Abstract
This study examines how stress-coping phenotypes influence the effect of prenatal \(\:\varDelta\:\)9-tetrahydrocannabinol (THC) exposure (PTE) on selectively bred Dominant (Dom) and Submissive (Sub) mice offspring exhibiting stress resilience and vulnerability, respectively. Pregnant Dom and Sub dams of generation 54 received THC (20 mg/kg, intraperitoneally) on gestation days 13, 15, and 17. Our findings indicate that PTE significantly reduced body weight (measured at postnatal day (PND) 7 and 30), increased anxiety-like behaviors in two-month-old Dom offspring, and enhanced sociability and reduced anxiety-like behaviors in Sub offspring. Brain mRNA expression analysis revealed phenotype-specific alterations in endocannabinoid (ECS) and dopaminergic (DAs) system genes. Remarkably, fatty acid amide hydrolase (FAAH) expression, responsible for anandamide degradation, was downregulated in the prefrontal cortex and hippocampus of Sub PTE offspring on PND 7 and 30, alongside changes in CB1, CB2, D1, D2, and D5 receptors expression. Our findings suggest that the intergenerational effect of PTE depends on individual temperament, with ECS modulation during prenatal development offering a potential intervention for stress vulnerability. However, we state that stress resilience does not necessarily confer protection against PTE’s adverse effects, emphasizing the complexity of cannabis exposure during pregnancy.
Similar content being viewed by others
Introduction
The increasing legalization and social acceptance of cannabis use have led to its growing consumption, including among pregnant women1. This trend raises concern about the potential impacts of prenatal exposure to \(\:\varDelta\:\)9-tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis, on offspring development2. The effects of THC on the body, including during embryonic development, are mediated by the endocannabinoid system (ECS), a physiological network that plays a crucial role in regulating various biological processes. The ECS consists of CB1 and CB2 receptors along with endogenous lipid ligands, anandamide (AEA), and 2-arachidonoylglycerol (2-AG). AEA is synthesized by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), and its catabolism is primarily mediated by FAAH3.
The ECS regulates key physiological processes during gestational and early postnatal development, including embryo implantation, placentation, and uterine homeostasis4. In the developing rodent brain, ECS components are expressed as early as gestation day (GD) 115, mediating cellular communication, inflammation, and smooth muscle function. Disruption of the ECS may lead to complications, including miscarriage and preeclampsia6. Postnatally, the ECS supports essential functions such as the initiation of feeding7. Elevated 2-AG levels in maternal milk stimulate neonatal suckling via CB1R activation, which is critical for survival. Blocking CB1R in neonates disrupts feeding and growth7 and impairs maternal behavior, offspring development, and social interactions8. Moreover, the ECS regulates neuronal differentiation and synaptic plasticity, both critical during early brain development and adaptation7.
Maternal cannabis exposure has been shown to cause long-term alterations in emotional reactivity, social behavior, and behavioral flexibility in rat offspring9,10. Prenatal THC exposure (PTE) can alter the normal functioning of the ECS, potentially contributing to neurodevelopmental disorders11 and altered behavior in offspring12,13.
A prospective longitudinal study found that prenatal cannabis exposure (PCE) or PTE may predispose children to a range of behavioral and emotional disorders14. PTE can adversely affect various physiological, psychological, and neurodevelopmental processes during fetal development15. However, short- and long-term outcomes vary across studies, including inconsistent impacts on growth, cognitive development, emotional reactivity, and social interaction12,14,16,17. High heterogeneity in experimental models, human populations, and environmental factors contributes to this variability. Stress-coping ability may play a critical role in modulating THC effects. Research suggests that stress resilience influences the behavioral and physiological outcomes of PTE, with some individuals exhibiting resilience to its adverse effects18. This variability likely reflects genetic and environmental interactions with neurobiological pathways involved in stress response and emotional regulation18. Numerous studies underscore the intricate relationship between stress resilience, ECS, and the dopaminergic system (DAs)15,19,20. PTE may disrupt this relationship, leading to intergenerational changes in brain function15,20. These alterations may stem from dysregulated dopaminergic signaling and altered neuronal architecture during critical periods of development21,22.
This study investigates the long-term intergenerational PTE using an animal model of social dominance (Dom) and social submissiveness (Sub)23,24. Using both biochemical and behavioral approaches, our previous research demonstrated that Dom and Sub mice exhibit stress resilience and vulnerability phenotypes, respectively25,26,27. These mice differentially respond to prenatal stressogenic stimuli, such as chronic mild stress26 and poly(I: C)-induced maternal immune activation28as well as antidepressants24,28,29mood stabilizers24and addictive compounds30. In addition, Dom and Sub mice exhibit differences in brain neurochemical profile28 and expression of main dopaminergic receptors30.
We hypothesize that the distinct inherent stress-coping abilities of Dom and Sub mice will result in differential responses to PTE, affecting developmental and behavioral outcomes in their offspring and modulation of endocannabinoid and dopaminergic systems.
Results
Prenatal exposure to THC reduces offspring body weight in a Strain-Dependent manner
In line with our previous findings31, Dom mice exhibited significantly higher body weights than their Sub counterparts from the early stages of postnatal development (Fig. 1). The disparity in body weight between Dom and Sub offspring in the vehicle-treated groups remained consistent (Fstrain (1, 45) = 43.45, p < 0.0001) throughout the experimental period (Fig. 1A), with Sub Veh exhibit 25.06% lower body weight at postnatal day (PND) 7 (p = 0.0002), and 23.13% at PND 30 (p < 0.0001), and 15.24% at PND 180 (p = 0.0034). However, in the Dom-PTE offspring, PTE significantly reduced their body weight compared to Dom Veh offspring (Ftreatment (1, 21) = 5.325, p = 0.0313), observed as 16.92% at PND 7 (p = 0.0082) and 19.76% at PND 30 (p = 0.0005), but by PND 180, this difference was no longer apparent. (Fig. 1B). Interestingly, PTE had no significant impact on body weight in Sub offspring across the same time points. Notably, the maternal behavior (pups’ retrieval test) conducted at PND 5 and 7 didn’t show a significant change among the groups (Fig. S8). These findings suggest a strain-dependent effect of PTE on offspring body weight, with Dom mice being more vulnerable to the adverse effects of PTE during early postnatal development.
Effect of PTE on Dom and Sub offspring body weight. (A) The significant weight difference between the Dom and Sub Veh treated groups at all three time points: PND 7, 30, and 180. (B) PTE resulted in a significant reduction in body weight in Dom offspring compared to Dom Veh offspring at PND 7 and PND 30. (C) No significant effect of PTE was observed in the Sub offspring. Data are presented as means ± SEM (n = 7–10 per group). Statistical significance is indicated as *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, analyzed by two-way ANOVA with Sidak’s post hoc correction.
