Abstract
Current climate change challenges, including rising temperatures, reduced water availability, and increased soil salinity, pose severe threats to global agricultural productivity. While plant growth promoting bacteria (PGPB) have been studied for their role in stress mitigation, their application in enhancing heat tolerance in Chilean landraces of common bean (Phaseolus vulgaris L.) remains largely unexplored. This study evaluated the interaction between native PGPB and genetically distinct Chilean common bean landraces, which are part of the Andean gene pool and represent a socio-cultural food heritage. Auxin production and root adhesion capacity were first validated for two native Bacillus strains. Subsequently, their effects on heat-stressed plants were assessed at physiological and biochemical markers associated with thermal stress tolerance. Although PGPB inoculation improved certain stress-related responses at 30–35 °C in the Tórtola and Sapito landraces, growth responses at 40 °C in Mantequilla were observed even in control plants, indicating an inherent thermotolerance rather than a treatment-specific effect. Furthermore, although individual PGPB strains positively influenced traits such as root development and oxidative stress mitigation, the bacterial consortium did not show additive or synergistic effects under the tested conditions. These findings highlight the importance of host genotype and microbial compatibility in shaping plant responses under thermal stress. This study contributes novel insights into the role of PGPB in Chilean bean landraces and provides a foundation for future efforts in developing climate-resilient agroecosystems through targeted microbial applications.
Similar content being viewed by others
Introduction
In the face of climate change and its far-reaching impact on global agricultural systems, the pursuit of sustainable and resilient crop production strategies has become the priority. Among the various innovative approaches gaining momentum is the utilization of plant growth-promoting bacteria (PGPB) to enhance plant growth and stress tolerance. PGPB residing in the rhizosphere have emerged as vital allies in facilitating sustainable agriculture practices by promoting nutrient acquisition, ameliorating abiotic stresses, and fostering beneficial interactions with their host plants1. In this context, it is crucial to contextualize the increasing temperature trends in Chile over the last decade within the broader context of global climate change. Chile, like many other regions worldwide, especially with its Mediterranean climate, has experienced notable temperature fluctuations and rising average temperatures, significantly impacting agriculture2. According to climate data and reports, Chile has witnessed a noticeable temperature increase over the past decade, with both short- and long-term heat waves. The last 10 years (2014–2023) have been the warmest on record, and in 2023, both land and ocean temperatures increased 1.79 °C and 0.91 °C, respectively2.
Increasing temperature due to climate change impacts have put the survival of many species at risk, which has resulted in progressive loss of biodiversity and thereby fluctuating the ecological structures3. The rise in temperature has led to various challenges in the agricultural sector too, particularly for crops like common bean. Temperature stress during critical growth stages, such as seed germination and early seedling development, can negatively impact crop yield and productivity, directly affecting food security in Chile4. The common bean holds significant agricultural importance in Chile, serving as a fundamental staple crop that sustains food security and supports rural livelihoods. Chilean native landraces of common beans represent a rich and diverse genetic resource that has been cultivated for centuries in various regions of the country5. These ecotypes, often referred to by unique local names (Sapito, Tórtola, Mantequilla, Palo, Bayo, Coscorrón, Pajarito, Burro, Pallar, etc.), have adapted to the specific environmental conditions prevailing in different areas of Chile. Each landrace carries distinct traits, such as seed size, shape, color, and growth habit, which contribute to their cultural significance and culinary versatility. The nutritional quality of Chilean native common beans is noteworthy as they serve as a vital source of dietary protein, fiber, essential amino acids, vitamins, and essential micronutrients for local communities6,7. The evolution of common bean cultivation in Chile reflects a dynamic interplay between human activity and natural selection. Over time, farmers have meticulously saved seeds from the best-performing plants, leading to the development of locally adapted ecotypes with enhanced resilience to various environmental stressors8. A region that heavily relies on common bean cultivation for sustenance and economic stability of small agricultural families, the increasing heat stress poses a significant threat to Chilean agriculture. Thus, ensuring the resilience of this crucial legume to temperature stress becomes an urgent necessity to maintain food security and agricultural sustainability in Chile9. In this context, the research on PGPB from the rhizosphere enhancing seed germination and growth under temperature stress has crucial relevance. Identifying and harnessing native PGPB strains that can bolster the heat tolerance of common beans offers a viable solution to alleviate the adverse impacts of heatwaves and ensure sustainable crop production10.
Recent studies have underscored the significance of PGPB in promoting plant growth and stress tolerance11,12,13. For instance, studies have demonstrated the positive impact of PGPB on the germination and growth of various crops, including maize (Zea mays L.)14rice (Oryza sativa L.)15and soybean (Glycine max (L.) Merr.)1. The application of PGPB has been shown to enhance nutrient availability, stimulate root development, and increase stress-related metabolites, thereby boosting plant defences against temperature extremes10,16. Studies have revealed that PGPB inoculation can improve antioxidant activities, alleviate oxidative stress, and modulate gene expression to enhance stress tolerance in plants17,18. Additionally, PGPB have been found to induce the production of osmoprotectants, phytohormones, and secondary metabolites, conferring enhanced resilience to temperature stress19,20. Physiological changes in crop plants under temperature stress, particularly alterations in chlorophyll and carotenoid content, oxygen radical absorbance capacity (ORAC), polyphenol content, membrane damage, and proline content, play critical roles in determining the plant’s response and adaptation to adverse environmental conditions. In response to temperature stress, plants often undergo a series of physiological adjustments to cope with the imposed challenges. Research have indicated that under heat stress, there is a decline in chlorophyll content and an accumulation of carotenoids as a protective mechanism against oxidative damage caused by reactive oxygen species (ROS)21,22. Additionally, increased temperature stress can lead to a significant reduction in ORAC and polyphenol content, which are crucial antioxidants involved in scavenging ROS and mitigating oxidative stress23,24. Alongside, temperature stress can induce membrane damage due to lipid peroxidation and electrolyte leakage25. But plants also accumulate proline, an osmoprotectant that helps maintain cellular integrity and regulates water balance, aiding in stress tolerance26,27. Understanding these physiological changes is essential for developing strategies to enhance crop resilience to temperature stress and ensure sustainable agriculture in the face of climate change.
