Abstract
This study aimed to explore the beneficial properties, antimicrobial, and potential anticancer effects of Lactobacillus bacteria isolated from traditional dairy products on oral cancer cell lines. Twenty-one Lactobacillus strains were obtained from Kermanshah traditional cheese in western Iran. Using the well-diffusion method, the antimicrobial activities of the bacterial extracts were assessed against several human pathogens, revealing significant inhibitory effects, particularly for strains C9 and C47. These strains demonstrated broad-spectrum activity against both gram-positive and gram-negative bacteria, with notable inhibition of Staphylococcus aureus and Klebsiella pneumoniae. Additionally, the MTT assay was used to evaluate the antiproliferative effects of these extracts against oral cancer cell lines (KB and OSCC) and normal fibroblast and HUVEC lines. The compounds extracted from strains C9 and C47 (predominantly proteins) effectively suppressed the survival and growth of KB and OSCC cancer cell lines in a dose- and duration-dependent manner without significant harm to normal cells. Acridine orange/ethidium bromide labeling and mRNA expression analysis confirmed apoptosis as the primary mechanism of cancer cell death. In summary, strains C9 (Limosilactobacillus fermentum KUMS-C9) and C47 (Lactiplantibacillus plantarum KUMS-C47) exhibited potent antimicrobial and anticancer activities, highlighting their potential as probiotic candidates for therapeutic applications.
Similar content being viewed by others
Introduction
Probiotic bacteria, particularly lactic acid bacteria (LAB), have garnered considerable scientific attention due to their potential to promote health through mechanisms such as modulating gut microbiota, regulating immune responses, and inhibiting pathogenic microorganisms1,2. Lactobacillus species, one of the most studied LAB groups, are known to produce beneficial compounds, including lactic and acetic acids, bacteriocins, hydrogen peroxide, and biosurfactants, which exhibit broad-spectrum antimicrobial properties3,4.
Beyond their gastrointestinal benefits, probiotics have recently emerged as promising adjunct therapies for cancer. Multiple in vitro and in vivo studies have demonstrated that certain LAB strains can induce apoptosis, suppress tumor growth, and reduce metastasis in various types of cancer, including colon, liver, and breast cancers5,6,7. However, most of these investigations have focused on commercial or laboratory strains, while indigenous strains from traditional fermented foods—which may harbor more potent bioactivities—remain underexplored6,8.
Oral cancer, especially oral squamous cell carcinoma (OSCC), accounts for over 90% of all oral malignancies and remains a significant cause of morbidity and mortality worldwide, particularly in developing countries9. The global incidence is approximately 377,000 new cases annually, with a 5-year survival rate of less than 60%, mainly due to late diagnosis and treatment resistance10. Current therapies often carry adverse side effects, highlighting the need for safer, more targeted alternatives. Probiotic-derived compounds represent one such alternative, capable of exerting anticancer effects through modulation of cell signaling and induction of apoptosis, with minimal toxicity to normal cells11,12.
In Iran, traditional dairy products such as cheese, yogurt, and tarkhineh provide a rich source of diverse LAB communities. Some studies have indicated that strains isolated from these foods may offer strong probiotic effects, yet their anticancer properties, particularly against oral cancer, have not been fully characterized13,14.
This Study aimed to isolate and identify Lactobacillus strains from traditional Iranian cheese and evaluate their survivability, antimicrobial activity, and anticancer potential. Special focus was placed on their effects against KB and OSCC oral cancer cell lines, with a comparative analysis of safety on normal cells. These findings could contribute to the development of novel probiotic-based interventions for cancer prevention or therapy.
Materials and methods
Isolation and culture of Lactobacillus strains.
Lactobacillus strains were isolated and identified from 50 traditional cheese samples collected randomly from remote areas in Kermanshah Province. One gram of sample was immersed in 9 cc of trisodium citrate solution and incubated at 4 °C for one hour to enhance bacterial strain isolation. Afterward, 1 mL of this solution was added to 10 mL of de Man Rogosa & Sharpe (MRS) culture medium to encourage the growth of the primary bacterial population. The cultures were incubated anaerobically at 37 °C for 24 h. The isolated bacterial strains were subsequently cultivated on MRS agar media under the same conditions. Basic morphological and biochemical tests, including cell morphology analysis, catalase testing, and Gram staining, were also conducted to characterize and differentiate the bacterial populations.
Simulated Gastrointestinal tract (GIT) tolerance test
To evaluate the tolerance of the isolated bacterial strains to adverse conditions, each culture underwent a series of treatments. Initially, a 10 mL bacterial culture was grown for 24 h and was subsequently centrifuged at 5000 × g for 4 min. The supernatant was discarded, and the pelleted cells were gently agitated for 3 h in 10 mL of low-pH MRS medium (pH 2.5 at 37 °C) and oral pH medium (pH 6.6 at 37 °C). Subsequently, the cells were exposed to 10 mL of growth medium containing a high concentration of bile salts (pH 6.8 at 37 °C with 0.3% w/v oxgall) for 4 h. The initial selection of bacterial types was based on optical density1,12. OD measurements for both treated and untreated strains were recorded at a wavelength of 600 nm using a spectrophotometer (Eppendorf, Germany). Bacterial resistance to the specified conditions was then calculated by determining the proportion of survival rates with the following equation:
Survival percentage = [OD after treatment/OD before treatment] ×100.
Two distinct survival rate calculations were utilized in this Study: OD-based estimates to determine initial acid and bile tolerance and CFU-based estimates under simulated gastric and intestinal conditions. All formulas provide a survival percentage; however, they are used in two settings and with two endpoints that differ.
Five bacterial strains exhibiting enhanced tolerance to low pH conditions and bile salts (> 70%) were chosen and exposed to simulated gastric and intestinal environments for further analysis to evaluate their viability within the digestive tract13.
For stomach simulation, the culture medium was supplemented with 5% (w/v) pepsin and maintained at a pH of 2.5 and a temperature of 37 °C. The cells were cultured at 400 × g for 2 h. To simulate intestinal conditions, a mixture of bile salts and pancreatin (0.3% and 0.1% w/v, respectively) was added to the MRS medium. The pH was adjusted to 6.0, and the mixture was incubated at 37 °C with agitation at 110 × g for 3 h. Bacterial growth was assessed by counting the colonies on MRS agar medium after 48 h at 37 °C, both before and after the experiment. Bacterial survival rates were calculated using the following equation:
Survival percentage = (log CFU N1/log CFU N0) × 100.
Where N1 represents the total number of bacterial clones after treatment, and N0 represents the total number of bacterial clones before treatment.
Antibiotic susceptibility
To assess antibiotic susceptibility, a key parameter of microbiological safety, we employed the agar diffusion method using a panel of widely used and clinically essential antibiotics.
The antibiotics tested included cefixime (5 µg), amoxicillin (25 µg), doxycycline (30 µg), trimethoprim-sulfamethoxazole (75 µg), azithromycin (15 µg), ciprofloxacin (5 µg), cephalexin (30 µg), amoxicillin-clavulanic acid (10 µg), and vancomycin (30 µg). Bacterial strains were cultured overnight on MRS agar medium at 37 °C, and antibiotic discs were placed on the inoculated plates. The inhibitory halos around each disc were measured using a digital caliper. Based on the Performance Standards for Antimicrobial Susceptibility Testing provided by the Clinical and Laboratory Standards Institute, Twenty-Third Information Supplement (CLSI 2013, Wayne, PA), the following zone diameters are indicative of antibiotic susceptibility: cefixime ≥ 19 mm, azithromycin ≥ 18 mm, amoxicillin ≥ 22 mm, doxycycline ≥ 14 mm, trimethoprim-sulfamethoxazole ≥ 30 mm, ciprofloxacin ≥ 21 mm, cephalexin ≥ 18 mm, amoxicillin-clavulanic acid ≥ 18 mm, and vancomycin ≥ 17 mm14.
Probiotic characterization
Antimicrobial activities
The antagonistic activities of the bacterial strains against common human pathogens were investigated using the well-diffusion method. The pathogens tested included Streptococcus sanguinis PTCC 1449, Streptococcus sobrinus PTCC 1601, Streptococcus salivarius PTCC 1448, Streptococcus mutans PTCC 1683, Yersinia enterocolitica ATCC 23,715, Pseudomonas aeruginosa PTCC 1181, Bacillus subtilis ATCC 19,652, Staphylococcus aureus ATCC 25,923, Klebsiella pneumoniae PTCC 1053, Listeria monocytogenes ATCC 13,932, and Shigella flexneri PTCC 1234.
The experimental procedure involved culturing each pathogen at a 0.5 McFarland concentration (1.5 × 10⁸ CFU/mL) on Mueller–Hinton agar medium. Subsequently, wells were created in the inoculated medium, and 70 µL of filtered cell-free supernatant from overnight probiotic cultures was added to each well. The plates were then incubated overnight at 37 °C, and the resulting inhibition halos around the wells were measured using a digital caliper. Additionally, cell-free extracts were separated by centrifugation at 4000 × g for 20 min (at 4 °C) to identify the nature of the antimicrobial compounds supernatant. After the pH was adjusted to 6.2, the extracts were treated with one mg/mL proteinase K and catalase for 2 h at 37 °C. Finally, the antagonistic activities following these treatments were evaluated using the agar-well diffusion method15.
