Abstract
We tested whether leg fat mass is associated with insulin sensitivity, metabolic syndrome components, inflammatory markers, and impaired pancreatic β-cell function in a setting where body composition is less related to socioeconomic status. Leg fat (relative to body fat) by DXA, surrogate measures of insulin sensitivity and pancreatic β cell function inferred from serum insulin kinetics during a 75 g oral glucose tolerance test (OGTT), metabolic syndrome components, and inflammatory markers, were measured in middle-aged Japanese parents of university students, whose BMI averaged < 23.0 kg/m2 and homeostasis model assessment insulin resistance (HOMA-IR) < 1.5. Low leg fat was associated with high HOMA-IR, adipose insulin resistance index (a product of fasting insulin and free fatty acid), OGTT-derived hepatic insulin resistance, and low Matsuda index. In addition, low leg fat was associated with a low disposition index (insulin resistance-adjusted insulin secretion) and high fasting and post-glucose glycemia. Further, it was related to high triglycerides, blood pressure, liver enzymes, and low HDL cholesterol. Finally, low leg fat was associated with high C-reactive protein and orosomucoid, acute phase proteins, and high leucocyte counts. The present study suggests that the percentage leg fat mass should be considered in assessing cardiometabolic risk stratification.
Similar content being viewed by others
Introduction
Insulin resistance and impaired insulin secretion are hallmarks of type 2 diabetes. Obesity in general, and abdominal (truncal) obesity in particular, is well-known to be associated with insulin resistance and cardiometabolic diseases. In addition, a low amount of leg (gluteofemoral) fat mass is reported to be associated with insulin resistance and cardiometabolic abnormalities1,2. We previously reported an inverse association of leg fat with post-breakfast lipemia even in young women who are of normal weight and insulin-sensitive after adjustment for trunk fat3. A recent Mendelian randomization study reported that the inability of subcutaneous fat depots to store energy substrates efficiently could be the causal factor underlying the association of visceral lipid deposition, lower leg (gluteofemoral) adipose tissue, with cardiometabolic health4. However, only a few studies have investigated the associations of leg fat with pancreatic β cell function.
It is recognized that chronic low-grade inflammation is an etiological link between obesity, insulin resistance, and type 2 diabetes5,6. It has been suggested that modified pro-inflammatory cytokine expression associated with a greater volume of adipose tissue may be a mechanism for low-grade inflammation6,7. In particular, increased inflammatory cytokine production may be one of the determinants of the increased cardiometabolic risk associated with visceral obesity8. However, only a few studies have investigated the associations of leg fat with inflammatory markers, as described later.
Dual-energy X-ray absorptiometry (DXA) has been used to assess fat mass in the arms, legs, trunk, and total body. We hypothesized that a lower ratio of leg fat to total body fat (LF/BF ratio) is related to high cardiometabolic risk, high markers of inflammation, and pancreatic β-cell dysfunction. Because there was an inverse association between socioeconomic status and body composition among adults and children in countries with high-income economies9, the present study was done on parents of female university students who were less heterogeneous in socioeconomic status10.
Methods
We examined, cross-sectionally, 144 middle-aged mothers and 58 fathers of female university students whose details have been previously reported10,11,12,13. We excluded those with clinically diagnosed acute or chronic diseases, those on hormonal contraception, and those on a diet to lose weight from the study. The present study was done between 2004 and 2007, and these individuals participated as volunteers as previously reported13. This research followed the tenets of the Declaration of Helsinki. After the experimental procedure had been explained, participants provided written informed consent. The study was approved by the Ethics Committees of the Mukogawa Women’s University (No. 07-28 on Feb. 19, 2008).
After a 12-h overnight fast, they underwent blood sampling, measurement of anthropometric indices, blood pressure, and body composition. Brachial blood pressure was measured using an automated sphygmomanometer (BP-203RV II, Colin, Tokyo, Japan) after participants were seated for 5 min. The measurements were repeated after 2–3 min, and the average of the measurements was used for analyses. Plasma glucose, serum aspartate aminotransferase, and alanine aminotransferase (AST and ALT, respectively), gamma-glutamyl transferase (GGT), insulin, triglycerides, cholesterol, high-density lipoprotein (HDL) cholesterol, apolipoproteins, adipokines, and free fatty acid (FFA) were measured as previously reported10,11,12,13. Two acute phase proteins, high-sensitivity C-reactive protein (hsCRP) and orosomucoid, and leukocytes, all of which have been reported to be associated with an increased risk of type 2 diabetes and cardiovascular diseases14, were also measured. Fasting glucose ≥ 100 mg/dL was defined as prediabetes15, triglycerides ≥ 150 mg/dL as hypertriglyceridemia16, and a serum ALT level ≥ 30 IU/L as elevated ALT17.
