Abstract
This study aimed to analyze the antibiotic resistance patterns and virulence profiles of Klebsiella pneumoniae, a prevalent gram-negative pathogen linked to CLABSI patients globally. Of a total of 185 microbial isolates, 51 (27.5%) were K. pneumoniae isolates. The results of antimicrobial susceptibility testing using the disk diffusion method were compared to those of the VITEK-2. Phenotypic analysis revealed that 88.3% were biofilm producers and 50.9% were extended-spectrum beta-lactamase (ESBL) producers. Enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR) revealed a discriminatory power of 0.7111 between ten selected isolates. The PCR detection of the virulence genes, including FimH, rmpA, iutA, and fyuA, revealed that the ten selected isolates harbored all these genes, except one without the fyuA gene. The presence of the rmpA and the iutA genes confirmed them as hypervirulent (hv) K. pneumoniae. The genes (EAST-1, CNF-1) were present in 20% and 60% of the isolates, respectively. All isolates had the blaTEM and blaSHV resistance genes, while 50% had the blaNDM carbapenemase resistance gene. In conclusion, all selected K. pneumoniae isolates were proven to be ESBL producers and carbapenem-resistant, highlighting significant multidrug resistance. We found a strong correlation between biofilm formation and multidrug resistance, as well as between phenotypic and genotypic detection of various virulence factors. Infections from hyKp strains represent a growing public threat. Our findings aim to enhance therapeutic options for patients and help reduce complications and mortality.
Similar content being viewed by others
Introduction
Central line-associated bloodstream infection (CLABSI) is among the most serious health-associated infections (HAIs). It has been associated with increased hospitalization, healthcare costs, and mortality, especially in developing countries1,2,3. Several pathogens may be probable causes of CLABSIs, but the primary pathogens of greatest concern are Klebsiella pneumoniae, followed by Staphylococcus spp., Acinetobacter baumannii, and Pseudomonas aeruginosa4. The increased occurrence of multidrug-resistant (MDR-GNB) bacteria, with the dramatic increase in the prevalence of extended-spectrum beta-lactamase (ESBL) producing K. pneumoniae and E. coli, as well as carbapenem-resistant Enterobacteriaceae (CRE), has become a serious challenge for healthcare professionals due to the limited treatment options available for patients5.
The diversity of clinical infections caused by K. pneumoniae is linked to various virulence factors, such as capsules, adhesions, biofilm formation, and iron acquisition systems. Capsule production is crucial for immune evasion during initial infection and persistence in the bloodstream. Adherence, mediated by fimbriae and other adhesins, may be essential in the earlier phases of bacteremia when pathogens bind to specific receptors at the initial infection site and disseminate to the blood. Biofilms promote the persistence of microorganisms and facilitate bacterial dissemination6,7. A new variant of K. pneumoniae called hypervirulent K. pneumoniae (hvKp) has been reported worldwide, causing more serious community and hospital-acquired infections. It can be distinguished from classical K. pneumoniae based on its hypermucoviscosity8. Therefore, this study focused on the phenotypic detection of several virulence profiles of K. pneumoniae, including biofilm formation, ESBL production, hypermucoviscosity, and multidrug resistance, along with the virulome analysis, in comparison with E. coli and A. baumannii, with emphasis on hypervirulent strains isolated from patients with CLABSIs.
Methods
Clinical specimens
Unidentified blood specimens were collected from the clinical microbiology laboratory of a tertiary care hospital from patients admitted to different ICUs from May 2019 to March 2022. The selection criteria included those with primary bacteremia who exhibited fever before reaching its peak, as well as patients presenting with hypotension and an elevated heart rate. The research was conducted as per the Ethical Code 04-Egypt-Code-of-Medical-Ethics-Ministry-of-Health-and-Population-238/2003-part four. This study was conducted following the ethical principles of the Declaration of Helsinki. The study was approved by the institutional ethical committee of the Faculty of Pharmacy, Ain Shams University (ACUC-FP-ASU RHDIRB2020110301).
Identification and antimicrobial susceptibility testing
The identification of gram-negative isolates was performed according to Bergy’s Manual using standard identification procedures. Antibiotic susceptibility was analyzed by the disc diffusion method as recommended by the Clinical and Laboratory Standards Institute 2020 (CLSI)9. The concentrations of the antibiotic discs used (expressed in µg) were as follows: amikacin (30), ampicillin (10), ampicillin/sulbactam (10/10), ceftriaxone (30), cefuroxime (30), cefepime (30), ciprofloxacin (5), gentamicin (10), levofloxacin (5), tetracycline (30), imipenem (10), meropenem (10), ertapenem (10), trimethoprim (5) and tigecycline (15). Antibiotic susceptibility profiling was also performed using the VITEK-2 system in accordance with the manufacturer’s instructions (BioMerieux®, France). Interpretative values obtained by VITEK-2 were compared to those obtained by the disk diffusion method. The following definitions were adopted: (1) Categorical agreement (CA) (VITEK-2 and manual MIC values agree using the interpretative CLSI criteria). (2) Minor errors (mE) (Manual is S or R and VITEK-2 is I; alternatively, Manual is I and VITEK-2 is S or R). (3) Major errors (ME) (Manual is S and VITEK-2 is R). (4) Very major errors (VME) (Manual is R, and VITEK-2 is S). Each assay used E. coli ATCC 25,922, K. pneumoniae ATCC 700,603, and A. baumannii ATCC 19,606 as controls.