Influence of PTE on behavioral performance in Dom and sub offspring
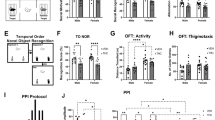
At two months of age (PND 60), the effects of PTE on the behavioral patterns of Dom and Sub offspring were evaluated. No intergenerational effects of THC were observed in the open field test (OF; Supplementary Fig. S1), elevated plus maze (EPM; Supplementary Fig. S2), hole board test (HB; Supplementary Fig. S3), or forced swim test (FST; Supplementary Fig. S4). However, the significant influence of PTE was observed in the marble burying test (MBT; Fig. 2A) and the three-chamber test (TCT; Fig. 2B, C).
MBT is a behavioral assay used to measure repetitive and anxiety-like behaviors in rodents. The number of marbles buried (at least two-thirds of their depth) is counted. A higher number of buried marbles is indicative of increased anxiety or compulsive-like behaviors, while decreased burying suggests reduced anxiety32. PTE had opposing effects on Dom and Sub offspring in this test (Ftreatment (3, 31) = 8.074, p = 0.0004). Sub-PTE offspring buried 65.39% less marbles than Sub Veh offspring (Fig. 2A, p = 0.007), suggesting that PTE reduced repetitive and anxiety-like behaviors in Sub mice. In contrast, Dom-PTE offspring had 68.33% higher buried marbles compared to Dom Veh offspring (p = 0.0469), indicating that PTE induced anxiety-like behavior in Dom offspring.
Impact of PTE on marble-burying behavior and sociability in Dom and Sub offspring. (A) MBT. One-way ANOVA with Sidak’s multiple comparisons test revealed that Sub-PTE offspring buried significantly fewer marbles compared to Sub Veh offspring, while Dom-PTE offspring buried significantly more marbles compared to Dom Veh offspring. (B-C) TCT. PTE-induced sociability in Sub offspring was reflected by increased (B) duration of time spent and (C) number of interactions (frequency) in the stranger room. Both Dom offspring groups (PTE and Veh) exhibited higher cumulative duration and frequency in the stranger room compared to the empty room. (S)—stranger, (E)—empty room. Data are presented as mean ± SEM (n = 7–10 per group). Statistical significance was determined using two-way ANOVA with Sidak’s multiple comparisons test and is indicated as *p < 0.05, **p < 0.01, and ****p < 0.0001. ns- non-significant.
Sub mice are characterized by reduced sociability, a phenotype previously demonstrated using the TCT27. Here, we found that exposure of Sub dams to THC resulted in increased sociability in their offspring, as shown in Fig. 2B, C. Thus, Sub-PTE offspring spent significantly more time near the stranger mouse (S) than with the empty room (E) and compared to Sub Veh offspring, as evidenced by both spent 28% higher (Fig. 2B, p = 0.0054) (Froom*treatment (3, 64) = 13.51, p < 0.0001, Froom (1, 64) = 171.1, p < 0.0001, Ftreatment (3, 64) = 4.415, p = 0.0069) and 93.69% higher frequency (Fig. 3C, p < 0.0001) (F (3, 64) = 15.65, p < 0.0001, Froom (1, 64) = 161.1, p < 0.0001, Ftreatment (3, 64) = 5.387, p = 0.0023) in stranger room (S) compared to Sub Veh offspring. No significant effect of PTE was observed on Dom. Thus, both Dom Veh and Dom-PTE offspring spent significantly more time near the stranger mouse than in the empty room (Fig. 2B, C).
PTE altered brain expression of key genes of ECS and dopaminergic signaling
PTE altered the HIP and PFC mRNA expression of key genes (CB1R, CB2R, FAAH, D1R, D2R, and D5R) involved in endocannabinoid and dopaminergic signaling in a phenotype-dependent manner. Thus, at PND 7, CB1R expression in the PFC was significantly downregulated in Sub-PTE offspring (Fig. 3D, t (6) = 2.618, p = 0.0397). Notably, CB2R (Fstrain*treatment (1, 14) = 3.489, p = 0.0829, Fstrain (1, 14) = 31.85, p < 0.0001, Ftreatment (1, 14) = 71.98, p < 0.0001) expression in the HIP was higher in Sub Veh compared to Dom Veh offspring (Fig. 3A, p = 0.0002) and was further reduced in prenatally THC-exposed Dom and Sub groups (Fig. 3A, p = 0.0011, p < 0.0001, respectively) and no changes in CB1R (Fig. S5A). Conversely, CB2R (Fstrain*treatment (1, 14) = 2.279, p = 0.1534, Fstrain (1, 14) = 1.218, p = 0.2884, Ftreatment (1, 14) = 15.01, p = 0.0017) was upregulated in the PFC of Sub-PTE offspring at PND 7 (Fig. 3E, p = 0.0057). Expression of FAAH enzyme, responsible for AEA degradation, was higher in Sub Veh compared to Dom Veh (Fstrain*treatment (1, 14) = 1.506, p = 0.24, Fstrain (1, 14) = 6.395, p = 0.0241, Ftreatment (1, 14) = 3.978, p = 0.066) in the HIP (Fig. 3B, p = 0.0373) and downregulated in Sub-PTE offspring in both the HIP (Fig. 3B, t(8) = 3.272, p = 0.0133) and PFC (Fig. 3F, t(6) = 2.497, p = 0.0467). For the dopaminergic receptors, D1R (Fstrain*treatment (1, 14) = 4.171, p = 0.0604, Fstrain (1, 14) = 3.724, p = 0.0742, Ftreatment (1, 14) = 3.882, p = 0.0689) and D2R (Fstrain*treatment (1, 14) = 9.746, p = 0.0075, Fstrain (1, 14) = 6.270, p = 0.0253, Ftreatment (1, 14) = 2.406, p = 0.1431) were downregulated in Dom-PTE offspring in the PFC (Fig. 3G and H, p = 0.0187, p = 0.007, respectively) at PND 7, and no changes in HIP (Fig. S5B, C), where Dom Veh expression levels were higher than Sub Veh (p = 0.0277, p = 0.0027, respectively). In contrast, D5R (Fstrain*treatment (1, 14) = 0.1769, p = 0.6805, Fstrain (1, 14) = 8.009, p = 0.0134, Ftreatment (1, 14) = 29.90, p < 0.0001) was downregulated in both Dom and Sub-PTE offspring in the HIP (Fig. 3C, p = 0.0088, p = 0.0012, respectively), while no changes in PFC (Fig. S5D). By PND 30, CB1R in the HIP was downregulated in Dom-PTE offspring relative to Dom Veh controls (Fig. 4A, t(7) = 2.772, p = 0.0276), while CB2R (Fstrain*treatment (1, 13) = 1.874, p = 0.1943, Fstrain (1, 13) = 8.761, p = 0.0111, Ftreatment (1, 13) = 6.906, p = 0.0209) was significantly lower in Sub-PTE offspring compared to Sub Veh (Fig. 4D, p = 0.0352). Sub offspring had higher CB2R levels than Dom offspring (Fig. 4D, p = 0.0293). Similar to PND 7, FAAH was downregulated in Sub-PTE offspring (Fig. 4G, t (5) = 2.853, p = 0.0357).