Emerging evidence points specifically to the functional relevance of free-living Bacillus species in conferring abiotic stress tolerance28. Bacillus safensis, for instance, has demonstrated the ability to significantly improve seed germination in common bean under high salinity conditions, tolerating up to 10% NaCl, while enhancing antioxidant content and reducing oxidative stress in maize plants exposed to salt stress29. This includes a notable reduction in H₂O₂, MDA, and electrolyte leakage, alongside increased levels of flavonoids and ascorbic acid30. Likewise, Bacillus proteolyticus has been reported to activate induced systemic resistance (ISR) in Arabidopsis thaliana, improving stress responses through enhanced deposition of callose, increased ROS generation, and modulation of plant hormone signalling pathways31,32. These physiological responses are aligned with the bacteria’s potential to enhance tolerance to environmental extremes, such as heat, salinity, and drought33,34. Their role is not only limited to individual inoculations but may be further potentiated in microbial consortia that mitigate potential negative trade-offs such as flavonoid reduction and altered wound responses30. In this context, we hypothesize that native auxin-producing PGPB isolated from the rhizosphere of Chilean common bean landraces can enhance plant resilience to elevated temperatures. This study explores their potential in promoting seed germination, early seedling development, and thermal stress tolerance, while also examining the influence of strain compatibility and plant genotype. The findings aim to advance the understanding of PGPB applications in climate-resilient agriculture.
Results
Qualitative and quantitative measurement of auxin production by bacteria
According to the colorimetric indicators, all three bacterial cultures (B. proteolyticus Cyn1, B. safensis Cyn2, and their consortium) showed the presence of auxin compounds, with the consortium showing the most intense red color (Fig. 1A). Alongside the commercial indole acetic acid (IAA) standard curve (Fig S1), the quantitative measurement confirmed higher auxin production in the consortium (90.67 µg mL− 1) compared to B. proteolyticus Cyn1 (46 µg mL− 1) and B. safensis Cyn2 (43.78 µg mL− 1) individually (Fig. 1B). The auxin production trend for the consortium actually demonstrated a steady increase at lower concentrations, reaching its peak at 0.5 mg mL−1 (~ 90.67 µg mL− 1), after which the production gradually declines reaching (67.56 µg mL− 1). This suggests that while moderate concentrations promote auxin synthesis, higher concentrations may not further enhance production or could have an inhibitory effect. Notably, the consortium produces more auxins than the PGPBs individually, indicating a synergistic effect of the bacteria in auxin production. While B. safensis Cyn2 shows slightly higher auxin production than B. proteolyticus Cyn1, the consortium outperforms both, highlighting its potential for enhanced plant growth promotion.
Seed bacterization and germination
The influence of the two PGPB strains B. proteolyticus Cyn1 and B. safensis Cyn2 individually and in consortium, was evaluated through a seed germination assay involving ten Chilean common beans landraces. Nine of the ten landraces showed germination, with the Araucano landrace displaying a 0% germination rate under all treatments. Germination parameters, including germination rate, relative germination percentage, toxicity tolerance index, and vigor index, were recorded for all landraces. However, only three landraces Mantequilla, Sapito, and Tórtola exhibited consistent and reproducible germination responses across treatments, allowing for detailed physiological and biochemical evaluation. The complete dataset, including results from all nine germinated landraces, is available in the Supplementary Material (Table S2).
Confocal microscopy analysis confirmed bacterial colonization in the roots of the nine germinated landraces. SYTO 9 and propidium iodide (PI) staining enabled simultaneous visualization of both viable bacterial cells and plant root cell integrity. Bacterial colonization was most extensive in the consortium treatment, with particularly high colonization observed in the Mantequilla, Tórtola, and Sapito landraces (Fig. 2, Fig S2). Based on these colonization patterns and their robust germination performance, these three landraces were selected for further experimental analyses.
Confocal microscopy images of roots with B. proteolyticus Cyn1, B. safensis Cyn2, consortium and control treatments in 9 bean landraces. Staining was performed with SYTO 9/Propidium Iodide (LIVE/DEAD BacLight kit, ThermoFisher, USA), and images were acquired at 60X magnification. All images share the same scale bar, represented by a white line in the lower corner of each panel, corresponding to 25 μm.
Total antioxidant activity and polyphenol content
The ORAC test revealed that antioxidant capacity was generally higher in roots than in leaves across the bacterial treatments. In leaves, Sapito (295.51 ± 94.35 µM TE/100 g) and Mantequilla (292.59 ± 62.39 µM TE/100 g) exhibited greater antioxidant activity compared to Tórtola (277.15 ± 94.51 µM TE/100 g), highlighting differential responses to bacterial treatments among landraces (Fig. 3A). In roots, Mantequilla showed the highest ORAC value (863.15 ± 153.19 µM TE/100 g), followed by Sapito (631.49 ± 124.39 µM TE/100 g) and Tórtola (542.15 ± 295.51 µM TE/100 g), suggesting that bacterial inoculation enhanced the antioxidant capacity of the root system (Fig. 3B).
Total phenolic content was highest in the Mantequilla (911.29 ± 114.19) and Sapito (661.29 ± 149.19) landraces compared to the other landraces Bayo, Arverjilla, and Manteca. The application of B. safensis Cyn2 in Mantequilla and B. proteolyticus Cyn1 in Sapito appeared to enhance polyphenol accumulation in these specific landraces (Fig. 3C). This elevated polyphenol content, along with the observed increase in antioxidant capacity, suggests that Mantequilla and Sapito may exhibit greater physiological resilience under stress conditions, supporting their potential for further investigation in stress-adaptive breeding and bioinoculant studies.
