Abstract
Dietary proteins are absorbed by the intestine as amino acids or peptides. Di- or tripeptides are absorbed via the intestinal peptide transporter 1 (PepT1); however, the intestinal target of oligopeptides larger than tetrapeptides remain unclear, posing a major contradiction in the current protein-peptide nutrition. This study aimed to explore the unidentified function of intestinal alkaline phosphatase (IAP) in regulating cholesterol metabolism using the dietary peptide IIAEK. In IAP-knockout (Akp3-/-) mice, the IIAEK-induced amelioration of cholesterol metabolism via the suppression of intestinal cholesterol absorption was absent. Furthermore, we found that IIAEK specifically interacts with the substrate recognition sites of mouse IAP (Akp3) and human IAP. The IIAEK-human IAP complex interacts with the transmembrane glycoprotein cadherin-17 (CDH17). In conclusion, we demonstrated that IAP is pivotal for the pentapeptide IIAEK-induced amelioration of cholesterol metabolism and serves not only as an enzyme (phosphatase) but also as a glycosylphosphatidylinositol-anchored specific receptor for oligopeptide IIAEK. In addition, we suggest that human cadherin-17 plays a key role in signal transduction in the IIAEK-human IAP complex. Our findings contribute to the understanding of the molecular mechanism of intestinal sensing of oligopeptides (IIAEK) and the development of novel functional foods and medicines targeting IAP to treat lifestyle-related diseases, such as dyslipidaemia.
Similar content being viewed by others
Introduction
Dyslipidaemia contributes to global health and economic burden and is often associated with lifestyle1. Medical treatment has been committed to improving dyslipidemia1; however, a definitive solution remains unachieved. Ischaemic heart disease remains the leading global cause of death2, with lifestyle-related diseases such as dyslipidemia playing a substantial role3. Consequently, research into food ingredients that improve cholesterol metabolism to prevent lifestyle-related diseases has been promoted. The improvement of cholesterol metabolism via food protein-derived bioactive peptides has recently garnered considerable attention owing to their notable efficacy, safety, lack of toxicity, and affordability4. Highly bioactive peptides derived from food proteins have been identified to control and prevent lifestyle-related diseases worldwide; however, most studies have focused on the in vitro effects of these peptides on improving cholesterol metabolism in cultured cells derived from the liver or adipose tissues5. Few studies have focused on improving cholesterol metabolism of food protein-derived bioactive peptides in the small intestine, the primary site of absorption of food protein-derived bioactive peptides5.
An important aspect of studying food-protein-derived bioactive peptides is their absorption in the small intestine. Dietary proteins are fragmented by digestive enzymes, such as pepsin or trypsin, and absorbed in the intestine as amino acids or peptides6,7. In the small intestine, peptide transporter 1 (PepT1) can only absorb di- or tripeptides and cannot absorb oligopeptides larger than tetrapeptides8. Cholesterol-lowering di- or tripeptides are absorbed by intestinal PepT1 to improve cholesterol metabolism in the small intestine and liver9,10. Thus, oligopeptides larger than tetrapeptides cannot improve cholesterol metabolism through intestinal absorption via PepT1. IIAEK (Ile-Ile-Ala-Glu-Lys) was the first identified hypocholesterolemic pentapeptide derived from bovine milk β-lactoglobulin by our group11. IIAEK can powerfully influence serum cholesterol levels and exhibit greater hypocholesterolemic activity than β-sitosterol in animal studies11. However, the precise mechanism underlying the hypocholesterolemic action of IIAEK remains unclear. To date, only seven food protein-derived oligopeptides larger than tetrapeptides including IIAEK (milk), HIRL (milk), VAWWMY (soy), GEQQQPGM (rice), VHVV (soy), VSEE (egg), and VFVRN (chickpea) effectively improve cholesterol metabolism in animal studies12. Although food protein-derived oligopeptides larger than tetrapeptides improve cholesterol metabolism, the intestinal-specific receptors for these peptides, such as PepT1, remain unclear, representing major contradictions in the current protein-peptide nutrition. Thus, identifying intestinal-specific receptors for food protein-derived oligopeptides larger than tetrapeptides is crucial for developing strategies to prevent and manage lifestyle-related diseases, including dyslipidemia. However, no established evaluation system exists for the identification of these receptors. Therefore, among the cholesterol-lowering oligopeptides that are effective in animal studies, we recently used photoaffinity labeling to provide in vitro evidence that the cholesterol-lowering pentapeptide IIAEK targets glycosylphosphatidylinositol (GPI) anchored receptor intestinal alkaline phosphatase (IAP) 13,14. However, it remains unclear whether the GPI-anchored receptor IAP acts as a specific receptor for the pentapeptide IIAEK to inhibit intestinal cholesterol absorption in vivo.
In this study, we hypothesised that a specific interaction between IIAEK and IAP is essential for IIAEK-induced suppression of cholesterol absorption in vivo. To verify our hypothesis, we investigated whether IAP functions as a specific receptor for the oligopeptide (IIAEK) and is essential for the pentapeptide IIAEK-induced amelioration of cholesterol metabolism. Furthermore, IAP has no transmembrane domain owing to its GPI-anchored receptor13 and cannot transduce cell signalling induced by IIAEK-IAP interactions. Therefore, we developed an experimental method to identify the transmembrane target protein involved in cell signalling induced by IIAEK-IAP interactions. Collectively, this study aimed to reveal the novel role of IAP in controlling cholesterol metabolism via the dietary peptide IIAEK.
Results
IIAEK-induced amelioration of cholesterol metabolism was absent in IAP-knockout (IAP-KO) (Akp3 -/-) mice
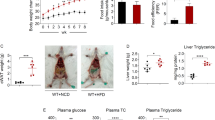
We first investigated the effect of IAP deficiency on IIAEK-induced amelioration of cholesterol metabolism in IAP-KO mice. No significant differences in body weight (B.W.), food intake, or liver weight were observed between the experimental groups (Supplementary Fig. 1a–f). Oral administration of IIAEK to wild-type (WT) mice significantly suppressed serum cholesterol (Fig. 1a, c), total liver lipids (Fig. 1b), liver cholesterol (Fig. 1b), liver triglycerides (Fig. 1b), and [3H]-cholesterol absorption (Fig. 1e) and significantly increased fecal weights (Supplementary Fig. 1 g) and steroid excretion (fecal cholesterol, acidic steroids, neutral steroids, and total steroids) (Fig. 1d) compared to that in the WT, Control groups. The IIAEK-induced amelioration of cholesterol metabolism was absent in IAP-KO mice (Fig. 1a–e; IAP-KO, Control vs. IAP-KO, IIAEK).
IIAEK-induced amelioration of cholesterol metabolism was absent in IAP-knockout (IAP-KO) (Akp3-/-) mice. Seven week-old wild-type (WT) and IAP-knockout (IAP-KO) (Akp3-/-) mice were orally administered the pentapeptide IIAEK (Ile-Ile-Ala-Glu-Lys) (600 mg/kg B.W./d) once a day for 14 d. At the end of the feeding period, the mice were orally administered the test solutions, including [3H]-cholesterol. One hour later, blood, liver, and intestinal samples were collected from the mice. (a, c) Serum cholesterol; (b) Liver lipids profile (Total liver lipids, Liver cholesterol, and Liver triglycerides); (d) Fecal steroid excretion (fecal cholesterol, coprostanol, acidic steroids, neutral steroid, total steroids). (e) [3H]-cholesterol absorption. (a) n = 6 mice/group; (b) n = 8 mice/group; (c) n = 6 mice in WT, Control group (WT, Control); n = 7 mice in WT, IIAEK group (WT, IIAEK); n = 5 mice in IAP-KO, Control group (IAP-KO, Control); n = 4 mice in IAP-KO, IIAEK group (IAP-KO, IIAEK); (d) n = 7 mice in WT, Control and IAP-KO, IIAEK; n = 8 mice in WT, IIAEK; n = 5 mice in IAP-KO, Control; (e) n = 7 mice in WT, IIAEK; n = 5 mice in IAP-KO, Control; n = 6 mice in WT, Control and IAP-KO, IIAEK. Data are presented as mean ± standard error of the mean (SEM). Statistical significance was calculated using a one-way ANOVA with Tukey’s post-hoc test. Asterisks indicate significant differences between the experimental groups (* p < 0.05, ** p < 0.01).
IIAEK-induced regulation of duodenal, jejunal, and hepatic mRNA levels of key genes related to cholesterol metabolism was absent in IAP-knockout (IAP-KO) (Akp3 -/-) mice
We measured the duodenal, jejunal, and hepatic mRNA levels of key genes associated with cholesterol metabolism to further investigate the effect of IIAEK on cholesterol metabolism. IIAEK significantly increased the duodenal and jejunal mRNA levels of Abcg5 (the key transporter for cholesterol excretion15), Abcg8 (the key transporter for cholesterol excretion15), and Akp3 (mouse IAP16) and significantly decreased the duodenal and jejunal mRNA levels of Hmgcr (the rate-limiting enzyme for cholesterol synthesis17), Npc1l1 (the pivotal transporter for cholesterol absorption18), and Srebf2 (the master regulator of cholesterol metabolism19) in WT mice compared to those in the WT, Control group (Fig. 2a–d; WT, Control vs.WT, IIAEK). In IAP-KO mice, the effect of IIAEK on the duodenal and jejunal mRNA levels of key genes associated with cholesterol metabolism was absent (Fig. 2a–d; IAP-KO, Control vs. IAP-KO, IIAEK).
IIAEK-induced regulation of duodenal, jejunal, and hepatic mRNA levels of key genes related to cholesterol metabolism was absent in IAP-knockout (IAP-KO) (Akp3-/-) mice. Seven-week-old wild-type (WT) and IAP-knockout (IAP-KO) (Akp3-/-) mice were orally administered the pentapeptide IIAEK (Ile-Ile-Ala-Glu-Lys) (600 mg/kg B.W./d) once a day for 14 d. After fasting for 8 h, the duodenum, jejunum, and liver were collected from the mice. (a–e) Total RNA was collected from the duodenum, jejunum, and liver of mice. Real-time quantitative PCR was performed to the mRNA level of (a) duodenal Akp3 mRNA level, (b) jejunal Akp3 mRNA level, (c, d) duodenal and jejunal mRNA levels of key genes associated with cholesterol metabolism (Abca1, Abcg5, Abcg8, Cd36, Hmgcr, Mttp, Npc1l1, Scarb1, Soat2, and Srebf2), and (e) Liver mRNA levels of key genes associated with cholesterol metabolism (Abca1, Abcg5, Abcg8, Cd36, Cyp7a1, Fxr, Hmgcr, Ldlr, Mttp, Npc1l1, Scarb1, Soat2, and Srebf2). (f–h) Western blot analyses were performed to measure the hepatic protein levels of NPC1L1 and CYP7A1. Original blots are presented in Supplementary Fig. 2. (a, b) n = 7 mice in WT, Control group (WT, Control); n = 8 mice in WT, IIAEK group (WT, IIAEK), IAP-KO, Control group (IAP-KO, Control), and IAP-KO, IIAEK group (IAP-KO, IIAEK); (c–h) n = 6 mice per group. Data are presented as the mean ± standard error of the mean (SEM). Statistical significance was calculated using a one-way ANOVA with Tukey’s post-hoc test. Asterisks indicate significant differences between the experimental groups (* p < 0.05, ** p < 0.01).