For the dopaminergic receptors at PND 30, D2R (Fstrain*treatment (1, 12) = 2.531, p = 0.1376, Fstrain (1, 12) = 4.830, p = 0.0483, Ftreatment (1, 12) = 38.82, p < 0.0001) expression in the HIP was downregulated in both Dom and Sub-PTE offspring upon PTE (Fig. 4C, p = 0.0118, p = 0.0003, respectively), while D1R and D5R showed no significant changes (Fig. S6A, C). In the PFC, similarly to HIP, CB1R (Fstrain*treatment (1, 15) = 1.428, p = 0.2506, Fstrain (1, 15) = 9.428, p = 0.0078, Ftreatment (1, 15) = 15.63, p = 0.0013) expression was reduced in Dom-PTE offspring (Fig. 4B, p = 0.0038), and CB2R (Fstrain*treatment (1, 15) = 6.865, p = 0.0193, Fstrain (1, 15) = 2.338, p = 0.147, Ftreatment (1, 15) = 46.09, p < 0.0001) was significantly lower in both Dom and Sub-PTE offspring (Fig. 4E, p = 0.0165, p < 0.0001, respectively). As in HIP, FAAH expression in the PFC was downregulated in Sub-PTE offspring (Fig. 4H, t (7) = 3.508, p = 0.0099). D2R (Fstrain*treatment (1, 15) = 24.82, p = 0.0002, Fstrain (1, 15) = 14.45, p = 0.0017, Ftreatment (1, 15) = 3.489, p = 0.0814) expression was reduced only in Sub-PTE offspring (Fig. 4F, p = 0.0006), while D5R (Fstrain*treatment (1, 15) = 7.334, p = 0.0162, Fstrain (1, 15) = 0.7168, p = 0.4105, Ftreatment (1, 15) = 89.81, p < 0.0001) was significantly downregulated in both Dom and Sub-PTE offspring (Fig. 4I, p = 0.0004, p < 0.0001, respectively) and no changes in D1R (Fig. S6B).
mRNA expression of ECS and dopaminergic-related genes in HIP and PFC of PTE offspring at PND 7. (A) CB2R mRNA expression in the HIP was significantly higher in Sub Veh compared to Dom Veh, but downregulated in both Dom-PTE and Sub-PTE offspring relative to their respective controls. (B) FAAH expression in the HIP was elevated in Sub Veh compared to Dom Veh and significantly reduced in Sub-PTE compared to Sub Veh. (C) D5R mRNA expression in HIP was downregulated in Dom-PTE and Sub-PTE offspring compared to Dom Veh and Sub Veh offspring, respectively. (D) In the PFC, CB1R expression was downregulated in Sub-PTE compared to Sub Veh. (E) CB2R expression in the PFC was significantly upregulated in Sub-PTE compared to Sub Veh. (F) FAAH expression in the PFC was downregulated in Sub-PTE compared to Sub Veh. D1R (G) and D2R (H) in PFC are higher in the Dom strain (Veh) compared to Sub and downregulated in Dom-PTE compared to Dom Veh offspring, respectively. Data are presented as mean ± SEM (n = 4–5/group). Statistical significance was determined using two-way ANOVA with Sidak’s correction and is denoted as * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Significant differences identified via student’s t-test are denoted by p-value as # at p < 0.05.
mRNA expression of ECS and dopaminergic-related genes in HIP and PFC of offspring exposed to prenatal THC at PND 30. (A) CB1R in HIP was significantly downregulated in Dom-PTE compared to Dom Veh. (B) CB1R in PFC is lower in Sub Veh than in Dom Veh and downregulated in Dom-PTE compared to Dom Veh. (C) D2R in HIP was downregulated in both Dom and Sub-PTE offspring compared to Dom and Sub Veh, respectively. (D) CB2R in HIP is lower in Dom Veh compared to Sub and downregulated in THC Sub compared to Sub Veh offspring. (E) CB2R in PFC is downregulated in both Dom-PTE and Sub-PTE offspring compared to Dom Veh and Sub Veh offspring, respectively. (F) D2R in PFC was downregulated in Sub-PTE offspring compared to Sub Veh. (G) FAAH gene in HIP was downregulated in Sub-PTE compared to Sub Veh. (H) FAAH is downregulated in the PFC of Sub-PTE compared to Sub Veh. (I) D5R in PFC has been downregulated in both Dom and Sub-PTE offspring compared to Dom Veh and Sub Veh offspring, respectively. Data represent means (± SEM); n = 3–5/group. Statistical significance is presented as * at p < 0.05, ** at p < 0.01, *** at p < 0.001, and **** at p < 0.0001, performed by two-way ANOVA (Sidak correction); # represents significance in a student’s t-test with p-value as # at p < 0.05, ## at p < 0.01.
Discussion
The rising demand for cannabis over the past two decades, along with its legalization in numerous countries, has raised public health concerns33. Evidence suggests that frequent cannabis use may contribute to mental health disorders, cognitive impairments, and higher substance dependence risk15. Furthermore, prenatal exposure to cannabinoids, particularly THC, may potentially lead to long-term effects on neurodevelopment and behavior15. Given the high heterogeneity within the human population, the effects of cannabis can vary significantly among individuals. Among environmental factors influencing THC’s effect, stress plays an important role. This study explored the interplay between prenatal THC exposure (PTE) and stress vulnerability, using two distinct stress phenotype models, Dom and Sub mice, to elucidate how stress-coping mechanisms influence PTE’s developmental and behavioral outcomes. To our knowledge, this is the first study exploring how inherited stress resilience and vulnerability affect responses to PTE.
Dom and Sub mice were developed through a selective breeding and social interaction-based food competition paradigm23. These models exhibit distinct behavioral25, physiological34, and metabolic31 profiles. Sub mice, with heightened stress sensitivity, are more prone to addictive behaviors, while Dom mice demonstrate greater stress resilience24,25,26,30. These phenotypes also respond differentially to stressogenic triggers, antidepressants, and mood stabilizers, reflecting distinct synaptic plasticity and aging-related cognitive decline35,36.
Dom and Sub dams were administered THC (20 mg/kg, i.p) on GD 13, 15, and 17, key stages for neurodevelopmental processes and ECS maturation19.
