Abstract
Osteoarthritis (OA) is a prevalent chronic degenerative joint disease. Ferroptosis, an iron-dependent form of programmed cell death, has been implicated as a crucial contributor to OA progression. Simiao Powder, a classical traditional Chinese medicine formula, has been widely used in clinical practice to treat inflammatory diseases such as gout and OA. However, its regulatory effects on ferroptosis remain unclear. This study investigates whether Simiao Powder could alleviate OA by regulating ferroptosis and analyzes its underlying mechanisms. We integrated multiple bioinformatics analyses along with in vivo and in vitro experiments to elucidate the mechanism by which DHT IIA treats OA. Potential therapeutic targets of Simiao Powder were identified through the TCMSP and GEO databases, and their potential biological functions were evaluated using GO enrichment, ROC curve analysis, molecular docking, and immune infiltration assessment via CIBERSORTx. OA was induced in SD rats using a high carbohydrate and fat diet, followed by a 16-week intervention with DHT IIA. Histological staining, OARSI scoring, and cartilage thickness measurements were performed. The expression levels of PPARγ, GPX4, and the apoptosis marker TUNEL in rat cartilage were evaluated using immunofluorescence and immunohistochemistry. In an LPS-induced ATDC5 chondrocyte injury model, Western blotting was used to assess the expression of PPARγ, COL2A1, ACSL4, and MMP13. Furthermore, the scTenifoldKnk algorithm was applied to perform a virtual knockout of PPARγ based on the GSE216651 single-cell RNA-seq dataset to explore its downstream regulatory effects. Bioinformatics analysis identified five key genes (CDKN1A, HMOX1, DPP4, GJA1, and PPARγ), among which PPARγ and HMOX1 exhibited strong predictive potential with AUC values > 0.8. Molecular docking analysis revealed that DHT IIA exhibited the lowest binding energy with PPARγ (-8.15 kcal/mol), indicating it as the most likely therapeutic target. In vitro and in vivo experiments demonstrated that DHT IIA significantly upregulated PPARγ expression reduced ACSL4, MMP13 and TUNEL levels, increased COL2A1 and GPX4 expression levels. Meanwhile computer simulated gene knockdown also laterally confirmed that PPARγ is a key gene affecting ferroptosis and OA development. The study confirmed that DHT IIA could improve the articular cartilage structure of OA rats by elevating PPARγ and inhibiting ferroptosis. This study reveals that DHT IIA, the active ingredient of Simiao Powder, may alleviate OA by inhibiting ferroptosis through the activation of PPARγ. These findings provide a new molecular mechanism basis for the treatment of OA by Simiao Powder, as well as scientific support for the subsequent drug development and clinical applications.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a common chronic degenerative joint disease characterized by progressive degeneration of articular cartilage, and abnormal subchondral bone remodeling, and may lead to pain, stiffness, and limited joint mobility, which can lead to severe disability in in severe cases1. Chronic pain is the most prevalent symptom of OA and has become a major factor affecting the physical and mental well-being of the elderly2. Globally, OA has a high prevalence, with an estimated 595 million cases in 2020, accounting for 7.6% of the global population. With increasing life expectancy and an aging population, the prevalence of OA continues to rise. Moreover, OA imposes a significant economic burden, with related healthcare expenditures accounting for 1–2.5% of the gross domestic product in high-income countries3. Currently, clinical treatments for OA are mainly focused on relieving symptoms, reducing pain and improving joint function, but its pathophysiological process needs to be further investigated4. Therefore, further investigation into the pathogenesis of OA is of great importance for disease prevention and treatment.
Ferroptosis is an iron-dependent form of programmed cell death primarily caused mainly by abnormal intracellular iron accumulation and lipid peroxidation5,6. This process differs from conventional apoptosis, necrosis, and autophagy and is characterized by glutathione peroxidase 4 (GPX4) inactivation, accumulation of lipid reactive oxygen species (LPO), and an abnormal increase in iron ions, which leads to irreversible damage to the cell membrane. Recently, it has been shown that chondrocyte ferroptosis plays an important role in the progression of OA, possibly through oxidative stress, redox imbalance and inflammation induction7,8. It has been found that HIF-2α expression can be induced by activation of NF-κB, which downregulates glutathione (GSH) production, inhibits GPX 4 activity, and promotes ferroptosis9. These findings suggest that ferroptosis may serve as a promising therapeutic target for OA treatment.
Simiao Powder is a well-known traditional Chinese medicine formula first documented in Danxi’s Mastery of Medicine. It is composed of Atractylodes chinensis rhizome, Cortex phellodendri, Radix Achyranthis Bidentatae, and Coicis Semen. Currently, the commonly adopted formulation ratio is Cortex phellodendri (2): Coicis Semen (2): Atractylodes chinensis rhizome (1): Radix Achyranthis Bidentatae (1). The components of this formula possess anti-inflammatory, analgesic, vasoconstrictive, and anti-exudative properties10. Due to its efficacy and minimal side effects, Simiao Powder has been widely used in clinical practice in China for the treatment of inflammatory diseases such as gout, OA, and hyperuricemia11,12. Modern pharmacological studies have further demonstrated that Simiao Powder effectively alleviates OA symptoms and reduces inflammatory cytokine levels in patients13,14. Therefore, we hypothesize that the therapeutic effects of Simiao Powder in OA may be associated with ferroptosis. However, the underlying mechanisms by which Simiao Powder regulates ferroptosis in OA remain unclear.
Therefore, we utilized an integrated approach combining bioinformatics, network pharmacology, molecular docking, and traditional experimental methodologies to investigate whether Simiao Powder alleviates OA by modulating chondrocyte ferroptosis15. Through in vitro and in vivo experiments, we further validated that its active component, Dehydrotanshinone II A (DHT IIA), exerts its therapeutic effects by activating PPARγ to inhibit chondrocyte ferroptosis, ultimately alleviating OA progression. These findings provide a scientific basis for identifying potential drug targets and supporting future clinical applications.
Materials and methods
Screening of active ingredients and effective targets of Simiao powder
The active components and potential targets of Simiao Powder (Atractylodes chinensis rhizome, Cortex phellodendri, Radix Achyranthis Bidentatae, and Coicis Semen) were retrieved from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP)16. Given the complex composition of traditional Chinese medicine, not all ingredients can be effectively absorbed and utilized by the human body. Oral bioavailability (OB) is a crucial parameter for determining whether a ingredient can be absorbed into systemic circulation and exert therapeutic effects at its target sites. Drug-likeness (DL) is another key indicator used to assess the pharmacokinetic properties of a ingredient. Therefore, screening criteria were set at OB ≥ 30%17 and DL ≥ 0.1818, resulting in the identification of active ingredients and their corresponding potential targets.