IIAEK significantly increased the hepatic mRNA levels of Hmgcr and Srebf2 and significantly decreased the hepatic Npc1l1 mRNA levels in WT mice compared to those in the WT, Control group (Fig. 2e; WT, Control vs.WT, IIAEK). Furthermore, IIAEK significantly increased the liver CYP7A1 (the rate-limiting enzyme for bile acid synthesis20) protein level and significantly decreased the liver NPC1L1 protein level in WT mice compared to the levels in the WT, Control group (Fig. 2f–h; WT, Control vs.WT, IIAEK). In IAP-KO mice, the effect of IIAEK on the hepatic mRNA and protein levels of key genes associated with cholesterol metabolism was absent (Fig. 2e–h; IAP-KO, Control vs. IAP-KO, IIAEK).
Oral administration of IIAEK had the greatest impact on the pathway related to cholesterol biosynthesis and metabolism in mouse liver
To understand the molecular mechanism by which IIAEK ameliorates hepatic cholesterol metabolism, we performed DNA microarray analysis using liver samples from the control and IIAEK groups of WT and IAP-KO mice. In this study, 1995 transcripts were identified in the livers of WT mice [WT, Control (WC) vs. WT, IIAEK (WI)] and 3802 in IAP-KO mice [IAP-KO, Control (KOC) vs. IAP-KO, IIAEK (KOI)] (Fig. 3a, c, e, ≥ 1.2 fold-change, p < 0.05). Of these, 1729 transcripts fluctuated only in the WT group (WC vs. WI) and 3536 fluctuated only in the IAP-KO group (KOC vs. KOI) (Fig. 3e). Comparison of the WC and WI group, pathway analysis of the liver of WT mice administered IIAEK revealed that ‘Cholesterol biosynthesis’ and ‘Cholesterol metabolism with Bloch and Kandutsch-Russell pathways’ were identified as the top significantly upregulated pathways (Fig. 3b). However, comparing the KOC and KOI groups, no pathways associated with cholesterol synthesis and metabolism were identified IAP-KO mice administered IIAEK (Fig. 3d). Furthermore, comparing the WC and WI groups, pathway analysis of 1729 transcripts exclusively fluctuating in WT mice administered with IIAEK revealed the identification of ‘Cholesterol biosynthesis’ and ‘Cholesterol metabolism with Bloch and Kandutsch-Russell pathways’ (Fig. 3f). These two pathways contained transcripts associated with cholesterol biosynthesis and metabolism, such as Cyp51, Ebp, Elovl5, Fads1, Fasn, Fdft1, Fdps, Hmgcs1, Idi1, Lss, Nsdhl, Pmvk, Sc5d, and Soat2 (Fig. 3g, ≥ 1.2 fold-change, p < 0.05). Our qPCR results showed that oral administration of IIAEK to WT mice significantly increased the hepatic mRNA level of genes related to cholesterol biosynthesis and metabolism (Cyp51, Ebp, Elovl5, Fads1, Fdft1, Fdps, Hmgcs1, Idi1, Lss, Nsdhl, Pmvk, and Sc5d) compared to those in the WT and Control groups (Fig. 3h; WT, Control vs. WT, IIAEK). Conversely, in IAP-KO mice, IIAEK significantly increased the hepatic mRNA levels of Lss; however, no significant differences were observed in the other mRNA levels (Cyp51, Ebp, Elovl5, Fads1, Fdft1, Fdps, Hmgcs1, Idi1, Nsdhl, Pmvk, and Sc5d) were observed (Fig. 3h; IAP-KO, Control vs. IAP-KO, IIAEK). Comparing the KOC and KOI groups, pathway analysis of 3536 transcripts that exclusively fluctuated in IAP-KO mice administered IIAEK revealed that no pathways associated with cholesterol synthesis and metabolism, observed in WT mice administered IIAEK, were identified (Fig. 3i).
Oral administration of IIAEK has the greatest impact on the pathways related to cholesterol biosynthesis and metabolism in mouse liver. Seven-week-old wild-type (WT) and IAP-knockout (IAP-KO) (Akp3-/-) mice were orally administered the pentapeptide IIAEK (Ile-Ile-Ala-Glu-Lys) (600 mg/kg B.W./d) once a day for 14 d. After fasting for 8 h, the livers were collected from the mice for DNA microarray analyses. Heatmap of upregulated transcripts in the liver of WT administered with IIAEK (a) or IAP-KO mice administered with IIAEK (c). Wikipathway analysis of the upregulated transcripts in the liver of WT mice administered IIAEK (b; red) or IAP-KO mice administered IIAEK (d; yellow). Venn diagram of the upregulated transcripts in the liver of WT mice administered with IIAEK (e, left; red) and IAP-KO mice administered with IIAEK (e, right; yellow). A total of 266 transcripts were shared between the liver of WT mice administered with IIAEK and the liver of IAP-KO mice administered with IIAEK (e; white). Wikipathway analysis using transcripts exclusively fluctuating in WT mice administered IIAEK (f; red) or IAP-KO mice administered IIAEK (i; yellow). (g) Heatmap of upregulated transcripts related to cholesterol biosynthesis and metabolism in the liver of WT mice administered IIAEK. (h) Real-time quantitative PCR was performed to measure the mRNA level of the upregulated hepatic transcripts related to cholesterol biosynthesis and cholesterol metabolism (related to Fig. 3 g). (a–g, i) n = 3/group, fold change ≥ 1.2. (h) n = 6/group. Statistical significance for (a–g, i) was calculated using a nonparametric Mann–Whitney U test (p < 0.05) between WT mice, Control (WC) and WT mice, IIAEK (WI) or between IAP-KO mice, Control (KOC) and IAP-KO mice, IIAEK (KOI). (h) Data are presented as the mean ± standard error of the mean (SEM). Statistical significance was calculated using a one-way ANOVA with Tukey’s post-hoc test. Asterisks indicate significant differences between the experimental groups (* p < 0.05, ** p < 0.01).
Next, we performed DNA microarray analyses of liver samples from the WC and KOC groups and WI and KOI groups. Compared to the WC group, 221 transcripts were significantly upregulated in the livers by IAP deficiency (WC vs. KOC; Supplementary Table 1, ≥ 1.2 fold-change, p < 0.05). In addition, 221 transcripts comparing KOC and WC were associated with embryonic stem cell (ESC) -related signaling pathways such as ‘ESC pluripotency pathways’, ‘Mechanism associated with pluripotency’, and ‘TGF-beta signaling pathway’ (Supplementary Fig. 3a). Furthermore, compared to the WI group, 120 transcripts were significantly upregulated in the livers by IAP deficiency (WI vs. KOI; Supplementary Table 1, ≥ 1.2 fold-change, p < 0.05). In addition, 120 transcripts comparing KOI and WI were associated with ‘Cholesterol metabolism with Bloch and Kandutsch-Russell pathways’, ‘Polyol pathway’, and ‘Mitochondrial gene expression’ (Supplementary Fig. 3b).
Oral administration of IIAEK affected the lipid metabolism pathway in the mouse duodenum and jejunum
We performed DNA microarray analysis using duodenal and jejunal samples from the control and IIAEK groups of WT or IAP-KO mice to understand the molecular mechanism by which IIAEK ameliorates intestinal cholesterol metabolism. In total, 1406 transcripts were identified in the duodenum and 3503 in the jejunum of WT mice, and 85 transcripts were shared between the duodenum and jejunum of WT mice (Fig. 4a–c, ≥ 1.2 fold-change, p < 0.05). Eighty-five transcripts shared between the duodenum and jejunum of WT mice were associated with ‘Eicosanoid synthesis’, ‘Eicosanoid metabolism’, and ‘Insulin signalling’ (Fig. 4d). Furthermore, pathway analysis demonstrated that upregulated transcripts in the duodenum of IIAEK-administered WT mice were associated with pathways related to lipid metabolism, such as ‘Electron transport chain’, ‘Oxidative phosphorylation’, ‘Insulin signalling’ and ‘TCA cycle’ (Fig. 4e). The upregulated transcripts in the jejunum of IIAEK-administered WT mice were associated with lipid metabolism, such as ‘Insulin signalling’ and ‘Cholesterol metabolism with Bloch and Kandutsch-Russell pathways’ (Fig. 4f). Conversely, we identified 777 transcripts in the duodenum and 1388 in the jejunum of IAP-KO mice and 20 transcripts were shared between the duodenum and jejunum of IAP-KO mice (Fig. 4g–i, ≥ 1.2 fold-change, p < 0.05). The pathways associated with lipid metabolism observed in IIAEK-treated WT mice were not identified in the duodenum or jejunum of IAP-KO mice (Fig. 4j–l).
Oral administration of IIAEK affected lipid metabolism pathways in the mouse duodenum and jejunum. Seven-week-old wild-type (WT) and IAP-knockout (IAP-KO) (Akp3-/-) mice were orally administered the pentapeptide IIAEK (Ile-Ile-Ala-Glu-Lys) (600 mg/kg B.W./d) once a day for 14 d. After fasting for 8 h, the duodenum and jejunum were collected from the mice for DNA microarray analysis. Venn diagram of the upregulated transcripts in the duodenum and jejunum of WT mice administered IIAEK (a; red) and IAP-KO mice administered IIAEK (g; yellow). Heatmap of upregulated transcripts in the duodenum and jejunum of WT mice administered IIAEK (b, c) or the duodenum and jejunum of IAP-KO mice administered IIAEK (h, i). Wikipathway analysis of the shared transcripts in the duodenum and jejunum of WT mice administered IIAEK (d; red) or IAP-KO mice administered IIAEK (j; yellow). Wikipathway analysis of the upregulated transcripts in the duodenum and jejunum of WT mice administered IIAEK (e, f; red) or the duodenum and jejunum of IAP-KO mice administered IIAEK (k, l; yellow). (a–l) n = 3/group, Fold change ≥ 1.2. Statistical significance was calculated using a nonparametric Mann–Whitney U test (p < 0.05) between WT mice, Control (WC) and WT mice, IIAEK (WI) or between IAP-KO mice, Control (KOC) and IAP-KO mice, IIAEK (KOI).
