Abstract
Acute kidney injury (AKI) is a frequent complication in acute heart failure (AHF) patients, yet few studies have examined the prognostic implications of different dynamic KDIGO AKI stages in this population. This retrospective cohort study aimed to rigorously investigate the impact of dynamic KDIGO AKI staging on outcomes in ICU patients with AHF using robust statistical methods like propensity score matching and doubly robust analysis. Utilizing the MIMIC-IV database of 5136 adult AHF patients from 2008 to 2022, doubly robust analysis revealed no significant differences in 28-day, 180-day, and 1-year mortality between non-AKI and mild AKI groups. However, moderate-to-severe AKI had significantly higher mortality at all timepoints compared to normal or mild AKI. Patients exhibiting mild AKI, termed “subclinical AKI” as their prognosis often parallels non-AKI, face a markedly worsened outlook if their condition progresses to moderate-to-severe AKI during hospitalization. However, it is worth noting that further sensitivity analysis showed that in patients with AHF as the first diagnosis, transient AKI caused by intensive decongestion therapy and other interventions did not have a significant impact on long-term outcomes.
Similar content being viewed by others
Introduction
Recent years have seen increased focus on the relationship between cardiovascular conditions, such as heart failure (HF), acute myocardial infarction (AMI), and cardiovascular surgery, and the development of acute kidney injury (AKI), due to the significant impact of AKI on patient outcomes and prognosis1. In a state of physiological homeostasis, the heart and kidneys engage in a synergistic interplay, collectively regulating vital parameters such as blood pressure, electrolyte balance, and fluid homeostasis. However, in pathological states, this intricate crosstalk can be disrupted, leading to a deleterious reciprocal exacerbation of cardiac and renal dysfunction, culminating in clinical decompensation. When acute cardiac decompensation precipitates AKI, this clinical entity is termed cardiorenal syndrome type 12.
The pathophysiology of cardiorenal syndrome (CRS) is driven by a complex interplay of hemodynamic and non-hemodynamic factors, resulting in reciprocal cardiac and renal injury. Key contributors include shared risk factors such as hypertension, diabetes, atherosclerosis, and chronic inflammation, all of which accelerate disease progression3. Hemodynamic disturbances, including venous congestion and increased intra-abdominal pressure, lead to reduced renal perfusion, impaired glomerular filtration, and activation of the renin–angiotensin–aldosterone system (RAAS), further deteriorating renal function4,5. Non-hemodynamic mechanisms involve neurohormonal dysregulation, oxidative stress, and inflammation, which promote chronic renal hypoxia, tissue injury, and fibrosis6,7,8. Proinflammatory cytokines such as TNF-α, IL-1, and IL-6 are pivotal in mediating cardiac and renal remodeling9. Endothelial dysfunction exacerbates this cycle by impairing vasodilation, increasing vascular permeability, and promoting thrombosis and atherosclerosis10. These processes collectively contribute to a self-perpetuating cycle of organ dysfunction, driving the progression of CRS11.
The incidence of AKI in heart failure patients varies significantly across studies, ranging from 25 to 70%. This variability may be attributed to differences in the definitions of worsening renal function (WRF) used in these studies12. There is currently no international consensus on the definition of AKI in patients with heart failure13. In the literature, AKI is typically defined using creatinine, serum cystatin C, and estimated glomerular filtration rate (eGFR) levels. However, there is considerable variability in how changes in these markers are quantified within the definitions of AKI14.
Recent expert consensus statements and multiple studies indicate that “kidney injury” is largely absent in most acute heart failure (AHF) patients. The more appropriate term is “kidney dysfunction,” which remains neutral regarding the presence or persistence of injury. This is because all definitions used to estimate kidney function assume a steady state, a condition that does not exist in the dynamic clinical setting of acute decompensated heart failure15. It is critical to note that the prognostic impact of kidney dysfunction depends on actual renal damage rather than the historical labeling of “kidney injury” based primarily on serum creatinine (sCr) concentration or estimated glomerular filtration rate (eGFR) changes14.
Currently, the most widely used criteria for diagnosing AKI include the RIFLE criteria16, AKIN criteria (a later version of the RIFLE classification)17, and KDIGO guidelines18. Some researchers have attempted to use three criteria to predict patient prognosis. Studies conducted across different populations have demonstrated that the KDIGO criteria offer a superior ability to predict mortality compared to the RIFLE and AKIN criteria19,20,21.
