibody. Peroxidase-conjugated proteins were detected using 2.8 mM 4-chloro-1-naphthol and 4.4 mM H2O2 in 10 mM sodium–potassium phosphate buffer (pH 7.4). Relative protein densities were analyzed using ImageJⓇ.
Statistical analyses
Data are presented as the means ± standard deviations. Continuous variables were compared using repeated-measures one-way analysis of variance and Tukey’s test. Two-sided p-value < 0.05 was considered statistically significant.
Results
Changes in hemodynamic variables during ECMO in the in vivo model
Table 1 shows the changes in the hemodynamic variables, including PaO2, PaCO2, blood pressure, heart rate, pH, and hemoglobin (Hb) concentration, in each group. Blood pressure and Hb levels significantly decreased during ECMO in both the ECMO and sham groups. The PaO2 levels were higher in the ECMO group than in the sham group. In contrast, no significant differences were found in PaCO2 levels or pH between these groups.
Changes in plasma cytokines and hepatic enzyme values during ECMO in the in vivo model
The plasma TNF-α and IL-6 levels in the ECMO group were substantially higher (Fig. 2a, b) than those in the sham group. The AST and ALT levels in the ECMO group were significantly higher than those in the sham group (p < 0.05) (Fig. 2c, d).
PK analysis
Forty-eight plasma samples were collected from six rats and analyzed for MDZ concentrations. The MDZ concentration profiles in the ECMO and sham groups are shown in Fig. 3. The mean individual PK parameters were determined for both groups using a noncompartmental approach. Compared with those in the sham group, the AUC0→180 min, t1/2, and Vdss in the ECMO group were higher, whereas the CLtot was lower (Table 2).
The best-fitting model for describing the PK profile of MDZ was a two-compartment model with a proportional error. The PK parameter estimates, interindividual variability, and residue errors for the final model are summarized in Table 3. The typical value of CL was 0.69 L/h. The typical values for V1 and V2 were 0.22 L and 0.49 L, respectively. The typical value of Q was 0.29 L/hr. The estimated interindividual variabilities (CV%) were 92.4 for CL and 49.1 for V1. ECMO significantly influenced the CL of MDZ, and V1 was 3.02 times higher in the presence of ECMO than in its absence.
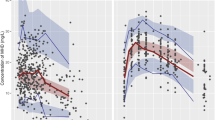
Following visual inspection of the prespecified covariates, their effects on PK parameters were assessed using ECMO. The basic GOF plots (Fig. 4a, b) revealed that the final model was acceptable because the predicted population and individual concentrations closely aligned with the observed concentrations. Additionally, most conditional weighted residual values were evenly distributed randomly around the line of unity (± 2 standard deviations of the mean), which indicated the suitability of the error model (Fig. 4c, d). A visual predictive check plot based on n = 500 simulations with the final model is shown in Fig. 5. The proportion of observations between the 5th and 95th simulated percentiles was > 90%, which was deemed acceptable.
Goodness-of-fit plots for the final pharmacokinetics model. The plots show the (a) population predictions and (b) individual predictions versus observations, (c) time, and (d) population predictions versus conditional weighted residuals. The dotted lines in the top panels (a and b) represent the line of identity. The dotted lines in the lower panels (c and d) represent zero lines.
Visual predictive check of the final population pharmacokinetics model for MDZ in six rats during ECMO. The lines represent concentrations at the designated quantile given by the number on the line (0.05, 0.25, 0.5, 0.75, and 0.95). Open cycles represent observed MDZ concentrations in ECMO-treated rats.
Simulation-based analysis
The simulation-based analysis offered a primary internal validation by simulating experimental doses (200, 400, and 800 µg/kg). The simulation profiles obtained using the PK model for all dosing strategies are shown in Fig. 6. MDZ concentration decreased in a dose-dependent manner. Plasma MDZ levels in the ECMO group were lower immediately after administration than those in the non-ECMO group at all doses.
Simulation of time-course profiles of plasma MDZ concentration obtained using the PPK model for different dosage regimens. A gradual increase in color intensity represents the level of the dose considered (200, 400, and 800 µg/kg). The top panels (a and b) represent the profiles of plasma MDZ concentration over 0–12 h. The lower panels (c and d) represent the profiles of plasma MDZ concentration over 0–3 h.
