Abstract
The growing challenge of drug-resistant pathogens has forced the urgent need for natural and sustainable antimicrobial alternatives. This research explores the green chemistry-based synthesis of zinc oxide quantum dots (ZnO-QDs) employing the ethanolic root extract of Brassica rapa (L.) to assist their formation and stabilization. Characterization of ZnO-QDs confirmed their hexagonal crystalline structure with a nanoscale size (0.8–2.6 nm) and their good stability. GC/MS assay identified β-phenylethyl isothiocyanate and hydroquinone as the main antimicrobial compounds in the essential oil, while LC-ESI-TOF-MS profiling pinpointed the existence of glucosinolates, flavonoids, and phenolic acids as potential active compounds contributing to nanoparticle formation and antimicrobial potential. The combination of B. rapa essential oil and ZnO-QDs exhibited the strongest antibacterial and antifungal activity among the all investigated samples, showing the highest inhibition against Listeria monocytogenes (30.5 mm), Aspergillus niger (20.5 mm), and Fusarium verticillioides (18.2 mm). Molecular docking revealed powerful binding affinities of key phytochemicals to fungal sterol demethylase CYP51B and bacterial DNA gyrase 2, reinforcing their antimicrobial activity. The study’s outcomes reveal that B. rapa-derived ZnO-QDs could be a novel, eco-friendly antimicrobial agent for controlling food spoilage bacteria and mycotoxigenic fungi in food products. Future research should focus on in-vivo efficacy and biocompatibility to fully utilize their potential in food preservation, the pharmaceutical industry, and biomedicine.
Similar content being viewed by others
Introduction
Bacterial and fungal infections pose considerable health concerns. Whereas antibiotics and antifungals serve as the primary lines of treatment, we are facing challenges such as resistance to medications and undesirable health effects promote the need for natural substitutes. Also, foodborne pathogens present a major public health risk, particularly for vulnerable groups, specifically infants, older individuals, and immunocompromised people. International health organizations have emphasized the identification and control of foodborne threats1, including bacterial pathogens (Cronobacter sakazakii, Listeria monocytogenes, Clostridium botulinum, Bacillus cereus, Campylobacter jejuni, Clostridium perfringens, Escherichia coli, Staphylococcus aureus, and Yersinia enterocolitica) and mycotoxigenic fungi (Fusarium spp., Aspergillus spp., and Penicillium spp.)2. The synthetic preservatives are commonly employed to control foodborne pathogens, but their long-term consumption has been linked to adverse health effects. These include possible relations to hepatic disorders, allergies, carcinogenicity, and genotoxicity3. Therefore, natural preservatives are gaining increasing attention in numerous sectors, including biomedicine, agriculture, and food preservation. They are being explored to enhance safety and shelf life of the prepared foods4. Numerous efforts are directed to using natural sources to find different safe antimicrobial agents.
Quantum dots (QDs) are nanoscale semiconductor particles with distinctive physicochemical properties, such as water solubility, low toxicity, small size, biocompatibility, and characteristic photoluminescence. Green synthesis of QDs utilizing plant extracts affords a cost-effective, eco-friendly, and sustainable approach5. Zinc oxide (ZnO) is a broadly studied semiconductor with significant pharmacological properties6. Nature-inspired synthesis of (ZnO-QDs) using plant-derived reducing agents provides a promising alternative for multiple applications.
Brassica rapa L. skeels is an economically important biennial herbaceous plant of the Brassicaceae family. In Egypt, its edible roots and leaves are broadly cultivated7. The root of the plant naturally produces wide and significant bioactive compounds, including flavonoids, phenolics, organic acids, and glucosinolates, which contribute to its diverse therapeutic properties8. These significant phytochemicals make B. rapa a potent candidate for green nanoparticle synthesis, as they can act as both reducing and stabilizing agents.
DNA gyrase is a type II topoisomerase and is exclusive to bacteria. It plays a vital role in DNA transcription and recombination by introducing negative supercoils into DNA. The DNA gyrase 2 (GyrA subunit) acts as a fundamental target for antibacterial agents as a result of its essential contribution in bacterial DNA metabolism and the fact that it is not present in humans9. Similarly, sterol demethylase (CYP51) is a cytochrome P450 enzyme required for ergosterol synthesis. It is considered a principal target for antifungal therapy. Ergosterol is essential for fungal cell membrane integrity, making CYP51 an ideal selective antifungal target10.
The objective of the current study was to establish a green, simple, and sustainable chemical technique for the fast formation of zinc oxide quantum dots (ZnO-QDs) using the ethanolic root extract of B. rapa. The root extract serves as both a reducing and capping agent throughout nanoparticle formation. The phytochemical constituents of B. rapa are expected to contribute to the formation of ZnO-QDs. Also, the structure, nature, form and appearance of the produced ZnO-QDs were investigated by the standard scientific methods, including transmission electron microscopy (TEM), UV analysis, zeta potential study, Fourier transform infra-red spectroscopy (FT-IR), and X-ray diffraction (XRD) analysis. Our work examines the ability of the ethanolic extract, the synthesized ZnO-QDs, the plant’s essential oil, and their combinations to inhibit the growth of particular pathogenic bacteria and mycotoxigenic fungi. Gas chromatography-mass spectrometry (GC/MS) was utilized to investigate the volatile constituents of B. rapa essential oil, while liquid chromatography-electrospray ionization time-of-flight mass spectrometry (LC-ESI-TOF-MS) was employed to determine the plant’s metabolic profile. Furthermore, docking simulations were conducted to assess the interactions of certain identified key phytochemicals with DNA gyrase 2 and sterol demethylase CYP51B, potentially elucidating their antimicrobial mechanisms.
Results
Identification of B. rapa root oil constituents by GC/MS
Gas chromatography coupled with mass spectrometry (GC/MS) was employed to examine the volatile constituents of B. rapa root oil cultivated in Egypt. A total of ten compounds were noticed and authenticated through assessing their fragmentation pattern with entries in the Wiley and NIST Mass Spectral Libraries (Table 1). The compounds detected through analysis, namely, Hydroquinone (61.15%), in addition to Ethyl pentyl (8.09%), Diacetone alcohol (7.42%), β-Phenylethyl isothiocyanate (5.72%), and Butyl propyl ether (5.66%). The remaining constituents are found in smaller amounts, comprising 11.9% of the identified volatile constituents. The GC/MS chromatogram of B. rapa root volatile constituents is presented in (Fig. S1).
LC–ESI-TOF–MS-based identification and profiling of B. rapa metabolites
Negative mode of electrospray ionization mass spectrometry (ESI-MS) was employed in the recognition of 49 compounds in the B. rapa root ethanolic extract. The main classes of detected compounds included glucosinolates, flavonoids, flavonoid glycosides, phenolic acids, fatty acids, and organic acids, ordered according to their retention time values (Rt). Compound identification was demonstrated via Mass Spectrometry- Data Independent Analysis (MS-DIAL) 4.8, an open-source software11, with the ReSpect Negative database (containing 1573 records), serving as a reference for comparison. Additionally, previously reported phytochemical profiles of B. rapa and other Brassicaceae species were consulted to support compound identification. The recognized metabolites are represented in (Table S1), where the base peak chromatogram in negative electrospray ionization mode is presented in (Fig. S2).