The KDIGO criteria build upon and integrate key components of both the RIFLE and AKIN systems, creating a more unified and comprehensive approach to defining AKI20. By harmonizing these frameworks, KDIGO reduces inconsistencies in diagnosis and enhances early AKI detection, with increased sensitivity particularly in the initial stages of the condition21. Its three-stage classification model simplifies practical use without compromising the diagnostic precision of earlier, more intricate systems such as RIFLE. While the diagnosis of AKI often depends on acute elevations in serum creatinine and reductions in urine output (UO), the latter is frequently underutilized in clinical practice, despite robust evidence supporting its critical diagnostic and prognostic significance22. Currently, no definitive pharmacological intervention has been identified for the prevention of AKI. Preventive strategies emphasize the importance of identifying and assessing individuals at high risk, mitigating potential triggers, and implementing dynamic monitoring. This approach facilitates the early detection and timely management of AKI23. The adoption of more dynamic KDIGO criteria may offer an advantage in detecting AKI in patients with AHF. Nevertheless, limited studies have investigated the influence of dynamic KDIGO staging on both short- and long-term outcomes in patients with AMI after discharge. Additionally, it remains unclear whether AKI in its early stages affects prognosis. Therefore, this study aims to thoroughly analyze outcomes in AHF patients across different dynamic KDIGO AKI stages to uncover the prognostic implications of each stage.
Methods
Study design
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, as outlined in the supplementary materials. Its primary objective is to evaluate both the short- and long-term prognostic impacts of mild and moderate-to-severe AKI on ICU patients with acute heart failure, using real-time monitoring of AKI based on KDIGO criteria. The MIMIC-IV KDIGO model dynamically assesses AKI stages: It first stages AKI based on creatinine levels by comparing the current serum creatinine with the lowest values in the past 7 days and 48 h, assigning stages based on relative increases: Stage 3 for creatinine at least three times the baseline or greater than 4.0 mg/dL with an acute increase, Stage 2 for at least double the baseline, and Stage 1 for an increase of at least 1.5 times the baseline or a rise of 0.3 mg/dL within 48 h. For urine output, stages are determined using rates over 6, 12, and 24 h, with Stage 3 defined as anuria for at least 12 h or less than 0.3 mL/kg/h for 24 h, Stage 2 as less than 0.5 mL/kg/h for 12 h, and Stage 1 as less than 0.5 mL/kg/h for 6 to 12 h. If a patient receives CRRT, they are automatically assigned Stage 3. The highest stage from the creatinine, urine output, and CRRT evaluations is chosen as the final AKI stage, and to address fluctuations due to missing data, a 6-h rolling maximum is applied to smooth the AKI stage over time. This method enhances the sensitivity of AKI detection, enabling earlier identification and more accurate classification of kidney injury. The project received approval from the institutional review boards of both the Massachusetts Institute of Technology (MIT) and Beth Israel Deaconess Medical Center (BIDMC), with informed consent waived.
This retrospective observational study utilized data from the Medical Information Mart for Intensive Care IV (MIMIC-IV, version 3.0) database, an updated iteration of MIMIC-III, which includes comprehensive critical care data for ICU patients at Beth Israel Deaconess Medical Center (BIDMC) from 2008 to 2022. The database contains detailed patient records, including laboratory measurements, medications administered, vital signs, and more. Author PG, after completing the necessary data usage agreement and Collaborative Institutional Training Initiative (CITI) certification, was granted access to the database. As all patient data is de-identified, informed consent was not required24.
Study population
Inclusion criteria: (1) Patients aged 18 years or older; (2) AHF listed among the top three discharge diagnoses. Exclusion criteria: (1) Non-first hospitalization; (2) Absence of ICU records; (3) ICU stay of less than one day.
The study is structured in two parts. In the first part, patients classified under KDIGO AKI stages 0 and 1 were grouped as Non-AKI and Mild-AKI, respectively. In the second part, all patients were included, with those in KDIGO AKI stages 0 and 1 categorized as Normal-or-mild-AKI, and those in stages 2 and 3 classified as Moderate-to-severe-AKI.
Data extraction and preprocessing
Data extraction was performed using PostgreSQL 14 and SQL queries (Berkeley, California, USA). The extracted dataset included patient demographics, ICU length of stay, complications, laboratory test results, treatments, and other relevant clinical information. All laboratory results were taken from the first tests conducted upon ICU admission, as these initial values are available sooner and facilitate timely assessment using clinical prediction models. The eGFR was calculated utilizing the 2012 CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula25. The parameter κ is 0.9 for males and 0.7 for females, while α is − 0.411 for males and − 0.329 for females. The sex coefficient is 1.018 for females and 1 for males, and the race coefficient is 1.159 for African Americans and 1 for other races. When SCr/κ ≤ 1, the α exponent is applied; when SCr/κ > 1, the exponent − 1.209 is used instead.
Endpoint
The endpoints were 28-day mortality, 180-day mortality, and 1-year all-cause mortality.