CYP3A activity and protein expression during ECMO
To verify the effect of ECMO on CYP3A enzyme activity, testosterone hydroxide concentrations measured using liver microsomes with or without ECMO are shown in Fig. 7a. The concentration of 6β-hydroxytestosterone was lower in the ECMO group than in the sham group. The results of comparing the protein expression levels of CYP3A in hepatic microsomes treated with ECMO are shown in Fig. 7b. The amount of CYP3A protein did not differ between the ECMO and sham groups.
Changes in CYP3A protein expression and testosterone 6β-hydroxylation activity induced by ECMO in rat livers. Hepatic microsomal protein is isolated from the livers of the rats. (a) Testosterone 6β-hydroxylation activity is determined using the high-performance liquid chromatography‒ultraviolet method as described in the Materials and methods section. The values represent the means ± standard deviations for three rats. Statistical significance is assessed using the Kruskal–Wallis nonparametric analysis of variance test. (b) Western blotting analysis and Coomassie Brilliant Blue R-250 staining are performed. Microsomal protein (2.5 μg) is subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and assayed for CYP3A apoprotein using antibodies against rat CYP3A, as described in the Materials and methods section. The values are expressed as percentages of the sham group and represent the means ± standard deviations of three rats.
Discussion
This is the first study to assess the PK of MDZ and CYP3A activity in an in vivo ECMO rat model. The use of sedatives in critically ill patients is usually complicated by the degree of interindividual variability in PK parameters. One factor that causes variability is ECMO, which induces considerable fluctuations in the concentration of sedatives, even at the same dosage18. This is especially important because the effectiveness of sedatives, such as MDZ, depends on achieving and maintaining therapeutic plasma concentrations. For patients in the intensive care unit, the target plasma trough concentration of MDZ is ≥ 150 ng/mL to ensure treatment efficacy, regardless of intraindividual PK variability3. Therefore, fluctuations in the PK profile of MDZ may significantly reduce the probability of obtaining a therapeutic effect.
Our study aimed to clarify the effect of ECMO on the PK of MDZ in a rat model through noncompartmental analysis and nonlinear mixed-effects modeling. Furthermore, a two-compartment model was selected for population PK analysis of MDZ19. According to Shaker et al. (2012), an increase in protein-unbound drugs and Vd reduces plasma levels in neonates20. Hemodilution of cardiac output and drug adsorption are considered factors that increase Vd. Some reports have demonstrated that the Vd of lipophilic drugs increases in patients on ECMO; however, no reports have been conclusive21. MDZ is a protein-bound drug. In plasma, 95% of MDZ is bound to albumin and α1-glycoprotein9.
In this study, ECMO induced an increase in V1 (0.66 vs. 0.22 L) according to the NONMEM approach, a decrease in total CL (0.69 vs. 1.05 L/h) and an increase in AUC according to noncompartmental analysis. MDZ is metabolized mainly by the liver, with a hepatic extraction ratio of 0.3822. The clearance of MDZ is dependent on hepatic-specific clearance and unbound fractions. A decrease in total clearance suggests a decrease in hepatic clearance. Therefore, if a patient is suspected to have reduced ECMO-related clearance, slight overexposure to MDZ can be expected. Hepatic-specific clearance is associated with variable and nonuniform reductions in CYP450 activities, which may not correlate with reduced hepatic blood flow.