The ethanolic extract from B. rapa revealed the existence of twelve distinct glucosinolates. These compounds are secondary metabolites predominantly distributed in the Brassicaceae family, where they serve as distinctive bioactive constituents7. Glucosinolates (GSLs) identification relied on the presence of characteristic product ions with a consistent neutral loss pattern during mass fragmentation. The fragment ions m/z 97 for (HSO4) ion, m/z 195, and m/z 259 for (a sulphated glucose moiety) are considered a signature fragment for glucosinolates12. Moreover, the identification of GSLs was confirmed using their retention time values and molecular weight. The mass ion peak at m/z 436.0397, fragmented mainly to m/z 372 because of the removal of the methyl sulphoxide moiety, was found to be glucoraphanin12. While the peak at m/z 450.0556 was characterized as glucolyssin13. The mass fragmentation at m/z 372 showed the loss of m/z 196, which is likely resulting from the removal of a neutral thioglucose moiety, yielding an ion at m/z 176 defined as a distinctive compound of gluconapin12. Indolic GSLs at m/z 561.08600, 447.0524, and 477.06342 were characterized by odd-mass ions for the aglycone fragments, which thus indicate a structure containing an even number of nitrogen atoms. These compounds were recognized as glucoisatisin14, glucobrassicin12, and neoglucobrassicin12, respectively. Peaks with [M − H]− at m/z 386.0576, 420.0443, 422.0577, 434.0603, and 402.0898 were detected as glucobrassicanapin12, glucoerucin15, gluconasturtiin12, glucoberteroin12, and hexyglucosinolate16, respectively. Glucotropeolin12 was detected at m/z 408.04175 and was distinguished by a fragment ion m/z 245.9900, representing the loss of sulphur trioxide and anhydroglucose.
Flavonoids are additional predominant metabolites present in B. rapa, with isorhamnetin, kaempferol, and quercetin being the most reported aglycons from this plant7. Flavonoids identification includes three flavonol aglycones, five flavonol glycosides, and one flavanone. The characteristic parent ion peaks in negative‑ion mode were identified at m/z 285.04172, 447.09121, and 577.1549 corresponding to kaempferol17 and its glycosides18,19. The existence of the ion peaks at m/z 315.04896, 477.1044, and 623.16071 is considered as base peaks for isorhamnetin17 and its derivatives20. However, quercetin and its glycosides17 were assigned to two characteristic parent ion peaks at m/z 301.03499 and 463.08801, respectively. Moreover, the parent ion peak at m/z 271.06271 for naringenin20 has been observed.
In most metabolic profiling studies of medicinal plants, phenolic acids and organic acids are frequently listed. Twelve compounds were identified in this study, among them one phenolic glycoside identified as sinapic acid O-glucoside12. Phenolic acids loss generally characteristic fragments ion [M‑H‑44] –, corresponding to loss of CO2 from the carboxylic acid group21. A diagnostic mass ion peak at m/z 147.04367 was attributed to cinnamic acid17, while the peak at m/z 163.04037 was characterized as P-coumaric acid17. The mass ion peak at m/z 165.05470 was recognized as phenyl acetic acid22. In addition, other metabolites with the mass ion peaks at m/z 137.02248 and m/z 151.0393 were annotated as salicylic acid23, and mandelic acid24, respectively.
The identified organic acids in the plant, including malic acid18, galactaric acid25, maleic acid20, citric acid18, gluconic acid25, and aconitic acid18, which have previously been recognized in diverse organs of the plant8. The plant exhibited the presence of sugars, which were recognized as glucuronic acid26, fructose27, and sucrose27.
Seven fatty acids were detected and annotated in the plant extract. Hydroxy fatty acid with the mass ion peaks at m/z 147.0296, which was assigned to citramalic acid25. Three unsaturated fatty acids were distinguished at m/z 277.2165, 279.2324, and 281.2477, corresponding to linolenic acid28, linoleic acid28, and oleic acid28, respectively. Furthermore, three saturated fatty acids were elucidated as heptadecanoic acid29, palmitic acid28, and stearic acid28 with mass ion peaks at m/z 269.2455, 255.2318, and 283.2645, respectively.
Six amino acids were also revealed from B. rapa extract27. Among them, two acidic amino acids were acknowledged as glutamic acid and pyroglutamic acid. Fructosylvaline is a neutral amino acid, was also detected. The recognition of L-arginine classified it among the basic amino acid group, which is essential for both protein synthesis and metabolic processes. Additionally, the aromatic amino acids phenylalanine and L-tryptophan were annotated in the plant extract.
Characterization of zinc oxide quantum dots mediated by the selected B. rapa root extract
UV–Vis characterization of synthesized ZnO quantum dots of B. rapa root extract
ZnO quantum dots (ZnO-QDs) synthesized using B. rapa root extract exhibited a prominent absorption peak at 371 nm, as shown in (Fig. S3). This characteristic peak confirms the successful formation of ZnO-QDs.
Visual analysis of synthesized ZnO quantum dots of B. rapa root extract
Figure 1A presents the visual analysis of ZnO quantum dots (ZnO-QDs) synthesized from B. rapa root extract. To confirm their fluorescence properties, commercial ZnO was used as a control sample. Upon UV light exposure, the ZnO-QDs exhibited a notable fluorescence effect, in contrast to the non-fluorescent control sample. The greenish fluorescence strongly indicates the excellent photoluminescence properties of the synthesized ZnO-QDs.
Examination of the fluorescence spectrum of synthesized ZnO quantum dots of B. rapa root extract
The emission spectrum of ZnO-QDs under excitation at a specific wavelength exhibits a prominent emission peak centered around 530 nm, as shown in Fig. 1B. These findings confirm the luminescent properties of synthesized ZnO-QDs.
Transmission electron microscopy (TEM) characterization of synthesized ZnO quantum dots of B. rapa root extract
The Transmission Electron Microscopy (TEM) study was performed to examine the appearance, nature, and crystallinity of ZnO-QDs. Figure 1C,D showed that ZnO-QDs exhibited a hexagonal shape and aggregated in clusters. The particle size distribution was examined with Image J software, revealing a homogeneous structure with a narrow size distribution. The particle size extended from 0.8 to 2.6 nm, with an average of 1.56 nm and a standard deviation (S.D.) of ± 0.6 nm.
Fourier transform infra-red spectroscopy FT-IR analysis of synthesized ZnO quantum dots and B. rapa root extract
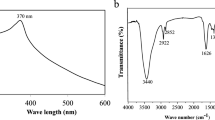
The Fourier Transform Infrared (FT-IR) spectroscopy analysis was performed in the 400–4000 cm−1 range to recognize the characteristic functional groups contributing to the formation of ZnO-QDs using B. rapa root extract. In Fig. 2B, a characteristic absorption band at 3387 cm−1 corresponds to the O–H stretching vibration of adsorbed water molecules. Peaks at 2962.66, 2924.09, and 2854.65 cm−1 are related to C–H stretching vibrations of alkane groups. Moreover, bands at 2372.44, 2345.44, 2299.15, 2283.72, and 2133.27 cm−1 indicate triple bond stretching. A peak at 1419.61 cm−1 was attributed to polyphenolic O–H and aromatic groups, while a peak at 1342.46 cm−1 corresponded to the C–O–H bending vibration. The distinctive ZnO stretching mode was confirmed at 412.77 cm−121. In Fig. 2A, a broad absorption band at 3444.87 cm−1 indicates the presence of phenolic, carboxylic O–H, or alcoholic hydroxyl (–OH) groups. The C=O stretching vibrations of carboxylic and ketonic groups presented in the 1600–1700 cm−1 region, attributed to π-electron delocalization and conjugation with C=C bonds in flavonoids and carboxylic acid compounds30. Also, the peak at 2935.66 cm−1 corresponded to C–H stretching of alkanes, while bands within the 1631–1377 cm−1 range were assigned to C=C or C–C stretching of polyphenolic compounds.
