Abstract
Fertilization with sex-sorted semen is the most used approach to increase the proportion of female calves in dairy farms; however, it is commonly characterized by a lower pregnancy rate. We provide new insights into embryo developmental morphokinetics following fertilization with sorted semen. We also examined whether morphokinetic parameters are sex specific and can be used to select embryos with a desired sex. The first experiment included in vitro fertilization with X- and Y-sorted or unsorted semen. Embryos were developed in a time-lapse system and their developmental morphokinetics was recorded; blastocysts were collected for DNA-PCR and their sex was determined by TSPY and BOV97M expression. In the second experiment, fertilization was performed with unsorted semen and the embryo sex was predicted by the differential kinetic parameters that were found in the first experiment. The proportion of embryos that developed to blastocysts was higher in the unsorted vs. the sex-sorted group; embryos in this group also had diverse morphokinetics. Moreover, male and female embryos displayed different developmental kinetics. The prediction rate for male embryos was relatively low (~ 59–67%), indicating a limited accuracy of sex predication based on the kinetic data. A larger-scale study with a higher number of embryos might clarify this point.
Similar content being viewed by others
Introduction
Shifting the proportion of newborn calves to the desired sex (male or female) is a big challenge and has high economic significance to farms. Nowadays, the commonly used approach is insemination with sex-sorted sperm, which enables approximately a 90% accuracy of the desired sex1. Insemination with sex-sorted semen possesses some significant advantages over the unsorted semen. These advantages include accelerating the genetic gain2,3 and herd expansion4. Despite its potential benefits, the utilization of sex-sorted semen presents significant challenges due to the reduced fertility outcomes compared with unsorted semen5. In support, meta-analysis conducted in 2021 indicated that artificial insemination with sex-sorted semen results in a lower pregnancy rate6.
Apart from artificial insemination with sex-sorted semen, embryo transfer with a known embryo sex is also possible; it considers the additional benefit of using the sorted semen through in vitro fertilization. Nevertheless, some studies reported a negative outcome following the transfer of embryos produced with sex-sorted semen, such as ~ 10% reduction in the pregnancy rate7 and an increase in early embryonic loss8. Although not clear, a negative impact might result from fertilization failure due to impaired fertilization competence through the sorting process or a carryover effect from the spermatozoa to the developed embryo.
From the time of fertilization and throughout early embryonic development, the embryo undergoes numerous cellular and molecular milestones. The first step is the transition of the zygote to the 2-cell-stage embryo, which is also referred to as the first cleavage. Next, the embryo undergoes several divisions, until the blastomeres are tightly formed and the gap junction for intercellular communication, known as the compaction step, takes place. The morula stage is defined as the stage of development during which the dividing cell mass has 20–30 cells9. In bovine, the onset of compaction occurs at the 16- to 32-cell stage10. Then, a cavity, i.e., a blastocoel is formed in a process called blastulation, which results in the formation of the blastocyst. These dynamic development steps can be evaluated by morphological scoring and division speed11. Morphological evaluation includes the blastomere number and size, the extent of fragmentation, the cytoplasmic granularity appearance, and the presence of multinucleation12. The division speed refers to the timing intervals between the stage of development and the subsequent one, for instance, from the zygote to the 2-cell stage. The timing of embryo growth has been previously associated with embryo quality11,13. All together, both the morphology and the developmental growth rate of the embryo are referred to as the embryo morphokinetics, which can be monitored and documented in a non-invasive manner using a time-lapse system.
The current study aimed to determine whether in vitro fertilization with sex-sorted semen affects the embryo developmental morphokinetics. In addition, we determined whether the morphokinetic parameters are associated with the embryo sex. For that purpose, we used an in vitro embryo production methodology while using an incubator equipped with a time-lapse system. Finally, we determined whether differential morphokinetic parameters can be used to predict the embryo sex.
Materials and methods
All reagents were acquired from Merck-Sigma (Rehovot, Israel) unless otherwise specified. All culture media in the laboratory were prepared as previously reported14,15. This included oocyte maturation medium (OMM) composed of TCM-199 with Earl’s salts supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Sartorius, Goettingen, Germany), 0.2 mM sodium pyruvate, 50 µg/µl gentamicin, 1.3 µg/ml porcine folltropin-V (Vetoquinol, Magny-Vernoi, France), and 2 µg/ml estradiol. HEPES–Tyrode’s lactate (HEPES–TL) was supplemented with 0.3% (w/v) bovine serum albumin (BSA), 0.2 mM sodium pyruvate, and 0.75 mg/ml gentamicin (HEPES-TALP). Sperm-TL (SP-TL) was supplemented with 0.6% BSA, 1 mM sodium pyruvate, and 0.2 mg/ml gentamicin (SP-TALP). In vitro fertilization-TL (IVF-TL) was supplemented with 0.6% essential fatty acid-free BSA, 0.2 mM sodium pyruvate, 0.05 mg/ml gentamicin, 0.01 mg/ml heparin (IVF-TALP), and potassium simplex optimized medium (KSOM).
In vitro embryo production
For all in-vitro embryo production processes, oocytes were aspirated from ovaries collected from slaughterhouse post-mortem cows only; thus, living animals were not used in the experiments. The in vitro model of bovine embryo production was approved by the Ethics Committee of the Hebrew University of Jerusalem (AG-22-16883-1). Ovaries were only obtained during the cold season (October-May) and transferred to the laboratory within 3 h in physiological saline solution (0.9% NaCl at 38 °C with 50-µg/ml penicillin-streptomycin). The cumulus oocyte complexes (COCs) were aspirated from 3-mm to 8-mm follicles using a 10-ml syringe attached to an 18-gauge needle into a 50-ml sterile tube with 10-ml HEPES-TALP solution preheated at 38 °C. Briefly, COCs were washed three times in HEPES –TALP; then, groups of 30 COCs were transferred to 500 µl droplets of OMM and incubated in humidified air with 5% CO2 for 22 h at 38.5 °C. At the end of maturation, the COCs were washed in HEPES–TALP and transferred in groups of 30 to four-well plates containing 600 µl of IVF–TALP per well and 25 µl PHE (0.5 mM penicillamine, 0.25 mM hypotaurine, and 25 µM epinephrine in 0.9% NaCl). Cryopreserved semen was used for the in vitro fertilization. Spermatozoa were co-incubated with COCs for 18 h at 38.5 °C in a humidified atmosphere of 5% CO2. After fertilization, putative zygotes were carefully pipetted to remove any remaining cumulus cells and adhering spermatozoa and individually cultured in 25 µl droplets of KSOM in a specially designed dish for the Miri® Time-Lapse Incubator (CultureCoin®; Esco Medical Group, Kringelled, Denmark). The CultureCoin dish was covered with mineral oil and inserted into the Miri® Time Lapse system (Esco Medical Group) for ∼190 h at 38.5 °C, with 5% CO2 and 5% O216.
Embryo developmental competence
Embryo developmental competence and embryo morphokinetics were evaluated as previously reported by our group16. Each individual embryo was monitored automatically every 5 min throughout the culture period (∼190 h). The images were taken through seven focal planes using a built-in Zeiss objective (×20) with a numerical aperture of 0.35 specialized for 635 nm illumination using red light. The individual time-lapse images were then assembled into AVI movies using Miri®TL software. All videos were assessed daily by the same person. Note that the morphokinetic analysis was conducted only on embryos that developed to the blastocyst stage and had a clear morphokinetic visualization throughout their development. Embryos that were out of the camera frame or moved out of the focal planes (i.e., had image distortion rather morphological degeneration) were excluded from the analysis.
