Abstract
Diabetes mellitus (DM) is a major global health concern associated with complications that significantly impair quality of life. Among these, the impact of DM on visual function and the underlying mechanisms, particularly the role of sleep quality, remain insufficiently understood. This cross-sectional study recruited 629 participants from Henan Provincial People’s Hospital to examine the relationships among DM, sleep quality, and visual function. Visual function was assessed using the National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25), while sleep quality was evaluated with the Pittsburgh Sleep Quality Index (PSQI). Compared to non-diabetic controls, individuals with DM exhibited significantly poorer visual function and sleep quality. Path analysis further revealed that sleep quality played a significant mediating role in the relationship between DM and visual function, accounting for 42.2% of the total effect. Subgroup analyses indicated that female and elderly patients with DM were more susceptible to the influence of sleep disturbances on visual outcomes. These findings underscore the critical role of sleep quality in diabetes-related visual impairment and highlight the importance of incorporating routine sleep assessments and targeted interventions into diabetes management to help preserve visual function and improve overall quality of life in diabetic populations.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) has emerged as one of the most critical global public health challenges, with its prevalence continuing to rise and projected to reach 783 million by 20451. The impact of DM on health and socioeconomic systems is profound2. The complexity of DM management primarily arises from its multiple severe complications, such as cardiovascular disease, renal failure, and diabetic retinopathy (DR). These complications not only significantly reduce patients’ quality of life but also increase healthcare burdens3.
Among these complications, DR is one of the most common and serious microvascular outcomes of diabetes and represents a leading cause of visual impairment worldwide4. In addition to DR, diabetes-related visual decline may result from a combination of systemic pathophysiological mechanisms, including blood glucose fluctuations, vascular endothelial dysfunction, and neurodegeneration5. Such impairments in visual function adversely affect daily activities such as reading, driving, and working, thereby contributing to reduced independence and socioeconomic productivity6.
In parallel, increasing attention has been given to the role of sleep disturbances in individuals with DM. Studies suggest a bidirectional relationship between poor sleep quality and diabetes progression—sleep disorders are not only more prevalent in patients with DM but may also exacerbate glycemic dysregulation through inflammatory and hormonal mechanisms7. Furthermore, poor sleep has been implicated in the worsening of DR, potentially accelerating retinal damage via metabolic imbalance, oxidative stress, and neurovascular compromise8.
Despite growing evidence linking sleep quality and diabetic outcomes, the indirect impact of sleep quality on visual function in patients with DM remains underexplored. Most existing studies have investigated these relationships in isolation, without examining sleep quality as a potential mediating factor in the pathway from DM to visual decline9. To address this gap, the present study aims to examine the association between DM and visual function, with a specific focus on whether sleep quality mediates this relationship. By comparing diabetic and non-diabetic individuals using validated instruments, we seek to elucidate how sleep disturbances contribute to diabetes-related visual dysfunction. Understanding this interplay may provide novel insights for comprehensive, multi-dimensional diabetes care—especially for early identification and intervention strategies aimed at preserving visual function and enhancing patient well-being.
Materials and methods
Study population
This cross-sectional study included participants who were patients or individuals undergoing routine health examinations at Henan Provincial People’s Hospital between January and June 2024. Henan Provincial People’s Hospital, as a regional comprehensive medical center, serves a diverse population encompassing various age groups, genders, geographic areas, and socio-economic backgrounds. By enrolling individuals from this setting, the study sample was designed to reflect both diabetic and non-diabetic populations within the region, thereby enhancing the representativeness and potential relevance of the findings. Inclusion criteria were: (1) age ≥ 18 years; (2) availability of complete clinical and examination data with a confirmed diagnosis of DM or confirmed non-diabetic status; (3) ability and willingness to provide written informed consent; and (4) cognitive ability to independently complete or respond to questionnaire items. Exclusion criteria included: (1) individuals missing key variables such as age, gender, or questionnaire scores; (2) patients with malignant tumors or severe organ failure (e.g., heart, liver, or kidney failure); (3) participants with more than 20% missing questionnaire data; and (4) responses identified as invalid based on trap questions. This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of Henan Provincial People’s Hospital (Approval No. HNEECKY-2022[22]). All participants received detailed written information outlining the study’s objectives, procedures, potential risks, and benefits. Informed consent was voluntarily signed prior to enrollment.
Sample size calculation was conducted using PASS software, based on a two-sided test for independent group means (formulas a and b). Where α = 0.05 (two-sided), β = 0.1 (power = 90%), \(\:{Z}_{\alpha\:}\:\)= 1.96,\(\:\:\:{Z}_{\beta\:}\:\)= 1.28, and matching ratio k = 1. Preliminary data indicated that the mean and standard deviation of visual function scores were \(\:{x}_{DM}\)=62.3, \(\:{S}_{DM}\)=18.6 for the DM group and \(\:{x}_{con}\)= 70.7, \(\:{S}_{con}\) = 16.9 for the control group, with a difference of \(\:\delta\:=|{x}_{DR}-{x}_{con}|=8.4\). Based on this, the minimum required sample size for each group was calculated to be 117 participants. To account for potential data invalidity, the sample size for each group was increased by 20%, resulting in a minimum required sample size of 141 participants per group.
