Abstract
The optimal fluid resuscitation strategy for managing sepsis remains unknown. We aimed to examine the independent and joint associations of infusion time and volume of fluid resuscitation on mortality in patients with sepsis. We analyzed adult patients with sepsis who received > 20 mL/kg of fluid for initial resuscitation within 6 h after the start of fluid resuscitation from prospectively collected nationwide data at 15 hospitals in South Korea between September 2019 and December 2022.The volume of administered fluid (mL/kg) was categorized into six groups (20 to < 25, 25 to < 30, 30 to < 35, 35 to < 40, 40 to < 45, and ≥ 45) and infusion time (h) was categorized into two groups (≤ 3 and > 3). Among a total of 1305 patients, a fluid volume of 40 to < 45 mL/kg with an infusion time of ≤ 3 h was significantly associated with a lower 28-day mortality rate compared with that in 20 to < 25 mL/kg (hazard ratio [HR], 0.18; 95% confidence interval [CI], 0.04–0.87). This nationwide cohort study of patients with sepsis showed that administering a higher (> 30%) volume of fluid than the current recommendation within 3 h was associated with a lower risk of mortality.
Similar content being viewed by others
Introduction
Sepsis is a global health challenge with high incidence and mortality1. Sepsis induces reduced tissue perfusion because of impaired microvascular blood flow caused by systemic vasodilatation and increased vascular permeability2. This can lead to organ dysfunction and death in patients with sepsis, therefore, initial fluid resuscitation is critical to increase circulating fluid volume and cardiac output3,4. In contrast, fluid overload from excess fluid administration can cause tissue edema resulting in iatrogenic death5,6,7.
Current guidelines recommend 30 mL/kg intravenous bolus of crystalloid fluids within 3 h. However, these guidelines are either based on expert opinions or studies with a retrospective single-center design, a small sample size, or a narrow range of volume and infusion time8. Although the optimal dose and rate of fluid administration have been determined by a balanced weighing of the benefits and risks of fluid resuscitation in these patients, the optimal fluid resuscitation strategy is still unclear because most studies have focused on volume or initiation time alone9,10,11,12,13. As the volume expansion kinetics after fluid resuscitation vary and change according to the time and amount of fluid administered, the ultimate effect of fluid resuscitation is dependent on the best coupling of both fluid volume and infusion time14. Therefore, this study aimed to examine the independent associations and combined associations between fluid infusion time and volume and 28-day mortality in patients with sepsis.
Methods
Study population and assessment of infusion time and volume of fluid resuscitation
We analyzed prospectively collected nationwide data from 14,027 patients with sepsis treated at 15 tertiary referral or university-affiliated hospitals in South Korea between September 2019 and December 2022 (the Korean Sepsis Alliance registry). The protocols for patient enrollment and data collection were described previously15. Diagnosis of sepsis in the registry was based on the third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)2. The registry only recorded the performance of resuscitation, hence, we separately calculated the infusion time and volume of fluid resuscitation (Figure S1 in the Supporting Information). Time zero (T0) was defined as the time when a patient first entered the emergency room triage or when a patient in the general ward was first screened by the rapid response team. Time of ICU admission (Ti) was defined as that when a patient arrived at the ICU. Time T0 and Ti were available in the registry data, and infusion time was computed as the interval between T0 and Ti. Fluid resuscitation volume was calculated as the total fluid input (including parenteral intake, enteral intake, and transfusion) from T0 to Ti. To ensure accuracy in the calculated volume and infusion time for resuscitation, we included adult patients (≥ 19 years) who received > 20 mL/kg of fluid resuscitation volume and were admitted to the intensive care unit (ICU) for sepsis treatment within 6 h after T0. Taking into consideration that the calculations for fluid resuscitation volume and infusion time for patients who have a small amount of fluid intake over a long duration could be under- or overestimated, we excluded patients who were administered < 20 mL/kg of fluid resuscitation volume. Patients not admitted to ICU within 6 h after T0, and those with missing data on fluid input before ICU admission or body weight were also excluded. The study protocol was approved by the Institutional Review Board of each participating hospital, including Asan Medical Center (approval no. 2018–0181), which waived the requirement for patient-informed consent because of the observational design and the de-identification of the data sets before analysis. The study was conducted following the Guidelines for Good Clinical Practice and the Declaration of Helsinki. All methods were performed in accordance with the relevant guidelines and regulations for research involving human participants.