Cell surface hydrophobicity analysis
To evaluate cell surface hydrophobicity, the bacterial strains were cultured overnight and then centrifuged at 6000 × g for 10 min. The resulting cell pellets were resuspended in 3 mL of phosphate-buffered saline (PBS) to achieve a concentration of 1 × 108 CFU/mL. The initial absorbance of the suspension was recorded at 600 nm (A0). Next, the bacterial suspension was vortexed and incubated with 1 mL of xylene (Merck, Germany) for 2 min at 37 °C to separate the bacteria into distinct phases. After phase separation, the absorbance of the aqueous phase was measured (A1). Finally, the cell surface hydrophobicity was calculated as a percentage using the formula (1 - A1/A0) × 1001.
Cell adhesion assay
To assess cell adherence, Caco2 cells were cultured on glass slides in 6-well tissue culture plates in RPMI-1640 medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum and 50 units/mL penicillin-streptomycin. After 24 h of incubation at 37 °C with 5% CO2, the monolayers were rinsed twice with PBS (pH 7.4). Subsequently, a 10 mL bacterial suspension containing 1 × 107 CFU/mL was added to each dish. The cell dishes were then incubated for 2 h at 37 °C, followed by three washes with PBS to remove nonadherent bacterial cells. Adherent bacterial cells were detached using a 0.05% trypsin-EDTA solution and suspended in 10 mL of NaCl solution. The adherence rate was determined by comparing the number of attached bacterial cells to the total number of bacterial cells16.
Cholesterol absorption capacity
To evaluate the cholesterol absorption capacity of the bacterial strains, they were cultured in MRS medium supplemented with 150 µg/mL water-soluble cholesterol (polyoxyethylcholesteryl sebacate; Sigma) and 0.3% oxgall bile salts at 37 °C. After incubation, the bacterial cells were centrifuged for 15 min at 4000 × g to separate them from the medium. The amount of cholesterol remaining in the aqueous phase was quantified using the o-phthalaldehyde method16.
Hemolytic activity
After 48 h of bacterial cell culture (1 × 108 CFU/mL) on blood agar (Oxoid) supplemented with 7% (v/v) sheep blood at 37 °C, hemolytic activity tests, which are indicative of microbiological safety, were assessed and categorized as follows:
β-Hemolysis was indicated by clear, well-defined zones of complete hemolysis around the bacterial colonies.
α-Hemolysis: characterized by a greenish discoloration or partial clearing around the colonies.
γ-Hemolysis: characterized by the absence of hemolysis or any change in the appearance of the blood agar around the colonies.
These classifications provide insights into the hemolytic potential of the bacterial strains under investigation17.
Autoaggregation and coaggregation analysis
For the autoaggregation analysis, the bacterial strains were harvested after 24 h of incubation (107–108 CFU/mL) by centrifugation at 4000 × g for 10 min. Subsequently, the cells were washed with sterile PBS and suspended in PBS at 37 °C for 4 h without agitation. Autoaggregation was quantified using the following formula:
(%) = (A0 – At)/At × 100.
Where A0 is the absorbance at time 0, and At is the absorbance at time t after incubation18.
For the coaggregation test, bacterial strains and three pathogens (E. coli ATCC 25922, L. monocytogenes ATCC 13932, and B. subtilis ATCC 19652) were coincubated at a concentration of 107–108 CFU/mL for 4 h at 37 °C without agitation. The coaggregation percentage was calculated using the following formula:
(%) = [(Ap + Ai)/2 - (Amix)/(Ap + Ai)/2] × 100.
Here, Ap, Ai, and Amix represent the absorbance of the pathogenic bacteria, the isolated strains, and their mixture after the incubation period, respectively18,19.
Cell culture conditions and treatments
Human epithelial carcinoma cells (KBs), oral squamous cell carcinoma (OSCC), fibroblasts, and human umbilical vein endothelial cell (HUVEC) cell lines were obtained from the Pasteur Institute in Iran. These cell lines were cultured in Eagle’s minimum essential medium (EMEM) sourced from Sigma‒Aldrich (catalog no. M4655) supplemented with 10% fetal bovine serum (FBS) from Gibco (catalog no. 10270-106) and 1% penicillin-streptomycin solution from PAN-Biotech (catalog no. P06-07100). The cells were maintained at 37 °C with 5% CO2. Upon reaching 80% confluence, the cells were detached using 0.25% trypsin/EDTA (PAN-Biotech; Catalog No.: P10-029100) and seeded into 96-well plates at a density of 2000 cells per well for a 24-hour incubation period. Subsequently, the EMEM was replaced with 200 µL of bacterial suspension at various concentrations (1–25 µg/mL) (without antibiotics) adjusted to pH 7. The cells were then cultured for 24, 48, or 72 h. The experimental design included several control groups: untreated cells, cells treated with standard growth medium (presumably MRS), cells treated with anticancer drugs (doxorubicin at 0.7 µg/mL and paclitaxel at 0.5 µg/mL), and cells treated with a commercial probiotic strain (Lactobacillus plantarum subsp. plantarum PTCC 1896 at 25 µg/mL)20.
Cytotoxicity test on cancer and normal cell lines
In the MTT assay, 180 µL of cells at a concentration of 2 × 104 cells/mL were seeded into each well of a 96-well plate and incubated for 24 h. Subsequently, different concentrations (20 µL) of metabolites extracted from bacteria (108 CFU/mL) (ranging from 1 to 25 µg/mL) were added and incubated at 37 °C. After 24, 48, and 72 h of further incubation, MTT solution (5 mg/mL, 20 µL; Sigma‒Aldrich, Catalog No. M5655-1G) was added to each well, after which the cells were incubated for an additional 3 h. The culture medium was then removed, and the formazan crystals produced by the metabolically active cells were dissolved in 150 µL of dimethyl sulfoxide (Sigma‒Aldrich, Catalog No. 472301–1 L). The absorbance of the MTT formazan product was measured using an ELISA reader (Bioteck, Synergy H1, USA) at wavelengths between 570 and 630 nm.
For comparison purposes, absorbance values were measured for several control groups: untreated cells, cells treated with MRS (as a reference medium), cells treated with a commercial probiotic strain (L. plantarum subsp. plantarum PTCC 1896 at 108 CFU/mL), and cells treated with anticancer drugs (doxorubicin at 0.7 µg/mL and paclitaxel at 0.5 µg/mL). To identify and characterize anticancer metabolites (active proteins), pronase (Roche Applied Science) was added to the bacterial supernatants at a concentration of 1 mg/mL. The mixture was then incubated for 30 min at 37 °C. The role of active proteins was evaluated by comparing absorbance values between samples treated commonly, pronase-treated samples, and untreated cells with drug-treated cell lines21. The percentage of viable cells for each sample was calculated using the following formula:
(%) = (Asample-Ablank)/(Acontrol-Ablank) × 100%.
Where A sample is the absorbance value of the sample, A blank is the absorbance value of the blank (medium without cells), and A control is the absorbance value of the control (untreated cells). This approach enabled the assessment of the potential anticancer activities of the bacterial metabolites, particularly the impact of active proteins after pronase treatment.
Maximum safe dose
Peripheral blood is a dependable and easily accessible source of normal human cells for determining the maximum safe dosage of anticancer agents. In this method, heparinized blood is gently mixed with an equal volume of Ficoll Hypaque solution (concentration 1.077 g/mL) and then centrifuged for 30 min at 2000 rpm. The resulting buffy layer containing peripheral blood mononuclear cells (PBMCs) was separated by washing in a phosphate saline buffer solution for 5 minutes at 2000 rpm. The PBMCs were subsequently resuspended in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% FBS. Viability was assessed using 0.5% trypan blue staining and a hemocytometer. For the cytotoxicity test, approximately 1 × 105 blood cells were placed into each well of a 96-well microtiter plate. Various treatments, including successive dilutions of bacterial extracts (108 CFU/mL) ranging from 100 to 3000 µg/mL and commonly used chemotherapeutic drugs such as doxorubicin (0.7 µg/mL) and paclitaxel (0.5 µg/mL), were added to the cells. Control treatments without extracts or drugs were also included. After 72 h of incubation in a 5% CO2 incubator, each well was treated with 20 µL of MTT solution (5 mg/ml in PBS, pH 7) at 37 °C for 4 h. Following centrifugation at 2000 rpm for 10 min, the MTT solution was removed, and the blue formazan crystals within the cells were dissolved in 150 µL of DMSO. Finally, the IC50 (the concentration causing 50% cellular death based on the absorbance at 570 nm) and the safe dose coefficient (EC100, the concentration sustaining 100% cell viability) were calculated using GraphPad InStat software22.