OA microarray dataset collection and processing
The GSE98918 and GSE178557 datasets were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), including 20 normal cartilage samples and 20 OA cartilage samples19,20. GSE98918, based on the GPL20844 platform (Agilent-072363 SurePrint G3 Human GE v3 8 × 60 K Microarray 039494), was primarily used for identifying overlapping genes, while GSE178557, based on the GPL13497 platform (Agilent-026652 Whole Human Genome The probes were converted to gene symbols according to the annotation documents, the data from the microarray were log2 transformed, and the samples were normalized by the “removebatcheffect” function in the “limma” package for R21. Differentially expressed genes (DEGs) between OA and normal cartilage tissues in the GSE98918 dataset were identified using the “limma” package21, with the significance threshold set at |logFC| ≥ 0.5 and adjusted p-value < 0.05. Heatmaps and volcano plots were visualized using the “ggplot2” package in R4.
Overlapping genes between OA, Simiao powder, and ferroptosis
Ferroptosis-related genes, including “driver genes”, “suppressor genes”, and “marker genes” were retrieved from the FerrDb database (http://www.zhounan.org/ferrdb/). To identify key genes associated with ferroptosis in OA, a Venn diagram analysis was performed using the “VennDiagram” package in R22. The overlapping genes among the DEGs from the GSE98918 dataset, the potential therapeutic targets of Simiao Powder, and ferroptosis-related genes were visualized to identify potential therapeutic targets for further investigation.
Construction of a drug active ingredient and disease target network
The obtained overlapping genes and the active ingredients of Simiao Powder and their corresponding active targets were imported into Cytoscape 3.10.3 software to draw the “active ingredient-target” network regulation map.
Enrichment analysis of GO and Cnetplot network
Gene Ontology (GO) enrichment analysis of overlapping genes in the Biological Process (BP) category helps to recognize their biological functions. GO functional annotation was performed using the “ClusterProfiler” package in R23. To further visualize the relationships between genes and enriched biological pathways, a category network plot (cnetplot) was constructed. This visualization aids in understanding the biological pathways in which these genes are involved.
Validation of the overlapping genes with receiver operating characteristic
To validate the accuracy of the overlapping genes, we performed ROC curve analysis on both the GSE98918 and GSE178557 datasets to assess their diagnostic accuracy. It is generally accepted that an AUC > 0.8 indicates good diagnostic performance24. Therefore, in subsequent analyses, we selected genes that exhibited an AUC greater than 0.8 in both datasets for further investigation.
Immune cell infiltration analysis using CIBERSORTx
To assess the role of the immune microenvironment in the progression of OA, we utilized CIBERSORTx (https://cibersortx.stanford.edu/index.php) to perform immune infiltration analysis on the combined dataset from GSE98918 and GSE178557, comparing OA tissues and normal tissues. We set the number of Permutations to 1000 to robustly estimate the proportions of various immune cell types. The results of the analysis were then visualized to gain insights into the immune landscape associated with OA.
Molecular docking of active ingredients with hub genes
The genes obtained from the previous analysis were imported into the UniProt database (https://www.uniprot.org/) to retrieve the corresponding gene identifiers. Protein structures encoded by these genes were then obtained from the RCSB PDB database (https://www.rcsb.org/), and the relevant receptors were selected for download in PDB format. These structures were then imported into PyMOL to remove water molecules and small molecules. Subsequently, the active ingredient from the previously generated"ingredient-target” network were retrieved, and their corresponding 3D structures were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) in SDF format. Molecular docking was performed using AutoDock 1.5.6 software. The active sites and docking parameters were set, and the binding energy values were calculated to rank the results. A lower binding energy indicates a more stable binding25. We screened out the active ingredients and targets with the lowest binding energy for subsequent experimental verification.
Experimental animals
This study was approved by the Ethics Committee of the School of Medicine, Jianghan University (NO: JHDXLL2025–002), and all experimental procedures were conducted under its supervision. The 18 6-week-old male Sprague-Dawley (SD) rats (180–200 g) used in the experiments were purchased from Beijing Vital River Laboratory Animal Technology Co. All animals were housed in an SPF-grade animal laboratory, with ambient temperatures controlled at 22 ± 1 °C and a light/dark cycle of 12 h. The SD rats were acclimatized for 1 week before the experiment, with free access to drinking water and experimental diet. The rats were divided into three groups, each consisting of six animals. The first group was fed a normal diet, the second group was fed a high carbohydrate and fat diet (HCFD) to induce OA26, and the third group was fed HCFD plus DHT IIA at a dose of 20 mg/kg. All experimental groups were fed continuously for 16 weeks.
Sampling and tissue Preparation
All SD rats were anesthetized for euthanasia, and the knee joints were dissected, fixed in 4% paraformaldehyde for 24 h, and then decalcified using 10% EDTA Decalcified Solution (G1105, Servicebio) for 4 weeks. Decalcified specimens were dehydrated in incremental grades of ethanol and paraffin embedded using standard protocols.
Histologic production of rat knee joints
Sagittal sections of the knee joint specimens (4 μm thick) were prepared and stained with Hematoxylin and Eosin (H&E) as well as Safranin O-Fast Green to analyze tissue structure and pathological changes. Safranin O-Fast Green staining was used to assess proteoglycan content in the articular cartilage. The thickness of the hyaline cartilage (HC) was measured for each group, and the severity of knee osteoarthritis in rats was evaluated using the Osteoarthritis Research Society International (OARSI) scoring system27.
Immunofluorescence staining
Paraffin-embedded knee joint tissue sections were routinely dewaxed and rehydrated through a graded ethanol series, followed by heat-induced antigen retrieval using citrate buffer (pH 6.0). After cooling to room temperature, endogenous peroxidase activity was blocked with 3% H₂O₂ followed by 5% BSA for 1 h. The sections were then incubated overnight at 4 °C with primary antibody against PPARγ (1:1000, GB12164, Servicebio). On the following day, after washing with PBS, the sections were incubated with Cy3-conjugated fluorescent secondary antibody (1:200, GB21301, Servicebio) for 1 h at room temperature in the dark. Nuclei were counterstained with DAPI, and the slides were mounted. Fluorescence images were captured using a fluorescence microscope, and quantitative analysis was performed using ImageJ software.
Immunohistochemistry
To evaluate the effects of DHT IIA intervention on ferroptosis and apoptosis in rat articular cartilage tissue, immunohistochemical staining for GPX4 and TUNEL was performed on paraffin-embedded knee joint sections. The sections were dewaxed, subjected to antigen retrieval and blocking, then incubated overnight with primary antibody against GPX4 (1:2000; GB115275, Servicebio). On the following day, secondary antibody was applied according to the manufacturer’s instructions, and the sections were observed and imaged under a microscope. For apoptosis detection, the DAB (SA-HRP) TUNEL Cell Apoptosis Detection Kit (G1507-50T, Servicebio) was used to stain paraffin-embedded sections. After dewaxing, gradient rehydration, incubation with proteinase K, and blocking of endogenous peroxidase with 3% H₂O₂, TUNEL labeling and DAB chromogenic reaction were performed following the kit protocol. Finally, the sections were counterstained with hematoxylin, dehydrated, cleared, and mounted. All stained sections were observed under a microscope and quantitatively analyzed using ImageJ software.