Next, we performed DNA microarray analyses between the WC and KOC groups and the WI and KOI groups using duodenal and jejunal samples. Compared to the WC group, 182 transcripts were significantly upregulated in the duodenum and 533 in the jejunum by IAP deficiency (WC vs. KOC; Supplementary Table 1, ≥ 1.2 fold-change, p < 0.05). Pathway analysis revealed that 182 transcripts were upregulated in the duodenum by IAP deficiency were associated with the pathways related to lipid metabolism, such as ‘Triacylglyceride synthesis’, ‘PPAR signalling’, ‘Fatty acid beta-oxidation’, ‘Acetylcholine synthesis’, and ‘SREBF and miR33 in cholesterol and lipid homeostasis’ (Supplementary Fig. 4a). In addition, duodenal pathway analyses upregulated 533 transcripts in the jejunum by IAP deficiency were associated with ‘Triacylglyceride synthesis’ (Supplementary Fig. 4c). Furthermore, compared to the WI group, 78 transcripts were significantly upregulated in the duodenum and 442 in the jejunum by IAP deficiency (WI vs. KOI; Supplementary Table 1, ≥ 1.2 fold-change, p < 0.05). The 78 transcripts upregulated in the duodenum by IAP deficiency were associated with ‘Focal adhesion-PI3K-Akt-mTOR signaling pathway’, ‘GPCRs, class A rhodopsin-like’, and ‘Glycogen metabolism’ (Supplementary Fig. 4b). In addition, upregulated 442 transcripts in the jejunum by IAP deficiency were associated with ‘Mitochondrial gene expression’, ‘EGFR1 signaling pathway’, ‘mRNA processing’, and ‘IL-7 signaling pathway’ (Supplementary Fig. 4d).
A specific interaction between the oligopeptide IIAEK and mouse IAP (Akp3) is pivotal for IIAEK-induced amelioration of cholesterol metabolism in vivo (Figs. 1–4).
Mechanism of specific interaction between IIAEK and mouse duodenum-specific IAP (Akp3)
This study showed that the IIAEK-induced amelioration of cholesterol metabolism via suppression of intestinal cholesterol absorption was absent in IAP-knockout mice. However, it remains unclear whether IIAEK interacts with mouse dIAP. Therefore, Discovery Studio was used to investigate the mechanism of the interaction between IIAEK and mouse duodenum-specific IAP (mouse dIAP, gene name: Akp3) in silico. IIAEK interacted with the substrate-binding site of mouse dIAP dimer (Fig. 5a, b). In addition to the interaction between IIAEK and mouse dIAP dimer, PLP (the natural substrate of hIAP) interacted with the substrate-binding site of mouse dIAP dimer (Fig. 5d, e). IIAEK and PLP interact with the substrate-binding pocket of the mouse dIAP dimer via a network of hydrophobic, electrostatic, and hydrogen bonds (Fig. 5c, f). Furthermore, we found that IIAEK interacted with mouse dIAP at the same binding site as its natural substrate, PLP (Fig. 5g). We observed that the -CDOCKER ENERGY values for the interaction between IIAEK and mouse dIAP were 1.85 times higher than those for the interaction of PLP and mouse dIAP, whereas the -CDOCKER INTERACTION ENERGY values for the interaction between IIAEK and mouse dIAP were 1.19 times higher than those for the interaction between PLP and mouse dIAP (Fig. 5h).
Mechanism of specific interaction between IIAEK and mouse IAP (Akp3). (a) Overall structure of the IIAEK (Ile-Ile-Ala-Glu-Lys) (salmon pink)–mouse IAP (gene name: Akp3) dimer (gray) complex. Green: Surface of the mouse IAP interacting with IIAEK. (b) Detailed view of the interaction between IIAEK (salmon pink) and mouse IAP. (c) 2D diagram of the network of IIAEK-mouse IAP interaction. (d) Overall structure of the natural substrate of IAP [Pyridoxal 5′-phosphate (PLP)] (silver)–human IAP dimer (gray) complex. Green: the surface of IAP interacting with PLP. (e) Detailed view of the interaction between PLP (yellow) and mouse IAP. (f) 2D diagram of the network of PLP-mouse IAP interaction. (g) Detailed view of overlapping IIAEK-mouse IAP interactions and PLP-mouse IAP interactions. (h) Virtual docking simulation of IIAEK-mouse IAP interactions or PLP-mouse IAP interactions.
Next, we used the KEGG PATHWAY Database 21,22 (https://www.genome.jp/kegg/pathway.html) to investigate the orthologue of mouse dIAP (Akp3) in humans. In this study, human IAP was an orthologue of mouse dIAP (Akp3) [35th, (entry) hsa: 248; (KO) K01077; (len) 528; (SW-score) 2732; (identity) 0.804; (overlap) 499; https://www.kegg.jp/ssdb-bin/ssdb_best?org_gene=mmu:11648].
IIAEK specifically interacts with human IAP
Our previous study identified hIAP and rat IAP as targets that interact with IIAEK using photoaffinity labeling with IIXEK (photoaffinity-labelled IIAEK) in the rat small intestinal mucosa and human intestinal lipid raft fractions of Caco-2 cells. However, the details of the interaction between IIAEK and hIAP, such as the dissociation constant and the mechanism of interaction between IIAEK and hIAP, remain unclear. Thus, we performed surface plasmon resonance (SPR) measurements (Fig. 6) and in silico molecular docking studues (Fig. 7) to observe the interactions between IIAEK and human IAP (hIAP). IIAEK interacted with hIAP in a concentration-dependent manner, with a dissociation constant (Kd) of 297.2 μM (Fig. 6a). Pyridoxal 5′-phosphate, the natural substrate of IAP, interacted with hIAP with a Kd value of 782.3 μM (Fig. 6b). The interaction between IIAEK and hIAP was 2.63 times stronger than that between PLP and hIAP. PLP compared with IIAEK and hIAP in a concentration-dependent manner (Fig. 6c). These results suggest that IIAEK interacts with the substrate-binding site of the hIAP. Subsequently, we measured the interaction between the control pentapeptide (GGGGG) and hIAP and between IIAEK alanine substitutes and hIAP to evaluate the specificity of IIAEK in its interaction with hIAP. The GGGGG and IIAEK alanine substitutes exhibited markedly fewer interactions with hIAP than with IIAEK (Fig. 6d–h). The Kd values for the interactions between the analytes and hIAP are shown in Fig. 6i.
IIAEK specifically interacts with human IAP. (a) SPR sensorgram of the interaction between IIAEK (Ile-Ile-Ala-Glu-Lys) and immobilised human intestinal alkaline phosphatase (hIAP). (b) SPR sensorgram of the interaction between the natural substrate of IAP [Pyridoxal 5′-phosphate (PLP)] and hIAP. (c) Competitive inhibition of IIAEK binding to hIAP by PLP. The running buffer was HBS-N buffer (pH 7.4) supplemented with PLP ranging from 0 to 2.0 mM (0, 1000, and 2000 μM). IIAEK (final concentration: 297 μM) and PLP (0, 1000, and 2000 μM) were injected into the immobilised hIAP. (d) SPR sensorgram of the interaction between control pentapeptide (GGGGG) and hIAP. (e–h) SPR sensorgram of the interaction between alanine substitution of IIAEK (AIAEK, IAAEK, IIAAK, and IIAEA) and hIAP. (i) Summary of the binding affinity (Kd value) between the analytes [IIAEK, PLP, GGGGG, and the alanine substitution of IIAEK (AIAEK, IAAEK, IIAAK, and IIAEA)] and hIAP.
Mechanism of specific interaction between IIAEK and human IAP. (a) Overall structure of the IIAEK (Ile-Ile-Ala-Glu-Lys) (pink)–human IAP dimer (gray) complex. Green: Surface of human IAP interacting with IIAEK. (b) Detailed view of the interaction between IIAEK (pink) and human IAP. (c) 2D diagram of the network of IIAEK-human IAP interaction. (d) Overall structure of the PLP (yellow)–human IAP dimer (gray) complex. Green: Surface of human IAP interacting with PLP. (e) Detailed view of the interaction between PLP (yellow) and human IAP. (f) 2D diagram of the network of PLP-hIAP interaction. (g) Detailed view of overlapping of IIAEK-human IAP interactions and PLP-human IAP interactions. (h) Virtual docking simulation of IIAEK-human IAP interactions or PLP-human IAP interactions.
Mechanism of specific interaction between IIAEK and human IAP
Discovery Studio was used to investigate the interaction mechanism between IIAEK and hIAP in silico. As shown in Fig. 7a and b, IIAEK interacts with the substrate-binding site of hIAP. In addition to the interaction between IIAEK and hIAP, PLP (the natural substrate of hIAP) interacted with the substrate-binding site of hIAP (Fig. 7d, e). IIAEK and PLP interact with the substrate-binding pocket of the hIAP dimer via a network of hydrophobic, electrostatic, and hydrogen bonds (Fig. 7c, f). Furthermore, we found that IIAEK interacted with hIAP at the same binding site as its natural substrate, PLP (Fig. 7g). The amino acid residues of IIAEK and the structure of PLP involved in binding to hIAP are shown in Supplementary Fig. 5. These results are consistent with the finding that PLP competes for the interaction between IIAEK and hIAP in a concentration-dependent manner (Fig. 6c). The -CDOCKER ENERGY values for the interaction between IIAEK and hIAP were 1.82 times higher than those for the interaction of PLP and hIAP, whereas the -CDOCKER INTERACTION ENERGY values for the interaction between IIAEK and hIAP were 1.57 times higher than those for the interaction between PLP and hIAP (Fig. 7h). These results were consistent with the observation that the interaction between IIAEK and hIAP was stronger than that between PLP and hIAP (Fig. 6a, b). To explain the similarities between mouse duodenum-specific IAP (mouse dIAP; gene name: Akp3) and hIAP, we performed multiple amino acid sequence alignments. As shown in Supplementary Fig. 6a, the amino acid sequences of mouse dIAP and hIAP were well conserved. Several amino acid residues involved in the interaction with IIAEK matched between hIAP and mouse dIAP (Supplementary Fig. 6a, mouse dIAP: blue square; hIAP: green square). Furthermore, we performed molecular docking studies to investigate the similarities between mouse dIAP-IIAEK and hIAP-IIAEK complexes. The mouse dIAP-IIAEK and hIAP-IIAEK complexes matched well (Supplementary Fig. 6b, c).
IIAEK-human IAP complex interacts with cadherin-17
As IAP is a GPI-anchored receptor, the transmembrane target protein interacting with IAP is essential for IIAEK-induced amelioration of cholesterol metabolism. Furthermore, despite the identification of transmembrane targets of other alkaline phosphatases (ALP) [mouse global IAP (Akp6) 23 and tissue non-specific ALP (Akp2) 24], no reports are available on transmembrane targets interacting with hIAP or mouse dIAP (Akp3). Therefore, we developed an experimental method to identify the targets that interact with hIAP using photoaffinity-labeled IIAEK14. Human cadherin-17 was identified as a transmembrane target protein that interacts with the human IAP-IIAEK complex (Fig. 8a, b). Furthermore, multiple sequence alignments showed that the amino acid sequences of cynomolgus monkey cadherin-17 and human cadherin-17 were well conserved (Supplementary Fig. 7). Thus, we conducted the same experiment using recombinant cynomolgus cadherin-17 to determine whether the IIAEK-hIAP complex interacted with cadherin-17. The IIAEK-hIAP complex also interacted with recombinant cynomolgus cadherin-17 (Fig. 8c). Furthermore, cadherin-17 interacted with hIAP in a concentration-dependent manner (Fig. 8d). Moreover, IIAEK inhibited the interaction between recombinant cadherin-17 and hIAP in a concentration-dependent manner (Fig. 8e). In summary, we identified human cadherin-17 as a target that interacts with the IIAEK-hIAP complex.
