Abstract
SLC20A2, encoding human type III sodium-dependent phosphate transporter 2 (hPiT2), is the gene most frequently associated with primary familial brain calcification (PFBC). The mechanism by which a SLC20A2 mutation causes phosphate transporter dysfunction may depend on the functional region of hPiT2 being affected. We presented clinical and brain imaging data of a patient with idiopathic brain calcification. Genetic testing detected a novel, de novo and in silico-predicted deleterious variant, c.1891 C > T (p.Pro631Ser), in SLC20A2. Computational simulations revealed that, compared to the wild type, this variant hPiT2 was associated with a higher root mean square deviation in molecular dynamics, a smaller value with a wider range for the kink angle of transmembrane helix 8 (TM8), and a less flexible TM8 structural conformation. These molecular characteristics were also observed in the known pathogenic missense variants in the TM8 of hPiT2. The pathogenicity of the novel SLC20A2 variant p.Pro631Ser is supported by the computational simulations for molecular characteristics of the variant hPiT2. The findings also highlight the role of TM8 helix in maintaining normal hPiT2 functions.
Similar content being viewed by others
Introduction
Primary familial brain calcification (PFBC), previously known as idiopathic basal ganglia calcification, bilateral striatopallidodentate calcinosis or Fahr’s disease, is a disorder characterized by abnormal brain calcification. The calcified lesions that are hyperdense on CT images are located primarily in the bilateral basal ganglia, with varying extents of involvement in the dentate nuclei, thalami, subcortical white matter and cerebral cortex1. The diagnosis of PFBC requires the exclusion of biochemical abnormalities, infectious diseases, exposure to toxic agents and inherited metabolic disorders (e.g., mitochondrial diseases) that may be associated with abnormal brain calcification.
PFBC is heterogeneous in its inheritance patterns and causative genes. Earlier studies identified four genes (SLC20 A2, PDGFB, PDGFRB, and XPR1) associated with PFBC in an autosomal dominant inheritance pattern, accounting for approximately 55% of PFBC patients2. Later studies have also suggested that biallelic mutations in MYORG3, JAM24, CMPK25 and NAA606 can lead to PFBC. SLC20A2 is the gene most frequently associated with PFBC (denoted PFBC-SLC20A2), with mutations accounting for 50% of all familial cases7,8.
SLC20A2 encodes type III sodium-dependent phosphate transporter 2 (PiT2), which plays a vital role in maintaining the homeostasis of inorganic phosphate (Pi). As a member of the PiT family, PiT2 consists of two domains, the intracellular soluble domain and the transmembrane (TM) domain. The TM domain of human PiT2 (hPiT2) shares high sequence homology with various species, such as Thermotoga maritima (TmPiT), with 39% identity and 62% similarity9. Previously, we modeled the hPiT2 TM domain on the basis of the TmPiT–Na/Pi complex structure9. The hPiT2 TM domain contains four highly conserved motifs, namely, GΦNDΦ, GxxxxGxxVxxT, PΦSxT and IxxxWΦ (x, any amino acid; Φ, hydrophobic residue), which are vital for the functional and conformational changes of PiT9,10,11. Loss-of-function mutations of the gene, especially those affecting the TM domain, tend to impair the transporter’s ability to take up Pi into the cell and cause abnormal depositions of calcium phosphate in the brain12. On the other hand, the mechanisms by which SLC20A2 mutations cause Pi transporter dysfunctions may depend on the functional regions of hPiT2 that are affected9. For instance, the pathogenic variant D28N affects the sodium-binding residue D28 in one of the Pi−2Na–binding sites, resulting in reduced Pi uptake while retaining Pi-binding affinity. In contrast, the variant W626R affects a tryptophan in the middle of TM8 helix, leading to dysfunction of TM8 conformational changes and impeding opening/closing of the PiT2 inner gate.
PFBC caused by de novo SLC20A2 mutations is rare. To our knowledge, only two PFBC cases with de novo single nucleotide mutations in SLC20A2 have been reported previously13,14. Here, we present a case of PFBC with a novel and de novo missense SLC20A2 variant that affects the TM8 helix. Given that a positive correlation for the variant among the affected familial members was unfeasible, we evaluated the effects of this variant on hPiT2 molecular characteristics using computational modeling and simulations. Four other SLC20A2 missense variants affecting TM8, which either caused PFBC clinically or impaired Pi uptake in TmPiT, were included for comparison. We found that the novel variant exhibited adverse effects on hPiT2 conformational changes and stability, similar to known pathogenic variants.