Data collection and outcomes
Data were retrieved from the registry. Body weight was recorded in the registry based on the value documented closest to the time zero. For patients who received weight-based medications, such as vasopressors, the body weight used for dosing was recorded instead. Comorbidity was determined as previously described16. An initial sequential organ failure assessment (SOFA) score was calculated to evaluate the illness severity at T0. Patients without pre-existing organ dysfunction were assigned a baseline SOFA score of zero. The infection origin was classified as community-acquired, long-term healthcare facility-related, or hospital-related. The primary infection site was categorized as pulmonary, abdominal, urinary, or other.
We categorized infusion time (h) in two groups (≤ 3 h and > 3 h) and infused volume (mL/kg) into six groups (20 to < 25, 25 to < 30, 30 to < 35, 35 to < 40, 40 to < 45, and ≥ 45). The primary study outcome was the association of fluid resuscitation infusion time and volume with 28-day mortality. The secondary outcomes were ventilator-free and ICU discharge at 28 days, and hospital mortality. These outcomes were separately compared according to infusion time, fluid resuscitation volume, and infusion time and resuscitation volume combined. We evaluated the dose–response relationship of fluid resuscitation volume with 28-day mortality.
Statistical analysis
Data are represented as numbers with percentages and medians with interquartile ranges (IQRs) for categorical and continuous variables, respectively. We applied chi-square or Fisher’s exact tests to compare categorical variables with normal or non-normal distributions, respectively. One-way analysis of variance or Kruskal–Wallis test to compare continuous variables with normal or non-normal distributions, respectively. To examine the independent associations of infusion time and fluid resuscitation volume with 28-day mortality, we performed Cox proportional hazards regression for categorized groups. Multivariable modeling was performed with adjustments for covariables with significant values of p < 0.10 in the univariable analysis. A final model was constructed considering multicollinearity and scientific importance using the following independent covariables: institution, age, sex, comorbidities (cirrhosis, cerebrovascular accidents, and hematologic malignancy), infection origin, infection site, initial SOFA score, vasopressor use, serum lactate level, bacteremia presence, and treatment (steroid and source control). The results were represented as hazard ratios (HRs) with 95% confidence intervals (CIs). The proportional hazard assumption was assessed by inspecting the Schoenfeld residuals. Multivariate Cox proportional hazards regression models adjusted for the same set of covariables were used to investigate the combined associations between the patients classified by infusion time (in h; ≤ 3 and > 3) and fluid resuscitation volume (in mL/kg; 20 to < 25, 25 to < 30, 30 to < 35, 35 to < 40, 40 to < 45, and ≥ 45) and the risk of 28-day mortality.
For secondary outcomes, multivariate Cox proportional hazards regression modeling with covariates was used to analyze hospital mortality. We estimated ventilator-free and ICU discharge at 28 days by competing-risks regression based on a clustered Fine and Gray’s proportional subhazards model with death before day 28 of the competing event. We selected the covariables for the subhazards model based on the results of univariate analyses. These results are shown as sub-hazard ratios (sHRs) and 95% CIs. To further examine the dose effects, we applied restricted cubic spline models with four knots to flexibly model the shape of the association of fluid resuscitation volume with 28-day mortality in patients who received fluid resuscitation within 3 h. We selected the 65th percentile of fluid resuscitation volume as the reference and performed multivariable Cox proportional hazards regression analysis. We separated the data into patients with and without septic shock to test the associations of volume and 28-day mortality for each cohort. Two-sided p < 0.05 indicated significance. All analyses were performed using R software version 4.1.2 (R Core Team).