Light scattering dynamics and zeta potential (ZP)
Zeta potential acts as a key parameter for evaluating the stability of ZnO-QDs, particularly during electrophoresis. The average particle size distribution was measured at 37.56 d.nm, with a polydispersity index (PdI) of 0.040 and an intercept value of 0.854 (Fig. 3A). Figure 3B shows that zeta potential peak at − 18.6 mV, with a conductivity of 0.0121 mS/cm and a zeta deviation of 5.98 mV. From these findings confirm that the ZnO-QDs have negative surface charge and are well dispersed throughout the medium. The recorded value of ZP suggests good colloidal stability, which is essential for maintaining the uniformity and functional performance of ZnO-QDs in various applications.
X-ray diffraction (XRD)
The synthesized ZnO-QDs structural features were examined using X-ray diffraction (XRD) to determine their shape, size, and crystal structure. The XRD pattern exhibited distinct diffraction peaks at 2θ values of 31.9081°, 34.5836°, 36.4419°, 47.6944°, 56.7476°, 62.8929°, 66.4492°, 68.0637°, 69.1527°, 72.7751°, 77.0498°, and 81.5715°, as shown in Fig. 4. These peaks correspond to the standard ZnO diffraction form (JCPDS card no. 96-900-8878), authorizing the formation of pure ZnO-QDs with a wurtzite hexagonal crystalline structure. Also, the absence of any additional peaks indicates that the synthesized ZnO-QDs are free from impurities. The well-defined peaks suggest that the ZnO-QDs possess high crystallinity, which is crucial for their use in biomedical applications.
Biological activity
Antibacterial activity
The inhibitory effects of B. rapa essential oil (I), ethanolic extract (II), ZnO-QDs (III), the combination of B. rapa essential oil with ZnO-QDs (IV), and the combination of ethanolic extract with ZnO-QDs (V) against six food-related pathogenic bacterial strains are revealed in Table 2. The highest inhibition zone, 30.5 mm, was recorded by the combination of B. rapa essential oil and ZnO-QDs (IV) against L. monocytogenes. Whereas the lowest inhibition zone value, 7.7 mm was recorded by B. rapa ethanolic extract (II) against L. monocytogenes. The combination of B. rapa essential oil and ZnO-QDs (IV) showed the highest antibacterial potency across all tested bacterial strains, with inhibition zone values ranging between 10.3 and 30.5 mm, followed by the combination of ethanolic extract and ZnO-QDs (V), with inhibition zones ranging from 10.2 mm against B. cereus and 16.8 mm against L. monocytogenes.
Figure 5. illustrates the minimum inhibitory concentration of B. rapa essential oil, ethanolic extract, ZnO-QDs and their different combinations against foodborne pathogenic bacteria. The highest activity of B. rapa essential oil (I) was recorded against Staph. aureus with an MIC of 1.17 mg/mL, while the lowest activity was observed against B. cereus and S. typhi with an MIC value of 1.83 mg/mL. Whereas B. rapa ethanolic extract (II) showed the highest activity against B. cereus, E. coli, and P. aeruginosa, with an MIC of 0.92 mg/mL. On the other hand, ZnO-QDs (III) showed stronger antibacterial activity than both B. rapa essential oil (I) and ethanolic extract (II), with the highest activity being recorded against E. coli at an MIC value of 0.13 mg/mL. The combinations of B. rapa essential oil with ZnO-QDs (IV) and ethanolic extract with ZnO-QDs (V) showed higher activity than the individual treatments alone against all tested bacterial strains, with MICs ranging from 0.58 mg/mL to 0.07 mg/mL.
Antifungal activity
Table 3 indicates the antifungal potential of B. rapa essential oil (I), ethanolic extract (II), ZnO-QDs (III), and their combinations against six toxin-producing fungal species. The greatest antifungal efficacy was showed by the combination of B. rapa essential oil and ZnO-QDs (IV) against A. niger and F. verticillioides, with inhibition zones of 20.5 and 18.2 mm, respectively. This combination showed superior antifungal activity against all tested fungi, in comparison to the individual treatments and the combination of B. rapa ethanolic extract with ZnO-QDs (V). In contrast, the lowest antifungal effect was observed by the ethanolic extract (II), with inhibition zone values varying from 7.5 to 8.7 mm against all the fungal species.
As shown in Fig. 6, the highest antifungal activity was recorded by the combination of B. rapa essential oil with ZnO-QDs (IV) against A. niger and F. proliferatium, as well as the combination of the ethanolic extract with ZnO-QDs (V) against (A) ocheracus, all with MIC of 0.58 mg/mL. Both (B) rapa essential oil/ZnO-QDs (IV) and ethanolic extract/ZnO-QDs (V) combinations showed higher antifungal activity compared to the individual treatments alone. On the other hand, the weakest potent effect was showed by B. rapa ethanolic extract (III) against P. verrcusum, followed by A. niger, and A. flavus with MIC values 3.33, 2.17, and 2.0 mg/mL, respectively.
Molecular modeling
The docking studies were operated as a computational approach to estimate the binding affinities of glucosinolate and flavonoid compounds, identified in the B. rapa root extract, toward two target proteins: DNA gyrase 2 (PDB ID: 2XCT) and sterol demethylase CYP51B (PDB ID: 5FRB). These proteins are involved in mediating antibacterial and antifungal properties, respectively.
The consequences suggest that astragalin binds to DNA gyrase 2 with a favorable binding affinity, with a ΔG value of − 9.19 kcal /mol as presented in Table 4. This binding is primarily driven by a combination of hydrogen bonds, pi-cation, pi-pi stacked, and pi-alkyl interactions (Fig. 7). The key connections stabilizing the complex between astragalin and the protein include hydrogen bonds formed between the phenolic hydroxyl group on ring B of the flavonol nucleus and GLU: 447. Furthermore, the interaction between the alcoholic hydroxyl group of the sugar moiety and ASP: 437. These interactions validate that astragalin is well-positioned within the protein’s binding pocket, resulting in the formation of a stable complex.
In the context of sterol demethylase, the screened compounds exhibited strong binding affinities to the enzyme’s catalytic domains, with calculated binding free energies (ΔG) ranging from − 6.05 to − 10.20 kcal/ mol (Table 5). Among these, narcissin demonstrated the highest binding affinity, with a ΔG value of − 10.20 kcal/ mol. This binding was attributed to multiple hydrogen bond interactions, including those between the carbonyl group of the C-ring and TYR: 122, the phenolic hydroxyl groups of the A and B rings with SER: 375 and ALA: 303, respectively, and the alcoholic hydroxyl groups of the sugar moiety with TYR: 136, HIS: 461, and PHE: 456. Also, the stability of the complex was further reinforced by hydrophobic interactions such as π–π stacking and π-alkyl interactions (Fig. 8).
Discussion
Nowadays, there has been a growing interest in utilizing natural pathogen-targeting agents in food applications to minimize spoilage caused by microbial contamination. B. rapa has been acknowledged as a potential source of substances with antimicrobial properties7. Various crude extracts and fractions of B. rapa have been evaluated for their antimicrobial potential to combat a wide spectrum of microorganisms, encompassing Gram-positive and Gram-negative bacteria, as well as fungi. Studies have proved that B. rapa extracts exhibit antimicrobial activity against Pseudomonas aeruginosa, Candida albicans, and Bacillus subtilis31. Also, the plant has been reported to possess broad-spectrum antifungal properties against various fungal pathogens. The ethanolic extract of B. rapa exhibited significantly higher antifungal activity than the aqueous extract against Candida albicans MTCC4748, Candida glabrata MTCC3814, and Candida tropicalis MTCC9038 in in-vitro assays using the agar well diffusion method32.
