Abstract
Chemotherapy-induced cardiotoxicity remains a major clinical concern, with Sorafenib (Sor) being among the agents associated with adverse cardiac effects. Vitamin B17 (VB17), known for its antioxidant and anti-inflammatory properties, has shown promise in mitigating cardiovascular damage. This study investigated the cardioprotective potential of VB17, alone or in combination with Sor, in a mouse model of Ehrlich Ascites Carcinoma (EAC)-induced cardiomyopathy. Seventy-two male Swiss albino mice were divided into 12 groups and treated with VB17, Sor, or their combination via intraperitoneal or oral routes. Assessments included tumor volume, viable EAC cell count, cardiac enzyme levels (cardiac troponin (cTn), CK-MB, LDH), oxidative stress markers (malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD)), gene expression (IL-1β, NF-κB, TGF-β, MMP-9, P53), histological analysis, and molecular docking. Combination therapy significantly reduced tumor burden and EAC cell viability while improving cardiac function. VB17 co-treatment lowered elevated troponin I levels and normalized creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH). Oxidative stress was reduced, evidenced by a 50% decrease in MDA and increases in GSH and SOD activities. Gene analysis showed reduced expression of pro-inflammatory and fibrotic markers (IL-1β, NF-κB, TGF-β, MMP-9) and enhanced P53 expression. Histopathological findings confirmed reduced myocardial damage in the combination group compared to Sor alone. These findings suggest that VB17 enhances the cardioprotective effects of Sor by modulating key inflammatory and apoptotic pathways. The combination therapy shows promise as a potential strategy to counteract chemotherapy-induced cardiomyopathy. Further research is needed to validate these results and explore clinical applications.
Similar content being viewed by others
Introduction
Cardiomyopathy remains a significant cause of morbidity and mortality worldwide, necessitating continuous exploration of novel therapeutic avenues1. Cancer chemotherapy-related cardiotoxicity is a major cause of mortality globally2. Among those chemotherapeutic drugs known to cause cardiotoxicity is Sorafenib (Sor), the very first approved medication, which has anti-tumor effects on hepatocellular carcinoma and renal cell carcinoma by reducing angiogenesis and, therefore, tumor progression3. Our previous studies have reported that Vitamin B17 (VB17) enhances the anti-tumor effect of Sor in both in vivo and in vitro4,5.
The protective effects of bitter apricot seeds containing elevated levels of VB17 on reducing the likelihood of cardiovascular disorders have previously been confirmed, and regular consumption of apricot seeds was theoretically described as beneficial in reducing the risk of heart diseases6. Moreover, VB17 has been demonstrated to alleviate the process of atherosclerosis in mouse models7. Notably, its mechanisms of action encompass intricate signaling pathways implicated in cardiac remodeling and inflammation, including P53/MMP-9 and NF-κB/TGF-β/IL-1β pathways4. Moreover, the fundamental anti-tumor molecular processes of VB17 can be primarily attributed to cell cycle restriction, apoptosis induction, cytotoxic stimulation, and immune system control within the human body8. On the other hand, Sor operates as an anti-cancer agent by blocking many proteins. Most are tyrosine kinase receptors, with the principal mechanism suppressing the MAPK/ERK pathway9. However, Sor’s long-term treatment may result in cardiovascular side effects, including hypertension, thrombosis, and cardiac toxicity. The severe cardiovascular effects of Sor may be attributable to the complicated interaction between the drug and the vasculature3.
Ehrlich Ascites Carcinoma (EAC), a spontaneous mouse mammary adenocarcinoma, is a well-known model in tumor biology. The EAC model has been widely utilized for investigating tumor etiology and the development of anti-tumorigenic drugs10. Moreover, EAC-induced cardiomyopathy serves as a valuable model for elucidating the pathophysiological mechanisms underlying cardiac dysfunction in the context of cancer11. The EAC-induced cardiomyopathy model can be considered a unique model system to explore breast cancer-induced cardiomyopathy and is ideal for assessing the effect of various chemotherapy treatments on cardiac function12. EAC-induced tumors were previously reported to cause cardiomyopathy in rodents and are associated with heart atrophy, fibrosis, and cardiac failure through the activation of fetal, lysosomal, and atrophy-related genes, which in turn cause cardiac muscle deterioration in these EAC tumor-bearing rodents12,13,14.
VB17 has been reported to modulate the P53 pathway, a critical regulator of cell cycle arrest, DNA repair, and apoptosis. Activation of P53 can induce apoptosis in cancer cells, contributing to the anti-cancer effects of VB17. P53 plays a dual role in cardiomyopathy, promoting apoptosis of damaged cardiomyocytes while contributing to cardiac remodeling15. By enhancing P53 expression, VB17 may promote apoptosis of EAC-induced cardiomyocytes, thereby attenuating cardiac dysfunction16.
Matrix metalloproteinase-9 (MMP-9) is implicated in extracellular matrix remodeling and fibrosis in cardiomyopathy17. VB17 has been revealed to inhibit MMP-9 activity, thereby mitigating cardiac fibrosis and preserving myocardial structure and function. By reducing MMP-9-mediated degradation of the extracellular matrix, VB17 may attenuate the progression of EAC-induced cardiomyopathy5. The P53/MMP-9 pathway is pivotal in regulating myocardial apoptosis, fibrosis, and extracellular matrix remodeling18. Recent studies have highlighted VB17’s ability to upregulate P53 expression, inducing apoptosis in cancer cells19. Additionally, VB17 has been exposed to inhibit MMP-9 activity, mitigating extracellular matrix degradation and fibrosis5,20. Sor, a multikinase inhibitor with established anti-cancer properties, has also demonstrated cardioprotective effects through modulation of P53 and MMP-9 expression, albeit through different mechanisms21.
Furthermore, the NF-κB/TGF-β/IL-1β axis is a central regulator of inflammation and fibrosis in cardiomyopathy. VB17 has been reported to suppress NF-κB activation, attenuating inflammatory responses and TGF-β-induced fibrosis22. Similarly, Sor exhibits anti-inflammatory properties by inhibiting NF-κB signaling and downstream IL-1β expression, consequently ameliorating cardiac remodeling and dysfunction induced by EAC23.
Despite the promising preclinical evidence supporting the cardioprotective effects of VB17 and its anti-tumor effect with SOR, their combination’s effect on cardiomyopathy in the context of EAC-induced cardiomyopathy warrants further investigation24,25. Several other challenges and unanswered questions, including safety concerns, optimal dosage, and potential drug interactions, necessitate careful consideration in translational studies.
The objective of this study is to examine the potential cardioprotective and anti-cancer effects of VB17 and Sor in a model of EAC-induced cardiomyopathy by elucidating their mechanisms of action through the modulation of the P53/MMP-9 and NF-κB/TGF-β/IL-1β signaling pathways, by integrating in vivo experimentation with computational in silico modeling. Overall, this study’s outcome may help improve the cardiac health of cancer patients undergoing Sor treatment. This approach offers a promising strategy for enhancing Sor’s therapeutic efficacy and tolerability.
