Abstract
A novel two-step wet-chemical synthesis produced neodymium-doped core/shell heterojunctions, Copper(II) oxide (neodymium)/Zinc oxide(CuO(Nd)/ZnO and Zinc oxide (neodymium)/Copper(II) oxide ZnO(Nd)/CuO), with tunable optoelectronic and photocatalytic properties. Structural characterization via x-ray diffraction (XRD) and transmission electron microscopy (TEM) reveals uniform crystallite sizes (6.3–31.4 nanometers- nm) and distinct morphologies: hexagonal for CuO(Nd)/ZnO and spherical for ZnO(Nd)/CuO. Incorporating neodymium induced lattice strain (7.8–10.2 × 10⁻⁴) and increased Urbach energies (5.0–15.3 millielectron volts-meV), enhancing defect states. Tauc analysis demonstrated bandgap narrowing to 2.49–3.17 eV for ZnO and 1.49–1.55 eV for CuO. ZnO(Nd)/CuO achieved a remarkable 94.6% degradation of malachite green (MG) under cost-effective 500 W (W) white light irradiation. This significantly surpasses the performance of undoped systems (by 5%) and generally exceeds that reported for other nanocomposites (≤ 90%) in the literature. Conversely, CuO(Nd)/ZnO exhibited 89.04% ultraviolet blocking efficiency at 280–315 nm (UV-B), outperforming polymethyl methacrylate (PMMA) with ZnO quantum dots (50–60%) and ZnO nanoparticles with polylactic acid composite films (15–75%). These enhancements stem from neodymium(III) (Nd³)-mediated trap states, optimized band alignment, and charge separation at the heterojunction interface. Integrating rare-earth doping with core/shell architecture provides a scalable route for high-performance photocatalytic and ultraviolet-protective materials.
Similar content being viewed by others
Introduction
Dye pollution, driven by synthetic colorants like malachite green (MG), poses severe environmental and health risks due to its carcinogenic, mutagenic, and bioaccumulative properties. Despite the textile industry’s reliance on MG for bright coloration and antimicrobial efficacy, improper disposal into aquatic systems necessitates advanced remediation strategies1,2,3,4,5,6,7.
Conventional biological, chemical, or physical methods suffer from inefficiency, high costs, and secondary pollution, underscoring the need for innovative alternatives such as photocatalytic nanomaterials8,9,10.
Metal oxide nanocomposites, such as CuO and ZnO, have emerged as multipurpose candidates due to their tunable optoelectronic properties, finding applications in photocatalysis, sensing, and energy storage11,12,13,14,15,16.
Beyond dye pollution, ultraviolet (UV) radiation poses another critical challenge, threatening ecosystems and human health by causing DNA damage, skin cancer, and material degradation. Conventional UV-blockers (e.g., organic sunscreens, bulk metal oxides) face limitations in efficacy and environmental compatibility. Nanomaterials with band gaps ≥ 3 eV, such as ZnO and TiO₂, are particularly effective for UV protection, leveraging quantum effects to attenuate UV-A (320–400 nm) and UV-B (280–320 nm) wavelengths while maintaining visible-light transparency. Recent advances in doped semiconductors (e.g., Ce³⁺-TiO₂) and heterostructures (e.g., ZnO/graphene) have achieved over 95% UV-blocking efficiency, revolutionizing protective textiles and eco-friendly coatings17,18,19,20,21.
Despite extensive research on CuO/ZnO heterojunctions, there remains a conspicuous lack of systematic investigations into how core/shell inversion and rare‑earth doping combine to tune interfacial charge dynamics and defect states. The research gap addressed in this work is that the prior studies have largely focused on undoped or fixed‑configuration architectures, neglecting the synergistic effects of Nd³⁺‑induced lattice distortion and core/shell ordering on photocatalytic and UV‑blocking performance22,23,24,25,26.
In this study, we bridge this gap via a novel two-step synthesis of Nd-doped heterojunctions with inverted core/shell architectures: (CuO(Nd)/ZnO and ZnO(Nd)/CuO), enabling programmable defect engineering and core/shell structure. By inverting the core/shell order and doping Nd³⁺ exclusively into the core lattice, we achieve unprecedented control over crystallinity, lattice strain (7.8–10.2 × 10⁻⁴), and energy-trap states (5.0–15.3 meV), as evidenced by XRD, TEM, and Urbach-energy analyses. This strategy achieves dual functionality unattained in prior studies: (i) 94.6% MG photodegradation under low-intensity white light (500 W), surpassing undoped systems by > 5% and literature benchmarks (≤ 90%); and (ii) 89.04% UV-B blocking efficiency (280–315 nm), outperforming polymer-composite alternatives (e.g., 50–75%).
Our work establishes the first scalable paradigm integrating rare-earth doping, core/shell inversion, and defect engineering for high-performance environmental and protective applications.
Materials and experimental aspects
Materials
Table 1 lists the chemicals used in this study, along with details about their grade, purity, and manufacturer.
Preparation of neodymium-doped core (metal oxide) nanoparticles
A similar synthesis procedure was applied to both ZnO(Nd) and CuO(Nd) nanoparticles. For the preparation of ZnO(Nd), zinc nitrate hexahydrate (Zn(NO₃)₂·6 H₂O) and neodymium(III) chloride hexahydrate (NdCl₃·6 H₂O) were dissolved in 80 mL of deionized water at a molar ratio of Zn: Nd = 0.09:0.01. The solution was stirred at 350 revolutions per minute (rpm) at 70 °C, while the pH was adjusted to 7.0 by the dropwise addition of 20 mL of 0.1 M NaOH, which immediately produced a white precipitate. The mixture was stirred for 4 h at room temperature, after which the precipitate was isolated via centrifugation at 4000 rpm for 10 min. The collected solid was washed with 30 mL of deionized water, followed by two ethanol washing cycles (20 mL each). Each wash cycle included 10 min of stirring at 4000 rpm to eliminate residual salts and organic impurities. The purified precipitate was dried at 80 °C for 24 h, finely ground using a mortar and pestle, and annealed in air at 400 °C for 1 h to obtain the ZnO(Nd) nanopowder (8.2% w/w). The final product was cooled to room temperature before further use.
Preparation of Nd-doped metal oxide core/shell heterojunctions
The ZnO(Nd)/CuO core/shell heterojunction was synthesized by dispersing the pre-synthesized ZnO(Nd) powder in an 80 mL aqueous solution of copper(II) nitrate trihydrate (Cu(NO₃)₂·3 H₂O) at a 1:1 weight ratio. The suspension was stirred at 300 rpm and 70 °C, and the pH was adjusted to 7.0 via the dropwise addition of 20 mL of 0.1 M NaOH. A precipitate formed immediately, which was isolated by centrifugation (4000 rpm, 10 min), washed repeatedly with deionized water and ethanol (following the aforementioned washing protocol), and dried at 60 °C for 24 h. The dried composite was calcined at 400 °C for 1 h to crystallize the heterojunction, yielding the final ZnO(Nd)/CuO product (13.8% w/w). An analogous procedure with reversed core and shell roles was employed to prepare the CuO(Nd)/ZnO heterojunction. Scheme 1 illustrates the preparation of the ZnO(Nd)/CuO heterojunction.
The characterization methods
Powder X-ray diffraction (XRD)
Structural investigations were performed using a Bruker D8 Discover X-ray diffractometer equipped with a Cu microfocus X-ray source (λ = 0.15406 nm) and a 2-dimensional Vantec 500 detector. The XRD system was operated at 40 kV and 40 mA, with a scattering angle range of 3° to 70° and a scanning step size of 0.02°.
Transmission electron microscopy (TEM)
The nano-heterojunction size and morphology were analyzed using a JEOL transmission electron microscope (model GEM-1010), operated at 70 kV. Samples were prepared by dispersing the nano-heterojunctions in distilled water, depositing the suspension onto a copper grid, and air-drying prior to imaging.
Ultraviolet–Visible (UV–Vis) spectroscopy
A Shimadzu UV-2800 spectrometer was used to estimate the optical properties of the samples, such as the absorption coefficient, energy band gap, and UV-blocking parameters. Preparing the sample for UV-Vis measurement was as follows: The sample was ground into powder using an agate mortar. The sample (50-100 mg) was placed in an integrating-sphere powder holder. The sample surface was leveled to produce a smooth layer. Excess powder around the holder rim was removed to ensure a smooth measurement surface. A BaSO₄ reference disk was utilized to create a baseline using the “Relative Diffuse Reflectance” mode. The diffuse reflectance spectrum was transformed to pseudo-absorbance using the Kubelka-Munk function, resulting in an absorption-like spectrum suited for analyzing UV-blocking capabilities.