Oocyte developmental competence was assessed by calculating the (1) cleavage rate, i.e., the proportion of oocytes that cleaved to 2- and 4-cell-stage embryos at 42–44 h post-fertilization (hpf) out of the total oocytes; and (2) blastocyst formation rate, i.e., the proportion of embryos that further developed to the early blastocyst and blastocyst stages at days 7–8 post-fertilization out of the total oocytes or out of the total number of cleaved embryos.
Embryo kinetics Embryos were monitored individually from fertilization (i.e., the time of co-culturing the oocyte with the spermatozoa; time = 0) to the 2-cell stage and throughout the subsequent developmental stages (i.e., 2-3-cell, 3 4-cell, 4-5-cell, 5-6-cell, 6- 7-cell, 7- 8-cell, and 8-cell to morula) (Fig. 1A). The time of blastulation was recorded, including the timing of an early blastocyst appearance, the timing of the blastocyst appearance, which is characterized by the distinct layers of the inner cell mass (ICM) and the trophectoderm cells as well as the time that the blastocyst started to expand (Fig. 1A).
Embryo morphology was defined for each developmental stage and evaluated based on three main morphological criteria, as previously reported by our group16: the percentage of fragmentation, the shape, and the size of blastomeres in each division. Based on these parameters, the embryo morphology was graded as good, fair, or poor. The morphology of each blastocyst relied on the arrangement and structure of the ICM and the trophectoderm cells. Regarding the ICM, a good morphology was defined as when there were many tightly packed cells; a fair morphology was defined as when a loose group of cells was presented, and poor morphology was defined as when a very small number of cells were presented. Regarding the trophectoderm, a good morphology was defined as when the trophectoderm cells had a cohesive epithelium, a fair morphology was defined as when the trophectoderm cells had a loose epithelium, and poor morphology was defined when there were a few large cells in the trophectoderm (Fig. 1B).
Embryo morphokinetic characterization. (A) Representative images illustrating the sequential stages of embryonic development in the time-lapse system. (1) 2-cell stage, (2) 3-cell stage, (3) 4-cell stage, (4) 5-cell stage, (5) 6-cell stage, (6) 7-cell stage, (7) 8-cell stage, (8) morula stage, (9) blastulation, i.e., the early blastocyst and blastocyst, and (10) with its distinct inner cell mass and trophoblast layers. (B) Embryo morphology. Representative image of good and poor morphological scoring throughout the embryonic developmental stages. A 2-cell-stage embryo with good (1) or poor (2) morphology; a 3-cell-stage embryo with good (3) or poor (4) morphology, a 4-cell-stage embryo with good (5) or poor (6) morphology, a 5-cell-stage embryo with good (7) or poor (8) morphology, a 6-cell-stage embryo with good (9) or poor (10) morphology, a 7-cell-stage embryo with good (11) or poor (12) morphology, a 8-cell-stage embryo with good (13) or poor (14) morphology; a blastocyst stage with good (15) or poor (16) morphology. Note that during embryonic development, not all embryos pass through all developmental stages (1–10).
Division Pattern was defined as either normal or abnormal as previously reported16. Normal cleavage was defined as when the cleavage resulted in equal blastomeres. In addition, two subgroups of normal division were characterized: the synchrony or asynchrony patterns. The synchronous cleavage pattern was characterized by synchronous divisions, i.e., from the zygote into a 2-cell-stage embryo, followed by division into 4- and 8–cell-stage embryos. The asynchronous cleavage pattern was characterized by at least one asynchronous cleavage event that resulted in 3, 5, 6, or 7 blastomeres16,17. Abnormal cleavage included three types of mitotic division: (1) unequal cleavage - when embryos divided into 2 uneven cells, one big cell, and the second is smaller; (2) direct cleavage - cleavage of zygotes into embryos with three or four blastomeres18,19and (3) reverse cleavage defined as two daughter blastomeres that recombine after complete or incomplete separation20.
Blastocyst sexing
Collection of blastocysts Each developed blastocyst was washed in droplets containing Dulbecco’s PBS with 1 mg/ml polyvinylpyrrolidone (PBS/PVP) and then incubated in acid Tyrode’s solution for 0.5 to 2 min until the zona pellucida was dissolved. Next, the blastocysts were rinsed again in PBS/PVP and placed individually into nuclease-free tubes with a minimum volume. Finally, the tube was placed in liquid nitrogen for snap freezing and stored at −80 °C until the DNA extraction procedure.
DNA extraction from blastocysts was performed using the High Pure PCR Template Preparation Kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Prior to DNA extraction, each sample of frozen blastocysts was examined under a stereomicroscope to ensure that it is located within the tube. First, samples were resuspended in 200 µl of PBS without Mg/Ca2++, along with 200 µl binding buffer and 40 µl of proteinase K and incubated at 70 °C for 10 min. The DNA was maintained at −20 °C until PCR was performed.
DNA extraction from somatic cells Samples from somatic tissues of male and female bovine kidneys were harvested at the slaughterhouse. In the laboratory, a sample of 25 milligrams from each tissue was carefully transferred into a sterile 2-ml microcentrifuge tube, and stored at −80 °C. DNA from the tissues was extracted using the DNeasy® Blood & Tissue Kit (Qiagen, Hilden, German), following the manufacturer’s protocol. The amount of DNA was measured using the Nanodrop device. The female kidney sample contained 100 ng/µL DNA, whereas the male counterpart contained 380 ng/µL. Thus, the male sample was equilibrated relative to the female sample.
Polymerase chain reaction (PCR) was conducted using the Biometra TProfessional Basic cycler (SelectSience®, Bath, Britain) in a final reaction volume of 25 µl containing 2.5 µl DNA, 1 µl (10 µM) of each primer, and 12.5 µl Taq PCR Mix (TIANGEN, Beijing, Chaina). A negative control with no DNA template was also included. The PCR program consisted of an initial denaturation step at 95 °C for 3 min, followed by 35 cycles at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 45 s, and a final extension at 72 °C for 10 min. The sexing assay was performed with primers for the Testis Specific Protein Y-Linked 1 (TSPY) and the Cattle Y-specific repeated DNA sequence (BOV97M). A reference autosomal (AUTO) was also used, as previously reported for the bovine embryo21,22. The primers were derived from bovine sequences found in Genbank and specific primer pairs were designed using Primer 3.0 software (Table 1). Then, an electrophoresis (Bio-Rad, California, USA) was conducted with 2% (w/v) agarose and 0.5 µg/ml ethidium bromide (Genesee Scientific, California, USA). The gel was photographed using an ultra-violet transillumination scanning imager, i.e., the Molecular imager, ChemiDoc™ XRS System with Image Lab™ Software (Bio-Rad). The sex of the blastocyst was classified as male if the two Y-linked amplicons were identified and as female if only the AUTO amplicon was presented.