Quantitative biofilm assay
Biofilm formation was carried out according to a previously established protocol10, with some modifications. The isolates were cultured overnight in Luria–Bertani Broth (LB) at 37 °C and adjusted to a 0.5 McFarland turbidity standard. One milliliter of the culture was added to 9 mL of LB. From each bacterial suspension, 200 µl was inoculated into three wells of a polyvinyl chloride 96-well microtiter plate and incubated without shaking at 35 °C for 24 h. After incubation, the wells were washed three times with tryptone water and left to air dry for 30 min. Then, 100 µl of 0.5% crystal violet stain was added and left for 15 min. Finally, the wells were washed three times with phosphate-buffered saline, traces of crystal violet were dissolved using 90% ethanol, and the optical density was measured at 600 nm using an ELISA auto reader. The degree of biofilm formation was calculated using the formula; SBF = (AB − CW)/G, where SBF is the specific biofilm formation index, AB is the optical density of the stained bacteria, G is the optical density of cell growth in media and CW is the optical density of the stained control wells containing absolute medium without bacteria. The isolates were classified as follows: SBF ≥ 1.10: strong biofilm formation, SBF = 0.70–1.09: moderate biofilm formation, SBF = 0.35–0.69: weak biofilm formation, and SBF < 0.35: negative biofilm formation.
Phenotypic detection of ESBL-producing isolates
Isolates were screened for ESBL production using the double disk synergy test (DDST) according to the CLSI 202011. An augmentin disc (30 µg) was placed in the middle of a Mueller-Hinton agar plate seeded with a suspension of an isolate equivalent to 0.5 McFarland turbidity standard. Three antibiotic discs were placed around the augmentin disc: cefepime (30 µg), ceftazidime (30 µg), and ceftriaxone (30 µg). The plate was incubated at 37 °C for 24 h. If the inhibition zone increased toward the augmentin disc, the isolate was considered an ESBL producer.
PCR conditions
Genomic DNA purification was done using a kit (Thermo Fisher Scientific, United States) according to the manufacturer’s protocol. Three pairs of primers listed in Table 1 were used for the amplification of 16–23 S ribosomal RNA (16–23 S rRNA), UIDA, and OMPA genes for the identification of K. pneumoniae, E. coli, and A. baumannii, respectively. Each PCR mixture contained 2 µl of each primer (forward and reverse), 10 µl of PCR master mix (Willow Fort, UK), and 4 µl of extracted chromosomal DNA, and 20 µl of each mixture was mixed with sterile nuclease-free water. DNA amplification was performed using a Horizontal Thermocycler (Biometra, Germany). As shown in Table 2, amplification was performed with cycling parameters including initial denaturation at 95˚C for 5 min, followed by 30 cycles each of denaturation at 94˚C for 30 s to 1 min, annealing at 58˚C for 30 s to 1 min, extension at 72˚C, and a final extension at 72˚C for 5 min. The PCR products were analyzed using 2% agarose gel electrophoresis.
Determination of genetic diversity using ERIC-PCR
Genotyping of the selected K. pneumoniae isolates was performed by Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR) using a pair of published primers (F: 50 -ATG TAA GCT CCT GGG GAT TCA C-30) (R: 50 -AAG TAA GTG ACT GGG GTG AGC-30)15. The PCR master mix was prepared according to the instructions of Emerald Amp GT PCR master mix (Takara Bio INC, Japan) Code No. RR310A kit. The thermal cycling protocol was performed as follows: initial denaturation of the target DNA sequence at 94 ◦C for 5 min, followed by 35 cycles of secondary denaturation at 94 ◦C for 30 s, annealing at 52 ◦C for 1 min, extension of the primers by thermostable polymerase at 72 ◦C for 2 min and a final extension step for 12 min at 72 ◦C, followed by cooling to 4 ◦C. Electrophoresis of PCR products was done using 1.5% agarose gel. The ERIC fingerprinting data were transformed into a binary code depending on the presence or absence of each band. Dendrograms were generated by the unweighted pair group method with arithmetic average (UPGMA) and Ward’s hierarchical clustering routine. Cluster analysis and dendrogram construction were performed with SPSS version 22 (IBM 2013)16. The discriminatory index (D-value) was calculated using an online discriminatory power calculator (http://insilico.ehu.es/mini_tools/discriminatory_power/). The similarity index (Jaccard/Tanimoto Coefficient and number of intersecting elements) between all samples was calculated using an online tool (https://planetcalc.com/1664/)17.
Phenotypic identification of hypervirulent Klebsiella pneumoniae
The selected K. pneumoniae isolates were cultured on MacConkey agar. The incubation was set at 37 °C for 24 h under aerobic conditions. A string test was carried out by gently stretching the colonies using the bacteriological loop. The test is considered positive, and the strain is if a mucoid string with > 5 mm in length is observed18.
Virulome screening by PCR
Profiling of the virulence genes among K. pneumoniae, E. coli, and A. baumannii was performed, including the EAST-1, CNF-1, FimH, rmpA, iutA, FyuA, bla SHV−1, bla TEM−1, bla KPC, and bla NDM genes. All primers used are listed in Table 3. As shown in Table 4, amplification was performed with cycling parameters including initial denaturation at 95˚C for 4–5 min, except for the bla KPC, and bla NDM genes were for 15 min, followed by 30 cycles each of denaturation at 94˚C for 30–90 s. Different annealing temperatures were employed depending on the detected gene, ranging from 50–63˚C for 40–90 s. Followed by extension at 72˚C, and a final extension at 72˚C for up to 10 min was employed. The PCR products were analyzed using 2% agarose gel electrophoresis.
Statistical analysis
Statistical analysis was performed with IBM Statistical Package for Social Sciences (SPSS) Statistics for Windows Version 23.0. Armonk, NY: IBM Corp. Fisher’s exact test was used to assess the correlation between MDR isolates and biofilm formation. Pearson’s chi-squared test was used to compare between the biofilm producing capacity of different isolates and analyze the difference between the DDST and VITEK-2 systems in the detection of ESBL producers. A probability value (P value) less than 0.05 was considered to indicate statistical significance.
Results
Microbial population
Among the 231 blood specimens, 185 were positive. Of these, 185 microbial isolates were recovered. Laboratory examination of the isolates revealed that 120 (64.4%), 56 (30.2%), and 9 (4.8%) were gram-negative, gram-positive, and Candida spp., respectively. Among the gram-negative isolates, the most common organisms identified were K. pneumoniae (51; 27.5%), followed by E. coli (46; 24.8%), A. baumannii (11; 5.9%), P. aeruginosa (7; 3.7%), Salmonella (3; 1.62%), and S. marcescens (2; 1%).