Methods
Genetic testing and in Silico analysis of variants
The study was approved by the Chang Gung Memorial Hospital Institutional Review Board (No.201702186B0) and all participants provided informed consent. All methods were performed in accordance with the relevant guidelines. The details of whole-exome sequencing and genetic variant analysis are described in Additional File 1. In brief, the exome library was captured via a Roche KAPA HyperExome Plus Kit (Roche Sequencing Solutions), and sequencing was performed on a NovaSeq X Plus (Illumina). After sequencing adaptors and low-quality bases were trimmed, read mapping and variant calling were performed using the Burrows–Wheeler Aligner and Genome Analysis Toolkit.
Variants of PFBC-causative genes, including SLC20A2, PDGFB, PDGFRB, XPR1, MYORG, JAM2, CMPK2 and NAA60, were prioritized. Next, only variants that were either reported as “pathogenic” or “likely pathogenic” in ClinVar or predicted to alter the coding proteins were considered. Variants with allele frequencies > 0.001 in any population of any population in the genome database were discarded. Multiple in silico prediction tools have been utilized to predict the pathogenicity of missense variants. The reported variant was confirmed by Sanger sequencing.
Initial structure of the hPiT2 model
The TM domain of hPiT2, which contains 12 TM helices, was modeled based on the TmPiT structure9. When the soluble domain region (residues 238 to 479) of hPiT2, located between TM5 and TM6 of the TM domain, was modeled using AlphaFold315, the secondary structure prediction revealed a per-residue confidence score (pLDDT) below 50. We therefore excluded the soluble domain and used only the remaining TM helices for the molecular dynamics (MD) simulation. The hPiT2 variants, including V624E, P631S, W626 A, W626R, and S637R, were prepared using the same approach. V624E, W626R and S637R are the missense variants which were identified previously in PFBC patients7,16,17,18 and all of them, like P631S, are located in the TM8 helix. The residue W626 in hPiT2 corresponds to W378 in TmPiT. The TmPiT mutant W378 A lacks Pi binding affinity9, suggesting that the ability of the W626 A variant of hPiT2 to bind Pi might also be disrupted.
The structural models of wild-type (WT) hPiT2 and its variants were embedded into the same membrane system using the Membrane Builder of CHARMM-GUI19,20,21,22. This system consisted of a lipid mixture of phosphatidylethanolamine (POPE) and phosphatidylcholine (POPC) in a 1:1 ratio with a water layer and 100 mM NaCl. The dimensions of the MD simulation system were 140 × 140 × 111 ų. The force fields applied were ff14SB23, and the water model TIP3P was used with tLEaP from AmberTools2224.
Molecular dynamics simulation
Each MD simulation of hPiT2 complexes was performed with AMBER2224 with three independent repeats. The first minimization constrains all atoms of the protein, and the water and lipids are minimized. In the second minimization, the backbone of the protein was constrained, and the side chains of the protein, water, and lipid were minimized. In the third minimization, the whole simulation system was optimized without any constraints. These minimizations are calculated in 2500 steps of the steepest descent and then 2500 steps of the conjugate gradient. The simulation systems were then heated from 0 K to 300 K in 100 ps and equilibrated via the NVT ensemble calculation method in 100 ps. Finally, the production is performed in 200 ns from the NPT ensemble calculation method, in which the total number of atoms is fixed at 300 K and 1 atm.
Measurement of the TM8 helix bending angle
The kink angles of TM8 in WT and hPiT2 variants were determined by calculating the angle between two center-of-mass vectors that represent the upper (residue 626–631) and lower (residue 632–637) halves of TM8. Kink angle analysis was performed using CPPTRAJ of AmberTool2224, and only the Cα atoms were considered for the measurement.
Results
Case presentation and genetic testing results
A 48-year-old Taiwanese woman with a history of anxiety disorder and insomnia presented with an insidious onset of generalized muscle stiffness, slow limb and trunk movements, and intermittent involuntary facial and limb movements for 1 year. Additional symptoms included cognitive decline, apathy, impaired verbal fluency and gait disturbance. She did not have any family history of neurodegenerative disorders (Fig. 1A).