A standard 75 g oral glucose tolerance test (OGTT) was done with multiple post-load glucose and insulin measurements over a 30–120-min period in 63 mothers and 58 fathers. Blood samples were taken at min 0 (fasting), 30, 60, and 120 for glucose and insulin analysis. Insulin resistance/sensitivity was determined by homeostasis model assessment (HOMA-IR) using fasting plasma glucose and insulin levels18, the adipose tissue insulin resistance (AT-IR) index calculated as a product of fasting insulin and FFA19, and the Matsuda insulin sensitivity index using glucose and insulin levels during OGTT20. Glucose-induced insulin secretion was evaluated by the insulinogenic index (IGI), which was calculated as incremental insulin concentrations (μU/mL) divided by incremental glucose concentrations (mg/dL) during the first 30 min of OGTT21. The oral disposition index (ODI) was calculated as the product of the IGI and Matsuda index22. The area under the glucose and insulin concentration curve during OGTT (AUCg and AUCi, respectively) was calculated using the trapezoidal method.
Fat mass, lean mass, and total mass of the arms, legs, and trunk in kilograms were measured using whole-body dual-energy X-ray absorptiometry (DXA) (Hologic QDR-2000, software version 7.20D, Waltham, MA) as previously reported13. The leg region included the entire hip, thigh, and legs. General adiposity was assessed using height-adjusted and weight-adjusted body fat; fat mass index (FMI) and body fat (BF) percentage (%), respectively. Leg fat (relative to BF, LF/BF%) and trunk fat (relative to BF, TF/BF%) were also calculated in each participant. Because lean mass in the arms and legs represents skeletal muscle mass, the sum of the two was used as the appendicular skeletal muscle mass (SM). The SM index (SMI) was calculated as SM in kilograms divided by the squared height in meters.
Data were presented as mean ± SD unless otherwise stated. Due to deviation from normal distribution, CRP, IGI, and ODI were logarithmically transformed for analyses. Comparisons between the two groups were made with a two-sample t-test. Since LF/BF% was higher in mothers than in fathers (Supplementary Tables 1 and 2), firstly, the data of mothers and fathers, including cardiometabolic variables, were divided into the respective LF/BF% tertile, and, secondly, they were combined for analyses (Table 2). Because of sex differences in body fat23, associations of LF/BF% with cardiometabolic variables were evaluated using Pearson’s correlation analyses in mothers and fathers separately. Differences among the three groups were analyzed using variance analysis and Bonferroni’s multiple comparison procedure. When an ANOVA p value was ≥ 0.05, the p values were presented in tables. When an ANOVA p<0.05, Bonferroni’s multiple comparison procedure results were presented. A two-tailed p<0.05 was considered statistically significant. All calculations were performed with SPSS system 23 (SPSS Inc, Chicago, IL).
Results
The middle-aged Japanese studied were, on average, not obese (Table 1) and had a mean HOMA-IR of 1.3 ± 0.8. Although fathers had higher BMI and waist circumference, they had lower FMI and BF percentages than mothers. As expected, fathers had lower serum leptin and adiponectin concentrations. They also had lower LF/BF% and higher TF/BF%.
Since LF/BF% was higher in mothers than in fathers, the data were divided into respective LF/BF% tertile (Supplementary Tables 1 and 2). The results of the two groups were combined to produce the LF/BF% tertile of parents (Table 2). Although parents in the low compared to high tertile had higher BMI and FMI, body fat% % and serum leptin did not differ. As LF/BF% increased, waist circumference decreased, and serum adiponectin increased in a stepwise fashion. SMI was elevated in those with low LF/BF%.