Cell culture
The chondrogenic cell line ATDC5, purchased from the Chinese Academy of Sciences, was cultured in DMEM (G4511, Servicebio) supplemented with 10% fetal bovine serum (FBS) at 37 °C in a humidified incubator with 5% CO₂. Prior to all cell-based experiments, the cells were pretreated with 1% insulin-transferrin-selenium (ITS) for 14 days to induce chondrogenesis28.
Cell viability assay
To determine the optimal drug concentration, ATDC5 cells were seeded into 96-well plates at a density of 2 × 10³ cells per well. After allowing the cells to adhere for 6 h, the medium was replaced with 100 µL of fresh medium containing different concentrations of DHT IIA. The cells were treated with lipopolysaccharide (LPS, 5 µg/mL) and varying concentrations of DHT IIA (0, 0.5, 1, 2, 4, 6, 8, and 10 µM) for 24 h. After treatment, 10 µL of CCK-8 solution (G4103, Servicebio) was added to each well, followed by 100 µL of fresh medium, and the plates were incubated for 2 h at 37 °C. The absorbance was measured at 450 nm using a microplate reader. Each drug concentration was tested in triplicate, and the cell viability of the control group was set to 100%.
Western blot
Total protein was extracted from LPS- and DHT IIA-treated ATDC5 cells using RIPA lysis buffer (G2002, Servicebio). Protein concentration was determined using the BCA protein assay kit (G2026, Servicebio). The extracted proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes. After blocking with 5% skim milk for 2 h, the membranes were incubated overnight at 4 °C with primary antibodies targeting PPARγ (1:500), MMP13 (1:500), COL2A1 (1:500), ACSL4 (1:2000), and GAPDH (1:3000). The next day, the membranes were washed three times with TBST and incubated with HRP-conjugated secondary antibodies for 2 h. Protein bands were visualized using ECL reagent (G2020, Servicebio) and detected using a chemiluminescence imaging system. Band intensities were quantified using ImageJ software.
Computer simulation of PPARγ knockout
To further validate the critical regulatory role of PPARγ in OA, this study used scTenifoldKnk, a simulated knockdown analysis tool based on single-cell RNA sequencing data, to perform virtual knockdown of PPARγ to infer its downstream functional impact29. This method has been shown to be an efficient and systematic tool for studying gene function, and is particularly suitable for research contexts where real KO experiments cannot be performed. We used the single-cell transcriptome dataset GSE216651, (4 healthy and 5 OA). We normalized and preprocessed this dataset, and constructed Gene Regulatory Network (GRN), which captures the regulatory relationships between genes, from the healthy and OA groups, respectively, using the scTenifoldKnk method. Unsupervised virtual knockdown was performed in the constructed GRN by approximating the deletion of PPARγ nodes to simulate their knockdown state, thus identifying genes whose regulatory relationships were significantly altered by PPARγ knockdown. We further screened the set of genes associated with ferroptosis among them, ranked them for significance by combining the Z-score and p-value, and visualized the change patterns of up and down regulated genes.
Statistical analysis
All data were expressed as mean ± SD and analyzed using R software (version 4.2). For data that met the normal distribution and variance chi-square, a one-way ANOVA was performed followed by multiple comparisons using Tukey’s post hoc test. For data that did not meet the assumption of normality, the Kruskal-Wallis H test was used. If significant differences were observed (p < 0.05), pairwise comparisons were performed using Dunn’s test with Bonferroni correction. p < 0.05 was considered statistically different.
Results
Screening the therapeutic targets and active ingredients of Simiao powder
We utilized the TCMSP database with the screening criteria OB ≥ 30% and DL ≥ 0.18, ultimately identifying 75 ingredients (Atractylodes chinensis rhizome with 9, Cortex phellodendri with 37, Radix Achyranthis Bidentatae with 20, and Coicis Semen with 9). The corresponding target proteins of these 75 ingredients were retrieved, and after ID conversion and removal of duplicate targets, a total of 208 therapeutic targets were obtained.
Identification of Ferroptosis-related genes in the OA microarray dataset by active ingredient of Simiao powder
Using the “Limma” package, we performed differential expression analysis on the GSE98918 OA microarray dataset, identifying a total of 1,690 DEGs, including 915 upregulated genes and 775 downregulated genes. Heatmaps and volcano plots were generated to visualize these findings (Fig. 1A, B). We retrieved 484 ferroptosis-related genes from the FerrDb database. Using the “VennDiagram” package, we identified the overlapping genes among Simiao Powder potential therapeutic targets, OA-DEGs, and ferroptosis-related genes, generating a Venn diagram (Fig. 1C). As a result, five overlapping genes (CDKN1A, HMOX1, DPP4, GJA1, and PPARγ) were identified. Finally, we constructed a network diagram to illustrate the relationship between active ingredients of Simiao Powder and disease targets (Fig. 1D).
Bioinformatics analysis of osteoarthritis, Simiao Powder, and ferroptosis related. (A) Heat map analysis of the GSE98918 dataset. (B) GSE98918 Volcano map. Red color represents genes whose expression is up-regulated in OA and blue color represents genes that are down-regulated. (C) Venn diagram of Simiao Powder and OA and ferroptosis. Overlapping genes (CDKN1A, HMOX1, DPP4, GJA1, and PPARγ). (D) Drug active ingredient-target network diagram. MOL002644 is phellopterin; MOL002651 is dehydrotanshinone II A; MOL000098 quercetin, MOL000173 is wogonin; MOL000422 is kaempferol; MOL002714 is baicalein; MOL000188 is 3β-acetoxyatractylone. Green is derived from Atractylodes macrocephala, Gray is derived from Radix Achyranthis Bidentatae, Yellow is derived from Cortex phellodendri; Orange is derived from a variety of herbs.
GO BP enrichment analysis and Cnetplot analysis results
These five overlapping genes were functionally enriched using the R package “clusterProfiler” and their GO BP and cnetplots were mapped (Fig. 2A, B) to predict the potential biological functions of these overlapping genes and associated pathways. Finally, 382 pathways were obtained, of which the top five pathways were regulation of smooth muscle cell proliferation, smooth muscle cell proliferation, muscle cell proliferation, negative regulation of smooth muscle cell proliferation, and negative regulation of cell migration.