Statistical analysis
Data were analyzed using JMP 17 software (JMP Statistical Discovery LLC, Cary, NC). Before analysis, a logarithmic transformation was applied. The data normality distribution was examined according to the Shapiro-Wilk test on all examined variables. Variance assumptions were verified based on Levene’s test. For normal variables (i.e., > 0.5), a one-way ANOVA, followed by Dunnett’s tests (multiple comparison correction), was conducted. The variables included the proportion of cleaved embryos, the proportion of blastocysts from the total oocytes, and the proportion of blastocysts from cleaved embryos. For that purpose, the unsorted group served as a control for comparison between unsorted vs. sex-sorted groups, and the X-sorted group served as a control for comparison between the X- vs. the Y-sorted groups. Data are presented as the mean ± SEM. Nonnormal variables were analyzed non-parametrically; the Kruskal Wallis test was performed, followed by Dunn’s post-hoc test with correction for multiple comparisons. This includes a comparison between the median time values of each developmental stage between groups. Data for embryo kinetics are presented in box and whisker plots, indicating the timing for 25, 50 (i.e., median), and 75% of the cleaved embryos.
The chi-squared test, followed by Fisher’s exact or Pearson tests for an overall comparison of the incidence data, was performed for the (i) division pattern distribution, (ii) embryo morphology distribution, and (iii) embryo sexing distribution.
For all analyses, a P-value < 0.05 was considered significant. P-values of 0.05 and 0.1 were also reported, showing a discernible tendency toward significance and noteworthy.
Experimental design
The study included two sets of experiments. All experiments were performed during the cold season (October-May) to avoid thermal stress on the oocytes. In all experiments, in vitro embryo production was performed, using an incubator equipped with a time-lapse system. In vitro fertilization was performed with different types of semen, i.e., X- or Y-sorted or unsorted semen, as described below.
Experiment 1
Developmental morphokinetics of embryos that developed following in vitro fertilization with sex-sorted vs. unsorted semen
Embryo developmental competence and morphokinetics were compared for embryos that developed following fertilization with sex-sorted vs. unsorted semen. For that purpose, COCs were aspirated from ovaries and in vitro matured. At the end of maturation, the COCs were randomly assigned into three groups and in vitro fertilized with cryopreserved semen from sexually mature bulls as follows: with X- or Y-sex-sorted semen (Semex, Guelph, ON, Canada) or with unsorted semen (SION, Israel). Semen from three different bulls were used in the study, i.e., one for each experimental group (unsorted, X-, and Y-sex sorted). Following fertilization, the putative zygotes were cultured and continuously monitored for ~ 190 h using the time-lapse system. Embryo developmental competence and morphokinetics were recorded.
Developmental competence evaluation included the proportion of cleaved embryos that developed to the 2- and 4-cell stage and the proportion of embryos that further developed to the blastocyst stage. Embryo morphokinetic evaluation included the following: (a) a cleavage pattern as normal or abnormal (direct, unequal, or reverse) cleavage; (b) embryo kinetics: the timing from fertilization to embryo division into the 2-, 3-, 4-, 5-, 6-, 7-, 8-cell stages, morula, and blastocyst formation; (c) morphology of the cleaved embryos at the 2-, 3-, 4-, 5-, 6-, 7-, 8-cell stages and blastocysts. Each embryo was evaluated and classified as good, fair, or poor morphology. Analysis included a total of n = 370 COCs for the unsorted and n = 420 for the sex-sorted group from at least 3 replicates. Blastocysts from both groups underwent DNA-PCR to validate their sex.
Statistical analysis was performed in three main steps: (1.1) to compare the developmental morphokinetics of embryos that developed following in vitro fertilization with unsorted semen vs. sorted semen, regardless the sex; (1.2) to compare the developmental morphokinetics of male and female embryos within the sex-sorted group; and (1.3) to compare the embryo developmental morphokinetics of male and female embryos within the unsorted group. The differences between the male and female morphokinetics served as sex prediction parameters for Experiment 2.
A two-tailed independent samples ttest (α = 0.05) with a moderate effect size of 0.5 (as applied in biological studies) and a power of 80% (a commonly accepted value). The required a sample size is n = 134.
Experiment 2
Prediction of the embryo sex based on its kinetic parameters
We aimed to determine whether the morphokinetic parameters that were found to be associated with the embryo sex in experiment 1 can be used for predicting the embryo sex. For that purpose, a new set of in vitro embryo production runs were conducted as described above. This included a total of n = 252 COCs that were in vitro matured and further fertilized with unsorted semen. Then, the putative zygotes were cultured in the time-lapse system and were continuously monitored. For each embryo, the sex was predicted according to its individual kinetic pattern while using the kinetic parameters that were found to differ between males and females. To confirm the embryo sex prediction, the developed blastocysts (n = 60) underwent DNA-PCR as described above. The proportion of embryos whose sex was predicted, based on the kinetic parameters, fit the sex-DNA validation that was calculated (i.e., the validated prediction value). An exact two-tailed test (α = 0.05) for a bivariate normal correlation with an effect size of 0.3 (i.e., a moderate effect when no prior data are available) and a power of 80%. The required sample size is n = 84.
An additional analysis was performed on the DNA-validated blastocysts to determine whether the association between the kinetic parameters and the embryo sex found in experiment 1 was also expressed in this set of embryos. Accordingly, a comparison of embryo morphokinetics was conducted between male vs. female validated embryos (n = 60 blastocysts).
Results
Experiment 1
Comparing the developmental morphokinetic of embryos that developed following in vitro fertilization with unsorted- vs. sorted semen, regardless of the sex
Embryo developmental competence
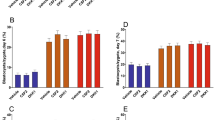
The proportion of cleaved oocytes that developed to the 2-4-cell-stage did not differ between the sex-sorted group relative to the unsorted group (P = 0.86; Fig. 2A). On the other hand, the proportion of embryos that further developed to blastocysts differed between groups, reflected by a higher proportion of blastocysts in the unsorted group relative to the sex-sorted group when calculated from the total number of oocytes (P = 0.03); it tended to be higher when calculated from the cleaved embryos (P = 0.08; Fig. 2A).
Embryonic development following fertilization with unsorted or sex-sorted semen. COCs were allocated to two experimental groups and fertilized with unsorted or sex-sorted semen. (A) Presented is the proportion of oocytes that cleaved to 2-4-cell-stage 42–44 hpf. Presented is the proportion of blastocysts calculated from the total oocytes and from the cleaved embryos. Data are presented as means ± SEM. An asterisques above the bar indicates a significant difference between groups. *P < 0.05. (B) Presented is the distribution of normally vs. abnormally cleaved embryos in the unsorted and sex-sorted groups. (B′) Presented is the distribution of normally cleaved embryos according to the number of asynchronous cleavage events in unsorted and sex-sorted groups. Data analyzed using the chi-squared test were followed by the Pearson test. (C) Presented is the embryo kinetics. Data are presented in whisker plots and indicate the maximum and minimum values within the acceptable range defined by the two quartiles. Boxes indicate the 25th and 75th percentiles, and the middle-horizontal line indicates the median. The line inside the box is the median; the upper whisker denotes the 95th percentile values and the lower whisker denotes the 10th percentile. Data were analyzed using the Kruskal Wallis test, followed by Dunn’s posthoc test, which were used to compare the median value in the different stages of embryonic development. Different letters above the bar indicate significant differences between groups within each developmental stage. *P < 0.05.