ORAC and polyphenols content in Chilean common beans landraces. (A) ORAC values in leaves. (B) ORAC values in roots. (C) Total polyphenol content in leaves. (D) Visual comparison of seeds from the 10 common beans landraces used in the study. All biochemical measurements were performed after bacterial inoculation (Control, B. proteolyticus Cyn1, B. safensis Cyn2, and consortium). Antioxidant activity is expressed in µM TE/100 g dry weight (Trolox Equivalents), and polyphenol content is expressed in mg GAE/g dry weight (Gallic Acid Equivalents). Values represent mean ± SD. *p < 0.05, **p < 0.01.
Correlation and principal component analysis (PCA)
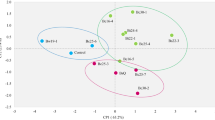
The Spearman’s correlation matrix presented in Fig. 4B revealed significant pairwise correlations between certain plant growth parameters. A positive strong correlation was found between the total plant length and plumule length and shoot length (Fig. 4B). There was also a positive strong correlation between the shoot length and plumule length, a weak negative relationship between the number of roots and shoot length. The other growth parameter correlations were very week and/or not significant (Fig. 4B). The PCA analysis showed a lack of significant separation between the growth-promoting bacterial treatments and the control, suggesting that these treatments did not have a uniform effect on growth parameters across the nine bean landraces evaluated (Fig. 4A). However, the Generalized Linear Mixed Models (GLMMs) revealed that PGPB treatments significantly affected several specific traits, including total plant length (GLMM, χ2 = 30.85, df = 8, p < 0.001), radicle length (GLMM, χ2 = 11.83, df = 8, p < 0.001), and number of roots (GLMM, χ2 = 181.77, df = 8, p < 0.001). In contrast, plumule length (GLMM, χ2 = 4.50, df = 3, p = 0.2115), shoot length (GLMM, χ2 = 6.04, df = 3, p = 0.1095), and root length (GLMM, χ2 = 2.65, df = 3, p = 0.448) did not show significant differences among treatments. Specifically, B. proteolyticus Cyn1 had a significant positive effect on radicle length. Both B. proteolyticus Cyn1 and B. safensis Cyn2 individually increased the number of roots compared to the control, while their consortium did not produce a significant effect on this parameter. Similarly, total plant length was significantly improved by individual strains but not by the consortium treatment. These results suggest that the combination of strains did not produce additive or synergistic effects under the tested conditions. All findings described above are visualized in Fig. 5, which illustrates the differential impact of each bacterial treatment on morphological growth parameters. Moreover, all measured growth parameters were significantly influenced by the landrace genotype, indicating that plant genetic background plays an important role in modulating the response to bacterial inoculation. The remaining comparisons were not statistically significant.
Multivariate analysis of growth parameters in nine bean landraces under bacterial treatments. (A) Principal Component Analysis (PCA) showing the distribution of treatments and growth parameters, with ellipses representing the 95% confidence interval. PC1 (46.76%) and PC2 (31.63%) together explain 78.39% of the variance. (B) Spearman’s correlation matrix among growth parameters of bean landraces. Correlation coefficients are represented by color intensity, positive values in orange, negative values in blue. Values with an “**” indicate significant correlations (P > 0.05).
Estimated marginal means (and 95% confidence intervals) for the GLMM models comparing the effect of different treatments (PGPB and control) on the growth parameters (plumule length, root length, radicle length, number of roots, total plant length, and shoot length) of the nine common bean landraces.
Temperature tolerance screening
Pot experiment
The interaction of three landrace of Chilean bean (Sapito, Mantequilla, and Tórtola) with bacterial treatments (B. proteolyticus Cyn1, B. safensis Cyn2, consortium, and Control) under high-temperature conditions was analyzed in pot experiments (Fig. 6). Growth parameters were measured at 25, 30, 35, and 40 °C (Table S1).
Effect of seed bacterization on morphology and growth of common beans in Mantequilla, Sapito, and Tórtola landraces under four temperature conditions (25 °C, 30 °C, 35 °C, and 40 °C). Treatments included control, B. proteolyticus Cyn1, B. safensis Cyn2, and their bacterial consortium. Representative images of seedlings were selected from biological replicates to illustrate the typical morphological response observed for each combination of landrace, temperature, and treatment.
A Principal Component Analysis (PCA) was conducted to explore the relationships between the treatments (PGPB and control) and the growth parameters in three landraces of common beans subjected to temperature stress. As shown in Fig. 7A, no distinct clustering or separation between treatment groups was observed, suggesting that the variation in growth parameters was primarily driven by temperature and genotype, rather than by the bacterial inoculation. This implies that the bacterial treatments did not induce strong or consistent shifts in the multivariate trait space under the tested thermal conditions, and that the landraces responded in a comparable manner across treatments. The Spearman’s correlation matrix allowed for the assessment of linear relationships among the different growth parameters. The results presented in Fig. 7B revealed significant correlations among temperature and certain growth parameters. For example, a strong positive correlation was observed between temperature stress conditions and the number of roots (r = 0.63), a moderate positive correlation between the plumule length and temperature (r = 0.57), and between the presence of symptoms and temperature (r = 0.42). Controversially, temperature stress had a negative had a negative strong effect on shoot length (r=-0.66), a moderate negative effect on the number of leaves (r=-0.5) and on the total plant length (r=-0.34).
The results of the Generalized Linear Mixed Models (GLMM) evaluating the effect of temperature variation (25, 30 and 35 °C) and PGPB treatments (control, B. proteolyticus Cyn1, B. safensis Cyn2 and their consortium) on the plant growth parameters of three common bean landraces (Mantequilla, Sapito and Tórtola) are visualized in Fig. 8. The models indicate that the PGPB treatments alone (B. proteolyticus Cyn1, B. safensis Cyn2, their consortium, and control) did not significantly affect any of the plant growth parameters. However, the interaction between treatment and temperature had a significant effect on the total plant length (GLMM, χ2=8.94, df = 3, p = 0.030) and root length (GLMM, χ2=7.80, df = 2, p = 0.020). These results indicate that bacterial treatments had little overall effect in promoting plant growth under heat stress conditions.