Data collection tools
Data were collected using a combination of the hospital’s electronic information system and a structured questionnaire developed following expert consultation. The questionnaire covered sociodemographic information, medical history, visual function assessment, and sleep quality assessment.
Visual function was assessed using the Chinese version of the National Eye Institute Visual Function Questionnaire-25 (NEI-VFQ-25). This tool has been previously validated in Chinese populations with diverse ocular conditions, demonstrating strong internal consistency (Cronbach’s α > 0.70 across domains) and structural validity10,11. The questionnaire includes 25 items across 12 domains, such as general health, general vision, ocular pain, near activities, distance activities, vision-specific social functioning, mental health, role difficulties, dependency on others, driving, color vision, and peripheral vision. Each item is scored on a five-point scale (0, 25, 50, 75, 100), with domain scores calculated as the average of the relevant items. The overall visual function questionnaire (VFQ) score is the mean of all 25 items, with higher scores indicating better visual function. Participants were categorized as having “good” or “poor” visual function based on whether their overall VFQ scores were above or below the median.
Sleep quality was assessed using the Chinese version of the Pittsburgh Sleep Quality Index (PSQI), a self-reported questionnaire measuring sleep quality and disturbances over a one-month period. The Chinese PSQI has been psychometrically validated in various Chinese populations, including patients with chronic illnesses, demonstrating high internal consistency (Cronbach’s α = 0.82) and test–retest reliability (intraclass correlation coefficient, ICC = 0.87)12,13. The PSQI consists of 19 self-reported items and 5 additional items rated by a bed partner or roommate, though only the self-reported items were used in this study. These items cover seven components: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. Each component is scored on a scale from 0 to 3, with 0 indicating the best and 3 indicating the worst outcomes. The global PSQI score, ranging from 0 to 21, is obtained by summing the component scores, with higher scores indicating worse sleep quality. A PSQI score greater than 5 is considered indicative of poor sleep quality, a threshold validated in multiple Chinese population studies14. Minor wording adaptations were made to accommodate regional dialects. These changes were reviewed by bilingual experts and did not alter the original scoring structure or conceptual content of the instruments.
DM was diagnosed according to the criteria of the World Health Organization and the Chinese Diabetes Society15,16including any of the following: (1) Fasting plasma glucose (FPG) ≥ 7.0 mmol/L after at least 8 h of fasting; (2) 2-hour plasma glucose ≥ 11.1 mmol/L during an oral glucose tolerance test (OGTT) with 75 g of anhydrous glucose; (3) Hemoglobin A1c (HbA1c) level ≥ 6.5%; (4) Random plasma glucose ≥ 11.1 mmol/L in the presence of typical DM symptoms (e.g., polyuria, polydipsia, polyphagia, and weight loss). Participants were categorized into the DM group (DM) or the control group (Con) based on their DM status. Additionally, participants were stratified into three age groups: young (< 45 years), middle-aged (45–60 years), and older (> 60 years).
Statistical analysis
Data analysis was conducted using SPSS 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism 8.0 (La Jolla, CA, USA). Continuous variables were presented as mean ± standard deviation (Mean ± SD), and comparisons between groups were performed using independent sample t-tests or one-way analysis of variance (ANOVA). Mann–Whitney U and Kruskal–Wallis H tests were used for non-normally distributed data. Categorical variables were reported as frequencies and percentages, with group comparisons conducted using the Chi-square test. Spearman correlation analysis was used to assess associations between PSQI and VFQ scores. Scatter plots with regression lines were created to visualize the relationship between sleep quality and visual function.
To investigate the mediating role of sleep quality, path analysis was conducted using Amos 24.0 (IBM, Armonk, NY, USA). The model included DM status (independent variable), VFQ score (dependent variable), and PSQI score (mediator), adjusting for age, gender, and education. Model fit was evaluated using the following indices: chi-square to degrees of freedom ratio (χ²/df < 3.0), Tucker-Lewis Index (TLI) and Comparative Fit Index (CFI) values greater than 0.90, and Root Mean Square Error of Approximation (RMSEA) less than 0.08. Modification indices > 10 were used to optimize model fit. Standardized path coefficients (β-values) were used to quantify the strength of the relationships between variables. All hypothesis tests were two-tailed, and a significance level of α = 0.05 was set for all analyses.
Results
General characteristics of study participants
A total of 629 participants were included in the study, with a mean age of 54.1 ± 13.5 years. Of these, 323 (51.4%) were male and 306 (48.6%) were female. The educational levels of the participants were categorized as follows: 173 (27.5%) had primary education or below, 322 (51.2%) had secondary education, and 134 (21.3%) had a college education or higher. Regarding marital status, 45 participants (7.2%) were single, 537 (85.3%) were married or cohabiting, and 47 (7.5%) were divorced/widowed/separated. In terms of residence, 340 participants (54.1%) lived in rural areas and 289 (45.9%) in urban areas. Among the participants, 313 (49.8%) were diagnosed with DM, of which 176 (56.2%) were male and 137 (43.8%) were female. Among the 313 control groups, there were 147 males and 169 females, respectively. The overall mean VFQ score was 65.6 ± 18.6, and the mean PSQI score was 6.08 ± 2.84.