Antimicrobial susceptibility testing
The antimicrobial susceptibility profiles of K. pneumoniae, E. coli, A. baumannii, and P. aeruginosa were determined using the disk diffusion method and the VITEK-2 system. Among the 120 recovered GNB strains, 76.6% (92/120) were MDR-GNB strains as they were resistant to more than three antibiotic classes, 57.5% (69/120) were CRE as they exhibited intermediate or resistance designations to more than one of the tested carbapenems. A comparison between the results of the VITEK-2 system and the disk diffusion method for K. pneumoniae and E. coli is presented in (S1). For the K. pneumoniae isolates, CA was 100% for cefepime, tetracycline, trimethoprim, and tigecycline; 98% for levofloxacin and gentamicin; 96% for amikacin, imipenem, and ciprofloxacin; and 94.1% for ampicillin, ceftriaxone, and cefuroxime.
For the E. coli isolates, CA was 100% for amikacin, trimethoprim, and tigecycline; 97.8% for imipenem, gentamicin, and levofloxacin; 95.6% for cefuroxime, cefepime, and tetracycline; and 93.4% for ampicillin, ampicillin + sulbactam, ertapenem, meropenem, and ciprofloxacin. A categorical agreement of 100% was found with the tested antibiotics for the A. baumannii and P. aeruginosa isolates.
Quantitative biofilm assay
As shown in Table 5, among the K. pneumoniae strains, 47% were strong biofilm producers, 31.3% were moderate producers, 9.8% were weak producers, and 11.7% were non-biofilm producers. The differences between biofilm production within K. pneumoniae strains were statistically significant (P-value < 0.001). For E. coli, 50% were strong biofilm producers, 30.4% were moderate, 9.8% were weak, and 8.6% were non-biofilm producers. The differences between biofilm production within E. coli strains were statistically significant (P-value < 0.001). For the A. baumannii isolates, 63.6% were strong biofilm producers, and 36.3% were moderate, with a non-statistically significant difference (P-value = 0.366). For P. aeruginosa, 42.85% were strong biofilm producers, 28.5% were moderate producers, and 28.5% were non-biofilm producers. The differences between biofilm production within P. aeruginosa strains were non-statistically significant (P-value = 0.368).
Comparison between strong biofilm-producing strains showed a statistically significant difference (P-value < 0.001). K. pneumoniae strains showed the highest prevalence among strong biofilm-producing strains, followed by E. coli, and then A. baumannii strains, while P. aeruginosa strains showed the lowest production of strong biofilm. Comparison between moderate biofilm-producing strains also showed a statistically significant difference (P-value = 0.005). K. pneumoniae strains showed the highest prevalence among moderate biofilm-producing strains, followed by E. coli, while A. baumannii and P. aeruginosa strains showed the same and the lowest production of moderate biofilm. While for weak and non-biofilm production, there was no statistically significant difference between strains (P-value = 1) and (P-value = 0.368), respectively. Overall, K. pneumoniae was consistently the most prevalent strain in both strong and moderate biofilm categories.
Association of biofilm formation and antibiotic resistance
Among the biofilm producers, GNB, 68.6% of the K. pneumoniae isolates were MDR (P value = 0.016), and 84.7% of the E. coli isolates were MDR (P value = 0.001). Thus, there was a significant correlation between MDR phenomena and biofilm formation among K. pneumoniae and E. coli isolates.
Phenotypic detection of ESBL-producing isolates
Using DDST, 50.9% of K. pneumoniae and 67.3% of E. coli strains were ESBL producers, while the results of the VITEK-2 system revealed that 56.8% of K. pneumoniae and 71.7% of E. coli strains were ESBL producers. Accordingly, no statistically significant difference was found between the two methods for K. pneumoniae and E. coli, with P values of 0.355 and 0.205, respectively.
Identification of selected K. pneumoniae isolates using PCR
PCR analysis of the ten isolates of K. pneumoniae, E. coli, and A. baumannii revealed the presence of the 16–23 S rRNA, UIDA, and OMPA genes, respectively, thus confirming their identification.
ERIC-PCR analysis
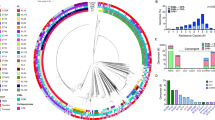
The variable banding pattern of ERIC-PCR gel electrophoresis revealed diversity among the 10 selected K. pneumoniae isolates. The DNA bands yielded from REP-PCR type amplification were thoroughly analyzed, and a phylogenetic tree for the isolated strains was designed by GelClust. The cluster analysis and related dendrogram are shown in Fig. 1. Based on the results shown in Fig. 1, the K. pneumoniae isolates were categorized into three clusters (C1–C3) with a discriminatory power of 0.7111, which is closer to 1.0 than 0, revealing wide heterogeneities among the tested isolates. Dendrogram analysis revealed an overall similarity of 73.3% among the three clusters. Furthermore, the similarity between each C1–C3 cluster member was 87.5%, 75.5%, and 57%, respectively.
String test
The ten selected K. pneumoniae isolates were positive for the string test as the string length was ≥ 0.5 mm
Prevalence of virulence and resistance genes among K. pneumoniae
As shown in Fig. 2, the genes responsible for toxin production in K. pneumoniae (EAST-1 and CNF-1) were present in 20% and 60% of the selected isolates, respectively. All isolates contained genes associated with biofilm formation (FimH), the iron acquisition gene (iutA), and the gene linked to hypermucoviscosity (rmpA). The other iron acquisition gene (FyuA) was found in 90% of the isolates. Additionally, all isolates harbored the two ESBL genes, blaTEM−1 and blaSHV−1, and the carbapenemase resistance gene blaNDM was found in 50% of the isolates. The other carbapenemase resistance gene, blaKPC, was not detected in any of the isolates. In comparison, E. coli and A. baumannii exhibited a lower prevalence of virulence genes than K. pneumoniae. Regarding resistance genes, both ESBL genes were present in all E. coli isolates, while the blaTEM−1 gene was found in 80% of A. baumannii isolates. The prevalence of blaNDM was 30% in E. coli and 20% in A. baumannii.