The interaction between temperature and landraces of beans (Mantequilla, Sapito, and Tórtola) were significant for plumule length (GLMM, χ2=30.47, df = 2, p < 0.001), radicle length (GLMM, χ2= 15.33, df = 2, p < 0.001) and root length (GLMM, χ2=13.67, df = 6, p = 0.033). The total plant length shows a general decreasing trend with increasing temperature in all three landraces (GLMM, χ2=17.21, df = 1, p < 0.001; Fig. 8). Regarding plumule length, Mantequilla and Tórtola landraces exhibit a positive trend, indicating greater growth at higher temperatures, while Sapito maintain a more stable trend (Fig. 8). For root length, only Sapito landrace display a general decrease as temperature rises (Fig. 8). However, the number of roots increased in all landraces at higher temperatures (Fig. 8). In general, Mantequilla and Sapito appear to be the least affected by temperature variations, while Tórtola experiences a more pronounced decline. The other comparisons were not significant.
The stress response indicators presented in Table 1 showed that the Tórtola and Sapito landraces exhibited greater tolerance and vigor at higher temperatures (35 °C). This effect was particularly pronounced in plants treated with B. proteolyticus Cyn1, which resulted in a vigor index of 19,800 and a tolerance value of 2.5 in Tórtola, indicating enhanced seedling growth. Although some treatments were toxic at lower temperatures (25 °C), the landraces improved their performance as the temperature increased. In contrast to the other two landraces, Mantequilla was more responsive to the PGPB treatments, showing better performance at extreme temperatures (40 °C). This suggests as temperature-dependent interaction between PGPBs and seed performance. These findings also highlight Mantequilla’s exceptional heat tolerance and the potential of PGPB to enhance thermotolerance in common beans landraces.
Growth response of three Chilean bean landraces to temperature stress and bacterial inoculation. (A) Principal Component Analysis (PCA) of bacterial treatments and growth parameters in beans landraces (Mantequilla, Sapito, and Tórtola) under three temperature conditions (25 °C, 30 °C, 35 °C). (B) Spearman’s correlation matrix showing the relationship among growth parameters under temperature variation. Significant correlations are color-coded; significant values are marked with an “**” (P > 0.05).
Estimated marginal means (and 95% confidence intervals) from a generalised linear mixed model (GLMM) evaluating the effects of temperature variation (25, 30 and 35 °C) and PGPB treatments (control, B. proteolyticus Cyn1, B. safensis Cyn2, and a consortium of the two bacteria) on plant growth parameters in three common bean landraces (Mantequilla, Sapito, and Tórtola). Asterisks (**) indicate a significant effect of temperature on a plant growth parameter (p < 0.05).
Photosynthetic pigments
Among the assayed landraces, chlorophyll a was the least abundant, followed by chlorophyll b, while carotenoids were the most abundant. In general, bacterial treatments increased chlorophyll a content compared to controls, suggesting a potential protective role of PGPB in photosynthetic efficiency under stress. Notably, B. safensis Cyn2 led to a significant increase in chlorophyll a in the Sapito landrace, reaching 30.56 ± 0.09 at 25 °C and 30.28 ± 0.04 at 30 °C, whereas B. proteolyticus Cyn1 enhanced chlorophyll a level in Mantequilla to 31.22 ± 0.036 at 35 °C. Similarly, chlorophyll b content was highest in Mantequilla treated with the bacterial consortium, reaching 52.27 ± 0.05 at 25 °C, but declined to 17.36 ± 0.09 at 35 °C, indicating a temperature-dependent reduction in chlorophyll stability. In Sapito, the consortium treatment resulted in chlorophyll b levels of 16.18 ± 0.82, suggesting a potential moderating effect of bacterial inoculation under heat stress. In contrast, carotenoid content remained relatively stable across all landraces and treatments, ranging between 0 and 7, suggesting that temperature stress and bacterial treatments had less influence on carotenoid levels (Fig. 9).
These suggest that PGPB treatment helps maintain chlorophyll content under moderate heat stress, but its protective effects may diminish as temperatures rise more. The relative stability of carotenoid levels indicates that these compounds may serve as a baseline photoprotective mechanism, while chlorophyll degradation reflects the temperature threshold at which stress begins to impact photosynthetic efficiency. This highlights the potential role of PGPB in delaying photosynthetic pigment breakdown, supporting their application in enhancing plant resilience under heat stress conditions.
Total antioxidant activity and polyphenol content
Consistent with previous ORAC results, thermal stress led to higher antioxidant capacity in leaves compared to roots, with a general decline as temperature increased. In leaves, the highest ORAC value was observed in Sapito at 25 °C with B. proteolyticus Cyn1 (57.901 ± 1.68), followed by Sapito at 30 °C with the consortium (31.158 ± 0.52) and Tórtola at 35 °C in the control treatment (50.194 ± 0.93). In roots, the highest ORAC value was recorded in Mantequilla at 25 °C with B. safensis Cyn2 (28.095 ± 0.46), followed by Tórtola at 30 °C with B. safensis Cyn2 (9.846 ± 0.08) and Sapito at 35 °C with B. safensis Cyn2 (18.732 ± 0.29) (Fig. 9). Similarly, polyphenol content was consistently higher in leaves than in roots, showing a decreasing trend with rising temperatures. In leaves, the highest values were recorded in Sapito at 25 °C with B. safensis Cyn2 (538 ± 0.56), in Sapito at 30 °C with the consortium (439 ± 0.24), and in Mantequilla at 35 °C with B. safensis Cyn2 (340 ± 0.37). In roots, the highest values were found in Sapito at 25 °C with B. safensis (282 ± 0.65), in Tórtola at 30 °C with the consortium (121 ± 0.47), and in Mantequilla at 35 °C with the consortium (186 ± 0.63) (Fig. 9). These results suggest that PGPB inoculation plays a role in modulating antioxidant responses under heat stress, with leaves exhibiting higher ORAC and polyphenol content compared to roots. However, as temperature increased, a decline in these protective compounds was observed, indicating a potential threshold beyond which stress adaptation mechanisms become less effective.