Fluorescence staining
To stain cells with dual acridine orange/ethidium bromide (AO/EB), the following protocol was used. The cells were initially cultured in 24-well plates and treated with the optimal concentration of the bacterial extracts (25 µg/mL, 108 CFU/mL). After 12 h of treatment, the cells were harvested and washed three times with cold phosphate-buffered saline (PBS). Subsequently, the cells were suspended in a 100 µL mixture of acridine orange and ethidium bromide, maintaining a ratio of 1:100 mg/mL. Finally, 10 µL of the stained cell suspension was placed on a slide, and both normal intact and apoptotic cells were visualized using a fluorescence microscope (Bioteck, CellCyation, USA)20.
Apoptotic gene expression
In this study, we investigated the role of apoptotic genes, specifically the second mitochondria-derived activator of caspases23 and baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5, also known as SURVIVIN), in regulating programmed cell death (apoptosis) in cancer cells. Cancer cells were seeded in 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h. Subsequently, the cells were treated with secretions from C9, C47, or pronase-treated C9 and C47 cells at a concentration of 25 µg/mL for 72 h. The control groups included untreated cancer cells and cells treated with a commercial probiotic strain (L. plantarum subsp. plantarum PTCC 1896 at 25 µg/mL, 108 CFU/mL).
Total RNA was extracted from both treated and untreated cancer cells using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, USA). After extraction, RNA quality and quantity were assessed using a NanoDrop device and electrophoresis on a 1% agarose gel. Subsequently, cDNA synthesis was performed according to the manufacturer’s instructions. Quantitative real-time PCR (qRT‒PCR) was used to analyze the mRNA expression of apoptosis-related genes (SMAC and SURVIVIN), with the β-actin gene serving as the internal control. The specific primers used were as follows:
The sequence of the β-actin gene was as follows: Forward primer: AGAGCTACGAGCTGCCTGAC; Reverse primer: AGCACTGTGTTGGCGTACAG.
The primers used for the SMAC gene were as follows: forward primer, CAGAGGAGGAAGATGAAGTGTG; reverse primer, GCGGTTATAGAGGCCTGATCTG.
SURVIVIN gene: Forward primer: CCCTTTCTCAAGGACCACCG, Reverse primer: GTTCCTCTATGGGGTCGTCA.
The PCR mixture included cDNA, PCR premix, and forward and reverse primers at specified ratios. The PCR protocol comprised an initial denaturation step at 94 °C for 1 min, followed by 40 cycles of denaturation at 94 °C for 20 s, annealing at 59 °C for 30 s, and extension at 72 °C for 30 s20.
Molecular identification
PCR amplification of the bacterial 16 S rRNA gene (1500 bp) for each strain was performed using Lactobacillus-specific primers (forward: 5′-AGAGTTTGATCMTGGCTCAG-3′ and reverse: 5′-TACCTTGTTAGGACTTCACC-3′). The amplification protocol consisted of an initial denaturation step at 95 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 60 s, annealing at 57 °C for 60 s, and extension at 72 °C for 120 s, with a final extension at 72 °C for 10 min.
After amplification, the PCR products were sequenced by the Macrogen DNA Sequencing Service (Korea), and the sequences were aligned using the ClustalW program. Sequence alignments were subsequently compared to reference bacterial strains in the GenBank database to identify and classify the isolated strains based on their 16 S rRNA gene sequences.
Statistical analysis
All the experiments were conducted in triplicate following a completely randomized design. This approach ensures the robustness and reliability of the experimental results by minimizing bias and accounting for variability. The obtained means and their corresponding standard deviations were reported. The data analysis was conducted using SPSS 18 software, employing Duncan’s test to determine the significance of the samples at a 95% confidence level (P < 0.05). This statistical method enables the comparison of multiple groups and the identification of significant differences among them based on the experimental data. Duncan’s multiple-range test was chosen for its increased sensitivity in detecting behavioral differences within treatment groups, a crucial attribute for exploratory biological studies involving probiotic strains. This statistical approach has been employed in other recent microbiological investigations.
Results and discussion
Biochemical and morphological analysis of Lactobacillus strains
Lactobacillus strains within the lactic acid bacteria group are regarded as the safest microorganisms for consumption or inclusion in food products due to their established health-promoting effects. Biochemical and morphological analyses were conducted using specific growth media (MRS) to isolate selected Lactobacillus strains from 50 samples of traditional cheese collected randomly from remote regions in Kermanshah Province. The isolated bacterial colonies exhibited a whitish-to-creamy hemispherical form, and 21 gram-positive, catalase-negative bacteria with bacillus-like morphology were identified as Lactobacilli. These 21 strains were subsequently cultured in MRS media under anaerobic conditions and selected for further characterization (Table 1).
Simulated Gastrointestinal tract (GIT) tolerance test
To evaluate the efficacy of isolated probiotic strains, a comprehensive characterization of their properties is crucial. This characterization entails subjecting the probiotics to various in vitro tests, including exposure to adjusted PBS supplemented with elevated bile salt concentrations and a low pH24, as well as assessment using a dynamic gastrointestinal model that simulates gastric and intestinal growth conditions25. Following a meal, the pH levels in the human stomach and oral cavity typically range from 1.5 to 4.5 and from 6.4 to 6.8, respectively, with food digestion lasting up to 3 h26. Additionally, the ability of probiotics to survive within the digestive tract after ingestion and colonization hinges on their capacity to withstand the 0.3% (w/v) bile salts secreted by the digestive system over 4 h. Consequently, initial in vitro tests were conducted using pH levels of 2.5 and 6.6 for 3 h, along with exposure to 0.3% (w/v) bile salts for 4 h.
Following exposure to bile salts and acidic conditions, all 21 selected strains exhibited varying growth rates. However, the results indicated that five Lactobacillus strains, specifically C8, C9, C21, C31, and C47, demonstrated robust survival rates (> 72%) under these challenging conditions (Table 1). Prior research has highlighted the difficulty that Lactobacillus species face in surviving within the harsh environment of the stomach and duodenum. Consequently, these strains must possess the capacity to withstand acidic, bile-saline, and enzymatic conditions to thrive, persist, and actively participate in gastrointestinal transit.
Table 2 presents the outcomes related to the viability of the Lactobacillus strains under simulated digestive conditions. According to the results, the isolated strains exhibited robust survival during the initial hour of incubation. Under gastric conditions, a modest decrease in log CFU/mL occurred after the first hour, ranging from 0.3 to 1.6, followed by a substantial decrease between 1 and 2 h, ranging from 2.5 to 6.5. By the end of the gastric digestion period, strains C8, C9, C21, C31, and C47 demonstrated survival rates of 30%, 71%, 21%, 20%, and 36%, respectively (Table 2). In contrast, under intestinal conditions, all the Lactobacillus strains exhibited greater viability during the first hour. However, a significant decrease in log CFU/mL occurred between 1 and 2 h, followed by a minor decrease between 2 and 3 h. According to our findings, C8, C9, C21, C31, and C47 exhibited survival rates of 41%, 75%, 34%, 36%, and 48%, respectively (Table 2).
The C9 and C47 strains exhibited favorable outcomes, with viabilities exceeding 72% in environments characterized by high bile salt concentrations and low pH. Additionally, these strains demonstrated more than 36% viability under both gastric and intestinal digestive conditions. Consistent with our findings, previous research has highlighted that Lactobacillus strains maintain their viability even after exposure to a pH of 6.6. However, the tested strains exhibited low resistance under gastric and intestinal digestive conditions (pH 2.5) and moderate resistance to a bile salt environment (0.3% w/v).
Antibiotic susceptibility and hemolysis activity
Considering antibiotic susceptibility is crucial when selecting probiotics. Probiotic bacteria inherently exhibit susceptibility to antibiotics due to their fixed and mobile genetic factors27. However, the widespread use of antibiotics has led to the emergence of novel antibiotic-resistant genes in probiotics. The transfer of these resistance genes to pathogens within the digestive system poses significant challenges to antibiotic resistance.
The susceptibility of Lactobacillus strains to nine important antibiotics is displayed in Table 3. Notably, all five strains were resistant to cefixime, trimethoprim, sulfamethoxazole, and vancomycin. Conversely, these strains exhibited low resistance to other antibiotics. The C9 strain performed exceptionally well, showing susceptibility or semi-susceptibility to six antibiotics: amoxicillin, doxycycline, azithromycin, ciprofloxacin, amoxicillin-clavulanic acid, and cephalexin (Table 3). Assessing the susceptibility of Lactobacillus strains, including the C9 strain, is critical for safety considerations28. Notably, some LAB probiotic strains, particularly those in the Lactobacillus genus, harbor resistance genes for trimethoprim, sulfamethoxazole, cefixime, and vancomycin, corroborating our findings14,15. The heightened antibiotic sensitivity of the C9 strain likely results from the limited use of antibiotics in livestock in the rural districts of Kermanshah Province, where we collected traditional cheese samples. However, other researchers have reported elevated antibiotic resistance in Lactobacillus strains isolated from traditional dairy products, contrary to our observations13,29 (Table 4).
Among the five tested strains, only C9 lacked α- or β-hemolytic properties and exhibited γ-hemolytic activity, as indicated in Table 5. The remaining strains, C8, C21, C31, and C47, demonstrated α-hemolytic activities. Notably, some Lactobacillus strains, similar to our strains, did not exhibit hemolytic activity, as reported in other studies. However, it is essential to recognize that the absence of blood cell hemolysis (γ-hemolytic) does not necessarily guarantee the safety of probiotic strains, as outlined in existing guidelines.