IIAEK-human IAP complex interacts with cadherin-17. (a) SDS-PAGE analysis to identify the transmembrane target protein interacting with the human IAP- IIAEK (Ile-Ile-Ala-Glu-Lys) complex. (b) Identification of the transmembrane target protein interacting with the human IAP-IIAEK complex using nano LC–MS/MS and a Mascot search engine. (c) SDS-PAGE analysis to observe the interaction between the IIAEK-human IAP complex and cynomolgus cadherin-17. (d) SPR sensorgram of the interaction between cynomolgus cadherin-17 and immobilised human intestinal alkaline phosphatase (hIAP). (e) Effect of the addition of IIAEK on the SPR sensorgram of the interaction between cynomolgus cadherin-17 and immobilised hIAP. The original gels are presented in Supplementary Fig. 8.
Effect of IIAEK on fluorescently labeled cholesterol uptake in Caco-2 cells introduced with human cadherin 17 (CDH17) siRNA
To evaluate whether human cadherin 17 participates in the IIAEK-induced amelioration of intestinal cholesterol metabolism, we investigated the effect of IIAEK on the uptake of fluorescently labeled cholesterol (NBD-cholesterol) in differentiated Caco-2 cells transfected with human cadherin 17 (CDH17) siRNA. CDH 17 mRNA levels were significantly decreased in Caco-2 cells introduced with CDH 17 siRNA compared to those in the negative control siRNA group (Fig. 9a). IIAEK significantly reduced the uptake of micellar NBD-cholesterol compared to that in the control group in differentiated Caco-2 cells transfected with negative control siRNA (Fig. 9b). The IIAEK-induced decrease in the uptake of micellar NBD-cholesterol disappeared after the introduction of CDH 17 siRNA into differentiated Caco-2 cells (Fig. 9c).
Effect of IIAEK on fluorescently labelled cholesterol uptake in Caco-2 cells with knockdown of human cadherin 17 (CDH17). Caco-2 cells were cultured in 6-well plates for 14 days post-confluence. CDH 17 (20 nM) or negative control siRNA (20 nM) was introduced into the cells for 72 h. After 72 h of introduction, the cells were reintroduced with these siRNAs for 72 h. Subsequently, the cells were treated with serum-free medium containing 2 mM IIAEK (Ile-Ile-Ala-Glu-Lys) or the vehicle for 24 h. After removing the medium, the cells were incubated with a serum-free medium containing fluorescently labeled cholesterol (NBD-cholesterol) micelle for 1 h. After incubation, the cells were washed and cultivated to measure the fluorescent intensity of NBD-cholesterol. (a) CDH 17 mRNA levels. (b) Effect of IIAEK on NBD-cholesterol uptake in Caco-2 cells introduced with negative control siRNA. (c) Effect of IIAEK on NBD-cholesterol uptake in Caco-2 cells introduced with CDH17 siRNA. (a–c) n = 3/group. Data are presented as mean ± standard error of the mean (SEM). Statistical significance was calculated using an unpaired Student’s t-test. Asterisks indicate significant differences between the experimental groups (* p < 0.05).
Discussion
This is the first study to demonstrate a specific interaction between the cholesterol-lowering pentapeptide IIAEK and the GPI-anchored receptor IAP (IIAEK receptor), resulting in improved cholesterol metabolism by suppressing intestinal cholesterol absorption in vivo. These findings represent a novel discovery that IAP (IIAEK receptor) not only catalyzes the dephosphorylation of the substrate but also specifically interacts with the oligopeptide (IIAEK) to improve cholesterol metabolism by suppressing intestinal cholesterol absorption. To our knowledge, membrane-bound enzymes that act as receptors for exogenous ligands have been reported only in plants [the interaction between gibberellins and their receptor (GID1)25,26] and insects [the interaction between Bacillus thuringiensis Cry toxins and insect midgut GPI-anchored receptors (alkaline phosphatase (AP) and aminopeptidase N)27], but not in mammals. Our findings represent the first evidence that the GPI-anchored receptor IAP (IIAEK receptor) acts as a specific receptor for the oligopeptide IIAEK in vivo.
In this study, IAP-KO mice did not exhibit IIAEK-induced suppression of intestinal cholesterol absorption. The mice harboured two IAP genes (Akp3 and Akp6)16. Despite the presence of these genes in mice, Akp3 was knocked out in our study because rat IAP II, which resembles mouse dIAP (Akp3)16, was identified as the primary target interacting with IIAEK in our previous photoaffinity labeling with IIXEK14. Duodenum-specific IAP (mouse dIAP, gene name: Akp3)16 has been implicated in improving lipid absorption28, metabolic syndrome29, and anti-aging30; however, most reports have concluded that IAP exerts its physiological functions by dephosphorylation catalytic action as the enzyme. However, the physiological function of mouse gIAP (gene name: Akp6) remains unknown31. gIAP (Akp6) shares 87% amino acid sequence identity with mouse dIAP (Akp3), with specific differences primarily in the crown domain sequence, which is critical for the structural stabilization of alkaline phosphatases16. The substrate-binding site is well conserved between dIAP and gIAP; however, the catalytic efficiency is much higher in dIAP than in gIAP by approximately 4-fold16. Furthermore, replacing the 317th amino acid residue of dIAP (arginine) with a glutamine residue abolishes ALP activity16. This substitution was also found in gIAP16, suggesting that the 317th residue of dIAP (arginine) is important for natural substrate-dIAP interactions. We identified a common IIAEK-binding amino acid sequence crucial for interaction with IAP (GFYLFVEGGR), located proximal to the 317th residue (arginine) of the IAP14. Therefore, mouse gIAP (Akp6) may interact with different substrates than mouse dIAP (Akp3), potentially leading to the lack of interaction between mouse gIAP (Akp6) and IIAEK. In this study, we investigsted the regulation of cholesterol metabolism through the interaction between IIAEK and IAP in vivo. Furthermore, IAP is associated with longevity30, gut barrier maintenance30, and regulation of lipid metabolism29. Thus, we will investigate the potential bioactivities of IIAEK, other than the regulation of cholesterol metabolism, such as longevity and gut barrier maintenance in the future.
Despite numerous scientific reports on the physiological functions of IAP, definitive evidence regarding its physiological significance remains elusive. In addition, Ghosh et al. reported that hyperlipidaemic mice overexpressing IAP exhibited decreased lipid absorption, which was attributed to the reduction in mRNA levels of lipid metabolism-related genes (duodenum: Cd36, Slc27a4, and Npc1l1; jejunum: Cd36, Slc27a4, Npc1l1, Fabp1, Fabp2, and Scp2)32. Furthermore, the IAP overexpressed in mice used in their report32 was a chimeric human IAP with a catalytic domain of human placental AP33. Owing to the use of mice with exogenous IAP-overexpressing chimeric human IAP33, it remains uncertain whether endogenous IAP ameliorates lipid metabolism-related diseases, such as atherosclerosis. Conversely, we demonstrated for the first time that endogenous IAP acts as specific receptors for the oligopeptide (IIAEK) using IAP-KO mice in vivo.
In this study, we used IAP-knockout (Akp3-/-) mice to reveal the novel role of IAP in controlling cholesterol metabolism via the dietary peptide IIAEK in vivo. More evidence using human intestinal models (e.g., organoids or epithelial cell lines) will be needed to add validation of the translational relevance to our findings that IAP (IIAEK receptor) specifically interacts with the oligopeptide IIAEK to improve intestinal cholesterol metabolism. Furthermore, the effects of IIAEK administration on cholesterol metabolism in humans should be examined in future studies. Related to our research, Kawase et al. reported that oral administration of fermented milk with a high whey protein content exerted both serum lipid improvement and hypotensive effects in humans and animals34. Pal et al. suggested that dietary whey protein exhibits hypocholesterolemic effects in humans35. Thus, oral administration of IIAEK derived from beta-lactoglobulin of whey protein may improve cholesterol metabolism in humans, as IIAEK is a hypocholesterolemic pentapeptide derived from a beta-lactoglobulin tryptic hydrolysate11. We will examine whether our findings using IAP-knockout mice can be applied to humans. To the best of our knowledge, no cases of IAP deficiency have been reported in humans. In addition, the physiological role of IAP, including human IAP (gene name: ALPI) remains unclear; however, it may play a notable role in the maintenance of gut homeostasis and response to dietary ingredients36 (starvation37, protein deficiency38, lipid intake16,28). Murine IAP deficiency leads to metabolic syndrome29 and a short life span30. In this study, we found that the IIAEK-induced amelioration of cholesterol metabolism was absent in IAP-knockout (Akp3-/-) mice. Thus, IAP deficiency in humans may be related to diseases, such as metabolic syndrome and aging. We will investigate using human biopsied tissues (small intestine and liver) whether these findings regarding the expression of mouse IAP and their function apply to human IAP.
Although extensive research has focused on the biological function of exogenous IAP39, a notable absence of comprehensive analyses of endogenous IAP or its interactions with ligands (pentapeptides) and their impact on lipid metabolism exist. Therefore, for the first time, we performed a comprehensive mRNA analysis using liver and intestine (duodenum and jejunum) samples from WT and IAP-KO mice orally administered IIAEK (Figs. 3, 4 and Supplementary Figs. 3, 4). In WT mice orally administered IIAEK, cholesterol biosynthesis and cholesterol metabolism were the most significantly upregulated pathways in the liver, whereas these two pathways (cholesterol biosynthesis and metabolism) were absent in IAP-KO mice administered IIAEK. This suggests that IIAEK inhibits intestinal cholesterol absorption, leading to reduced cholesterol influx into the liver and enhanced cholesterol biosynthesis and metabolism to maintain cholesterol homeostasis. Supporting this notion, the cholesterol-lowering drug ezetimibe decreases liver cholesterol levels by increasing hepatic mRNA levels of Hmgcr and Srebf2 in the mouse liver40. IIAEK markedly suppressed the hepatic mRNA levels of Npc1l1 in WT mice; however, IAP-KO mice administered IIAEK showed no significant decrease in the hepatic mRNA levels of Npc1l1(Fig. 3e). Hepatic Npc1l1 aggravates hepatic steatosis, and hepatic NPC1L1-induced hepatic lipids accumulation is eliminated by the NPC1L1‐selective inhibitor ezetimibe41,42. These results show for the first time that prominently lowering liver cholesterol by IIAEK is due to a reduction in cholesterol influx to the liver by inhibiting intestinal cholesterol absorption and due to a reduction in hepatic cholesterol absorption through IIAEK-induced downregulation of hepatic NPC1L1. Collectively, we found that the IIAEK-IAP interaction suppresses cholesterol absorption by targeting NPC1L1 in the small intestine and liver. Focusing on the duodenum and jejunum, IIAEK activates insulin signalling in both the mouse duodenum and jejunum. Intestinal insulin signalling pathways are potential new therapeutic targets for diabetes-associated nonalcoholic steatohepatitis, hepatocellular carcinoma, and gut barrier formation43. We suggest that intestinal insulin signalling pathways participate in the improvement of cholesterol metabolism via the interaction between IIAEK and IAP in the duodenum and jejunum of mice, which requires detailed investigation. In future studies, we will perform ATAC-seq to further investigate the molecular mechanism of IIAEK-induced regulation of cholesterol metabolism via intestinal alkaline phosphatase using Caco-2 cells or IAP-KO (Akp3-/-) mice models. In summary, the interaction between the oligopeptide IIAEK and IAP ameliorates cholesterol metabolism in vivo not only by directly regulating intestinal cholesterol metabolism but also indirectly or directly by regulating hepatic cholesterol metabolism (Figs. 1–4).