X-ray photoelectron spectroscopy (XPS)
Elemental composition and chemical states were determined via XPS (Thermo Scientific K-Alpha) using monochromatic Al Kα radiation (1486.6 eV). Measurements were conducted at a base pressure of 10⁻⁹ mbar with an X-ray spot size of 400 μm. Survey scans (200–1350 eV) and high-resolution scans (50 eV window) were acquired for qualitative and quantitative analysis.
Evaluation of malachite green photodegradation efficiency
The photocatalytic activity of CuO(Nd)/ZnO and ZnO(Nd)/CuO heterojunctions was evaluated in two stages:
-
1-
Adsorption Selectivity Screening:
-
Aqueous solutions (20 mL, 10 mg/L) of five dyes were prepared.
-
Each dye solution was mixed with 10 mg of the prepared sample and stirred in the dark for 1 h.
-
UV absorbance was measured before and after treatment to assess adsorption efficiency.
-
2-
Photodegradation under illumination:
-
The dye showing highest adsorption (malachite green, MG) was selected for photodegradation.
-
A 500 W white light lamp irradiated the MG–nano-heterojunction mixture for 2 h.
-
At 30-minute intervals, suspended particles were removed via centrifugation, and UV absorbance was recorded.
-
Dye removal efficiency (%) was calculated using:
where Co and Ce are the initial and equilibrium concentrations (mg/L) of the MG dye aqueous solution, respectively.
Results and discussion
The nano-heterojunction formation mechanism
The synthesis mechanism for CuO(Nd)-doped nanoparticles and CuO(Nd)/ZnO nano-heterojunctions26,27,28,29,30,31 follows the experimental protocols outlined in Sects. 2−1 and 2–2. The process can be summarized in two main steps:
Synthesis of CuO(Nd)-doped core nanoparticles
- Precursor Dissolution: Copper(II) nitrate trihydrate [Cu(NO3)2⋅3H2O] and neodymium(III) chloride hexahydrate [NdCl3⋅6H2O] were dissolved in deionized water at a Cu: Nd molar ratio of 0.09:0.01.
- Coprecipitation of Hydroxides: Addition of 0.1 M Sodium hydroxide to the solution induced the formation of a mixed hydroxide precipitate:
- Aging and Dehydration: The mixture was stirred for 4 h to ensure homogeneous growth of hydroxide precursors. Subsequent drying at 80 °C removed physisorbed water:
- Annealing for Crystallization: Calcination at 400 °C for 1 h facilitated crystallization of the CuO lattice, with Nd³⁺ ions substituting Cu²⁺ sites. Charge compensation occurred via oxygen vacancy (\(\:{V}_{O}^{**}\)) formation:
Formation of CuO(Nd)/ZnO core/shell nano-heterojunction
- Core activation: The synthesized CuO(Nd) nanopowder was dispersed in deionized water, generating surface hydroxyl groups to promote Zn²⁺ adsorption:
- Adsorption and hydrolysis of Zn²⁺: Zinc nitrate [Zn(NO₃)₂·6 H₂O] dissociated in solution, and Zn²⁺ ions adsorbed onto the hydroxylated CuO(Nd) surface. The addition of NaOH (pH 7) induced hydrolysis:
- Heteroepitaxial shell growth: Heating at 70 °C facilitated dehydration of Zn(OH)₂ into a crystalline ZnO shell:
- Core/shell structure formation: The final core/shell structure formed as
- Interfacial band alignment (conduction band-CB and valence band-VB): The Type-II heterojunction between p-type CuO and n-type ZnO enabled efficient charge separation:
- Calcination for Stability: Final annealing at 400 °C enhances interfacial bonding and crystallinity:
XRD analysis
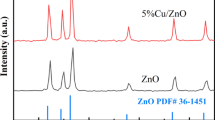
The XRD patterns (Fig. 1) revealed the presence of two distinct crystallographic phases: CuO with a monoclinic structure (JCPDS 48-1548, space group C2/c) and ZnO with a hexagonal structure (JCPDS 36-1451, space group P6₃mc)32,33. When noise in the patterns was considered, secondary phases (e.g., Nd₂O₃) could be neglected, suggesting successful Nd³⁺ incorporation into the host lattices without phase separation34. The absence of impurity peaks underscored the phase purity of the samples, which is critical for reproducible optoelectronic behavior35.
In the ZnO(Nd)/CuO sample (red trace), the three most intense peaks at 2θ = 31.80° (100), 34.45° (002), and 36.29° (101) corresponded to the wurtzite ZnO planes, while weaker features at 35.58°, 38.84°, 48.60°, 58.36°, and 66.20° matched the (–111), (111), (–202), (202), and (113) planes of CuO reflecting a ZnO shell over an Nd-doped CuO core. Conversely, in CuO(Nd)/ZnO (black trace), the CuO reflections (–111 at 35.5°, 111 at 38.8°, − 202 at 48.8°, etc.) dominated, and the ZnO peaks at 31.82°, 34.48°, and 36.24° appeared only as minor shoulders, consistent with a thin ZnO shell on a predominantly CuO(Nd) core.
In both heterojunctions, the ZnO peaks were slightly broadened and shifted to lower angles relative to undoped ZnO, indicating Nd³⁺ incorporation and nanoscale crystallite sizes36. From Fig. 1b, three benchmark peaks for standard CuO (32.56°, 35.59°, 38.79°) and ZnO (31.80°, 34.45°, 36.29°) served as references for our samples. The observed shifts were attributed to core/shell architecture and Nd³⁺ doping (Table 2)37,38.
Lattice constants and unit-cell volumes were calculated using PEAKFIT(version 4.12, 2003) and ChekCell(version 3, 2000)43,44,45. Peak-broadening analysis revealed symmetric broadening in ZnO(Nd)/CuO, characteristic of spherical nanoparticles, whereas asymmetric broadening in CuO(Nd)/ZnO suggested polyfaceted morphologies (Fig. 1b)46,47. Table 3 presents the refined lattice parameters for hexagonal ZnO and monoclinic CuO systems, which are visualized in Fig. 2 below using Visualization of Electronic and STructural Analysis-VESTA(version3, 2006–2021) software48,49. This highlights Nd-induced distortions and interfacial strain.
Nd³⁺ incorporation increased lattice volumes by ~ 0.5% in ZnO(Nd) and ~ 0.2% in CuO(Nd), aligning with Vegard’s law and lanthanide substitution trends50,51. Core/shell structures exhibited further strain-driven adjustments: the ZnO shell on CuO(Nd) expanded under tensile stress, while the CuO shell on ZnO(Nd) compressed. Bond-length analysis confirmed Nd doping shortens metal-oxygen (M–O) distances in cores, offset by oxygen vacancies in ZnO and cation vacancies in CuO52,53.
Crystallinity and deformation parameters like crystal size (D), lattice strain (ξ), and stacking fault probability (α)are crucial for demonstrating the effects of rare earth doping in the samples. These parameters for both CuO and ZnO phases at their characterized XRD peaks were calculated using the PEAKFIT program and based on the following equations21,54:
where λ is the X-ray incident wavelength, β is the XRD peak width at half maximum, θ is the X-ray scattering angle via crystal planes in the samples, and Δ(2θ) is the difference between the samples’ scattering angle and the standard.
Scherrer analysis yielded crystallite sizes of 14.89–16.02 nm for undoped phases, reduced by ~ 1–5% with Nd³⁺ doping, consistent with TEM data (Fig. 3).
For photodegradation applications, these cell parameter variations in the synthesized heterojunction lead to modulating band structure, enhancing charge separation efficiency, and accelerating photocatalytic reactive species generation. Moreover, this results in reduced band alignment recombination rates, improved spatial separation of electrons and holes, and increased surface area for pollutant adsorption. Tensile stress increases defect density and UV light absorption efficiency60,61,62,63,64,65.
In UV-blocking applications, the strain-induced adjustment of the bandgap in the (e.g., ZnO) shell enhances the optical absorption cross-section in the UV-B region, resulting in higher blocking efficiencies against 280–315 nm irradiation. Additionally, interfacial strain promotes denser crystalline packing and smoother interfaces that reduce light scattering losses in the visible region, preserving transparency while maximizing UV attenuation in CuO(Nd)/ZnO coatings. Such deformation-mediated enhancements have been correlated with up to 20–30% increases in degradation rates and UV-blocking efficiencies in comparable metal-oxide heterostructures, underscoring strain as a critical design parameter for multifunctional nanomaterials66,67,68,69.