GC-MS investigation of B. rapa essential oil in the current research marked hydroquinone as the dominant constituent. Hydroquinone is broadly recognized for its potent bacteria-fighting properties against Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), and extended-spectrum β-lactamase (ESBL)-producing S. aureus. The mode of action of the antibacterial effect of hydroquinone involves disruption of bacterial cell outer layers, increased permeability, leakage of intracellular substances, inhibition of protein synthesis, and alteration of gene expression. In addition, β-phenylethyl isothiocyanate was identified as the principal isothiocyanate in B. rapa, demonstrating antimicrobial potential against harmful pathogens transmitted through food consumption, including Vibrio parahaemolyticus, Staphylococcus aureus, and Bacillus cereus33. This compound also exhibits strong antimicrobial activity against Pseudomonas aeruginosa, Escherichia coli, Listeria monocytogenes and Staphylococcus aureus, by disrupting bacterial cell membranes and altering bacterial cell hydrophobicity. The antibacterial properties of isothiocyanates are influenced by their chemical structure, particularly the aromatic electron-donating benzene ring, which enhances their antimicrobial effectiveness34. According to previous research, plants from the Brassicaceae family are important natural sources of β-phenylethyl isothiocyanate, which has demonstrated strong antimicrobial activity against MRSA. Due to its potent antibacterial properties, this compound holds promise for use in pharmacological formulations, either alone or in combination with conventional antibiotics, for the treatment of MRSA infections35.
LC–ESI-TOF–MS revealed the existence of glucosinolates, flavonoids, fatty acids, phenolic compounds, and organic acids. GSLs have exhibited several biological actions, including anti-carcinogenic, antioxidant, and antimicrobial characteristics, and are considered potent natural agents to prevent food spoilage36. These activities are principally attributed to volatile compounds created upon glucosinolate hydrolysis, particularly isothiocyanates, that can inhibit a broad range of microorganisms at low concentrations37. Flavonoids are potent microbe-fighting compounds in vitro against a wide variety of pathogenic microbes. They can converse the antibiotic resistance and enhance the effectiveness of recent antibiotic drugs38. Mandelic acid has been shown to inhibit the growth of various numbers of bacteria, such as Staphylococcus aureus, Proteus sp., Escherichia coli, and Klebsiella aerogenes. Additionally, salicylic acid is used in the formulation of antiseptics and cosmetics due to its antimicrobial properties24. Organic compounds such as malic and citric acid show a wide spectrum of antimicrobial action against Gram-positive bacteria, Gram-negative bacteria, yeasts, and fungi, and are commonly used as food preservative39. Also, the antimicrobial properties of fatty acids are well recognized, and there is a strong association between their chemical structure and activity40. The literature reported that the bioactivity of these compounds displays enhancement when linked to amino acid residues41.
The key phytochemicals in B. rapa extract, mainly glucosinolates and flavonoids, are possibly responsible for the stabilization and reduction of the synthesized ZnO-QDs42. GSLs can be hydrolyzed to form reactive intermediates such as isothiocyanates and nitriles43. These compounds are known to exhibit strong redox activity and have been reported to reduce oxidative stress in biological systems44. Owing to their redox potential, they may act as effective reducing agents and can facilitate the transformation of Zn2+ ions to zinc, which react with water in the solution to form zinc hydroxide. After thermal treatment in the presence of base, ZnO nanoparticles are immediately formed45. Additionally, flavonoids such as kaempferol and quercetin possess hydroxyl and carbonyl groups capable of chelating metal ions and donating electrons, supporting the formation of ZnO-QDs46. These structural features also allow them to adsorb onto nanoparticle surfaces, serving as natural capping agents that prevent aggregation and enhance stability47. The dual action of these phytochemicals results in a controlled and eco-friendly synthesis of stable ZnO-QDs.
The unique features of QDs, including wide spectral range (from ultraviolet to infrared), excellent crystallinity, and consistent particle size48. These physicochemical characteristics making them appropriate for numerous technological and biomedical applications49. The successful synthesis of ZnO-QDs in our study was confirmed through multiple characterization techniques. UV–Vis analysis showed a maximum absorption peak at 371 nm, indicating ZnO-QD formation. TEM study exhibited hexagonal crystal structures and a particle size varying from 0.8 to 2.6 nm. This size distribution and uniform morphology aligns with prior work in which the prepared QDs exhibited controlled shape and size within the range of 3.4 to 5.3 nm50. Additionally, XRD analysis authorized the existence of a wurtzite hexagonal crystalline structure, consistent with patterns reported in previous studies of nanocrystalline CdS quantum dots, where a broad diffraction pattern indicated their nanoscale size and wurtzite structure51. FT-IR spectroscopy supported the successful synthesis of ZnO-QDs, and zeta potential analysis demonstrated their stability. Moreover, a notable greenish fluorescence and emission absorption around 530 nm indicate the excellent photoluminescence properties. The strong photoluminescence indicates that QDs have a nearly uniform size distribution and well dispersibility52. These outcomes support our results, confirming the structural uniformity, integrity, and stability of ZnO-QDs.
Zinc is a critical mineral for humans and is distributed in different parts of the body. It performs a principal role in defense against cancer and exhibits anti-inflammatory and antioxidant activities5. Zinc oxide (ZnO) has dominant antimicrobial activity53and it is used as an active ingredient in various preparations, including antibacterial creams, ointments and lotions, because of its capability to liberate zinc ions in aqueous suspension54. Beyond healthcare, zinc oxide is increasingly utilized in nanoelectronics, optics, cosmetics, food preservation, pharmaceuticals, and chemical industries. The U.S. Food and Drug Administration recognizes zinc oxide as typically safe55. The substantial impact of plant species and their phytochemical profile on nanoparticle features and bioactivity has been investigated via numerous recent researches. ZnO nanoparticles are highly effective antibacterials at small concentrations (0.16–5.00 mmol/L), against a wide series of bacterial strains, targeting both Gram-positive and Gram-negative bacterial varieties. Their mechanism of action includes interruption of the cell structure, binding to proteins, induction of reactive oxygen species (ROS), and modification of gene expression56. Also, they have antifungal properties, effectively inhibiting fungal species such as Aspergillus niger and Candida albicans57. The application of ZnO-QDs in food applications can be valuable in restricting the microbial contaminants, including Salmonella Enteritidis, Listeria monocytogenes and Escherichia coli55. The synthesized ZnO-QDs using Eclipta alba leaf extract exhibited a remarkable antibacterial effect targeting E. coli58supporting their use in food preservation and biomedical implementations. Vitis vinifera stem extract has been used to biosynthesize (ZnONPs), showing potent antimicrobial activity, which is related in part to their high colloidal stability, with a zeta potential at − 29.49mV. A synergistic effect was observed when these ZnONPs combined with an antibiotic (Ampicillin), increasing its effectiveness against the pathogenic microbes, and minimizing its MIC value59. Likewise, our findings show that ZnO-QDs exhibited a zeta potential of − 18.6 mV and their antimicrobial efficacy, enhanced upon their combination with the essential oil of B. rapa, indicating a promising synergistic interaction. Pelargonium zonale leaf extract was shown to effectively synthesize ZnONPs with an average particle size of about 5.5 nm. The combination of these formed nanoparticles with the extract demonstrated enhanced antiviral activity against human coronavirus 229E60. In comparison, our paper uses B. rapa extract for synthesizing ZnO-QD, which accomplishes stable, smaller particle sizes (0.8–2.6 nm). This likely contributed to the strong observed antimicrobial effects, particularly in combination with the essential oil. Smaller ZnO nanoparticles exhibit greater biological efficacy as a result of their increased (surface-area-to-volume) ratio and more effective interaction with microbial membranes61.