Cleavage pattern Overall, the proportion of normally cleaved embryos was higher than the abnormally cleaved embryos in both the unsorted and sex-sorted groups (64.8 and 66.1% vs. 35.2 and 33.9%, respectively). Within the normally cleaved embryos, the proportion of embryos that further developed to the blastocyst stage did not differ between the unsorted and the sex-sorted groups (Fig. 2B). Within the normally cleaved embryos, the cleavage pattern did not differ between the unsorted and the sex-sorted groups (P = 0.8); in both groups, most embryos displayed asynchronous divisions. Within the asynchronously embryos that further developed to the blastocyst stage, a higher number of asynchronic events was recorded in the unsorted group (Fig. 2B′). For instance, within the unsorted-group, most embryos (68.9%) displayed at least 3 asynchronous events during embryonic development, whereas within the sex-sorted group, most embryos displayed no more than 2 events (31.0%). Considering the abnormally cleaved embryos, only a small number of embryos reached the blastocyst stage; thus, further evaluations were conducted only on normally cleaved embryos.
Embryo kinetics Overall, embryos that developed from unsorted semen displayed an earlier development than those that developed from sex-sorted semen (P < 0.05). This was manifested by earlier division timing into the 2-, 5-, 7-, and 8-cell, morula, and blastocyst stages (P < 0.05; Fig. 2C). In addition, the division timing into the 6-cell stage tended to be earlier in the unsorted group relative to the sex-sorted group (P = 0.1).
Embryo morphology In general, no differences were recorded in embryo morphology throughout all the developmental stages regarding the unsorted vs. the sex-sorted group (Table 2).
Comparing the developmental morphokinetics of male and female embryos within the sex-sorted group
Only blastocysts whose gender was in accordance with the type of the sorted semen (i.e., males from Y-sorted or females from X-sorted, respectively) were included in the statistical analysis. The examined parameters are cleavage pattern, embryo kinetics, embryo morphology, and the association with the embryo sex; n = 85 cleaved embryos and n = 12 blastocysts from the Y-sorted group and n = 219 cleaved embryos and n = 13 blastocysts from the X-sorted semen, collected from at least 3 replicates.
Embryonic competence Embryo developmental competence for the X- and the Y-sorted groups was compared. No difference was found in the proportion of 2-4-cell embryos (P = 0.88), nor in the proportion of the developed blastocysts in the X- and Y-sorted groups when calculated out of total oocytes and out of cleaved embryos (P = 0.53 and P = 0.83, respectively; Fig. 3A).
Cleavage pattern The proportion of normally cleaved embryos from the totally cleaved embryos tended to be higher in the Y-sorted vs. the X-sorted group (Fig. 3B). Among embryos that further developed to the blastocyst stage, no difference was found between the X- and Y-sorted groups (P = 0.59). Only one embryo in the X-sorted and two embryos in the Y-sorted groups developed to the blastocyst stage among the abnormally cleaved embryos. Thus, further evaluation was conducted only on embryos that exhibited a normal cleavage pattern.
Embryo kinetics Embryos that developed from Y-sorted semen differ in their kinetics from those that developed from X-sorted semen. This was reflected by an earlier cleavage to the 8-cell stage (P = 0.04) and to the morula (P = 0.01) in the Y-sorted group (Fig. 3C). In addition, the division timing to the 6-cell stage tended (P = 0.06) to be early in the Y-sorted vs. the X-sorted group.
Embryo morphology With respect to the morphology of the developed embryos, no differences were found between those that developed from the Y- vs. the X-sorted groups (Fig. 3D). This was reflected by a similar distribution of embryos into morphological scores (i.e., good, fair, and low) in all the examined developmental stages.
Embryonic development and the morphokinetics of DNA-validated blastocysts that developed from Y- and X-sorted semen. (A) Presented is the proportion of oocytes that cleaved to 2-4-cell-stage 42–44 hpf; the proportion of blastocysts calculated from the total oocytes and from cleaved embryos. Data are presented as means ± SEM. (B) Morphokinetic pattern. Presented is the distribution of embryos into normal and abnormal patterns of X- and Y-sorted groups. Data were analyzed using the chi-squared test followed by Fisher’s exact test. (C) Presented is embryo kinetics. Data are presented in whisker plots and indicate the maximum and minimum values within the acceptable range defined by the two quartiles. Boxes indicate the 25th and 75th percentiles, and the middle-horizontal line indicates the median. The line inside the box is the median; the upper whisker denotes the 95th percentile values and the lower whisker denotes the 10th percentile. Data were analyzed using the Kruskal Wallis test, followed by Dunn’s posthoc test, which were used to compare the median value in the different stages of embryonic development. An asterisk above the bar indicates a significant difference between groups. *P < 0.05. (D) Morphological evaluation. Presented are the distributions into three morphological categories (good and fair) of 2-cell-stage embryos (n = 17), 4-cell-stage embryos (n = 21, and 8-cell-stage embryos (n = 21). Note that a different number of embryos between stages is due to different patterns of division (synchronized vs. unsynchronized). Data were analyzed using the chi-squared test, followed by Fisher’s exact test.
Comparing the developmental competence and morphokinetics of male and female embryos within the unsorted group
Analysis for this part was conducted only on embryos that developed to the blastocyst stage. Blastocysts were subjected to DNA extraction, followed by PCR for sex determination. The proportion of male and female embryos, based on DNA-PCR, was 51.61 and 48.39% of the developed blastocysts (Fig. 4A). Accordingly, embryos were divided into male or female subgroups, and the embryonic development and morphokinetics were retrospectively evaluated. The analysis included n = 246 cleaved embryos and n = 31 sex-validated blastocysts, collected from at least 3 replicates.
Embryonic development following fertilization with unsorted semen of DNA-validated blastocysts. (A) Blastocyst sexing by PCR using DNA as a template. The examined genes were the AUTOZOMAL gene (AUTO; 217 bp), TSPY (153 bp), and BOV97M (143 bp). Presented is a cropped gel including the following: a DNA ladder (line 1); DNA extracted from female (lines 2–4 and 8–10) and male (lines 5–7 and 11–13) blastocysts, respectively; DNA extracted from female (lines 14–16) and male (lines 17–19) tissues, respectively; a negative control for AUTO, TSPY and BOV97M (i.e., without a DNA template) (lines 20, 21 and 22, respectively). The original gel is presented in Supplementary Fig. 1. (B) Presented is the division pattern of the cleaved embryos that developed from blastocysts into normal and abnormal embryos from the total blastocyst population and within the male and female subcategory. (C) Presented is the embryo kinetics. Boxes indicate the 25th and 75th percentiles, and the middle-horizontal line indicates the median. The line inside the box is the median; the upper whisker denotes the 95th percentile values and the lower whisker denotes the 10th percentile. Data were analyzed using the Kruskal Wallis test, followed by Dunn’s posthoc test, which were used to compare the median value in the different stages of embryonic development. An asterisk above the bar indicates a significant difference between groups. *P < 0.05.
Cleavage patterns The distribution of embryos into normal or abnormal division patterns differed between male and female blastocysts. This was reflected by a higher proportion of female blastocysts that displayed a normal cleavage (P = 0.03; Fig. 4B). Within the normally cleaved embryos, most embryos displayed asynchronous divisions in both the female and male groups (100 and 96.1%, respectively). Considering the number of asynchronous events, no difference was found between male and female groups, with most embryos displaying 3 events (P = 0.2; data not shown). Further analysis was conducted only on embryos that displayed a normal division pattern and that further developed to the blastocyst stage.