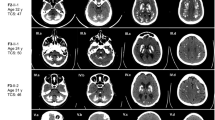
Clinical information and the detected SLC20A2 mutation in the patient. (A) Pedigree with the proband indicated by an arrow. Squares represent male subjects, and circles represent female subjects. The affected individual is shown with solid symbols, and unaffected individuals or carriers are denoted with open symbols. (B) Brain CT image showing symmetric calcification in the bilateral dentate, basal ganglia and periventricular and subcortical white matter. (C) Electropherogram of the identified SLC20A2 variant. wt, wild type; m, c.1891 C > T (p.Pro631Ser). (D) Conservation of the affected residue across different species.
On neurological examination, the patient was alert and oriented. A brief cognitive function screening was performed. The Montreal Cognitive Assessment score was 20/30 (normal ≥ 26), the Mini-Mental State Examination score was 29/30, and the Cognitive Abilities Screening Instrument score was 87.4/100. Multiple primitive reflexes were present. Her speech was slow, hypophonic and aprosodic. Neurological examination revealed unsmooth eye pursuit, generalized hyperreflexia, and hypomimia but orolingual dyskinesia, axial and appendicular rigidity, global hypokinesia and limb dystonia. The plantar reflexes were flexor bilaterally. The cerebellar and sensory systems were normal. Her gait was narrow and stable, but her stride length and arm swings were decreased. Brain CT revealed symmetric calcification in the dentate, bilateral basal ganglion and periventricular and subcortical white matter (Fig. 1B). Blood tests excluded acquired metabolic (including parathyroid hormone, calcium, phosphate and lactate), autoimmune, RPR, HIV antibody, and toxic (lead, mercury, cadmium, manganese and arsenic) causes of brain calcification.
Because of the characteristic brain features and lack of evidence of abnormalities in calcium or phosphate metabolism, PFBC was suspected. A genetic study with whole-exome sequencing was performed after informed consent was obtained from the patient. After the variants in the known PFBC-related genes were reviewed, a heterozygous missense variant, c.1891 C > T (p.Pro631Ser), in SLC20A2, which was not registered in gnomAD, dbSNP, ClinVar or the Taiwan Biobank, was detected and confirmed by Sanger sequencing (Fig. 1C). This missense variant affects a highly evolutionarily conserved amino acid residue (Fig. 1D). High probabilities of disruptive or deleterious effects on protein structure or function by residue substitution were predicted by CADD (score 25.9), SIFT (score 0, pathogenic), PolyPhen-2 (score 0.994, probably damaging) and AlphaMissense (score 0.9957). The proband’s parents and her brother exhibited normal neurological function and brain images and did not carry this SLC20A2 variant (Fig. 1A). Paternity was confirmed via a panel of 15 microsatellite polymorphisms. Thus, the variant was interpreted as “likely pathogenic” (PS2, PM2, PP3, PP4) based on ACMG-AMP guidelines.
Secondary structures and molecular dynamics simulations
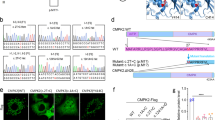
On the basis of the structure of TmPiT, which is homologous to the hPiT2 protein, we mapped the novel variant P631S and other variants in the TM8 helix, including V624E, W626 A, W626R, and S637R, to the hPiT2 TM domain (Fig. 2A).
Initial structure and molecular dynamics of hPiT2. (A) The transmembrane domain of hPiT2 constructed on the basis of TmPiT, with residues V624, W626, P631, and S637 mapped to the structure. (B) Root mean square deviation (RMSD) values of the wild-type and V624E, W626A, W626R, P631S, and S637R mutant hPiT2 structures.