Statistical methods
The normality of continuous variables was evaluated using the Kolmogorov–Smirnov test. Variables with a normal distribution were expressed as mean ± standard deviation, while those with a non-normal distribution were presented as median and interquartile range [IQR, M (P25, P75)]. Levene’s test was applied to assess the homogeneity of variances. For comparisons between two groups, Student’s t-test was used if the continuous variables exhibited both normality and homogeneity of variances; otherwise, the Mann–Whitney U test was employed. Fisher’s exact test was used for categorical variables when the sample size was below 40, and the Chi-square test was applied for larger sample sizes. Categorical variables were reported as frequencies and percentages. Multiple imputation using the ‘mice’ package in R was performed to handle missing data, excluding variables with over 20% missing values from imputation and model construction. To ensure the robustness of imputation, 100 imputations were performed.
The doubly robust estimation method was applied to determine the independent associations between the occurrence of AKI in patients with AHF and their prognosis. This approach, also referred to as survey-weighted generalized linear models, combines a multivariate regression model with a propensity score model to assess both the correlation and causal effects of an exposure on an outcome26. When using either a regression model or a propensity score model individually, unbiased causal estimates are only achieved if the respective model is correctly specified. The doubly robust estimator, however, ensures an unbiased effect estimate even if only one of the models is correctly specified.
The gradient boosted model (GBM) was employed to estimate propensity scores for AKI, aiming to reduce covariate imbalance between the Non-AKI and Mild-AKI groups, as well as between the Normal-or-mild-AKI and Moderate-to-severe-AKI groups. GBM, a machine learning algorithm, iteratively builds and integrates multiple models into an ensemble to improve the accuracy of response variable predictions. The core principle of GBM is the sequential construction of models that are strongly correlated with the negative gradient of a specified loss function. In this study, regression trees served as the base learners for GBM, incorporating a total of 39 covariates27. To identify variables significantly associated with the study outcome, the Boruta feature selection method was employed. This technique determines the importance of variables by comparing actual features with randomly generated “shadow features.” Specifically, the Boruta package in R was used to perform this selection. This method ensures that the selected features hold statistical significance while minimizing noise or redundancy that could arise from including excessive variables in the model.
An Inverse Probability of Treatment Weighting (IPTW) model was utilized to create a weighted cohort based on the estimated propensity scores. Covariate imbalances between the original and adjusted cohorts were evaluated to determine the propensity score model’s effectiveness in balancing the compared groups, with standardized mean differences (SMDs) calculated to quantify group differences. Logistic regression or Cox regression was then applied to this weighted cohort, adjusting for variables that remained unbalanced. This analysis, referred to as doubly robust, was performed using the ‘survey’ package, with logistic regression conducted through the ‘stats’ package. The Cox proportional hazards models were fitted using the ‘survival’ package, which also facilitated testing the proportional hazards (PH) assumption via functions like cox.zph. When time-dependent covariates violated the PH assumption, appropriate transformations, such as time-dependent covariate effects or stratification, were implemented to allow for more accurate hazard ratio estimation and improved model fit.
Statistical analyses were conducted using R software (version 4.4.1; R Foundation for Statistical Computing, Vienna, Austria). All tests were two-tailed, with a significance level set at P < 0.05.
Sensitivity analysis
We performed a series of sensitivity analyses to assess the robustness of the study findings and to explore the potential impact of using different association inference models on our conclusions. For the outcome of 28-day mortality, we employed several models, including a Log-rank test, a multivariate logistic regression adjusted for Boruta selected covariates, a multivariate logistic regression adjusted for unbalanced covariates, and a survey-weighted GLM adjusted for Boruta selected covariates and unbalanced covariates using inverse probability of treatment weighting (IPTW). For the outcomes of 180-day mortality and 1-year mortality, we applied a Log-rank test, a multivariate Cox regression adjusted for Boruta selected covariates, a multivariate Cox regression adjusted for unbalanced covariates, and a survey-weighted Cox model adjusted for Boruta selected covariates and unbalanced covariates using IPTW. The effect sizes and p-values from all these models were reported and compared. Additionally, to further explore whether there were differences in prognosis between patients with AHF as their first primary diagnosis and the main patient population of this study, we conducted a separate prognostic analysis on the subgroup of patients with AHF as their first primary diagnosis.
Results
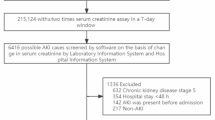
A total of 5,136 patients were included in the study, as depicted in Fig. 1. During the first phase, 979 patients were classified into the Non-AKI group, and 1,179 were allocated to the Mild-AKI group. After propensity score matching (PSM), each group comprised 576 patients, as detailed in Table 1 and Supplementary Tables S1–S4. In the second phase, 2,158 patients were grouped into the Normal-or-mild-AKI category, while 2,978 were assigned to the Moderate-to-severe-AKI category. Following PSM, both groups included 1,323 patients, as presented in Table 2. Overall, 4,157 patients (80.93% of the cohort) developed AKI. In the AHF first subgroup, 649 patients (75.82% of the subgroup) developed AKI.