The effective concentrations of oral administration of IIAEK used in this study (600 mg/kg B.W./d for 14 days) were based on a previous study44. According to the ‘dietary Reference Intakes for Japanese (2020)’ from the Ministry of Health, Labour and Welfare, Japan, the recommended dietary allowance of protein intake is 65 g/day (18–65 years old adult males). If the average B.W. of an adult male was 65 kg, the daily protein intake was calculated as 1 g/kg B.W./day. If the protein source is beta-lactoglobulin (molecular weight: 18 kDa), the recommended dietary allowance of IIAEK ((molecular weight: 573 Da) is 31.3 mg/kg B.W./day. Thus, 31.3 mg.kg B.W./day. may be the physiological relevance of IIAEK concentrations in humans. The effective concentration of IIAEK in the mouse model in this study (600 mg/kg B.W./d) was 19.2 times higher than the calculated concentration (31.3 mg/kg B.W./d). However, whether the calculated concentrations based on the recommended dietary allowance of protein intake in humans are physiologically relevant in mice, remains unclear. Related to this discussion, Dybdahl et al. reported that the no-observed-adverse effect level (NOAEL) for Lacprodan® BLG (the protein fraction isolated from bovine whey that contains over 90% pure beta-lactoglobulin) in a 90-d toxicity study was established as 1000 mg/kg B.W./day, corresponding to the highest dose level administered45. However, in an animal study of Dybdahl et al., beta-lactoglobulin was administered orally while feeding a standard diet including protein, because they assumed that beta-lactoglobulin was ingested as a supplement to their normal diet45. Thus, if the supplement is IIAEK (pentapeptide derived from a beta-lactoglobulin trypsin hydrolysate), it may be expected to be nontoxic at doses up to 1000 mg/kg B.W./day. Furthermore, as shown in Supplementary Fig. 1, no negative effects on body weight, food intake, or liver weight were observed in WT or IAP-knockout mice orally administered IIAEK (600 mg/kg B.W./day). Collectively, the concentrations of IIAEK used in this study did not significantly deviate from the physiological relevance in mice. We will conduct toxicity tests, such as acute and subacute toxicity tests, to further clarify the physiological relevance of IIAEK concentration using a mouse model. IIAEK is a hypocholesterolemic pentapeptide derived from a beta-lactoglobulin tryptic hydrolysate located at amino acid residues 71–75 of bovine milk beta-lactoglobulin11. In addition, IIAEK is not degraded by digestive enzymes such as pepsin, trypsin, or chymotrypsin. Thus, pentapeptide IIAEK may occur naturally in the gut after the digestion of bovine beta-lactoglobulin. Related to this discussion, beta-lactoglobulin tryptic hydrolysate has more hypocholesterolemic activity than casein tryptic hydrolysate in rats11. Furthermore, beta-lactoglobulin is known to be an important source of biologically active peptides, including IIAEK46. Given the possibility that bioactive peptides other than IIAEK may act, we will investigate the intake of bovine milk beta-lactoglobulin to yield IIAEK at effective concentrations.
Despite extensive research on IAP, there is no SPR-based information on the interaction between human IAP and natural substrates. Our findings provide the first evidence of an interaction between exogenous ligands, such as IIAEK, and the GPI-anchored receptor human IAP. Moreover, the interaction between IIAEK and human IAP was 2.63 times stronger than that between pyridoxal 5′-phosphate and IAP (Fig. 6a, b). Furthermore, pyridoxal 5′-phosphate competed with the interaction between IIAEK and IAP (Fig. 6c). These results suggest that IIAEK specifically interacts with the substrate recognition site of IAP. Furthermore, our findings suggest that the interaction between IAP and IIAEK is not strong, which may be appropriate considering the interactions between enzymes and biomolecules that are highly expressed in the duodenum. For example, DPP4, which is highly expressed in the duodenum alongside IAP, interacts with GLP-1 with a Kd value of 153 μM, indicating a low binding affinity47. In addition, given that DPP4 reacts with several substrates other than GLP-1, the interactions between DPP4 and its substrates are expected to have a low affinity48. Furthermore, AP, including IAP, has various natural substrates, such as vitamin B6, lipopolysaccharides, flagellin, CpG DNA motifs, and extracellular nucleotides49,50,51.
In this study, we performed an IIAEK alanine substitution assay to understand the sequence specificity of IIAEK in its interaction with IAP. This substitution resulted in a distinctly different interaction compared to that of the original peptide IIAEK (Fig. 6e–h). This finding implies that a specific amino acid sequence (IAEK) is required for the interaction between IAP. Alanine substitution is a powerful tool for identifying the key amino acid residues required for binding between a ligand and target protein52. In the future, we aim to investigate whether the effects of IIAEK alanine substitution on cholesterol absorption and lipid metabolism-related genes in the small intestine are like those observed with IIAEK alanine substitution in this study.
We provided evidence for the specificity of the interaction between IIAEK and human IAP (Figs. 6, 7). Furthermore, our molecular docking study showed that IIAEK interacted with mouse dIAP (Akp3), however, we did not provide sufficient evidence for the specificity of the interaction between IIAEK and other intestinal alkaline phosphatase, such as mouse dIAP (Akp3) (Fig. 5). We will investigate the specificity of the interaction between IIAEK and other intestinal alkaline phosphatases using other validation methods, such as SPR. Regarding the limitation of our study, we could not quantitatively investigate the interactions between other peptides and human IAP because of the vast number of peptides (even in simple amino acid sequence combinations alone). Thus, to investigate the possibility of interaction between other peptides and human IAP, we will examine the amino acid sequence and structural features of peptides essential for IIAEK-IAP interaction using IAP and IIAEK as templates in competitive binding assays. Furthermore, in this study, it remained to be determined if IIAEK is special in its interaction with IAP. We will perform a general screen regarding the interaction between dietary oligopeptides and IAP to determine if IAP functions as a general oligopeptide sensor or if the interaction between IAP and IIAEK is a special case in the future.
To the best of our knowledge, only the crystal structure of the rat IAP-homodimer has been reported52, necessitating the prediction of hIAP structures. Therefore, AlphaFold2, a highly accurate in silico tool that constructs a 3D structural model of the target protein using only the amino acid sequence as the input53,54, was used to model the hIAP 3D structure. The 3D structural model of hIAP was used to investigate the mechanism of interaction between IIAEK and IAP using Discovery Studio. Our molecular docking analysis showed that IIAEK interacted more strongly with the substrate-binding site of hIAP than with PLP, the natural substrate (Fig. 7j). These results are consistent with the data obtained in this study regarding the IIAEK-hIAP interaction using SPR (Fig. 6). Furthermore, our molecular docking results suggested that all amino acid residues of IIAEK interacted with the substrate-binding site of hIAP (Fig. 7i, Supplementary Fig. 5). These results are consistent with the findings of this study, which showed that the IIAEK alanine substitution resulted in a distinctly different interaction compared to the original peptide IIAEK. Our SPR and molecular docking results suggest that the E residue of IIAEK interacts with the substrate-binding site of hIAP as a substitute for the PLP phosphate group. In addition, residue A of IIAEK interacted with the region around the substrate-binding site of hIAP [His320 (chain A of hIAP) and Tyr367 (chain B of hIAP)] as a substitute for the PLP pyridoxal structure. In addition, the stronger interaction of IIAEK than PLP was attributed to the interaction of Ile and K residues of IIAEK with hIAP. Although natural hIAP substrates, such as PLP, possess phosphate groups, our study revealed for the first time that oligopeptides without phosphate groups (IIAEK) can interact with hIAP.
Lynes et al. reported that CD36 interacts with mouse global IAP (gene name: Akp6) but not with mouse dIAP (gene name: Akp3)23. In addition, tissue-non-specific ALP interacts with low-density lipoprotein receptor-related protein 6, subsequently affecting the canonical Wnt/β-catenin pathway24. Thus, transmembrane proteins that interact with human IAP or mouse dIAP are likely to be present. However, no studies have identified the transmembrane proteins that interact with human IAP or mouse dIAP. Therefore, we developed an experimental method to identify target proteins interacting with IAP using photoaffinity-labelled IIAEK (IIXEK)14 and successfully identified cadherin-17 as a transmembrane target protein interacting with the human IAP-IIAEK complex (Fig. 8). Cadherin-17, a member of the cadherin superfamily, is expressed only in the intestinal epithelial cells of humans and mice55. Unlike classic cadherins, such as E-cadherin, N-cadherin, and P-cadherin, cadherin-17 has seven cadherin repeats, instead of five, within the extracellular domain and a markedly short cytoplasmic domain55. Furthermore, cadherin-17 plays a key role in intestinal homeostasis by limiting intestinal permeability, with its signalling pathways linked to the Hippo-Yap1 pathway but not the Wnt signaling pathway 56. LATS2 (a central member of the Hippo pathway) inhibits the activity of the transcription factor SREBP2, a master regulator of cholesterol homeostasis, to suppress hepatic cholesterol accumulation in the liver57. However, whether the inhibition of SREBP2 activity by LATS2 occurs in the small intestine remains unclear. Collectively, we hypothesize that the IIAEK-IAP-cadherin-17 tripartite complex regulates intestinal cholesterol metabolism via the LATS2-mediated Hippo pathway. The interaction of the IIAEK-IAP complex with cadherin-17 leads to reduced cholesterol absorption and its relationship with the LATS2-mediated Hippo pathway will be studied.
In this study, IIAEK reduced the interaction between recombinant cadherin-17 and hIAP in a concentration-dependent manner (Fig. 8e). These results suggest that cadherin-17 interacts with hIAP at the same binding site as IIAEK. However, reducing the hIAP-cadherin-17 interaction does not necessarily mean they share the same binding site as IIAEK does. Thus, to further investigate the structural and functional roles of the IIAEK-IAP complex or the IIAEK-IAP-cadherin-17 tripartite, the native structures of these complexes must be determined. Hoylaerts et al. reported that completely metalated mammalian alkaline phosphatases (ALP), including IAP, are noncooperative allosteric enzymes; however, the stability and catalytic properties of each monomer are controlled by the conformation of the second ALP subunit58. Thus, the above allosteric modulation, which may cause a conformational change that reduces the binding affinity of the interaction between human IAP and cadherin-17, may explain IIAEK-induced reduction in the interaction between recombinant cadherin-17 and hIAP in a concentration-dependent manner. In addition, steric hindrance, where IIAEK may physically block cadherin-17 from binding to IAP, or other indirect effects, may also be involved.