TEM analysis
Transmission electron microscopy (TEM) was employed to examine the morphology and particle size of the synthesized CuO(Nd)/ZnO and ZnO(Nd)/CuO nano-heterojunctions. TEM images at various magnifications are presented in Fig. 3, confirming the successful fabrication of core/shell nano-heterojunctions via a two-step synthesis. ImageJ(version 1.54 g, 2023)70 software was used to quantify particle dimensions.
In Fig. 3a, the CuO(Nd)/ZnO heterojunction (highlighted in yellow) displays a darker, polyhedral CuO(Nd) core measuring approximately 12–15 nm, enveloped by a lighter ZnO shell to give a total diameter of about 20–25 nm. The hexagonal habit of the ZnO shell, which grows uniformly along all facets of the CuO(Nd) core, clearly defines the core–shell interface. By contrast, the ZnO(Nd)/CuO heterojunction (marked in red) consists of roughly spherical to slightly elongated nanoparticles, ~ 15–25 nm in size, that assemble into short chains. Each features an 8–12 nm ZnO(Nd) core surrounded by a continuous, darker CuO shell, demonstrating a clear, defect-free interface. In both samples, particles are well dispersed with minimal aggregation, indicating effective colloidal stabilization during synthesis71.
As shown in Fig. 3a, no significant aggregation was observed; particles appear well separated, indicating strong surface compatibility between core and shell. Such dispersion is critical for efficient charge separation at the heterojunction, supporting the superior photocatalytic and UV-blocking performance detailed below72,73,74.
Figure 3b reports average diameters of 13.39 ± 3.76 nm for CuO(Nd)/ZnO and 18.58 ± 4.69 nm for ZnO(Nd)/CuO, consistent with XRD measurements (crystallite sizes). The narrow (monodisperse) size distributions result from a rapid nucleation burst, followed by a controlled crystallization phase in which all nuclei grew at comparable rates, minimizing size variance75,76.
Uniformity in both size and crystallinity confers exceptionally consistent optical properties: stable band-edge positions and low, uniform defect densities enhance light absorption and charge separation. Consequently, electron-hole pairs migrate to the particle surface with minimal recombination, maximizing reactive-oxygen-species generation for photocatalysis. Likewise, identical bandgap energies and plasmon-resonance frequencies across the sample produce a sharp, high-intensity UV absorption band, bolstering partially UV-blocking performance. In contrast, polydisperse or defect-rich samples exhibit uneven charge dynamics and weaker, broadened optical responses77,78,79,80,81. In Fig. 3b, the size distribution of ZnO(Nd)/CuO nanoparticles demonstrates greater uniformity compared to CuO(Nd)/ZnO, which explains their enhanced photocatalytic activity.
UV-Absorption analysis
Figure 4 displays the UV-absorption spectra of the prepared CuO(Nd)/ZnO and ZnO(Nd)/CuO nano-heterojunctions.
Both ZnO(Nd)/CuO and CuO(Nd)/ZnO exhibit clear characteristics with two absorption regions: one in the UV region (~ 200–400 nm) attributed to ZnO’s wide band gap and a second in the visible region (~ 400–800 nm) from CuO’s narrower band gap. Nd³⁺ doping introduces discrete trap states within the band gap, manifesting as a redshift of the ZnO edge from ~ 3.17 eV to ~ 2.49 eV and of the CuO edge from ~ 1.55 eV to ~ 1.49 eV, as listed in Table 5. Similar redshifts have been reported in Cu-doped ZnO systems, where transition-metal inclusion creates impurity levels that broaden visible-light absorption26,82. These spectral shifts confirm the TEM measurements, which indicated larger crystallite sizes for ZnO(Nd)/CuO compared to CuO(Nd)/ZnO.
Bandgap determination
-
1-
Absorption Cut-Off Method: To compute the bandgap (Eg) for each metal oxide in the heterojunction, we used the sudden drop in UV absorption in each spectrum’s two wavelength regions. We calculated Eg using the formula:
where λc is the wavelength at the abrupt UV-absorption drop. For each sample, the core and shell band gaps were calculated and listed in Table 5, confirming gap narrowing upon Nd³⁺ doping. This phenomenon arises from trap states created by Nd³⁺ ions, which temporarily capture charge carriers and promote lower-energy electronic transitions from the valence band (VB) to the conduction band (CB)30,83.
-
2-
Tauc Plot Analysis: The bandgap of the heterojunction samples was also determined using the Tauc relationship as the second method84:
where \(\rho\) is the UV absorption coefficient, A is the maximum absorption, t is the sample thickness (≈1 mm), T is the Tauc number, the Planck constant is h, the electromagnetic wave frequency is v, and C is the Tauc constant. The band gap value was estimated by plotting (ρhv)1/T against hv, using the absorption edge’s linear range, and finding the intercept with the energy axis (T = ½ for direct transitions). Figure 5 demonstrates the direct Tauc plots for the synthesized heterojunctions.
The bandgap of heterojunctions (CuO(Nd)/ZnO and ZnO(Nd)/CuO) is primarily affected by two factors: Nd³⁺ doping (a direct factor) reduces the energy bandgap, while nanoparticle size variation (an indirect factor) influences interfacial strain. The direct Tauc method suggests two energy band gaps in each heterojunction, consistent with the absorption cut-off method (Table 5)85,86.
Direct Tauc plots [(αhν)2 vs. hν] for bandgap determination.
When plotting the indirect bandgap (Fig. 6), the effect of Nd³⁺ doping was observed as a reduction in Eg, corroborating trends across methods.
Table 5 lists the bandgap values for each heterojunction, obtained using various methods, including cut-off, direct, and indirect Tauc plots.
This consistency enhances the reliability of the results and supports the conclusion that Nd³⁺ doping modifies the electronic band structure of ZnO and CuO87. The ZnO(Nd)/CuO heterojunction exhibited a more pronounced Eg reduction in ZnO compared to CuO(Nd)/ZnO, inversely correlating with photocurrent efficiency88.
Urbach energy and disorder analysis
To confirm the abundance of these impurities’ energy levels and their effect on the bandgap structure, the Urbach energy (Eu) of the samples was quantified using the formula89 that relates the incident photon energy to the logarithm of the UV-Vis absorption coefficient (ρ), as follows:
In this equation, C is a constant in the relationship, which mainly depends on the bandgap. The reciprocal of the slope in the linear region of lnρ vs. hv yields Eu.
From Fig. 7, the study reveals that ZnO(Nd)/CuO exhibits Eu = 15.31 × 10−3 eV, three times higher than CuO(Nd)/ZnO (4.99 × 10−3 eV). This suggests the ZnO(Nd)/CuO configuration has a higher degree of disorder and more localized states, likely due to lattice strain or a higher defect concentration at the interface between the Nd-doped ZnO core and the CuO shell77,90.
Energy-band alignment
We constructed the heterojunction band alignment as follows:
- Baseline values: We used reported conduction-band energies for CuO (4.07 eV) and ZnO (4.35 eV), neglecting the slight Fermi-level shifts from Nd³⁺ due to its low concentration91,92.
- Bandgap inputs: The energy band construction was based on the average energy band gap values for ZnO and CuO (Table 5).
- Band bending: Upon contact, charge transfer across the interface generates an internal field, causing Fermi levels to equilibrate and bands to bend until a common Fermi energy is reached. Figure 8 shows the resulting alignment93.
- Work function validation: The calculated work function values of ZnO, ZnO(Nd), CuO, and CuO(Nd) were 5.19, 4.82, 4.91, and 4.54 eV, respectively, in agreement with the literature94.
The tailored optical properties reflect efficient interfacial charge transfer characteristic of type-II heterojunctions: photoexcited electrons in CuO migrate to ZnO, and holes in ZnO transfer to CuO, with Nd-induced trap states prolonging carrier lifetimes. This alignment enhances photocatalytic and UV-blocking performance95,96.
UV-blocking properties of the samples
The UV-Vis absorption measurements were used to evaluate the UV-blocking parameters of the ZnO(Nd)/CuO and CuO(Nd)/ZnO nano-heterojunctions in the wavelength range of 280 nm to 400 nm (UV-A and UV-B). For each wavelength λ, the transmittance T(λ) was calculated from the measured absorbance A(λ) using Beer’s law97:
where A(λ) is the absorbance. The UV-transmission and UV-blocking percentages of the prepared samples for (UV-A) and (UV-B) regions were calculated21,98:
Δλ is explicitly the width of the wavelength range (Δλ = 85 nm for UV-A & Δλ = 35 nm for UV-B). The spectral transmission of the samples is T(λ). For each of the corresponding spectral regions (A & B), the average transmittance percentage is denoted by T(UV−A) and T(UV−B).