Doubly robust analysis and Boruta feature selection
A propensity score model was initially developed using 39 covariates through GBM. Figure 2 illustrates the relative contributions of each covariate to the calculated propensity scores. Figure 2A highlights that the most significant covariates distinguishing the Non-AKI and Mild-AKI groups include eGFR, SOFA score, vasopressor use, loop diuretics use, and BUN, all closely associated with the onset of AKI. Figure 2B shows that the key covariates differentiating the Normal-or-mild-AKI group from the Moderate-to-severe-AKI group are vasopressor use, SOFA score, eGFR, heart rate, and anticoagulant, all of which are strongly linked to the progression to Moderate-to-severe AKI.
Using the estimated propensity scores, IPTW was applied to standardize differences between the Non-AKI and Mild-AKI groups, as well as between the Normal-or-mild-AKI and Moderate-to-severe-AKI groups. Details are presented in Table 1, Fig. 3A,B. In the first analysis, most covariates in the weighted cohorts were comparable or balanced between the Non-AKI and Mild-AKI groups, with some exceptions: SOFA Score, Antiplatelet use, Loop Diuretic use, Vasopressor use, Renal Disease, Potassium, BUN, Creatinine and eGFR. In the second analysis, most covariates were similarly balanced between the Normal-or-mild-AKI and Moderate-to-severe-AKI groups, with exceptions for SOFA Score, Antiplatelet use, β-Blocker use, Loop Diuretic use, Vasopressor use, MAP and WBC.
To address the residual imbalance in covariates within the weighted cohorts, several regression models were constructed using doubly robust estimation.
In the Non-AKI and Mild-AKI groups, Boruta feature selection identified variables such as eGFR, BUN, chloride, bicarbonate, potassium, sodium, platelet count, hemoglobin, WBC, heart rate, renal disease, vasopressor use, IABP use, SOFA score, and age. For the Normal-or-mild-AKI and Moderate-to-severe-AKI groups, the selected variables included CK, TNT, eGFR, BUN, chloride, bicarbonate, potassium, sodium, platelets, hemoglobin, WBC, heart rate, MAP, malignancy, stroke, renal disease, vasopressor use, positive inotropic agents, β-blockers, antiplatelet agents, ACEI/ARB, IABP use, continuous renal replacement therapy, coronary artery bypass grafting, SOFA score, and age, as detailed in Fig. 4.
Outcomes and sensitivity studies
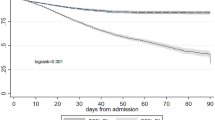
The doubly robust analysis, employing a survey-weighted GLM/Cox model adjusted with Boruta-selected covariates and using IPTW as the primary focus, found no significant differences in short- or long-term outcomes between Non-AKI and Mild-AKI patients (28-day mortality: OR 1.01, 95% CI 0.71–1.44, p = 0.96; 180-day mortality: HR 1.03, 95% CI 0.84–1.26, p = 0.79; 1-year mortality: HR 1.00, 95% CI 0.84–1.18, p = 0.96). In contrast, patients with Moderate-to-severe-AKI had a significantly worse prognosis compared to the Normal-or-mild-AKI group (28-day mortality: OR 2.00, 95% CI 1.74–2.30, p < 0.001; 180-day mortality: HR 1.08, 95% CI 1.04–1.11, p < 0.001; 1-year mortality: HR 1.04, 95% CI 1.02–1.07, p < 0.01). These findings were consistently supported by sensitivity analyses across all estimation models, as detailed in Table 3, Supplementary Table S5–S30, and Fig. 5.
We conducted a comprehensive analysis of this subgroup of patients with AHF as their first diagnosis. The results indicated that there was no significant difference in prognosis between the Non-AKI and Mild-AKI groups (28-day mortality: OR 1.05, 95% CI 0.74–1.48, p = 0.80; 180-day mortality: HR 1.05, 95% CI 0.86–1.27, p = 0.66; 1-year mortality: HR 1.00, 95% CI 0.85–1.19, p = 0.96). When comparing the Normal-or-mild-AKI group with the Moderate-to-severe-AKI group, all five models consistently demonstrated that the Moderate-to-severe-AKI group had a significantly higher risk of 28-day all-cause mortality than the Normal-or-mild-AKI group (28-day mortality: OR 1.85, 95% CI 1.14–3.01, p < 0.05). However, no significant differences were observed between these two groups in terms of 180-day and 1-year mortality analyses (180-day mortality: HR 1.02, 95% CI 0.94–1.10, p = 0.65; 1-year mortality: HR 0.98, 95% CI 0.92–1.04, p = 0.52), as detailed in Table 4.
Subgroup analysis
A subgroup analysis was performed based on age (< 65 or ≥ 65 years), SOFA score (< 5 or ≥ 5), CAD, renal disease status, and whether AHF was the first diagnosis. In the comparison between Non-AKI and Mild-AKI patients, in the renal disease–free subgroup, the risk of 28-day and 180-day all-cause mortality was higher in the Mild-AKI group than in the Non-AKI group. No significant prognostic differences were observed across the other subgroups. When comparing patients with Normal-or-mild-AKI to those with Moderate-to-severe-AKI, the latter group consistently demonstrated poorer outcomes across all subgroups. These results are illustrated in Fig. 6.