In our work, the combination of B. rapa essential oil and ZnO-QDs exhibited the highest antibacterial and antifungal activity among the tested samples. This formulation demonstrated the strongest inhibition against Listeria monocytogenes (30.5 mm) and significant antifungal effects against Aspergillus niger (20.5 mm) and Fusarium verticillioides (18.2 mm). In contrast, the extract of B. rapa lonely exhibited the least inhibitory effects against both bacterial and fungal growth. These outcomes align with prior studies and highlight the cooperative efficacy of the combination of zinc oxide nanoparticles with B. rapa plant extracts, contributing to the enhancement of an effective, natural and eco-friendly strategy for controlling harmful phytopathogenic bacteria and fungi. Furthermore, the nanoscale size, the unique physicochemical properties, and stability of ZnO-QDs synthesized from B. rapa root extract and essential oil offer promising opportunities for numerous commercial, clinical and fluorescence labeling applications in antimicrobial research and biotechnology. Additional in-vivo validation is necessary to assess their toxicity and effectiveness in biological systems. So, future research should emphasize formulation optimization to fully investigate their therapeutic activity and their application in food- related products.
Computational analysis demonstrated that glucosinolate and flavonoid compounds from B. rapa exhibited potent binding affinities toward DNA gyrase 2 and sterol demethylase CYP51B, which are considered the key enzymes for bacterial and fungal survival, respectively. The resulting scores revealed that these plant-derived compounds could be potential antimicrobial agents by disrupting bacterial and fungal survival mechanisms. These findings align with the antimicrobial activity observed in the study.
Materials and methods
Plant material
The roots of B. rapa used in this study were obtained from the Agriculture Research Centre, Cairo, Egypt, in November 2024. A reference sample was preserved in the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Cairo University (Giza, Egypt), under representative number (1-12-2024-F) for future inquiries.
Preparation of the plant extract
Fresh B. rapa roots (1 kg) were blended with ethanol using 100 g of root per one liter of ethanol. The mixture was successively filtered and evaporated under reduced pressure at 40 °C to get a sticky residue, which was stored in the dark at 4 °C for further examination.
Gas chromatography-mass spectrometry analysis (GC/MS)
A fresh sample of B. rapa root was prepared for GC/MS analysis62. In detail, 50 g of fresh root was blended with 500 mL of ethanol, filtered, and mixed with 50 mL of water. The resulting filtrate was partitioned using n-hexane21. The n-hexane extract was then subjected to evaporation using a rotary evaporator at 35 °C for 25 min to obtain volatile oil. The volatile constituents were examined using a gas chromatograph (Agilent 7890B) linked to a mass spectrometer (5977 A). This analysis was carried out at the Central Laboratories Network, which is part of the National Research Centre in Cairo, Egypt. Separation was performed on an HP-5MS column (which is 30 m in length, has an internal diameter of 0.25 mm, and a film thickness of 0.25 μm). Helium gas was used as carrier gas at a flow rate of 1.0 ml/min. The sample was split into a ratio of 1:50 before entering the column, and the injection volume was 1 μm. The temperature profile was set as follows: 40 °C for one minute, gradually increased by 4 °C every minute until it reached 150 °C, where it was kept for another six minutes. Finally, the temperature increased again by 4 °C per minute until it reached 210 °C, and was maintained for one minute. The injector was set at a constant temperature of 280 °C, while the detector part was maintained at 220 °C. Mass spectral data were acquired using electron ionization (EI) at an energy level of 70 eV, detecting ions with mass-to-charge ratios (m/z) varying from 50 to 900. The analysis began five minutes after the injection to permit the solvent to pass through. Compound identification of the volatile constituents in the n-hexane fraction constituents was performed by comparing the resulting spectral fragmentation patterns with the entries in the Wiley and NIST Mass Spectral Library databases.
Metabolic profiling
Metabolic characterization of the root ethanolic extract of B. rapa was done by using a High-performance liquid chromatography coupled with electrospray ionization-quadruple-time of flight-mass spectrometry (LC-ESI-TOF-MS)63.
Sample preparation
A 50 mg portion of the sample was blended with 1 mL of a special liquid (reconstitution solvent). This liquid was a mixture of water, methanol, and acetonitrile in a 50:25:25 (v/v/v) ratio. The prepared solution was forcefully mixed using a vortex mixer and subjected to ultrasonication at a frequency of 30 kHz for 10 min. After this, the solution was centrifuged at a high speed of 10,000 rpm for another 10 min. From the stock solution, a small amount (50 µL) was taken and further diluted to a total volume reached 1000 µL. This resulted in a final concentration of 2.5 2.5 µg/µL. A 10 µL of the prepared sample was introduced for analysis in the negative ionization approach. For quality assurance, control samples and reference standards were also analyzed to ensure experimental reliability.
Instruments and acquisition method
The separation of small molecules was done by using an Exion LC procedure (AB Sciex in Framingham, Massachusetts, USA) equipped with an automated sample. The process included a pre-column in-line filter disk (0.5 μm wide and 3.0 mm diameter; Phenomenex in Torrance, California, USA) and a special column called an Xbridge C18 column (3.5 μm inside and measures 2.1 mm wide and 50 mm long; Waters Corporation, Milford, MA, USA). The column was held at a constant temperature of 40 °C with a flow rate of 300 µL/min. The mobile phase is composed of two different solutions: solution (A) containing 5 mM ammonium formate in 1% methanol, with the acidity adjusted to a pH of 3.0 using formic acid and solution (B) also containing 5 mM ammonium formate in 1% methanol, but with the alkalinity adjusted to a pH of 8.0 using sodium hydroxide. Gradient elution was performed as follows: 0–20 min, 10% of solution B and 90% of solution A; 21–25 min, the solution B was rapidly increased to 90%, while solution A decreased to 10%.; 25.01–28 min, switched back to the initial condition of 10% solution B and 90% solution A.; followed by re-equilibration at 90% solution B. Mass spectrometry (MS) analysis was conducted using a Triple TOF 5600 + mass spectrometer (AB SCIEX in Concord, Ontario, Canada), prepared with a Duo-Spray source operating in negative electrospray ionization (ESI). The sprayer capillary voltage was set to − 4500 V, and the declustering ability was − 80 V. The ionization source was set at a constant temperature of 600 °C, with a curtain gas pressure of 25 psi, and two other gases (gas 1 and gas 2) both at a pressure of 40 psi. Collision-induced energy was set to − 35 V with a spread of 20 V. An ion tolerance of 10 ppm was applied for accurate mass matching. High-resolution survey spectra were acquired over the range of m/z from 50 to 1100, with full detection taking only 50 milliseconds.
LC-MS data processing
MS-DIAL 4.8, open-source software, was utilized for untargeted and extensive investigation of small molecules in the sample. The spectral library negative database (containing 1573 records) was employed as the reference database, associated with the acquisition mode. To ensure the detected peaks in the total ion chromatogram (TIC), the Master View 1.1 software (AB SCIEX) was applied. The selection measures included detected features with a signal-to-noise (S/N) ratio higher than 5 and an intensity exceeding three times that observed in blank samples.