The resistance to vancomycin and cefixime observed in strains such as C9 and C47 raises reservations about the clinical use of these strains. Resistance to vancomycin in Lactobacillus spp. is typically considered to be intrinsic, chromosomally encoded, and non-transferrable and, therefore, is not an actual biosafety issue1. However, resistance to cefixime may be due to acquired and potentially mobile genetic elements. Probiotic strains that are intended for treatment should, therefore, be assessed with whole genome sequencing to establish the presence and mobility of antibiotic resistance genes that might confer a risk of resistance spreading from the probiotic strain to pathogenic bacteria. Infection control procedures will be crucial in preventing the possibility of horizontal gene transfer to pathogenic bacteria and ensuring the safe application of probiotics in populations with increased susceptibility. Leaving aside these possible limitations, the use of these strains in a clinical context should be kept only to non-treatment contexts unless and until analyses have been performed.
Probiotic characterization
In multiple studies, specific probiotic strains have been shown to have protective effects against infections caused by pathogenic microorganisms30,31,32. Lee et al. (2013) highlighted the potential of probiotics to eradicate harmful bacteria, thereby enhancing their health-promoting properties. To meet the criteria for probiotics, selected bacterial strains must exhibit the ability to eliminate both gram-positive and gram-negative pathogenic microorganisms33.
Table 4 presents the inhibitory effects of five isolated strains against eleven pathogens across varying pH conditions (neutral and natural pH) and treatment scenarios involving catalase and proteinase K. The findings indicate that the C9 strain exhibits significant antipathogenic activity, effectively preventing the growth of all the indicator pathogens (Table 4). In contrast, the C47 strain demonstrated moderate antagonistic activity, effectively suppressing the growth of five pathogens, namely, S. aureus, Y. enterocolitica, K. pneumonia, B. subtilis, and S. flexneri (Table 4).
Upon adjusting the pH to 6.8, the C9 strain exhibited no antipathogenic activity against several pathogens, including S. sanguinis, S. salivarius, S. sobrinus, S. mutans, S. aureus, K. pneumoniae, and S. flexneri. Similarly, strains C8, C21, C31, and C47 were unable to suppress the growth of K. pneumoniae or S. aureus. Further investigation revealed that the ability of the strains to inhibit the aforementioned pathogens was attributed to their production of organic acids. Additionally, C9 was ineffective against B. subtilis and L. monocytogenes, while C8 and C47 failed to inhibit B. subtilis, and the C21 strain was ineffective against Y. enterocolitica after treatment with the catalase enzyme. Consequently, the observed pathogen-preventing capability of these strains is linked to their production of hydrogen peroxide. Following treatment with proteinase K, the C9, C8, C47, C21, and C31 strains were found to be ineffective against specific pathogens, such as Y. enterocolitica, P. aeruginosa, S. flexneri, S. sanguinis, and L. monocytogenes. This finding suggested that the proteinaceous extracts (bacteriocins) from these probiotic strains can inhibit the growth of the abovementioned pathogens.
Lactobacillus strains derived from fermented dairy products have demonstrated robust antipathogen activity against various pathogens. The ability of these bacteria to produce organic acids such as lactic, acetic, and hydrochloric acids, along with biosurfactants, bacteriocins, and hydrogen peroxide, contributes to this effect34. Notably, S. aureus and K. pneumoniae were the most susceptible pathogenic bacteria. Additionally, gram-negative pathogens exhibited greater susceptibility to the extracted metabolites than gram-positive pathogens due to the structure of their outer membrane, which includes a thinner peptidoglycan layer in their cell walls35. S. aureus is associated with bacteremia, infective endocarditis, and skin and soft-tissue infections when skin or mucous barriers are compromised. Conversely, K. pneumoniae causes pneumonia and urinary tract infections, particularly in elderly individuals with compromised immune systems. Given the significant drug tolerance observed in both types of bacteria, this Study suggested that isolated lactobacilli with a safe origin and potent antagonistic activity against antibiotic-resistant forms of these pathogens could be recommended, especially for individuals with weakened immune systems.
Assessing the ability of bacteria to adhere to the intestinal epithelium involves evaluating their binding affinity to the hydrophobic phase of fluids, known as surface hydrophobicity. This characteristic is critical because it helps prevent pathogens from attaching to the gut lining, thereby mitigating potential digestive issues. Hydrophobicity rates vary among different Lactobacillus strains, and strain C9 was observed to exhibit a significantly greater hydrophobic rate (55%) than the other tested Lactobacillus strains (Table 5). Surface proteins are pivotal for influencing the diverse ranges of cell surface hydrophobicity observed in Lactobacillus strains. Previous studies have established a positive correlation between cell surface hydrophobicity and bacterial adhesion ability, particularly in potentially probiotic bacteria such as the C9 isolate36. These results emphasize the importance of surface hydrophobicity in the ability of probiotics to prevent pathogen attachment, highlighting strain-specific differences in hydrophobicity.
During the selection of potential probiotic strains, evaluating their ability to bind to intestinal epithelial cells is crucial for determining their suitability. Adherence capacity serves as a fundamental criterion for identifying probiotics. According to the research findings, strains C9 (52%) and C47 (31%) exhibit robust adhesion to human intestinal Caco2 cells. In contrast, the bacterial strains C21 (10%) and C31 (14%) exhibited weak adherence (Table 5). For successful gut colonization, probiotics must adhere to the intestinal mucosa and resist elimination during bowel movements. These results align with previous studies, which highlight that only a subset of Lactobacillus strains effectively adhere to Caco2 cells37.
An essential consideration in probiotic selection is the capacity of probiotics to absorb cholesterol. Probiotics may achieve cholesterol reduction through either binding cholesterol to the external surface of bacterial cells or internalizing it. According to the results, strain C9 exhibited the highest cholesterol uptake, absorbing more than 72% of the total cholesterol after 20 h of incubation. In contrast, strains C8 and C31 absorbed only 16% and 12%, respectively, of the total cholesterol. Key mechanisms involved in probiotic-mediated cholesterol reduction include enzymatic conversion of cholesterol to coprostanol, cholesterol absorption within the cell wall, and disruption of cholesterol micelle formation in the gut via deconjugated bile salts38.
Autoaggregation and coaggregation play crucial roles in preventing pathogen colonization on surfaces39. Autoaggregation involves the self-assembly of microorganisms of the same species, which is common among microorganisms that attach to the intestinal mucosa40. Coaggregation occurs when cells from different microbial strains adhere to each other and is associated with their ability to interact with pathogens. It serves as a potential defense mechanism for hosts against infections41. In the autoaggregation assay, each strain exhibited unique grouping behavior. Among the potential Lactobacillus strains, strain C9 demonstrated the highest autoaggregation percentage (71 ± 1.8%) (Table 5). Additionally, coaggregation was assessed for these five Lactobacillus strains, considering three different pathogens at various time points. All the tested strains could aggregate with pathogenic bacteria; however, the coaggregation patterns were strain-specific and time-dependent. Notably, compared to the other strains, strain C9 exhibited the highest percentage of coaggregation with E. coli (67%), L. monocytogenes (55%), and B. subtilis (46%). These findings highlight the diverse auto- and coaggregation capabilities of the selected Lactobacillus strains, with C9 showing the highest observed aggregation ability (> 46%). The aggregation capability of probiotics is closely linked to their antimicrobial activity. The intricate interactions between surface molecules, including secreted factors and proteins, likely contribute to the antibacterial and aggregation abilities of C9. Previous research has established that aggregation abilities are influenced by environmental and internal factors42. During the release of antimicrobial substances, probiotics engage in close contact with pathogenic bacteria, and their high aggregation capacity can effectively suppress pathogens43.
Prescreening cytotoxicity test on KB cancer cells
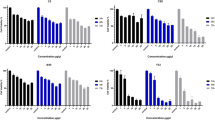
Probiotics offer numerous health benefits, including potential anticancer properties. Extensive research has demonstrated the therapeutic effects of specific Lactobacillus strains, which play a protective role against various cancers, such as liver, breast, gastric, bladder, and colon cancers. In this Study, in vitro cytotoxic analysis was conducted to assess the anticancer effects of the Lactobacillus strains C9 and C47. The present Study involved prescreening tests at different concentrations of the bacterial extract (ranging from 0 to 25 µg/mL) for 24, 48, or 72 h. The results revealed dose- and time-dependent cytotoxic effects of the extracted metabolites on KB cancer cell lines (Fig. 1; Table 6). Notably, the metabolites from strains C9 and C47 exhibited significantly lower cell viability (p < 0.05) at a concentration of 25 µg/mL than at other concentrations (0, 1, 5, 10, 15, and 20 µg/mL). Consequently, the effective anticancer concentration was determined to be 25 µg/mL. Furthermore, the highest anticancer activities and lowest cell viability were observed after 72 h of incubation at this concentration, with both strains showing less than 18% cell viability (Fig. 1).