Embryo kinetics The male blastocysts exhibited an earlier division time into the 6-cell stage relative to their female counterparts (P = 0.02; Fig. 4C). More specifically, the median time from fertilization to the 6-cell-stage for the male blastocysts was 44.1 vs. 51.8 hpf for the female blastocysts.
Embryo morphology The morphological scores of male and female embryos were found to differ in some of the developmental stages. The male embryos displayed a higher number of 3-cell-stage embryos with good morphology relative to the female embryos (Fig. 4D). In addition, a tendency toward a higher number of bad-quality embryos was found at the 7-cell stage in the female relative to the male embryos (P = 0.06). However, blastocyst morphology did not differ between male and female embryos, with a similar proportion of good morphology in both groups (33.33%).
Experiment 2
Prediction of embryo sex based on the morphokinetic parameters
Based on the different kinetic parameters found in experiment 1 (see Sect. 1.2 in the Results section), an attempt to predict the embryo sex was conducted on a new set of embryos, developed from unsorted semen. The predictions were conducted only on embryos that developed to the blastocyst stage and exhibited normal cleavage patterns. The kinetic parameters included the division time into the 6-, 7-, 8-cell stage, and the morula. To determine the cut-off line for sex prediction, a Z-score was calculated for each division time. Accordingly, the cut-off values were 55.24, 91.22, 96.3, and 123.3 hpf for the 6-, 7-, 8-cell, and the morula stages, respectively. Accordingly, embryos that displayed division times earlier than the cut-off value at each division time were predicted as male, otherwise as female. Embryos with unclear cut-off values for definition were excluded from prediction. Table 3 presents the prediction rates for each embryo. Presented are a single parameter or a combination of two-, three-, and four parameters. The proportion of analyzed embryos was higher (i.e., 100%) when a single parameter was predicted and decreased as more kinetic parameters were combined, with a lower proportion (53%) for four parameters. In general, based on the morphokinetic parameters, a higher proportion of embryos was predicted as male embryos (Table 3).
Validating the sex prediction
To confirm the prediction by kinetic parameters, the sex of the blastocysts was further validated by DNA-PCR; the suitability between the two methods (i.e., kinetic and PCR) was examined. Collectively, a higher matching was found for male rather than female embryos. The highest matching (66.7%) was found when three kinetic parameters (i.e., the division to 6-, 8-cells, and the morula stages) were combined, whereas the lowest matching was around 59% (Table 4). With respect to the female embryos, a relatively small number were predicted and validated.
Morphokinetics of the DNA-validated blastocyst
Further analysis was performed on DNA-validated blastocysts to examine the morphokinetics of embryos from Exp.2. This was done to confirm the findings from Exp.1 indicating that male and female embryos differ in their kinetics, while using another set of embryos. The proportion of normally cleaved embryos did not differ between male and female embryos (85.4 vs. 80.7%, respectively). In support of the findings from Exp. 1, the kinetic patterns of embryos differed between male and female, manifested by earlier division timing into the 3-, 7-, and 8-cell stages (P < 0.05). A tendency (P = 0.06) toward the 5-cell stage was also found (Table 5). No difference was found in the number of asynchronous events between male and female embryos nor in the morphology of normally cleaved embryos (data not shown).
Discussion
By using an in vitro model and a time-lapse system, we provide new insights into bovine embryo development following fertilization with sex-sorted sperm. Interestingly, although the fertilization rate, determined by the first cleavage, did not differ between the sorted and unsorted groups, the proportion of embryos that developed to blastocysts was higher in the unsorted groups, suggesting a reduced developmental competence following fertilization with sorted semen. Notably, the reduced blastocyst formation was associated with differential morphokinetics, mainly manifested by slow embryo divisions. In addition, using the time-lapse system, we were able to differentiate between the embryo developmental morphokinetics and its sex. This was confirmed by two different approaches: One included an in vitro fertilization with sex-sorted sperm, whereas in the second approach, fertilization was performed with unsorted semen and embryo sex was determined by PCR. Both approaches revealed that the male embryos cleaved earlier throughout the first mitotic divisions, suggesting a potential kinetic feature for embryo sex prediction.
Nowadays, there is a growing use of sorted semen in the dairy and beef industry, with an accuracy of ~ 90% in predetermining the calf sex. Nonetheless, the conception rate following insemination with sorted semen is relatively low. This reduction can be explained by alterations in the spermatozoa throughout the sorting process itself in which the cells are exposed to a variety of stressors. These stressors include exposure to chemicals (e.g., Hoechst 33342, buffers, and cryoprotectants), high-pressure systems, physical stress (centrifugation), laser irradiation, and thermal fluctuations23,24. In addition, a recently published review suggests that the outcome of in vitro fertilization with sex-sorted semen depends on the sorting methodology used25. In support of this notion, previous studies provided evidence that the spermatozoa quality is compromised through the sex-sorting process, manifested by reduced motility and viability23membrane permeability26and an impaired acrosome reaction27. Moreover, in vitro studies reported a negative impact on fertilization outcomes, as well as a reduction in the cleavage rate and the formation of blastocysts23,28,29,30. Here we provide evidence that the fertility capability, determined by the first cleavage, did not differ between the sex-sorted vs. the unsorted groups. However, a reduction in the blastocyst formation rate was found for the unsorted group. Our findings suggest that sorting-induced alterations in the spermatozoa not only directly affect the spermatozoa fertilization competence—they also carry over to the developing embryo.
A decline in bovine blastocyst formation was previously reported when in vitro fertilization was performed with sorted semen23,28,29,30. Bermejo-Alvarez et al.31,32 reported that embryos that developed from unsorted semen underwent earlier first divisions (i.e., before 48 hpf) relative to those that developed from sex-sorted semen. Nevertheless, it should be noted that in the latter a time-laps system was not used and the data were evaluated by a restricted observation time rather than continuous monitoring, which provides additional kinetic details. In the current study, by using the time-lapse system, we were able to provide new sights into the deleterious effects of fertilization with sorted semen on the embryo developmental morphokinetics. Embryo morphokinetics possess several embryonic developmental features; some features enable the prediction of reproductive outcomes, such as blastocyst formation or the implantation success13,33,34. For instance, early cleaved embryos potentially develop to blastocysts; similarly, normally cleaved embryos rather than abnormally cleaved embryos develop to blastocysts16. Our findings provide clear evidence that embryos that developed from unsorted semen display an earlier development than those that developed from sex-sorted semen, in association with the embryo developmental competence. In agreement, Magata et al.30 reported that the kinetics of bovine embryos was impaired following in vitro fertilization with sorted relative to unsorted semen, as reflected by a slower division time to the first cleavage, morula stage, blastocyst stage, and blastocyst hatching following fertilization with the sorted semen. Steele et al.35 reported that in vitro fertilization with sex-sorted semen resulted in a higher percentage of arrested zygotes and a higher proportion of 4-cell arrested embryos, presumably due to a failure of the transition from the totipotency of the cells to pluripotency36; however, this phenomenon was not found in the current study. On the other hand, in the current study, embryos that developed from the unsorted semen were characterized by more asynchronic events during their development. This phenomenon was previously reported to correlate with higher blastocyst formation16. Note that no difference was found in the proportion of abnormally cleaved embryo following in vitro fertilization with sex-sorted or unsorted sperm. Collectively, alterations in embryo kinetics can explain at least in part the decline in the blastocyst formation rate if those embryos were fertilized with sex-sorted semen.