Validation of overlapping genes from the GSE98918 and GSE178557 datasets with receiver operating characteristic
In order to verify whether the above five overlapping genes have predictive significance, we introduced an external validation dataset (GSE178557). we analyzed the ROC curves for the GSE98918 and GSE178557 datasets (Fig. 2C, D). It was observed that the AUC of almost all overlapping genes was greater than 0.5, representing that all genes had some predictive value. However, in order to AUC > 0.8 is considered to have strong discriminatory power30, we selected only the genes with AUC > 0.8 in both datasets for further analysis—namely, PPARγ and HMOX1.
Functional enrichment and diagnostic value of hub genes. (A) GO enrichment analysis of the top 10 biological processes associated with the five identified hub genes. The x-axis indicates the gene ratio, and bubble size reflects the number of genes involved in each term. Bubble color corresponds to adjusted p-values. (B) GO network analysis showing the relationships between the five hub genes (CDKN1A, PPARγ, GJA1, DPP4, and HMOX1) and the top enriched biological processes. (C) ROC curves of the five hub genes in the training cohort (GSE98918). (D) ROC curves of the five hub genes in the validation cohort (GSE178557).
Results of immune cell infiltration analysis by CIBERSORTx
By using CIBERSORTx to identify immune cell types involved in the pathogenesis of OA, we obtained the proportion of 22 immune cell types in OA versus normal synovial tissue (Fig. 3A). The proportion of activated mast cells was elevated and statistically different in OA compared to normal synovium (Fig. 3B, p < 0.05). And there was a significant positive correlation with dendritic cell activated, which indicated that mast cell activation and dendritic cells were jointly involved in the development of OA (Fig. 3C).
Molecular docking results
According to Fig. 1D, we can know that the drug active ingredients corresponding to PPARγ and HMOX1 are MOL002651 (Dehydrotanshinone II A), MOL000098 (quercetin), MOL000173 (wogonin), MOL000422 (kaempferol). We performed molecular docking and showed that they all have good binding affinity for each other. Among them, the PPARγ-Dehydrotanshinone II A docking model had the lowest binding free energy (−8.15 kcal/mol), which was considered as the best binding model (Fig. 4), and the specific binding free energy values of each docking model are detailed in Table 1.
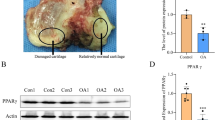
DHT IIA alleviates articular cartilage degeneration in OA rats
We induced OA in SD rats by HCFD, and after 16 weeks of treatment with DHT IIA, H&E staining and Safranin O-fast green staining were performed on the knee joint sections of the rats, and OARSI scores and knee cartilage thickness measurements were performed to objectively reflect the OA status of the rats. H&E staining of knee joint sections showed that the knee joints of the Normal group were normal (Fig. 5A), while the articular cartilage structure of the OA group was severely damaged, such as the irregularity of the articular cartilage surface (Fig. 5B), and the DHT IIA group was able to improve the structure of the knee joints of the rats (Fig. 5C). Safranin O-fast green staining showed that the articular surface of the Normal group was smooth. showed that the joint surface of the Normal group was smooth, the cartilage structure was clear and proteoglycan was abundant (Fig. 5D); obvious cartilage degradation and loss of proteoglycan were observed in the OA group (Fig. 5E); the changes in the knee joints of the rats in the DHT IIA group were significantly smaller than those in the OA group, with the cartilage being smoother and proteoglycan content being more abundant, but poorer than that in the Normal group (Fig. 5F). This suggests that DHT IIA has the ability to maintain proteoglycan production and delay the process of OA, but cannot reverse the development of osteoarthritis.
Histologic results of the knee joints of rats in each group. (A-C) H&E staining (×4). (D-F) Safranin O-fast green staining (×4). Normal group (A and D); OA group (B and E); DHT IIA group (C and F); (G) Rat OARSI scores for each group (mean ± SD, n = 5). (H) Hyaline cartilage (HC) Thickness of articular cartilage in each group of rats (mean ± SD, n = 5). *p < 0.05; **p < 0.01; ***p < 0.001.
The OARSI score and the surface thickness of rat articular hyaline cartilage were measured by histology of rat knee joints. The OARSI score of the OA group was 3.5 ± 0.67 significantly higher than that of the Normal group (0.42 ± 0.51) (p < 0.001), and the OARSI score of the DHT IIA group was significantly reduced to 2.09 ± 0.67 compared to that of the OA group (p < 0.05). Also hyaline cartilage thickness in the OA group (14.33 ± 2.90 μm) was significantly decreased compared to the Normal group (65.83 ± 5.25 μm), while the articular hyaline cartilage thickness in the DHT IIA group (42.75 ± 4.56 μm) was significantly improved (p < 0.001) (Fig. 5G, H).
DHT IIA attenuates ferroptosis and restores PPARγ in rat chondrocytes
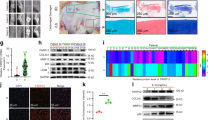
We examined the expression of PPARγ in cartilage tissues using immunofluorescence staining. The results showed that PPARγ was highly expressed in the chondrocytes of the normal group. In the OA model group, PPARγ expression was significantly reduced, whereas DHT IIA treatment markedly restored the fluorescence intensity, approaching normal levels (Fig. 6A). Quantitative analysis confirmed that the average fluorescence intensity of PPARγ in the OA group was significantly lower than in the normal group (p < 0.001), and was notably increased after DHT IIA treatment (p < 0.01) (Fig. 6B), suggesting that PPARγ may play a key role in the anti-ferroptotic mechanism.
We further assessed the expression of the ferroptosis-inhibitory factor GPX4 and the apoptosis marker TUNEL by immunohistochemical staining. GPX4 was broadly expressed in the cartilage of the normal group but was markedly downregulated in the OA group, with a noticeable reduction in positive cells. DHT IIA treatment significantly restored GPX4 expression, as evidenced by the increased number of positively stained cells (Fig. 6C). Statistical analysis showed that the proportion of GPX4-positive cells was significantly lower in the OA group compared to the normal group (p < 0.01), and DHT IIA treatment reversed this trend (p < 0.01) (Fig. 6D). TUNEL staining revealed significantly elevated levels of chondrocyte apoptosis in the OA group, with a higher number of TUNEL-positive cells than in the normal group (p < 0.01). Although DHT IIA reduced the TUNEL-positive rate (Fig. 6E, F), the difference was not statistically significant compared to the OA group, indicating that apoptosis was indeed present in OA, and while DHT IIA could alleviate ferroptosis, its effect on apoptosis might be limited.
DHT IIA alleviates chondrocyte ferroptosis via activation of PPARγ in OA rats. (A) Representative immunofluorescence images of PPARγ (red) and DAPI (blue) staining in articular cartilage tissues from Normal, OA, and DHT IIA-treated rats (Scale bar: 100 μm). (B) Quantification of mean fluorescence intensity of (n = 3). (C) Immunohistochemical staining of GPX4 in rat articular cartilage (Scale bar: 100 μm). (D) Quantification of GPX4-positive cells in different groups (n = 3). (E) Representative TUNEL staining showing apoptotic chondrocytes in articular cartilage (Scale bar: 100 μm). (F) Quantification of TUNEL-positive cells in different groups (n = 3). Data are presented as mean ± SD. *p < 0.05, *p < 0.01, *p < 0.001 as indicated.