The results of this study indicate that PTE effects on offspring body weight were dependent on the animal’s behavioral phenotype. Specifically, Dom’s offspring exhibited significant weight reduction, suggesting impaired early growth. Interestingly, body weight reductions in Dom offspring diminished with aging, consistent with studies indicating PTE long-term effects on body weight37,38. At PND 30, CB1R mRNA expression in the HIP and PFC was downregulated in Dom-PTE offspring, potentially contributing to reduced neonatal growth via impaired milk-sucking behavior. ECS is critical for initiating feeding behaviors in neonates, with elevated 2-AG levels in maternal milk activating CB1 receptors to promote suckling. Disruption of CB1R signaling impairs feeding, growth, maternal behavior, and social interactions7,8.
In contrast, Sub offspring exhibited no changes in body weight at PND 7 and 30, suggesting resilience to PTE’s physiological impacts. Research suggests that a stress-coping strategy influences neurobiological responses to stress and environmental challenges39. Sub offspring, employing passive coping strategies such as disengagement or avoidance, may be buffered against the harmful physiological consequences of prenatal stressors like THC39. Importantly, consistent with stable body weight, CB1R mRNA expression remained unchanged in the HIP and PFC of Sub-PTE offspring after weaning. This lack of alteration in CB1R expression may explain the absence of birth weight changes in Sub-PTE offspring, as CB1R signaling influences milk-sucking behavior. This finding further aligns with Dom-PTE offspring, where CB1R downregulation is associated with reduced birth weight.
In the subsequent phase of this study, we assessed the impact of PTE on the behavioral performances of Dom and Sub offspring. Maternal administration of THC has been associated with subtle behavioral effects in offspring, including reduced activity across several behavioral domains. Specifically, cognitive behavior is moderately affected, while locomotor activity and emotional behavior show slight impairments following prenatal cannabis exposure16,40,41.
It is worth mentioning that even a single neonatal THC administration can elicit long-term effects, including impaired cognitive function in adulthood42. We found that PTE differentially influenced behavioral traits such as repetitive, anxiety-like, and social behaviors in Dom and Sub offspring. Thus, Dom-PTE offspring exhibited induced repetitive and anxiety-like behaviors, whereas Sub-PTE offspring demonstrated reduced anxiety-like behaviors and enhanced sociability.
We assume that these differences may arise from distinct ECS responses to PTE. Remarkably, a significant reduction in FAAH gene expression was observed only in Sub-PTE offspring in both the HIP and PFC compared to Sub Veh offspring. Notably, this effect persisted at PND 7 and PND 30 FAAH, a critical enzyme for AEA catabolism, is integral to endocannabinoid signaling. Many studies link FAAH and AEA levels to behavioral performance43,44,45. Thus, FAAH inhibition or genetic downregulation has been shown to improve social behavior43,44, while elevated AEA levels are associated with anxiolytic effects and improved reward processing, both essential for promoting social interaction45. Therefore, our findings further suggest that in submissive individuals, PTE during critical development stages may modulate neurochemical architecture and ECS signaling, leading to FAAH reduction, increased AEA levels, and, consequently, decreased anxiety and enhanced sociability. These results imply that PTE produces divergent behavioral effects in stress-resilient and stress-vulnerable individuals due to different modulations of endocannabinoid signaling.
Our hypothesis is supported by numerous studies highlighting the substantial role of the ECS in the mediation of stress-coping responses46,47,48,49,50,51,52,53,54. For example, Hohmann et al. demonstrated that ECS activation contributes to stress-induced analgesia55. Furthermore, several research groups have suggested that ECS activation enhances the stress-coping abilities of experimental animals56,57. Moreover, AEA modulates acute stress responses in the amygdala, a brain region critical for stress regulation47,51,58. Interestingly, stressful events, such as fear conditioning, elevate AEA levels in the amygdala51, while FAAH inhibition enhances stress-coping abilities and produces anxiolytic and antidepressant-like effects49,58,59.
Interestingly, at PND 7, no differences were observed in CB1R expression in HIP between Dom and Sub mice or following prenatal THC treatment. However, CB2R expression in HIP was significantly higher in Sub mice compared to Dom mice and was reduced following PTE in both experimental groups. This trend was also observed at PND 30. We may suggest that elevated CB2R levels in Sub offspring may result from the low availability of endogenous endocannabinoid ligands and, consequently, hypoactivation of ECS, although this phenomenon should be further evaluated in future studies using biochemical approaches. It is plausible that PTE partially compensates for this deficit, leading to at least temporary restoration of ECS activity in Sub mice during critical gestational stages. It is important to underline that CB2R expression is important for proper dopaminergic neurotransmission60. In line with this statement, we found that changes in the CB2R were associated with the downregulation of main dopaminergic receptors, specifically D2R and D5R. Previous studies demonstrated that hyperactivation of the ECS through exposure to THC presumably attenuates dopaminergic neurotransmission, which is critical for proper brain development during the late prenatal stages60,61. We assume that the hippocampal downregulation of CB2R in both Dom and Sub offspring results from compensatory regulation to counteract the reduction in dopaminergic neuron firing. The study has shown that CB2R agonists significantly reduce neuronal firing in the ventral tegmental area (VTA), where dopamine is projected. This effect was blocked by the coadministration of CB2R antagonists and was not observed in CB2 knockout mice62.
Given the high density of D2R and CB2R in developmental neurons60, D2R activation may buffer the effects of prenatal Dom-PTE exposure by reducing CB2R-mediated signaling during critical periods of neurodevelopment.
Interestingly, a reduction in D2R-mediated inhibition has been linked to increased reward-seeking behavior and reduced risk aversion63,64,65. Studies have shown that dopaminergic modulation affects marble-burying behavior , a measure of anxiety-like behavior. For example, D2R antagonists have been found to reduce marble burying, potentially lowering anxiety levels66,67while D2R agonists, such as pramipexole, increase marble burying and can lead to compulsive behaviors68. In alignment with this data, in this study, we showed that PTE led to increased marble burying in Sub offspring, suggesting positive ECS-mediated modulation of the DAs. In contrast, PTE-impaired marble burying in Dom offspring, hinting at a negative influence. Thus, these results suggest that the impact of PTE depends on individual temperament and stress-coping abilities, potentially leading to changes in brain neurochemistry, particularly the DAs.
Therefore, activation of ECS may modulate the dopaminergic pathway in Sub offspring exposed to PTE, as evidenced by altered mRNA expression levels of dopamine-related genes and behavioral outcomes. Strong support for this hypothesis stems from the well-established recognition that deficient endocannabinoid signaling contributes to neuropsychiatric and metabolic disorders69. The ECS is essential for maintaining homeostasis by regulating physiological and behavioral processes, including mood, stress responses, energy balance, appetite, pain perception, and immune function70. Reduced availability of AEA and 2-AG disrupts these systems, potentially exacerbating neurodevelopmental vulnerabilities71. Many pathologies associated with the deficiency of ECS are seen and comprehensively documented in Sub mice, including altered immune profiles28, growth restriction34,72, metabolic abnormalities31,73, depression, anxiety-related behavioral changes24,27, and reduced stress-coping abilities26,27. Our findings align with these observations, suggesting that PTE may transiently restore ECS functionality in Sub offspring, mitigating neurochemical and behavioral deficits associated with endocannabinoid signaling deficiencies.