Results
Study population
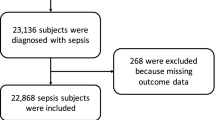
Among 14,027 patients recruited into the Korean Sepsis Alliance registry from September 2019 to December 2022, a total of 1,305 patients who met all inclusion criteria were included in the final analysis, after excluding 12,722 due to no ICU admission (n = 8,623), missing data on fluid administration or body weight (n = 542), no fluid resuscitation (n = 772), delayed ICU admission beyond 6 h (n = 2,337), or insufficient fluid volume (< 20 mL/kg; n = 448) (Figure S2 in the Supporting Information). The descriptive characteristics of all patients are discussed in the Table S1 in the Supporting Information.
Patient characteristics
Table 1 compares the descriptive patient characteristics according to the administered volume. The six groups showed similar demographics, body mass index, comorbidities, SOFA, infection site, microbiological characteristics, and treatment. Patients who received 20 to < 25 mL/kg (14.8%; p = 0.032) fluid volume had a greater frequency of chronic kidney disease. Patients administered ≥ 45 mL/kg fluid volume were more likely to have hematologic malignancy (9.4%; p = 0.033), other infection sites (16.9%; p = 0.006), and steroid therapy (27.5%; p = 0.016) than that in other groups. Patients who received 40 to < 45 mL/kg fluid resuscitation volume had the lowest initial SOFA score (6.0, IQR 5.0–9.0; p = 0.037).
Outcomes according to fluid resuscitation volume and infusion time
The 28-day mortality was 24.3% (317 patients). The HR for 28-day mortality among patients who received 25 to < 30 mL/kg of fluid volume was 0.80 (95% CI 0.53–1.21), 30 to < 35 mL/kg was 0.82 (95% CI 0.55–1.24), 35 to < 40 mL/kg was 0.98 (95% CI 0.64–1.50), 40 to < 45 mL/kg was 0.83 (95% CI 0.52–1.31), and ≥ 45 mL/kg was 1.19 (95% CI 0.85–1.66), compared with those who received 20 to < 25 mL/kg of fluid volume (Table 2). Even after adjusting for covariates, administered fluid volume per se was not associated with 28-day mortality. Regarding infusion time, the 28-day mortality did not differ significantly between patients who received fluid resuscitation for over 3 h or within 3 h (HR, 0.89; 95% CI 0.69–1.15). In addition, shorter fluid infusion times did not yield additional benefit (≤ 1 h: HR 0.88, 95% CI 0.48–1.61; > 1 h to ≤ 2 h: HR 0.84, 95% CI 0.55–1.28; > 2 h to ≤ 3 h: HR 0.93, 95% CI 0.67–1.28). After adjusting for confounding factors, infusion time was not statistically significantly associated with 28-day mortality.
Combined associations of fluid resuscitation volume and infusion time with outcomes
The stratified analysis by infusion time dichotomized the cohort into patients in whom fluid resuscitation was completed within 3 h (n = 354, 36.4%) or beyond 3 h (n = 951, 63.6%) (Figure S3 in the Supporting Information). Of patients who completed fluid resuscitation within 3 h, 71 (20.1%) received 20 to < 25 mL/kg fluid volume, 58 (16.8%) received 25 to < 30 mL/kg, 69 (19.5%) received 30 to < 35 mL/kg, 48 (13.6%) received 35 to < 40 mL/kg, 33 (9.3%) received 40 to < 45 mL/kg, and 75 (21.2%) received ≥ 45 mL/kg (Table S2 in the Supporting Information). Among patients who completed fluid resuscitation beyond 3 h, 125 (13.1%) received 20 to < 25 mL/kg fluid volume, 147 (15.5%) received 25 to < 30 mL/kg group, 136 (14.3%) received 30 to < 35 mL/kg, 108 (11.4%) received 35 to < 40 mL/kg, 107 (11.3%) received 40 to < 45 mL/kg, and 328 (34.5%) received ≥ 45 mL/kg. In the combined analyses, the combination of 40 to < 45 mL/kg of fluid volume and infusion time within 3 h (HR, 0.18; 95% CI, 0.04–0.87) was associated with lower 28-day mortality compared to those in 20 to < 25 mL/kg of fluid volume within 3 h (Fig. 1 and Table S3 in the Supporting Information). Similar associations were observed for patients who were ventilator-free at 28 