Cytotoxic effects of bacterial metabolites from strains C9 and C47 on KB cancer cells after 24, 48, and 72 h at various concentrations. Data are presented as mean ± SD (n = 6). IC50 values for each strain at each time point are indicated on the corresponding bars. Control: untreated cancer cell line.
Cytotoxicity test on cancer and normal cell lines
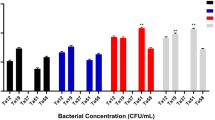
In this Study, the objective was to assess the impact of metabolites extracted from strains C9 and C47 on both oral cancer cells (KB and OSCC) and normal cells (fibroblasts and HUVEK). The evaluation involved comparing treated cells with untreated cells, as well as cells treated with MRS (control medium) and drug-treated cell lines (doxorubicin and paclitaxel) as negative and positive controls (Fig. 2). The MTT assay was used to measure the ability of the cells to convert yellow tetrazolium to blue formazan. The evaluation was conducted at a concentration of 25 µg/mL after 72 h of incubation, which was selected based on prescreening cytotoxicity tests performed on KB cancer cells44.
Cytotoxicity of bacterial metabolites on KB and OSCC cancer cell lines, as well as normal fibroblast and HUVEC cells after 72 h incubation. Data are presented as mean ± SD (n = 6). *p < 0.05, **p < 0.01 vs. untreated control group (Duncan’s test). Control: untreated cancer cell line. MRS: MRS-treated cell lines. Dla: cells treated with an in-market probiotic strain (Lactobacillus plantarum subsp. plantarum PTCC 1896). C9 + pronase: pronase-treated C9 secretion. C47 + pronase: pronase-treated C47 secretion. Dox: cells treated with the doxorubicin anticancer drug. Pac: cells treated with the anticancer drug paclitaxel.
Based on these findings, strains C9 and C47 demonstrated pronounced anticancer effects on KB and OSCC cancer cell lines. After 72 h of incubation, compared with those in the control groups, the mean differences in cancer cell viability between cells treated with the C9 and C47 strains were significantly lower (less than 22%) (p < 0.05). These results highlight the efficacy of these strains in combating cancer. Notably, when the C47 strain was compared to positive controls on KB and OSCC cell lines, cell viability was significantly lower at the 0.05 level, indicating superior anticancer activity compared to commonly used chemical drugs such as doxorubicin and paclitaxel (Fig. 2).
After 72 h of incubation, compared with drug-treated cells, normal cells (fibroblasts and HUVEKs) treated with strains C9 and C47 exhibited significantly (p < 0.05) greater cell viability. These findings support the safety profile of these strains, as they did not cause adverse effects. In contrast, doxorubicin and paclitaxel significantly inhibited the proliferation of rapidly dividing normal cell lines during the same incubation period (Fig. 2). Notably, the secreted compounds from strains C9 and C47 have been approved as safe and cost-effective anticancer agents, demonstrating minimal impact on normal cell lines despite the heightened mortality associated with other anticancer treatments targeting delicate cells and tissues. Interestingly, the C47 strain exhibited superior results and exerted the most pronounced cytotoxic effects on oral cancer cell lines. Furthermore, the cytotoxicity of the C9 and C47 strains in normal cells after 72 h of incubation was lower than that observed in cancer cells, highlighting the specificity of their toxicity effects at this particular incubation time (72 h) for cancerous cells (Fig. 2).
Maximum safe dose
The cytotoxic potential of the probiotic extracts and conventional anticancer drugs was assessed using normal human peripheral blood mononuclear cells (PBMCs). Table 7 shows that the probiotic strains C9 and C47 yielded safer outcomes than commonly used chemotherapy drugs such as doxorubicin and paclitaxel. These chemical drugs exhibited high toxicity against human PBMCs. The IC50 values for C9, C47, paclitaxel, and doxorubicin, which represent the concentrations at which 50% of the normal cells were affected, were 2453.6, 2153.3, 29.8, and 24.2, respectively. Notably, the safe doses for C9, C47, paclitaxel, and doxorubicin were 1218.8, 1078.02, 14.4, and 11.6, respectively. These findings indicate that probiotic extracts, even at high concentrations, maintained 100% cell viability compared to the control drugs. Consequently, probiotics can be considered a safe alternative to traditional chemotherapy in cancer management.
PBMCs, which include monocytes and lymphocytes, are isolated from blood and characterized by their round nuclei. These cells play a crucial role in immune responses and are commonly employed in preclinical and clinical studies related to safety and cytotoxicity45. Cancer is a prevalent global disease that affects individuals of all ages and is rapidly spreading worldwide46. Recent research has highlighted probiotics, particularly LAB, as promising nonchemotherapeutic alternatives for cancer treatment and prevention47. Unlike expensive chemical drugs such as doxorubicin and paclitaxel, which often cause severe side effects and nonspecific toxicity against normal cells (including PBMCs), probiotics, similar to our results, offer a safer approach. Therefore, investigating new anticancer agents, such as probiotic strains, is essential for developing effective biological strategies to control cancer48. The isolation and characterization of new native Lactobacillus strains from dairy products, such as traditional Iranian cheese, which exhibit robust probiotic and anticancer properties as evidenced by our findings, represent a more precise and effective therapeutic approach for the prevention and treatment of prevalent cancers in the community. Based on the findings of this Study, these indigenous strains demonstrate notable advantages over commercial Lactobacillus strains, including increased stability and survival rates in gastrointestinal conditions, superior safety profiles, high antipathogenic activity, compatibility with the host microbiota, and applicability in the treatment of common diseases, including cancer. Consequently, these strains possess significant potential for future commercialization.
Fluorescence staining
The mode of cell death provides insights into the interactions between anticancer agents and neighboring tissues during drug treatment. Necrosis, characterized by oxidative stress and the secretion of proinflammatory cytokines49, contrasts with apoptosis and is a tightly regulated process associated with minimal loss of membrane integrity that occurs prior to secondary necrosis or later stages50.
Following staining with AO/EB fluorescent dyes, viable cells appeared green and intact, while apoptotic cells exhibited an orange hue and displayed signs of shrinkage. Based on the cancer assay results, strain C47 exhibited the highest anticancer effects and the lowest cytotoxicity on normal cell lines. Additionally, this strain demonstrated a more favorable IC50 and safe dose compared to strain C9. Therefore, strain C47 was selected for further investigation of the mode of cell death at its optimal concentration. Notably, C47 secretion significantly reduced the number of viable cancer cells. The mean number of apoptotic KB cancer cells treated with secreted C47 metabolites (25 µg/mL) during different incubation periods (24, 48, and 72 h) was markedly greater (p < 0.05) than that of viable and necrotic cells. Notably, the most significant number of apoptotic cells was observed after 72 h of incubation (Fig. 3).
Dual acridine orange/ethidium bromide (AO/EB) fluorescence staining following incubation with the secreted C47 metabolite (25 µg/mL) in the KB cancer cell line. The panels are as follows: (A) Treated KB cancer cell line after 24 h of incubation, (B) Treated KB cancer cell line after 48 h of incubation, (C) Treated KB cancer cell line after 72 h of incubation, and (D) Untreated KB cancer cell line serving as the control. a: normal intact cells, b: apoptotic cells.
Furthermore, treated KB cancer cells exhibited typical apoptotic features, including condensed chromatin, cell shrinkage, membrane blebbing, and the formation of apoptotic bodies. Interestingly, the red blood cell count remained unchanged, indicating that the predominant mode of cancer cell death was apoptosis rather than necrosis (Fig. 3). Our findings align with recent research demonstrating that AO/EB staining, when observed under a fluorescence microscope, effectively distinguishes apoptosis-related changes in cancer cells. Overall, our results support the notion that the cytotoxic effects of isolated probiotic strains, such as C47, on treated cancer cells primarily occur via apoptosis.
Apoptotic gene expression
The characteristic traits of cancer cells include resistance to apoptosis and unchecked proliferation51. Compounds that promote apoptosis, such as probiotics, are being investigated as promising anticancer agents52. Apoptosis is a crucial pathway in cancer progression, characterized by the activation of proapoptotic genes and the inhibition of antiapoptotic genes. Through modulation of this pathway, probiotics and other therapeutic interventions could trigger apoptosis and impede the growth of cancer cells53.
In the present study, extracts from the probiotic strains C9 and C47 demonstrated significant upregulation of the SMAC gene (a proapoptotic gene) in both KB and OSCC cancer cell lines. Simultaneously, there was a substantial downregulation of the SURVIVIN gene (an antiapoptotic gene) in oral cancer cell lines treated with secreted metabolites from C9 and C47 (Fig. 4). These findings suggest that the metabolites extracted from these probiotic strains may modulate apoptosis-related genes in oral cancer cells. This is consistent with broader evidence that noncoding RNAs act as crucial regulators of apoptotic and inflammatory pathways in various disease contexts, including liver injury during sepsis54. In particular, in oral squamous cell carcinoma (OSCC), the NF-κB signaling pathway has been implicated in promoting cell proliferation and survival, and its inhibition has been shown to suppress tumor growth55. The probiotic-induced regulation of apoptotic genes observed in our study may involve modulation of similar molecular pathways.