Materials and methods
Animal experimentation
Animal experiments were ethically approved by the research ethical committee of Tanta University, Egypt (IACUC-SCI-TU-00216 A). Moreover, this study is performed in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Animal model and housing
Male Swiss albino mice, aged 5–7 weeks and weighing 20–25 g, were used for the study. The mice were housed under standardized conditions, including controlled temperatures and a 12-hour light-dark cycle, and were acclimatized to the housing environment for two weeks before the experiments. A standard diet, water, and ad libitum were provided. To ensure animal welfare, mice were monitored daily for signs of pain or distress using standardized criteria, such as changes in behavior, grooming, and activity levels. Appropriate interventions were made in consultation with a veterinarian if discomfort was observed. All experimental procedures were conducted under sterile conditions using isoflurane anesthesia to minimize discomfort.
EAC model
The study used mice bearing EAC provided by Cairo’s Al-Kaser Al-Eini Hospital’s Cancer Biology Unit. We maintained and propagated EAC cells in our laboratory through serial aseptic intraperitoneal (IP) transplantation in mice. Untreated injections of EAC cells set off rapid proliferative growth within the peritoneal cavity, resulting in ascites fluid formation and eventual morbidity within 17–18 days.
Experimental design
There were 72 mice divided into 12 groups (n = 6/group) as follows: Normal (Control) group: normal saline was injected intraperitoneally (0.9% w/v, 300 mg). In the VB17 group, VB17 was administered. In the Sor intraperitoneal (SorIP) group, Sor was injected intraperitoneally. The Oral Sor (SorO) group was treated orally with Sor. In the VB17 + SorIP group, VB17 and Sor were administered intraperitoneally together. The VB17 + SorO group received both VB17 and Sor orally. For the EAC group, 200 mL of 1 × 106 EAC cells were injected IP and left untreated for 14 days. EAC + VB17 group: injected with EAC cells and administered with VB17 24 h later. EAC + SorIP group: injected with EAC cells and treated with Sor via intraperitoneal injection for 24 h. EAC + SorO group: injected with EAC cells and treated with SorO 24 h later. EAC + VB17 + SorIP group: injected with EAC cells and co-dosed with VB17 and Sor via intraperitoneal injection 24 h later. EAC + VB17 + SorO group: injected with EAC cells and co-administered with VB17 and SorO 24 h later. All treatments were performed for 14 days, and the doses used were selected based on previous literature. Specifically, VB17 was administered at a dose of 300 mg/kg, a dosage that has been revealed to exert anti-inflammatory and cardioprotective effects in murine models without inducing toxicity4,18. Sor was used at a dose of 30 mg/kg, a standard and well-tolerated dose in preclinical studies assessing anticancer and cardiotoxic effects, known to produce plasma levels comparable to therapeutic concentrations in humans. The chosen dosages align with established pharmacokinetic profiles and efficacy data in mice and were selected to balance therapeutic effectiveness4,18.
Sampling
At the end of the 14-day experimental period, overnight-fasted mice were euthanized using isoflurane. Blood samples were then collected from the tail vein for serum biochemical analysis. Heart specimens were also collected for histopathological examination, biochemical assays, and RNA extraction.
Assessment of tumor volume and EAC count
The Ascitic tumor volume in EAC mice was measured by carefully collecting ascitic fluid from the peritoneal cavity using a sterile syringe under aseptic conditions. The total volume is determined by measuring the fluid in a graduated cylinder. The total, viable, and non-viable EAC cells were counted using a Neubauer hemocytometer after suspension in sterile isotonic saline as previously described26; in brief, Each mouse’s ascitic fluid was diluted with 1% trypan blue and incubated for 5 min at 37 °C before counting the number of viable (unstained) and non-viable (stained) cells using the hemocytometer. Viable cells were counted using the following formula: The viable cell count is calculated by multiplying the number of cells by the dilution factor and dividing the area by the thickness of the liquid film27.
Biochemical analysis
Serum levels of creatine kinase (CPK), creatine kinase-MB (CK-MB), cardiac troponin (cTn), and lactate dehydrogenase (LDH) were measured using commercially available kits (Biomed, Egypt)28. Heart homogenates were prepared to measure the lipid peroxidation marker malondialdehyde (MDA) and the antioxidant markers reduced glutathione (GSH) and superoxide dismutase (SOD) using colorimetric assays at their respective wavelengths. The MDA levels were determined by measuring the absorbance of the thiobarbituric acid reactive substances (TBARS) complex at 532 nm. GSH levels were quantified using Ellman’s reagent, with absorbance measured at 412 nm. SOD activity was assessed by monitoring the inhibition of Nitroblue tetrazolium (NBT) reduction, with absorbance measured at 560 nm, as previously described29,30, respectively.
qRT-PCR analysis
The mRNA expression levels of interleukin-1β (IL-1β), tumor growth factor-β (TGF-β), Nuclear factor kappa B (NF-κB), MMP-9, and Tumor protein-53 (P53) in heart tissues from each group were assessed. Total RNA was reverse-transcribed using kits obtained from Thermo Scientific, USA, to produce cDNA. At 42 °C/60 minutes, the mixture was incubated, then heated for 10 min at 70 °C to stop it. In the heart tissue, mRNA expressions of target genes were evaluated with SYBR Green and gene-specific primers using the Step OnePlus real-time PCR system (Applied GeneXpert, USA). The primer sequences are presented in (Supplementary Table S1). For the reaction cycle, 95 °C was used for 10 min, followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. Gene expression is measured using the Ct value analysis4.
Histological examination
Heart specimens were processed for histological analysis following a standardized paraffin-embedding protocol. The tissues were dehydrated through a graded ethanol series: 70% ethanol for 1 h, 80% ethanol for 1 h, 95% ethanol for 1 h, and 100% ethanol for 2 h (changed twice). Following dehydration, samples were cleared in xylene for 2 × 30 min, then embedded in paraffin wax at 60 °C for 2 h. Paraffin-embedded blocks were sectioned at 5 μm thickness using a rotary microtome. Sections were mounted on slides, deparaffinized, and stained with hematoxylin for 5 min and eosin for 3 min. After staining, slides were dehydrated, cleared, and mounted using DPX. The stained sections were examined under a light microscope, and representative images were captured at ×100 magnification for histopathological assessment31.