From Table 6, CuO(Nd)/ZnO exhibits superior UV-B blocking (89.04%) and enhanced UV-A attenuation (80.68%) compared to ZnO(Nd)/CuO, indicating its stronger UV-filtering capability, particularly in the high-energy UV-B band. The CuO(Nd)/ZnO nano-heterojunctions’ UV-blocking mechanism arises from (i) a wider band gap (ZnO ~ 3.17 eV) restricts electron excitation to high-energy UV-B photons, enhancing UV-B blocking. (ii) Smaller nanoparticles size (∼13.38 nm) amplifies light scattering and absorption due to increased surface-to-volume ratios. (iii) Neodymium incorporation reduces impurity energy states (Eu = 4.99 × 10−3 eV), suppresses charge recombination, and improves photocurrent generation via enhanced charge separation99,100,101,102. Table 7, below, presents a comparative analysis with various reported metal oxide-based UV-blocking materials. This comparison allows us to benchmark the performance of our synthesized CuO(Nd)/ZnO and ZnO(Nd)/CuO heterojunctions against existing materials, highlighting their strengths and limitations within the broader field.
From Table 7, the CuO(Nd)/ZnO nano-heterojunction outperforms ZnO/propolis composites (75.4% UV-B) and CMC/CuO@ZnO systems (67–99% UV-B) but is slightly less effective than CuO/propolis (91.3% UV-B). Its balanced absorption in both UV-A (80.68%) and UV-B (89.04%) spectra highlights its potential for use in sunscreen additives and protective coatings.
XPS analysis
Figure 9 presents the XPS spectra of the ZnO(Nd)/CuO and CuO(Nd)/ZnO heterojunctions. Elemental composition and oxidation states are determined by analyzing photoelectron binding energies calibrated against the C 1 s peak at 284.8 eV (corrected from 285.6 eV). Core–shell structural integrity is confirmed by higher atomic percentages of shell elements (e.g., Zn in CuO(Nd)/ZnO and Cu in ZnO(Nd)/CuO), as evidenced by their elevated peak intensities in survey scans (Fig. 9a). High-resolution scans for each element appear in Figs. 9b–e.
Table 8 compares the dominant binding-energy peaks of the samples to standard values for Cu, Zn, Nd, and O obtained from survey scans110,111,112.
The sample peaks are shifted to higher energies relative to the standards, indicating reduced electron density and confirming that the elements are bonded within a lattice rather than existing as free metal. Moreover, this reduced electron density arises from metal-oxide rather than metallic bonding113,114.
-
Zn 2p region (Fig. 9b) displays the Zn scan spectra, Zn 2p1/2 and Zn 2p3/2, with a spin–orbit splitting of approximately 23.1 eV. It shows a significant shift (blue shift, ~ 0.5 eV) in binding energies for the ZnO(Nd)/CuO heterojunction compared to CuO(Nd)/ZnO. This peak shifts (~ 0.5 eV) to higher binding energy, indicating an increased electron density around Zn atoms due to Nd³⁺ incorporation and possible Zn–Nd–O bonding115,116,117.
-
Cu 2p region (Fig. 9c), the peaks for Cu 2p₃/₂ (approximately 932.6 eV) and Cu 2p₁/₂ (approximately 952.5 eV) are clearly dominant, indicating the presence of Cu⁺ states. The pronounced satellite features at around 941, 943, and 961 eV unequivocally confirm the existence of Cu²⁺. The ZnO(Nd)/CuO sample exhibits significantly larger positive shifts in both the main and satellite peaks compared to the CuO(Nd)/ZnO sample, demonstrating that Nd effectively modifies the Cu–O bonding environment111,118,119.
-
O 1 s region (Fig. 9d) clearly presents a primary peak at approximately 530.0 eV, directly linked to lattice O²⁻ in Zn–O or Cu–O bonds. Additionally, the higher-energy shoulders in the range of 531–533 eV are distinctly attributed to oxygen vacancies, metal-oxygen interstitials, and adsorbed hydroxyl species. Notably, the ZnO(Nd)/CuO heterojunction exhibits a significant high-energy shoulder, demonstrating a greater density of defect states, which undeniably aligns with its increased Urbach energy120,121.
-
Nd 3 d region (Fig. 9e), the Nd 3d₅/₂ and Nd 3d₃/₂ peaks are definitively observed at approximately 980–982 eV and 1001–1002 eV, respectively. The spin–orbit splittings measured are 18.6 eV for ZnO(Nd)/CuO and 21.2 eV for CuO(Nd)/ZnO. Additionally, there are unmistakable satellites around 988–990 eV, which are attributed to Nd₂O₃-like environments and Nd–metal–oxygen bonding. The lower binding energies noted in CuO(Nd)/ZnO indicate a distinct local coordination and oxidation state equilibrium compared to ZnO(Nd)/CuO83,112,122,123,124.
The photodegradation study
Figure 10 compares the adsorption-based removal efficiencies of five organic dyes, Congo Red (CR), Methylene Blue (MB), Malachite Green (MG), Methyl Orange (MO), and Rhodamine B (RHB), on ZnO(Nd)/CuO and CuO(Nd)/ZnO under identical pH and contact-time conditions. Both heterojunctions exhibit dye-specific selectivity, with MG showing the highest removal percentage and ZnO(Nd)/CuO achieving the greatest overall adsorption capacity.
The photocurrent is influenced by the dynamics of surface charges associated with dye decomposition, which vary significantly depending on the specific type of shell nanoparticles used. Impurities, especially Nd in the core nanoparticles, enhance the photocurrent by effectively slowing down the recombination of hole-electron pairs. In the ZnO(Nd)/CuO configuration, Nd³⁺ ions act as electron donors, increasing the surface electron concentration. Conversely, in the CuO(Nd)/ZnO structure, the electrons donated by Nd³⁺ ions are captured by copper vacancies (holes), limiting their impact on surface charge accumulation125,126,127,128,129,130. Charge transfer occurred based on the junction’s energy alignment band, as described in Sects. 3–3.
Figure 11 clearly demonstrates that ZnO(Nd)/CuO achieves greater photolysis activity than CuO(Nd)/ZnO, with photodegradation ratios of 94.6% versus 84.99%. The optimized core–shell ordering in ZnO(Nd)/CuO reduces the valence-band offset (ΔEv), facilitating charge separation and transfer. Additionally, the host oxide for Nd³⁺ influences defect-state formation, narrowing the local band gap and prolonging carrier lifetimes. The energy-band diagrams in Fig. 8 corroborate these findings, showing that the smaller valence-band difference in ZnO(Nd)/CuO underpins its superior photocurrent generation and photolysis performance131,132,133,134,135. Dye photodegradation also depends on nanoparticle size and band gap136.
The photodegradation mechanism can be summarized into three main steps. The first step is that the incident light generates charge carriers (electron-hole pairs) in the heterojunction. Next, water, containing nanoparticles and dye, is ionized into three main species: the superoxide anion (•O2‒), hydrogen peroxide (H2O2), and the hydroxyl radical (•OH). The final step involves electrochemical reactions between the generated ions and the dye’s active groups, which enhance dye decomposition137. The nanoparticle photocatalysis process for the chemical decomposition of the dye can be demonstrated in detail as follows:
- When light rays, including ultraviolet rays, strike these nanoparticles, they absorb energy exceeding the bandgap, which generates electron-hole pairs (e/h).
- This study presents a type II heterojunction, where electron and hole transfer occurs due to electrons accumulating in the region with the highest positive conduction band. Conversely, holes migrate to the area with the lowest positive or highest negative valence band. This movement enhances catalyst photolysis efficiency by promoting charge separation and reducing electron-hole recombination.
- These charges accumulate on the nanoparticle surface over time. Dissolved oxygen molecules in water capture the electrons, generating highly reactive anionic radicals in a process known as reduction:
- The holes react with water to generate hydroxyl anions, which subsequently react with holes to produce hydroxyl radicals. These reactions are termed oxidation.
- The superoxide radicals (·O2−) combine with two protons of hydrogen to create hydrogen peroxide.
- Hydrogen peroxide molecules react with the generated electrons and superoxide radicals to produce highly reactive hydroxyl radicals, as follows:
- These radicals and the resulting anions react with the dye (inorganic pollutants) to generate non-toxic chemicals containing carbon dioxide, water, and inorganic compounds:
Figure 12: Proposed photocatalytic degradation mechanism of dye molecules on the heterojunction surface.