days, discharged from the ICU at 28 days, and hospital mortality (Table 3). Patients who received 40 to < 45 mL/kg of fluid resuscitation volume had a higher probability of being ventilator-free at 28 days (sHR, 2.55; 95% CI 1.24–5.27), discharged from the ICU at 28 days (sHR, 1.61; 95% CI 1.06–2.45), and a lower risk of hospital mortality (HR, 0.18; 95% CI 0.03–0.93) compared to those who received 20 to < 25 mL/kg fluid volume. In the subgroup analysis based on fluid resuscitation volume, fluid resuscitation time ≤ 3 h was significantly associated with a lower risk of 28-day mortality only in the patients who received 20 to < 25 mL/kg fluid volume (HR, 0.43; 95% CI 0.21–0.88) and 40 to < 45 mL/kg fluid volume (HR, 0.11; 95% CI 0.02–0.57) (Fig. 2).
Combined Associations between Fluid Resuscitation Time and Volume and 28-day mortality. Hazard ratios (solid circle symbols) with 95% CIs (error bars) of combined associations of volume and time of fluid for 28-day mortality. (A) Fluid resuscitation time within 3 h, (B) Fluid resuscitation time beyond 3 h. The 28-day mortalities were adjusted for the institution, age, sex, comorbidities (cirrhosis, cerebrovascular accidents, and hematologic malignancy), infection origin, infection site, initial sequential organ failure assessment score, vasopressor use, lactate level, bacteremia, treatments (steroid and source control) using multivariate Cox proportional regression models.
Hazard Ratios of Fluid Resuscitation Time and 28-day Mortality Stratified by Fluid Resuscitation Volume. A forest plot of fluid resuscitation with 3 h versus beyond 3 h in subgroups of fluid resuscitation volume with respect to death at 28 days. Model adjusted for age, sex, comorbidities (cirrhosis, cerebrovascular accidents, and hematologic malignancy), infection origin, infection site, initial sequential organ failure assessment score, lactate level, bacteremia, and treatments (steroid and source control).
Dose–response relationship
The restricted cubic spline model adjusted for covariance to determine the dose–response association between the fluid volume and outcomes revealed a significant non-linear association in patients who received fluid resuscitation volume within 3 h (p = 0.046) (Fig. 3). The risk of 28-day mortality increased substantially in patients who received fluid volume between 25 to < 30 mL/kg but then decreased gradually until approximately 50 mL/kg. Patients with shock showed a stronger association than patients without shock (p = 0.032).
Restricted Cubic Spline Models of Hazard Ratios of Fluid Resuscitation Volume Within 3 h and 28-day Mortality. (A) All patients. (B) Patients with septic shock. (C) Patients without septic shock. Knots set at the 5th, 35th, 65th, and 95th percentiles of fluid resuscitation volume. Reference is the 65th percentile. Solid lines, hazard ratios; shadow, 95% confidence interval. Model adjusted for age, sex, comorbidities (cirrhosis, cerebrovascular accidents, and hematologic malignancy), infection origin, infection site, initial sequential organ failure assessment score, lactate level, bacteremia, and treatments (steroid and source control).
Discussion
In this study, patients with sepsis who received 40 to < 45 mL/kg of fluid volume within 3 h were more likely to survive, discontinue mechanical ventilation, and get discharged from the ICU compared with those administered 20 to < 25 mL/kg fluid volume within 3 h. Moreover, among those who received 40 to < 45 mL/kg of fluid, those who received fluid resuscitation for a shorter infusion time (≤ 3 h) had a lower mortality risk than those who received it for a longer infusion time (> 3 h). The findings of this large cohort study of patients with sepsis suggested the importance of the combined effect of volume and infusion time in fluid resuscitation in patients with sepsis.