Relative expression levels of apoptotic genes (SMAC and SURVIVIN) in KB and OSCC cell lines treated with bacterial metabolites from C9 and C47 strains, with or without pronase. Data are normalized to β-actin and expressed as fold-changes relative to untreated controls. Mean ± SD of six independent experiments. Data are presented as mean ± SD (n = 6). *p < 0.05, **p < 0.01 vs. untreated control group (Duncan’s test). Control: untreated cancer cell line. Dla: cells treated with an in-market probiotic strain (Lactobacillus plantarum subsp. plantarum PTCC 1896). C9 + pronase: Pronase-treated C9 secretion. C47 + pronase: Pronase-treated C47 secretion.
Interestingly, untreated cancer cell lines and those treated with an in-market probiotic strain (L. plantarum subsp. plantarum PTCC 1896) as a control did not exhibit significant changes in apoptotic gene expression. This finding suggested that alternative cell death mechanisms operate in these control groups and that apoptosis is not the primary cytotoxic factor in oral cancer cell lines (Fig. 4). Furthermore, when comparing pronase-treated C9 and C47 metabolites to those of normal C9 and C47 extracts, the mean differences were significantly lower for the SMAC gene and notably greater for the SURVIVIN gene. This highlights the crucial role of effective proteins in mediating the apoptotic effects of C9 and C47 secretions (Fig. 4).
Consistent with our results, other studies have suggested that probiotics exert anticancer effects by modulating the expression of apoptosis-related genes, including MAPK, PTEN, SMAC, SURVIVIN, and BCL-220,56. Various components of probiotic-derived metabolites contribute to cancer cell death by regulating apoptosis-related gene expression. Notably, our findings emphasize the importance of proteins in driving the apoptotic effects of probiotic strains C9 and C47, in contrast to previous research suggesting that other components (such as polysaccharides and peptidoglycans) in the supernatant are potential mediators of probiotic anticancer activity. The involvement of specific proteins in the apoptotic mechanisms triggered by probiotic metabolites, as well as the secreted bacteriocins of the C9 and C47 strains, aligns with findings from other studies, highlighting the significance of understanding the protein-based nature of probiotic activities20,57. Although these results are promising, it is crucial to recognize the complexity of apoptotic pathways and the necessity for further mechanistic studies to identify the specific molecules and pathways involved. Furthermore, in vivo studies and clinical trials are crucial for translating these findings into practical applications.
The upregulation of SMAC and the downregulation of SURVIVIN were observed in treated cancer cells from the in vitro trials, which could plausibly be the result of host-pathogen interaction pathways activated by probiotic-derived proteins. One possible way this may have occurred is if the probiotic proteins recruited and activated host pattern recognition receptors, for example, by identifying bacterial-derived components via their toll-like receptors (TLR) to promote signaling pathways. The added probiotic-derived peptides or bacteriocins bind to TLR on cancer cells or on immune cells involved in the tumor microenvironment, which can activate new signaling pathways to lead to the inhibition of positive regulation via NF-κB, promoting the expression of proapoptotic genes and inhibiting SURVIVIN1,2. Previous reports have shown that inhibition of NF-κB activity increases SMAC expression and facilitates apoptosis of cancer cells3. It is a strong possibility that the proteinaceous compounds from the Lactobacillus strains C9, and C47 alter the networks regulating intracellular apoptosis in cancer cells if we can solidify the immunomodulatory and receptor-mediated alterations.
Numerous investigations have shown that bacteriocins produced by strains of L. plantarum and L. fermentum may induce apoptosis in cancer cells. For instance, plantaricin—a bacteriocin from L. plantarum that has been characterized in detail—has been shown to induce physiological effects in the colorectal and oral cancer cells through mitochondria-mediated apoptosis and caspase activation1,2. Likewise, proteinaceous products from L. fermentum have shown apoptotic effects in vitro. This mechanism may be related to the regulation of proapoptotic gene expression and reactive oxygen species3,4. These findings are consistent with our observation that anticancer activity was negatively impacted by pronase treatment. This suggests that proteins, most likely bacteriocin-like peptides, secreted by strains C9 and C47 may have played a crucial role as mediators of apoptosis in the oral cancer cells.
Molecular identification
Sequencing of the 16 S rRNA gene confirmed the accuracy of the phenotypic descriptions for the selected bacterial strains. Amplification of the 16 S rRNA genes revealed that all five chosen strains belonged to the Lactobacillus genus. Specifically, strains C8 and C9 were identified as Limosilactobacillus fermentum, while strains C21 and C31 were classified as Lactiplantibacillus pentosus. Strain C47 was assigned to the Lactiplantibacillus plantarum species. These sequences were subsequently deposited in the NCBI GenBank under accession numbers OQ891483, OQ891482, OQ891486, OQ891487, and OQ891485.
The 16 S rRNA sequencing method, known for its robustness, generates easily interpretable patterns. It offers simplicity in execution, rapidity, and reproducibility. Based on these findings, this molecular technique, with its strong discriminatory ability, holds promise as an effective tool for distinguishing and characterizing dairy-associated Lactobacillus strains.
Sequence data confirmed the identity of the isolates at the species level. BLAST analysis demonstrated the following similarity percentages to the reference sequences in the NCBI GenBank database:
C8: Limosilactobacillus fermentum – 99.86% similarity.
C9: Limosilactobacillus fermentum – 99.87% similarity.
C21: Lactiplantibacillus pentosus – 100% similarity.
C31: Lactiplantibacillus pentosus – 100% similarity.
C47: Lactiplantibacillus plantarum – 99.93% similarity.
These high similarity values help confirm the molecular identity of the strains and align with the previously reported phenotypic and functional characteristics.
Overview of isolated lactobacilli
The probiotic characteristics of Lactobacillus strains isolated from traditional Iranian cheese have been extensively studied, revealing numerous beneficial properties. These strains exhibit high stability and survival rates under gastrointestinal conditions, which are crucial for their efficacy as probiotics. Similar results have been observed in other traditional Iranian dairy sources, such as tarkhineh and curd1,58,59. The antimicrobial effects of these Lactobacillus strains are notable, as they produce organic acids, hydrogen peroxide, and bacteriocins, contributing to their broad-spectrum antimicrobial activity. Consistent with other studies, these strains have demonstrated effectiveness against common pathogens, including Escherichia coli, Salmonella spp., and Staphylococcus aureus60,61. Their ability to inhibit the growth of these pathogens highlights their potential as natural preservatives in food and as therapeutic agents in the prevention and treatment of infections62.
This Study has shown that the isolated indigenous Lactobacillus strains, similar to commercialized probiotic strains, can adhere well to intestinal epithelial cells, a key factor for colonization and exerting their health benefits63,64. Additionally, they demonstrate significant cell surface hydrophobicity, autoaggregation, and coaggregation properties, which are essential in probiotic strains and associated with their ability to form biofilms and compete with pathogenic bacteria65,66.
The anticancer effects of the isolated Lactobacillus strains have been a focal point of recent research67,68. These strains exhibit high anticancer potential and effectively suppress the survival and growth of KB and OSCC cancer cell lines. Similar to our results, other probiotic strains have demonstrated cytotoxicity against various cancer cell lines, including those of the colon, breast, and liver69,70. Mechanistic studies suggest that their anticancer activity is mediated through the induction of apoptosis and cell cycle arrest, as well as the production of metabolites that inhibit the proliferation of cancer cells. Furthermore, the expression of apoptotic genes in cancer cells treated with these probiotic strains underscores their potential as adjuncts in cancer therapy71.
Safety is a paramount concern when evaluating probiotic strains for human consumption72. The Lactobacillus strains isolated from traditional Iranian cheese have undergone rigorous safety assessments. As gold standards in probiotics, hemolytic activity tests and cytotoxicity assays on normal cell lines have confirmed their safety profile, showing no adverse effects73. Additionally, the determination of the maximum safe dose further supports their safety for human use. These findings are consistent with the general recognition of Lactobacillus strains from dairy sources as safe for consumption74,75.
When compared to commercial Lactobacillus strains, these indigenous strains demonstrate several advantages. They exhibit higher stability and survival rates under gastrointestinal conditions, superior antipathogenic properties, high anticancer properties, and better compatibility with the local microbiota. These characteristics make them more effective and reliable for use in the local population, where they can be easily incorporated into the traditional diet. This investigation has revealed their promising probiotic characteristics, antimicrobial and anticancer effects, as well as their safety.
Recent psychological interventions, such as cognitive behavioral therapy (CBT), have demonstrated benefits for cancer patients beyond pharmacological treatments76, suggesting that integrating biological approaches like probiotics with psychosocial care may enhance therapeutic outcomes. Similarly, acceptance and commitment therapy (ACT), particularly in web-based formats, has shown promise in improving psychological outcomes among cancer patients77, highlighting the value of integrating digital and biological tools in supportive cancer care. Beyond oncology, probiotics and plant-derived bioactives have both demonstrated immunomodulatory and microbiota-modifying effects, as shown in animal studies using Litsea cubeba essential oil78. Additionally, AI-based diagnostic frameworks such as deep learning models for cancer detection are emerging rapidly79, and may complement biological strategies like probiotic therapy in personalized oncology. These indigenous strains offer significant therapeutic potential and can be considered valuable resources for the development of functional foods and probiotic supplements.