To highlight the impact of Nddoping and core–shell ordering, below is a scientifically revised comparison of our Malachite Green (MG) photodegradation results against selected literature reports. Table 9 summarizes catalyst compositions, reaction conditions, and MG-removal efficiencies under comparable experimental setups.
Conclusion
This study successfully demonstrates the synthesis and multifunctional efficacy of neodymium-doped CuO/ZnO and ZnO/CuO core/shell heterojunctions, establishing a novel and promising strategy for environmental remediation and ultraviolet (UV) protection. Through a two-step wet-chemical method, Nd³⁺ doping was selectively confined to the core phase by controlling the synthesis conditions, inducing lattice strain (7.8–10.2 × 10⁻⁴), reducing crystallite size (6.3–31.4 nm), and tailoring optoelectronic properties. Structural analyses (XRD, TEM) confirmed distinct morphologies: hexagonal CuO(Nd)/ZnO and spherical ZnO(Nd)/CuO, with well-defined interfaces that facilitate efficient charge separation. Bandgap engineering via Nd³⁺ incorporation narrowed the bandgap values of ZnO and CuO to 2.49–3.17 eV and 1.49–1.55 eV, respectively, while the Urbach energy analysis (4.99–15.3 meV) revealed defect-mediated trap states that prolonged carrier lifetimes.
The ZnO(Nd)/CuO heterojunction exhibited exceptional photocatalytic activity, achieving 94.6% malachite green (MG) degradation under irradiation from 500 W white light source, surpassing undoped systems by 5% and prior nanocomposites (typically ≤ 90%). This enhancement stems from optimized type-II band alignment, where electrons migrate to CuO and holes to ZnO, along with Nd³⁺-induced oxygen vacancies suppressing recombination, thereby enhancing photocatalytic activity. Conversely, CuO(Nd)/ZnO demonstrated superior UV-B blocking (89.09%) and UV-A attenuation (85.99%), exceeding conventional UV filters like PMMA/ZnO composites (50–60%) and polylactic acid/ZnO films (15–75%) due to their tailored morphology and enhanced UV absorption. The hexagonal ZnO shell in CuO(Nd)/ZnO minimized light scattering losses, while tensile strain amplified the UV absorption cross-section, highlighting structure-dependent performance.
XPS analyses validated interfacial charge transfer and Nd³⁺ integration, with work function adjustments (4.54–5.19 eV) aligning with theoretical band diagrams, further supporting the proposed charge transfer mechanism. The interplay of doping, core/shell ordering, and defect engineering underscores the versatility of rare-earth-modified heterojunctions. These findings bridge critical gaps in photocatalytic efficiency and UV protection, offering potentially scalable solutions for industrial dye detoxification and eco-friendly coatings. Future work should explore scalability, long-term stability under real-world conditions, and synergistic effects of multi-rare-earth doping to further optimize multifunctionality. This study not only advances nanomaterial design but also provides actionable insights for sustainable environmental technologies.
Given their enhanced UV attenuation and defect-engineered photocatalytic behavior, these heterojunctions also hold promise for biomedical applications, such as UV-protective films for skin or antimicrobial coatings for medical devices. Future studies should evaluate their biocompatibility and functional integration in health-related technologies.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ardila-Leal, L. D., Poutou-Piñales, R. A., Pedroza-Rodríguez, A. M. & Quevedo-Hidalgo, B. E. A brief history of colour, the environmental impact of synthetic dyes and removal by using laccases. Molecules 26, 3813 (2021).
Gürses, A. et al. Dyes and pigments: their structure and properties. Dye Pigment 13–29 (2016).
Benkhaya, S. & M’rabet, S. El harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 115, 107891 (2020).
Kooravand, M., Asadpour, S., Haddadi, H. & Farhadian, S. An insight into the interaction between malachite green oxalate with human serum albumin: molecular dynamic simulation and spectroscopic approaches. J. Hazard. Mater. 407, 124878 (2021).
Etezad, S. M. & Sadeghi-Kiakhani, M. Decolorization of malachite green dye solution by bacterial biodegradation. Prog Color. Color. Coat. 14, 79–87 (2021).
Abu-Hussien, S. H. et al. Microbial degradation, spectral analysis and toxicological assessment of malachite green dye by streptomyces exfoliatus. Molecules 27, 6456 (2022).
Muthu, S. S. & Khadir, A. Dye Biodegradation, Mechanisms and Techniques (Springer, 2022).
Rane, A. & Joshi, S. J. Biodecolorization and biodegradation of dyes: a review. Open Biotechnol. J 15, (2021).
Gupta, V. K., Khamparia, S., Tyagi, I., Jaspal, D. & Malviya, A. Decolorization of mixture of dyes: A critical review. (2015).
Ruan, W. et al. Removal of dyes from wastewater by nanomaterials: a review. Adv. Mater. Lett. 10, 9–20 (2019).
Rana, A. et al. Investigation of photocatalytic, antibacterial and antioxidant properties of environmentally green synthesized zinc oxide and yttrium doped zinc oxide nanoparticles. Nano-Structures Nano-Objects. 38, 101188 (2024).
Phiwdang, K., Phensaijai, M. & Pecharapa, W. Study of antifungal activities of cuo/zno nanocomposites synthesized by co-precipitation method. Adv. Mater. Res. 802, 89–93 (2013). (Trans Tech Publ.
Kabbara, H., Ghanbaja, J., Noël, C. & Belmonte, T. Synthesis of cu@zno core–shell nanoparticles by spark discharges in liquid nitrogen. Nano-Structures Nano-Objects. 10, 22–29 (2017).
Mansournia, M. & Ghaderi, L. CuO@ ZnO core-shell nanocomposites: novel hydrothermal synthesis and enhancement in photocatalytic property. J. Alloys Compd. 691, 171–177 (2017).
Li, H. et al. Synthesis and investigation of novel ZnO–CuO core-shell nanospheres. Mater. Lett. 174, 99–101 (2016).
Marzuki, M., Rusdi, N. M., Zain, M. Z. M. & Izaki, M. Multi-staged sol–gel synthesis of Mg doped zno/cuo core-shell heterojunction nanocomposite: Dopant induced and interface growth response. J. Sol-Gel Sci. Technol. 100, 388–403 (2021).
Cerrito, L. & Cerrito, L. Natural sources of radiation. Radiat Detect. Introd Phys. Radiat. Detect. Devices 19–36 (2017).
Blatchley, I. I. I. E. R. Photochemical Reactors: Theory, Methods, and Applications of Ultraviolet Radiation (Wiley, 2022).
Robinson, J., Begum, R. & Maqbool, M. Ultraviolet radiation: benefits, harms, protection. Introd Non-Ionizing Radiat. 2, 62 (2023).
Khan, A. et al. A review of UV radiation protection on humans by textiles and clothing. Int. J. Cloth. Sci. Technol. 32, 869–890 (2020).
Alqubati, M. et al. Nonlinear effects of the biosynthesis temperature of ZnO nanoparticles on their structural, optical, and ultraviolet blocking parameters. J Opt 1–18 (2023).
Costas, A., Florica, C., Preda, N., Kuncser, A. & Enculescu, I. Photodetecting properties of single CuO–ZnO core–shell nanowires with p–n radial heterojunction. Sci. Rep. 10, 18690 (2020).
Bekru, A. G. et al. Green synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-Nitrophenol. ACS Omega. 7, 30908–30919 (2022).
Biswal, H. J., Vundavilli, P. R. & Gupta, A. Fabrication of heterogeneous cuo/zno nanocomposite immobilized Cu and Ni tubular Lms through pulse electrodeposition having enhanced photocatalytic and antibacterial performance.
Widiarti, N., Sae, J. K. & Wahyuni, S. Synthesis CuO-ZnO nanocomposite and its application as an antibacterial agent. in IOP Conference Series: Materials Science and Engineering vol. 172 12036IOP Publishing, (2017).
Das, S. & Srivastava, V. C. An overview of the synthesis of CuO-ZnO nanocomposite for environmental and other applications. Nanotechnol Rev. 7, 267–282 (2018).
Phiwdang, K., Suphankij, S., Mekprasart, W. & Pecharapa, W. Synthesis of CuO nanoparticles by precipitation method using different precursors. Energy Procedia. 34, 740–745 (2013).
Medhi, R., Marquez, M. D. & Lee, T. R. Visible-light-active doped metal oxide nanoparticles: review of their synthesis, properties, and applications. ACS Appl. Nano Mater. 3, 6156–6185 (2020).
Rahman, M. H. et al. Understanding the role of rare-earth metal doping on the electronic structure and optical characteristics of ZnO. Mol. Syst. Des. Eng. 7, 1516–1528 (2022).