Lipid peroxidation test via malondialdehyde (MDA) and proline content
MDA content increased across most treatments in the Mantequilla landrace as the temperature rose from 35 °C to 40 °C. In the control, MDA levels increased from 1.25 ± 0.009 to 1.85 ± 0.03. A slight decrease was observed in the B. proteolyticus Cyn1 treatment (1.57 ± 0.02 to 1.54 ± 0.04), whereas B. safensis Cyn2 showed a substantial increase (0.59 ± 0.01 to 1.36 ± 0.03). The consortium exhibited the highest increase, rising from 0.71 ± 0.01 to 2.33 ± 0.03, suggesting increased oxidative stress at higher temperatures (Fig. 10A). In contrast, proline content decreased sharply across all treatments in the Mantequilla landrace from 35 °C to 40 °C, indicating a reduced osmoprotectant response at extreme heat of 40|°C. In the control, proline levels dropped from 2.37 ± 0.06 to 0.26 ± 0.01. The most pronounced decline was observed in B. proteolyticus Cyn1 (2.14 ± 0.1 to 0.12 ± 0.004) and B. safensis Cyn2 (0.47 ± 0.03 to 0.10 ± 0.001). The consortium also showed a significant reduction, from 1.06 ± 0.02 to 0.24 ± 0.01 (Fig. 10B). These results indicate that while PGPB inoculation influences oxidative stress responses, extreme heat (40 °C) compromises the protective role of proline in Mantequilla. The consortium treatment, despite its promising effects at lower temperatures, exhibited higher oxidative stress markers at 40 °C, suggesting potential limitations under severe heat wave.
Chlorophyll, carotenoid, polyphenol, and ORAC contents under different bacterial treatments (B. proteolyticus Cyn1, B. safensis Cyn2, consortium, and control) across three Chilean bean landraces (A) Tórtola, (B) Sapito, and (C) Mantequilla, evaluated under temperature stress (25 °C, 30 °C, 35 °C, and 40 °C).
Oxidative stress (MDA) and osmoprotectant (proline) levels in bean landraces under thermal stress and bacterial treatments. (A) MDA content and (B) Proline content in Mantequilla landrace from 35 °C to 40 °C under different treatments (Control, B. proteolyticus Cyn1, B. safensis Cyn2, consortium). (C) MDA and proline content in Sapito and Tórtola landraces under normal temperature conditions. Different letters above the bars indicate statistically significant differences between treatments (p < 0.05). The asterisk (*) denotes significant differences among temperaturetreatments within the 'Mantequilla' variety (p < 0.05).
Discussion
This study builds upon previous work by Meza et al. (2022)35in which B. proteolyticus Cyn1 and B. safensis Cyn2 were initially characterized as PGPB capable of improving seed germination and growth in local Chilean common bean varieties under saline conditions. This research expands the understanding of these strains by exploring their potential to confer thermotolerance in native landraces of common bean under elevated temperature regimes. This work provides a new perspective on the versatility of these native PGPB strains, demonstrating their relevance in mitigating different types of abiotic stress and offering broader applications in the context of agriculture resilient to extreme climatic conditions. Climate change is a reality and its effects in Chile are notorious; in the last 100 years, the average temperature has increased and will continue to increase over the years36. The stress caused by the high temperatures can have important effects on the production of common beans. Among them it has been shown to reduce the germination percentage, increase the number of abnormal seedlings, decrease the efficiency of nitrogen fixation, reduce the stability of the membranes, which affects photosynthetic activity and consequently decreases growth37. Various studies recognize that microorganisms associated with plants improve growth, facilitate the acquisition of nutrients and reduce different types of stress, including protection against various pathogens38,39,40. This significant role that microorganisms play in plants is an indication of potential for a more sustainable agricultural production system29. Legumes are essential for food security and agricultural sustainability due to their ability to fix nitrogen in symbiosis with rhizobacteria. These bacteria form root nodules in legumes, enhance metabolic efficiency depending on the presence of the bacterial strain41. In a recent study, coinoculation of Rhizobium spp. and Azospirillum brasilense further enhanced growth and physiological performance in soybean and common bean42. In soybean, the combination of Bradyrhizobium japonicum and A. brasilense increased grain yield and nodulation, while in common bean, coinoculation with Rhizobium tropici and A. brasilense boosted yield from 8.3% with R. tropici alone to 19.6% when both bacteria were applied43. While the role of rhizobia in legumes is well-established, recent trends highlight the importance of free-living PGPB in enhancing abiotic stress tolerance, particularly through seed biopriming strategies44. These free-living bacteria, often associated with the seed microbiome, may be vertically transmitted and contribute to early-stage stress resilience. Recent work demonstrated that seed-associated microbiota from forage legumes such as alfalfa and pitch clover include culturable strains capable of enhancing plant tolerance to abiotic stress, highlighting their potential as inherited bioinoculants in stress-adaptive agriculture45. Unlike rhizobia, which require nodulation for nitrogen fixation, free-living PGPB exert their effects by producing phytohormones, enhancing nutrient uptake, and promoting root development via production of biofilm and creating a safe microenvironment surrounding the root46. In this study, we focused on evaluating the potential of native PGPB isolates to improve heat stress resilience in common bean landraces.