Discussion
HF patients experience a complex interplay between cardiac and renal dysfunction, characterized by a bidirectional detrimental interorgan crosstalk28. Concomitant renal impairment among HF patients portends a poor prognosis, with increased hospitalization expenditures, heightened complication rates, and elevated mortality29. The AKI syndrome, defined as an abrupt decline in renal function in HF patients, independently predicts adverse short- and long-term outcomes30. The pathogenesis of AKI is multifactorial, attributable not only to the natural disease trajectory and underlying pathophysiology but also potentially iatrogenic, such as premature decongestion strategies or delayed diuretic cessation31. Consequently, dynamic cardio-renal phenotyping is imperative in HF management to elucidate pathophysiological underpinnings, optimize therapeutic approaches, and risk-stratify patients susceptible to adverse events. Scant evidence exists illuminating the prognostic ramifications of granular, dynamic AKI phenotyping among patients with acute decompensated heart failure. Herein, we sought to bridge this critical knowledge chasm by harnessing robust statistical methodologies, including propensity score matching and doubly robust analyses, to meticulously elucidate the impact of sensitively stratified AKI trajectories on outcomes in this vulnerable population.
Existing literature has documented a conspicuous heterogeneity in the reported incidence of AKI among heart failure patients, with estimates ranging from 25% to an alarming 70%. However, our investigation unveiled a strikingly elevated AKI incidence of 80.93% in patients with acute decompensated heart failure. Furthermore, to mitigate the impact of high patient heterogeneity in the ICU population, we analyzed the incidence of AKI specifically in the subgroup with AHF as the primary diagnosis. The observed AKI incidence rate of 75.82% was comparable to that of the overall ICU cohort. Our analysis suggests that the primary reason may be that as this study includes a cohort of ICU patients, the complexity of their conditions might lead to greater fluctuations in renal function evaluation indices such as creatinine and urine output compared with those in ordinary cohort patients. Secondarily, we postulate that this striking discrepancy from prior findings may be attributable to our use of a more sensitive and granular methodology for AKI identification. Specifically, our study meticulously monitored each laboratory test result and dynamically tracked every fluctuation in fluid intake and output throughout the entire hospitalization period, thereby enabling earlier detection of renal insults and more comprehensive capture of AKI events. This heightened sensitivity in AKI ascertainment, as meticulously delineated in our robust study design, likely constituted a pivotal contributing factor to the elevated incidence rate observed in our cohort.
Emerging evidence indicates a markedly higher incidence of acute cardiorenal syndrome type 1 (CRS-1) episodes identified by the KDIGO criteria in comparison to the RIFLE or AKIN criteria32. Notably, the AKI cases overlooked by the RIFLE or AKIN criteria have been established as independent predictors of in-hospital mortality. These findings underscore the superior prognostic utility of the KDIGO criteria in predicting short-term outcomes in early CRS-1, rendering it a more robust diagnostic tool relative to the RIFLE and AKIN criteria19. The findings accentuate the pivotal role of implementing more sensitive diagnostic modalities, such as the KDIGO criteria, in the timely detection of AKI among this vulnerable patient population.
Compared to patients with Mild-AKI, those Non-AKI used loop diuretics and vasopressors more frequently, and they exhibited lower eGFR, higher SOFA scores, and elevated BUN levels. These five variables were identified as the most significant during the PSM process. The use of loop diuretics, lower eGFR, higher BUN levels, and elevated SOFA scores all indicate poorer renal function. Additionally, the frequent use of vasopressors suggests a higher incidence of hypotensive states, which can lead to renal ischemia and further kidney function deterioration33.
In the PSM process comparing the Normal-or-mild-AKI and Moderate-to-severe-AKI groups, the five most significant variables were vasopressor use, SOFA score, eGFR, heart rate, and anticoagulant use. The first three variables were consistent with the findings in the Non-AKI group. Resting heart rate (RHR) has been established as a significant predictor of both heart failure and renal dysfunction. Firstly, tachycardia serves as a recognized indicator of autonomic dysregulation, characterized by heightened sympathetic activation and attenuated vagal tone. Moreover, the pathogenesis of atherosclerosis and the consequent development of nephrosclerosis are intrinsically linked to alterations in endothelial oxidative stress, a process that exhibits deleterious effects in response to reductions in RHR34. A positive correlation between anticoagulant administration and acute kidney injury has been established. Anticoagulation-related nephropathy (ARN) is an underdiagnosed yet clinically significant complication of anticoagulation therapy, associated with elevated renal morbidity and increased all-cause mortality. ARN represents a recently identified subtype of AKI arising from over-anticoagulation, characterized by profuse glomerular hemorrhage. This pathological entity manifests on renal biopsy as numerous renal tubules replete with erythrocytes and erythrocytic casts35.