An early analysis of the survival sepsis campaign database reported no association between 20 mL/kg of initial fluid resuscitation volume during 6 h with adjusted-risk assessment for hospital mortality17. The recent guidelines recommend 30 mL/kg of fluid volume within 3 h for initial resuscitation in patients with sepsis-induced hypoperfusion or septic shock3. However, our results suggested that resuscitation with fluid volume > 30 mL/kg within 3 h was associated with a better prognosis in patients with sepsis. A retrospective study showed that a higher proportion of total fluid amount in the first 3 h after 20 m L/kg of fluid resuscitation was significantly associated with a decreased risk of hospital mortality13. Similarly, children with septic shock who received 40 mL/kg fluid in the first hour had lower mortality than those who received < 20 mL/kg fluid volume18. Another retrospective study reported that higher fluid volumes with a plateau of 35–45 mL/kg by 3 h were correlated with decreased mortality10. A more recent study suggested that a fluid resuscitation rate of 0.25–0.5 mL/kg/min, which is equivalent to 45–90 mL/kg within 3 h was optimal11. Findings from these previous studies and our results suggest an association between > 30 mL/kg of fluid infusion within 3 h and survival in patients with sepsis.
Fluid resuscitation aims to restore intravascular volume and increase cardiac output, thereby improving tissue perfusion in patients with sepsis19. Fluid resuscitation comprises four dynamic phases: resuscitation, optimization, stabilization, and evacuation20. Most studies reporting the harmful effect of fluid overload analyzed the association between positive fluid balance after the stabilization phase21,22. Whereas, studies comparing restrictive versus liberal fluid administration focused on the resuscitation phase, and consequently showed no survival benefit of the restrictive fluid strategy23,24,25. Another study did not detect a difference in intubation incidence between guideline-based and restrictive fluid resuscitation in patients at a high risk of acute respiratory failure26. A recent study demonstrated the potential harm of early vasopressor use without sufficient fluid resuscitation in patients with septic shock27. Our results showed the best outcome for patients administered > 40 mL/kg of fluid within 3 h but not beyond 3 h. These findings collectively suggest that liberal fluid therapy in sepsis is important during the resuscitation phase, but not beyond that phase.
The curve of the restricted cubic spline model examining the dose–effect relationship between fluid resuscitation volume and mortality in this study showed a hormetic effect characterized by a dose-dependent variable response28. We observed increased odds of mortality in patients who received 25 to < 30 mL/kg fluid compared to the lower volumes, and the odds of survival benefit were higher with increased fluid volume, plateauing at 45–50 mL/kg. The paradoxically deleterious effects of fluid resuscitation could be attributable to the cardiovascular physiology during sepsis29. Studies have demonstrated the potential pathways through which fluid resuscitation may cause cardiovascular dysfunction including vasodilatation, cardiotoxicity, glycocalyx degradation, and inflammatory response30,31,32,33,34,35. Two randomized controlled trials highlighted the cardiovascular harm associated with fluid resuscitation36,37. Based on the findings from the two randomized trials the possible mechanism underlying the hormetic effect of fluid volume in our study could be explained. Although the cardiac output and blood pressure may appear to be restored at lower fluid volumes (25–40 mL/kg) during resuscitation efforts in patients with septic shock, these increases do not necessarily indicate restoration of tissue perfusion. However, a higher volume of fluid resuscitation (40–50 mL/kg) may offset cardiovascular dysfunction with a greater fluid volume, leading to better patient prognosis..