Green chemistry-based synthesis of zinc oxide quantum dots
ZnO-QDs were synthesized with the ethanol-based extract of B. rapa roots, which performed as both a reducing and stabilizing agent. One gram of the ethanolic extract was dissolved in hydro alcohol (100 mL total volume), then blended with 5 g of zinc acetate dissolved in bi-distilled water. The resulting solution was heated in a hot water bath at a temperature of 100 °C for 20 min. The pH values of the reaction medium were adjusted to 12 by gradual addition of droplets of ammonium hydroxide. A white precipitate of ZnO-QDs was generated, and the combination was kept at a temperature of 100 °C for another 20 min to ensure complete reduction of zinc acetate to ZnO-QDs. The resulting ZnO-QDs were utilized to centrifugation at a speed of 4000 rpm for 10 min. The pellets of ZnO-QDs were washed thoroughly, involving two cycles using distilled water and another two cycles using pure ethanol to get rid of any impurities. Finally, the purified pellets were freeze-dried and preserved at room temperature for further purposes.
Characterization of zinc oxide quantum dots
UV–Vis spectral analysis
A UV spectrophotometer model UV-1601(Shimadzu Corporation, Kyoto, Japan) was used to evaluate ZnO-QDs preparations. UV spectra were recognized for wavelengths ranging from 200 to 800 nanometers.
Visual assessment of zinc oxide quantum dots
Visual assessment of the ZnO-QDs was performed by using UV light source.
Examination of the fluorescence spectrum of zinc oxide quantum dots
The fluorescence spectrum was recorded using a fluorescence spectrometer (Lumina, Model LF1201005) to analyze the photoluminescent properties of the synthesized ZnO quantum dots.
FT-IR analysis
The functional moieties and phytochemical constituents involved in the formation and stabilization of ZnO-QDs were identified using a Shimadzu FT-IR Affinity-1 Spectrometer (Shimadzu Corporation, Kyoto, Japan) operated in attenuated total reflectance (ATR) mode.
Zeta-Sizer measurements
ZnO-QDs size distribution was performed using a Zeta-sizer Nano-ZS laser diffractometer (Malvern, Worcestershire, UK).
Transmission electron microscopy (TEM) analysis
The morphology and particle dimension of ZnO-QDs were observed using the TEM technique (JEOL-JEM-1011, JEOL Ltd., Tokyo, Japan). A small drop of the liquid containing (ZnO-QDs) was put onto a copper grid that had a thin layer of carbon on it, then left to dry naturally at room temperature before examination.
X-ray diffraction (XRD)
X-ray Diffraction of dry powder of ZnO-QDs was investigated by using a Bruker D8 Advance Diffract meter (Bruker AXS, Karlsruhe, Germany) and Cu Ka radiation (k = 1.54).
Antimicrobial assay
Antibacterial assay
The inhibitory effects of all tested treatments were evaluated against six species of foodborne bacterial pathogens: Bacillus cereus EMCC-1080, Staphylococcus aureus ATCC-13565, Listeria monocytogenes, Escherichia coli 0157H7 ATCC 51659, Salmonella typhi ATCC-25566, and Pseudomonas aeruginosa NRRL B-272. The extracts were formulated at a concentration of 10 mg/ml by dissolving them in dimethyl sulfoxide (DMSO). The antibacterial effectiveness of these prepared extracts was tested using the disc diffusion method on nutrient agar plates. Strains of bacteria were cultured on nutrient agar plates for 24 h at 37 °C. For testing their susceptibility to antibacterial agents, a small amount of each bacterial culture was inoculated into a test tube containing 5 ml of Mueller-Hinton broth (MHB) and incubated at 37 °C for 4 h. This incubation lasted until the bacterial suspension reached a cloudiness matched to 0.5 McFarland standards, which is equivalent to approximately 1 × 108 colony-forming units per milliliter (CFU/mL). A uniform layer of bacteria was spread on the surface of solid nutrient agar plates using sterile cotton swabs. These plates were allowed to dry for 30 min. Discs that had been soaked in the test extract were then placed on the surface of the dried agar plates. For comparison, discs soaked in a ceftriaxone solution (at a concentration of 1.0 mg/ml) served as positive controls. Subsequently, all the plates were maintained at 37 °C for 24 h. After incubation, the antibacterial activity was assessed by measuring the diameter of the clear area (in millimeters) around each disc, where bacterial growth was inhibited (inhibition zone)64.
Antifungal assay
All tested treatments were assessed for their antifungal activity against six mycotoxin-producing fungal species: Aspergillus flavus NRRL-3357, A. ochraceus ITAL-14, A. niger ATCC-16888, Fusarium verticillioides ITEM-10027, F. proliferatum MPVP-328, and Penicillium verrucosum BFE-500. The ability to inhibit fungal growth was experimented with by employing the disc diffusion method on potato dextrose agar. Fungal spores were inoculated on the agar surface, then sterile discs impregnated with the extract were applied on the surface, and inhibition zones were measured after 24 to 48 h at 28 °C. A miconazole solution (at a concentration of 1.0 mg/ml) was used as a positive control65.
Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration of all tested treatments was determined via the Microdilution technique66. The samples were serially diluted twofold in Mueller-Hinton Broth (MHB) to produce a concentration gradient from 10 to 0.1 mg/mL. Then, 100 µL of each dilution was transferred into a 96-well microplate. Bacterial suspensions were adjusted to 108 CFU/mL, corresponding to 0.5 McFarland standards, and 100 µL of each was added to the wells containing the treatments. After 24 h of incubation at 37 °C, the optical density at 600 nm was evaluated, and wells including only growth medium without bacteria were used as a negative control. The minimum inhibitory concentration (MIC) was identified as the lowest concentration of the treatment that suppressed bacterial growth67.
Molecular modeling
Several key bioactive compounds identified in the extract of B. rapa using LC-ESI-MS/MS were selected for molecular docking studies via the Molecular Operating Environment (MOE 2015.10). Docking was performed at the catalytic domains of two important microbial enzymes: DNA gyrase 2 (PDB ID: 2XCT)68 and sterol demethylase CYP51B (PDB ID: 5FRB)69. The proper conformations of both proteins were retrieved from the Protein Data Bank (PDB), with unnecessary solvents and cofactors excluded. Residues of the protein chain were prepared as outlined in prior work70. The Site Finder tool within MOE was used to identify optimal binding pockets, focusing on regions containing key amino acid residues. Both the plant-derived compounds and the reference drugs (tetracycline for DNA gyrase 2 and nystatin for sterol demethylase CYP51B) were prepared for docking by protonation and energy minimization. The prepared ligands were converted into MOE database files (mdb) and docked within the identified binding sites. Docking results were assessed based on binding free energy (ΔG, kcal/mol) and visualized using 2D and 3D ligand interaction representations. The best favorable ligand poses were selected based on strong binding affinities (low ΔG values) and root-mean-square deviation (RMSD) values of ≤ 2 Å, ensuring their accuracy.
Statistical analysis
All analyses were applied in three separate trials throughout the study, and the resulting data are presented as the mean ± standard error (SE), calculated from no fewer than three independent experiments (n ≥ 3). One-way analysis of variance ANOVA was used to evaluate statistical significance in Statistica Version 9, with a significance threshold set at p < 0.05. To compare groups that displayed significant differences, post-hoc analysis was conducted using Fisher’s Least Significant Differences (LSD) test.