Correlation between the string test and RmpA and ItuA genes
All isolates were positive for the string test and harbored the rmpA and ituA genes, thus confirming their identification as hvPk.
Correlation between biofilm formation, ESBL production, and their related genes
All isolates were found to be both biofilm-forming and ESBL-producing and harbored their related genes, the FimH gene (for biofilm formation) and the blaTEM−1 and blaSHV−1 genes (for ESBL production).
Discussion
Globally, bloodstream infections (BSIs) are among the leading and most life-threatening conditions in hospital settings, particularly in immunocompromised patients25. Monitoring antibiotic susceptibility patterns and virulence genes in pathogens causing BSIs is crucial for determining the optimum antimicrobial therapy for patients26. Among the Gram-negative pathogens, K. pneumoniae is one of the frequent causes of BSIs associated with increased mortality and commonly occurring in patients with severe underlying conditions such as malignancies and trauma27,28. Therefore, this study focused on virulome analysis and antibiotic resistance pattern of K. pneumoniae in comparison with E. coli and A. baumannii, with emphasis on hypervirulent strains of K. pneumoniae.
Our results revealed the predominance of gram-negative bacteria (64.8%), with K. pneumoniae followed by E. coli being the most isolated pathogens from BSIs, with percentages of 27.5% and 24.8%, respectively. Similar findings were previously reported in other countries29,30. Such findings could be attributed to being in the hospital for more than one week with a catheter inserted for more than 3 days, undergoing surgery, or receiving treatment with antimicrobial agents such as beta-lactams31. Moreover, our results were in accordance with other studies conducted in Egypt, revealing the predominance of K. pneumoniae among BSIs over the past few decades32,33.
Biofilm formation is an important virulence factor in Gram-negative pathogens, significantly enhancing resistance to external stressors. This mechanism allows pathogens to evade host immune responses and the antibacterial effects of antibiotics, contributing to the challenges in treating various diseases and potentially leading to therapeutic failures. Biofilm formation can also result in the emergence of MDR strains, which are linked to poorer clinical outcomes and increased mortality rates, especially among immunocompromised patients34,35. The results of the quantitative biofilm assay revealed that among GNB, 88.3% of K. pneumoniae, 91.4% of E. coli, 100% of A. baumannii, and 71.5% of P. aeruginosa were biofilm producers. Previous literature reported that around 84% of Gram-negative bacteria isolated from CVC-related infections were biofilm producers36.
In this study, the overall proportion of MDR-GNB was 76.6% (92/120). The significant multidrug-resistant (MDR) patterns observed in various Gram-negative pathogens pose a major challenge in managing infectious diseases. Therefore, optimizing antibiotic use through stewardship programs is essential, as these initiatives are key components in the battle against antimicrobial resistance37. Numerous studies indicate that using a combination of antibiotics can help prevent the development of new resistant strains, as treatment failures are often seen in patients receiving only single antibiotic therapy. Additionally, collaboration between clinicians and microbiologists is crucial for effective infection management, as emphasized by the Rational Use of Medicine Program. K. pneumoniae is one of the most important causes of MDR infections worldwide. This high prevalence is not surprising, as this pathogen is known for its ability to transfer its resistance determinants and its association with BSIs38. This study proved that a significant correlation was found between MDR phenomena and biofilm formation among K. pneumoniae and E. coli isolates, with P values of 0.016 and 0.001, respectively. The correlation between biofilm formation and antibiotic resistance is of great concern, especially in healthcare facilities for patients with device-related infections. These results were in line with a previous study conducted by Nirwati et al. (2019), where antibiotic resistance was greater among biofilm producers of K. pneumoniae than non-biofilm producers38.
The widespread occurrence of MDR isolates, especially ESBL producers, has presented a global threat to public health39. In our study, the prevalence of ESBL production was detected by DDST and VITEK-2, revealing a high prevalence among K. pneumoniae and E. coli, with no significant difference between the two methods. Such a high prevalence could be attributed to the empirical use of antibiotics, resulting in positive pressure on GNB, leading to the selection of resistant strains40.
ERIC-PCR analysis demonstrated a discriminatory power of 0.7111 among the selected isolates, confirming significant heterogeneity among the tested strains. Consequently, these isolates were assessed for hypervirulence, as hvKp poses a growing threat as a pathogen responsible for life-threatening infections and is crucial to differentiate from classical K. pneumoniae41. As previously mentioned by Emam et al. (2023), hvKp can be identified phenotypically using the string test. All our isolates were found to be hvKp since hypermucoviscosity is one of the typical features of hvKp42.
Several previous studies from different countries focused on evaluating the prevalence of important virulence genes in K. pneumoniae43,44. Consequently, the study focused on investigating the prevalence of selected crucial virulence genes encoding for biofilm formation (FimH), the gene associated with hypermucoviscosity (rmpA), the iron acquisition genes (iutA) and (fyuA), as well as the genes encoding toxin production (EAST-1, CNF-1). According to Anis et al. (2021), the FimH gene is strongly linked to biofilm formation45. We detected this gene and confirmed that all K. pneumoniae possessed the FimH gene, indicating they are biofilm producers. A slightly lower prevalence of this gene was found in E. coli and A. baumannii. Our results revealed that K. pneumoniae strains showed the highest prevalence among strong and moderate biofilm-producing strains, with a statistically significant difference. Moreover, research shows that when comparing the biofilm-forming abilities of E. coli and K. pneumoniae, Klebsiella is generally more effective at forming biofilms, especially in clinical settings, which plays a significant role in its virulence and resistance to treatment46.