A previous comparative study conducted in bovine reported that embryos that developed from Y- vs. X-sorted semen did not differ in their kinetic development throughout the first 48 hpf (i.e., corresponding to the 2- and 4-cell embryonic stages) nor in the timing of the blastocyst formation31,32. On the other hand, other studies in bovine reported that the male embryos develop earlier than the female embryos37,38,39,40. However, most of these reports were based on static observations rather than continuous monitoring of embryo development. For instance, Yadav et al.41 concluded that male embryos cleave earlier, since the sex ratio of embryos was 3.6:l.0, favoring the male embryos when the embryos were collected between 24 and 30 hpf. Similarly, the number of embryos that developed from Y-sorted sperm was greater than those that developed from X-sorted sperm, when 8-cell-stage embryos were flushed from the uterine horn42. Apparently, this was because the female embryos were delayed in their development and did not reach the horn at the flushing time. In the current study we used two experimental approaches to explore differences in morphokinetics between male and female bovine embryos. In the first, the fertilization was performed with X- or Y-sorted semen. Embryos that developed from Y-sorted semen differ in their kinetics from those that developed from X-sorted semen, reflected by an earlier cleavage to the 7- and 8-cell stages and to the morula stage in the Y-sorted group. However, the proportion of the embryos that developed to blastocysts was relatively low in both the X- and Y-sorted groups and did not differ between groups. In contrast, Trigal et al.43 reported that the embryo developmental rate from the 5-cell stage up to the blastocyst stage did not differ between Y- and X-sorted sperm, but it was lower than that of the unsorted sperm. Nonetheless, most of the observations are related to in vitro- rather than to in vivo-derived embryos, which represent a true biological benchmark for embryonic development. A comparison between the in vivo embryo kinetics and the embryo sex is very interesting and requires further investigation.
Although the embryo kinetics differ between male and female embryos, no differences were detected in their morphology. In our previous study, we concluded that the morphological criteria by themselves are not sufficient to predict the blastocyst development16. In support, a previous study in human embryos also reported that the blastocyst morphology was not correlated with the embryo sex44. In the second approach, fertilization was performed with unsorted semen and the embryo sex was determined by PCR. Using this approach enabled us to exclude the sorting effect on the embryo morphokinetics discussed above. Importantly, we found that male and female embryos differ in their developmental kinetics regardless of the semen type used for fertilization. Similar to the findings from the first approach (i.e., using X- and Y-sorted semen), the male embryos were found to cleave earlier than the female embryos throughout the preimplantation period. In contrast, a previous study in bovine that also used a time-lapse system did not find differences in embryo kinetics between male and female embryos when fertilization was conducted with unsorted sperm45. Differences between the current study and that of Holm et al.45 might be due to differences in the resolution of the imaging system (the Miri time-lapse incubator vs. the MultiControl 2000 Scanning stage), the timing of capturing intervals (5 vs. 30 min), the culturing methodology (individual vs. a group of zygotes), respectively, or a different definition of the division timing.
Taken together, both the first and the second approaches, our findings indicated that male and female embryos differ in their developmental kinetic parameters. Using a time-lapse system enabled us to accurately determine the kinetic differences between male and female embryos, in particular, the precise division times by which the differences are expressed. Although not examined here, there are some possible explanations for the kinetic differences between male and female embryos. These include transcriptional differences44,46,47glucose metabolism48DNA content49and the culture environment50.
In humans, various methods are used to determine the embryo sex. These include karyotyping methods such as the fluorescence in situ hybridization (FISH)51 and preimplantation genetic diagnosis (PGD) combined with PCR52. Previous studies in bovine reported that using the FISH methodology with the BtY2 probe (a bovine Y-chromosome-specific sequence DNA probe) resulted in an accuracy of > 98% for males and > 93% for females53. A bovine embryo transfer study reported a pregnancy rate of 51.3% with 92% prediction of the newborn calf, while using the FISH methodology54. Other non-invasive methods such as detection of X-linked enzymes55 and H-Y antigens56,57 have also been suggested. Nevertheless, although various invasive and non-invasive methods exist, none of them have been used in the livestock industry, most likely due to the high cost and the need for professional manpower. In the current study we attempted to determine whether the kinetic dissimilarities between male and female embryos can be used for predicting the sex of the embryo. We used the morphokinetically recorded data of embryos that developed from unsorted semen and utilized a few morphokinetic parameters that were found to differ between the X- and Y-sorted embryos. In this way, about 73–100% of the embryos were predicted as males, depending on the number of parameters that were used (i.e., one or more). Nevertheless, although the morphokinetic parameters were associated with the embryo’s sex, the level of accuracy was relatively low (~ 59-66.7%) and insufficient for reliable applications. Further validation with larger datasets is necessary before these markers can be considered for prediction. The obtained prediction rate was higher than the common ratio (i.e., 54%) reported for in vitro-derived male embryos58. However, the accuracy was relatively lower than expected and raised some questions. Given that embryos that developed from unsorted semen were found to differ in their kinetics relative to those that developed from sex-sorted semen (Exp. 1), it is possible that some of the parameters used for sex prediction were not suitable for use (Exp. 2). To confirm this assumption, we used the division time into the 6-cell-stage embryo, which was found to be a prominent kinetic parameter to distinguish between male and female embryos that developed from unsorted semen. Using it as a single parameter resulted in a prediction and accuracy of 63.3%, the highest rate compared with other kinetic parameters. However, it is still considered a low accuracy to determine a desired sex. Another explanation might be the small number of embryos that underwent analysis (i.e., the sample size) in the second experiment. Taken together, another study with a larger scale of embryos is required to confirm which of the kinetic markers suggested here is reliable for sex prediction.
We can conclude that in vitro fertilization with sorted semen impairs embryo development, which in turn, leads to reduced blastocyst developmental competence in association with impaired developmental kinetics, apparently due to the lower quality of the sperm. Thus, efforts in the field must be conducted to minimize the negative impact during the sorting process. An association exists between the embryo developmental kinetics and its sex. This suggests that the sex of in vitro-derived blastocysts can be predicted, based on the embryo morphokinetics, which could serve as a potential method for embryo transfer programs. However, there are some limitations of the study that should be considered. These include the relatively low number of embryos used for sex prediction, in vitro fertilization was conducted with one bull for each experimental group, and only one culture medium (KSOM) was used. Therefore, it is not clear whether the medium favors a specific sex. In addition, the findings of the current study are limited to in vitro-derived embryos and are mostly relevant to an embryo transfer program. Further studies with a larger scale of embryos might explore the accurate kinetic parameters needed for embryo prediction. The authors believe that this knowledge might be useful for developing an intensive reproductive management program based on transferring sexed embryos.
Data availability
All data generated or analyzed during this study are included in the published article.
References
Wellmann, R., Rolfes, A., Rensing, S. & Bennewitz, J. Economic benefits of herd genotyping and using sexed semen for pure and beef-on-dairy breeding in dairy herds. J. Dairy. Sci. 107, 2983–2998. https://doi.org/10.3168/jds.2023-23297 (2024).