Conclusion
Hydroquinone and β-phenylethyl isothiocyanate are the major constituents in the essential oil of B. rapa root that were identified through the GC/MS technique. Both constituents are exhibiting significant antimicrobial efficacy. The metabolic profiling identified various bioactive compounds, including glucosinolates, flavonoids, phenolic, organic acids and fatty acids in the ethanolic root extract. The root extract was successfully employed in a green and sustainable synthesis for ZnO-QDs preparation, as authorized by XRD, UV analysis, zeta potential, TEM, and FT-IR spectroscopy. Our work represents a novel report on the green fabrication of ZnO-QDs utilizing B. rapa and their promising antimicrobial properties. The antimicrobial study demonstrates the potent antibacterial and antifungal activity of ZnO-QDs, remarkably in combination with B. rapa essential oil, which showed superior efficacy against various pathogenic bacteria and all tested toxigenic fungi. Also, it presents promising docking scores, demonstrating potential targets for antimicrobial treatment. Our green synthesis offers a powerful starting point for developing sustainable antimicrobial agents from plant-based sources. Subsequently, future studies should focus on in-vivo trials to validate the safety, efficacy, and biocompatibility of B. rapa-derived ZnO-QDs in living systems. Emphasis on how they can be utilized in food safety, pharmaceutical disciplines, and preservative products. As well as developing unique antimicrobial agents, adjuvants to conventional therapies and natural food preservatives.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Van de Venter, T. Emerging food-borne diseases: a global responsibility. Food Nutr. Agric. 4–13 (2000).
Wood, G. E., Trucksess, M. W. & Henry, S. H. Major fungal toxins of regulatory concern. In Food Science and Technology-New York-Marcel Dekker, 423–444 (2003).
Helal, E. G., Mustafa, R. A., Mohamed, A. & El-Gamal, M. S. Adverse effects of two kinds of food additive mixtures (flavor enhancer, food preservative or food coloring agent) on physiological parameters in young male albino rats. Egypt. J. Hosp. Med. 67, 344–351 (2017).
Aziz, M. & Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 58, 486–511 (2018).
Hamed, R., Obeid, R. Z. & Abu-Huwaij, R. Plant mediated-green synthesis of zinc oxide nanoparticles: an insight into biomedical applications. Nanatechnol. Rev. 12, 20230112 (2023).
Flora, R. M. N., Palani, S., Sharmila, J. & Chamundeeswari, M. Green synthesis and optimization of zinc oxide quantum dots using the Box–Behnken design, with anticancer activity against the MCF-7 cell line. Appl. Phys. A. 128, 359 (2022).
Paul, S., Geng, C. A., Yang, T. H., Yang, Y. P. & Chen, J. J. Phytochemical and health-beneficial progress of turnip (Brassica rapa). J. Food Sci. 84, 19–30 (2019).
Cao, Q., Wang, G. & Peng, Y. A critical review on phytochemical profile and biological effects of turnip (Brassica rapa L). Front. Nutr. 8, 721733 (2021).
Spencer, A. C. & Panda, S. S. DNA gyrase as a target for quinolones. Biomedicines. 11, 371 (2023).
Singh, A. et al. Recent advances in antifungal drug development targeting lanosterol 14α-demethylase (CYP51): A comprehensive review with structural and molecular insights. Chem. Biol. Drug Des. 102, 606–639 (2023).
Tsugawa, H. et al. MS-DIAL: data-independent MS/MS Deconvolution for comprehensive metabolome analysis. Nat. Methods. 12, 523–526 (2015).
Francisco, M. et al. Simultaneous identification of glucosinolates and phenolic compounds in a representative collection of vegetable Brassica rapa. J. Chromatogr. A. 1216, 6611–6619 (2009).
Padilla, G., Cartea, M. E., Velasco, P., de Haro, A. & Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry. 68, 536–545 (2007).
Fabre, N. et al. Characterisation of glucosinolates using electrospray ion trap and electrospray quadrupole time-of‐flight mass spectrometry. Phytochem. Anal. Int. J. Plant. Chem. Biochem. Tech. 18, 306–319 (2007).
Lee, J. G. et al. Evaluation of glucosinolate variation in a collection of turnip (Brassica rapa) germplasm by the analysis of intact and Desulfo glucosinolates. J. Agric. Food Chem. 61, 3984–3993 (2013).
Sun JiangHao, S. J. et al. Metabolomic assessment reveals an elevated level of glucosinolate content in CaCl2 treated broccoli microgreens (2015).
Cartea, M. E., Francisco, M., Soengas, P. & Velasco, P. Phenolic compounds in brassica vegetables. Molecules. 16, 251–280 (2010).
Fernandes, F. et al. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. Rapa L). Food Chem. 105, 1003–1010 (2007).
Ibrahim, L. F. et al. Flavonoid investigation, LC–ESIMS profile and cytotoxic activity of Raphanus raphanistrum L.(Brassicaceae). J. Chem. Pharm. Res. 8, 786–793 (2016).
Alotaibi, B. et al. Antimicrobial activity of Brassica rapa L. flowers extract on Gastrointestinal tract infections and antiulcer potential against indomethacin-induced gastric ulcer in rats supported by metabolomics profiling. J. Inflamm. Res. 7411–7430 (2021).
Attia, G. H. et al. Antiviral zinc oxide nanoparticles mediated by hesperidin and in Silico comparison study between antiviral phenolics as anti-SARS-CoV-2. Colloids Surf. B. 203, 111724 (2021).
Miceli, N. et al. Phytochemical profile and antioxidant activity of the aerial part extracts from Matthiola Incana subsp. Rupestris and subsp. Pulchella (Brassicaceae) endemic to Sicily. Chem. Biodivers. 18, e2100167 (2021).
Stratil, P., Klejdus, B. & Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 54, 607–616 (2006).
Przybylska-Balcerek, A. & Stuper-Szablewska, K. Phenolic acids used in the cosmetics industry as natural antioxidants. EJMT. 4, 24–32 (2019).
Farid, M. M. et al. Comprehensive phytochemical characterization of Raphanus raphanistrum L.: in vitro antioxidant and antihyperglycemic evaluation. Sci. Afr. 16, e01154 (2022).
Hua, H. et al. Healthy regulation of Tibetan Brassica rapa L. polysaccharides on alleviating hyperlipidemia: A rodent study. Food Chem. Mol. Sci. 6, 100171 (2023).
Yang, J. et al. Chemical profile of turnip according to the plant part and the cultivar: a multivariate approach. Foods. 12, 3195 (2023).
Sharafi, Y., Majidi, M. M., Goli, S. A. H. & Rashidi, F. Oil content and fatty acids composition in brassica species. Int. J. Food Prop. 18, 2145–2154 (2015).
Aly, A. & Morsy, H. Evaluation of fatty and amino acids profile, sensory and microbial loud of chicken luncheon prepared with lentil powder, turnip plant and cauliflower. J. Food Dairy. Sci. 10, 165–170 (2019).
Bryce, D. L., Webster, F. X., Silverstein, R. M. & Kiemle, D. J. (Wiley, 2014).
Beltagy, A. M. Investigation of new antimicrobial and antioxidant activities of Brassica rapa L. Int. J. Pharm. Pharm. Sci. 6, 84–88 (2014).
Sangray, A., Singh, A. P. & Singh, A. P. Phytochemical evaluation and investigation of anti-fungal activity of turnip top extracts. Indian J. Pharm. Pharmacol. 8, 248–253 (2021).