The rmpA gene is a mucoid regulator that mediates the increased capsular polysaccharide production and is one of the genotypic markers for identifying hvKp. This direct correlation has been mentioned before by Neumann et al. (20230) and by Khattab and Hager (2022)47,48. Our results confirmed the presence of the rmpA gene in all selected isolates, which is considered one of the key virulent features of K. pneumoniae. Although this gene has been found in all E. coli isolates, the rmpA gene is not considered part of the core genome of E. coli. However, its presence contributes to the capsule formation and can enhance the ability of bacteria to resist certain host defenses49. As previously reported by Shankar et al. (2021), the aerobactin (iutA) gene is another frequent and stable genotypic marker for hvKp isolates; our investigations also detected the (iutA) gene and confirmed its presence in all selected isolates50. Together, these genes significantly enhance the hypervirulent phenotype of K. pneumoniae, particularly in central line-associated bloodstream infections (CLABSIs). Increased biofilm formation, effective iron acquisition, and strong adherence capabilities allow K. pneumoniae to establish and maintain infections, leading to severe clinical outcomes46.
As iron is one of the essentials for bacterial survival and reproduction, we also assessed the presence of the yersiniabactin receptor gene (fyuA), another important iron uptake gene besides the iutA gene51. The FyuA gene has been identified in 90% of K. pneumoniae isolates, although several studies have reported finding it at lower frequencies52. Iron is essential for the survival, growth, and pathogenicity of A. baumannii. To thrive in the iron-limited environment of the host, this bacterium has developed multiple iron acquisition systems, including the production of siderophores, heme uptake mechanisms, and TonB-dependent transport systems53. This clarifies the reason for the high prevalence of the FyuA and the iutA genes in A. baumannii. Moreover, the iron acquisition genes are a crucial factor contributing to the pathogenicity of E. coli, accordingly, a high prevalence of these genes was found among the E. coli isolates, which was also previously reported by Guo et al. (2024)54.
The prevalence of virulence genes responsible for toxin production, such as the EAST-1 and CNF-1 genes, has been assessed. Although no previous study detected the EAST-1 gene among K. pneumoniae, our results confirmed its low prevalence. Moreover, a DNA search in the GenBank database carried out using BLAST (Basal Local Alignment Research Tool) records the presence of the gene’s sequence in several K. pneumoniae strains (https://www.ncbi.nlm.nih.gov/nucleotide/1890420180). The CNF-1 gene was detected in 60% of our K. pneumoniae isolates. Similarly, Lateef et al. (2012) identified the CNF-1 gene in 57.1% of K. pneumoniae isolates55. In the case of E. coli, 50% of the isolates carried the CNF-1 gene, whereas 90% of A. baumannii isolates possessed it. A study conducted by Sheikh et al. (2019) found the CNF-1 gene in 22.8% of E. coli isolates56. As for A. baumannii isolates, it was reported by Al-Kadmy et al. (2018) that the CNF-1 gene was detected in 47.6% of their isolates57. The high prevalence of the CNF-1 gene underscores the importance of this gene in the virulence of A. baumannii strains.
The detection of the resistance determinants blaSHV−1 and blaTEM genes in all K. pneumoniae and E. coli isolates confirmed them as ESBL producers. Moreover, both conventional antimicrobial susceptibility testing and the VITEK-2 system verified these isolates as carbapenem-resistant. However, detection of the carbapenemase resistance genes blaNDM and blaKPC, showed that none of the isolates harbored the blaKPC, while the prevalence of the blaNDM among K. pneumoniae, E. coli, and A. baumannii was 50%,30%, and 20%, respectively.
The alarming prevalence of beta-lactamase enzymes, along with the global emergence of carbapenemase enzymes in K. pneumoniae strains, is a major public concern due to limited treatment options and emerging resistance to last-resort antibiotics like colistin and tigecycline. Genomic studies show the clonal expansion of high-risk strains, such as ST11, contributing to healthcare outbreaks. The combination of hypervirulence and multidrug resistance poses an even greater threat. While advances in genomic surveillance, phage therapy, and new antibiotics like cefiderocol provide hope, addressing MDR K. pneumoniae requires antimicrobial stewardship and global collaboration58,59. By implementing effective infection control measures, screening for the microbial source, optimizing antimicrobial use, and discovering novel therapeutic options, it is possible to mitigate these challenges and improve patient outcomes.
Limitations
This study has several limitations that must be acknowledged. The sample size is relatively small, and the research was conducted at a single center, which may impact the generalizability of the findings. Additionally, the lack of clinical correlation with the study results is another limitation. These challenges arose from logistical issues related to the collection of blood specimens from hospitals and the follow-up of patients. Future studies should aim for larger sample sizes and multi-center involvement to improve the robustness and generalizability of the findings.
Conclusion
K. pneumoniae is a frequent and critical cause of BSIs. As shown in our study, all selected isolates were found to be both ESBL producers and CRE, emphasizing the high level of multidrug resistance encountered nowadays. In comparison to E. coli and A. baumannii, K. pneumoniae was found to be the most persistent biofilm producer. The findings of this study also revealed a significant correlation between biofilm formation and multidrug resistance. Also, a direct correlation has been found between the phenotypic and genotypic detection of various virulence factors, including biofilm formation, hypermucoviscosity, and ESBL production.
Bloodstream infections caused by hyKp strains pose an increasing public health threat in healthcare settings. Consequently, the data collected from our study will contribute to addressing this challenge and identifying better therapeutic alternatives for patient treatment, while also aiming to reduce complications, mortality, and financial burdens.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to confidentiality and privacy concerns but are available from the corresponding author upon reasonable request.
References
Kharduit, P. B. et al. Monitoring and outcomes of central Line-Associated bloodstream infections in a tertiary care intensive care unit. Cureus 16 (6). https://doi.org/10.7759/cureus.63428 (2024).