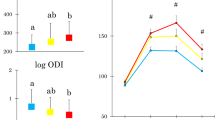
Glucose concentrations at four time points and hence AUCg were higher in the low compared to the high LF/BF% tertile (Fig. 1). Although IGI did not differ, HOMA-IR and AT-IR were higher. Matsuda index was lower (Table 3), and log ODI (Fig. 1) was lower in the low tertile. Parents with low LF/BF% had higher serum liver enzymes, TG, apolipoprotein B, and blood pressure. The prevalence of prediabetes, hypertriglyceridemia, elevated blood pressure, and elevated ALT was also higher (Fig. 2). Although HbA1c levels did not differ, prediabetes defined by HbA1c of 5.7 % or greater15 increased from the high to low LF/BF% (from 14.7, through 20.9 to 37.3 %, p=0.007). Although they had lower HDL cholesterol and apolipoprotein A1, their HDL cholesterol concentrations averaged 62 mg/dL (Table 3). The prevalence of low HDL cholesterol16 was low in the total population (4.8 %) and did not differ among the three groups (data not shown). Serum orosomucoid, log CRP, and leukocyte counts decreased from the low through medium to high LF/BF% tertile (Fig. 3).
Left column (mean ± SD): area under the glucose concentration curve (AUCg) and log oral disposition index (ODI). Means not sharing common alphabetical letters are significantly different from each other at p < 0.05 or less by Bonferroni’s multiple comparison procedure. Right column (mean ± SE): Glucose response curves during 75-g oral glucose tolerance testing. The data of mothers and fathers were divided into respective leg fat relative to the body fat percentage tertile and then combined for analyses. Red, yellow, and green symbols indicate the low, medium, and high groups, respectively. #: p < 0.05 or less versus the low group.
Serum orosomucoid, log C-reactive protein (CRP), and white blood cell (WBC) counts in parents with low (in red), medium (in yellow), and high (in green) leg fat relative to body fat percentage tertile. Mean ± SD. Means not sharing common alphabetical letters are significantly different from each other at p < 0.05 or less by Bonferroni’s multiple comparison procedure.
In a multivariable linear regression analysis, Matsuda index (standardized β: −0.314, p=0.001) and LF/BF% (standardized β: 0.204, p=0.02) emerged as determinants of log IGI independently of age and sex and explained 8 % of its variability.
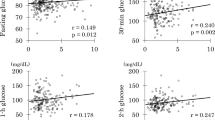
Table 4 shows Pearson’s correlation coefficient (r) of LF/BF% with cardiometabolic variables in parents, mothers, and fathers. The majority of associations were significant in parents. However, almost all the correlations became stronger in women but became insignificant in men. The association with the Matsuda index was particularly the strongest (r=0.648) in mothers as previously reported12, suggesting that the whole-body insulin sensitivity may be associated with leg fat mass in nonobese Japanese women. However, there was no association with log IGI.
In a multivariable linear regression analysis, LF/BF% (standardized β: 0.359) and age (standardized β: 0.293, both p<0.001) emerged as determinants of the Matsuda index independently of TF/BF% and sex and explained 6 % of its variability. A multivariable linear regression analysis for LF/BF% as the dependent variable revealed that age (standardized β: 0.459), sex (standardized β: 0.396), and the Matsuda index (standardized β: 0.293, all p<0.001) were determinants of LF/BF% independently of log IGI and explained 61.7% of its variability.
Discussion
The present study confirmed associations of low leg fat (relative to body fat) with low insulin sensitivity and metabolic syndrome components as previously reported1,24,25,26,27. In addition, low leg fat was associated with pancreatic β-cell dysfunction (a low ODI, a predictor of future development of diabetes above and beyond fasting and 2-h glucose levels)22, high glucose excursion (AUCg), and a high prevalence of HbA1c-defined prediabetes. Further, there was a direct positive association between insulin secretion (log IGI) and leg fat. Finally, low-grade inflammation (elevated CRP, orosomucoid, and leukocyte count) was found in middle-aged Japanese with low leg fat, possibly associated with lipodystrophy-like phenotypes1. It is to be noted that these observations were found in middle-aged Japanese whose BMI averaged <25 kg/m2 and HOMA-IR<1.5. In other words, a higher amount of leg fat in Japanese women might be related to better insulin sensitivity. However, this relationship may be less applicable to men.
We and others reported that decreased leg fat was associated with higher 2-h postload glucose12,28,29,30. However, little is known about the direct relationship between leg fat mass and pancreatic β-cell function. Stefan et al.1 reported insulin secretion failure in middle-aged Caucasians with metabolically unhealthy normal weight, characterized by low leg fat. The current study may be the first to demonstrate pancreatic β-cell dysfunction in middle-aged Japanese with low leg fat. We have reported an inverse association of AT-IR with β-cell dysfunction31. Because middle-aged Japanese with low leg fat had high AT-IR, a product of fasting insulin and FFA19, lipotoxicity to pancreatic β-cells may explain the association32. Chronic systemic low-grade inflammation may be another likely explanation, as discussed later.