Hong, E. & Kim, G. H. Anticancer and antimicrobial activities of β-phenylethyl isothiocyanate in Brassica rapa L. Food Sci. Technol. Res. 14, 377–377 (2008).
Borges, A. et al. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J. Food Sci. Technol. 52, 4737–4748 (2015).
Dias, C., Aires, A. & Saavedra, M. J. Antimicrobial activity of isothiocyanates from cruciferous plants against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 15, 19552–19561 (2014).
Saavedra, M. J. et al. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 6, 174–183 (2010).
Lewis, J. & Papavizas, G. Effect of sulfur-containing volatile compounds and vapors from cabbage decomposition on aphanomyces euteiches. Phytopathology. 61, 208 (1971).
Górniak, I., Bartoszewski, R. & Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272 (2019).
Coban, H. B. Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst. Eng. 43, 569–591 (2020).
McGaw, L., Jäger, A. & Van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. South. Afr. J. Bot. 68, 417–423 (2002).
Shivakumara, K., Prakasha, K., Suresha, G., Suhas, R. & Gowda, D. Synthesis and antimicrobial study of amino acids conjugated benzylpiperazine derivatives. MyScience. 2, 100–106 (2007).
Chaudhary, M. et al. Pharmaceutical orientation and applications of silver/zinc oxide nanoparticles developed from various fruit Peel extracts: an emerging sustainable approach. Discover. Sustain. 6, 7 (2025).
Fahey, J. W., Zalcmann, A. T. & Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 56, 5–51 (2001).
Ochar, K. et al. The potential of glucosinolates and their hydrolysis products as inhibitors of cytokine storms. Molecules. 29, 4826 (2024).
Bandeira, M., Giovanela, M., Roesch-Ely, M. & Devine, D. M. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sustain. Chem. Pharm. 15, 100223 (2020).
Ahmed, S., Ahmad, M., Swami, B. L. & Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 9, 1–7 (2016).
Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 13, 2638–2650 (2011).
Ratnesh, R. K., Singh, M. K. & Singh, J. Enhancing zno/si heterojunction photodetector performance for ultra high responsivity across wide spectral range. J. Mater. Sci.: Mater. Electron. 35, 756 (2024).
Ratnesh, R. K. & Mehata, M. S. Tunable single and double emission semiconductor nanocrystal quantum dots: a multianalyte sensor. Methods Appl. Fluoresc. 6, 035006 (2018).
Ratnesh, R. & Mehata, M. S. Synthesis and optical properties of core-multi-shell cdse/cds/zns quantum dots: surface modifications. Opt. Mater. 64, 250–256 (2017).
Ratnesh, R. Hot injection blended tunable cds quantum dots for production of blue LED and a selective detection of Cu2 + ions in aqueous medium. Opt. Laser Technol. 116, 103–111 (2019).
Ratnesh, R. & Mehata, M. S. Investigation of biocompatible and protein sensitive highly luminescent quantum dots/nanocrystals of cdse, cdse/zns and cdse/cds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 179, 201–210 (2017).
Yadav, A., Kumar, H., Kumar, P., Rani, G. & Maken, S. Syzygium cumini leaf extract mediated green synthesis of ZnO nanoparticles: A sustained release for anticancer, antimicrobial, antioxidant, and anti-corrosive applications. J. Mol. Struct. 1325, 141017 (2025).
Pasquet, J. et al. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A. 457, 263–274 (2014).
Jin, T., Sun, D., Su, J., Zhang, H. & Sue, H. J. Antimicrobial efficacy of zinc oxide quantum Dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J. Food Sci. 74, M46–M52 (2009).
Gudkov, S. V. et al. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 9, 641481 (2021).
Pillai, A. M. et al. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 1211, 128107 (2020).
Singh, A. K. et al. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum Dots using eclipta Alba. Mater. Chem. Phys. 203, 40–48 (2018).
Mohammed, M. A., Elgammal, E. W., Gaara, A. H. & El Raey, M. A. Synergistic effect of silver and ZnO nanoparticles green synthesized by vitis vinifera stem extract with ampicillin against some pathogenic microbes. Egypt. J. Chem. 65, 697–709 (2022).
Alqahtani, A. A. et al. The biosynthesized zinc oxide nanoparticles’ antiviral activity in combination with pelargonium Zonale extract against the human Corona 229E virus. Molecules. 27, 8362 (2022).
Raghupathi, K. R., Koodali, R. T. & Manna, A. C. Size-dependent bacterial growth Inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 27, 4020–4028 (2011).
Alqahtani, A. et al. Mechanistic action of Linalyl acetate: acyclic monoterpene isolated from bitter orange leaf as anti-inflammatory, analgesic, antipyretic agent: role of TNF-α, IL1β, PGE2, and COX-2. Ind. Crops Prod. 203, 117131 (2023).
Mohammed, H. A. et al. Phytochemical profiling, in vitro and in Silico anti-microbial and anti-cancer activity evaluations and Staph gyraseb and h-TOP-IIβ receptor-docking studies of major constituents of zygophyllum coccineum L. Aqueous-ethanolic extract and its subsequent fractions: an approach to validate traditional phytomedicinal knowledge. Molecules. 26, 577 (2021).
Matuschek, E., Brown, D. F. & Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 20, O255–O266 (2014).
Marrez, D. A., Naguib, M. M., Sultan, Y. Y. & Higazy, A. M. Antimicrobial and anticancer activities of scenedesmus obliquus metabolites. Heliyon. 5 (2019).
Andrews, J. M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48, 5–16 (2001).
Sokmen, A. et al. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic thymus Spathulifolius. Food Control. 15, 627–634 (2004).
Bax, B. D. et al. Type IIA topoisomerase Inhibition by a new class of antibacterial agents. Nature. 466, 935–940 (2010).
Hargrove, T. Y. et al. Crystal structure of the new investigational drug candidate VT-1598 in complex with Aspergillus fumigatus sterol 14α-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrob. Agents Chemother. 61, 00570–00517. https://doi.org/10.1128/aac (2017).
Essa, A. F. et al. Integration of LC/MS, NMR and molecular Docking for profiling of bioactive diterpenes from Euphorbia mauritanica L. with in vitro Anti-SARS‐CoV‐2 activity. Chem. Biodivers. 20, e202200918 (2023).
Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through a small group research project under Grant number RGP1/68/46.
Funding
Deanship of Research and Graduate Studies at King Khalid University.
Author information
Authors and Affiliations
Contributions
Conceptualization, D.K.A. and S.S.A.; methodology, D.K.A., M.A.E., A.K.E. and D.A.M.; software, D.K.A., M.A.E. and A.F.E.; validation, D.K.A., M.A.E., G.F.A. and D.A.M.; formal analysis, D.K.A., investigation, D.K.A and M.A.E,; ,resources, D.K.A and S.S.A.; data curation, D.K.A, M.A.E, A.K.E., M.A.R., S.A.A. writing—original draft preparation, D.K.A.,; writing—review and editing, D.K.A, S.S.A, M.A.E, A.F.E., G.F.A., M.A.R., S.A.A. and D.A.M.; visualization, D.K.A., S.S.A., M.A.E., and A.K.E. Funding, M.A.R. supervision, S.S.A.; M.A.E, A.K.E. and G.F.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Alsayed, D.K., Elhawary, S.S., El Raey, M.A. et al. Eco-friendly fabrication of ZnO quantum dots using Brassica rapa (L.): metabolomic profiling and antimicrobial efficacy against foodborne pathogens supported by in-silico insights. Sci Rep 15, 28738 (2025). https://doi.org/10.1038/s41598-025-13925-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13925-0