Cottle, D. J., Wallace, M., Lonergan, P. & Fahey, A. G. Bioeconomics of sexed semen utilization in a high-producing Holstein-Friesian dairy herd. J. Dairy. Sci. 101, 4498–4512. https://doi.org/10.3168/jds.2017-13172 (2018).
Holden, S. A. & Butler, S. T. Review: applications and benefits of sexed semen in dairy and beef herds. Animal 12(s1), s97–s103. https://doi.org/10.1017/S1751731118000721 (2018).
Hossein-Zadeh, N. G., Nejati-Javaremi, A., Miraei-Ashtiani, S. R. & Kohram, H. Bio-economic evaluation of the use of sexed semen at different conception rates and herd sizes in Holstein populations. Anim. Reprod. Sci. 121, 17–23 (2010).
Vishwanath, R. & Moreno, J. F. Review: semen sexing - current state of the art with emphasis on bovine species. Animal 12(s1), s85–s96. https://doi.org/10.1017/S1751731118000496 (2018).
Reese, S., Pirez, M. C., Steele, H. & Kölle, S. The reproductive success of bovine sperm after sex-sorting: a meta-analysis. Sci. Rep. 11, 17366. https://doi.org/10.1038/s41598-021-96834-2 (2021).
Mikkola, M., Andersson, M. & Taponen, J. Transfer of cattle embryos produced with sex-sorted semen results in impaired pregnancy rate and increased male calf mortality. Theriogenology 84, 1118–1122. https://doi.org/10.1016/j.theriogenology.2015.06.012 (2015).
Rasmussen, S. et al. Pregnancy rates of lactating cows after transfer of in vitro produced embryos using X-sorted sperm. Theriogenology 79, 453–461. https://doi.org/10.1016/j.theriogenology.2012.10.017 (2013).
Brison, D. R., Sturmey, R. G. & Leese, H. J. Metabolic heterogeneity during preimplantation development: the missing link? Hum. Reprod. Update. 20, 632–640. https://doi.org/10.1093/humupd/dmu018 (2014).
Koyama, H., Suzuki, H., Yang, X., Jiang, S. & Foote, R. H. Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biol. Reprod. 50, 163–170 (1994).
Rienzi, L. et al. Significance of morphological attributes of the early embryo. Reprod. Biomed. Online. 10, 669–681. https://doi.org/10.1016/s1472-6483(10)61676-8 (2005).
Nasiri, N. & Eftekhari-Yazdi, P. An overview of the available methods for morphological scoring of pre-implantation embryos in in vitro fertilization. Cell. J. 16, 392–405. https://doi.org/10.22074/cellj.2015.486 (2015).
Yaacobi-Artzi, S., Kalo, D. & Roth, Z. Morphokinetics of in vitro-derived embryos—A lesson from human and bovine studies. Dairy 5, 419–435. https://doi.org/10.3390/dairy5030033 (2024).
Kalo, D. & Roth, Z. Effects of mono(2-ethylhexyl)phthalate on cytoplasmic maturation of oocytes–The bovine model. Reprod. Toxicol. 53, 141–151. https://doi.org/10.1016/j.reprotox.2015.04.007 (2015).
Gendelman, M., Aroyo, A., Yavin, S. & Roth, Z. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 140, 73–82. https://doi.org/10.1530/REP-10-0055 (2010).
Yaacobi-Artzi, S., Kalo, D. & Roth, Z. Association between the morphokinetics of in-vitro-derived bovine embryos and the transcriptomic profile of the derived blastocysts. PLoS One. 17, e0276642. https://doi.org/10.1371/journal.pone.0276642 (2022).
Prados, F. J., Debrock, S., Lemmen, J. G. & Agerholm, I. The cleavage stage embryo. Hum. Reprod. 27, i50–i71 (2012).
Suzuki, R. et al. Direct cleavage during the first mitosis is a sign of abnormal fertilization in cattle. Theriogenology 200, 96–105. https://doi.org/10.1016/j.theriogenology.2023.01.028 (2023).
Magata, F. Time-lapse monitoring technologies for the selection of bovine in vitro fertilized embryos with high implantation potential. J. Reprod. Dev. 69, 57–64. https://doi.org/10.1262/jrd.2022-131 (2023).
Liu, Y., Chapple, V., Roberts, P. & Matson, P. Prevalence, consequence, and significance of reverse cleavage by human embryos viewed with the use of the embryoscope time-lapse video system. Fertil. Steril. 102, 1295–1300e2. https://doi.org/10.1016/j.fertnstert.2014.07.1235 (2014).
Carneiro, M. C. et al. Sexing single bovine blastomeres using TSPY gene amplification. Genet. Mol. Res. 10, 3937–3941. https://doi.org/10.4238/2011 (2011).
Park, J. H. et al. Rapid sexing of preimplantation bovine embryo using consecutive and multiplex polymerase chain reaction (PCR) with biopsied single blastomere. Theriogenology 55, 1843–1853. https://doi.org/10.1016/s0093-691x(01)00526-x (2001).
Leme, L. O. et al. Impact of sperm sex sorting on sperm quality and in vitro embryo production in bovine. Anim. Reprod. Sci. 270, 107604. https://doi.org/10.1016/j.anireprosci.2024.107604 (2024).
Neculai-Valeanu, A. S. & Ariton, A. M. Game-changing approaches in sperm sex-sorting: microfluidics and nanotechnology. Anim. (Basel). 11, 1182. https://doi.org/10.3390/ani11041182 (2021).
Álvarez Gallardo, H., Urbán Duarte, D., Velázquez Roque, A. & Torre Sánchez, J. F. D. L. Use and evolution of sperm sexing in cattle. https://doi.org/10.22319/rmcp.v15i3.6372 (2024)
Balao da Silva, C. M. et al. Sex sorting increases the permeability of the membrane of stallion spermatozoa. Anim. Reprod. Sci. 138, 241–251. https://doi.org/10.1016/j.anireprosci.2013.02.021 (2013).
Mocé, E., Graham, J. K. & Schenk, J. L. Effect of sex-sorting on the ability of fresh and cryopreserved bull sperm to undergo an acrosome reaction. Theriogenology 66, 929–936 (2006).
Palma, G. A., Olivier, N. S., Neumüller, C. & Sinowatz, F. Effects of sex-sorted spermatozoa on the efficiency of in vitro fertilization and ultrastructure of in vitro produced bovine blastocysts. J. Vet. Med. Ser. C Anat. Histol. Embryol. 37, 67–73 (2008).
Wilson, R. D., Fricke, P. M. & Leibfried-Rutledge, M. L. In vitro production of bovine embryos using sex-sorted sperm. Theriogenology 65, 1007–1015 (2006).
Magata, F., Urakawa, M., Matsuda, F. & Oono, Y. Developmental kinetics and viability of bovine embryos produced in vitro with sex-sorted semen. Theriogenology 161, 243–251. https://doi.org/10.1016/j.theriogenology.2020.12.001 (2021).
Bermejo-Alvarez, P., Rizos, D., Rath, D., Lonergan, P. & Gutiérrez-Adán, A. Can bovine in vitro-matured oocytes selectively process X- or Y-sorted sperm differentially? Biol. Reprod. 79, 594–597. https://doi.org/10.1095/biolreprod.108.070169 (2008).