Molecular docking assessment
To investigate the molecular interactions and calculate the affinity scores of VB17 and Sor with the target genes, the three-dimensional (3D) structures of these target proteins were obtained from the RCSB Protein Data Bank (https://www.rcsb.org/), which provides high-resolution structural data of biomolecules. The molecular structures of VB17 and Sor were retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/), a comprehensive resource for chemical compound information. Before docking, the target proteins were preprocessed using UCSF Chimera software to ensure optimal simulation conditions; this step included the removal of water molecules and non-relevant binding ligands to refine the protein structure for docking. The molecular docking simulations were carried out using AutoDock 4.2 software integrated with UCSF Chimera. AutoDock employs a Lamarckian genetic algorithm (LGA) to predict binding affinities. Docking grid boxes were created around the active sites of the proteins, with dimensions adjusted to encompass all potential ligand-binding regions. Standard docking parameters were applied, including a population size of 150, 2,500,000 energy evaluations, and a maximum of 27,000 generations per docking run. After docking, the interactions between VB17, Sor, and the target genes were further analyzed to identify key binding residues and interaction patterns, such as hydrogen bonds and hydrophobic interactions. These interactions were visualized in detail using BIOVIA Discovery Studio, a specialized software for interpreting and presenting molecular interactions in a user-friendly graphical format, enabling an in-depth understanding of the binding mechanisms. Binding affinities were calculated as the negative binding free energy (ΔG) values in kcal/mol, with lower values indicating stronger binding affinities.
Statistical analysis
Data were analyzed using GraphPad Prism version 9.5.2. All results are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used to compare differences between groups, followed by Tukey’s post-hoc test for multiple comparisons. A significance threshold of p ≤ 0.05 was considered statistically significant. Sample size per group was n = 6. Statistical comparisons were performed with 95% confidence intervals (CI) to strengthen interpretation of significance. All statistical assumptions for ANOVA (e.g., homogeneity of variance) were verified before testing.
Results
Tumor volume and EAC cell count in response to VB17 and/or Sor
To assess the potential anti-cancer effects of VB17 and/or Sor treatment on EAC-bearing mice, the ascetic tumor volume and the number of viable EAC cells were measured. Compared with other treated groups, the untreated EAC group had a significantly higher tumor volume (ascitic fluid volume) (Fig. 1a). A group consisting of EAC + VB17 + SorIP showed the lowest ascitic fluid volume, followed by a group comprised of EAC + SorIP, EAC + VB17 + SorO, and then a group consisting of EAC + VB17. VB17 and Sor (IP or O) treatment significantly decreased total and live tumor cell counts compared to the EAC group. The lowest counts were found in EAC + VB17 + SorIP, EAC + SorIP, and EAC + VB17 + SorO, followed by EAC + VB17, EAC + SorO, and finally EAC + VB17. However, EAC + SorIP and EAC + VB17 + SorO produced significantly more dead cells than their counterparts (Fig. 1b).
(A) Ascitic fluid volume and (B) total, viable, and non-viable Ehrlich Ascites Carcinoma (EAC) cell counts following treatment with VB17 and/or Sorafenib (Sor) in EAC-bearing mice. Treatments included monotherapy and combination therapy via intraperitoneal (IP) or oral (O) routes. Data are presented as mean ± SEM (n = 6 per group). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. Significance is indicated as: ns (not significant), p ≤ 0.05 (*), p < 0.01 (**), p ≤ 0.0001 (****).
The outcome of VB17 and/or Sor on biochemical functions
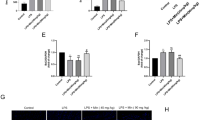
Following treatment, the most significant improvements in these serum biochemical parameters were observed in the EAC + SorIP and EAC + VB17 + SorIP groups. The decrease in the levels of cTn and LDH in the EAC + SorIP and EAC + VB17 + SorIP groups suggests improved myocardial function (Fig. 2a-b). These results demonstrate that the combination therapy of SorIP and VB17 had a synergistic effect in reducing cardiac damage and improving cardiac function. CPK and CK-MB activity levels were significantly increased in the EAC group compared to the control group. Compared to the EAC group, the most significant improvement was observed in the group cotreated with VB17 + SorIP and/or SorO. However, no significant differences were found between the group treated with SorIP and those treated with SorO (Fig. 3a-b).
(A) Serum cardiac troponin (cTn) levels and (B) lactate dehydrogenase (LDH) activity in EAC-bearing mice treated with VB17 and/or Sorafenib (Sor). Groups included untreated EAC mice and mice receiving monotherapy or combination therapy via intraperitoneal (IP) or oral (O) routes. Data are presented as mean ± SEM (n = 6 per group). Statistical analysis was performed using one-way ANOVA followed by Tukey’s post hoc test. Significance is denoted as: ns (not significant), p ≤ 0.05 (*), p < 0.01 (**), p ≤ 0.001 (***). p ≤ 0.0001 (****).
(A) Creatine phosphokinase (CPK) and (B) creatine kinase-MB (CK-MB) levels in the serum of EAC-bearing mice treated with Vitamin B17 (VB17) and/or Sorafenib (Sor), administered either intraperitoneally (IP) or orally (O). Data are expressed as mean ± SEM (n = 6 per group). Statistical comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test. Significance is indicated as: ns (not significant), p ≤ 0.05 (*), p < 0.01 (**), p ≤ 0.0001 (****).
Enhanced oxidative and antioxidative markers with VB17 and/or Sor
To assess the potential effect of treatments on oxidative stress status, MDA levels and GSH and SOD activities were measured in all treated groups. As compared with all control groups, untreated EAC-bearing mice had significantly higher heart levels of MDA, a marker of lipid peroxidation, and lower levels of antioxidant markers (GSH and SOD), both markers of antioxidant function (Fig. 4a-c). In EAC + VB17 + SorIP and EAC + VB17 + SorO groups, these markers were restored to levels near control groups (lowest MDA, highest GSH, and highest SOD), with the most significant improvements (lowest MDA, highest GSH, and highest SOD). Finally, EAC + VB17 + SorO, EAC + SorO, and EAC + VB17 were considered the most desirable. This indicates that these combinations had a potentiating effect in reducing oxidative damage.
Effects of Vitamin B17 (VB17) and/or Sorafenib (Sor) on oxidative stress and antioxidant defense markers in heart tissues of EAC-bearing mice. (A) Malondialdehyde (MDA) levels, (B) reduced glutathione (GSH), and (C) superoxide dismutase (SOD) activity. Treatments were administered via intraperitoneal (IP) or oral (O) routes. Data are presented as mean ± SEM (n = 6 per group). Statistical analysis was conducted using one-way ANOVA with Tukey’s post hoc test. Significance indicators: ns (not significant), p ≤ 0.05 (*), p < 0.01 (**), p ≤ 0.001 (***). p ≤ 0.0001 (****).
Effect of VB17 and/or Sor on gene expression
The data (Fig. 5a-e) demonstrated that the expression of IL-1β, TGF-β, NF-κB, and MMP-9 in the heart was significantly higher in the EAC group than in the control groups. In contrast, the expression of P53 was considerably lower. However, the administration of VB17 and/or Sor had a beneficial effect as it brought the expression levels of these genes back to normal levels seen in the control groups for both IP and O treatment. The groups that revealed the most prominent benefits were EAC + VB17 + SorIP, EAC + VB17 + SorO, EAC + SorIP, EAC + SorO, and EAC + VB17. In these groups, the levels of IL-1β, TGF-β, NF-κB, and MMP-9 were at their lowest, while the levels of P53 were at their highest. This suggests that using VB17 and/or Sor in conjunction with (IP or O) substantially impacts restoring gene expression levels more than using either treatment alone.