Gopalakrishnan, R. & Ashokkumar, M. Rare Earth metals (Ce and Nd) induced modifications on structural, morphological, and photoluminescence properties of CuO nanoparticles and antibacterial application. J. Mol. Struct. 1244, 131207 (2021).
Bano, K. et al. Fabrication of cuo/zno heterojunction photocatalyst for efficient photocatalytic degradation of Tetracycline and Ciprofloxacin under direct sun light. Environ. Nanatechnol. Monit. Manag. 20, 100863 (2023).
Åsbrink, S. & Norrby, L. J. A refinement of the crystal structure of copper (II) oxide with a discussion of some exceptional esd’s. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 26, 8–15 (1970).
Xu, Y. N. & Ching, W. Y. Electronic, optical, and structural properties of some wurtzite crystals. Phys. Rev. B. 48, 4335 (1993).
Zhou, Y., Wu, B., Wang, J. & Wang, H. Effect of signal-to‐noise ratio on the automatic clustering of X‐ray diffraction patterns from combinatorial libraries. Mater. Genome Eng. Adv. 2, e27 (2024).
Hu, Y. et al. Identifying and controlling phase purity in 2D hybrid perovskite thin films. J. Mater. Chem. A. 6, 22215–22225 (2018).
Baskakov, A. O. et al. Magnetic and interface properties of the core-shell Fe3O4/Au nanocomposites. Appl. Surf. Sci. 422, 638–644 (2017).
Galil, M. S. A. et al. Effect of single and combined (core/shell) biosynthesis of nanoparticles (Ag and ZnO) on their photocatalytic and antimicrobial activities. Kuwait J. Sci. 52, 100356 (2025).
Ma, W. et al. Simple synthesis and sodium storage analysis of Ultra-High Nitrogen‐Doped Core‐Shell mesoporous carbon nanospheres. Adv Funct. Mater 2421790 (2025).
Sahu, J. et al. Electrochemical and electronic structure properties of high-performance supercapacitor based on Nd-doped ZnO nanoparticles. J. Energy Storage. 59, 106499 (2023).
Gopinath, K. et al. Fabrication of neodymium (Nd), cadmium (Cd) and nd: cd doped hybrid copper oxide nanocomposites: evaluation of their antibacterial activity and cytotoxicity against human L132 cell line. Ceram. Int. 49, 29933–29947 (2023).
Miao, Y., Zhao, Y., Zhang, S., Shi, R. & Zhang, T. Strain engineering: a boosting strategy for photocatalysis. Adv. Mater. 34, 2200868 (2022).
He, Y., Zhang, L., Xiong, H. W. & Kang, X. Evolution of lattice defects in nickel ferrite spinel: oxygen vacancy and cation substitution. J. Alloys Compd. 917, 165494 (2022).
Chen, R., Jakes, K. A. & Foreman, D. W. Peak-fitting analysis of cotton fiber powder X‐ray diffraction spectra. J. Appl. Polym. Sci. 93, 2019–2024 (2004). https://systatsoftware.com/download-peakfit-software/
Barowy, D. W., Gochev, D., Berger, E. D. & Checkcell Data debugging for spreadsheets. in Proceedings of the ACM International Conference on Object Oriented Programming Systems Languages & Applications 507–523 (2014). 507–523 (2014). (2014).
Bochu, J. L. et B. LMGP–Suite Suite of Programs for the Interpretation of X-ray Experiments, ENSP/Laboratoire des Matériaux et du Génie Physique, BP 46, 38042 Saint Martin dHres, France. http://www.ccp14.ac.uk/tutorial/lmgp/
Hassanzadeh-Tabrizi, S. A. Precise calculation of crystallite size of nanomaterials: A review. J. Alloys Compd. 968, 171914 (2023).
Redhammer, G. J. & Structure Scattering techniques. Met. Oxide Nanopart. Form. Funct. Prop. Interfaces. 1, 303–347 (2021).
Kumar, N., Singh, D., Kumar, P. & Gangwar, J. Crystallographic representation of polymorphs ZrO2 using VESTA software. in AIP Conference Proceedings vol. 2142AIP Publishing, (2019).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Tian, Z., Zhang, P., Sun, W., Yan, B. & Sun, Z. Vegard’s law deviating Ti2 (SnxAl1 – x) C solid solution with enhanced properties. J. Adv. Ceram. 12, 1655–1669 (2023).
Nandi, C. et al. Phase relations and lattice parameter trends in Nd3 + – substituted UO2 system under reducing and oxidizing conditions. J. Nucl. Mater. 579, 154396 (2023).
Thien, T. D. et al. Characteristics of the incorporation of Yb defect States in cuo: ZnO nanocomposite. Ceram. Int. 50, 27573–27585 (2024).
Datta, S., Maneesha, P., Mishra, P. K. & Sen, S. Role of defects in metal oxide nanostructures. Opt Prop. Met. Oxide Nanostructures 189–221 (2023).
Scherrer, P. Nachr Ges Wiss goettingen. Math. Phys. 2, 98–100 (1918).
Zhao, Q., Tian, X., Ren, L., Su, Y. & Su, Q. Understanding of Lanthanide-Doped Core–Shell structure at the nanoscale level. Nanomaterials 14, 1063 (2024).
Zhao, J., Chen, B. & Wang, F. Shedding light on the role of misfit strain in controlling core–shell nanocrystals. Adv. Mater. 32, 2004142 (2020).
Alhaddad, M., Ismail, A. A., Alghamdi, Y. G., Al-Khathami, N. D. & Mohamed, R. M. Fabrication of novel neodymium oxide coupled mesoporous Titania for effective visible light-induced photocatalyst for decomposition of Ciprofloxacin. Opt. Mater. (Amst). 131, 112579 (2022).
Alabdulaal, T. H. et al. One-pot synthesis of multifunctionalized Nd2O3 dispersed ZnO nanocomposites for enhancing electrical, optical, and photocatalytic applications. J. Mater. Res. Technol. 19, 967–988 (2022).
Vijayaprasath, G. et al. Structural, optical and antibacterial activity studies of neodymium doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 26, 7564–7576 (2015).
Liu, T. et al. Interfacial engineering in two-dimensional heterojunction photocatalysts. Int. J. Hydrogen Energy. 48, 12257–12287 (2023).
Lin, M., Chen, H., Zhang, Z. & Wang, X. Engineering interface structures for heterojunction photocatalysts. Phys. Chem. Chem. Phys. 25, 4388–4407 (2023).
Xue, S. et al. Interfacial engineering of lattice coherency at ZnO-ZnS photocatalytic heterojunctions. Chem. Catal. 2, 125–139 (2022).
Balapure, A., Dutta, J. R. & Ganesan, R. Recent advances in semiconductor heterojunctions: a detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces. 1, 43–69 (2024).
Ismail, A. & Abdullah, M. J. The structural and optical properties of ZnO thin films prepared at different RF sputtering power. J. King Saud Univ. - Sci. 25, 209–215 (2013).
Liu, X. et al. Review on fundamental and recent advances of strain engineering for enhancing photocatalytic CO2 reduction. Adv Energy Mater 2405320 (2024).
Xue, J. & Bao, J. Interfacial charge transfer of heterojunction photocatalysts: characterization and calculation. Surf. Interfaces. 25, 101265 (2021).
Rahmadiawan, D. et al. Enhanced UV blocking, tensile and thermal properties of bendable TEMPO-oxidized bacterial cellulose powder-based films immersed in pva/uncaria gambir/zno solution. J. Mater. Res. Technol. 26, 5566–5575 (2023).
Chouhan, S., Bajpai, A. K., Bajpai, J., Katare, R. & Dhoble, S. J. Mechanical and UV absorption behavior of zinc oxide nanoparticles: reinforced Poly (vinyl alcohol-g-acrylonitrile) nanocomposite films. Polym. Bull. 74, 4119–4141 (2017).
Tan, X., Ng, S., Mohamed, A. R. & Ong, W. Point-to‐face contact heterojunctions: interfacial design of 0D nanomaterials on 2D g‐C3N4 towards photocatalytic energy applications. Carbon Energy. 4, 665–730 (2022).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH image to imageJ: 25 years of image analysis. Nat. Methods. 9, 671–675 (2012). http://imagej.org
Khan, M. A. Synthesis and characterization of zinc oxide/copper oxide & core-shell heterojunction nanowires grown by vapor deposition. (2017).
Zhang, J., Wang, X., Wang, X. & Li, C. Heterophase junction effect on photogenerated charge separation in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 58, 787–798 (2025).