DHT IIA attenuates LPS-induced chondrocyte injury and ferroptosis
To investigate the effect of DHT IIA in regulating chondrocytes, we first measured its cytotoxic effect. ATDC5 cells were treated with DHT IIA for 24 h and cell viability was assessed using the CCK8 assay. Figure 7A shows that no cytotoxic effect was observed at concentrations of DHT IIA below 8 µM. Subsequently, we examined the effect of DHT IIA on LPS-induced ATDC5 cell activity, and concentrations of 2, 4, and 8 µM of DHT IIA restored chondrocyte activity to varying degrees, but the most significant therapeutic effect was observed at a DHT IIA concentration of 6 µM (Fig. 7B).
To investigate whether DHT IIA modulates the production of LPS-induced ATDC5 cell injury by affecting PPARγ and ferroptosis, we treated LPS-treated ATDC5 cells with DHT IIA, and then assessed the expression of PPARγ, ACSL4, MMP13, and COL2A1. Western blot analysis showed that, compared to the control group, DHT IIA significantly restored PPARγ expression while inhibiting the ferroptosis marker acyl-CoA synthetase long-chain family member 4 (ACSL4). Additionally, DHT IIA was found to reduce MMP13 expression and restore COL2A1 protein levels (Fig. 7C, D). These findings suggest that DHT IIA alleviates LPS-induced chondrocyte damage by activating PPARγ and inhibiting ferroptosis.
DHT IIA alleviates LPS-induced osteoarthritis through ferroptosis in a cellular model. (A) ATDC 5 cells were treated with different doses of DHT IIA for 24 h and cell viability was measured using the CCK 8 assay. (B) LPS-treated ATDC 5 cells were treated with different DHT IIA concentrations for 24 h and cell viability was measured using the CCK 8 assay. ### p < 0.001 vs. Vehicle group; * p < 0.05, ** p < 0.01, *** p < 0.001 vs. LPS group. (C, D) Western blot was performed to quantify the expression of MMP13, PPARγ, ACSL4 and COL2A1 in three groups of ATDC5 cells with different treatments. Data are represented as means ± SD. *p < 0.05; ** p < 0.01; *** p < 0.001.
Simulated knockout of PPARγ reveals its regulatory network in ferroptosis
To further validate the regulatory role of PPARγ in OA, we conducted a simulated knockout analysis using the scTenifoldKnk algorithm based on the GSE216651 single-cell transcriptomic dataset. Gene regulatory networks (GRNs) were constructed for both healthy and OA groups, and virtual knockout of PPARγ was performed to assess its perturbation effects under OA conditions. In the healthy group, PPARγ knockout resulted in significant regulatory changes in 70 genes (|Z| > 2, adjusted p < 0.01) (Fig. S1A). Differential gene expression analysis in the OA group identified 38 overlapping genes that were also significantly perturbed by PPARγ deletion (Fig. S1B), suggesting that PPARγ downregulation may causally contribute to OA pathogenesis at the computational level.
Furthermore, we conducted simulated PPARγ knockout within the OA group and compared the perturbed genes with a curated list of 484 ferroptosis-related genes. Among them, 309 genes were found to be significantly dysregulated following PPARγ deletion in the OA context (Fig. S2A). Z-score analysis and visualization revealed enhanced regulatory activity for several key ferroptosis-associated genes, including CREB5, YAP1, EGFR, NOX4, ALOX5, and FTH1, all of which exhibited significantly elevated positive Z-scores (Z > 0). These genes have been previously implicated in critical ferroptotic processes such as lipid peroxidation, iron metabolism, and glutathione depletion (Fig. S2B).
Collectively, these results indicate that downregulation of PPARγ may promote ferroptosis, thereby contributing to the onset and progression of OA. The findings from both healthy and OA group simulations consistently highlight the central role of PPARγ in maintaining redox homeostasis and chondrocyte viability.
Discussion
OA is one of the most common arthritic diseases among the elderly. According to statistics, 300 million people around the world are currently suffering from OA, and it is also currently the most common cause of disability in the elderly study4. Ferroptosis has been proven to play a crucial role in OA progression, and blocking chondrocyte ferroptosis has been shown to alleviate OA symptoms8. Traditional Chinese medicine, particularly Simiao Powder, has demonstrated anti-inflammatory and analgesic effects in OA treatment, though its precise mechanisms remain unclear. This study integrates bioinformatics, network pharmacology, molecular docking, and in vitro/in vivo experiments to explore the therapeutic effects of Simiao Powder in OA, with a focus on ferroptosis regulation. The results showed that DHT IIA, the active ingredient of Simiao Powder, could alleviate OA and realize its protective effect by activating PPARγ and inhibiting chondrocyte Ferroptosis.
TCMSP screening identified 75 active ingredients and 208 potential targets of Simiao Powder. Differential analysis in GSE98918 revealed 1,690 DEGs, and FerrDb integration identified five key genes (CDKN1A, HMOX1, DPP4, GJA1, and PPARγ) linked to OA and ferroptosis. Among them, PPARγ and HMOX1 had strong predictive value. Molecular docking showed DHT IIA had the lowest binding energy with PPARγ, suggesting its anti-ferroptosis role via PPARγ. In an HCFD-induced OA rat model, DHT IIA improved cartilage structure, reduced OARSI scores, and increased cartilage thickness. Through immunofluorescence and immunohistochemistry, we found that DHT IIA increased the expression of PPARγ in rat articular cartilage, along with an upregulation of GPX4. A reduction in GPX4 leads to the accumulation of intracellular peroxides, thereby triggering ferroptosis. Additionally, TUNEL expression was decreased after DHT IIA treatment; however, there was no statistically significant difference compared to the OA group. This indirectly suggests that both ferroptosis and apoptosis are involved in the progression of OA. DHT IIA may reduce apoptosis by alleviating OA progression, thereby lowering TUNEL expression, rather than mitigating apoptosis through the suppression of ferroptosis. In LPS-induced ATDC5 cells, DHT IIA upregulated PPARγ, downregulated ACSL4 and MMP13, and increased COL2A1, indicating its protective role in chondrocytes by inhibiting ferroptosis via PPARγ.