Arunan, B. et al. Central Line-Associated bloodstream infections: effect of patient and pathogen factors on outcome. J. Glob Infect. Dis. 15 (2), 60. https://doi.org/10.4103/jgid.jgid_213_22 (2023).
Selby, L. M., Rupp, M. E. & Cawcutt, K. A. Prevention of central-line associated bloodstream infections: 2021 update. Infect. Dis. Clini. 35 (4), 841–856 (2021).
Tufa, T. B. et al. Prevalence and characterization of antimicrobial resistance among gram-negative bacteria isolated from febrile hospitalized patients in central Ethiopia. Antimicrob. Resist. Infect. Control. 11 (1), 1–12. https://doi.org/10.1186/s13756-022-01053-7 (2022). https://link.springer.com/article/
Vance, M. K. et al. Risk factors for bloodstream infections due to ESBL-Producing Escherichia coli, Klebsiella spp., and Proteus mirabilis. Pharm 11 (2), 74. https://doi.org/10.3390/pharmacy11020074 (2023).
Holmes, C. L., Anderson, M. T., Mobley, H. L. & Bachman, M. A. Pathogenesis of gram-negative bacteremia. Clin. Microbiol. Rev. 34 (2), 234–220. https://doi.org/10.1128/CMR.00234-20 (2021). https://journals.asm.org/doi/full/10.1128/CMR.00234-20 DOI.
Cepas, V. & Soto, S. M. Relationship between virulence and resistance among gram-negative bacteria. Antibiot 9 (10), 719. https://doi.org/10.3390/antibiotics9100719 (2020). https://www.mdpi.com/2079-6382/9/
Emam, S. M., Abdelrahman, S., Hasan, A. A. & EL-Melouk, M. S. Hypervirulent Klebsiella pneumoniae at Benha university hospitals. Egy J. Hospit Med. 90 (2), 3592–3597. https://doi.org/10.21608/ejhm.2023.292752 (2023).
Lubbers, B. V., Diaz-Campos, D. & Schwarz, S. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. 5th Edn. CLSI supplement VET01S. (2020).
Teh, K. H., Flint, S. & French, N. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int. J. Food Microbiol. 143, 118–124. https://doi.org/10.1016/j.ijfoodmicro.2010.07.037 (2010). https://www.sciencedirect.com/science/article/abs/pii/S0168160510004472
AL-Hasnawy, H. H., Saleh, R. H. & Hadi, B. H. Existence of ESBL genes in Escherichia coli and Acinetobacter baumannii isolated from different clinical specimens. J. Pharm. Sci. Res. 10, 1112–1117 (2018). https://www.proquest.com/openview/7ebb838a3fd380b140eb6506ac0ae 041/1?pq-origsite=gscholar&cbl=54977
Derakhshan, S., Najar Peerayeh, S. & Bakhshi, B. Association between presence of virulence genes and antibiotic resistance in clinical Klebsiella Pneumoniae isolates. Lab. Med. 47, 306–311. https://doi.org/10.1093/labmed/lmw030 (2016). https://academic.oup.com/labmed/article/47/4/306/2949531 DOI.
Joy, N. B., Cajethan, O. E., Munachimso, E. N. & Tolulope, S. A. Prevalence of integrons in Enterobacteriaceae obtained from clinical samples. J. Microbiol. Antimicrob. 13, 1–10. https://doi.org/10.5897/JMA2020.433 (2021).
Bardbari, A. M. et al. Correlation between ability of biofilm formation with their responsible genes and MDR patterns in clinical and environmental Acinetobacter baumannii isolates. Microb. Pathog. 108, 122–128. https://doi.org/10.1016/j.micpath.2017.04.039 (2017).
Versalovic, J., Koeuth, T. & Lupski, R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial Enomes. Nucleic Acids Res. 19, 6823–6831. https://doi.org/10.1093/nar/19.24.6823 (1991).
Hunter, P. R. Reproducibility and indices of discriminatory power of microbial typing methods. J. Clin. Microbiol. 28, 1903–1905. https://doi.org/10.1128/jcm.28.9.1903-1905.1990 (1990).
Anjay, A. K. et al. Molecular typing of Salmonella typhimurium and S. enteritidis serovars from diverse origin by ERIC-PCR. J. Pure Appl. Microbiol. 9, 2627–2634 (2015).
Neumann, B. et al. Detection and characterization of putative hypervirulent Klebsiella pneumoniae isolates in Microbiological diagnostics. Sci. Rep. 13 (1), 19025. https://doi.org/10.1038/s41598-023-46221-w (2023).
Palma, N. et al. Virulence factors profiles and ESBL production in Escherichia coli causing bacteremia in Peruvian children. Diagn. Microbiol. Infect. Dis. https://doi.org/10.1016/j.diagmicrobio.2016.05.017 (2016). : 86, 70 – 5 https://www.sciencedirect.com/science/article/abs/pii/S0732889316301419
Momtaz, H., Morteza, S. & Tavakol, M. Determining the prevalence and detection of the most prevalent virulence genes in Acinetobacter Baumannii isolated from hospital infections. Int. J. Med. Lab. 2 (2), 87–97 (2015). https://ijml.ssu.ac.ir/article-1-52-fa.html
El Fertas-Aissani, R., Messai, Y., Alouache, S. & Bakour, R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol. Biol. (Paris). 61, 209–216. https://doi.org/10.1016/j.patbio.2012.10.004 (2013). https://www.sciencedirect.com/science/article/abs/pii/S036981141200185X
Ahmed, A. J. A. & Alaa, H. A. Virulence factors and antibiotic susceptibility patterns of multidrug resistance Klebsiella pneumoniae isolated from different clinical infections. Afr. J. Microbiol. Res. 10 (22), 829–843. https://doi.org/10.5897/AJMR2016.8051 (2016). https://academicjournals.org/journal/AJMR/article-full-text-pdf/EC412E358948
Awoke, T. et al. Detection of Bla KPC and Bla NDM carbapenemase genes among Klebsiella pneumoniae isolates in addis ababa, ethiopia: dominance of Bla NDM. PLoS One. 17 (4), e0267657. https://doi.org/10.1371/journal.pone.0267657 (2022).