Although transcriptional changes in ECS and dopaminergic markers observed in this study align with behavioral phenotypes and are supported by prior studies linking similar gene expression changes to functional outcomes74,75further studies should be performed to confirm functional alterations in receptor activity, endocannabinoid tone, or downstream signaling dynamics.
In conclusion, this work demonstrates that enhancing ECS activity during early prenatal development may offer therapeutic potential for alleviating stress-induced behavioral abnormalities in stress-vulnerable individuals. On the other hand, stress resistance, whether genetic or environmental, does not necessarily protect against the adverse effects of PTE. These findings emphasize the importance of a deeper understanding of the ECS and its influence on neurodevelopment and behavioral responses to stressogenic environments.
Methodology
Animals
In this study, mice with strong features of dominance and submissiveness were used. These distinct populations of animals were produced using outbred Sabra strain76 (Envigo, Ness Ziona, Israel) based on a selective breeding approach and food competition Dominance-Submissiveness Relationship (DSR) behavioral paradigm23which assesses social interactions within fixed pairs of mice23,24,27. In this test, more than 99% of selectively bred Dom and Sub mice consistently develop robust and stable dominant or submissive relationships. To ensure phenotypic consistency, all breeding animals were assessed using the DSR test (Fig. S7) before mating, confirming the persistence of temperament-related traits in the current cohort. Generation 54 was used in this study (in-house breeding).
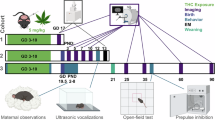
After weaning, mice were housed in a colony room (12:12 L: D cycle with lights on 07:00–19:00, 25 ± 2 °C, 55 ± 5% humidity) in groups of five per cage and provided with standard laboratory chow and water, ad libitum. At delivery, litter size was culled to eight pups per cage. For the maternal attachment experiments and mRNA expression analysis were conducted at postnatal day (PND) 7, pups were randomly selected from different litters based on their body weight and maternal treatment group. Pups were weaned from dams at PND 24 and subsequently housed together according to treatment type and sex. We made efforts to minimize litter-related confounding by randomizing pups based on treatment group, sex, and body weight. Behavioral and mRNA-based experiments in adult mice were conducted on separate cohorts of animals. Mice were sacrificed by asphyxiation with CO2 in a 3 × 8 cm plastic restrainer. This method was selected for its rapidity, reproducibility, and minimal impact on stress77. All euthanasia procedures were performed at the same time of day under identical conditions to ensure consistency across experimental groups. The study design is illustrated in Fig. 5.
The experiments were conducted by NIH/USDA guidelines under the approval of the Ariel University Institutional Animal Care and Use Committee (permission numbers: AU-IL-2112-105-3 and AU-IL-2112-108-3) in accordance with ARRIVE guidelines.
A timeline of the study design. 20 mg/kg THC was administered (i.p) to pregnant mice at GD 13, 15, and 17. Pup retrieval was conducted at PND 5 and 7. The tissues of interest from the dams and offspring were extracted on PND 7 and 30 for molecular and physiological analyses. Behavioral experiments were conducted between PND 60 and 75.
Pharmacological materials
∆9-THC Preparation
To produce ∆9-THC from cannabidiol (CBD), 3.14 g of CBD was dissolved in 100 ml dry dichloromethane, where 400 mg pre-heated MgSO4 was dispersed, 25 µl of BF3 diethyl etherate was added, and the reaction was mixed at −15 °C for 1.5 h in an N2 atmosphere78. Then, the reaction was quenched with 20 ml ice-cold NaHCO3 saturated solution, separated phases, and the aqueous phase was extracted with two portions of 30 ml dichloromethane (DCM). The combined organic phase was washed to neutral with a saturated NaCl solution, dried over MgSO4, and evaporated. The yield of ∆9-THC was 82.3%, and the rest of the material remained unreacted CBD. The purity of THC used for treatment was > 97%, as confirmed using LC-MS.
THC administration
To test the intergenerational effects of prenatal THC upon Dom and Sub mice, two-month-old females were bred with males of their corresponding behavioral phenotype. The day on which the vaginal plug appeared was considered GD 1. On GD 10, females were single-housed and randomly assigned to a treatment group. Dom or Sub dams were intraperitoneal (i.p) injected with 200 µl ethanotl:Tween-80:saline (1:1:18) solution (n = 8 each) or 20 mg/kg synthetic THC (n = 8 each) on GD 13, 15, and 17 between 09:00 and 12:00 h.
The dose was selected based on prior preclinical studies demonstrating that high-dose THC elicits robust behavioral effects on both Dom and Sub models29. Given our focus on persistent neurodevelopmental alterations, we administered THC on GD 13, 15, and 17, a critical window for fetal brain development marked by rapid synaptogenesis and ECS maturation79,80.
Pup retrieval test
In rodents, pup retrieval provides insight into parental care behavior. The experiments were conducted on PND 3, 5, and 7. The original cage was transferred to the testing room for habituation one hour before the experiment. The pups were moved into a clean, preheated 35 °C cage with new bedding while the dam remained in her original cage.
Each time, one pup was placed in the farthest corner relative to the nest of the original cage. Dam retrieval behavior was scored manually from the moment the dam picked up the pup until she placed it in the nest81. The maximum time for a trial is 90 s. Pups not retrieved within 90 s were placed back into the original nest.
Behavioral experiments
Behavioral experiments were conducted between PND 60–75 (Fig. 6). The experiments were performed between 8 AM and 3 PM. For habituation, the animals were placed in the experimental room one hour before the behavioral tests. All tests were conducted under minimal lighting to minimize stress levels. The tests were executed in the following order: marble burying test (MBT), open field (OF), elevated plus maze (EPM), hole board (HB), three-chamber test (TCT), and forced swim test (FST).
The behavioral battery test commenced at postnatal day (PND) 60, beginning with the MBT. On PND 61, offspring were sequentially tested in the OF, EPM, and HB tests. Following a four-day washout period, the TCT was conducted on PND 66. After an additional seven-day washout, the FST was performed on PND 74.
Marble burying test (MBT)
MBT measures repetitive and anxiety-like behavior in rodents82. The test was performed in polycarbonate mice cages (36 × 20 × 14 cm). Fresh, unscented mouse bedding material was placed to a depth of 5 cm and briefly flattened uniformly. At the beginning of each session, 15 standard black glass toy marbles (5.2 g) were placed gently on the surface of the arena, 3 marbles in each row. The mouse was placed carefully into the middle of the arena containing marbles, and a filter top was placed over the cage in the opposite orientation. After 30 min, the mouse was returned to the home cage. A marble is considered buried if two-thirds (2/3) of its surface area is covered by bedding. The bedding was discarded at the end of each session, and all marbles were retrieved. The arena and marbles were cleaned with mild, unscented laboratory detergent and rinsed 3x with distilled water between trials.