PPARγ is Peroxisome proliferator-activated receptor gamma, and current studies have confirmed that PPARγ can be involved in the regulation of lipid metabolism, inflammation, and oxidative stress, and affect the ferroptosis process31,32,33,34. Our study found that PPARγ expression is significantly reduced in OA cartilage compared to normal cartilage35. PPARγ plays an important role in the regulation of chondrocyte homeostasis, and its mechanism involves multiple signaling pathways. It has been suggested that PPARγ can induce chondrocyte autophagy through the Akt/mTOR pathway, thereby maintaining cell viability and delaying cartilage degeneration36. In addition, another study found that PPARγ can also play a protective role in the pathological process of OA by inhibiting the activation of the MAPK and NF-κB signaling pathways, reducing the release of inflammatory factors, inhibiting chondrocyte apoptosis, and reducing the local inflammatory response37. Notably, PPARγ has also been reported to activate the AMPK-SIRT1 pathway in human articular cartilage, which not only helps to enhance the energy metabolism and antioxidant capacity of chondrocytes, but also further attenuates inflammatory injury and maintains the functional integrity of cartilage tissue38.
According to the Cnetplot enrichment analysis, PPARγ is involved in five pathways: regulation of smooth muscle cell proliferation, smooth muscle cell proliferation, muscle cell proliferation, negative regulation of smooth muscle cell proliferation, and negative regulation of cell migration. Angiogenesis plays a key role in OA progression, promoting inflammation and osteoclast activity, leading to cartilage degeneration39,40. PPARγ can regulate vascular smooth muscle cell (VSMC) proliferation, affecting synovium and subchondral bone vascularization41,42, and by reducing VEGF expression, it decreases angiogenesis, thus alleviating synovitis and cartilage damage43,44. Additionally, abnormal proliferation of VSMCs exacerbates synovitis and promotes the release of inflammatory factors such as IL-1β and TNF-α45. PPARγ may inhibit VSMC abnormal proliferation through the PI3K/Akt and MAPK signaling pathways, alleviating synovial inflammation and delaying OA progression46. PPARγ also enhances skeletal muscle metabolism through the AMPK-SIRT1 pathway, inhibiting inflammation and oxidative stress, and maintaining joint function47. Moreover, it promotes mitochondrial biogenesis and autophagy to prevent muscle atrophy. PPARγ can also reduce the release of inflammatory factors such as IL-6 and TNF-α, reducing oxidative stress and cartilage degradation48. During OA progression, the abnormal migration of synovial cells, macrophages, and osteoclasts accelerates cartilage degradation and bone erosion49,50. PPARγ can slow cartilage degeneration and bone destruction by downregulating matrix metalloproteinases (MMP-3, MMP-13)51.
It has been shown that PPARγ inhibits ACSL4 expression and reduces lipid peroxidation, thereby preventing ferroptosis. During ferroptosis, mitochondria undergo morphological changes that are significantly different from other forms of cell death. For example, mitochondria undergo fragmentation, increased density, and rupture of the outer membrane, while a large number of mitochondrial cristae appear lost or disorganized32. Therefore, it is likely that PPARγ regulates ferroptosis in OA chondrocytes by affecting intracellular mitochondrial autophagy and thereby regulating ferroptosis. Previous studies have also found that OA can be attenuated by activating PPARγ to promote Pink1/Parkin-dependent mitochondrial autophagy, which leads to the inhibition of ferroptosis in chondrocytes32,52. These studies have demonstrated that PPARγ is likely to be an important target for OA treatment, and that it can achieve multiple protective effects on chondrocyte survival, inflammation regulation and metabolic homeostasis by regulating multiple signaling pathways.
Previous studies have found that an imbalance between innate and adaptive immune responses may also contribute to the onset and development of OA53,54. Meanwhile ferroptosis may have a close connection with immune cells55. Therefore, we analyzed immune cell infiltration in OA tissues using CIBERSORTx, and we found that activated mast cells were significantly elevated in OA synovium compared to normal tissues. Mast cells have been reported to promote cartilage degradation and inflammatory responses during the pathogenesis of OA by can be activated and release a variety of inflammatory mediators, such as histamine, chemokines and matrix metalloproteinases (MMPs), IL-1, IL-6, and TNF-α, in which histamine plays a major role. Especially during the interaction between MCs and other inflammatory cells, the mediators released from MCs activation can affect a variety of inflammatory cells, exacerbate local inflammatory responses, and further promote disease progression56,57. This study suggests that Simiao Powder may alleviate OA progression by modulating the immune microenvironment, reducing mast cell activity, and decreasing histamine release.
To the best of our knowledge, most previous studies have focused on the bioinformatics aspects of Simiao Powder in the treatment of OA, with limited in vitro and in vivo experimental validation. Meanwhile, earlier research has identified PPARγ as a potential therapeutic target in OA. Our study comprehensively integrates bioinformatics, network pharmacology, molecular docking, and both in vitro and in vivo experiments, demonstrating that Simiao Powder exerts its therapeutic effects on OA through the regulation of PPARγ. These findings provide scientific evidence supporting the application of traditional Chinese medicine in OA treatment. However, this study has several limitations. First, although we identified and validated PPARγ as a key target through bioinformatics and experimentation, we did not perform knockdown or overexpression studies in cell lines to directly confirm a causal relationship between PPARγ activity and inhibition of chondrocyte ferroptosis. Second, although DHT IIA was identified as a major active compound, potential synergistic or antagonistic effects of other components in Simiao Powder were not explored. Third, although the study used a rat model and the ATDC5 cell line to mimic the OA situation, these models may not fully reflect the complexity of human OA pathology and drug metabolism. Fourth, the exact signaling pathway downstream of PPARγ that mediates the inhibition of ferroptosis remains to be elucidated. Fifth, we lacked the ability to observe changes through direct use of ferroptosis inhibitors. Finally, traditional Chinese medicine formulations are highly individualized for drug administration, and the standardized Simiao Powder ratios sold on the market may not fully represent their clinical efficacy. Future research is necessary to fill these gaps in the real world through gene knockout or overexpression and personalized herbal models in multiple ways and dimensions.
Conclusion
This study demonstrates that DHT IIA, the active ingredient of Simiao Powder, can alleviate the progression of osteoarthritis (OA) by inhibiting chondrocyte ferroptosis through the activation of PPARγ. Additionally, Simiao Powder may further exert anti-OA effects by modulating the immune microenvironment and reducing inflammatory cell infiltration. These findings offer novel insights into traditional Chinese medicine interventions for OA and lays foundation for future clinical applications.
Data availability
The datasets used in this study are publicly accessible. The datasets included in this study can be downloaded from the GEO database (GSE98918: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE98918; GSE178557: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE178557), whose corresponding data are cited in references [18, 19]. Further inquiries can be directed to the corresponding author.
References
Lu, J. et al. Positive-Feedback regulation of subchondral H-Type vessel formation by chondrocyte promotes osteoarthritis development in mice. J. Bone Min. Res. 33 (5), 909–920. https://doi.org/10.1002/jbmr.3388 (2018).
Vina, E. R. & Kwoh, C. K. Epidemiology of osteoarthritis: literature update. Curr. Opin. Rheumatol. 30 (2), 160–167. https://doi.org/10.1097/bor.0000000000000479 (2018).