One of the key mechanisms through which PGPB enhance plant resilience under stress conditions is the production of phytohormones, particularly auxins. As a physiologically active auxin, IAA plays a crucial role in promoting root elongation, increasing lateral root formation, and improving nutrient and water uptake features that are particularly beneficial under high-temperature stress47,48. The property of synthesizing auxin is considered to be an effective tool to improve plant growth49. The two strains of bacteria used in this study gave positive results as producers of auxins (Fig. 1A) with the maximum concentration being in the consortium (Fig. 1B). To study the effect of these bacteria and the consortium on nine bean landraces, confocal microscopy experiments were carried out. However, the Araucano variety failed to germinate, likely due to factors such as poor seed quality, inappropriate storage conditions, environmental stress, seed dormancy, or potential contamination by pathogens or pests50. In order to study the interaction, structure and spatial distribution of these two bacteria alone and in consortium on the roots, root colonization by these bacteria, as well as their effects on the growth of the plants is shown in Fig. 2 where a higher count of bacterial cells are observed when they are in a consortium. Another study carried out to understand the effect of bacteria on bean landraces by analyzing the polyphenol content and antioxidant activity. The authors Jung et al. (2011)51 indicated that the ORAC content is usually higher in roots than in leaves, as our results demonstrate in Fig. 3B, 3 C, and Fig. 9 since they are usually rich in antioxidant compounds. Furthermore, it is noteworthy to mention that the ORAC content can vary depending on the landrace of the plant and the conditions, and in our case, this is where the results vary between landraces and treatments (Fig. 3B C) and between landraces, treatments, and temperature conditions. For example, Mantequilla exhibited higher ORAC values in roots under B. safensis Cyn2, while Sapito had greater antioxidant capacity in leaves under B. proteolyticus Cyn1, and Tórtola showed moderate responses in both tissues. However, ORAC levels are normal when the plants are at their optimal temperature and increase in other conditions (Fig. 9). Polyphenols are antioxidant molecules that are found to a greater or lesser extent in almost all plants52. They are the molecules produced naturally by the metabolic processes of plants as a defense barrier against attacks by external stimuli. It is also mentioned before that the content of polyphenols in leaves can vary widely between different plants or even within the same species due to environmental conditions and genetic variability53. This is coincident with our results in Fig. 3A where the content of polyphenols varies depending on the bean landraces. Together, these findings suggest that PGPB treatments contribute to stress adaptation by enhancing root colonization, antioxidant responses, and phytohormone production, all of which play a role in plant resilience. However, to further understand how these physiological changes translate into plant growth differences, we performed PCA and GLMM analyses to assess the broader impact of bacterial inoculation on the morphological traits of common bean landraces. Notably, GLMM analysis showed that Mantequilla responded with greater improvements in total plant length under B. proteolyticus Cyn1, whereas Sapito displayed increased root number particularly under B. safensis Cyn2, and Tórtola responded more variably depending on the temperature condition.
The PCA analysis in Fig. 4A indicated PGPB treatments had little effect on the growth parameters of the evaluated landraces not subjected to temperature stress and that the Chilean bean landraces responded similarly under the temperature stress conditions of the study. Moreover, the lack of distinct clustering suggests that the treatments did not result in strong or consistent multivariate effects on the measured variables. However, the results of the GLMMs in Fig. 5 show that bacterial treatments, especially B. proteolyticus Cyn1 and the bacterial consortium, have a significant positive effect on total plant length of the nine bean landraces evaluated, suggesting an impact on aerial growth, which aligns with studies linking PGPB to the production of phytohormones such as IAA54. Additionally, B. proteolyticus Cyn1 improves root length in all landraces, which may be associated with enhanced nutrient and water absorption, crucial for resilience in adverse conditions55. It is also observed that these bacteria promoted radicle length in the common bean landraces, potentially facilitating seedling germination and early growth establishment31. The ability of B. proteolyticus Cyn1 and B. safensis Cyn2 to increase the number of roots in the beans landraces highlights their potential to enhance root branching and the efficiency of resource acquisition from the soil. Notably, the consortium of B. proteolyticus Cyn1 and B. safensis Cyn2 did not increase the number of roots. Similarly, B. proteolyticus Cyn1 and B. safensis Cyn2 applied separately affected the total plant length in the bean landraces, however, the consortium did not affect plant length. This may suggest inexistence of a cumulative synergistic effect from these microorganisms. Finally, the effects on root development in the landraces reinforce the idea that PGPB may influence aerial growth through mechanisms such as hormonal regulation and protection against oxidative stress56. Similar responses among the different local landraces suggest that plants respond uniformly to the treatments. However, the type of landraces also explained variations among growth parameters, appearing to benefit differently from bacterial inoculation treatments, possibly due to better adaptation for interacting with beneficial microorganisms. The combination of bacterial strains in consortium did not produce additive effects on root number or plant length compared to individual strains. This finding is intriguing, as the interaction of multiple PGPBs was expected to have a synergistic effect, enhancing plant growth benefits. Although bacterial consortium did not show a significantly additive effect under normal conditions, their positive impact under salinity stress in the Sapito landrace suggests that they may play a key role in plant tolerance to adverse conditions35. Competition for resources and possible antagonistic interactions among bacteria could be limiting the expected effects under standard conditions57but their positive impacts under stress conditions highlight the importance of considering the resilience of bacterial consortium in promoting plant growth in a changing climate58. The correlation matrix analysis in Fig. 4B showed significant relationships between certain germination parameters, such as the positive correlation between plumule length/shoot length and total plant height, and between plumule length and shoot length. These results imply that the aerial growth of seedlings follows a consistent pattern, regardless of PGPB application59,60. While these findings suggest that plant growth patterns are relatively stable across treatments; however, environmental factors such as temperature play a crucial role in shaping germination success and early seedling development61. Since temperature stress is reported to be a major constraint in P. vulgaris L. cultivation62it is important to assess how germination and root growth respond to increasing temperatures and whether PGPB can mitigate these effects.