Previous studies demonstrated lower mortality in patients who received faster than 3 h of infusion times9,11,12. By contrast, in this study, the infusion time of fluid per se was not associated with 28-day mortality unlike the fluid resuscitation volume. These differences may be due to the pharmacokinetics of fluid administration14. The context-sensitive half-time of the expansion of plasma volume after intravenous infusion of crystalloids varies over time38. The peak volume expansion of the intravenous infusion of 1 L of crystalloids is higher over 1 h than 3 h; however, the amount of volume expansion between these two cases is reversed at 2 h. Finally, volume expansions beyond 3 h are similar between intravenous infusions over 1 h and 3 h. Thus, the net effect of fluid resuscitation seems to be determined by the dynamics of ‘infusion volume and rate’ rather than the isolated effects of either administered volume or rapidity of infusion.
In this study, only approximately one-third of the patients met the criteria for septic shock, but a substantial volume of fluid was administered across the entire cohort. This is likely to reflect the characteristics of the study population, many of whom may correspond to severe sepsis under the Sepsis-2 criteria39. We included only patients who received at least 20 mL/kg of fluid resuscitation within the first 6 h. Given this inclusion criterion, most of these patients likely exhibited clinical signs of initial hypotension or hypoperfusion requiring fluid resuscitation, although they ultimately did not require vasopressors or meet the criteria for septic shock after fluid resuscitation. Interestingly, in our study population, the volume of fluid resuscitation was positively correlated with initial lactate levels (Figure S4 in the Supporting Information). In the multivariable linear regression analysis adjusting for covariates (body mass index, chronic kidney disease, hematologic malignancy, pulmonary infection, bacteremia, and steroid use), elevated lactate levels remained significantly associated with an increase in fluid volume. These findings may reflect protocol-based decision-making guided by lactate levels, as recommended in international guidelines. However, the strength of this association was modest, and the effect size was too small to suggest any meaningful clinical impact. Therefore, the actual influence of lactate elevation on fluid administration in clinical practice is likely to have been limited.
The strengths of this study include the nationwide, multicenter data, detailed information on covariables, and examination of both separate and joint associations of infusion time and fluid volume with mortality. Furthermore, we conducted a multivariate analysis to mitigate the potential reduction in statistical power due to the relatively small sample size. Covariate selection was controlled by clearly defined criteria to minimize the risk of overfitting. This approach helped improve the precision and robustness of our findings.The study limitations include an observational design, which limits findings of causality associations between fluid resuscitation volume and mortality. Second, although our study used data from a well-constructed registry, the possibility of inevitable measurement errors in fluid intake cannot be overlooked. We included all fluid intake such as oral intake, transfusion, and intravenous fluid after fluid resuscitation, which could result in an over-estimation of the fluid volume. However, the amount of other fluid intake except for resuscitation fluid was likely to account for a very small portion of all fluid intake because all patients were admitted to the ICU within 6 h of initiating fluid resuscitation. Third, there was a potential for selection bias. This study analyzed fewer than 10% of all patients in the registry due to inclusion and exclusion criteria, which limited to the cohort to patients with sepsis admitted to the ICU who received > 20 mL/kg of intravenous fluids within the first 6 h. Although statistical adjustments were performed, this might have influenced the outcomes of this study. Furthermore, the relatively small number of included patients after exclusion necessitated a cautious interpretation of the findings. Finally, due to the lack of guidelines supported by high-quality evidence, the volume and time of fluid administration were determined at the discretion of the attending physician at each participating center.
In conclusion, infusion time and fluid resuscitation volume were not significantly associated with 28-day mortality in this nationwide cohort study of patients with sepsis. However, compared with 20 to < 25 mL/kg of fluid volume within 3 h, 40 to < 45 mL/kg of initial fluid resuscitation (at least > 30% of the current recommendation) administered within 3 h was associated with decreased odds of 28-day mortality and increased odds of the patient being ventilator-free and discharged from the ICU at 28 days. Additionally, among patients who received 40 to < 45 mL/kg of fluid, those with a shorter infusion time (3 h or shorter) had a lower mortality risk. Our findings suggest that our findings suggest that careful coordination of administered volume and infusion time of fluid resuscitation may be associated with improved outcomes in patients with sepsis.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Rudd, K. E. et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 395, 200–211 (2020).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 (2016).
Evans, L. et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 47, 1181–1247 (2021).