Numerous investigations have explored the influence of AKI on the prognosis of patients with AHF. Aletras G et al.36 conducted a prospective study involving 218 AHF patients, defining acute cardiorenal syndrome (ACRS) as an increase in serum creatinine of ≥ 0.3 mg/dL or ≥ 1.5 times the baseline. Their findings indicated that ACRS was associated with higher in-hospital mortality, extended hospitalization, increased use of vasodilators, worsening renal function, and elevated six-month all-cause readmission and mortality rates. Similarly, Ru et al.37 performed a meta-analysis revealing that HF patients who developed AKI had a threefold increased risk of in-hospital death. This elevated risk persisted over a one-year follow-up, and multiple inpatient factors suggested that AKI was common among HF patients. They emphasized that early intervention and treatment are vital to reduce AKI incidence and improve outcomes. Banerjee D et al.38 noted that AKI frequently occurs in patients hospitalized with AHF, often related to renal hypoperfusion and venous congestion. Decongestion therapy during hospitalization may lead to reversible AKI due to hemodynamic changes, but this has a weak association with adverse events such as mortality, consistent with our findings. Thanapongsatorn P et al.39 followed 250 AHF patients over 12 months and found that pseudo-CRS (defined as reversible renal dysfunction) had long-term cardiovascular and renal prognoses comparable to patients without AKI.
Unlike the aforementioned studies, we employed a more sensitive dynamic assessment method based on the KDIGO criteria to evaluate the occurrence of AKI in patients with AMI. As depicted in Table 3, no significant differences in prognosis were observed between the Non-AKI and Mild-AKI groups in the multivariable-adjusted models. Further subgroup analysis identified that patients with Mild-AKI without pre-existing renal disease had worse short-term prognosis at 28 days and 180 days compared to the Non-AKI group. This finding suggests that patients without baseline renal disease may be more susceptible to fluctuations in kidney function. However, it is important to note that this observation was derived from a univariate analysis without adjustment for potential confounding variables. Across all models for the three outcome events (28 days, 180 days, and 1 year), patients with Moderate-to-severe AKI consistently exhibited worse outcomes compared to those with Normal-or-mild AKI. Sensitivity analyses confirmed the robustness of these findings, reinforcing the validity and reliability of our results.
It is important to emphasize that our study focused on an ICU population, which comprises patients with AHF resulting from various etiologies. This mixed-disease cohort inherently exhibits substantial heterogeneity. When AKI arises from causes such as sepsis, nephrotoxic medications, or extended and severe haemodynamic compromise, it may become irreversible and is linked to a worse prognosis38. Therefore, we conducted a comprehensive analysis of patients with AHF as their first diagnosis. Our findings revealed that the Moderate-to-severe-AKI group demonstrated significantly higher 28-day mortality risk compared to the Normal-or-mild-AKI group; however, the mortality rates between these groups were comparable at 180 days and 1 year (Table 4). These findings directly corroborate the recent expert consensus statement which notes that “In patients with acute heart failure, short-term changes in renal function may not accurately predict clinically relevant long-term outcomes”15. This pattern may be attributed to the fact that when AHF is the primary diagnosis, there is typically less interference from comorbidities, and aggressive decongestion therapy is often required. Although these patients may meet the diagnostic criteria for AKI, they may not have experienced actual kidney injury or adverse consequences. For instance, heart failure patients receiving diuretic therapy may exhibit elevations in creatinine (meeting AKI diagnostic criteria) due to reduced blood volume, yet demonstrate normal renal structure on ultrasound examination and present without oliguria or electrolyte disturbances. In such cases, the AKI diagnosis may merely reflect changes in blood volume rather than true kidney injury40. In the subgroup analysis based on whether AHF was the first diagnosis, we observed that, regardless of whether AHF was the first diagnosis or not, there was no significant difference in short- and long-term prognosis between the Mild-AKI group and the Non-AKI group, which is consistent with our results in Table 4. However, when comparing the Moderate-to-severe-AKI group with the Normal-or-mild-AKI group, it appears that whether AHF was the first diagnosis significantly affects both short- and long-term outcomes. It is important to note that this is a univariate result; our Table 4 presents multivariable-adjusted findings (which only significantly impact short-term prognosis). This suggests that confounding factors have a substantial influence on long-term outcomes, so we should not evaluate prognosis solely based on mortality or similar indicators but instead consider the overall condition of each patient for a more comprehensive assessment.