MDZ is metabolized by CYP3A4, and ECMO may induce postoperative pathophysiological changes associated with systemic inflammatory response syndrome and/or multiorgan dysfunction syndrome. These changes may result from exposure of blood to nonphysiological surfaces, coagulopathy, surgical trauma, anesthesia, ischemia/reperfusion disorders, hypotension, and several interrelated mechanisms, such as multiorgan damage. Khabar et al.23 discussed the association between endotoxins, proinflammatory cytokines (such as TNF-α and IL-6), and the contribution of potential ECMO phenomena. Rapid increases in the plasma concentrations of TNF-α and IL-8 almost immediately (within 15 min) after the initiation of ECMO suggest their role as key mediators in ECMO-related inflammation24,25. Animal models have shown that the IL-6 concentration in the lungs increases after venovenous-ECMO26. In other studies, plasma concentrations of IL-10 were significantly higher in the bronchoalveolar lavage fluid in venovenous-treated animals27. Several clinical studies have reported that cytokines can alter CYP P450 isoenzyme activity during ECMO28, which can impair the metabolism of clinically important drugs, leading to their accumulation in the body and causing side effects. Our rat ECMO model showed elevated levels of inflammatory cytokines (TNF-α and IL-6) and liver enzymes. During ECMO, liver function may decrease owing to inflammation. In addition, the protein expression level of CYP3A, the main metabolic enzyme of MDZ, did not change, but its enzyme activity decreased. In the in vivo rat ECMO model, inflammation caused a reduction in liver function and CYP3A activity. The decrease in CYP3A activity probably reduced the hepatic clearance of MDZ, leading to a decrease in systemic clearance.
The PK parameters obtained in this study were used to simulate plasma concentration profiles at each dose. Differences in the plasma concentration were observed immediately after administration, depending on ECMO status. The decrease in plasma concentration immediately after MDZ administration in the ECMO group may be due to adsorption to the circuit or blood dilution during priming. This aligns with the finding that the population parameter V1 is greater in the ECMO group than in the sham group. ECMO might cause a temporary decrease in plasma concentrations and may have effect on affect PK, regardless of the dose. The clinical efficacy of MDZ depends on its plasma concentration, and the plasma trough concentrations are considered important. If a patient is suspected of having reduced ECMO-related clearance, overexposure to MDZ can be expected. These results suggested that ECMO may affect the efficacy or lead to an increased risk of side effects.
Our study has some limitations. First, this was a pilot study with a small sample size. Future studies will allow us to elucidate the sequential mechanisms underlying pathological conditions by evaluating the histopathological severity of these disorders. Second, we evaluated the PK during short-term ECMO. Further studies are required to assess the systemic inflammatory response and organ damage during long-term ECMO and after weaning off ECMO.
This study provides preliminary data on the PK of MDZ in an ECMO model. PK models were developed, showing an increase in the V1 and AUC but a decrease in the CL associated with decreased CYP3A activity.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Milla, J. E., Fanning, J. P., McDonald, C. I., McAuley, D. F. & Fraser, J. F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit. Care 20, 387–396 (2016).
Hyland, R. et al. In vitro and in vivo glucuronidation of midazolam in humans. Br. J. Clin. Pharmacol. 67(4), 445–454 (2009).
van der Maaten, J. M., Epema, A. H., Huet, R. C. & Hennis, P. J. The effect of midazolam at two plasma concentrations of hemodynamics and sufentanil requirement in coronary artery surgery. J. Cardiothorac. Vasc. Anesth. 10(3), 56–63 (1996).
Shekar, K. et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit. Care 16(5), R194–R200 (2012).
Shekar, K. et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: result from an ex vivo study. Crit. Care 19(1), 164–171 (2015).
Nasr, V. G. et al. Sedative and analgesic drug sequestration after a single bolus injection in an Ex vivo extracorporeal membrane oxygenation infant circuit. ASAIO J. 65(2), 187–191 (2019).
Wagner, D., Pasko, D., Phillips, K., Waldvogel, J. & Annich, G. In vitro clearance of dexmedetomidine in extracorporeal membrane oxygenation. Perfusion 28(1), 40–46 (2013).
Michihara, A. et al. Change of dexmedetomidine and midazolam concentrations by simultaneous injection in an in vitro extracorporeal circuit. Perfusion 39(6), 1222–1230 (2024).
Yamasaki, K. et al. Possible involvement of protein binding inhibition in changes in dexmedetomidine concentration in extracorporeal circuits during midazolam Use. Biol. Pharm. Bull. 47(2), 389–393 (2024).
Sherwin, J., Heath, T. & Watt, K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: A Review of the Current Literature. Clin. Ther. 38, 1976–1994 (2016).