The optimal germination temperature in P. vulgaris L. depend on the landraces and environmental conditions63; in general, it is considered that the optimal temperature for seed growth is in the range of 20 to 30° C, this is because exceeding these temperatures can increase evaporation and dehydration, hindering their growth, causing damage to plant tissues, affecting their ability to perform vital functions such as photosynthesis and nutrient absorption64. Our results show that the reduction in number of roots and root growth occurred when it exceeds its optimal temperature (30–35 °C) as shown in Fig. 6. Among the tested landraces, Mantequilla maintained greater root length and number under elevated temperatures, suggesting a better capacity to cope with heat-induced dehydration. In contrast, Sapito exhibited a sharp decline in root parameters beyond 30 °C, while Tórtola showed intermediate sensitivity. In addition, a beneficial effect was observed in those landraces under treatment of PGPBs either individually or in a consortium, as they have the ability to increase their tolerance to different types of stress, including high temperatures35. The Principal Component Analysis (PCA) in Fig. 7A, conducted under different levels of temperature stress, did not indicated a separation between treatments (PGPB and control) nor between the bean landraces evaluated, suggesting that the plants, regardless of the applied treatment, responded similarly to thermal stress. This result indicate that extreme temperature conditions exert a uniform pressure on the cultivars, minimizing the differential impact that PGPB might have had on the plants under optimal temperature conditions. This finding aligns with the study of Li et al. (2022)65 which indicates that the appropriate combination of inoculants should be chosen according to the level of drought stress and the inoculant’s drought tolerance capacity. However, the correlation matrix in Fig. 7B revealed some important relationships between growth parameters and temperature, highlighting the influence of thermal conditions on plant development. Our results indicate a positive correlation (r = 0.63) between temperature and root number, suggesting that elevated temperatures within the tested range may stimulate root development. This response could be linked to increased metabolic activity and auxin signalling, which play a crucial role in root initiation and elongation. Studies have shown that moderate temperature can increases enhance root growth by accelerating cell division and expansion, facilitating greater water and nutrient uptake66. However, while an increase in root number under higher temperatures may initially benefit plant establishment and resource acquisition, prolonged exposure to elevated temperatures can lead to detrimental effects, such as root damage, reduced water use efficiency, and oxidative stress56,67,68. Likewise, the correlation between plumule length and temperature suggests that thermal stress may have affected the growth of the aerial parts of the plants, a phenomenon frequently observed under abiotic stress conditions. Finally, the positive correlation between stress symptoms and temperature reinforces the idea that plants, regardless of treatment, exhibited signs of thermal stress. The lack of significant differences between treatments suggests that under extreme temperature conditions, the effects of PGPB may not be pronounced enough to counteract stress-induced damages. This shows that PGPB are generally beneficial for the plants; however, their effectiveness may depend on the severity of the environmental stress the plants face69. The results show that increasing temperature significantly affects several growth parameters (plumule length, total plant length, number of roots and leaves and shoot length) in the bean landraces (Mantequilla, Sapito, and Tórtola). Overall, a decrease in total plant length is observed as temperature increases, suggesting that higher temperatures may inhibit growth in all landraces70,71. However, there are significant differences in each landrace’s response to temperature changes to specific parameters. The Mantequilla and Tórtola landraces show a positive trend in plumule length as temperature rises, indicating a potential adaptive advantage in warmer environments72while Sapito maintains more stable trends. Regarding root length, all landraces display a general decrease as temperature increases, which could limit the plants’ ability to absorb water and nutrients73. Mantequilla appears to be most tolerant to temperature fluctuations, with less decline in growth parameters compared to Tórtola. This suggests that Mantequilla could be a preferable option under high-temperature conditions, while Sapito sensitivity highlights the importance of considering thermal stress in its cultivation. These findings provide useful information for selecting landraces for cultivation based on environmental conditions74. Beyond their impact on growth parameters, high temperatures also influence key physiological processes such as photosynthesis and oxidative stress responses75. Additionally, oxidative stress markers such as MDA and proline serve as indicators of cellular damage and stress resilience76complementing the observed growth responses.
Both chlorophylls and carotenoids are crucial for photosynthesis and their levels can be influenced by environmental conditions77including thermal stress75 as also observed in our study under increasing temperature conditions (Fig. 9). High temperatures cause a decrease in the chlorophyll content in plant tissues. This is usually associated with its degradation. A reduction in chlorophyll levels affect photosynthetic efficiency and overall plant growth78. Carotenoids, including compounds such as beta-carotene, are also sensitive to high temperatures79. While some studies suggest that carotenoid levels may increase as a protective response to stress, where prolonged exposure to high temperatures may lead to carotenoid degradation80. Among the tested landraces, Mantequilla retained the highest levels of chlorophyll a and b under moderate to high temperatures, particularly when treated with B. proteolyticus Cyn1, suggesting greater stability of photosynthetic pigments. In contrast, Sapito showed the most pronounced reduction in both pigment types at 35–40 °C, indicating limited capacity to buffer photosynthetic damage. Tórtola maintained intermediate levels, with modest chlorophyll retention under the consortium treatment, potentially reflecting a partial protective effect. Carotenoid content remained relatively stable across all landraces, though Mantequilla again demonstrated slightly higher retention at elevated temperatures. Thus, the measurements of MDA and proline is essential for assessing oxidative damage and the osmoprotective response of plants under thermal stress81. While MDA serves as a biomarker of lipid peroxidation and cellular membrane deterioration, proline functions as a key antioxidant and osmoprotectant in stress adaptation82,83. Figure 10 shows the decrease in proline and the increase in MDA in PGPB inoculated plants. It suggests that under certain conditions the stress response was probably not sufficient enough to mitigate oxidative damage84,85. However, with B. proteolyticus Cyn1 treatment, stable MDA levels were maintained despite the temperature increase, indicating a potential protective effect by reducing lipid peroxidation and enhancing thermal stress tolerance86. Additionally, we have further addressed the physiological mechanisms underlying the observed proline and MDA variations. The increase in MDA content, particularly at 40 °C, suggests enhanced lipid peroxidation and membrane destabilization due to oxidative stress. This is a typical response in plants subjected to high temperature, reflecting cellular damage caused by excessive reactive oxygen species (ROS)87. In contrast, the sharp decline in proline levels under extreme heat indicates a compromised osmoprotective response. Proline acts as a multifunctional osmolyte and antioxidant, stabilizing proteins and membranes and scavenging ROS. The decrease may reflect impaired biosynthesis or accelerated degradation, suggesting the plant’s metabolic capacity to mitigate stress becomes overwhelmed. Recent studies in maize confirm that proline accumulation under heat stress is tightly regulated by complex genetic and metabolic pathways, and its role in sustaining turgor and cellular hydration is critical for survival88.