To explore the effects of the hPiT2 variants on the TM8 helix, we modeled the structures of the hPiT2 variants V624E, W626A, W626R, P631S, and S637R. We then performed MD simulations on these mutants for 200 ns with three independent equilibrium simulations. We measured the root mean square deviation (RMSD) values to assess how these variants impact protein stability. Our results revealed that the average RMSD of the overall structure was 1.4 ± 0.1 Å for the WT protein and 1.4 ± 0.1 Å, 1.6 ± 0.2 Å, 1.8 ± 0.2 Å, 1.5 ± 0.2 Å, and 1.6 ± 0.1 Å for the V624E, W626A, W626R, P631S, and S637R mutant proteins, respectively (Fig. 2B). Since these variant residues are all embedded in the membrane, their overall structures remain similar. Nevertheless, the higher RMSD values for the hPiT2 variants suggested lower stabilities than those of the WT protein.
We next measured the kink angle of the TM8 helix by defining the upper (residues 626–631) and lower (residues 632–637) halves of TM8 as two representative vectors to evaluate the conformational change in TM8 (Fig. 3A). In TmPiT, TM8 serves as a gate with a closed conformation to transport Pi within the membrane and an open conformation to allow the intracellular release of Pi9. We first measured the kink angles for TM8 of TmPiT as 20.7° and 27.6° for the closed and open forms, respectively (Fig. 3B). After the MD simulations, we calculated the kink angles for the TM8 s of the WT and mutants of hPiT2. The most likely kink angle was 27.0° for the WT protein and 25.0°, 20.0°, 20.1°, 19.0°, and 22.0° for the five V624E, W626A, W626R, P631S, and S637R mutant proteins, respectively (Fig. 3B).
Measurement of the TM8 helix kink angles. (A) The kink angle of the TM8 helix is measured by defining two representative vectors. The upper half is defined with residues 626 to 631, and the lower half is defined with residues 632 to 637. (B) The measured TmPiT kink angles in the open (27.6°) and closed (20.7°) states and the wild-type and mutant hPiT2 kink angles were observed using molecular dynamic simulations. The most likely kink angle of the P631S mutant is 19.0°, which is the lowest among all the hPiT2 mutants. This mutant also has the widest range of possible kink angles.
We subsequently compared the residual environments of these variants of hPiT2 within a 5 Å distance, with Pi shown as sticks and spheres in the binding site (Fig. 4). For wild-type hPiT2 (Fig. 4A), there is an important π-π interaction between W626 and H596 during Pi binding, which is conserved and found in TmPiT9. In the V624E variant, the π-π interaction is absent because of the conformational change in the HP2 and TM8 helices. Moreover, the side chain of E624 may form a hydrogen bond with N621, potentially interfering with helix stability. As expected, the π-π interaction between W626 and H596 was lost in W626A and W626R mutants (Fig. 4C, D), and the hydrophobic environment around W626 was disrupted under these conditions (Fig. 4D). Furthermore, as W626R forms a hydrogen bond with S113, which corresponds to one of the Pi/Na binding residues in TmPiT9, the W626R mutation may perturb this critical role of S113. For P631S, the variant present in our case, the replacement of a pyrrolidine ring by a hydroxymethyl side chain, which resists approaching the lipid environment, may result in TM8 toward a closed conformation, and stacking the additional π-π interactions between H116 and H596 (Fig. 4E). In the S637R mutant protein, the π-π interaction between H596 and W626 is retained, and the mutated arginine forms a hydrogen bond with E91 (Fig. 4F). Although E91 is not a highly conserved residue in the PiT family, this interaction may flip F125 outward from the HP1 helix (Fig. 4F).
Residual environments of wild-type and mutant hPiT2. (A-F) An important π-π interaction between W626 and H596 during Pi binding in wild-type hPiT2 is lost in the V624E, W626A, and W626R mutants. A new hydrogen bond is formed between the mutated residue in the V624E mutant and N621, and a similar situation is observed between the mutated residue S637R and E91. A pyrrolidine ring side chain pointed toward the lipid environment in the P631S mutant is lost.
In summary, these mutants of hPiT2, including P631S, may destabilize the protein, reduce the kink angle of TM8, disturb the hydrophobicity of TM8 and its associated TM helices, and potentially cause dysfunctional Pi binding and transport for hPiT2.