The general characteristics of the study participants are summarized in Table 1. There were no statistically significant differences in overall VFQ scores across gender (t = 1.132, P = 0.258), marital status (F = 2.883, P = 0.057), household monthly income per person (F = 1.268, P = 0.282), or residential location (t = 0.862, P = 0.389). However, significant differences in overall VFQ scores were observed across different age groups (F = 4.252, P = 0.015) and educational levels (F = 8.599, P < 0.001).
The impact of DM on visual function
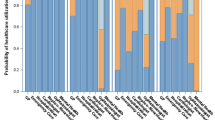
To evaluate the impact of DM on visual function and examine whether this effect varies by gender and age, group comparisons and stratified analyses were conducted. The overall VFQ score in the DM group (59.9 ± 19.7) was significantly lower than that in the control group (71.3 ± 15.2), with t = 8.090 and P < 0.001. This finding supports the hypothesis that DM negatively affects visual performance (Fig. 1A). This difference remained statistically significant across both males and females (Fig. 1B–C), as well as across all age categories—young (< 45 years), middle-aged (45–60 years), and older adults (> 60 years) (Fig. 1D–F). These results suggest that the detrimental impact of DM on visual function is robust across demographic subgroups.
Comparison of overall VFQ scores and classification between control and DM groups. (A) Comparison of overall VFQ scores between the control and DM groups shows a significant difference, with lower scores in the DM group, indicating impaired visual function. (B) In male participants, overall VFQ scores are significantly lower in the DM group compared to the control group, suggesting a negative impact of DM on visual function in men. (C) In female participants, overall VFQ scores are significantly lower in the DM group, indicating that DM negatively affects visual function in women. (D) In younger participants (< 45 years), overall VFQ scores significantly decline in the DM group compared to the control group, suggesting the possibility of early visual impairment in this age group. (E) In middle-aged participants (45–60 years), overall VFQ scores remain significantly lower in the DM group, indicating the persistent negative impact of DM on vision. (F) In older participants (> 60 years), the DM group continues to show significantly lower overall VFQ scores, emphasizing the exacerbated impact of DM on visual health in the elderly. (G) Distribution of participants with good and poor visual function in the control and DM groups, showing that the proportion of participants with poor visual function is higher in the DM group across all age and gender subgroups. VFQ: visual function questionnaire; DM: diabetes mellitus; Con: control; * P < 0.05; ** P < 0.01; *** P < 0.001.
Analysis of categorical VFQ classifications further supported these findings. The proportion of participants with “good” visual function was significantly higher in the control group than in the DM group (62.7% vs. 37.4%; χ2 = 40.191, P < 0.001). Stratified by gender, 65.3% of males in the control group reported good visual function compared to 44.3% in the DM group (χ2 = 14.198, P < 0.001), while among females the contrast was even more pronounced (60.4% vs. 28.5%; χ2 = 30.965, P < 0.001) (Fig. 1G). Age-stratified analysis similarly demonstrated significantly lower VFQ scores in the DM group across all age strata (P < 0.05), confirming that visual impairment is consistently more severe among diabetic participants regardless of age (Fig. 1G).
Dimension-specific analyses revealed that participants in the control group scored significantly higher than those in the DM group across multiple dimensions, including general health, ocular pain, near activities, distance activities, vision-specific (social functioning, mental health, role difficulties, and dependency), driving, color vision, and peripheral vision (P < 0.05), which indicated that the influence of DM on visual function involves many aspects. However, no statistically significant difference was found between the two groups in the general vision dimension (t = 1.437, P = 0.151) (Table 2), suggesting that certain core perceptual abilities may be preserved in early or moderate stages of diabetes.
Collectively, these findings demonstrate that visual function is significantly compromised in individuals with DM and that the impairment extends across multiple vision-related domains. The impact appears more pronounced in females and older adults, underscoring the need for targeted visual and systemic health interventions in these subgroups.
The impact of DM on sleep quality
To evaluate the impact of DM on sleep quality and to explore differences across genders and age groups, group comparisons and stratified analyses were conducted. The results showed that the PSQI scores were significantly higher in the DM group compared to the control group, with scores of 6.86 ± 2.99 and 5.31 ± 2.46, respectively (t = 7.139, P < 0.001) (Fig. 2A).