Conclusion
The experimental design ensured that the traditional cheese samples collected from Kermanshah province in Iran were representative of the entire population. Although lactobacilli from dairy sources are generally considered safe, additional safety tests were conducted for certainty. The efficacy and probiotic potential of the isolated strains were evaluated using various tests and different control groups. In summary, among the examined Lactobacillus strains, L. fermentum C9 and L. plantarum C47 demonstrated probiotic potential in several attributes. These include antipathogenic activity, cholesterol assimilation, tolerance to high bile salt concentrations and low pH, hydrophobicity, auto- and coaggregation capabilities, and safety aspects of isolates, such as antibiotic susceptibility and hemolytic activity. Moreover, the bacteriocin-like substances derived from these strains, which are composed of proteins, effectively suppressed the growth of KB and OSCC cancer cell lines.
Nevertheless, a crucial limitation of this Study is the absence of in vivo safety data. Although the in vitro tests confirmed the non-hemolytic characteristics and also confirmed the safety of the strains on human PBMCs, further toxicity testing in an animal model would be recommended to confirm the clinical potential of the strains. As a result, L. fermentum C9 and L. plantarum C47 have emerged as promising candidates for potential oral cancer therapies.
Data availability
Sequence data that support the findings of this study have been deposited in the NCBI Archive with the primary accession codes OQ891483, OQ891482, OQ891486, OQ891487, and OQ891485.
References
Kiani, A. et al. Tarkhineh as a new microencapsulation matrix improves the quality and sensory characteristics of probiotic Lactococcus lactis KUMS-T18 enriched potato chips. Sci. Rep. 11 (1), 12599 (2021).
Nami, Y. et al. Impacts of alginate–basil seed mucilage–prebiotic microencapsulation on the survival rate of the potential probiotic Leuconostoc mesenteroides ABRIINW. N18 in yogurt. Int. J. Dairy Technol. 76 (1), 138–148 (2023).
Nami, Y. et al. Administration of microencapsulated Enterococcus faecium ABRIINW. N7 with fructo-oligosaccharides and Fenugreek on the mortality of tilapia challenged with Streptococcus agalactiae. Front. Veterinary Sci. 9, 938380 (2022).
Sung, W. W. et al. Favorable lip and oral cancer mortality-to-incidence ratios in countries with high human development index and expenditures on health. Int. J. Environ. Res. Public Health. 18 (11), 6012 (2021).
Ghantous, Y. & Elnaaj, A. Global incidence and risk factors of oral cancer. Harefuah 156 (10), 645–649 (2017).
Hema, K. et al. Epigenetics in oral squamous cell carcinoma. J. Oral Maxillofacial Pathol. 21 (2), 252–259 (2017).
Mahjoory, Y. et al. Antifungal activity of potential probiotic Limosilactobacillus fermentum strains and their role against toxigenic aflatoxin-producing aspergilli. Sci. Rep. 13 (1), 388 (2023).
Nami, Y. et al. A newly isolated probiotic Enterococcus faecalis strain from vagina microbiota enhances apoptosis of human cancer cells. J. Appl. Microbiol. 117 (2), 498–508 (2014).
Haghshenas, B. et al. Anti-proliferative effects of Enterococcus strains isolated from fermented dairy products on different cancer cell lines. J. Funct. Foods. 11, 363–374 (2014).
Abedi, J. et al. Selenium-enriched Saccharomyces cerevisiae reduces the progression of colorectal cancer. Biol. Trace Elem. Res. 185, 424–432 (2018).
Nami, Y. et al. The prophylactic effect of probiotic Enterococcus lactis IW5 against different human cancer cells. Front. Microbiol. 6, 1317 (2015).
Yang, M. et al. Study of the probiotic properties of lactic acid bacteria isolated from Chinese traditional fermented pickles. J. Food Process. Preserv. 41 (3), e12954 (2017).
Haghshenas, B. et al. Isolation and characterization of probiotics from dairies. Iran. J. Microbiol. 9 (4), 234 (2017).
Sadeghi, M. et al. Screening of potential probiotic lactic acid bacteria with antimicrobial properties and selection of superior bacteria for application as biocontrol using machine learning models. LWT 162, 113471 (2022).
Shahverdi, S. et al. In-vitro and in-vivo antibacterial activity of potential probiotic Lactobacillus paracasei against Staphylococcus aureus and Escherichia coli. Heliyon, 9(4). (2023).
Kiani, A. et al. Application of Tarkhineh fermented product to produce potato chips with strong probiotic properties, high shelf-life, and desirable sensory characteristics. Front. Microbiol. 12, 657579 (2021).
Shivani, T. M. & Sathiavelu, M. Probiotic evaluation, adherence capability and safety assessment of Lactococcus lactis strain isolated from an important herb Murraya koenigii. Sci. Rep. 14 (1), 15565 (2024).
Alizadeh Behbahani, B. et al. Exploring the probiotic potential of Lactiplantibacillus pentosus SM1: resistance, antimicrobial activity, anti-biofilm, cytotoxic activity, and safety properties. LWT 210, 116850 (2024).
Nami, Y. et al. Application of unsupervised clustering algorithm and heat-map analysis for selection of lactic acid bacteria isolated from dairy samples based on desired probiotic properties. LWT 118, 108839 (2020).
Nami, Y. et al. Anti-oral cancer properties of potential probiotic lactobacilli isolated from traditional milk, cheese, and yogurt. Sci. Rep. 14 (1), 6398 (2024).
Rahbar Saadat, Y. et al. Prophylactic role of Lactobacillus paracasei exopolysaccharides on colon cancer cells through apoptosis not ferroptosis. Pharm. Sci. 27 (2), 251–261 (2020).
Abd Ellatif, S. A. et al. Assessment of probiotic efficacy and anticancer activities of Lactiplantibacillus plantarum ESSG1 (MZ683194.1) and Lactiplantibacillus pentosus ESSG2 (MZ683195.1) isolated from dairy products. Environ. Sci. Pollut. Res. 29 (26), 39684–39701 (2022).
Gobbetti, M. et al. Production of angiotensin-I-converting-enzyme-inhibitory peptides in fermented milk started by Lactobacillus delbrueckii subsp. Bulgaricus SS1 and Lactococcus lactis subsp. Cremoris FT4. Appl. Environ. Microbiol. 66 (9), 3898–3904 (2000).
Park, J. H. et al. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell. Immunol. 219 (1), 22–27 (2002).
Fernández, M. F., Boris, S. & Barbes, C. Probiotic properties of human lactobacilli strains to be used in the Gastrointestinal tract. J. Appl. Microbiol. 94 (3), 449–455 (2003).
Jacobsen, C. N. et al. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. By in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 65 (11), 4949–4956 (1999).
Zheng, M. et al. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front. Microbiol. 8, 908 (2017).
Guo, L. et al. Probiotic properties of Enterococcus strains isolated from traditional naturally fermented cream in China. Microb. Biotechnol. 9 (6), 737–745 (2016).
Nami, Y., Haghshenas, B. & Khosroushahi, A. Y. Molecular identification and probiotic potential characterization of lactic acid bacteria isolated from human vaginal microbiota. Adv. Pharm. Bull. 8 (4), 683 (2018).
Arqués, J. L. et al. Antimicrobial activity of lactic acid bacteria in dairy products and gut: effect on pathogens. Biomed. Res. Int. 2015 (1), 584183 (2015).
Campana, R., van Hemert, S. & Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathogens. 9, 1–12 (2017).
V. J, R.K., et al., Putative probiotic Lactobacillus spp. From Porcine Gastrointestinal tract inhibit transmissible gastroenteritis coronavirus and enteric bacterial pathogens. Trop. Anim. Health Prod., 42: pp. 1855–1860. (2010).
Lee, J. S., Chung, M. J. & Seo, J. G. Vitro evaluation of antimicrobial activity of lactic acid bacteria against Clostridium difficile. Toxicol. Res. 29 (2), 99–106 (2013).
Azizi, F., Habibi Najafi, M. B., Edalatian, M. R. & Dovom The biodiversity of Lactobacillus spp. From Iranian Raw milk motal cheese and antibacterial evaluation based on bacteriocin-encoding genes. AMB Express. 7 (1), 176 (2017).
Sharma, C. et al. Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech. 7 (1), 31 (2017).
Nami, Y. et al. Hypocholesterolemic activity of a novel autochthonous potential probiotic Lactobacillus plantarum YS5 isolated from yogurt. LWT 111, 876–882 (2019).
Javanshir, N. et al. Evaluation of the function of probiotics, emphasizing the role of their binding to the intestinal epithelium in the stability and their effects on the immune system. Biol. Procedures Online. 23, 1–17 (2021).
Vourakis, M., Mayer, G. & Rousseau, G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 22 (15), 8074 (2021).