To date, the physiological role of cadherin-17 is in the intestinal barrier function and inhibition of intestinal tumour formation. However, the physiological function of cadherin-17 remain largely unelucidated. Whether cadherin-17 plays a significant role in the regulating cholesterol metabolism remains unknown. This study showed that the IIAEK-induced decrease in the uptake of micellar fluorescently labeled cholesterol disappeared after the introduction of CDH 17 siRNA into differentiated Caco-2 cells (a human intestinal model). Based on these results, it was partially demonstrated in vitro that human cadherin-17 is required for IIAEK-induced inhibition of intestinal cholesterol absorption; however, further in vitro and in vivo evidence is needed. To completely understand the novel signaling pathway mediated by the IIAEK-IAP complex, we will perform an animal study using cadherin-17 knockout mice to evaluate whether cadherin- 17 participates in the induction of intestinal cholesterol metabolism by the IIAEK-IAP complex. The cadherin-17 mediated signaling pathways have not been fully elucidated in this study. We will investigate the downstream intracellular signaling pathway, such as the Hippo-Yap1 pathway56, to fully understand the novel signaling pathway of intestinal cholesterol metabolism mediated by the IIAEK-IAP-cadherin-17 tripartite complex (Fig. 10).
Hypothesis of how the specific interaction of IIAEK and IAP (IIAEK receptor) ameliorates cholesterol metabolism. The cholesterol-lowering pentapeptide IIAEK (Ile-Ile-Ala-Glu-Lys) specifically interacts with the GPI-anchored receptor intestinal alkaline phosphatase (IAP). Subsequently, the IIAEK-IAP dimer complex interacts with cadherin-17 to ameliorate cholesterol metabolism by suppressing cholesterol absorption, liver cholesterol, and serum cholesterol levels and promoting fecal cholesterol excretion.
We have highlighted that GPI-anchored IAP is pivotal for the pentapeptide IIAEK-induced amelioration of cholesterol metabolism and serves not only as an enzyme (phosphatase) but also as the intestinal membrane receptor for the oligopeptide IIAEK, as depicted in Fig. 10: (1) cholesterol-lowering pentapeptide IIAEK specifically interacts with the GPI-anchored receptor IAP. (2) Subsequently, the IIAEK-IAP dimer complex interacts with cadherin-17 to ameliorate cholesterol metabolism by suppressing cholesterol absorption via a novel signaling pathway. Our findings strongly contribute to the understanding of the molecular mechanism of oligopeptide sensing, including oligopeptide (IIAEK), in the intestine, the development of functional foods and new medicines targeting IAP to treat lifestyle-related diseases, such as dyslipidaemia and NAFLD, and offer insight into the novel role of IAP (oligopeptide IIAEK receptor) on the intestinal surface. Furthermore, our results may aid in maximising the use of underutilized peptides in functional foods and medical applications.
Methods
Chemicals
IIAEK [Ile-Ile-Ala-Glu-Lys: purity (> 95%)], the control pentapeptide [Gly-Gly-Gly-Gly-Gly: purity (> 95%)], and the alanine-substituted of IIAEK analogues [AIAAK, Ala-Ile-Ala-Glu-Lys; IAAEK, Ile-Ala-Ala-Glu-Lys; IIAAK, Ile-Ile-Ala-Ala-Lys; IIAEA, Ile-Ile-Ala-Glu-Ala; purity (> 95%)] were obtained from the peptide Institute, Inc. (Osaka, Japan). Pyridoxal 5′-phosphate (PLP) was purchased from Sigma Aldrich (P9255).
Animals and diet
All animal studies were performed as previously described9,44. Seven-week-old male C57BL6J WT and IAP global knockout (KO) (Akp3-/-) mice (BSRC, Shizuoka, Japan) were housed in individual cages under environmentally controlled conditions (22 ± 2 °C and a 12-h light/dark cycle (8:00–20:00)) with ad libitum access to food and water. Following acclimation to a commercially available non-purified diet (MF, Oriental Yeast, Tokyo, Japan) for 6–8 d, 7-week-old mice with a B.W. of approximately 22 g were divided into four groups (WT control group: WT, Control; WT IIAEK group: WT, IIAEK; IAP-KO control group: IAP-KO, Control; and IAP-KO IIAEK group: IAP-KO, IIAEK) based on body their weight. The period of intake of the high-cholesterol diet was 14 d in all experimental groups. The high-cholesterol diet composition (%) was as follows: casein, 20.0; lard, 5.00; corn oil, 1.00; cellulose, 5.00; AIN-93G mineral mixture, 3.5; AIN93 vitamin mixture, 1.00; cholesterol, 1.00; sodium cholate, 0.25; choline chloride, 0.20; sucrose, 21.02; and corn starch, 42.03. The pentapeptide IIAEK (600 mg/kg B.W./d) was dissolved in a 5 g/L solution of carboxymethylcellulose sodium salt (Sigma Aldrich) and orally administered to mice once a day using a disposable zonde (CL-4596; CLEA Japan, Inc., Shizuoka, Japan) at 11:00 a.m. for cholesterol absorption experiments or 9:00 a.m. for RNA preparation from the duodenum, jejunum, and liver of mice and DNA microarray for 14 d. All mice were euthanised under isoflurane anaesthesia by collecting blood from the heart, as previously reported9. All experimental protocols were conducted in accordance with the guidelines and regulations of Gifu University and were approved by the Ethics Committee on Animal Experiments at Gifu University (permit number: 2021-228, 2022-154). This study was conducted in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
Cholesterol absorption experiments
Cholesterol absorption experiments in mice were performed using previously described procedures44. At the end of the feeding period, the mice were orally administered the test solutions using a polyethylene zonde. One hour later, blood, liver, and intestinal samples were collected from the mice. Blood was centrifuged at 845 × g at 4 °C for 15 min to obtain serum. The liver was rinsed with ice-cold saline, and the luminal contents of the small intestine were flushed with ice-cold saline containing 2 mmol/L taurocholic acids (Sigma Aldrich). The test solutions included 1 mmol/L monoolein (Sigma Aldrich), 5 mmol/L taurocholic acid (Sigma Aldrich), 37 kBq [1,2-3H]-cholesterol (1972.1 GBq/mmol, NEN) in 15 mmol/L phosphate buffer (pH 7.4). All test solutions were emulsified by sonication (Ultrasonic Homogenizer, Model VP-5, Taitec, Tokyo, Japan). [3H]-Cholesterol in the serum, liver, and intestines of mice was extracted using hexane following saponification with KOH-ethanol and measured via scintillation counting.
Serum and liver lipid analyses
Cholesterol levels in the serum and liver of mice were measured using a commercial kit (439-17501, Wako Pure Chemical Industries, Osaka, Japan). Liver triglyceride levels were measured using a commercial kit (432-40201, Wako Pure Chemical Industries). Total liver lipids were extracted as previously described9. A portion of the liver lipid was used to measure liver cholesterol and triglyceride levels.
Fecal steroids analysis
Fecal steroid analysis was performed as previously described44. Total fecal steroids were calculated as the sum of fecal acidic and neutral steroids. Fecal acidic steroids were extracted and quantified using a commercial kit (431-15001, Wako Pure Chemical Industries, Osaka, Japan). Fecal neutral steroids were extracted using hexane following saponification with KOH-ethanol. Fecal neutral steroids (fecal cholesterol and coprostanol) were analysed using TMS-HT (T0274, Tokyo Chemical Industry Co., LTD, Tokyo, Japan) on a GC-14A instrument (Shimadzu, Kyoto, Japan). The internal standard used for measuring fecal neutral steroids was 5α-cholestane (C8003, Sigma Aldrich).
RNA preparation from the duodenum, jejunum, and liver
After fasting for 8 h, the duodenum, jejunum, and liver were collected from mice and immediately immersed in RNAlater (Qiagen, Hilden, Germany). The duodenum and jejunum of mice were collected as previously reported59. Total RNA was extracted from the duodenum, jejunum, and the liver using an RNeasy Plus Universal Mini Kit (Qiagen, Hilden, Germany).
DNA microarray analyses
Microarray analysis was performed by the Division of Genomics Research, Life Science Research Center, Gifu University, as previously reported60,61. The quality of total RNA was measured using a 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, California, USA). Total RNA samples from the duodenum were used with a RIN ≥ 6 and total RNA samples from the jejunum and liver with RIN ≥ 7. Total RNA samples were labeled using a Low RNA Input Linear Amplification Kit (Agilent Technologies). Targets comprising amplified and fluorescently labelled complementary RNA were hybridised to a microarray slide (SurePrint G3 Mouse GE Microarray 8 × 60 K Ver. 2.0; Agilent Technologies) using an Agilent Gene Expression Hybridization Kit (Agilent Technologies). Slides were scanned using a microarray scanner (ArrayScan, Agilent Technologies) and Agilent Feature Extraction software (Agilent Technologies) for spot identification and quantification. Data analyses, including gene expression and pathway analyses, were performed according to the manufacturer’s instructions using the GeneSpring GX 11.5 Expression Analysis Software (Agilent Technologies), as previously reported61. Pathway analysis involved enriching genes significantly affected by oral administration of IIAEK to WT or IAP-KO mice with p < 0.05 and fold change ≥ 1.2, analysed using a nonparametric Mann–Whitney U test as previously reported62 or one-way ANOVA with Tukey’s post hoc test.
Real-time quantitative PCR
Real-time quantitative PCR (RT-qPCR) was performed using a StepOnePlus™ Real-time PCR system (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol with SYBR® Premix Ex Taq (Takara Bio Inc., Shiga, Japan). RT-qPCR was performed using the primers listed in Supplementary Table 2.
Western blot analyses
Protein preparation from mouse liver and western blot analyses were performed as previously reported63. For western blot analyses, the following specific primary antibodies were used: anti-CYP7A1 (sc-518007, Santa Cruz Biotechnology), anti-NPC1L1 (sc-166802, Santa Cruz Biotechnology), and anti-β-actin (sc-47778, Santa Cruz Biotechnology). Goat anti-mouse IgG (H + L)-HRP conjugated antibodies (Bio-Rad, 1706516) were used as a secondary antibody. Western blot analyses were performed using an ImmunoStar LD system (Wako Pure Chemical Industries) and a Typhoon biomolecular imager (Cytiva). Protein levels were quantified using ImageJ 1.51 (National Institute of Health), as previously reported14,63.