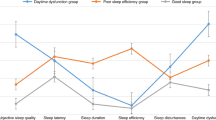
Comparison of PSQI scores and sleep quality categories between control and DM groups. (A) Comparison of overall PSQI scores between the control and DM groups, showing that the DM group has significantly higher PSQI scores, indicating poorer sleep quality in DM patients. (B) In male participants, PSQI scores are significantly higher in the DM group compared to the control group, suggesting a negative impact of DM on sleep quality in men. (C) In female participants, the DM group shows significantly higher PSQI scores than the control group, indicating that the negative impact of DM on sleep quality is consistent across genders. (D) In younger participants (< 45 years), PSQI scores are significantly higher in the DM group, suggesting that younger diabetic patients experience poorer sleep quality. (E) Among middle-aged participants (45–60 years), the DM group also has significantly higher PSQI scores compared to the control group, indicating a significant impact of DM on sleep quality in this age group. (F) In older participants (> 60 years), PSQI scores remain significantly higher in the DM group, emphasizing a pronounced negative impact of DM on sleep quality in the elderly. (G) Proportion of participants with good and poor sleep quality based on PSQI scores in the control and DM groups. The results show that the proportion of participants with poor sleep quality is higher in the DM group across all age and gender subgroups. (H) Comparison of PSQI subscale scores between the control and DM groups, including sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleeping medications use, and daytime dysfunction. The results show that, except for the sleeping medications use, the DM group scores significantly worse on all subscales, suggesting a broader negative impact of DM on multiple dimensions of sleep quality. PSQI: pittsburgh sleep quality index; DM: diabetes mellitus; Con: control; * P < 0.05; ** P < 0.01; *** P < 0.001.
In gender-stratified analyses, both males and females with DM showed significantly higher PSQI scores than their control counterparts. In male, DM patients had a PSQI score of 6.72 ± 2.95, which was significantly higher than that of the control group (5.26 ± 2.31, t = 4.863, P < 0.001). Similarly, in female, DM patients had a PSQI score of 7.04 ± 3.03, significantly higher than control group (5.34 ± 2.58, t = 5.602, P < 0.001) (Fig. 2B-C). Similarly, age-stratified analysis showed significantly elevated PSQI scores in the DM group across all age categories. In the young (< 45 years), middle-aged (45–60 years), and older (> 60 years) groups, PSQI scores in the DM group were significantly higher than that in the control group (t = 4.287, P < 0.001; t = 5.300, P < 0.001; t = 3.126, P = 0.002, respectively) (Fig. 2D-F).
Additionally, the proportion of participants with good sleep quality was significantly higher in the control group compared to the DM group (63.0% vs. 38.7%, χ2 = 37.203, P < 0.001). Among males, good sleep quality was observed in 62.6% of controls versus 36.9% of DM patients (χ2 = 21.101, P < 0.001). Among females, the corresponding proportions were 63.3% versus 40.9% (χ2 = 15.302, P < 0.001) (Fig. 2G). Across all age groups, a significantly smaller proportion of DM participants reported good sleep quality compared to controls (P < 0.05 for all subgroups) (Fig. 2G).
A further analysis of the six dimensions of the PSQI scale (including sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, and daytime dysfunction) revealed significant differences between the control and DM groups, with the DM group performing worse in each dimension. However, no significant difference was found between the two groups in the use of sleep medications (Z = 1.114, P = 0.265) (Fig. 2H).
These findings demonstrate that individuals with DM experience significantly impaired sleep quality compared to non-diabetic individuals. This effect is consistent across both genders and all age categories, with the most pronounced impact observed among female patients. These patterns highlight the importance of addressing sleep disturbances as part of comprehensive diabetes care.
The relationship between sleep quality and visual function
To investigate the association between sleep quality and visual function, we examined the relationship between PSQI and VFQ scores using both regression and correlation analyses. The analysis revealed that for every one-point increase in PSQI score, the overall VFQ score decreased by 3.40 points in the overall study population (R2 = 0.261) (Fig. 3A). Subgroup analyses showed similar trends. In the control group, each additional PSQI point corresponded to a 2.62-point decrease in VFQ score (R2 = 0.174), while in the DM group, the decrease was 3.26 points (R2 = 0.243) (Fig. 3B–C), suggesting greater sensitivity of visual function to sleep disturbances among diabetic patients.
Scatter plots of PSQI scores and overall VFQ scores. (A) Scatter plot showing the relationship between PSQI scores and overall VFQ scores across all participants. A significant negative correlation is observed, indicating that higher PSQI scores are associated with lower overall VFQ scores (R2 = 0.2607, P < 0.001), suggesting that poorer sleep quality is linked to worse visual function. (B) In the control group, a negative correlation between PSQI scores and overall VFQ scores is also observed (R2 = 0.1739, P < 0.001). This suggests that even in non-DM individuals, reduced sleep quality adversely affects visual function. (C) In the DM group, the negative correlation between PSQI scores and overall VFQ scores is more pronounced (R2 = 0.2427, P < 0.001). Compared to the control group, the impact of poor sleep quality on visual function is greater in the DM group, indicating that DM patients are more sensitive to the effects of sleep quality on visual function. PSQI: pittsburgh sleep quality index; VFQ: visual function questionnaire; DM: diabetes mellitus; Con: control.