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthr. Lancet 393(10182) 1745–1759. https://doi.org/10.1016/S0140-6736(19)30417-9. (2019).
Wang, X. et al. Metformin inhibits knee osteoarthritis induced by type 2 diabetes mellitus in rats: S100A8/9 and S100A12 as players and therapeutic targets. Open. Chem. 22 (1). https://doi.org/10.1515/chem-2024-0013 (2024).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U S A. 113 (34), E4966–E4975. https://doi.org/10.1073/pnas.1603244113 (2016).
Stockwell, B. R., Jiang, X. & Gu, W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell. Biol. 30 (6), 478–490. https://doi.org/10.1016/j.tcb.2020.02.009 (2020).
Yao, X. et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Translat. 27, 33–43. https://doi.org/10.1016/j.jot.2020.09.006 (2021).
Xue, X. et al. PPARgamma activation suppresses chondrocyte ferroptosis through mitophagy in osteoarthritis. J. Orthop. Surg. Res. 18 (1), 620. https://doi.org/10.1186/s13018-023-04092-x (2023).
Zhou, X. et al. D-mannose alleviates osteoarthritis progression by inhibiting chondrocyte ferroptosis in a HIF-2α-dependent manner. Cell. Prolif. 54 (11), e13134. https://doi.org/10.1111/cpr.13134 (2021).
Liu, H. et al. Efficacy and mechanism of the Ermiao San series of formulas for rheumatoid arthritis based on chinmedomics strategy. Phytomedicine 132, 155903. https://doi.org/10.1016/j.phymed.2024.155903 (2024).
Shi, L. et al. Suppressive effect of modified Simiaowan on experimental gouty arthritis: an in vivo and in vitro study. J. Ethnopharmacol. 150 (3), 1038–1044. https://doi.org/10.1016/j.jep.2013.10.023 (2013).
Xu, H. et al. Network Pharmacology and in vivo experiments reveal the Pharmacological effects and molecular mechanisms of Simiao powder in prevention and treatment for gout. BMC Complement. Med. Ther. 22 (1), 152. https://doi.org/10.1186/s12906-022-03622-0 (2022).
Xu, Z. et al. Exploring the mechanism of action of modified Simiao powder in the treatment of osteoarthritis: an in-silico study. Front. Med. (Lausanne). 11, 1422306. https://doi.org/10.3389/fmed.2024.1422306 (2024).
Huang, Z. et al. Network Pharmacology approach to uncover the mechanism governing the effect of Simiao powder on knee osteoarthritis. Biomed. Res. Int. 2020, 6971503. https://doi.org/10.1155/2020/6971503 (2020).
Huang, Z. et al. Network Pharmacology approach to uncover the mechanism governing the effect of Simiao powder on knee osteoarthritis. Biomed. Res. Int. 2020 (1), 6971503. https://doi.org/10.1155/2020/6971503 (2020).
Ru, J. et al. TCMSP: a database of systems Pharmacology for drug discovery from herbal medicines. J. Cheminform. 6, 13. https://doi.org/10.1186/1758-2946-6-13 (2014).
Xu, X. et al. A novel chemometric method for the prediction of human oral bioavailability. Int. J. Mol. Sci. 13 (6), 6964–6982. https://doi.org/10.3390/ijms13066964 (2012).
Lucas, A. J., Sproston, J. L., Barton, P. & Riley, R. J. Estimating human ADME properties, Pharmacokinetic parameters and likely clinical dose in drug discovery. Expert Opin. Drug Discov. 14 (12), 1313–1327. https://doi.org/10.1080/17460441.2019.1660642 (2019).
Brophy, R. H. et al. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthr. Cartil. 26 (3), 422–432. https://doi.org/10.1016/j.joca.2017.12.004 (2018).
Liu, Y. et al. N(6) -methyladenosine-modified circrna RERE modulates osteoarthritis by regulating β-catenin ubiquitination and degradation. Cell. Prolif. 56 (1), e13297. https://doi.org/10.1111/cpr.13297 (2023).
Ritchie, M. E. et al. Smyth, Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47. https://doi.org/10.1093/nar/gkv007 (2015).
Chen, H. & Boutros, P. C. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 12, 35. https://doi.org/10.1186/1471-2105-12-35 (2011).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16 (5), 284–287. https://doi.org/10.1089/omi.2011.0118 (2012).
Bahn, E. & Alber, M. On the limitations of the area under the ROC curve for NTCP modelling. Radiother. Oncol. 144, 148–151. https://doi.org/10.1016/j.radonc.2019.11.018 (2020).
Dias, R., de Azevedo, W. F. & Algorithms, M. D. Curr. Drug Targets 9(12) 1040–1047. https://doi.org/10.2174/138945008786949432. (2008).
Sekar, S. et al. Saturated fatty acids induce development of both metabolic syndrome and osteoarthritis in rats. Sci. Rep. 7, 46457. https://doi.org/10.1038/srep46457 (2017).
Glasson, S. S., Chambers, M. G., Van Den Berg, W. B. & Little, C. B. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 18 Suppl. (3), S17–23. https://doi.org/10.1016/j.joca.2010.05.025 (2010).
Atsumi, T., Miwa, Y., Kimata, K. & Ikawa, Y. A chondrogenic cell line derived from a differentiating culture of AT805 teratocarcinoma cells. Cell. Differ. Dev. 30 (2), 109–116. https://doi.org/10.1016/0922-3371(90)90079-c (1990).
Osorio, D. et al. ScTenifoldKnk: an efficient virtual knockout tool for gene function predictions via single-cell gene regulatory network perturbation. Patterns (N Y). 3 (3), 100434. https://doi.org/10.1016/j.patter.2022.100434 (2022).
White, N., Parsons, R., Collins, G. & Barnett, A. Evidence of questionable research practices in clinical prediction models. BMC Med. 21 (1), 339. https://doi.org/10.1186/s12916-023-03048-6 (2023).
Dörr, D. et al. C/EBPβ regulates lipid metabolism and Pparg isoform 2 expression in alveolar macrophages. Sci. Immunol. 7 (75). https://doi.org/10.1126/sciimmunol.abj0140 (2022).
Geng, Q. et al. PPARG-mediated autophagy activation alleviates inflammation in rheumatoid arthritis. J. Autoimmun. 146, 103214. https://doi.org/10.1016/j.jaut.2024.103214 (2024).
Wang, X. et al. Propionate alleviates fatty acid-induced mitochondrial dysfunction, oxidative stress, and apoptosis by upregulating PPARG coactivator 1 alpha in hepatocytes. J. Dairy. Sci. 105 (5), 4581–4592. https://doi.org/10.3168/jds.2021-21198 (2022).