Boisen, N. et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J. Infect. Dis. 205, 431–444. https://doi.org/10.1093/infdis/jir757 (2012). https://academic.oup.com/jid/article/205/3/431/850950 DOI.
Deku, J. G. et al. W. and The epidemiology of bloodstream infections and antimicrobial susceptibility patterns: A nine-year retrospective study at st. Dominic hospital, akwatia, Ghana. J Trop Med https://www.hindawi.com/journals/jtm/2019/6750864/ (2019). https://doi.org/10.1155/2019/6750864
Schöneweck, F. et al. The epidemiology of bloodstream infections and antimicrobial susceptibility patterns in thuringia, germany: a five-year prospective, state-wide surveillance study (AlertsNet). Antimicrob. Resist. Infect. Cont. 10 (1), 1–9. https://doi.org/10.1186/s13756-021-00997-6 (2021). https://aricjournal.biomedcentral.com/articles/
Kot, B. et al. Virulence analysis and antibiotic resistance of Klebsiella pneumoniae isolates from hospitalised patients in Poland. Sci. Rep. 13 (1), 4448 (2023).
Anis, R. H., Ahmed, S. M. & Esmaeel, N. E. Virulence determinants associated with biofilm formation by Klebsiella pneumoniae causing hospital-acquired bloodstream infection. Microbes Infect. Dis. 2 (2), 317–325. https://doi.org/10.21608/MID.2021.62223.1117 (2021). https://mid.journals.ekb.eg/article_154177.html
Baja, A. et al. Prevalence of gram-negative septicemia in a tertiary care center. J. Med. Sci. Health (2019). https://pesquisa.bvsalud.org/portal/resource/pt/sea-215698
Leal, H. F. et al. Bloodstream infections caused by multidrug-resistant gram-negative bacteria: epidemiological, clinical and Microbiological features. BMC Infecti Dis. 19, 1–1. https://doi.org/10.1186/s12879-019-4265-z (2019).
Ripa, M. et al. Short-term peripheral venous catheter-related bloodstream infections: evidence for increasing prevalence of gram-negative microorganisms from a 25-year prospective observational study. Antimicrob. Agents Chemother. 62 (11), e00892–e00818. https://doi.org/10.1128/AAC.00892-18 (2018). https://journals.asm.org/doi/full/10.1128/AAC.00892-18
Fahim, N. A. E. Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care units’ patients at Ain Shams university hospitals in Egypt-a retrospective study. J. Egypt. Public. Health Assoc. 96, 7. https://doi.org/10.1186/s42506-020-00065-8 (2021). https://jepha.springeropen.com/articles/10.1186/s42506-020-00065-8 DOI.
Halim, M. M., Eyada, I. K. & Tongun, R. M. Prevalence of multidrug drug resistant organisms and hand hygiene compliance in surgical NICU in Cairo University Specialized Pediatric Hospital. Egypt Pediatr Assoc Gaz, 66(4):103 – 11.10.21608/ejmm.2019.282666 (2018).
Guerra, M. E. et al. Klebsiella pneumoniae biofilms and their role in disease pathogenesis. Front. Cell. Infect. Microbiol. 12, 877995. https://doi.org/10.3389/fcimb.2022.877995 (2022).
Swedan, S. F. & Aldakhily, D. B. Antimicrobial resistance, biofilm formation, and molecular detection of efflux pump and biofilm genes among Klebsiella pneumoniae clinical isolates from Northern Jordan. Heliyon 10 https://doi.org/10.1016/j.heliyon.2024.e34370 (2024).
Cangui-Panchi, S. P. et al. Biofilm-forming microorganisms causing hospital-acquired infections from intravenous catheter: A systematic review. Curr. Res. Microb. Sci. 3, 100175. https://doi.org/10.1016/j.crmicr.2022.100175 (2022).
Elena, C., Michela, C., Marianna, M. & Cristina, M. The role of antimicrobial stewardship in preventing KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemo. 76 (1), i12–i18. https://doi.org/10.1093/jac/dkaa493 (2021).
Nirwati, H. et al. Biofilm formation and antibiotic resistance of Klebsiella pneumoniae isolated from clinical samples in a tertiary care hospital, Klaten, Indonesia. InBMC proceedings, 13: 1–8). BioMed Central. (2019). https://doi.org/10.1186/s12919-019-0176-7
Raphael, E., Glymour, M. M. & Chambers, H. F. Trends in prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolated from patients with community- and healthcare-associated bacteriuria: results from 2014 to 2020 in an urban safety-net healthcare system. Antimicrob. Resist. Infect. Cont. 10 (1), 1–13. (2021). https://aricjournal.biomedcentral.com/articles/10.1186/s
Hamam, S. S., El Kholy, R. M. & Zaki, M. E. Study of various virulence genes, biofilm formation and Extended-Spectrum β-lactamase resistance in Klebsiella pneumoniae isolated from urinary tract infections. Open. Microbiol. J. 13 (1). https://doi.org/10.2174/1874285801913010249 (2019). https://openmicrobiologyjournal.com/VOLUME/13/PAGE/249/FULLTEXT/
Russo T A, Alvarado C L, Davies C J, Drayer Z J, Carlino-MacDonald, Hutson A., … Lebreton F. (2024). Differentiation of hypervirulent and classical Klebsiella pneumoniae with acquired drug resistance. Mbio, 15(2), e02867-23.DOI: 10.1128/mbio.02867-23.
Emam, S. M., Abdelrahman, S., Hasan, A. A. & EL-Melouk, M. S. Hypervirulent Klebsiella pneumoniae at Benha university hospitals. Egypt. J. Hosp. Med. 90 (2), 3592–3597. https://doi.org/10.21608/ejhm.2023.292752 (2023).