Saputra, I. S. et al. Green decorated of Au/TiO2 nanocomposites as superior photocatalyst in methylene blue dye degradation under sodium light irradiation. Int. J. Environ. Res. 18, 8 (2024).
Annas, D. et al. Phytosynthesis of CeO2-ZnO nanocomposites mediated by Parkia speciosa Hassk bark aqueous extract as superior photocatalyst in reduction of nitroaromatic compound. Water Air Soil. Pollut. 235, 374 (2024).
Suárez-López, R., Puntes, V. F., Bastús, N. G., Hervés, C. & Jaime, C. Nucleation and growth of gold nanoparticles in the presence of different surfactants. A dissipative particle dynamics study. Sci. Rep. 12, 13926 (2022).
Das, A., Yadav, N., Manchala, S., Bungla, M. & Ganguli, A. K. Mechanistic investigations of growth of anisotropic nanostructures in reverse micelles. ACS Omega. 6, 1007–1029 (2021).
Mubeen, K. et al. Band structure tuning of zno/cuo composites for enhanced photocatalytic activity. J. Saudi Chem. Soc. 27, 101639 (2023).
Said, M. I. & Othman, A. A. Structural, optical and photocatalytic properties of mesoporous CuO nanoparticles with tunable size and different morphologies. RSC Adv. 11, 37801–37813 (2021).
Radwan, A., Mohamed, S. O., Khalil, M. M. H. & El-Sewify, I. M. Effective adsorption of fluorescent congo red Azo dye from aqueous solution by green synthesized nanosphere zno/cuo composite using propolis as bee byproduct extract. Sci. Rep. 14, 9061 (2024).
Veerabhadraiah, S. R., Maji, S. & Panneerselvam, A. Solvent influence on the formation of ZnO nanoparticles by sonochemical technique and evaluation of UV-blocking efficiency. J. Cryst. Growth. 579, 126430 (2022).
Mcoyi, M. P., Mpofu, K. T. & Sekhwama, M. & Mthunzi-Kufa, P. Developments in localized surface plasmon resonance. Plasmonics 1–40 (2024).
Abebe, B., Tsegaye, D., Sori, C., Prasad, R. & Murthy, H. C. R. C. kuppe A. Cu/CuO-doped ZnO nanocomposites via solution combustion synthesis for catalytic 4-nitrophenol reduction. ACS omega 8, 9597–9606 (2023).
Talapatra, S. et al. Enhanced opto-electronic properties of Nd doped CuO thin film. Ceram Int (2024).
Jubu, P. R. et al. Dispensability of the conventional tauc’s plot for accurate bandgap determination from UV–vis optical diffuse reflectance data. Results Opt. 9, 100273 (2022).
El-Hagary, M., Shaaban, E. R., Moustafa, S. H. & Gad, G. M. A. The particle size-dependent optical band gap and magnetic properties of Fe-doped CeO2 nanoparticles. Solid State Sci. 91, 15–22 (2019).
Kayani, Z. N., Chaudhry, W., Sagheer, R., Riaz, S. & Naseem, S. Effect of Ce doping on crystallite size, band gap, dielectric and antibacterial properties of photocatalyst copper oxide Nano-structured thin films. Mater. Sci. Eng. B. 283, 115799 (2022).
Ardekani, S. R., Rouhaghdam, A. S. & Nazari, M. N-doped ZnO-CuO nanocomposite prepared by one-step ultrasonic spray pyrolysis and its photocatalytic activity. Chem. Phys. Lett. 705, 19–22 (2018).
Mejia-Bernal, J. R., Gómez-Solís, C., Juárez-Ramírez, I. & Ortiz-Rabell, G. Díaz-Torres, L. A. Photocurrent generation using zno/cuo/ag heterojunction films. J. Mater. Sci. Mater. Eng. 20, 38 (2025).
Archana, K. J., Preetha, A. C. & Balasubramanian, K. Influence of Urbach energy in enhanced photocatalytic activity of Cu doped ZnO nanoparticles. Opt. Mater. (Amst). 127, 112245 (2022).
Makuła, P., Pacia, M. & Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 9, 6814–6817 (2018).
Wu, F., Cao, F., Liu, Q., Lu, H. & Li, L. Enhancing photoelectrochemical activity with three-dimensional p-CuO/n-ZnO junction photocathodes. Sci. China Mater. 59, 825–832 (2016).
Shao, G. Work function and electron affinity of semiconductors: doping effect and complication due to fermi level pinning. Energy Environ. Mater. 4, 273–276 (2021).
Pahi, S., Mahapatra, B., Behera, A., Singh, S. K. & Patel, R. K. Fermi level induced band edge alignment and band bending in Ag3PO4/Cu2O Pn heterojunction for proficient photocatalytic applications. Mater. Chem. Phys. 305, 127992 (2023).
Thu, C. et al. Role of the metal-oxide work function on photocurrent generation in hybrid solar cells. Sci. Rep. 8, 3559 (2018).
Sumanth, A., Mishra, V., Ramachandra Rao, M. S. & Dixit, T. Interface analysis of cuo/zno heterojunction for optoelectronic applications: an experimental and simulation study. Phys. Status Solidi. 220, 2300256 (2023).
Sharumathi, S. et al. Copper oxide-anchored ZnO nanoflakes for the enhanced photocatalytic degradation performance and mechanistic investigations. Phys. B Condens. Matter. 670, 415336 (2023).
Tolbin, A. Y., Pushkarev, V. E. & Tomilova, L. G. A mathematical analysis of deviations from linearity of beer’s law. Chem. Phys. Lett. 706, 520–524 (2018).
Murshed, M. N. et al. The study of copper oxide nanoparticles based on the pH varying during propolis-mediated synthesis: structure, optical properties, UV-block ability, and malachite green photodegradation. Appl. Nanosci. 14, 585–602 (2024).
Zhang, J. et al. Design and synthesis of a UV–vis-NIR response heterostructure system: for efficient solar energy conversion and BPA photocatalytic degradation. Appl. Surf. Sci. 653, 159346 (2024).
Anku, W. W., Agorku, E. S., Oppong, S. O. B. & Karikari, A. Y. MWCNTs attached neodymium doped-ZnO photocatalysts for efficient removal of dyes from wastewater. SN Appl. Sci. 2, 972 (2020).
Badry, R., El-Nahass, M. M., Nada, N., Elhaes, H. & Ibrahim, M. A. Structural and UV-blocking properties of carboxymethyl cellulose sodium/cuo nanocomposite films. Sci. Rep. 13, 1123 (2023).
Silva, M. R. F. et al. Nanostructured transparent solutions for UV-shielding: recent developments and future challenges. Mater. Today Phys. 35, 101131 (2023).
Badry, R., El-Nahass, M. M., Nada, N., Elhaes, H. & Ibrahim, M. A. UV filters and high refractive index materials based on carboxymethyl cellulose sodium and cuo@ ZnO core/shell nanoparticles. Sci. Rep. 13, 21159 (2023).
Hess, S. C. et al. Direct synthesis of carbon quantum Dots in aqueous polymer solution: one-pot reaction and Preparation of transparent UV-blocking films. J. Mater. Chem. A. 5, 5187–5194 (2017).
Xing, Q. et al. Biodegradable UV-blocking films through core–shell lignin–melanin nanoparticles in Poly (butylene adipate-co-terephthalate). ACS Sustain. Chem. Eng. 7, 4147–4157 (2019).
Motakef-Kazemi, S. H. Green synthesis of zinc oxide nanoparticles using parsley extract. Nanomed. Res. J. 3, 44–50 (2018).
Irede, E. L. et al. Cutting-edge developments in zinc oxide nanoparticles: synthesis and applications for enhanced antimicrobial and UV protection in healthcare solutions. RSC Adv. 14, 20992–21034 (2024).
El–Sayed, N. S., Hasanin, M. & Kamel, S. Wood By-Products as UV protection: A consequence review. J Renew. Mater 12, (2024).
Moustafa, H., El-Sayed, S. M. & Youssef, A. M. Synergistic impact of Cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable Poly (butylene adipate-co-terephthalate)/clay platelets nanocomposites. J. Thermoplast Compos. Mater. 36, 96–117 (2023).
Ren, Y. et al. Promoting adsorption-diffusion-deposition of Zn2 + via a highly reactive modulator for dendrite-free and kinetics-enhanced Zn metal anode. Chem Eng. J 155245 (2024).
Biesinger, M. C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 49, 1325–1334 (2017).
Chastain, J. & King, R. C. Jr Handbook of X-ray photoelectron spectroscopy. Perkin-Elmer Corp. 40, 221 (1992).