Relative mRNA Expression of the selected inflammatory genes in rat heart tissues. The relative expression levels of (A) IL-1β, (B) TGF-β, (C) NF-kB, (D) P53, and (E) MMP-9 that obtained by qRT-PCR. The graphs of genes are plotted as 2−∆∆Ct folds change. One-way ANOVA followed by post-hoc Tukey’s multiple comparisons test was employed to determine the significant differences (P < 0.05). Data were expressed as the mean ± SEM. ns (non-significant), * (p ˂0.05), ** (p ˂ 0.01), and **** (p ˂ 0.0001).
Histopathology studies among the different groups
A histopathological investigation was performed to investigate the effect of treatments on the histology of the heart in all treated groups of mice. The normal control group shows epicardial cell layer (arrowhead), cardiac blood vessel (asterisk), and branched cardiomyocytes (arrows) (Fig. 6a). The Heart of the EAC group shows shrinkage of cardiac muscle fibers (arrows), a large area of necrosis of (tailed arrow), and an increase in the number of connective tissue fibers (arrowhead) (Fig. 6b). Heart of VB17 group showing normal cardiac muscle fibers (arrows) separated by connective tissue fibers (tailed arrow) and congestion of cardiac blood vessels (arrowheads) (Fig. 6c). Heart of Sor IP group showing normal cardiac muscle fibers (arrows) separated by connective tissue fibers (tailed arrow) and mild congestion of cardiac blood vessels (arrowheads) (Fig. 6d). Heart of SorO group showing normal cardiac muscle fibers (arrows), congestion of cardiac blood vessels with hemolysis (arrowheads) and mononuclear cell infiltrations (tailed arrow) (Fig. 6e).
Effect of VB17 and/or Sor on histopathology studies among the different groups: (A) Normal control group: Epicardial cell layer (arrowhead), cardiac blood vessel (asterisk), and branched cardiomyocytes (arrows). (B) EAC group: Shrinkage of cardiac muscle fibers (arrows), large area of necrosis (tailed arrow), and increased number of connective tissue fibers (arrowhead). (C) VB17 group: Normal cardiac muscle fibers (arrows) separated by connective tissue fibers (tailed arrow) and congestion of cardiac blood vessels (arrowheads). (D) Sor IP group: Normal cardiac muscle fibers (arrows) separated by connective tissue fibers (tailed arrow) and mild congestion of cardiac blood vessels (arrowheads). (E) SorO group: Normal cardiac muscle fibers (arrows), congestion of cardiac blood vessels with hemolysis (arrowheads), and mononuclear cell infiltrations (tailed arrow). (F) VB17 + SorIP group: Normal branched cardiac muscle fibers (arrows) with intact and regular-sized cardiac blood vessels (arrowhead). (G) VB17 + SorO group: Congestion and hemolysis inside cardiac blood vessels (arrowhead) in addition to mild vacuolar degeneration of cardiac muscles (arrow). (H) EAC + VB17 group: Shrinkage and mild degeneration in cardiac muscles (arrow) with dilation and congestion of cardiac blood vessels (arrowheads). (I) EAC + SorIP group: Severe congestion and hemolysis inside cardiac blood vessels with normal cardiac muscle architecture (arrows). (J) EAC + VB17 + SorIP group: Mild degeneration in cardiomyocytes (arrows) and increased connective tissue fibers (arrowheads). (K) EAC + SorO group: Severe hemorrhage (arrowheads) and necrosis of cardiac muscle fibers (arrow). (L) EAC + VB17 + SorO group: Degeneration (arrow) and shrinkage (tailed arrow) in cardiac muscle fibers with mild hemorrhage (arrowhead).
Heart of VB17 + SorIP group showing normal branched cardiac muscle fibers (arrows) with intact and regular-sized cardiac blood vessels (arrowhead) (Fig. 6f). Heart of VB17 + SorO group showing congestion and hemolysis inside cardiac blood vessels (arrowhead) in addition to mild vacuolar degeneration of cardiac muscles (arrow) (Fig. 6g). Heart of EAC + VB17 showing shrinkage and mild degeneration in cardiac muscles (arrow) with dilation and congestion of cardiac blood vessels (arrowheads) (Fig. 6h). Heart of EAC + SorIP showing severe congestion and hemolysis inside cardiac blood vessels with normal cardiac muscle architecture (arrows) (Fig. 6i). Heart of EAC + VB17 + SorIP shows mild degeneration in cardiomyocytes (arrows) and increased connective tissue fibers (arrowheads) (Fig. 6j). Heart of EAC + SorO showing severe hemorrhage (arrowheads) and necrosis of cardiac muscle fibers (arrow) (Fig. 6k). Heart of EAC + VB17 + SorO showing degeneration (arrow) and shrinkage (tailed arrow) in cardiac muscle fibers with mild hemorrhage (arrowhead) (Fig. 6L).
Drug docking assessment (Hypothesis-Generating in Silico Analysis)
To complement our experimental findings and explore potential molecular interactions, we conducted a molecular docking study of VB17 and Sor. The target proteins—IL-1β (PDB ID: 2MIB), TGF-β (3KFD), NF-κB (1VKX), MMP-9 (1L6J), and P53 (1HU8)—were retrieved from the Protein Data Bank. Proteins were preprocessed by removing water molecules, adding polar hydrogens, and energy minimization through PyRx. Docking grids were positioned over active sites, and an exhaustiveness value of 8 ensured robust sampling. Binding energies (ΔG in kcal/mol) and interacting residues for IL-1β, TGF-β, NF-κB, MMP-9, and P53 are presented in Table 1. Interaction types include hydrogen bonding (H-bond), van der Waals (VDW), π-alkyl, π–π stacking, halogen bonding, and others, also accessible in Table 1. Additionally, Supplementary Fig. S1a–b illustrates the interaction of Sor and VB17 with IL-1β, with VB17 notably binding Lys65, a residue involved in integrin recognition, suggesting a potential—but not confirmed—modulatory role. Supplementary Fig. S2a–b shows distinct interaction patterns with TGF-β, while Supplementary Fig. S3a–b depicts engagement with NF-κB. Sor and VB17 revealed variable interaction residues, possibly reflecting differential biological effects. Supplementary Fig. S4a–b highlights MMP-9 binding, where VB17 exhibits slightly enhanced interaction energy. Supplementary Fig. S5 presents VB17’s binding to P53, particularly at residues (Cys173, Cys239) near zinc-binding motifs. These computational predictions suggest plausible modes of interaction and provide a structural basis for further validation. However, we underscore that molecular docking alone does not establish mechanistic causality; experimental binding assays and functional studies are essential for confirmation.
The binding affinities (ΔG, kcal/mol) were calculated using AutoDock Vina and AutoDock 4.2 (Lamarckian Genetic Algorithm). Interaction types, including Van der Waals, hydrogen bonds, electrostatic forces, and pi-related interactions, were analyzed via PyMOL and Discovery Studio. Docking validation involved RMSD checks (< 2 Å) against reference ligands to ensure methodological reliability. It is essential to emphasize that these docking studies serve as hypothesis-generating tools, offering preliminary insight into possible ligand-target interactions. While they highlight favorable binding conformations and energy profiles, they do not confirm biological activity or therapeutic mechanisms.