Spearman correlation analysis was conducted to further evaluate the relationship between PSQI scores and overall VFQ scores, as well as their individual dimensions. As shown in Table 3, the Spearman correlation coefficient between PSQI scores and overall VFQ scores was − 0.467 in the DM group and − 0.382 in the control group. Both correlations were statistically significant and indicated a moderate negative association, with a stronger effect observed in the diabetic population. This pattern supports the hypothesis that visual function in individuals with DM may be more adversely affected by declines in sleep quality, potentially due to underlying metabolic and neurovascular vulnerabilities. In the control group, PSQI scores were significantly correlated with 10 out of 12 dimensions of quality of life, excluding General Health and Ocular Pain. In contrast, in the DM group, PSQI scores were significantly correlated with 11 out of 12 dimensions, except for the General Vision dimension. These results indicated that poorer sleep quality is associated with lower overall VFQ scores. However, this correlation did not significantly differ between the DM and control groups.
The findings underscore the impact of sleep quality on visual function and highlight the potential significance of this relationship in DM patients. These results suggest that clinicians should consider the influence of sleep quality on visual function when managing patients with DM.
The mediating role of sleep quality in the relationship between DM and visual function
To further investigate the mediating role of sleep quality in the relationship between DM and visual function, a path model was constructed. In this model, DM status was included as the independent variable, overall VFQ score as the dependent variable, and PSQI score as the mediating variable. Gender, age, and educational level were included as covariates. The model was adjusted based on modification indices. The optimal path model is shown in Fig. 4. The path analysis indicated good model fit, with χ2/df = 1.135, TLI = 0.995, CFI = 0.999, and RMSEA (95% CI) = 0.015 (0.001–0.064).
Standardized path model illustrating the relationships among DM, PSQI scores, and overall VFQ scores. This standardized path model illustrates the relationships among DM, PSQI scores, and overall VFQ scores, while controlling for gender, age, and educational level. Path coefficients are displayed alongside the arrows. The diagram shows that DM has a direct negative effect on overall VFQ scores (β = -0.170) and an indirect negative effect mediated by PSQI scores (β=-0.453 * 0.274 = -0.124). Error terms (e1, e2, e3) are included to account for the unexplained variance in PSQI and VFQ scores. *P < 0.05, ** P < 0.01, *** P < 0.001.
The analysis revealed that both DM (β = -0.170, P = 0.001) and poor sleep quality (β = -0.453, P < 0.001) had significant negative effects on visual function. Additionally, PSQI scores were significantly higher in the DM group compared to the control group, indicating a notable reduction in sleep quality among DM patients (β = 0.274, P < 0.001). Further analysis showed that the total effect of DM on visual function was − 0.294, with a direct effect of -0.170 and an indirect effect of -0.124 mediated through sleep quality. The mediating effect accounted for 42.2% of the total effect. This model explained 29.7% of the total variance in overall VFQ scores.
These findings suggest that individuals with DM experience both reduced sleep quality and impaired visual function, and that sleep quality significantly mediates the adverse impact of DM on vision. In other words, poor sleep quality amplifies the negative effects of DM on visual outcomes. This underscores the importance of improving sleep quality, in addition to routine glycemic control, as a key factor in mitigating the decline in visual function in diabetic patients.
Discussion
This study demonstrates that DM has a significant negative impact on visual function and that sleep quality plays a critical mediating role in this relationship. Individuals with DM exhibited lower VFQ scores than non-diabetic controls, indicating a decline in vision-related quality of life. Moreover, poor sleep quality significantly exacerbated this decline, highlighting a synergistic interaction between metabolic and neurobehavioral factors in visual impairment. These findings contribute to the growing body of literature by offering novel evidence of the indirect pathway through which sleep disturbances influence diabetes-related visual dysfunction.
This study identified sleep disturbances as a significant modulating factor. Our data indicate that diabetic patients with poor sleep quality have significantly lower overall VFQ scores than those with good sleep quality. Path analysis further revealed that sleep disturbances mediate the relationship between DM and visual function. A decline in sleep quality not only directly impacts overall health but may also exacerbate DM-related visual function decline through complex pathological mechanisms.
Biologically, persistent hyperglycemia induces retinal damage through multiple synergistic pathways, increases vascular permeability, and promotes retinal edema and neurodegeneration through chronic low-grade inflammation17,18. Firstly, activation of the polyol pathway increases sorbitol accumulation, triggering osmotic stress and endothelial cell apoptosis. Secondly, advanced glycation end products bind to their receptors, activating NF-κB signaling and amplifying pro-inflammatory cytokines (e.g., IL-6, TNF-α) that disrupt blood-retinal barrier integrity. Concurrently, chronic hyperglycemia promotes mitochondrial dysfunction in retinal ganglion cells, leading to excessive reactive oxygen species production and caspase-3-mediated apoptosis. These cascades collectively contribute to microvascular leakage, neuroinflammation, and progressive visual field loss17,18,19. The density of retinal ganglion cells is significantly reduced in diabetic patients, which closely correlates with visual function decline20. This neurovascular damage extends to the optic nerve and central pathways, impairing visual signal transmission.