Discussion
The clinical features of PFBC are heterogeneous. The pathogenesis of neurological and psychiatric symptoms in PFBC-SLC20A2 patients could be multifactorial. Several plausible mechanisms, including cerebral hypoperfusion due to microvasculopathy18, impairment of dopaminergic pathways25, cellular energy deficiency due to mitochondrial dysfunction26, and reactivation of astrocytes and microglia27, have been implicated in brain damage. hPiT2 is expressed ubiquitously in various tissues, with the highest levels noted in the basal ganglia, thalamus and cerebellum28. In combination with the other human PiT family member hPiT1, hPiT2 maintains cerebral Pi homeostasis. In the choroid plexus, hPiT2 is localized mainly in the apical microvillar membranes facing the cerebrospinal fluid, contributing to the transport of Pi from the cerebrospinal fluid to the blood29. The perturbation of hPiT2 functions leads to impaired intracellular Pi import and subsequent anomalous calcium phosphate deposition due to extracellular Pi accumulation12.
On the basis of the model we established previously, TmPiT is a dimer in which each subunit includes a transport domain and a scaffold domain composed of 12 TM helices. The transport domain is formed by two inverted repeats, N-PD001131 and C-PD0011319. The TM8 helix is located in the C-PD001131 repeat. By adopting conformation changes, the helix governs the opening of the transporter inner gate and plays an important role in controlling intracellular Pi entry, highlighting the role of TM8 in hPiT2 function. A highly conserved residue W378 of TmPiT (corresponding to W626 in hPiT) located in the middle of TM8 is hypothesized to be involved in TM8 bending for inner gate opening9. In support of this, TmPiT with the W378A mutation loses its Pi-binding ability, and the SLC20A2 variant W626R is associated with PFBC9,17.
By providing important information on how molecular characteristics of the protein are affected upon mutation, computational simulations of molecular dynamics can serve as supplementary investigations for cellular or animal models to predict the pathogenicity of mutant protein30. To assess the pathogenicity of the variant P631S, the stability of the entire TM domain was first tested via MD simulation. We showed that variant P631S had a greater RMSD, which implied a lower molecular stability than that of WT hPiT2. Additionally, the simulation of the TM8 kink angle revealed that the P631S variant had a wider predicted kink angle range than WT hPiT2 did (Fig. 3B). This finding suggests that serine may introduce a more malleable structure compared to proline. However, the π-π stacking interactions among H116, H596, and W626 restricted the kink angle and maintained in the closed conformation of TM8. Next, as the P631 residue is located in the TM8 helix of hPiT2, the influence of the variant on the kink angle of TM8 was evaluated. Among the amino acids, proline and glycine are known to disrupt helix formation in the secondary structure31. A lack of an amide hydrogen prevents proline from forming a helix, which facilitates the introduction of a kink in an amino acid chain32. This property is significant in many membrane proteins, where proline acts as a ligand-binding site for cations33. Moreover, proline commonly serves as a molecular switch involved in conformational changes, as observed in transporters such as NaDT and LeuT34. The P631 residue is positioned centrally within the membrane and located in the middle of the TM8 helix, suggesting that it might be a bending site of this helix. Our study revealed that the simulated TM8 kink angle of WT hPiT2 is 27.0°, whereas that of the P631S variant is only 19.0°, suggesting that the flexion of the TM8 helix may be reduced by this amino acid change. Finally, scrutiny of the sequence homology and helical wheel projection of TM8 (Additional File 2) revealed only two polar residues, T629 and S637, indicating that TM8 is a very hydrophobic helix. TM8 is located between the substrate binding site and the membrane lipid. Residue P631 and a series of spatially adjacent residues form a highly hydrophobic region composed of 624(I/M/V)−628(L/V)−631P-635(A/V/L)−639 A on the lipid side of TM8. Replacement of the hydrophobic residue by a charged or polar residue, as in the cases of V624E and P631S, may alter the integrity of the interface between the hydrophobic region and the lipid membrane. We conclude that the P631S mutation may alter the flexibility of the TM domain and the conformation of the TM8 helix, potentially impairing the Pi transport functions of hPiT2. The pathogenicity of the novel variant P631S is further supported by similar changes in TM domain flexibility and the TM8 kink angle, which are also present in the missense variants in TM8 associated with PFBC clinically (V624E, W626R, S637R) or impaired Pi transport of hPiT2 in vitro (W626A) (Figs. 2B, 3B and 4).
It should be noted that our study is limited to computational modeling and molecular dynamics simulation for effects of amino acid substitution on protein 3D structure and stability. Additional in vitro or in vivo studies are needed to confirm the findings and their associations with hPiT2 functions.