Surface plasmon resonance
SPR experiments were performed using a Biacore X100 instrument (Cytiva, Tokyo, Japan) at 37 ℃. The running buffer used was the HBS-N buffer (10 mM HEPES, 150 mM NaCl, pH 7.4) as previously described64. All analytes (IIAEK, PLP, GGGGG, and the alanine substitution of IIAEK (AIAEK, IAAEK, IIAAK, and IIAEA)) were injected into flow cells 1 and 2 at concentrations between 31.3 and 2000 μM) at a flow rate of 30 μL/min. Each analyte injection had a contact time of 180 s, followed by a 120 s dissociation time. Recombinant hIAP (13225-H08H, Sino Biological) was diluted to 50 μg/mL in 10 mM sodium acetate (pH 4.5) (Cytiva, BR100350) and immobilised on a CM5 sensor chip (BR100012, Cytiva) via amine coupling using an amine coupling kit (BR100050, Cytiva) at chip densities of approximately 9000 response units on flow cell 2. Flow cell 1 was amino-activated and blocked in the absence of any protein using the same amino coupling kit as for the reference surface, as previously described65. The dissociation constant of the interaction between each analyte and immobilised hIAP was determined by assuming a 1:1 binding model using BIAevaluation Software (Cytiva), as previously described66. The running buffer used for the competition study was HBS-N buffer (pH 7.4) supplemented with PLP ranging from 0 to 2.0 mM (0, 1.0, or 2.0 mM) according to an experimental method described previously67. IIAEK (297 μM) and various concentrations of PLP (0, 1.0, or 2.0 mM) were applied as analytes to the immobilised IAP according to the experimental procedure described in a previous study67. To measure the interaction between cadherin-17 and hIAP, hIAP was immobilised on a CM5 sensor chip via amine coupling at chip densities of approximately 1000 response units on flow cell 2. The recombinant cynomolgus cadherin-17 (CA7-C52H4, ACRO Biosystems) was injected into flow cells 1 and 2 between 0 and 1.0 μM at a flow rate of 30 μL/min, with a contact time of 120 s followed by a dissociation time of 120 s, according to a previously reported experimental method with minor modification68. To assess the interaction between the mixture of cadherin-17 and IIAEK and hIAP, 0.5 μM cadherin-17 and various concentrations of IIAEK (0, 297, 594, and 1188 μM) were added to the immobilised IAP at a flow rate of 30 μL/min, with a contact time of 60 s followed by a dissociation time of 120 s.
Molecular docking study using the combination of AlphaFold2 and Discovery Studio
AlphaFold2_advanced Notebook69 was used to generate a structural model for the two chains of full-length human IAP (amino acid residues: 20–503) or full-length mouse dIAP (Akp3) (amino acid residues: 20–499) as previously reported70. The parameters for the model building were as follows: MSA_method, mmseqs2_uniref_env; template_mode, none; and default for the other parameters as previously reported69. Briefly, the PDB coordinate file obtained from the AlphaFold2_advanced Notebook was used to conduct molecular docking using the Discovery Studio Client. The IAP docking site was selected from the predicted structure of human IAP or mouse dIAP (Akp3) using PyMOL, based on the rat IAP-I crystal structure (PDB ID: 4KJG)70. The radius of the final docking active site was set at 10 Å. The predicted structure was prepared by adding hydrogen atoms and eliminating irrelevant atoms to the interaction according to the manufacturer’s instructions before docking. Discovery Studio Client was used to draw the 3D structure of IIAEK and dock it onto the predicted structure of human IAP or mouse dIAP, as previously reported71. The 3D structure of PLP was downloaded from Het-PDB Navi272 using Het ID PLP. The 3D structures of IIAEK and PLP were optimised using dynamic simulation, and the ligand protocol was prepared before docking to the predicted structure of human IAP or mouse dIAP, as previously reported71. The docking protocol was calculated using the CDOCKER algorithm to obtain the values of -CDOCKER ENERGY and -CDOCKER INTERACTION ENERGY.
Extraction of membrane proteins from Caco-2 cells
Caco-2 cells were procured from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained as previously reported14. Cells were seeded in a 60 mm dish (430166, Corning, Inc., Corning, NY, USA) at a density of 1.2 × 105 cells/dish and incubated at 37 °C in a 5% CO2 humid atmosphere until reaching confluence, and maintained for 14 d. Following two washes, the cells were centrifuged at 1000 × g at 4 °C for 15 min, and the resulting cell pellet was collected to prepare membrane proteins. Membrane proteins were extracted from Caco-2 cells using Extraction Buffer 2B from the ProteoExtract® Transmembrane Protein Extraction Kit (Novagen, Darmstadt, Germany, 71772), as previously reported14.
Identification of targets of IIAEK-IAP complexes
Photoaffinity labeling with IIXEK was performed as previously reported14. Briefly, human IAP (Sino Biological, 13225-H08H) was photoaffinity-labelled with IIXEK, followed by a 30-min incubation at 37 °C with membrane proteins derived from Caco-2 cells or cadherin-17 (CA7-C52H4, ACRO Biosystems). Thereafter, the mixture was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis for fluorescence scanning. The BenchMark™ Fluorescent Protein Standard (Invitrogen, Carlsbad, CA) was used as a molecular weight marker. To identify the target protein interacting with the IIXEK-IAP complexes, nano LC–MS/MS (Q Exactive Plus, Thermo Scientific, Waltham, MA, USA) and the Mascot search engine (www.matrixscience.com) were used as previously reported14. Among the identified target proteins interacting with the IIXEK-IAP complexes, those proteins expressing at the cell membrane were selected as transmembrane target proteins that interacted with the human IAP-IIAEK complex.
Multiple sequence alignment
The amino acid sequence of cynomolgus cadherin 17 (UniProt accession No. A0A2K5X8I8-1) was aligned with human cadherin 17 (UniProt accession No. Q12864) using ClustalW as previously reported70. Similarly, the amino acid sequence of mouse dIAP (UniProt accession No. P24822) was aligned with human IAP (UniProt accession No. P09923) using ClustalW software.
siRNA introduction
Small interfering RNA (siRNA) was introduced as previously described73. Caco-2 cells were seeded in 6-well plates (3516, Corning, Inc., NY, USA) at a density of 2 × 105 cells/well and grown for 14 d post-confluence for siRNA transfection. Subsequently, equal amounts of serum-free DMEM containing 240 nM CDH17 siRNA (Silencer® Select Pre-designed siRNA Product, Ambion, 4392420, Thermo Fisher Scientific) or 240 nM Control siRNA (Silencncer® Select Negative Control #1 siRNA, Ambion, 439084, Thermo Fisher Scientific) and serum-free DMEM containing siLentFectTM Lipid Reagent (BIO-RAD, 170–3361) were mixed and incubated at room temperature for 30 min to generate siRNA-siLentFect complexes. Caco-2 cells were treated with each siRNA-siLentFect complex and incubated for 72 h for transfection. After 72 h transfection, the cells were re-treated with each siRNA-siLentFect complex and incubated for 72 h for re-transfection.
Cholesterol absorption experiment in vitro using fluorescent-labelled cholesterol
Cholesterol absorption experiments in vitro using fluorescent-labelled cholesterol were performed as previously reported74. Micellar solutions containing 0.2 mM 22(- N(- 7-Nitrobenz-2-Oxa-1, 3-Diazol-4-yl)Amino)-23, 24-Bisnor-5-Cholen-3β-Ol (NBD-cholesterol: N1148, Thermo Fisher Scientific) and 5 mM sodium taurocholate (T4009, Sigma-Aldrich) were prepared using a sonicator (Ultrasonic Homogenizer, Model VP-5; Taitec, Tokyo, Japan) and incubated at 37 °C for 24 h with shaking as previously reported14. Caco-2 cells grown to confluence in 6-well plates (3516, Corning, Inc., NY, USA) were treated with or without 2 mM IIAEK for 24 h. Following treatment for 24 h, the cells were washed twice with serum-free DMEM and then were incubated with micelles at 37 °C for one hour. The cells were harvested by scraping with the addition of 0.1% SDS solution. Aliquots of cell homogenate were used to measure NBD-cholesterol using a Multimode microplate reader Spark® 10 M (TECAN) and 96 well plate (3991, Corning, Inc., NY, USA).
RNA preparation from Caco-2 cells
RNA preparation from Caco-2 cells was performed as previously reported14. Briefly, total RNA was isolated from Caco-2 cells using a NucleoSpin® RNA Kit (MACHEREY–NAGEL, Düren, Germany) and reverse-transcribed into cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems) for real-time quantitative PCR.
Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM). Statistical significance among the four experimental groups was calculated using one-way ANOVA with Tukey’s post hoc test (* for p < 0.05, ** for p < 0.01). Statistical significance between the two experimental groups was calculated using a nonparametric Mann–Whitney U test (p < 0.05) or unpaired Student’s t-test (* for p < 0.05).
Data availability
The data supporting the findings reported herein are available upon request, from the corresponding author [Satoshi Nagaoka (email: nagaoka@gifu-u.ac.jp)]. Our microarray raw data using the mouse liver, duodenum, and jejunum have been deposited in NCBI’s Gene Expression Omnibus with the GEO Series accession number (mouse liver microarray data: GSE290764; mouse duodenum microarray data: GSE290758; mouse jejunum microarray data: GSE290759).
References
Chen, X. et al. Dietary inflammation index is associated with dyslipidemia: Evidence from national health and nutrition examination survey, 1999–2019. Lipids Health Dis. 22, 149 (2023).
Moran, A. E. et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: The Global Burden of Disease 2010 study. Circulation 129, 1483–1492 (2014).
Akasaki, Y. & Ohishi, M. How should we manage hypertension and dyslipidemia to maintain cognitive function in older adults?. Hypertens. Res. 46, 1880–1882 (2023).
Guo, J. et al. The modulatory effects on enterohepatic cholesterol metabolism of novel cholesterol-lowering peptides from gastrointestinal digestion of Xuanwei ham. Food Res. Int. 173, 113391 (2023).
Nagaoka, S., Takeuchi, A. & Banno, A. Plant-derived peptides improving lipid and glucose metabolism. Peptides 142, 170577 (2021).
Matthews, D. M. & Laster, L. Absorption of protein digestion products: a review. Gut 6, 411–426 (1965).
Ballegaard, A. R. & Bøgh, K. L. Intestinal protein uptake and IgE-mediated food allergy. Food Res. Int. 163, 112150 (2023).
Wang, C. Y., Liu, S., Xie, X. N. & Tan, Z. R. Regulation profile of the intestinal peptide transporter 1 (PepT1). Drug Des. Dev. Ther. 11, 3511–3517 (2017).
Banno, A. et al. Identification of a novel cholesterol-lowering dipeptide, phenylalanine-proline (FP), and its down-regulation of intestinal ABCA1 in hypercholesterolemic rats and Caco-2 cells. Sci. Rep. 9, 19416 (2019).
Mijiti, M. et al. Anti-obesity and hypocholesterolemic actions of protamine-derived peptide RPR (Arg-Pro-Arg) and protamine in high-fat diet-induced C57BL/6J mice. Nutrients 13, 2501 (2021).
Nagaoka, S. et al. Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem. Biophys. Res. Commun. 281, 11–17 (2001).
Nagaoka, S. Structure-function properties of hypolipidemic peptides. J. Food Biochem. 43, e12539 (2019).