Against this pathological backdrop, DM-related sleep quality decline may further worsen visual function. Diabetic patients frequently suffer from sleep disorders, including obstructive sleep apnea, insomnia, and circadian rhythm disturbances21. These sleep disorders not only impact overall health but also exacerbate retinal damage through various mechanisms. It was reported that patients with insufficient sleep and poor sleep quality experience more severe retinal microvascular damage22,23. Poor sleep exacerbates retinal hypoxia, oxidative stress, and systemic inflammation24,25. Sleep-related autonomic dysregulation and elevated cortisol further aggravate glycemic instability and vascular dysfunction, compounding the burden of diabetic retinopathy26.
Gender and age subgroup analyses revealed that females and older adults were particularly vulnerable to sleep-related visual impairment. Hormonal fluctuations, particularly in estrogen and progesterone, are known to impair both sleep and retinal neuroprotection27. A decline in estrogen levels, such as during menopause, can lead to poorer sleep quality28. The neuroprotective effects of estrogen on the retina suggest that its reduction can accelerate visual function decline29. The higher prevalence of mood disorders among women may further intensify sleep problems and associated visual decline30.
The increased sensitivity of visual function to sleep quality in elderly diabetic patients likely relates to age-associated physiological degeneration. Retinal function deteriorates with age, marked by reduced blood flow, fewer ganglion cells, and lower photoreceptor density31,32. This degeneration reduces elderly patients’ resilience to external factors, such as poor sleep quality. Common changes in sleep architecture in the elderly, such as reduced slow-wave and rapid eye movement sleep, decrease the restorative functions of sleep, exacerbating visual impairment33. Metabolic functions in elderly diabetic patients decline, leading to more pronounced blood glucose fluctuations, further affecting retinal health. These gender- and age-related findings suggest that elderly and female diabetic patients should be considered high-risk subgroups. Regular monitoring of sleep quality and timely intervention may be essential in preserving visual function in these populations.
Compared with previous studies, this research provides further evidence for the co-occurrence of visual function decline and impaired sleep quality in individuals with DM. This multidimensional perspective suggest that diabetes affects not only retinopathy-related domains but also broader aspects such as general health and social functioning, potentially reflecting the systemic pathological effects of DM. While prior studies have linked sleep problems with both DM and visual issues independently34,35our study extends these findings by identifying the mediating role of sleep quality in the diabetes-visual function relationship using path analysis, offering novel mechanistic insights into diabetes-related complications.
From a clinical perspective, these results advocate for the routine assessment of sleep quality in diabetic patients—particularly those presenting with visual symptoms. Interventions such as sleep hygiene education, cognitive-behavioral therapy for insomnia (CBT-I), and pharmacologic agents like melatonin may serve as adjunct strategies to prevent vision decline36,37,38. In ophthalmology, incorporating sleep and glycemic history into visual impairment workups may improve diagnostic accuracy and individualize treatment strategies. From a public health standpoint, integrating sleep health into diabetes management protocols may reduce the burden of diabetic visual impairment. Community-based sleep education and screening initiatives could enhance awareness and early detection of at-risk individuals, especially among aging and female populations.
Despite these contributions, several limitations should be acknowledged. First, the cross-sectional design prevents causal inference. Second, recruitment from a single tertiary hospital in central China may limit generalizability. Third, although major confounders were controlled for, residual bias from unmeasured variables (e.g., glycemic control, comorbid conditions) may remain. Fourth, the absence of objective ophthalmic evaluations such as optical coherence tomography (OCT) limits pathophysiologic interpretation. Lastly, reliance on self-reported PSQI and VFQ instruments may introduce response bias.
To address these limitations, future research should employ longitudinal designs, include multi-center and multi-ethnic samples, and integrate objective sleep and vision measures. Mechanistic studies investigating biomarkers (e.g., inflammatory cytokines, oxidative stress indicators), neurovascular coupling, and autonomic function are also warranted. Moreover, interventional trials targeting sleep improvement in diabetic patients could help determine whether such strategies directly reduce the risk of visual deterioration.
Conclusion
This study provides robust evidence that diabetes mellitus significantly impairs visual function and that sleep quality is a key mediating factor in this relationship. Patients with DM and poor sleep experienced more severe visual deterioration, highlighting sleep as a modifiable risk factor in diabetes-related visual impairment. These findings emphasize the need to incorporate sleep assessment and intervention into routine diabetes care. Addressing sleep disturbances may not only improve overall health but also preserve visual function and enhance quality of life. Future research should validate these insights through longitudinal and interventional studies and explore their generalizability across diverse populations.
Data availability
All relevant data are included in the papers. Contact to corresponding author for additional information regarding data access.
Abbreviations
- DM:
-
Diabetes mellitus
- DR:
-
Diabetic retinopathy
- NEI-VFQ-25:
-
National Eye Institute Visual Function Questionnaire-25
- VFQ:
-
Visual function questionnaire
- PSQI:
-
Pittsburgh Sleep Quality Index
- OGTT:
-
Oral glucose tolerance test
- HbA1c:
-
Hemoglobin A1c
- ANOVA:
-
One-way analysis of variance
- TLI:
-
Tucker-Lewis Index
- CFI:
-
Comparative Fit Index
- RMSEA:
-
Root Mean Square Error of Approximation
References
International diabetes federation diabetes atlas. 10th Edition. [Internet] (2021, accessed 13 Aug 2024). http://www.diabetesatlas.org/.