Open field (OF) test
The OF test was used to estimate spontaneous locomotor (horizontal) and exploratory (vertical) activity. Light intensity was adjusted to ~ 7 lx to avoid hyperlocomotion artifact. Each mouse was placed individually in the center of the apparatus (40 × 40 cm black plastic chamber). The total distance traveled, velocity, move duration, mobility, and rearing frequency were calculated for 5 min using a digital camera operated by the EthoVision video tracking system (EthoVision 11, Noldus Information Technology, the Netherlands). Between trials, the arena is cleaned with 70% ethanol.
Elevated plus maze (EPM)
EPM is a widely used test for measuring anxiety-like behavior and assessing emotional behavior in rodents by measuring general exploratory performance and avoidance of the aversive open arms of the maze83. Arranged in a “+” shape, the EPM apparatus (54 cm in height and 66 cm in length) has two closed and two open arms, as well as an open center. A mouse was placed in the center area of the maze with its head directed toward a closed arm. Animals’ behavior was recorded by tracking software (EthoVision 11.5) for 5 min. The locomotor activity (distance traveled and speed) and exploratory behavior (the number of entries and the time spent in each arm) were also recorded and analyzed. Anxiety-like behavior was determined by open/closed arm time and entry ratio.
Hole board test (HBT)
HBT is used to assess anxiety-like and exploratory behavior in rodents84. The apparatus consists of a square-shaped, clear polycarbonate box (40 cm x 40 cm x 35 cm) with an inserted elevated black polycarbonate floor arena (15 cm above box bottom) with 16 equally spaced holes (3 cm diameter). The mouse was placed in the center of the hole board apparatus for 5 min. All sessions were video recorded, and the frequency of spontaneously elicited hole-poking behavior (number of head dips) was manually counted. The head-dipping behavior indicates the mice’s exploratory activity84. Decreased head-dipping behavior is assumed to be a low exploratory activity, while increased head-dipping is interpreted as a high level of exploration.
Three chamber test (TCT)
The TCT test is used to evaluate the social behavior of rodents. The apparatus consists of three chambers. In the first 5 min of the test, the mouse was placed for habituation. In the second part, a stranger mouse was added and placed in a cylinder inside a random lateral room for 10 minutes85. An identical cylinder will be placed in the opposing room. The mouse movements will be video captured by a camera connected to computer software (EthoVision 11.5 Noldus). Assess the sociability by measuring the time spent in the stranger and empty room/zone, and the number of entrances to the stranger and empty room/zone.
Forced swimming test (FST)
FST is a widely used test for screening potential antidepressants as well as characterizing depression-like behavior86. Mice were placed individually into an inescapable transparent glass cylinder (30 cm in height, 10 cm in diameter) filled 25 cm high with water (25± 2 °C). All animals were recorded and forced to swim for 6 min, during which immobility (floating in the water with only minor movements to keep afloat) was counted. Animals that failed to stay afloat were removed immediately.
Molecular studies
Brain regions (hippocampus and prefrontal cortex) were extracted at PND 7 and 30 (as shown in Fig. 5) and stored at −80 °C until use. Total RNA was extracted using the RNeasy Micro Kit (Qiagen Cat. No./ID: 74104), and its concentration and purity were assessed using a NanoDrop spectrophotometer. Reverse transcription was performed using the GoScript™ Reverse Transcription System (Promega, A3801, Madison, WI) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was carried out using the SYBR™ Green PCR Master Mix (Applied Biosystems, Cat. No. 4309155) on a QuantStudio Real-Time PCR System. Primer efficiencies were determined using a five-point serial dilution of pooled cDNA samples to generate standard curves. Only primers with amplification efficiencies between 90 and 110% and correlation coefficients (R²) above 0.99 were used for gene expression analysis. Melt curve analyses were conducted for all reactions to confirm amplification specificity and the absence of primer-dimer artifacts. Primer sequences are provided in Table S1.
Statistical analysis
The comparative analysis between Dom and Sub mice were performed by two-tailed Student’s t-test. For multiple groups, one- or two-way ANOVA was employed for comparisons, such as time, type, and treatment, followed by either Tukey multiple comparisons (to evaluate the comparison between all groups) or Sidak (for comparison between fixed pairs within groups) means separation test. Data represented as mean ± SEM. ANOVA statistical significance of the difference between groups is presented as (*) for p < 0.05, (**) for p < 0.01, (***) for p < 0.001, and (****) for p < 0.0001. Student’s t-test-based significance of the difference between groups is presented as (#) for p < 0.05, (##) for p < 0.01, (###) for p < 0.001, and (####) for p < 0.0001.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Brian, H. U. N. O. D. C. & World Drug Report 2022 Highlights Trends on Cannabis Post-Legalization, Environmental Impacts of Illicit Drugs, and Drug Use among Women and Youth. (2022). https://www.unodc.org/unodc/en/press/releases/2022/June/unodc-world-drug-report-2022-highlights-trends-on-cannabis-post-legalization--environmental-impacts-of-illicit-drugs--and-drug-use-among-women-and-youth.html
Sujan, A. C., Pal, A., Avalos, L. A. & Young-Wolff, K. C. A systematic review of in utero cannabis exposure and risk for structural birth defects. Front. Pediatr. 11, 1149401 (2023).
Cravatt, B. F. et al. Supersensitivity to Anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. 98, 9371–9376 (2001).
Kozakiewicz, M. L., Grotegut, C. A. & Howlett, A. C. Endocannabinoid system in pregnancy maintenance and labor: A Mini-Review. Front. Endocrinol. 12, 699951 (2021).
Buckley, N. E., Hansson, S., Harta, G. & Mezey, É. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience 82, 1131–1149 (1997).
Feduniw, S. et al. Placental cannabinoid receptor expression in preterm birth. J. Pregnancy. 2024, 1–10 (2024).
Fride, E. The endocannabinoid-CB receptor system: importance for development and in pediatric disease. Neuro Endocrinol. Lett. 25, 24–30 (2004).
Schechter, M., Pinhasov, A., Weller, A. & Fride, E. Blocking the postpartum mouse dam’s CB1 receptors impairs maternal behavior as well as offspring development and their adult social–emotional behavior. Behav. Brain Res. 226, 481–492 (2012).
Penman, S. L. et al. Vaporized ∆9-tetrahydrocannabinol exposure in utero has negative effects on attention in a dose- and sex-dependent manner. Pharmacol. Biochem. Behav. 242, 173808 (2024).
Roeder, N. M. et al. Vaporized ∆9-THC in utero results in reduced birthweight, increased locomotion, and altered wake-cycle activity dependent on dose, sex, and diet in the offspring. Life Sci. 340, 122447 (2024).