Pancreatic β-cell dysfunction in non-obese Japanese with low leg fat may be consistent with the recent observation that β-cell dysfunction and lipodystrophy could be the driving pathological pathways in type 2 diabetes in Chinese individuals with normal weight33. Smith et al.34 found that the East Asian population has a higher proportion of polygenic risk related to lipodystrophy.
Mendelian randomization studies supported a causal relation between higher BMI and acute phase proteins (CRP and orosomucoid)35,36,37. In addition to general obesity, studies demonstrated a positive association of abdominal obesity with inflammatory biomarkers38,39. However, studies on the association of leg fat with inflammatory biomarkers were limited. We found one study that showed a negative association of leg fat with CRP40. Nonobese Japanese people with low leg fat had high inflammatory biomarkers, CRP, orosomucoid, and leukocyte counts, all of which had been reported to be risk factors for type 2 diabetes and cardiovascular disease14.
The strength of this study includes a homogeneous study population with scarce confounding factors less related to socioeconomic status and accurate and reliable measures of body composition by DXA. Several limitations of this study include a relatively small sample size and a single measurement of biochemical variables. We used many surrogates in the present study, which may be less accurate. Statistical power was not calculated. Finally, as we studied middle-aged Japanese, results may not be generalized to other ages, sex, races, or ethnicities.
In conclusion, impaired pancreatic β-cell function found in nonobese Japanese with low leg fat whose HOMA-IR averaged 1.3 may explain why type 2 diabetes develops at a much lower BMI and predominates insulin secretion failure in Japanese people. The present study suggests that the percentage leg fat mass should be considered in assessing cardiometabolic risk stratification.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Stefan, N., Schick, F. & Häring, H. U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 26, 292–300 (2017).
Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 8, 616–627 (2020).
Takeuchi, M., Tsuboi, A., Kurata, M., Kazumi, T. & Fukuo, K. Associations of postprandial lipemia with trunk/leg fat ratio in young normal weight women independently of fat mass and insulin resistance. Asia Pac. J. Clin. Nutr. 27, 293–299 (2018).
Gagnon, E., Paulin, A., Mitchell, P. L. & Arsenault, B. J. Disentangling the impact of gluteofemoral versus visceral fat accumulation on cardiometabolic health using sex-stratified Mendelian randomization. Atherosclerosis 386, 117371 (2023).
Ridker, P. M. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr. Rev. 65, S253–S259 (2007).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150 (2014).
Espinola-Klein, C., Gori, T., Blankenberg, S. & Munzel, T. Inflammatory markers and cardiovascular risk in the metabolic syndrome. Front. Biosci. (Landmark Ed.) 16, 1663–1674 (2011).
Tchernof, A. & Després, J. P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93, 359–404 (2013).
Bridger Staatz, C. et al. Life course socioeconomic position and body composition in adulthood: a systematic review and narrative synthesis. Int. J. Obes. (London) 45, 2300–2315 (2021).
Tanaka, M., Yoshida, T., Bin, W., Fukuo, K. & Kazumi, T. FTO, abdominal adiposity, fasting hyperglycemia associated with elevated HbA1c in Japanese middle-aged women. J. Atheroscler. Thromb. 19, 633–642 (2012).
Minato-Inokawa, S. et al. The different associations of serum gamma-glutamyl transferase and alanine aminotransferase with insulin secretion, β-cell function, and insulin resistance in non-obese Japanese. Sci. Rep. 14, 19234 (2024).
Wu, B. et al. Different associations of trunk and lower-body fat mass distribution with cardiometabolic risk factors between healthy middle-aged men and women. Int. J. Endocrinol. 2018, 1289485 (2018).
Takeuchi, M. et al. Weight trajectory since birth, current body composition, dietary intake, and glucose tolerance in young underweight Japanese women. Womens Health Rep. (New Rochelle) 3, 215–221 (2022).
Bao, X. et al. Comparing the inflammatory profiles for incidence of diabetes mellitus and cardiovascular diseases: a prospective study exploring the ‘common soil’ hypothesis. Cardiovasc. Diabetol. 17, 87 (2018).