Han, L. et al. PPARG-mediated ferroptosis in dendritic cells limits antitumor immunity. Biochem. Biophys. Res. Commun. 576, 33–39. https://doi.org/10.1016/j.bbrc.2021.08.082 (2021).
Afif, H. et al. Peroxisome proliferator-activated receptor gamma1 expression is diminished in human Osteoarthritic cartilage and is downregulated by interleukin-1beta in articular chondrocytes. Arthritis Res. Ther. 9 (2), R31. https://doi.org/10.1186/ar2151 (2007).
Wang, Z. J., Zhang, H. B., Chen, C., Huang, H. & Liang, J. X. Effect of PPARG on AGEs-induced AKT/MTOR signaling-associated human chondrocytes autophagy. Cell. Biol. Int. 42 (7), 841–848. https://doi.org/10.1002/cbin.10951 (2018).
Zhang, H. B. et al. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-κB. Apoptosis 21 (10), 1082–1093. https://doi.org/10.1007/s10495-016-1280-z (2016).
Huang, H. et al. The function of PPARγ/AMPK/SIRT-1 pathway in inflammatory response of human articular chondrocytes stimulated by advanced glycation end products. Biol. Pharm. Bull. 42 (8), 1303–1309. https://doi.org/10.1248/bpb.b19-00036 (2019).
Peng, Y., Wu, S., Li, Y. & Crane, J. L. Type H blood vessels in bone modeling and remodeling. Theranostics 10 (1), 426–436. https://doi.org/10.7150/thno.34126 (2020).
Su, W. et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. 5 (8). https://doi.org/10.1172/jci.insight.135446 (2020).
Marsboom, G. & Archer, S. L. Pathways of proliferation: new targets to inhibit the growth of vascular smooth muscle cells. Circ. Res. 103 (10), 1047–1049. https://doi.org/10.1161/circresaha.108.188003 (2008).
Salazar, G. et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy 16 (6), 1092–1110. https://doi.org/10.1080/15548627.2019.1659612 (2020).
Carpi, S. et al. The Extra-Virgin Olive oil polyphenols oleocanthal and Oleacein counteract Inflammation-Related gene and MiRNA expression in adipocytes by attenuating NF-κB activation. Nutrients 11 (12). https://doi.org/10.3390/nu11122855 (2019).
Moretti, L. et al. Towards precision medicine for osteoarthritis: focus on the synovial fluid proteome. Int. J. Mol. Sci. 23 (17). https://doi.org/10.3390/ijms23179731 (2022).
Han, L. et al. Interleukin-1β-Induced senescence promotes osteoblastic transition of vascular smooth muscle cells. Kidney Blood Press. Res. 45 (2), 314–330. https://doi.org/10.1159/000504298 (2020).
Gorowska-Wojtowicz, E., Duliban, M., Kotula-Balak, M. & Bilinska, B. Modulatory effects of estradiol and its mixtures with ligands of GPER and PPAR on MAPK and PI3K/Akt signaling pathways and tumorigenic factors in mouse testis explants and mouse tumor Leydig cells. Biomedicines 10 (6). https://doi.org/10.3390/biomedicines10061390 (2022).
Chyau, C. C. et al. Antrodan alleviates High-Fat and High-Fructose Diet-Induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 21 (1). https://doi.org/10.3390/ijms21010360 (2020).
Eymard, F. et al. Contribution of adipocyte precursors in the phenotypic specificity of intra-articular adipose tissues in knee osteoarthritis patients. Arthritis Res. Ther. 21 (1), 252. https://doi.org/10.1186/s13075-019-2058-9 (2019).
Zhao, K., Ruan, J., Nie, L., Ye, X. & Li, J. Effects of synovial macrophages in osteoarthritis. Front. Immunol. 14, 1164137. https://doi.org/10.3389/fimmu.2023.1164137 (2023).
Yuan, Z. et al. Emerging roles of macrophage polarization in osteoarthritis: mechanisms and therapeutic strategies. Orthop. Surg. 16 (3), 532–550. https://doi.org/10.1111/os.13993 (2024).
Forrester, S. J. & Eguchi, S. Vascular matrix metalloproteinase inhibition, a new mechanism for how peroxisome Proliferator-Activated Receptor-γ protects target organ damage. Hypertension 67 (1), 36–37. https://doi.org/10.1161/hypertensionaha.115.06532 (2016).
Shin, H. J. et al. Pink1-Mediated chondrocytic mitophagy contributes to cartilage degeneration in osteoarthritis. J. Clin. Med. 8 (11). https://doi.org/10.3390/jcm8111849 (2019).
Nedunchezhiyan, U. et al. Obesity, inflammation, and immune system in osteoarthritis. Front. Immunol. 13, 907750. https://doi.org/10.3389/fimmu.2022.907750 (2022).
Woodell-May, J. E. & Sommerfeld, S. D. Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 38 (2), 253–257. https://doi.org/10.1002/jor.24457 (2020).
Kim, R., Taylor, D., Vonderheide, R. H. & Gabrilovich, D. I. Ferroptosis of immune cells in the tumor microenvironment. Trends Pharmacol. Sci. 44 (8), 542–552. https://doi.org/10.1016/j.tips.2023.06.005 (2023).
Hao, G. et al. Synovial mast cells and osteoarthritis: current Understandings and future perspectives. Heliyon 10 (24), e41003. https://doi.org/10.1016/j.heliyon.2024.e41003 (2024).
Zhao, X. et al. RNA-seq characterization of histamine-releasing mast cells as potential therapeutic target of osteoarthritis. Clin. Immunol. 244, 109117. https://doi.org/10.1016/j.clim.2022.109117 (2022).
Funding
This study was supported by the Chinese Medicine Research Project of the Hubei Provincial Administration of Chinese Medicine (2023–2024) (No: ZY2023F143); by Scientific Research Projects from Wuhan Municipal Health Commission. (WX23A15); by the Graduate Scientific Research Foundation of Jianghan University (No. KYCXJJ202330); by the University-Industry Collaborative Education Program (Project Nos.: 22097040141133, 231104794215900).
Author information
Authors and Affiliations
Contributions
Conceptualization: W. G. and F.Y.; Methodology: W.G., F.Y. and X.W.; Software: X.W. and F.Y.; Visualization: W.G., and X.W.; Writing –original draft preparation: W.G., and X.W.; Writing– review and editing: F.Y. and X.W.; Funding acquisition: F.Y. and X.W.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal studies were conducted in accordance with the regulations and guidelines of the Department of Medicine, Jianghan University, and approved by the Ethics Committee of the Department of Medicine, Jianghan University (No: JHDXLL2025–002).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Guan, W., Yuan, F. & Wang, X. Dehydrotanshinone II A alleviates osteoarthritis via activating PPARγ to inhibit ferroptosis in chondrocytes. Sci Rep 15, 29602 (2025). https://doi.org/10.1038/s41598-025-14896-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-14896-y