Conclusions
We present a novel and rarely found de novo SLC20A2 variant, c.1891 C > T (p.Pro631Ser), in a PFBC patient. Based on computational modeling and simulations, its pathogenicity is supported by comparisons with wild-type hPiT2 as well as known disease-causing or functionally deleterious variants in the same functional region. Our findings also highlight the role of the TM8 helix in hPiT2 functions.
Data availability
The datasets generated and/or analyzed during the current study are available in the figshare repository at https://figshare.com/articles/figure/A_SLC20A2_novel_and_de_novo_variant_associated_with_PFBC/27215568.
Abbreviations
- ACMG-AMP:
-
American College of Medical Genetics and Genomics and Association of Molecular Pathology
- hPiT2:
-
Human sodium-dependent phosphate transporter 2
- MD:
-
Molecular dynamics
- PFBC:
-
Primary familial brain calcification
- Pi :
-
Inorganic phosphate
- PiT2:
-
Type III sodium-dependent phosphate transporter 2
- RMSD:
-
Root mean square deviation
- TM:
-
Transmembrane
- TmPiT:
-
Thermotoga maritima sodium-dependent phosphate transporter
- WT:
-
Wild type
References
Carecchio, M., Mainardi, M. & Bonato, G. The clinical and genetic spectrum of primary Familial brain calcification. J. Neurol. 270 (6), 3270–3277 (2023).
Ramos, E. M., Oliveira, J., Sobrido, M. J. & Coppola, G. in Primary Familial Brain Calcification in GeneReviews®. (eds Adam, M. P.) (University of Washington, 2004).
Yao, X. P. et al. Biallelic mutations in MYORG cause autosomal recessive primary Familial brain calcification. Neuron 98 (6), 1116–1123e5 (2018).
Schottlaender, L. V. et al. Bi-allelic JAM2 variants lead to Early-Onset recessive primary Familial brain calcification. Am. J. Hum. Genet. 106 (3), 412–421 (2020).
Zhao, M. et al. Loss of function of CMPK2 causes mitochondria deficiency and brain calcification. Cell. Discov. 8 (1), 128 (2022).
Chelban, V. et al. Biallelic NAA60 variants with impaired n-terminal acetylation capacity cause autosomal recessive primary Familial brain calcifications. Nat. Commun. 15 (1), 2269 (2024).
Yamada, M. et al. Evaluation of SLC20A2 mutations that cause idiopathic basal ganglia calcification in Japan. Neurology 82 (8), 705–712 (2014).
Balck, A. et al. Genotype-Phenotype relations in primary Familial brain calcification: systematic MDSGene review. Mov. Disord. 36 (11), 2468–2480 (2021).
Tsai, J. Y. et al. Structure of the sodium-dependent phosphate transporter reveals insights into human solute carrier SLC20. Sci. Adv. 6 (32), eabb4024 (2020).
Salaün, C., Rodrigues, P. & Heard, J. M. Transmembrane topology of PiT-2, a phosphate transporter-retrovirus receptor. J. Virol. 75 (12), 5584–5592 (2001).
Bøttger, P. & Pedersen, L. Mapping of the minimal inorganic phosphate transporting unit of human PiT2 suggests a structure universal to PiT-related proteins from all kingdoms of life. BMC Biochem. 12, 21 (2011).
Wang, C. et al. Mutations in SLC20A2 link Familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat. Genet. 44 (3), 254–256 (2012).
Ferreira, J. B. et al. First report of a de Novo mutation at SLC20A2 in a patient with brain calcification. J. Mol. Neurosci. 54 (4), 748–751 (2014).
Sellami, L., Verret, L., Poulin, S. & Laforce, R. Fahr’s disease due to a novel SLC20A2 gene mutation in a Canadian patient: early neurocognitive, structural and metabolic changes (P2.175). Neurology (2018). 90 (15_supplement).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with alphafold 3. Nature 630 (8016), 493–500 (2024).
Ramos, E. M. et al. Primary brain calcification: an international study reporting novel variants and associated phenotypes. Eur. J. Hum. Genet. 26 (10), 1462–1477 (2018).