Aguado, T. et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 26, 1551–1561 (2006).
Richardson, K. A., Hester, A. K. & McLemore, G. L. Prenatal cannabis exposure - The first hit to the endocannabinoid system. Neurotoxicol Teratol. 58, 5–14 (2016).
Spano, M. S., Ellgren, M., Wang, X. & Hurd, Y. L. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol. Psychiatry. 61, 554–563 (2007).
El Marroun, H. et al. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Int. J. Epidemiol. 48, 287–296 (2019).
Nashed, M. G., Hardy, D. B. & Laviolette, S. R. Prenatal cannabinoid exposure: emerging evidence of physiological and neuropsychiatric abnormalities. Front. Psychiatry. 11, 624275 (2021).
Kong, K. L. et al. Prenatal tobacco and cannabis co-exposure and offspring obesity development from birth to mid-childhood. Pediatr. Obes. 18, e13010 (2023).
Shisler, S. et al. Prenatal tobacco and tobacco-cannabis co-exposure: relationship with attention and memory in middle childhood. Neurotoxicol Teratol. 104, 107371 (2024).
Hurd, Y. L. et al. Cannabis and the developing brain: insights into its Long-Lasting effects. J. Neurosci. Off J. Soc. Neurosci. 39, 8250–8258 (2019).
Wu, C. S., Jew, C. P. & Lu, H. C. Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 6, 459–480 (2011).
Rodrigues, R. J., Marques, J. M. & Köfalvi, A. Cannabis, endocannabinoids and brain development: from embryogenesis to adolescence. Cells 13, 1875 (2024).
Sarikahya, M. H. et al. Prenatal THC Exposure Induces Sex-Dependent Neuropsychiatric Endophenotypes in Offspring and Long-Term Disruptions in Fatty-Acid Signaling Pathways Directly in the Mesolimbic Circuitry. eneuro 9, ENEURO.0253-22.2022 (2022).
DiNieri, J. A. et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry. 70, 763–769 (2011).
Feder, Y. et al. Selective breeding for dominant and submissive behavior in Sabra mice. J. Affect. Disord. 126, 214–222 (2010).
Nesher, E. et al. Differential responses to distinct psychotropic agents of selectively bred dominant and submissive animals. Behav. Brain Res. 236, 225–235 (2013).
Gross, M., Romi, H., Miller, A. & Pinhasov, A. Social dominance predicts hippocampal glucocorticoid receptor recruitment and resilience to prenatal adversity. Sci. Rep. 8, 9595 (2018).
Gross, M. & Pinhasov, A. Chronic mild stress in submissive mice: marked polydipsia and social avoidance without hedonic deficit in the sucrose preference test. Behav. Brain Res. 298, 25–34 (2016).
Pinhasov, A. et al. Development of a Selectively-Bred mouse model of dominance and submissiveness: technical considerations. In Psychiatric Vulnerability, Mood, and Anxiety Disorders Vol. 190 (eds Harro, J. et al.) 353–377 (Springer US, 2023).
Murlanova, K. et al. Double trouble: prenatal immune activation in stress sensitive offspring. Brain Behav. Immun. 99, 3–8 (2022).
Kardash, T. et al. Link between personality and response to THC exposure. Behav. Brain Res. 379, 112361 (2020).
Yanovich, C., Kirby, M. L., Michaelevski, I., Yadid, G. & Pinhasov, A. Social rank-associated stress vulnerability predisposes individuals to cocaine attraction. Sci. Rep. 8, 1759 (2018).
Agranyoni, O. et al. Gut microbiota determines the social behavior of mice and induces metabolic and inflammatory changes in their adipose tissue. Npj Biofilms Microbiomes. 7, 28 (2021).
Sur, D. et al. Nurture outpaces nature: fostering with an attentive mother alters social dominance in a mouse model of stress sensitivity. Mol. Psychiatry. 28, 3816–3828 (2023).
Farrelly, K. N. et al. The impact of recreational cannabis legalization on cannabis use and associated outcomes: A systematic review. Subst. Abuse Res. Treat. 17, 11782218231172054 (2023).
Bairachnaya, M., Agranyoni, O., Antoch, M., Michaelevski, I. & Pinhasov, A. Innate sensitivity to stress facilitates inflammation, alters metabolism and shortens lifespan in a mouse model of social hierarchy. Aging 11, 9901–9911 (2019).
Gross, M. et al. Early onset of cognitive impairment is associated with altered synaptic plasticity and enhanced hippocampal GluA1 expression in a mouse model of depression. Neurobiol. Aging. 36, 1938–1952 (2015).
Malatynska, E., Pinhasov, A., Crooke, J. J., Smith-Swintosky, V. L. & Brenneman, D. E. Reduction of dominant or submissive behaviors as models for antimanic or antidepressant drug testing: technical considerations. J. Neurosci. Methods. 165, 175–182 (2007).
Natale, B. V. et al. ∆9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci. Rep. 10, 544 (2020).
Breit, K. R., Rodriguez, C. G., Lei, A. & Thomas, J. D. Combined vapor exposure to THC and alcohol in pregnant rats: maternal outcomes and Pharmacokinetic effects. Neurotoxicol Teratol. 82, 106930 (2020).
Boersma, G. J., Moghadam, A. A., Cordner, Z. A. & Tamashiro, K. L. Prenatal stress and stress coping style interact to predict metabolic risk in male rats. Endocrinology 155, 1302–1312 (2014).
Ramírez, S. et al. Behavioral effects on the offspring of rodent mothers exposed to tetrahydrocannabinol (THC): A meta-analysis. Front. Psychol. 13, 934600 (2022).
Fearby, N., Penman, S. & Thanos, P. Effects of ∆9-Tetrahydrocannibinol (THC) on obesity at different stages of life: A literature review. Int. J. Environ. Res. Public. Health. 19, 3174 (2022).
Philippot, G., Nyberg, F., Gordh, T., Fredriksson, A. & Viberg, H. Short-term exposure and long-term consequences of neonatal exposure to ∆9-tetrahydrocannabinol (THC) and ibuprofen in mice. Behav. Brain Res. 307, 137–144 (2016).
Wei, D., Allsop, S., Tye, K. & Piomelli, D. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 40, 385–396 (2017).
Wei, D. et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc. Natl. Acad. Sci. 112, 14084–14089 (2015).
Gunduz-Cinar, O., Hill, M. N., McEwen, B. S. & Holmes, A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol. Sci. 34, 637–644 (2013).
Bortolato, M. et al. Anxiolytic-like properties of the Anandamide transport inhibitor AM404. Neuropsychopharmacol. Off Publ Am. Coll. Neuropsychopharmacol. 31, 2652–2659 (2006).
Gaetani, S., Cuomo, V. & Piomelli, D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol. Med. 9, 474–478 (2003).