Discussion
Cardiac disease is a complex and progressive process that is associated with the accumulation of detrimental changes over time32. These changes reduce physiological functions such as cardiovascular, renal, neurological, and endocrine, increasing the risk of disease and death33. One of the most debilitating consequences of cardiac disease is the loss of myocardial function, characterized by decreased cardiac elasticity and an inability to respond to changes in pressure within the arterial system34. This decline in heart function is particularly prevalent in older adults35. As such, our study aims to assess the potential benefits of co-administering VB17 and/or Sor for alleviating cardiomyopathies in an EAC-mice model.
The current study showed that LDH and cTn levels significantly decreased, especially after the combined treatment with VB17 and Sor, compared to the untreated EAC group. High serum levels of LDH were previously reported as a predictor of cardiac insufficiency in patients with acute myocardial infarction36. Similarly, troponin was previously described as the biomarker of choice for detecting cardiac injury37. VB17 may reduce LDH release by stabilizing cell membranes and mitigating damage. This effect is attributed to its potential antioxidant properties, which reduce oxidative stress and protect cells38. On the other hand, the antioxidant and anti-inflammatory effects of VB17, which could help protect cardiac tissue from damage caused by oxidative stress39, are expected to reduce the release of cTn into the bloodstream, which was confirmed by the current report.
Recent studies have shown that rats that have been administered EAC are likely to develop several forms of cardiomyopathy, including myocardial infarction (MI)40,41. This is because EAC causes myocardial necrosis, leading to cardiac dysfunction, increased levels of myocardial lipids, altered cardiac enzymes and antioxidant activities, and increased lipid peroxidation42. The proposed mechanisms that explain how EAC-induced cardiomyopathy occurs include the generation of highly cytotoxic free radicals through the autoxidation of catecholamines12. These free radicals may then attack polyunsaturated fatty acids (PUFAs) within the membranes, forming peroxyl radicals, which can then attack adjacent fatty acids43. This causes a chain reaction of MDA, which ultimately leads to the formation of harmful lipid hydroperoxides. These end products may contribute to increased membrane permeability, ultimately leading to the development of cardiomyopathy44.
Our study revealed a significant decrease in ascitic fluid volume in the VB17 group compared to the EAC group. Additionally, VB17/SorIP showed a highly significant reduction compared to VB17 + SorO. This could be due to a potentiating action between VB17 and SorIP, which substantially reduces the ascitic fluid volume more than the combined effect of VB17 and SorO. Furthermore, various studies have indicated that natural products can help relieve the development and proliferation of EAC cells, decreasing the volume, viable percentage, and total cell count and increasing the rate of dead cells45,46.
CK and CK-MB are the best-known markers for identifying acute myocardial infarction47. Our study revealed that VB17 treatment significantly reduced cardiac enzyme activity. However, the EAC + VB17 + SorIP group had substantially lower CK and CK-MB levels than the others. This indicates that VB17 significantly attenuated cardiotoxicity caused by EAC. This is likely because VB17 blocked the inflammatory signaling pathways induced by EAC, reducing cardiac enzyme production48. In addition, VB17 may have also inhibited the damage caused by EAC by reducing oxidative stress. The results of this study are consistent with the findings of other researchers who have also found that VB17 can alleviate cardiac toxicity49,50,51.
The development and progression of cardiomyopathies are closely linked to oxidative mechanisms52,53. Our data found that the EAC group had increased levels of MDA, a marker of oxidative stress, and decreased levels of GSH and SOD. In contrast, the lipid peroxidation content decreased after administering VB17; however, the concomitant administration of VB17 and Sor decreased MDA levels further. Cardiomyopathies generate more reactive oxygen species (ROS) than normal cells, leading to increased oxidative stress that damages cell components54. The notable increase in the levels of cardiac MDA in the EAC group observed in this study could be attributed to the excess free radicals generated by cancer cells, destroying lipids and possibly lipid peroxidation28,55. The potential benefits of VB17 and/or Sor as a dual treatment for oxidative stress-induced cardiotoxicity in our study were consistent with previous studies that revealed decreased MDA and increased antioxidants (SOD and GSH) levels in the liver and kidneys4,5.
The study reveals significant alterations in the expression of key genes—IL-1β, TGF-β, NF-κB, MMP-9, and P53—that are critically involved in inflammation, fibrosis, and apoptosis, all of which are commonly dysregulated in cardiac disorders56,57. IL-1β expression correlates with atherosclerotic plaque development, with low expression in healthy coronary arteries, increasing expression in simple plaques, and high expression in complex plaques58,59. TGF-β seems to cause cardiac fibrosis, cardiomyocyte apoptosis, and hypertrophy. TGF-β signaling requires interaction with the TGF-βRII receptor, which recruits the TGF-βRI receptors, also known as ALK, in five distinct isoforms60. Inhibitors include TGF-β neutralizing antibodies, anti-receptor antibodies, and receptor ectodomain proteins that trap ligands. Small chemical inhibitors have been produced to interfere with the kinase activity of type I TGF-β receptor61.
NF-κB activity increases during cardiovascular illness and is linked to cardiac fibrosis, hypertrophy, and heart failure62. Using NF-κB inhibitors in rodent models of heart disease can suppress this system globally and provide cardioprotection63,64. In late-onset cardiotoxicity in mice produced by low-dose doxorubicin therapy, p53 protects the heart by preserving mitochondrial activity65. A study targeting diverse features of p53 in heart and tumor tissues from the doxorubicin-treated mice suggested a potentially promising strategy for p53-mediated cardioprotection66. Finally, high levels of MMP-9 over time are a powerful predictor of cardiovascular death and heart failure.MMP-9 plasma concentrations were found to be a predictor of future death from cardiovascular disease and myocardial infarction67.
VB17 and Sor treatment normalized the expression of key cardiac-related genes to control levels, indicating a protective molecular effect. Notably, the combination therapies (VB17 + SorIP and/or VB17 + SorO) were more effective than monotherapies, highlighting their superior potential in mitigating cardiotoxic gene dysregulation.
Certain treatment combinations, particularly EAC + VB17 + SorIP, EAC + VB17 + SorO, EAC + SorIP, EAC + SorO, and EAC + VB17, showed the most pronounced reductions in pro-inflammatory and profibrotic gene expression. These findings align with previous studies highlighting the cardioprotective mechanisms of VB17 in acute myocardial infarction models68,69 involving the regulation of IL-1β70, TGF-β71, NF-κB72, P5373, and MMP-970. These findings are essential for advancing research to develop more effective treatment strategies for cardiac disorders28.