Azharuddin, M., Kapur, P., Adil, M., Ghosh, P. & Sharma, M. Health-related quality of life and sleep quality among North Indian type 2 diabetes mellitus patients: evidence from a cross-sectional study. Sleep Med. 73, 93–100. https://doi.org/10.1016/j.sleep.2020.04.022 (2020).
Birhanu, T. T., Hassen Salih, M. & Abate, H. K. Sleep quality and associated factors among diabetes mellitus patients in a Follow-Up clinic at the university of Gondar comprehensive specialized hospital in gondar, Northwest ethiopia: a Cross-Sectional study. Diabetes Metabolic Syndrome Obesity: Targets Therapy. 13, 4859–4868. https://doi.org/10.2147/dmso.s285080 (2020).
Morjaria, R. et al. Impact of diabetic retinopathy on sleep, mood, and quality of life. Investig. Ophthalmol. Vis. Sci. 60, 2304–2310. https://doi.org/10.1167/iovs.18-26108 (2019).
Ponomareva, M., Petrova, Y. & Gribanova, E. Visual prognosis and sleep quality in patients with chronic kidney disease. Med. Sci. Educ. Ural. 22, 63–66. https://doi.org/10.36361/1814-8999-2021-22-3-63-66 (2021).
Liao, K. M., Wu, W. C., Jang, Y., Su, F. Y. & Tsai, L. T. Impacts of monocular, binocular, and functional visual acuity on vision-related quality of life in patients with type 2 diabetes. Sci. Rep. 11, 298. https://doi.org/10.1038/s41598-020-79483-9 (2021).
Ibrahim, H., Hashim, Z. & Desher, Y. Sleep quality assessment in correlation to autonomic nerve function in type 2 diabetic patients. Sleep. Med. Res. 14, 141–148. https://doi.org/10.17241/smr.2023.01802 (2023).
Promsod, O. et al. Impact of irregular sleep pattern, and sleep quality on glycaemic parameters and endothelial function in adolescents and young adults with type 1 diabetes. J. Sleep Res. 33, e14110. https://doi.org/10.1111/jsr.14110 (2024).
Zhang, A. The role of sleep quality in the pathogenesis and management of type 2 diabetes. Theoretical Nat. Sci. https://doi.org/10.54254/2753-8818/15/20240472 (2023).
Lee, J. S., Lin, K. K., Hou, C. H., Li, P. R. & See, L. C. Chinese version of the Vision-Related quality of life (NEI-VFQ-25) among patients with various ocular disorders: A pilot study. Med. (Kaunas Lithuania). 58, 859. https://doi.org/10.3390/medicina58050602 (2022).
Wang, C. W., Chan, C. L. & Jin, H. Y. Psychometric properties of the Chinese version of the 25-item National eye Institute visual function questionnaire. Optometry Vis. Science: Official Publication Am. Acad. Optometry. 85, 1091–1099. https://doi.org/10.1097/OPX.0b013e31818b9f23 (2008).
Ho, K. Y. et al. Psychometric properties of the Chinese version of the Pittsburgh sleep quality index (PSQI) among Hong Kong Chinese childhood cancer survivors. Health Qual. Life Outcomes. 19, 176. https://doi.org/10.1186/s12955-021-01803-y (2021).
Zheng, B., Li, M., Wang, K. L. & Lv, J. Analysis of the reliability and validity of the Chinese version of Pittsburgh sleep quality index among medical college students. Beijing Da Xue Xue Bao Yi Xue ban = J. Peking Univ. Health Sci. 48, 424–428 (2016).
Backhaus, J., Junghanns, K., Broocks, A., Riemann, D. & Hohagen, F. Test-retest reliability and validity of the Pittsburgh sleep quality index in primary insomnia. J. Psychosom. Res. 53, 737–740. https://doi.org/10.1016/s0022-3999(02)00330-6 (2002).
Li, Y. et al. Prevalence of diabetes recorded in Mainland China using 2018 diagnostic criteria from the American diabetes association: National cross sectional study. BMJ (Clinical Res. ed.). 369, m997. https://doi.org/10.1136/bmj.m997 (2020).
Jia, W. et al. Standards of medical care for type 2 diabetes in China 2019. Diab./Metab. Res. Rev. 35, e3158. https://doi.org/10.1002/dmrr.3158 (2019).
Aiello, L. P., Cahill, M. T. & Wong, J. S. Systemic considerations in the management of diabetic retinopathy. Am. J. Ophthalmol. 132, 760–776. https://doi.org/10.1016/s0002-9394(01)01124-2 (2001).
Li, H. et al. Research progress on the pathogenesis of diabetic retinopathy. BMC Ophthalmol. 23, 586. https://doi.org/10.1186/s12886-023-03118-6 (2023).
Stitt, A. W. et al. The progress in Understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 51, 156–186. https://doi.org/10.1016/j.preteyeres.2015.08.001 (2016).