Ballén, V. et al. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 1, 11:738223 (2021).
Davoudabadi, S., Goudarzi, M. & Hashemi, A. Detection of virulence factors and antibiotic resistance among Klebsiella pneumoniae isolates from Iran. BioMed. Res. Inter. 1, 3624497 (2023).
Anis, R. H., Ahmed, S. M. & Esmaeel, N. E. Virulence determinants associated with biofilm formation by Klebsiella pneumoniae causing hospital-acquired bloodstream infection. Microbes Infect. Dis. 2(2), 317–325 (2021).
Li, Y. & Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 14, 1238482. https://doi.org/10.3389/fmicb.2023.1238482 (2023).
Neumann, B. et al. Detection and characterization of putative hypervirulent Klebsiella pneumoniae isolates in Microbiological diagnostics. Sci. Rep. 13 (1), 19025. https://doi.org/10.3201/eid2402.170957 (2023).
Khattab, M. A. & Hager, R. Detection of RmpA and MagA genes in hypervirulent Klebsiella pneumoniae isolates from tertiary care hospitals. Egypt. J. Med. Microbiol. 31 (3), 43–50. https://doi.org/10.21608/ejmm.2022.247186 (2022).
Nassrf, X., Honore, N., Vasselon, T., Cole, S. T. & Sansonetti, P. J. Positive control of Colanic acid synthesis in Escherichia coli by RmpA and rmpb, two virulence-plasmid genes of Klebsiella pneumoniae. Mol. Microbiol. 3 (10), 1349–1359. https://doi.org/10.1111/j.1365-2958.1989.tb00116.x (1989).
Shankar C, Basu S, Lal B, Shanmugam S, Vasudevan K, Mathur P, … Veeraraghavan, B.(2021) Aerobactin seems to be a promising marker compared with unstable RmpA2 for the identification of hypervirulent carbapenem-resistant Klebsiella pneumoniae: in silico and in vitro evidence. Front Cell Infect Microbi, 11, 709681. https://doi.org/10.3389/fcimb.2021.709681.
Bautista-Cerón, A. et al. Hypervirulence and multi-resistance to antibiotics in Klebsiella pneumoniae strains isolated from patients with hospital-and community-acquired infections in a Mexican medical center. Micro 10 (10), 2043. https://doi.org/10.3390/microorganisms10102043 (2022).
Kumar, A., Harjai, K. & Chhibber, S. Early cytokine response to lethal challenge of Klebsiella pneumoniae averted the prognosis of pneumonia in FyuA immunized mice. Microb. Pathog. 144, 104161. https://doi.org/10.1016/j.micpath.2020.104161 (2020). https://www.sciencedirect.com/science/article/abs/pii/S0882401020304484
Zhang, R. et al. Iron-dependent mechanisms in Acinetobacter baumannii: pathogenicity and resistance. JAC-Antimicrobial Res. 7 (2), dlaf039. https://doi.org/10.1093/jacamr/dlaf039 (2025).
Guo, Y. et al. Distribution of virulence genes and antimicrobial resistance of Escherichia coli isolated from hospitalized neonates: A multi-center study across China. Heliyon.10(16). (2024).
Lateef, L. & Abdul-Razzaq, M. S. Molecular detection of some virulence gene associated with pathogenicity of Klebsiella pneumoniae. Al-Kufa Uni J. Biol. 4 (2). https://doi.org/10.13005/bpj/1048 (2012).
Sheikh, A. F. et al. Virulence-associated genes and drug susceptibility patterns of uropathogenic Escherichia coli isolated from patients with urinary tract infection. Infection and drug resistance, 12 :.2039. (2019). https://doi.org/10.2147/IDR.S199764
Al-Kadmy, I. M. S., Ali, A. N. M., Salman, I. M. A. & Khazaal, S. S. Molecular characterization of Acinetobacter baumannii isolated from Iraqi hospital environment. New. Microbes New. Infect. 21, 51–57. https://doi.org/10.1016/j.nmni.2017.10.010 (2018).
Devanga Ragupathi, N. K. et al. The influence of biofilms on carbapenem susceptibility and patient outcome in device associated K. pneumoniae infections: insights into phenotype vs genome-wide analysis and correlation. Front. Microbiol. 11, 591679 (2020).
Jomehzadeh, N., Rahimzadeh, M. & Ahmadi, B. Molecular detection of extended-spectrum β‐lactamase‐and carbapenemase‐producing Klebsiella pneumoniae isolates in Southwest Iran. Trop. Med. Inter Health. 29 (10), 875–881 (2024).
Acknowledgements
The authors express their appreciation to the International Medical Center, Clinical Microbiology Laboratory, Cairo, Egypt, for administrative and laboratory support, including clinical specimens and utensils used for the experiments.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MIE, MSM, and MGS: Conceptualization; MIE and MGS have designed the protocol of the study. MGS performed all experiments incorporated in the manuscript under the supervision and guidance of MIE and MSM. HME has written the first draft of the manuscript. MIE and MGS were involved in manuscript editing and data interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted by the ethical principles of the Declaration of Helsinki. The study was approved by the institutional ethical committee of the Faculty of Pharmacy, Ain Shams University (ACUC-FP-ASU RHDIRB2020110301). A waiver of the patient consent was obtained from the Ain Shams University Research Ethics Committee, as the collected isolates were obtained from discharged clinical specimens of unidentified patients, and there was no contact with patients.
Consent for publication
Not applicable, as unidentified blood specimens were collected from the microbiology laboratory of a tertiary care hospital in Egypt.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayed, M.G., Mansy, M.S., El Borhamy, M.I. et al. Exploring virulence factors, virulome, and multidrug resistance of Klebsiella pneumoniae strains isolated from patients with central Line-associated bloodstream infections. Sci Rep 15, 20230 (2025). https://doi.org/10.1038/s41598-025-07493-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-07493-6