Our docking analysis revealed that VB17 exhibits strong binding affinities to MMP-9, NF-κB, and IL-1β, with binding energies of −103.82, −95.2, and − 84.39 kcal/mol, respectively. Notably, VB17 binds to Lys65 of IL-1β, a residue implicated in integrin interaction, which may potentially interfere with inflammatory signaling cascades74,75. Sor also revealed notable docking with IL-1β, interacting with residues such as Trp120 and Val132, which are critical for IL-1β signaling76,77. These findings suggest potential anti-inflammatory effects of Sor through modulation of IL-1β activity78. Moreover, VB17 was found to interact with Lys37 of NF-κB—a known SUMOylation site that plays a regulatory role in transcription factor activation—suggesting that VB17 may influence NF-κB signaling via SUMO-modulated regions79,80. While these findings are preliminary and based on molecular docking, they offer hypothesis-generating insights into the synergistic anti-inflammatory and cardioprotective potential of VB17 and Sor81,82.
Despite all the promising results in the current study, some limitations should also be stated concerning this study. Since the study was conducted on Swiss albino mice, which may not fully replicate the complexity of human cardiomyopathy and tumor biology, it must be noted that these findings require further validation in clinical settings. The study also investigated the P53/MMP-9 and NF-κB/TGF-β/IL-1β signaling pathways. Other potentially relevant pathways or mechanisms involved in cardiomyopathy still need to be explored. Future research should focus on validating the cardioprotective effects of VB17 in clinically relevant large-animal models, optimizing dosing regimens, and investigating their long-term safety and efficacy. Studies should also explore their mechanisms of synergy, compare them with existing therapies, and assess their potential in diverse etiologies of cardiomyopathy. Finally, human clinical trials are essential to establish their translational applicability for cardiac disorders.
Conclusion
In conclusion, this study demonstrates that VB17 and Sor significantly mitigate EAC-induced cardiomyopathy by regulating critical pathways, including P53/MMP-9 and NF-κB/TGF-β/IL-1β. The combination therapy revealed superior efficacy in reducing tumor burden, normalizing cardiac enzyme levels, alleviating oxidative stress, restoring gene expression profiles, and preserving cardiac histology compared to monotherapy. Our docking analysis suggests that VB17 exhibits strong binding affinities to MMP-9, NF-κB, and IL-1β. Notably, VB17’s interaction with IL-1β—a residue implicated in integrin binding—indicates a potential for disrupting IL-1β–integrin interactions. While these in silico findings provide valuable hypothesis-generating insights into the anti-inflammatory properties of VB17, further experimental validation is required to confirm their biological relevance. Additional research is needed to validate these results in diverse preclinical models, optimize dosing strategies, and ensure safety and efficacy in long-term studies.
Data availability
The datasets generated and/or analysed during the current study are available upon request from the corresponding author.
References
Sapna, F. N. U. et al. Advancements in heart failure management: a comprehensive narrative review of emerging therapies, Cureus, vol. 15, no. 10, (2023).
Anthony, F. Y., Steingart, R. M. & Fuster, V. Cardiomyopathy associated with cancer therapy. J. Card Fail. 20 (11), 841–852 (2014).
Li, J. et al. Understanding Sorafenib-Induced cardiovascular toxicity: mechanisms and treatment implications. Drug Des. Devel Ther. 18 (12), 829–843 (2024).
Attia, A. A. et al. Amygdalin potentiates the anti-cancer effect of Sorafenib on Ehrlich Ascites carcinoma and ameliorates the associated liver damage. Sci. Rep. 12 (1), 1–9 (2022).
El-Sewedy, T. et al. Hepatocellular carcinoma cells: activity of amygdalin and Sorafenib in targeting ampk/mtor and BCL-2 for anti-angiogenesis and apoptosis cell death. BMC Complement. Med. Ther. 23 (1), 1–17 (2023).
Kop ekov, J. et al. Influence of long-term consumption of bitter apricot seeds on risk factors for cardiovascular diseases. J. Environ. Sci. Heal Part. B. 53 (5), 298–303 (2018).
Lv, J. et al. Amygdalin ameliorates the progression of atherosclerosis in LDL receptor–deficient mice. mol. Med. Rep. 16 (6), 8171–8179 (2017).
Spanoudaki, M. et al. Amygdalin as a promising Anti-cancer agent: molecular mechanisms and future perspectives for the development of new nanoformulations for its delivery. Int. J. Mol. Sci. 24 (18), 14270 (2023).
Cervello, M. et al. Molecular mechanisms of Sorafenib action in liver cancer cells. Cell. Cycle. 11 (15), 2843–2855 (2012).
Egawa, J., Ishioka, K. & Ogata, T. Effect of irradiation and chemotherapeutic agents on the capillaries of Ehrlich Ascites carcinoma of mice. Acta Radiol. Oncol. Radiat. Phys. Biol. 18 (6), 535–543 (1979).
Vudatha, V. et al. Review of mechanisms and treatment of cancer-induced cardiac cachexia, Cells, vol. 11, no. 6, p. 1040, (2022).
Mishra, S. et al. Subcutaneous Ehrlich Ascites carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 8 (1), 1–11 (2018).
Ameen, A. et al. Doxorubicin-Induced cardiotoxicity in adult male albino rats and the ameliorative effects of Achillea fragrantissima crude extract, its combined Ethyl acetate/n. Butanol fraction, and vitamin E. Bull. Egypt. Soc. Physiol. Sci. 43 (2), 81–107 (2023).
Dubois-Deruy, E., Peugnet, V., Turkieh, A. & Pinet, F. Oxidative stress in cardiovascular diseases, Antioxidants, vol. 9, no. 9, p. 864, (2020).
Men, H. et al. The regulatory roles of p53 in cardiovascular health and disease. Cell. Mol. Life Sci. 78 (5), 2001–2018 (2021).
Patil, P. P. et al. Effect of theobroma cacao L. On the efficacy and toxicity of doxorubicin in mice bearing ehrlich ascites carcinoma, Antioxidants, vol. 11, no. 6, p. 1094, (2022).
Halade, G. V., Jin, Y. F. & Lindsey, M. L. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol. Ther. 139 (1), 32–40 (2013).
Nasser, H. M., El-Naggar, S. A., El-Sayed Rizk, M. E. S. R., Elmetwalli, A. & Salama, A. F. Effect of Sorafenib on liver biochemistry prior to vitamin B17 coadministration in Ehrlich Ascites carcinoma mice model: preliminary phase study. Biochem. Lett. 17 (1), 40–49 (2021).
Abboud, M. M., Al Awaida, W., Alkhateeb, H. H. & Abu-Ayyad, A. N. Anti-tumor action of amygdalin on human breast cancer cells by selective sensitization to oxidative stress. Nutr. Cancer. 71 (3), 483–490 (2019).
Lin, S. et al. Amygdalin induced Mitochondria-Mediated apoptosis of lung Cancer cells via regulating NF κ B-1/NF κ B signaling cascade in vitro and in vivo. Am. J. Chin. Med. 50 (05), 1361–1386 (2022).
Elmetwalli, A. et al. Diarylheptanoids/sorafenib as a potential anti-cancer combination against hepatocellular carcinoma: the p53/MMP9 axis of action. Naunyn Schmiedebergs Arch. Pharmacol. 396 (10), 1–17 (2023).