Based on our findings, we propose that in patients with acute heart failure, those with mild AKI, which may be referred to as “subclinical AKI,” only experience stage 1 AKI during hospitalization and do not encounter adverse prognostic effects. Conversely, many patients initially diagnosed with stage 1 AKI progress to stages 2–3, resulting in significantly worse outcomes compared to those who remain at AKI stages 0–1. The dynamic assessment of AKI stages is clinically important, as it facilitates early identification of “subclinical AKI” and enables timely interventions to prevent progression to moderate or severe AKI. It is important to emphasize that this phenomenon is particularly prominent in ICU patients, who often have multiple comorbidities. Sensitivity analysis focusing on patients with AHF as the first diagnosis indicates that only those with progression of AKI to stages 2–3 have a significantly higher 28-day mortality risk compared to those remaining at AKI stages 0–1.
Limitation
While this retrospective analysis from a single center provides valuable insights, the findings may have limited external validity. The retrospective design could potentially introduce selection bias and compromise data reliability. Since this study was conducted on a single-center ICU patient population, the generalizability of the results to broader populations and diverse demographic groups requires further validation. Multi-center prospective studies are necessary to confirm these findings and improve their applicability across different patient groups.
Conclusion
Within the ICU setting, AHF patients presenting with mild AKI demonstrate a clinical course more consistent with “subclinical AKI,” as their outcomes are comparable to those observed in patients who maintain normal renal function. However, the prognostic outlook for those with moderate-to-severe AKI is significantly worse compared to patients with normal renal function or mild AKI. This observation underscores that should “subclinical AKI” transition to moderate-to-severe AKI during the course of hospitalization, the patient’s prognosis would deteriorate markedly. Consequently, the dynamic and sensitive early detection of “subclinical AKI” and its potential progression to moderate-to-severe AKI assumes paramount importance for timely therapeutic intervention and optimized clinical outcomes. Notably, in patients with AHF as the first diagnosis, where transient renal dysfunction may occur due to intensive decongestion therapy, the occurrence of moderate-to-severe AKI did not demonstrate significant impact on intermediate to long-term outcomes.
Data availability
The dataset, code, algorithm files, and de-identified results used in this study are not publicly available. However, the data for this study can be shared upon reasonable request to the corresponding author.
References
Tao, F. et al. Acute kidney injury prediction model utility in premature myocardial infarction. Iscience 27, 109153 (2024).
Rangaswami, J. et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 139, e840–e878 (2019).
McCallum, W. & Testani, J. M. Updates in cardiorenal syndrome. Med. Clin. North Am. 107, 763–780 (2023).
Mullens, W., Verbrugge, F. H., Nijst, P. & Tang, W. Renal sodium avidity in heart failure: From pathophysiology to treatment strategies. Eur. Heart J. 38, 1872–1882 (2017).
Mullens, W. et al. Elevated intra-abdominal pressure in acute decompensated heart failure: A potential contributor to worsening renal function?. J. Am. Coll. Cardiol. 51, 300–306 (2008).
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T. & Kwong, J. Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13 (2019).
Milkovic, L., Cipak, G. A., Cindric, M., Mouthuy, P. A. & Zarkovic, N. Short overview of Ros as cell function regulators and their implications in therapy concepts. Cells 8, 793 (2019).
Gallo, G., Lanza, O. & Savoia, C. New insight in cardiorenal syndrome: From biomarkers to therapy. Int. J. Mol. Sci. 24, 5089 (2023).
McWilliam, S. J. et al. The complex interplay between kidney injury and inflammation. Clin. Kidney J. 14, 780–788 (2021).
Akhter, M. S. & Goodwin, J. E. Endothelial dysfunction in cardiorenal conditions: Implications of endothelial glucocorticoid receptor-Wnt signaling. Int. J. Mol. Sci. 24, 14261 (2023).
Lisa, A., Carbone, F., Liberale, L. & Montecucco, F. The need to identify novel markers for early renal injury in cardiorenal syndrome. Cells 13, 1283 (2024).
Gottlieb, S. S. et al. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J. Card. Fail. 8, 136–141 (2002).
Ronco, C., Bellasi, A. & Di Lullo, L. Implication of acute kidney injury in heart failure. Heart Fail. Clin. 15, 463–476 (2019).
Damman, K., Tang, W. H., Testani, J. M. & McMurray, J. J. Terminology and definition of changes renal function in heart failure. Eur. Heart J. 35, 3413–3416 (2014).
Lala, A. et al. Standardized definitions of changes in kidney function in trials of heart failure: Jacc expert panel from the Hf-arc. J. Am. Coll. Cardiol. 85, 766–781 (2025).
Bellomo, R., Kellum, J. A. & Ronco, C. Defining and classifying acute renal failure: From advocacy to consensus and validation of the rifle criteria. Intensive Care Med. 33, 409–413 (2007).
Mehta, R. L. et al. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 11, R31 (2007).
Kdigo, K. D. & Outcomes, I. G. Acute kidney injury work group: Kdigo clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Li, Z. et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: Kdigo is superior to rifle or akin. PLoS ONE 9, e114369 (2014).