Giocondi, M. C., Besson, F., Dosset, P., Milhiet, P. E. & Le Grimellec, C. Temperature-dependent localization of GPI-anchored intestinal alkaline phosphatase in model rafts. J. Mol. Recognit. 20, 531–537 (2007).
Takeuchi, A. et al. IIAEK targets intestinal alkaline phosphatase (IAP) to improve cholesterol metabolism with a specific activation of IAP and downregulation of ABCA1. Nutrients 12, 2859 (2020).
Duan, L. P., Wang, H. H. & Wang, Q. H. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J. Lipid Res. 45, 1312–1323 (2004).
Narisawa, S. et al. A novel phosphatase upregulated in Akp3 knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol. 293, 1068–1077 (2007).
Schumacher, M. M. & DeBose-Boyd, R. A. Posttranslational regulation of HMG CoA reductase, the rate-limiting enzyme in synthesis of cholesterol. Annu. Rev. Biochem. 90, 659–679 (2021).
Altmann, S. W. et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Eberlé, D., Hegarty, B., Bossard, P., Ferré, P. & Foufelle, F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86, 839–848 (2004).
Chiang, J. Y. L. & Ferrell, J. M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol. Cell. Endocrinol. 548, 111618 (2022).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, 457–462 (2016).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Lynes, M., Narisawa, S., Millán, J. L. & Widmaier, E. P. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1738–R1747 (2011).
Liu, W. et al. Alkaline phosphatase controls lineage switching of mesenchymal stem cells by regulating the LRP6/GSK3β complex in hypophosphatasia. Theranostics 8, 5575–5592 (2018).
Ueguchi-Tanaka, M. et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature 437, 693–698 (2005).
Shimada, A. et al. Structural basis for gibberellin recognition by its receptor GID1. Nature 456, 520–523 (2008).
Peña-Cardeña, A. et al. The C-terminal protoxin region of Bacillus thuringiensis Cry1Ab toxin has a functional role in binding to GPI-anchored receptors in the insect midgut. J. Biol. Chem. 293, 20263–20272 (2018).
Narisawa, S. et al. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol. Cell. Biol. 23, 7525–7530 (2003).
Kaliannan, K. et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc. Natl. Acad. Sci. U. S. A. 110, 7003–7008 (2013).
Kühn, F. et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 5, e134049 (2020).
Halling Linder, C., Englund, U. H., Narisawa, S., Millán, J. L. & Magnusson, P. Isozyme profile and tissue-origin of alkaline phosphatases in mouse serum. Bone 53, 399–408 (2013).
Ghosh, S. S. et al. Over-expression of intestinal alkaline phosphatase attenuates atherosclerosis. Circ. Res. 128, 1646–1659 (2021).
Ghosh, S. S. et al. Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates western diet-induced barrier dysfunction and glucose intolerance. Physiol. Rep. 6, e13790 (2018).
Kawase, M., Hashimoto, H., Hosoda, M., Morita, H. & Hosono, A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J. Dairy Sci. 83, 255–263 (2000).
Pal, S., Ellis, V. & Dhaliwal, S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br. J. Nutr. 104, 716–723 (2010).
Noda, S. et al. Characterization and structure of alternatively spliced transcript variant of human intestinal alkaline phosphatase (ALPI) gene. J. Nutr. Sci. Vitaminol. 68, 284–293 (2022).
Hamarneh, S. R. et al. A novel approach to maintain gut mucosal integrity using an oral enzyme supplement. Ann. Surg. 260, 706–714 (2014).
Montoya, C. A., Leterme, P. & Lalles, J. P. A protein-free diet alters small intestinal architecture and digestive enzyme activities in rats. Reprod. Nutr. Dev. 46, 49–56 (2006).
Lallès, J. P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 77, 710–724 (2019).
Muraoka, T. et al. Ezetimibe decreases SREBP-1c expression in liver and reverses hepatic insulin resistance in mice fed a high-fat diet. Metabolism 60, 617–628 (2011).
Toyoda, Y. et al. Identification of hepatic NPC1L1 as an NAFLD risk factor evidenced by ezetimibe-mediated steatosis prevention and recovery. FASEB Bioadv. 1, 283–295 (2019).
Toyoda, Y., Takada, T., Yamanashi, Y. & Suzuki, H. Pathophysiological importance of bile cholesterol reabsorption: hepatic NPC1L1-exacerbated steatosis and decreasing VLDL-TG secretion in mice fed a high-fat diet. Lipids Health Dis. 18, 234 (2019).
Soeda, K. et al. Gut insulin action protects from hepatocarcinogenesis in diabetic mice comorbid with nonalcoholic steatohepatitis. Nat. Commun. 14, 6584 (2023).
Takeuchi, A. et al. Pentapeptide IIAEK ameliorates cholesterol metabolism via the suppression of intestinal cholesterol absorption in mice. Biosci. Biotechnol. Biochem. 87, 1345–1353 (2023).
Dybdahl, M., Selesko, D. B. & Mikkelsen, U. R. Safety evaluation of whey derived beta-lactoglobulin, Lacprodan® BLG. Toxicol. Rep. 8, 617–626 (2021).
Hernández-Ledesma, B., Recio, I. & Amigo, L. Beta-lactoglobulin as source of bioactive peptides. Amino Acids 35, 257–265 (2008).
Hinke, S. A. et al. Metformin effects on dipeptidylpeptidase IV degradation of glucagon-like peptide-1. Biochem. Biophys. Res. Commun. 291, 1302–1308 (2002).
Huang, J. et al. Emerging role of dipeptidyl peptidase-4 in autoimmune disease. Front. Immunol. 13, 830863 (2022).
Fedde, K. N. & Whyte, M. P. Alkaline phosphatase (tissue-nonspecific isoenzyme) is a phosphoethanolamine and pyridoxal-5’-phosphate ectophosphatase: Normal and hypophosphatasia fibroblast study. Am. J. Hum. Genet. 47, 767–775 (1990).
Komazin, G. et al. Substrate structure-activity relationship reveals a limited lipopolysaccharide chemotype range for intestinal alkaline phosphatase. J. Biol. Chem. 294, 19405–19423 (2019).
Millan, J. L. & Whyte, M. P. Alkaline Phosphatase and Hypophosphatasia. Calcif. Tissue Int. 98, 398–416 (2016).
Struck, A. W., Axmann, M., Pfefferle, S., Drosten, C. & Meyer, B. A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 94, 288–296 (2012).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Ebihara, A. et al. Mapping the protein binding site of the (pro)renin receptor using in silico 3D structural analysis. Hypertens. Res. 46, 959–971 (2023).
Su, M. C., Yuan, R. H., Lin, C. Y. & Jeng, Y. M. Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod. Pathol. 21, 1379–1386 (2008).
Chang, Y. Y. et al. Deletion of cadherin-17 enhances intestinal permeability and susceptibility to intestinal tumour formation. J. Pathol. 246, 289–299 (2018).
Aylon, Y. et al. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30, 786–797 (2016).
Hoylaerts, M. F., Manes, T. & Millán, J. L. Mammalian alkaline phosphatases are allosteric enzymes. J. Biol. Chem. 272, 22781–22787 (1997).
Tsukahara, T. et al. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim. Sci. J. 84, 54–59 (2013).
Goto, T., Saito, Y., Morikawa, K., Kanamaru, Y. & Nagaoka, S. Epigallocatechin gallate changes mRNA expression level of genes involved in cholesterol metabolism in hepatocytes. Br. J. Nutr. 107, 769–773 (2012).
Takashima, S., Takemoto, S., Toyoshi, K., Ohba, A. & Shimozawa, N. Zebrafish model of human Zellweger syndrome reveals organ-specific accumulation of distinct fatty acid species and widespread gene expression changes. Mol. Genet. Metab. 133, 307–323 (2021).
Killedar, S. J. et al. Early pathogenic events associated with Sjögren’s syndrome (SjS)-like disease of the NOD mouse using microarray analysis. Lab. Invest. 86, 1243–1260 (2006).
Ye, Y. et al. Rose polyphenols exert antiobesity effect in high-fat-induced obese mice by regulating lipogenic gene expression. Nutr. Res. 119, 76–89 (2023).
Kim, K. et al. Surface plasmon resonance studies of the direct interaction between a drug/intestinal brush border membrane. Pharm. Res. 21, 1233–1238 (2004).
Wang, N. et al. The IGF-trap: Novel inhibitor of carcinoma growth and metastasis. Mol. Cancer Ther. 14, 982–993 (2015).
Iannucci, A. et al. Toll-like receptor 4-mediated inflammation triggered by extracellular IFI16 is enhanced by lipopolysaccharide binding. PLoS Pathog. 16, e1008811 (2020).
Kinouchi, H., Matsuyama, K., Kitagawa, H. & Kamimori, H. Surface plasmon resonance assay of inhibition by pharmaceuticals for thyroxine hormone binging to transport proteins. Anal. Biochem. 492, 43–48 (2016).
Dash, S. et al. Heterophilic recognition between E-cadherin and N-cadherin relies on same canonical binding interface as required for E-cadherin homodimerization. Arch. Biochem. Biophys. 727, 109329 (2022).
Mirdita, M. et al. ColabFold: Making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Ghosh, K. et al. Crystal structure of rat intestinal alkaline phosphatase–role of crown domain in mammalian alkaline phosphatases. J. Struct. Biol. 184, 182–192 (2013).
Yu, Z. et al. Novel ACE inhibitory tripeptides from ovotransferrin using bioinformatics and peptidomics approaches. Sci. Rep. 9, 17434 (2019).
Yamaguchi, A. et al. Het-PDB Navi.: A database for protein-small molecule interactions. J. Biochem. 135, 79–84 (2004).
Zanka, K., Kawaguchi, Y., Okada, Y. & Nagaoka, S. Epigallocatechin gallate induces upregulation of LDL receptor via the 67 kDa laminin receptor-independent pathway in HepG2 cells. Mol. Nutr. Food Res. 64, e1901036 (2020).
Sparrow, C. P. et al. A fluorescent cholesterol analog traces cholesterol absorption in hamsters and is esterified in vivo and in vitro. J. Lipid Res. 40, 1747–1757 (1999).
Acknowledgements
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (grant no. 20H00570 and 24H00676 to S.N.).
Author information
Authors and Affiliations
Contributions
S.N. developed the study design and wrote the manuscript. A.T. wrote the manuscript and conducted the experiments. N.O., K.T., R.M., T.A., A.B., and A.E. performed the experiments and contributed to this manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Takeuchi, A., Oda, N., Takada, K. et al. Intestinal alkaline phosphatase is a receptor for cholesterol-lowering pentapeptide IIAEK and regulates cholesterol homeostasis in mice. Sci Rep 15, 20345 (2025). https://doi.org/10.1038/s41598-025-04722-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-04722-w
This article is cited by
-
Tissue nonspecific and intestinal alkaline phosphatase crosstalk: a missing link in hypophosphatasia pathophysiology?
Journal of Translational Medicine (2026)