Besse, S., Nadaud, S., Balse, E. & Pavoine, C. Early protective role of inflammation in cardiac remodeling and heart failure: Focus on TNFα and resident macrophages, Cells, vol. 11, no. 7, p. 1249, (2022).
Kumar, A. R., Devan, A. R., Nair, B. & Nath, L. R. Anti-VEGF mediated Immunomodulatory role of phytochemicals: scientific exposition for plausible HCC treatment. Curr. Drug Targets. 22 (11), 1288–1316 (2021).
Salama, A. F. et al. Gingerol and/or Sorafenib attenuates the DAB-induced HCC and hepatic portal vein dilatation via ATG4/CASP3 and COIIV/COX-2/NF-κB expression. Med. Oncol. 41 (2), 57 (2024).
Jridi, I., Canté-Barrett, K., Pike-Overzet, K. & Staal, F. J. T. Inflammation and Wnt signaling: target for Immunomodulatory therapy? Front. Cell. Dev. Biol. 8, 615131 (2021).
Salama, W. M., El-Naggar, S. A. & ALRashdi, B. M. In vivo anti-tumor effect of Egyptian Scorpion Leiurus quinquestriatus venom in Ehrlich Ascites carcinomabearing mice. Trop. J. Pharm. Res. 22 (8), 1635–1643 (2023).
Bala, A., Kar, B., Haldar, P. K., Mazumder, U. K. & Bera, S. Evaluation of anti-cancer activity of Cleome gynandra on ehrlich’s Ascites carcinoma treated mice. J. Ethnopharmacol. 129 (1), 131–134 (2010).
Elmalla, A., Elmetwalli, A., Rizk, M. E. S. & Salama, A. F. The effect of vitamin B17 on cardiomyopathy against Ehrlich tumor development in female mice. Biochem. Lett. 17 (1), 69–76 (2021).
Elmetwalli, A. et al. Novel phloretin-based combinations targeting glucose metabolism in hepatocellular carcinoma through GLUT2/PEPCK axis of action: in Silico molecular modelling and in vivo studies. Med. Oncol. 41 (1), 12 (2023).
Elmetwalli, A. et al. Modulation of the oxidative damage, inflammation, and apoptosis-related genes by dicinnamoyl-L-tartaric acid in liver cancer. Naunyn Schmiedebergs Arch. Pharmacol. 396 (11), 1–13 (2023).
Al, H. N. et al. Cor triatriatum sinister (divided left atrium): histopathologic features and clinical management. Ann. Thorac. Surg. 110 (4), 1380–1386 (2020).
Izzo, C. et al. The role of oxidative stress in cardiovascular aging and cardiovascular diseases, Life 11 (1), 60 (2021).
Mehdizadeh, M., Aguilar, M., Thorin, E., Ferbeyre, G. & Nattel, S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat. Rev. Cardiol. 19 (4), 250–264 (2022).
Congdon, J. M. Cardiovascular disease. Canine Feline Anesth. Co-Existing Dis. 1 (1), 1–85 (2022).
Groenewegen, A., Rutten, F. H., Mosterd, A. & Hoes, A. W. Epidemiology of heart failure. Eur. J. Heart Fail. 22 (8), 1342–1356 (2020).
Zhang, H. et al. High serum lactate dehydrogenase as a predictor of cardiac insufficiency at follow-up in elderly patients with acute myocardial infarction. Arch. Gerontol. Geriatr. 117, 105253 (2024).
Babuin, L. & Jaffe, A. S. Troponin: the biomarker of choice for the detection of cardiac injury, Cmaj, vol. 173, no. 10, pp. 1191–1202, (2005).
Wang, Z., Du, H., Wan, H., Yang, J. & Wan, H. Amygdalin prevents multidrug-resistant Staphylococcus aureus-induced lung epithelial cell injury by regulating inflammation and oxidative stress. PLoS One. 19 (9), e0310253 (2024).
Mani, J. et al. Cyanide and lactate levels in patients during chronic oral amygdalin intake followed by intravenous amygdalin administration. Complement. Ther. Med. 43, 295–299 (2019).
Bei, W., Jing, L. & Chen, N. Cardio protective role of Wogonin loaded nanoparticle against isoproterenol induced myocardial infarction by moderating oxidative stress and inflammation. Colloids Surf. B Biointerfaces. 185, 110635 (2020).
Aldubayan, M. A. Evaluation of the cardiac protection conferred by proanthocyanidins in grape seeds against development of ehrlich solid tumors in mice, Biomed Res. Int., vol. 2020. (2020).
Farkhondeh, T., Folgado, S. L., Pourbagher-Shahri, A. M., Ashrafizadeh, M. & Samarghandian, S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 127, 110234 (2020).
Patil, P. P. et al. Computational and experimental Pharmacology to Decode the efficacy of Theobroma cacao L. against doxorubicin-induced organ toxicity in EAC-mediated solid tumor-induced mice. Front. Pharmacol. 14, 1174867 (2023).
Lankin, V. Z., Tikhaze, A. K. & Melkumyants, A. M. Malondialdehyde as an important key factor of molecular mechanisms of vascular wall damage under heart diseases development. Int. J. Mol. Sci. 24 (1), 128 (2022).
Islam, M. S. et al. In vivo anti-cancer activity of Basella alba leaf and seed extracts against Ehrlich’s ascites carcinoma (EAC) cell line, Evidence-Based Complement. Altern. Med., vol. 2018. (2018).
Alam, A. H. M. K. et al. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NFκB in EAC cells. PLoS One. 11 (12), e0167536 (2016).
Aydin, S., Ugur, K., Aydin, S., Sahin & Yardim, M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag. 2 (15), 1–10 (2019).
Tousson, E., Hafez, E., Gazia, M. M. A., Salem, S. B. & Mutar, T. F. Hepatic ameliorative role of vitamin B17 against Ehrlich Ascites carcinoma–induced liver toxicity. Environ. Sci. Pollut Res. 27 (9), 9236–9246 (2020).
El-Masry, T., Al-Shaalan, N., Tousson, E., Buabeid, M. & Al-Ghadeer, A. Potential therapy of vitamin B17 against Ehrlich solid tumor induced changes in interferon gamma, nuclear factor kappa B, DNA fragmentation, p53, Bcl2, survivin, VEGF and TNF-α expressions in mice. Pak J. Pharm. Sci. 33, 393–401 (2020).
El-Masry, T. A., Al-Shaalan, N. H., Tousson, E., Buabeid, M. & Alyousef, A. M. The therapeutic and antineoplastic effects of vitamin B17 against the growth of solid-form Ehrlich tumours and the associated changes in oxidative stress, DNA damage, apoptosis and proliferation in mice. Pak J. Pharm. Sci. 32 (6), 2801–2810 (2019).
Khan, T. et al. Anti-cancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects, Biomolecules, vol. 10, no. 1, p. 47, (2019).
Hare, J. M. Oxidative stress and apoptosis in heart failure progression, Circulation research, vol. 89, no. 3. Am Heart Assoc, pp. 198–200, (2001).