Antonetti, D. A., Klein, R. & Gardner, T. W. Diabetic retinopathy. N. Engl. J. Med. 366, 1227–1239. https://doi.org/10.1056/NEJMra1005073 (2012).
Reutrakul, S. & Van Cauter, E. Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab. Clin. Exp. 84, 56–66. https://doi.org/10.1016/j.metabol.2018.02.010 (2018).
Jee, D., Keum, N., Kang, S. & Arroyo, J. G. Sleep and diabetic retinopathy. Acta Ophthalmol. 95, 41–47. https://doi.org/10.1111/aos.13169 (2017).
Zheng, Z. et al. Meta-Analysis of relationship of sleep quality and duration with risk of diabetic retinopathy. Front. Endocrinol. 13, 922886. https://doi.org/10.3389/fendo.2022.922886 (2022).
Chiang, J. F. et al. Association between obstructive sleep apnea and diabetic macular edema in patients with type 2 diabetes. Am. J. Ophthalmol. 226, 217–225. https://doi.org/10.1016/j.ajo.2021.01.022 (2021).
Zhu, Z. et al. Relationship of obstructive sleep Apnoea with diabetic retinopathy: a Meta-Analysis. Biomed. Res. Int. 2017, 4737064. https://doi.org/10.1155/2017/4737064 (2017).
Khandelwal, D., Dutta, D., Chittawar, S. & Kalra, S. Sleep disorders in type 2 diabetes. Indian J. Endocrinol. Metabol. 21, 758–761. https://doi.org/10.4103/ijem.IJEM_156_17 (2017).
Kische, H. et al. Sex hormones and sleep in men and women from the general population: A Cross-Sectional observational study. J. Clin. Endocrinol. Metab. 101, 3968–3977. https://doi.org/10.1210/jc.2016-1832 (2016).
Freeman, E. W. et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch. Gen. Psychiatry. 61, 62–70. https://doi.org/10.1001/archpsyc.61.1.62 (2004).
Vingerling, J. R. et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology 102, 205–210. https://doi.org/10.1016/s0161-6420(95)31034-2 (1995).
Bangasser, D. A. & Cuarenta, A. Sex differences in anxiety and depression: circuits and mechanisms. Nat. Rev. Neurosci. 22, 674–684. https://doi.org/10.1038/s41583-021-00513-0 (2021).
Chen, M., Luo, C., Zhao, J., Devarajan, G. & Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 69, 159–172. https://doi.org/10.1016/j.preteyeres.2018.10.003 (2019).
Arnold, E., Thébault, S., Aroña, R. M., de la Martínez, G. & Clapp, C. Prolactin mitigates deficiencies of retinal function associated with aging. Neurobiol. Aging. 85, 38–48. https://doi.org/10.1016/j.neurobiolaging.2019.10.002 (2020).
Li, J., Vitiello, M. V. & Gooneratne, N. S. Sleep in normal aging. Sleep Med. Clin. 13, 1–11. https://doi.org/10.1016/j.jsmc.2017.09.001 (2018).
Briançon-Marjollet, A. et al. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol. Metab. Syndr. 7, 859. https://doi.org/10.1186/s13098-015-0018-3 (2015).
Shan, Z. et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 38, 529–537. https://doi.org/10.2337/dc14-2073 (2015).
van Straten, A. et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med. Rev. 38, 3–16. https://doi.org/10.1016/j.smrv.2017.02.001 (2018).
Irish, L. A., Kline, C. E., Gunn, H. E., Buysse, D. J. & Hall, M. H. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med. Rev. 22, 23–36. https://doi.org/10.1016/j.smrv.2014.10.001 (2015).
Pourhanifeh, M. H., Hosseinzadeh, A., Dehdashtian, E., Hemati, K. & Mehrzadi, S. Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol. Metab. Syndr. 12, 526. https://doi.org/10.1186/s13098-020-00537-z (2020).
Acknowledgements
We thank all members of our study team for their whole-hearted cooperation and data collection.
Funding
This work was funded by the Ministry of Science and Technology of the People’s Republic of China [grant number 2018YFC0114500]; the Henan Provincial Medical Science and Technology Research Joint Co-construction Project [grant number LHGJ20210071 and LHGJ20220085]; the Basic Science Project of Henan Eye Institute/Henan Eye Hospital [grant number 22JCQN004 and 21JCZD001]. No specific grants from public, commercial, or non-profit funding agencies were received for this research.
Author information
Authors and Affiliations
Contributions
Y.Y. and Z.L. conceived the study. Y.Y. and P.X. contributed to data interpretation, data analysis, and manuscript drafting. M.X., J.S., L.T., and Y.K. were responsible for data collection. Z.L. provided oversight and contributed to the writing, review, and editing processes. All authors have read and approved the final manuscript. All authors have consent for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Y., Pei, X., Sun, J. et al. Mediating role of sleep quality in the relationship between diabetes mellitus and visual function: a cross-sectional study. Sci Rep 15, 20566 (2025). https://doi.org/10.1038/s41598-025-06641-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-06641-2