Shekar, K., Fraser, J. F., Smith, M. T., & Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J. Crit. Care 27, e9–e18 (2012).
Fujii, Y., Abe, T. & Ikegami, K. Diabetic pathophysiology enhances inflammation during extracorporeal membrane oxygenation in a rat model. Membranes (Basel). 11(4), 283–294 (2021).
Cui, W., Liu, Q., Xiong, S. & Qiao, L. LC-MS/MS method for simultaneous quantification of dexmedetomidine, dezocine, and midazolam in rat plasma and its application to their pharmacokinetic study. J. Anal. Methods Chem. 2018, 3184759 (2018).
US Department of Health and Human Services, Food and Drug Administration, Guidance for Industry, Bioanalytical Method Validation Available online:https://www.regulations.gov/document/FDA-2013-D-1020-0002 (accessed on July 18th, 2024)
Smith, P. K. et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150, 76 (1985).
van der Hoeven, T. Assay of hepatic microsomal testosterone hydroxylases by high-performance liquid chromatography. Anal. Biochem. 138, 57–65 (1984).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259), 680–685 (1970).
Dzierba, A. L., Abrams, D. & Brodie, D. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit. Care. 21, 66–72 (2017).
Ahsman, M. J., Hanekamp, M., Wildschut, E. D., Tibboel, D. & Mathot, R. A. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin. Pharmacokinet. 49(6), 407–419 (2010).
Shekar, K. et al. Antibiotic sedative and analgesic pharmacokinetics during extracorporeal membrane oxygenation: a multicenter study to optimize drug therapy during ECMO. BMC Anesthesiol. 12, 29–37 (2012).
Unger, J. K., Kuehlein, G., Schroers, A., Gerlach, J. C. & Rossaint, R. Adsorption of xenobiotics to plastic tubing incorporated into dynamic in vitro systems used in pharmacological research limits and progress. Biomaterials 22(14), 2031–2037 (2001).
Pentikäinen, P. J., Välisalmi, L., Himberg, J. L. & Crevoisier, C. Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J. Clin. Pharmacol. 29, 272–277 (1989).
Khabar, K. S. et al. Circulating endotoxin and cytokines after cardiopulmonary bypass: Differential correlation with duration of bypass and systemic inflammatory response/multiple organ dysfunction syndromes. Clin. Immunol. Immunopathol. 85, 97–103 (1997).
Millar, J. E., Fanning, J. P., McDonald, C. I., McAuley, D. F. & Fraser, J. F. The Inflammatory response to extracorporeal membrane oxygenation (Ecmo): A Review of the pathophysiology. Crit. Care 20, 387 (2016).
McILwain, R. B. et al. Plasma concentrations of inflammatory cytokines rise rapidly during Ecmo-related sirs due to the release of performed stores in the intestine. Lab. Investig. 90, 128–139 (2010).
Shi, J. et al. Continuous renal replacement therapy reduces the systemic and pulmonary inflammation induced by venovenous extracorporeal membrane oxygenation in a porcine model. Artif. Organs. 38, 215–223 (2014).
Golej, J. et al. Impact of extracorporeal membrane oxygenation modality on cytokine release during rescue from infant hypoxia. Shock 20, 110–115 (2003).
Adiraju, S. K. S. et al. Effect of cardiopulmonary bypass on cytochrome P450 enzyme activity: Implications for pharmacotherapy. Drug Metab. Rev. 50, 109–124 (2018).
Acknowledgments
The authors would like to thank all the members of the Department of Pharmaceutical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences at Fukuyama University.
Author information
Authors and Affiliations
Contributions
Y.S. and Y.F. conceived and designed the study. Y.S., E.M., R.K., T.A., and Y.F. performed the experiments and analyzed the data. Y.S. wrote the manuscript. Y.N. and Y.F. revised the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
All the authors agree on the publication of the results of the present manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sato, Y., Motoishi, E., Kakuba, R. et al. Pharmacokinetics of midazolam and hepatic cytochrome P450 3A activity in an in vivo extracorporeal membrane oxygenation rat model. Sci Rep 15, 19363 (2025). https://doi.org/10.1038/s41598-025-03949-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03949-x