Münzel, T. et al. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J. Am. Coll. Cardiol. 70 (2), 212–229 (2017).
Panth, N., Paudel, K. R. & Parajuli, K. Reactive oxygen species: a key hallmark of cardiovascular disease, Adv. Med., vol. 2016. (2016).
Abbas, M. & Mahmoud, A. H. Evaluation of antioxidant and Anti-tumor activities of Moringa extract in mice. Egypt. J. Radiat. Sci. Appl. 35 (1), 29–37 (2022).
Goswami, S. K., Ranjan, P., Dutta, R. K. & Verma, S. K. Management of inflammation in cardiovascular diseases. Pharmacol. Res. 173, 105912 (2021).
Cabral-Pacheco, G. A. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21 (24), 9739 (2020).
Dewberry, R., Holden, H., Crossman, D. & Francis, S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler. Thromb. Vasc Biol. 20 (11), 2394–2400 (2000).
Bujak, M. & Frangogiannis, N. G. The role of IL-1 in the pathogenesis of heart disease. Arch. Immunol. Ther. Exp. (Warsz). 57, 165–176 (2009).
Liu, G., Ma, C., Yang, H. & Zhang, P. Y. Transforming growth factor and its role in heart disease. Exp. Ther. Med. 13 (5), 2123–2128 (2017).
Pardali, E. & Ten Dijke, P. TGF signaling and cardiovascular diseases. Int. J. Biol. Sci. 8 (2), 195 (2012).
Gaspar-Pereira, S. et al. The NF- B subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 180 (3), 929–939 (2012).
Timmers, L. et al. Targeted deletion of nuclear factor B p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ. Res. 104 (5), 699–706 (2009).
Kawano, S. et al. Blockade of NF- B improves cardiac function and survival after myocardial infarction. Am. J. Physiol. Circ. Physiol. 291 (3), H1337–H1344 (2006).
Li, J. et al. p53 prevents doxorubicin cardiotoxicity independently of its prototypical tumor suppressor activities, Proc. Natl. Acad. Sci., vol. 116, no. 39, pp. 19626–19634, (2019).
Saleme, B. et al. Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci. Transl Med. 11 (478), eaau8866 (2019).
Riba, A. et al. Doxycycline protects against ROS-induced mitochondrial fragmentation and ISO-induced heart failure. PLoS One. 12 (4), e0175195 (2017).
Boshen, Y. et al. Cardioprotective effects of amygdalin, a promising antioxidant, on acute myocardial infarction and underlying mechanisms. J. Funct. Foods. 107, 105684 (2023).
Liczbiński, P., Bukowska, B. Molecular mechanism of amygdalin action in vitro: review of the latest research. Immunopharmacol Immunotoxicol, 40 (3), 212–218. https://doi.org/10.1080/08923973.2018.1441301 (2018).
Molière, S., Jaulin, A., Tomasetto, C. L. & Dali-Youcef, N. Roles of matrix metalloproteinases and their natural inhibitors in metabolism: insights into health and disease. Int. J. Mol. Sci. 24 (13), 10649 (2023).
Zhang, A. et al. Amygdalin alleviated TGF- -induced epithelial-mesenchymal transition in bronchial epithelial cells. Chem. Biol. Interact. 369, 110235 (2023).
Zeng, Q. et al. Amygdalin [corrigendum]elays cartilage [corrigendum]ndplate [corrigendum]egeneration and [corrigendum]mproves [corrigendum]ntervertebral [corrigendum]isc [corrigendum]egeneration by [corrigendum]nhibiting NF- B signaling pathway and [corrigendum]nflammatory [corrigendum]esponse [Corrigendum]. J. Inflamm. Res. 17, 2655–2656 (2024).
El-Desouky, M. A., Fahmi, A. A., Abdelkader, I. Y. & Nasraldin, K. M. Anti-cancer effect of amygdalin (Vitamin B-17) on hepatocellular carcinoma cell line (HepG2) in the presence and absence of zinc. Anti-Cancer Agents Med. Chem. (Formerly Curr. Med. Chem. Agents). 20 (4), 486–494 (2020).
Liczbiński, P. & Bukowska, B. Molecular mechanism of amygdalin action in vitro: review of the latest research. Immunopharmacol. Immunotoxicol. 40 (3), 212–218 (2018).
Pyrillou, K., Burzynski, L. C. & Clarke, M. C. H. Alternative pathways of IL-1 activation, and its role in health and disease. Front. Immunol. 11, 613170 (2020).
Edwards, J. P. & Emens, L. A. The multikinase inhibitor Sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. Int. Immunopharmacol. 10 (10), 1220–1228 (2010).
Guadagni, F. et al. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anti-cancer therapeutic strategy, In Vivo (Brooklyn)., vol. 21, no. 2, pp. 147–161, (2007).
Zaafar, D., Khalil, H. M. A., Rasheed, R. A., Eltelbany, R. F. A. & Zaitone, S. A. Hesperetin mitigates sorafenib-induced cardiotoxicity in mice through Inhibition of the TLR4/NLRP3 signaling pathway. PLoS One. 17 (8), e0271631 (2022).
Varejão, N., Lascorz, J., Li, Y. & Reverter, D. Molecular mechanisms in SUMO conjugation. Biochem. Soc. Trans. 48 (1), 123–135 (2020).
Silva, V. S. et al. Thiophenacetamide as a potential modulator to NF-κB: structure and dynamics study using in Silico and molecular biology assays. J. Biomol. Struct. Dyn. 37 (16), 4395–4406 (2019).
Serasanambati, M. & Chilakapati, S. R. Function of nuclear factor kappa B (NF-kB) in human diseases-a review. South. Indian J. Biol. Sci. 2 (4), 368–387 (2016).
Singh, S. & Singh, T. G. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr. Neuropharmacol. 18 (10), 918–935 (2020).
Acknowledgements
NA.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, Afrah Fatthi Salama, Alyaa Elmalla, Alaa Elmetwalli,; Formal analysis, Alaa Elmetwalli, Hewida Hassan Fadel, Afrah Fatthi Salama; Investigation, Alyaa Elmalla, Mohammed Abu El-Magd, Hewida Hassan Fadel, Afrah Fatthi Salama; Project administration, Alyaa Elmalla, Mohammed Abu El-Magd,; Validation, Alaa Elmetwalli, Hewida Hassan Fadel,; Visualization, Alaa Elmetwalli, Hewida Hassan Fadel; Writing—original draft, Alaa Elmetwalli; Writing—review and editing, Alaa Elmetwalli; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures follow Tanta University’s animal care guidelines and the National Science Council’s Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Salama, A.F., Elmetwalli, A., Elmalla, A. et al. Vitamin B17 alleviates Sorafenib-induced cardiotoxicity in Ehrlich Ascites Carcinoma mice via modulation of inflammatory and fibrotic pathways. Sci Rep 15, 33980 (2025). https://doi.org/10.1038/s41598-025-11643-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-11643-1