Sharma, K., Attri, S. & Goel, G. Selection and evaluation of probiotic and functional characteristics of autochthonous lactic acid bacteria isolated from fermented wheat flour dough bamboo. Probiotics Antimicrob. Proteins. 11, 774–784 (2019).
Lukic, J. et al. Aggregation factor as an inhibitor of bacterial binding to gut mucosa. Microb. Ecol. 68, 633–644 (2014).
Zhang, W., Liu, M. & Dai, X. Biological characteristics and probiotic effect of Leuconostoc lactis strain isolated from the intestine of black porgy fish. Brazilian J. Microbiol. 44, 685–691 (2013).
Goh, Y. J. & Klaenhammer, T. R. Functional roles of aggregation-promoting-like factor in stress tolerance and adherence of Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 76 (15), 5005–5012 (2010).
Arena, M. P. et al. Immunobiosis and probiosis: Antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl. Microbiol. Biotechnol. 102, 9949–9958 (2018).
Prinsloo, S., Pieters, R. & Bezuidenhout, C. C. A cell viability assay to determine the cytotoxic effects of water contaminated by microbes. South Afr. J. Sci. 109 (7), 1–4 (2013).
Kleiveland, C. R. et al. Peripheral Blood Mononuclear Cells, in The Impact of Food Bioactives on Health: in vitro and ex vivo models, K. Verhoeckx, Editors. Springer International Publishing: Cham. pp. 161–167. (2015).
Fidler, M. M. et al. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 18 (12), 1579–1589 (2017).
Zhong, L., Zhang, X. & Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 20 (24), 7878–7886 (2014).
Haghshenas, B. et al. Cytotoxic effect of potential probiotic Lactiplantibacillus plantarum KUMS-Y8 isolated from traditional dairy samples on the KB and OSCC human cancer cell lines. Heliyon 9 (9), e20147 (2023).
Anderson, H. et al. Oxidative stress inhibits the phagocytosis of apoptotic cells that have externalized phosphatidylserine. Cell. Death Differ. 9 (6), 616–625 (2002).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit Proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 101 (4), 890–898 (1998).
Loftus, L. V., Amend, S. R. & Pienta, K. J. Interplay between cell death and cell proliferation reveals new strategies for Cancer therapy. Int. J. Mol. Sci., 23(9). (2022).
Kumar, R. & Dhanda, S. Mechanistic insight of probiotics derived anticancer pharmaceuticals: A road forward for Cancer therapeutics. Nutr. Cancer. 69 (3), 375–380 (2017).
Kumar, R. & Raghava, G. P. ApoCanD: database of human apoptotic proteins in the context of cancer. Sci. Rep. 6, 20797 (2016).
Wang, J. et al. Noncoding RNAs in sepsis-associated acute liver injury: roles, mechanisms, and therapeutic applications. Pharmacol. Res., : p. 107596. (2025).
Cheng, Y. et al. The Investigation of Nfκb Inhibitors To Block Cell Proliferation in OSCC Cells Lines (Current Medicinal Chemistry, 2024).
Ha, S., Zhang, X. & Yu, J. Probiotics intervention in colorectal cancer: from traditional approaches to novel strategies. Chin. Med. J. 137 (1), 8–20 (2024).
Martín, I. et al. Study of lactic acid bacteria isolated from traditional ripened foods and partial characterization of their bacteriocins. LWT 173, 114300 (2023).
Haghshenas, B. et al. Probiotic properties and antimicrobial evaluation of silymarin-enriched Lactobacillus bacteria isolated from traditional curd. Sci. Rep. 13 (1), 10916 (2023).
Kiani, A. et al. Application of Tarkhineh fermented product to produce potato chips with strong probiotic properties, high Shelf-Life, and desirable sensory characteristics. Front. Microbiol., 12. (2021).
Nami, Y. et al. Novel autochthonous lactobacilli with probiotic aptitudes as a main starter culture for probiotic fermented milk. LWT 98, 85–93 (2018).
Goudarzi, F. et al. Potential probiotic Lactobacillus delbrueckii subsp. Lactis KUMS-Y33 suppresses adipogenesis and promotes osteogenesis in human adipose-derived mesenchymal stem cell. Sci. Rep. 14 (1), 9689 (2024).
Erfani, A. et al. Specific species of probiotic bacteria as bio-preservative cultures for control of fungal contamination and spoilage in dairy products. Int. Dairy J. 151, 105863 (2024).
Walsh, C. et al. HMO-primed bifidobacteria exhibit enhanced ability to adhere to intestinal epithelial cells. Front. Microbiol. 14, 1232173 (2023).
Jayashree, S. et al. Genome-wide identification of probiotic Escherichia coli Nissle 1917 (EcN) fitness genes during adhesion to the intestinal epithelial cells Caco-2. Gene 803, 145890 (2021).
Chantanawilas, P., Pahumunto, N. & Teanpaisan, R. Aggregation and adhesion ability of various probiotic strains and Candida species: an in vitro study. J. Dent. Sci. 19 (4), 2163–2171 (2024).
Piwat, S., Sophatha, B. & Teanpaisan, R. An assessment of adhesion, aggregation and surface charges of Lactobacillus strains derived from the human oral cavity. Lett. Appl. Microbiol. 61 (1), 98–105 (2015).
Ahmadi, M. A. et al. Antimutagenic and anticancer effects of lactic acid bacteria isolated from Tarhana through Ames test and phylogenetic analysis by 16S rDNA. Nutr. Cancer. 66 (8), 1406–1413 (2014).
Riaz Rajoka, M. S. et al. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk. Food Funct. 9 (5), 2705–2715 (2018).
Srikham, K. et al. Characterization of Streptococcus salivarius as new probiotics derived from human breast milk and their potential on proliferative Inhibition of liver and breast Cancer cells and antioxidant activity. Front. Microbiol. 12, 797445 (2021).
Budu, O. et al. A combination of two probiotics, Lactobacillus sporogenes and Clostridium butyricum, inhibits Colon cancer development: an in vitro study. Microorganisms, 10(9). (2022).
Dehghani, N., Tafvizi, F. & Jafari, P. Cell cycle arrest and anticancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. Bioimpacts 11 (4), 245–252 (2021).
Fleming, P. F., Berrington, J. E. & Jacobs, S. E. Addressing safety concerns of probiotic use in preterm babies. Early Hum. Dev. 135, 72–74 (2019).
Gu, J. et al. Safety evaluation of Bifidobacterium animalis subsp. Lactis BLa80 under in vitro, and in vivo conditions. Microb. Pathog. 194, 106809 (2024).
Barzegar, H., Alizadeh Behbahani, B. & Falah, F. Safety, probiotic properties, antimicrobial activity, and technological performance of Lactobacillus strains isolated from Iranian Raw milk cheeses. Food Sci. Nutr. 9 (8), 4094–4107 (2021).
Rahmati, F. Characterization of lactobacillus, Bacillus and Saccharomyces isolated from Iranian traditional dairy products for potential sources of starter cultures. AIMS Microbiol. 3 (4), 815–825 (2017).
Duan, L. et al. Comparative efficacy of different cognitive behavior therapy delivery formats for depression in patients with cancer: A network Meta-Analysis of randomized controlled trials. Psycho-Oncology 34 (1), e70078 (2025).
Zhang, Y. et al. Effects of Web-Based acceptance and commitment therapy on Health‐Related outcomes among patients with lung cancer: A feasibility randomized controlled trial. Psycho-Oncology 33 (12), e70045 (2024).
Chen, F. et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 14, 1166022 (2023).
Liu, R. et al. SIR-3DCNN: A Framework of Multivariate time Series Classification for Lung Cancer Detection (IEEE Transactions on Instrumentation and Measurement, 2025).
Acknowledgements
The authors gratefully acknowledge the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant Number 4030369), for their financial support.
Funding
This research was supported by the Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant Number 4030369).
Author information
Authors and Affiliations
Contributions
B.H. and A.B. conceived and designed the research and wrote the manuscript and M.S. and O.T. conducted the experiments and analyzed the data and Y.N. and A.S. contributed new reagents or analytical tools.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tavallaei, O., Barghi, A., Nami, Y. et al. Survivability, antimicrobial and anticancer effects, and cytotoxicity of Lactobacillus strains isolated from traditional Iranian cheese. Sci Rep 15, 25449 (2025). https://doi.org/10.1038/s41598-025-11344-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11344-9
Keywords
This article is cited by
-
Metabolomic and functional profiling of Lactiplantibacillus plantarum strains reveals distinct probiotic, immunomodulatory, and antimicrobial signatures
Applied Microbiology and Biotechnology (2026)
-
Comparative Genome Analysis and Characterization of Lacticaseibacillus Paracasei NKN344 Strain Isolated from Curd of Buffalo Milk Reared on Brackish Water Lagoons of the Eastern Indian Coast
Probiotics and Antimicrobial Proteins (2025)
-
Purine-Degrading Lactic Acid Bacteria Isolated from Fermented Foods and Fig Fruit: Evaluation of Probiotic and Anti-Hyperuricemic Properties
Probiotics and Antimicrobial Proteins (2025)