American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care. 48(Supplement 1), S27–49 (2025).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120, 1640–5 (2009).
Lee, J. et al. Optimal ALT threshold for the automated diagnosis of MASLD: A population-based study using iLFT. Ann. Hepatol. 29, 101280 (2024).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Søndergaard, E., Espinosa De Ycaza, A. E., Morgan-Bathke, M. & Jensen, M. D. How to measure adipose tissue insulin sensitivity. J. Clin. Endocrinol. Metab. 102, 1193–9 (2017).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Stumvoll, M. et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23, 295–301 (2000).
Utzschneider, K. M. et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 32, 335–341 (2009).
Kelly, T. L., Wilson, K. E. & Heymsfield, S. B. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 4, e7038 (2009).
Zhang, X., Hu, E. A., Wu, H., Malik, V. & Sun, Q. Associations of leg fat accumulation with adiposity-related biological factors and risk of metabolic syndrome. Obesity (Silver Spring) 21, 824–830 (2013).
Han, E. et al. Anatomic fat depots and cardiovascular risk: a focus on the leg fat using nationwide surveys (KNHANES 2008–2011). Cardiovasc. Diabetol. 16, 54 (2017).
Kim, H. M. & Lee, Y. H. The leg fat to total fat ratio is associated with lower risks of non-alcoholic fatty liver disease and less severe hepatic fibrosis: Results from Nationwide Surveys (KNHANES 2008–2011). Endocrinol. Metab. (Seoul) 36, 1232–1242 (2021).
Visaria, A., Lo, D., Maniar, P., Dave, B. & Joshi, P. Leg and arm adiposity is inversely associated with diastolic hypertension in young and middle-aged United States adults. Clin. Hypertens. 28, 3 (2022).
Snijder, M. B. et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 27, 372–377 (2004).
Kosmiski, L. A. et al. Study of fat redistribution and metabolic change in HIV Infection (FRAM). Association of increased upper trunk and decreased leg fat with 2-h glucose in control and HIV-infected persons. Diabetes Care 34, 2448–53 (2011).
Tanaka, S. et al. Associations of lower-body fat mass with favorable profile of lipoproteins and adipokines in healthy, slim women in early adulthood. J. Atheroscler. Thromb. 18, 365–372 (2011).
Minato-Inokawa, S. et al. Associations of adipose insulin resistance index with pancreatic β cell function (inverse) and glucose excursion (positive) in young Japanese women. Sci. Rep. 14, 18590 (2024).
Ye, R., Onodera, T. & Scherer, P. E. Lipotoxicity and β cell maintenance in obesity and type 2 diabetes. J. Endocr. Soc. 3, 617–631 (2019).
Yu, G. et al. Type 2 diabetes pathway-specific polygenic risk scores elucidate heterogeneity in clinical presentation, disease progression and diabetic complications in 18,217 Chinese individuals with type 2 diabetes. Diabetologia https://doi.org/10.1007/s00125-024-06309-y (2024).
Smith, K. et al. Multi-ancestry polygenic mechanisms of type 2 diabetes. Nat. Med. 30, 1065–1074 (2024).
Timpson, N. J. et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int. J. Obes. (London). 35, 300–308 (2011).
Würtz, P. et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 11, e1001765 (2014).
Palaniswamy, S. et al. Could vitamin D reduce obesity-associated inflammation? Observational and Mendelian randomization study. Am. J. Clin. Nutr. 111, 1036–1047 (2020).
Lapice, E. et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care 32, 1734–1736 (2009).
Sam, S. et al. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 32, 932–937 (2009).
Lee, M. et al. Associations between trunk, leg and total body adiposity with arterial stiffness. Am. J. Hypertens. 25, 1131–1137 (2012).
Acknowledgements
We are indebted to all the participants for their dedicated and conscientious collaboration.
Author information
Authors and Affiliations
Contributions
SMI, MH, ATK, and MT collected data and SMI prepared figures. KK, MK, and BW analyzed data and prepared tables. TK wrote the manuscript, and KF reviewed and edited it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committees of the Mukogawa Women’s University (No. 07-28 on 19/02/2008).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Minato-Inokawa, S., Honda, M., Tsuboi-Kaji, A. et al. Low leg fat mass is associated with low insulin sensitivity, inflammatory markers, and β-cell dysfunction in non-obese Japanese people. Sci Rep 15, 21194 (2025). https://doi.org/10.1038/s41598-025-05949-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-05949-3