Gupta, R. G., Hartigan, S. M., Kashiouris, M. G., Sessler, C. N. & Bearman, G. M. Early goal-directed resuscitation of patients with septic shock: current evidence and future directions. Crit. Care. 19, 286 (2015).
Sadaka, F., Juarez, M., Naydenov, S. & O’Brien, J. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J Intensive Care Med. 29, 213–217 (2014).
Macdonald, S. Fluid resuscitation in patients presenting with sepsis: Current insights. Open Access Emerg. Med. 14, 633–638 (2022).
Sirvent, J. M., Ferri, C., Baró, A., Murcia, C. & Lorencio, C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am. J. Emerg. Med. 33, 186–189 (2015).
Perner, A. et al. The intensive care medicine research agenda on septic shock. Intensive Care Med. 43, 1294–1305 (2017).
Leisman, D. et al. Association of Fluid resuscitation initiation within 30 minutes of severe sepsis and septic shock recognition with reduced mortality and length of stay. Ann. Emerg. Med. 68, 298–311 (2016).
Kuttab, H. I. et al. Evaluation and predictors of fluid resuscitation in patients with severe sepsis and septic shock. Crit. Care Med. 47, 1582–1590 (2019).
Hu, B. et al. Effect of initial infusion rates of fluid resuscitation on outcomes in patients with septic shock: A historical cohort study. Crit. Care. 24, 137 (2020).
Wang, H. L. et al. Initial fluid resuscitation (30 mL/kg) in patients with septic shock: More or less?. Am. J. Emerg. Med. 50, 309–315 (2021).
Lee, S. J. et al. Increased fluid administration in the first three hours of sepsis resuscitation is associated with reduced mortality: A retrospective cohort study. Chest 146, 908–915 (2014).
Hahn, R. G. Volume kinetics for infusion fluids. Anesthesiology 113, 470–481 (2010).
Hyun, D. G. et al. Mortality of patients with hospital-onset sepsis in hospitals with all-day and non-all-day rapid response teams: a prospective nationwide multicenter cohort study. Crit. Care. 26, 280 (2022).
Li, A. et al. Epidemiology, management, and outcomes of sepsis in ICUs among countries of differing national wealth across Asia. Am. J. Respir. Crit. Care Med. 206, 1107–1116 (2022).
Levy, M. M. et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 36, 222–231 (2010).
Oliveira, C. F. et al. Time- and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr. Emerg. Care. 24, 810–815 (2008).
Byrne, L. & Van Haren, F. Fluid resuscitation in human sepsis: Time to rewrite history?. Ann. Intensive Care. 7, 4 (2017).
Malbrain, M. et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care. 8, 66 (2018).
Acheampong, A. & Vincent, J. L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care. 19, 251 (2015).
Shim, H. J., Jang, J. Y., Lee, S. H. & Lee, J. G. The effect of positive balance on the outcomes of critically ill noncardiac postsurgical patients: A retrospective cohort study. J. Crit. Care. 29, 43–48 (2014).
Shapiro, N. I. et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N. Engl. J. Med. 388, 499–510 (2023).
Meyhoff, T. S. et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 386, 2459–2470 (2022).
Kjær, M. N. et al. Long-term effects of restriction of intravenous fluid in adult ICU patients with septic shock. Intensive Care Med. 49, 820–830 (2023).
Khan, R. A. et al. Association between volume of fluid resuscitation and intubation in high-risk patients with sepsis, heart failure, end-stage renal disease, and cirrhosis. Chest 157, 286–292 (2020).
Yeo, H. J. et al. Vasopressor initiation within 1 hour of fluid loading is associated with increased mortality in septic shock patients: Analysis of national registry data. Crit. Care Med. 50, e351–e360 (2022).
Calabrese, E. J. & Mattson, M. P. How does hormesis impact biology, toxicology, and medicine?. NPJ Aging Mech. Dis. 3, 13 (2017).
Marik, P. E., Byrne, L. & van Haren, F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J. Thorac. Dis. 12, S37-s47 (2020).