Hill, M. N. et al. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J. Neurosci. Off J. Soc. Neurosci. 30, 14980–14986 (2010).
Kathuria, S. et al. Modulation of anxiety through Blockade of Anandamide hydrolysis. Nat. Med. 9, 76–81 (2003).
Lutz, B. Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 9, 46–52 (2009).
Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 (2002).
Patel, S. & Hillard, C. J. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304–311 (2006).
Patel, S. & Hillard, C. J. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur. J. Neurosci. 27, 2821–2829 (2008).
Steiner, M. A. et al. Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology 33, 54–67 (2008).
Hohmann, A. G. et al. An endocannabinoid mechanism for stress-induced analgesia. Nature 435, 1108–1112 (2005).
Bortolato, M. et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol. Psychiatry. 62, 1103–1110 (2007).
Gobbi, G. et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by Blockade of Anandamide hydrolysis. Proc. Natl. Acad. Sci. U S A. 102, 18620–18625 (2005).
Gunduz-Cinar, O. et al. Convergent translational evidence of a role for Anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry. 18, 813–823 (2013).
Hill, M. N., Froese, L. M., Morrish, A. C., Sun, J. C. & Floresco, S. B. Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacol. (Berl). 187, 245–259 (2006).
López-Ramírez, G. et al. D2 autoreceptor switches CB2 receptor effects on [3 H]‐dopamine release in the striatum. Synapse 74, e22139 (2020).
Zhang, H. Y. et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. 111, (2014).
Ma, Z. et al. Mechanisms of cannabinoid CB2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine 42, 225–237 (2019).
De Jong, J. W. et al. Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation. Neuropsychopharmacology 40, 2085–2095 (2015).
Blum, K., Thanos, P. K. & Gold, M. S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 5, 919 (2014).
Zalocusky, K. A. et al. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646 (2016).
Bruins Slot, L. A., Bardin, L., Auclair, A. L., Depoortere, R. & Newman-Tancredi, A. Effects of antipsychotics and reference monoaminergic ligands on marble burying behavior in mice. Behav. Pharmacol. 19, 145–152 (2008).
Graybiel, A. M. & Rauch, S. L. Toward a neurobiology of Obsessive-Compulsive disorder. Neuron 28, 343–347 (2000).
Jimenez-Gomez, C., Osentoski, A. & Woods, J. H. Pharmacological evaluation of the adequacy of marble burying as an animal model of compulsion and/or anxiety. Behav. Pharmacol. 22, 711–713 (2011).
Lu, H. C. & Mackie, K. Review of the endocannabinoid system. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 6, 607–615 (2021).
Jung, K. M. & Piomelli, D. Lipids | The Endocannabinoid System. in Encyclopedia of Biological Chemistry III 776–784 (Elsevier, 2021). https://doi.org/10.1016/B978-0-12-809633-8.21365-4
Kasatkina, L. A., Rittchen, S. & Sturm, E. M. Neuroprotective and Immunomodulatory action of the endocannabinoid system under neuroinflammation. Int. J. Mol. Sci. 22, 5431 (2021).
Ben-Shachar, M. et al. Inherited stress resiliency prevents the development of metabolic alterations in diet‐induced obese mice. Obesity 31, 2043–2056 (2023).
Agranyoni, O. et al. Colon impairments and inflammation driven by an altered gut microbiota leads to social behavior deficits rescued by hyaluronic acid and celecoxib. BMC Med. 22, 182 (2024).
Rafiei, D. & Kolla, N. J. Elevated brain fatty acid amide hydrolase induces Depressive-Like phenotypes in rodent models: A review. Int. J. Mol. Sci. 22, 1047 (2021).
Huang, R. et al. N6-Methyladenosine modification of fatty acid amide hydrolase messenger RNA in circular RNA STAG1–Regulated astrocyte dysfunction and Depressive-like behaviors. Biol. Psychiatry. 88, 392–404 (2020).
Nesher, E., Peskov, V., Rylova, A., Raz, O. & Pinhasov, A. Comparative analysis of the behavioral and biomolecular parameters of four mouse strains. J. Mol. Neurosci. MN. 46, 276–284 (2012).
Valentine, H., Williams, W. O. & Maurer, K. J. Sedation or inhalant anesthesia before euthanasia with CO2 does not reduce behavioral or physiologic signs of pain and stress in mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS. 51, 50–57 (2012).
Bassetti, B., Hone, C. A. & Kappe, C. O. Continuous-Flow synthesis of ∆9-Tetrahydrocannabinol and ∆8-Tetrahydrocannabinol from Cannabidiol. J. Org. Chem. 88, 6227–6231 (2023).
Basavarajappa, B. S., Nixon, R. A. & Arancio, O. Endocannabinoid system: emerging role from neurodevelopment to neurodegeneration. Mini Rev. Med. Chem. 9, 448–462 (2009).
Harkany, T. et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 28, 83–92 (2007).
Winters, C. et al. Author correction: automated procedure to assess pup retrieval in laboratory mice. Sci. Rep. 12, (2022).
Angoa-Pérez, M., Kane, M. J., Briggs, D. I., Francescutti, D. M. & Kuhn, D. M. Marble burying and nestlet shredding as tests of repetitive, Compulsive-like behaviors in mice. J. Vis. Exp. 50978 https://doi.org/10.3791/50978-v (2013).
Walf, A. A. & Frye, C. A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328 (2007).
Brown, G. R. & Nemes, C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav. Processes 78, 442–448 (2008).
Kaidanovich-Beilin, O., Lipina, T., Vukobradovic, I., Roder, J. & Woodgett, J. R. Assess. Social Interact. Behav. J. Vis. Exp. 2473 doi:https://doi.org/10.3791/2473-v. (2011).
Yankelevitch-Yahav, R., Franko, M., Huly, A. & Doron, R. The forced swim test as a model of Depressive-like behavior. J. Vis. Exp. 52587 https://doi.org/10.3791/52587-v (2015).
Funding
This work was supported by the internal fund of Ariel University and performed in partial fulfillment of the requirements for a Ph.D. degree of Mohamed Mari, Department of Molecular Biology and Adelson School of Medicine, Ariel University, Ariel, Israel.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design were carried out by MM, AP, and DS. NZ and NMK synthesized THC. THC injections were carried out by DB, with help from MM. MM carried out behavior experiments with help from AB and BGSR. Gene expression was carried out by MM with the help of AB and BGSR. Data analysis by MM with the help of AB and DS. KB, PKT, and AbB reviewed the manuscript and provided critical comments and suggestions for results interpretation. MM, NMK, and AP wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mari, M., Bagaev, A., Sur, D. et al. Personality-based intergenerational effects of prenatal THC exposure in an inherited mouse model of social dominance and submissiveness. Sci Rep 15, 30624 (2025). https://doi.org/10.1038/s41598-025-15528-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15528-1