Dupin, J. C., Gonbeau, D., Vinatier, P. & Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2, 1319–1324 (2000).
Greczynski, G. & Hultman, L. X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Prog Mater. Sci. 107, 100591 (2020).
Zhang, G. H., Deng, X. Y., Xue, H. & Xiang, G. Engineering of electronic and optical properties of ZnO thin films via Cu doping. Chin. Phys. B. 22, 47803 (2013).
Kamarulzaman, N., Kasim, M. F. & Rusdi, R. Band gap narrowing and widening of ZnO nanostructures and doped materials. Nanoscale Res. Lett. 10, 1–12 (2015).
Xia, W. et al. Improved visible-light photocurrent based on zno/zns core–shell nanorods via interfacial engineering. J. Phys. D Appl. Phys. 52, 35501 (2018).
Miola, M. & Verné, E. Bioactive and antibacterial glass powders doped with copper by ion-exchange in aqueous solutions. Mater. (Basel). 9, 405 (2016).
Piñon-Espitia, M. et al. Charge transfer effects and O2-vacancies in pure CuO nanofibers and enriched with 3.0% Mn. Mater. Chem. Phys. 295, 126989 (2023).
Siddiqui, H., Parra, M. R., Pandey, P., Qureshi, M. S. & Haque, F. Z. Utility of copper oxide nanoparticles (CuO-NPs) as efficient electron donor material in bulk-heterojunction solar cells with enhanced power conversion efficiency. J. Sci. Adv. Mater. Devices. 5, 104–110 (2020).
Wang, H. et al. Direct evidence of subsurface oxygen formation in oxide-derived Cu by x‐ray photoelectron spectroscopy. Angew Chemie Int. Ed. 61, e202111021 (2022).
Zhen, Z., Song, J., Zheng, J. & Lian, J. Optical properties and photocatalytic activity of Nd-doped ZnO powders. Trans. Nonferrous Met. Soc. China. 24, 1434–1439 (2014).
Zhang, J. et al. Preparation and photocatalytic activity of Nd doped ZnO nanoparticles. Mater. Technol. 29, 262–268 (2014).
Carabineiro, S. A. C. et al. Ethyl acetate abatement on copper catalysts supported on ceria doped with rare Earth oxides. Molecules 21, 644 (2016).
Shanmuganathan, R. et al. Core/shell nanoparticles: synthesis, investigation of antimicrobial potential and photocatalytic degradation of Rhodamine B. J. Photochem. Photobiol B Biol. 202, 111729 (2020).
Podurets, A. et al. Enhanced visible-light photocatalytic activity of core–shell oxide nanoparticles synthesized by wet chemical precipitation and atomic layer deposition. Appl. Surf. Sci. 533, 147520 (2020).
Ji, X., Lu, J. F., Wang, Q. & Zhang, D. Impurity doping approach on bandgap narrowing and improved photocatalysis of Ca2Bi2O5. Powder Technol. 376, 708–723 (2020).
Liu, F., Leung, Y. H., Djurisic, A. B., Ng, A. M. C. & Chan, W. K. Native defects in zno: effect on dye adsorption and photocatalytic degradation. J. Phys. Chem. C. 117, 12218–12228 (2013).
Siddiqui, H., Parra, M. R., Qureshi, M. S., Malik, M. M. & Haque, F. Z. Studies of structural, optical, and electrical properties associated with defects in sodium-doped copper oxide (CuO/Na) nanostructures. J. Mater. Sci. 53, 8826–8843 (2018).
Devi, L. V. et al. Synthesis, defect characterization and photocatalytic degradation efficiency of Tb doped CuO nanoparticles. Adv. Powder Technol. 28, 3026–3038 (2017).
Pramanik, M. B., Rakib, A., Siddik, M. A., Bhuiyan, S. & M. A. & Doping effects and relationship between energy band gaps, impact of ionization coefficient and light absorption coefficient in semiconductors. Eur. J. Eng. Technol. Res. 9, 10–15 (2024).
Islam, M. R. et al. Effect of al doping on the structural and optical properties of CuO nanoparticles prepared by solution combustion method: experiment and DFT investigation. J. Phys. Chem. Solids. 147, 109646 (2020).
Akhtar, M. J., Alhadlaq, H. A., Alshamsan, A., Majeed Khan, M. A. & Ahamed, M. Aluminum doping tunes band gap energy level as well as oxidative stress-mediated cytotoxicity of ZnO nanoparticles in MCF-7 cells. Sci. Rep. 5, 13876 (2015).
Morales-Mendoza, J. E. et al. Synthesis, structural and optical properties of Cu doped ZnO and CuO–ZnO composite nanoparticles. Nano-Structures Nano-Objects. 34, 100967 (2023).
Rao, B. N. et al. Exploring the optical and biological aspects of sodium-doped CuO nanoparticles. Mater. Chem. Phys. 308, 128174 (2023).
Ali, L. I., El-Molla, S. A., Ibrahim, M. M., Mahmoud, H. R. & Naghmash, M. A. Effect of Preparation methods and optical band gap of ZnO nanomaterials on photodegradation studies. Opt. Mater. (Amst). 58, 484–490 (2016).
Kaur, K., Badru, R., Singh, P. P. & Kaushal, S. Photodegradation of organic pollutants using heterojunctions: A review. J. Environ. Chem. Eng. 8, 103666 (2020).
Saravanan, R. et al. Enhanced photocatalytic activity of zno/cuo nanocomposite for the degradation of textile dye on visible light illumination. Mater. Sci. Eng. C Mater. Biol. Appl. 33, 91–98 (2013).
Gu, L. et al. Wavelength dependent photoinduced charge carrier dynamics of heterojunction materials: the case of cuo/zno. J. Alloys Compd. 904, 163934 (2022).
Zhan, H. et al. Photocatalytic O2 activation and reactive oxygen species evolution by surface BN bond for organic pollutants degradation. Appl. Catal. B Environ. 310, 121329 (2022).
Liu, Y., Zhao, Y. & Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: advances and prospects. J. Hazard. Mater. 404, 124191 (2021).
Chanu, L. A., Singh, K. J. & Devi, K. N. UV light illuminated photodegradation of malachite green dye using zno/cuo nanocomposites. Mater. Today Proc. 65, 2865–2870 (2022).
Yadav, J. & Qanungo, K. A review: On malachite green; synthesis, uses and toxic effects. in AIP Conference Proceedings vol. 2535AIP Publishing, (2023).
Modwi, A., Ghanem, M. A., Al-Mayouf, A. M. & Houas, A. Lowering energy band gap and enhancing photocatalytic properties of cu/zno composite decorated by transition metals. J. Mol. Struct. 1173, 1–6 (2018).
Yadav, R., Chundawat, T. S., Surolia, P. K. & Vaya, D. Photocatalytic degradation of textile dyes using β-CD-CuO/ZnO nanocomposite. J. Phys. Chem. Solids. 165, 110691 (2022).
Madiha, B., Zahid, Q., Nida, M. & Abdul, S. S. Studie on Malachite Green Dye Degradation by Biogenic Metal Nano Cuo And Cuo/Zno Nano Composites. Arch Nano Op Acc J 1 (4). ANOAJ. MS. ID 119. (2018).
Meena, S., Vaya, D. & Das, B. K. Photocatalytic degradation of malachite green dye by modified ZnO nanomaterial. Bull. Mater. Sci. 39, 1735–1743 (2016).
Acknowledgements
The authors dedicate this work to the soul of the deceased Dr. Attia Sayed Attia, for his support, Department of Chemistry, Faculty of Science, Ain Shams University.
Author information
Authors and Affiliations
Contributions
S.O.M. conducted the experiments on the sample preparation and their application (MG removal and UV-blocking ability).A.R. contributed to the analysis, drawing, and writing of the first draft of the manuscript. I.M.E.‘s contribution was to analyze and revise the first draft of the manuscript. M.M.H.K. proposed the research topic, oversaw every stage of the work, and evaluated the completed manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mohammed, S.O., Khalil, M.M.H., El-Sewify, I.M. et al. Nd-doped CuO/ZnO and ZnO/CuO heterojunctions for simultaneous UV blocking and malachite green detoxification. Sci Rep 15, 34063 (2025). https://doi.org/10.1038/s41598-025-13945-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-13945-w
Keywords
This article is cited by
-
Efficient dye removal in sunlight using biosynthesized nickel/nickel oxide nanoparticles from Ficus cordata: an optimal production strategy
Chemical Papers (2026)
-
A prototype integrated approach for sustainable treatment of organic dyes system using ZnO–CuO–AgO heterostructure as photocatalyst
Scientific Reports (2025)