Chen, S. et al. Underestimated disease prevalence and severe phenotypes in patients with biallelic variants: A cohort study of primary Familial brain calcification from China. Parkinsonism Relat. Disord. 64, 211–219 (2019).
Kimura, T. et al. Familial idiopathic basal ganglia calcification: histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology 36 (4), 365–371 (2016).
Anashkin, V. A. & Baykov, A. A. A lumenal loop associated with catalytic asymmetry in plant vacuolar H+-Translocating pyrophosphatase. Int. J. Mol. Sci. 22 (23), 12902 (2021).
Jo, S., Kim, T. & Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS One 2(9), e880 (2007).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29 (11), 1859–1865 (2008).
Shah, N. R. et al. Insights into the mechanism of membrane pyrophosphatases by combining experiment and computer simulation. Struct. Dyn. 4 (3), 032105 (2017).
Maier, J. A. et al. ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11 (8), 3696–3713 (2015).
Case, D. A. et al. AmberTools. J. Chem. Inf. Model. 63 (20), 6183–6191 (2023).
Koyama, S. et al. Clinical and radiological diversity in genetically confirmed primary Familial brain calcification. Sci. Rep. 7 (1), 12046 (2017).
Sun, H. et al. Golgi damage caused by dysfunction of PiT-2 in primary Familial brain calcification. Biochem. Biophys. Res. Commun. 642, 167–174 (2023).
Nahar, K. et al. Astrocyte-microglial association and matrix composition are common events in the natural history of primary Familial brain calcification. Brain Pathol. 30 (3), 446–464 (2020).
da Silva, R. J., Pereira, I. C. & Oliveira, J. R. Analysis of gene expression pattern and neuroanatomical correlates for SLC20A2 (PiT-2) shows a molecular network with potential impact in idiopathic basal ganglia calcification (Fahr’s disease). J. Mol. Neurosci. 50 (2), 280–283 (2013).
Guerreiro, P. M., Bataille, A. M., Parker, S. L. & Renfro, J. L. Active removal of inorganic phosphate from cerebrospinal fluid by the choroid plexus. Am. J. Physiol. Ren. Physiol. 306 (11), F1275–F1284 (2014).
Mir, Y. R. et al. Identification and structural characterization of a pathogenic ARSA missense variant in two consanguineous families from Jammu and Kashmir (India) with late infantile metachromatic leukodystrophy. Mol. Biol. Rep. 51 (1), 30 (2023).
Cordes, F. S., Bright, J. N. & Sansom, M. S. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323 (5), 951–960 (2002).
Wilman, H. R., Shi, J. & Deane, C. M. Helix Kinks are equally prevalent in soluble and membrane proteins. Proteins 82 (9), 1960–1970 (2014).
Sansom, M. S. Proline residues in transmembrane helices of channel and transport proteins: a molecular modelling study. Protein Eng. 5 (1), 53–60 (1992).
Kahn, E. S. & Pajor, A. M. Determinants of substrate and cation affinities in the Na+/dicarboxylate cotransporter. Biochemistry 38 (19), 6151–6156 (1999).
Acknowledgements
We are grateful to the patient and family members who participated in this study. This work was supported by the National Science and Technology Council, Taiwan (NSTC 111-2326-B-007-001- and 113-2311-B-007-007-MY3 to Y.-J. S.)
Funding
This work was supported by the National Science and Technology Council, Taiwan (NSTC 111-2326-B-007-001- and 113-2311-B-007-007-MY3 to Y.-J. S.)
Author information
Authors and Affiliations
Contributions
S.C.L., Y.J.S., and Y.Y.C.: study conceptualization and design. Y.S.H., J.Y.T., M.Y.L., and Y.Y.C.: data collection. S.C.L. and M.Y.L.: genetic testing and data analysis. Y.S.H., J.Y.T. and Y.J.S.: molecular structure modeling, molecular dynamics simulation and data analysis. S.C.L., Y.S.H., J.Y.T. and Y.Y.C.: writing of the first draft. M.Y.L. and Y.J.S.: manuscript review and critique.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lan, SC., Huang, YS., Tsai, JY. et al. Computational molecular characterization of a novel SLC20A2 variant associated with primary familial brain calcification. Sci Rep 15, 18682 (2025). https://doi.org/10.1038/s41598-025-03953-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-03953-1