Bermejo-Alvarez, P., Lonergan, P., Rath, D., Gutiérrez-Adan, A. & Rizos, D. Developmental kinetics and gene expression in male and female bovine embryos produced in vitro with sex-sorted spermatozoa. Reprod. Fertil. Dev. 22, 426–436. https://doi.org/10.1071/RD09142 (2010).
Meseguer, M. et al. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 26, 2658–2671. https://doi.org/10.1093/humrep/der256 (2011).
Giménez, C., Conversa, L., Murria, L. & Meseguer, M. Time-lapse imaging: morphokinetic analysis of in vitro fertilization outcomes. Fertil. Steril. 120, 218–227 (2023).
Steele, H., Makri, D., Maalouf, W. E., Reese, S. & Kölle, S. Bovine sperm sexing alters sperm morphokinetics and subsequent early embryonic development. Sci. Rep. 10, 6255. https://doi.org/10.1038/s41598-020-63077-6 (2020).
Ishiuchi, T. & Torres-Padilla, M. E. Towards an understanding of the regulatory mechanisms of totipotency. Curr. Opin. Genet. Dev. 23, 512–518. https://doi.org/10.1016/j.gde.2013.06.006 (2013).
Avery, B. Impact of asynchronous ovulations on the expression of sex-dependent growth rate in bovine preimplantation embryos. J. Reprod. Fertil. 87, 627–631. https://doi.org/10.1530/jrf.0.0870627 (1989).
Avery, B., Madison, V. & Greve, T. Sex and development in bovine in-vitro fertilized embryos. Theriogenology 35, 953–963. https://doi.org/10.1016/0093-691x(91)90306-x (1991).
Beyhan, Z., Johnson, L. A. & First, N. L. Sexual dimorphism in IVM-IVF bovine embryos produced from X and Y chromosome-bearing spermatozoa sorted by high speed flow cytometry. Theriogenology 52, 35–48. https://doi.org/10.1016/s0093-691x(99)00108-9 (1999).
Sidrat, T. et al. Difference in developmental kinetics of y-specific monoclonal antibody sorted male and female in vitro produced bovine embryos. Int. J. Mol. Sci. 21, 244. https://doi.org/10.3390/ijms21010244 (2019).
Yadav, B. R., King, W. A. & Betteridge, K. J. Relationships between the completion of first cleavage and the chromosomal complement, sex, and developmental rates of bovine embryos generated in vitro. Mol. Reprod. Dev. 36, 434–439. https://doi.org/10.1002/mrd.1080360405 (1993).
Wang, J. et al. Study of Developmental Differences between Female and Male Embryos Developed in Cattle. https://ssrn.com/abstract=4695519 (2024).
Trigal, B. et al. In vitro and in vivo quality of bovine embryos in vitro produced with sex-sorted sperm. Theriogenology 78, 1465–1475. https://doi.org/10.1016/j.theriogenology.2012.06.018 (2012).
Wang, A., Kort, J., Behr, B. & Westphal, L. M. Euploidy in relation to blastocyst sex and morphology. J. Assist. Reprod. Genet. 35, 1565–1572. https://doi.org/10.1007/s10815-018-1262-x (2018).
Holm, P. et al. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology 50, 1285–1299. https://doi.org/10.1016/s0093-691x(98)00227-1 (1998).
Lowe, R., Gemma, C., Rakyan, V. K. & Holland, M. L. Sexually dimorphic gene expression emerges with embryonic genome activation and is dynamic throughout development. BMC Genom. 16, 295. https://doi.org/10.1186/s12864-015-1506-4 (2015).
Bermejo-Alvarez, P., Rizos, D., Lonergan, P. & Gutierrez-Adan, A. Transcriptional sexual dimorphism during preimplantation embryo development and its consequences for developmental competence and adult health and disease. Reproduction 141, 563–570 (2011).
Tiffin, G. J., Rieger, D., Betteridge, K. J., Yadav, B. R. & King, W. A. Glucose and glutamine metabolism in pre-attachment cattle embryos in relation to sex and stage of development. J Reprod. Fertil. 93, 125–132. https://doi.org/10.1530/jrf.0.0930125 (1991).
Sharma, M., Singh, A., Sharma, N. & Rawat, S. Embryo sexing in cattle: review. Int. J. Curr. Innov. Res. 3, 955–960 (2017). (2017).
Kochhar, H. P., Peippo, J. & King, W. A. Sex related embryo development. Theriogenology 55, 3–14. https://doi.org/10.1016/s0093-691x(00)00441-6 (2001).
Griffin, D. K., Handyside, A. H., Penketh, A. H., Winston, R. J. A., Delhanty, J. D. & R. M. L., & A. Fluorescent in-situ hybridization to interphase nuclei of human preimplantation embryos with X and Y chromosome specific probes. Hum. Reprod. 6, 101–105. https://doi.org/10.1093/oxfordjournals.humrep.a137241 (1991).
Martinhago, C. et al. Development of a real-time PCR method for rapid sexing of human preimplantation embryos. Reprod. Biomed. Online. 20, 75–82. https://doi.org/10.1016/j.rbmo.2009.10.008 (2010).
Lee, J. H., Park, J. H., Lee, S. H., Park, C. S. & Jin D. I. Sexing using single blastomere derived from IVF bovine embryos by fluorescence in situ hybridization (FISH). Theriogenology 62, 1452–1458. https://doi.org/10.1016/j.theriogenology.2004.02.012 (2004).
Cenariu, M. et al. Bovine embryo sexing using the fluorescence in situ hybridization (FISH). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca, Hortic. https://doi.org/10.15835/buasvmcn-vm:65:2:1538
Sachan, V., Kumar, B., Agrawal, K., Saxena, A. & J., & Methods of embryo sexing in cattle breeding: a review. Iran. J. Appl. Anim. Sci. 10, 1–8 (2020).
White, K. L., Anderson, G. B. & Bondurant, R. H. Expression of a male-specific factor on various stages of preimplantation bovine embryos. Biol. Reprod. 37, 867–873. https://doi.org/10.1095/biolreprod37.4.867 (1987).
Avery, B. & Schmidt, M. Sex determination of bovine embryos using H-Y antibodies. Acta Vet. Scand. 30, 155–164. https://doi.org/10.1186/BF03548052 (1989).
Hasler, J. F. et al. Production, freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology 43, 141–152 (1995).
Acknowledgements
This research was funded by the Israeli Dairy Board Foundation (IDBF; Project No: 820-03550). The funder is not involved in the design of the study or collection, analysis, and interpretation of data. The authors wish to express their great appreciations to Dr. Hilary Voett for kind assistance in statistical consulting.
Author information
Authors and Affiliations
Contributions
D Kalo, study conception, analysis and interpretation of results, writing & review the manuscript; S Manovitz, performed the experiments, analysis and interpretation of results; S Yaacobi-Artzi, assisted in experiment performance; Z Roth, study conception, supervision and writing & review the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kalo, D., Manovich, S., Yaacobi-Artzi, S. et al. Association between sex and the developmental morphokinetics of in vitro derived bovine embryos. Sci Rep 15, 28631 (2025). https://doi.org/10.1038/s41598-025-14017-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14017-9