Pan, H. C. et al. Acute kidney injury classification for critically ill cirrhotic patients: A comparison of the kdigo, akin, and rifle classifications. Sci. Rep. 6, 23022 (2016).
Tsai, T. Y. et al. Comparison of rifle, akin, and kdigo classifications for assessing prognosis of patients on extracorporeal membrane oxygenation. J. Formos. Med. Assoc. 116, 844–851 (2017).
Malbrain, M. et al. Urine output is an early and strong predictor of acute kidney injury and associated mortality: A systematic literature review of 50 clinical studies. Ann. Intensive Care 14, 110 (2024).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Johnson, A. et al. Mimic-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1 (2023).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Funk, M. J. et al. Doubly robust estimation of causal effects. Am. J. Epidemiol. 173, 761–767 (2011).
Feng, M. et al. Transthoracic echocardiography and mortality in sepsis: Analysis of the Mimic-III database. Intensive Care Med. 44, 884–892 (2018).
Kociol, R. D. et al. Long-term outcomes of medicare beneficiaries with worsening renal function during hospitalization for heart failure. Am. J. Cardiol. 105, 1786–1793 (2010).
Damman, K. et al. Worsening renal function and prognosis in heart failure: Systematic review and meta-analysis. J. Card. Fail. 13, 599–608 (2007).
Damman, K. et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 35, 455–469 (2014).
Rossignol, P., Hernandez, A. F., Solomon, S. D. & Zannad, F. Heart failure drug treatment. Lancet 393, 1034–1044 (2019).
Roy, A. K. et al. A comparison of traditional and novel definitions (rifle, akin, and kdigo) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal. Med. 3, 26–37 (2013).
Marenzi, G. et al. Acute kidney injury in St-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit. Care Med. 38, 438–444 (2010).
Abdin, A. & Bohm, M. Resting heart rate: A valuable marker for preventing kidney disease. J. Am. Heart Assoc. 12, e032580 (2023).
Xu, Q. et al. Acute kidney injury in different anticoagulation strategies: A large-scale pharmacoepidemiologic study using real-world data. Cardiovasc. Drugs Ther. 8, 1–10 (2024).
Aletras, G. et al. Unraveling acute cardiorenal syndrome: Predictors and consequences in acute heart failure. J. Clin. Med. 14, 65 (2025).
Ru, S. C., Lv, S. B. & Li, Z. J. Incidence, mortality, and predictors of acute kidney injury in patients with heart failure: A systematic review. Esc Heart Fail. 10, 3237–3249 (2023).
Banerjee, D., Ali, M. A., Wang, A. Y. & Jha, V. Acute kidney injury in acute heart failure-when to worry and when not to worry?. Nephrol. Dial Transplant. 40, 10–18 (2024).
Thanapongsatorn, P., Tanomchartchai, A. & Assavahanrit, J. Long-term outcomes of acute kidney injury in acute decompensated heart failure: Identifying true cardiorenal syndrome and unveiling prognostic significance. Kidney Res. Clin. Pract. 43, 480–491 (2024).
Chavez-Iniguez, J. S., Ivey-Miranda, J. B., De la Vega-Mendez, F. M. & Borges-Vela, J. A. How to interpret serum creatinine increases during decongestion. Front. Cardiovasc. Med. 9, 1098553 (2022).
Acknowledgements
We thank the Ascetic Practitioners in Critical Care (APCC) team, and the easy Data Science for Medicine (easyDSM) team for sharing their knowledge and codes in big data of critical care, along with the cross-platform Big Data Master of Critical Care (BDMCC) software (https://github.com/ningyile/BDMCC_APP). We especially appreciate the MIMIC-IV database official team’s efforts to open-source the database and codes. Meanwhile, the authors wish to thank the Home for Researchers editorial team (www.home-for-researchers.com) for their language editing assistance.
Funding
This work was supported by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-055B) and Tianjin Science and Technology Plan Project (21JCZDJC00600, JF).
Author information
Authors and Affiliations
Contributions
YJ participated in the study conception and design, data analysis, and interpretation, manuscript drafting. CF, HTJ contributed to the study conception, manuscript drafting, and critical revision. BR, YYZ were involved in data analysis and interpretation. JCL participated in manuscript drafting. JPF led the study conception and design, critically revised the manuscript and funding acquisition. PG participated in the study conception and design, data access and verification, data analysis, and interpretation, critically revised. We confirm that both YJ and PG have directly accessed and verified the underlying data reported in the manuscript. All authors reviewed the manuscript and met the authorship of ICMJE criteria.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, Y., Feng, C., Jiang, H. et al. Prognostic value of dynamic KDIGO staging in acute kidney injury after acute heart failure: a doubly robust analysis. Sci Rep 15, 22920 (2025). https://doi.org/10.1038/s41598-025-07118-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07118-y