Byrne, L. et al. Unintended consequences: Fluid resuscitation worsens shock in an ovine model of endotoxemia. Am. J. Respir. Crit. Care Med. 198, 1043–1054 (2018).
Byrne, L. et al. An ovine model of hyperdynamic endotoxemia and vital organ metabolism. Shock 49, 99–107 (2018).
Monge García, M. I. et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 41, 1247–1255 (2015).
Berg, S., Engman, A., Hesselvik, J. F. & Laurent, T. C. Crystalloid infusion increases plasma hyaluronan. Crit Care Med. 22, 1563–1567 (1994).
Hippensteel, J. A. et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit. Care. 23, 259 (2019).
Rhee, P. et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit. Care Med. 28, 74–78 (2000).
Maitland, K. et al. Mortality after fluid bolus in African children with severe infection. N. Engl. J. Med. 364, 2483–2495 (2011).
Andrews, B. et al. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: A randomized clinical trial. JAMA 318, 1233–1240 (2017).
Hahn, R. G. & Lyons, G. The half-life of infusion fluids: An educational review. Eur. J. Anaesthesiol. 33, 475–482 (2016).
Dellinger, R. P. et al. Surviving Sepsis Campaign Guidelines Committee including the pediatric subgroup. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637 (2013).
Acknowledgements
The following persons and institutions participated in the Korean Sepsis Alliance (KSA): Steering committee–Chae-Man Lim (Chair), Sang-Bum Hong, Dong Kyu oh, Su Yeon Lee, Gee Young Suh, Kyeongman Jeon, Ryoung-Eun Ko, Young-Jae Cho, Yeon Joo Lee, Sung Yoon Lim, Sunghoon Park; Participating persons and centers–Kangwon National University Hospital–Jeongwon Heo; Korea University Anam Hospital–Jae-myeong Lee; Daegu Catholic University Hospital–Kyung Chan Kim; Seoul National University Bundang Hospital–Yeon Joo Lee; Inje University Sanggye Paik Hospital–Youjin Chang; Samsung Medical Center–Kyeongman Jeon; Seoul National University Hospital–Sang-Min Lee; Asan Medical Center–Chae-Man Lim, Suk-Kyung Hong; Pusan National University Yangsan Hospital–Woo Hyun Cho; Chonnam National University Hospital–Sang Hyun Kwak; Jeonbuk National University Hospital–Heung Bum Lee; Ulsan University Hospital–Jong-Joon Ahn; Jeju National University Hospital–Gil Myeong Seong; Chungnam National University Hospital–Song-I Lee; Hallym University Sacred Heart Hospital–Sunghoon Park; Hanyang University Guri Hospital–Tai Sun Park; Severance Hospital–Su Hwan Lee; Yeungnam University Medical Center–Eun Young Choi; Chungnam National University Sejong Hospital–Jae Young Moon; Inje University Ilsan Paik Hospital–Hyung Koo Kang
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (fund code 2019E280500, 2020E280700, 2021–10-026). The funding body had no role in the design of the study, data collection and analysis, or manuscript preparation.
Author information
Authors and Affiliations
Consortia
Contributions
Conception and design of the work were performed by DH, JHA, SYL, JWH, SH, YK and CL. Data acquisition was performed by DH, DKO, SYL, MHP, HL, JWH, and SH. Analysis was performed by DH and CL. The draft was written by DH. Review and revision of the draft were performed by JHA, SYL, JWH, SH, and YK. All authors have approved the final manuscript and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study protocol was approved by the Institutional Review Board of each participating hospital, including Asan Medical Center (approval no. 2018-0181), which waived the requirement for patient-informed consent because of the observational design and the de-identification of the data sets before analysis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hyun, Dg., Ahn, J.H., Huh, J.W. et al. Optimal time and volume of fluid resuscitation in patients with sepsis: a nationwide multicenter cohort study. Sci Rep 15, 30465 (2025). https://doi.org/10.1038/s41598-025-14854-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-14854-8