This finding highlights the importance of selecting landraces with greater thermal tolerance in areas facing high temperatures due to climate change89. While PGPB inoculation demonstrated potential in enhancing plant resilience to thermal stress, its effects varied across landraces and specific growth parameters, highlighting the complexity of plant-microbe interactions under high-temperature conditions. The observed differences among Mantequilla, Sapito, and Tórtola further reflect genetic variability in stress tolerance, emphasizing the need for integrating microbial inoculants into breeding and agricultural management strategies. Understanding these interactions is essential for developing climate-resilient legume production systems, aligning with the goals of Sustainable Development Goal 2 (SDG 2: Zero Hunger), which calls for sustainable food production systems and resilient agricultural practices to ensure food security amid global climate challenges. This research contributes to the broader effort of paving the way for sustainable agricultural practices in a warming world. However, several limitations must be acknowledged. First, the localized nature of the experimental setting may have increased the likelihood of biotic contamination, such as pest infestations or pathogen interference, which are more frequent in open or semi-controlled environments and may influence plant performance independently of bacterial inoculation. Additionally, the response to PGPB treatments may differ under field conditions due to environmental variability, microbial competition in natural soils, and plant genotype–environment interactions not fully captured in this study. The short duration of the seedling-stage evaluation may also limit the extrapolation of results to later developmental stages or full yield potential. Additionally, limitation of this study lies in the use of non-commercial native landraces, which, despite their cultural and genetic richness, often present challenges such as variable germination and inconsistent physiological performance under controlled conditions. As a result, detailed analyses were focused on the three landraces (Tórtola, Mantequilla, and Sapito) that showed both robust germination and clear root colonization by PGPB, as confirmed by confocal microscopy. Future studies should incorporate standardized or commercial cultivars as comparative controls to further elucidate genotype-specific versus generalizable plant-microbe interactions under thermal stress, and also, field-scale validations to confirm the consistency and agronomic relevance of the observed effects.
Conclusion
High temperatures induce anatomical, morphological, and physiological changes in plants that can severely compromise growth and productivity. A sustainable alternative for mitigating these effects is the use of PGPB, which can enhance stress resilience through multiple mechanisms. This study evaluated the response of nine P. vulgaris L. landraces inoculated with native PGPB strains under thermal stress conditions. Among them, three landraces ‘Mantequilla’, ‘Sapito’, and ‘Tórtola’ were selected for detailed analysis due to their superior bacterial colonization and germination performance. Our results indicate that these landraces exhibited improved physiological responses under bacterial inoculation, particularly at moderate temperatures (up to 35°C). Notably, Mantequilla’ demonstrated the ability to grow even at 40 °C in the absence of inoculation, suggesting an inherent thermotolerance in this genotype. The consortium treatment significantly enhanced bacterial adhesion on root surfaces, as visualized by confocal microscopy, potentially strengthening plant–microbe interactions. PGPB inoculation led to distinct physiological effects depending on the treatment and landrace. For instance, B. proteolyticus Cyn1 notably increased radicle length and total plant length across all three landraces. ‘Sapito’ treated with B. safensis Cyn2 at 25 °C showed the highest ORAC activity in leaves, while ‘Mantequilla’ treated with B. safensis Cyn2 had the highest ORAC activity in roots at the same temperature. Furthermore, polyphenol accumulation was most pronounced in ‘Sapito’ at 25 °C with B. safensis Cyn2, and in ‘Mantequilla’ at 35 °C under the same treatment, indicating that PGPB can enhance antioxidant responses in a landrace and temperature dependent manner. In terms of stress markers, inoculated ‘Mantequilla’ plants under B. proteolyticus Cyn1 maintained more stable MDA levels despite rising temperatures, suggesting a protective role in reducing lipid peroxidation. However, proline levels declined sharply across all treatments at 40 °C, implying that the osmoprotective response becomes compromised under extreme heat. While PCA and correlation analyses confirmed some significant associations, not all trends reached statistical significance, indicating variability in plant responses and the need for further validation. Future research should extend these findings to field conditions, where PGPB consortia can be evaluated under naturally variable environmental factors. It will also be critical to assess the performance of these bacterial strains under multiple simultaneous abiotic stressors such as drought, salinity, and nutrient deficiency to determine their full potential in climate-resilient agriculture. Long-term and multi-site trials will help establish the consistency and reliability of PGPB applications, supporting their integration into sustainable farming systems.
Materials and methods
Plant materials and microorganisms
Ten Chilean landraces of common beans (Sapito, Mantequilla, Tórtola, Bayo, Cimarrón, Manteca, Arverjilla, Palo, Cabrito, and Araucano) were obtained from the Centro de Estudios en Alimentos Procesados (CEAP), Talca, Chile. Rhizospheric soil samples from the Chilean landrace of P. vulgaris L. were collected in April 2021 from agricultural land in Tonguao, Maule region, Chile (− 35.4236°S, 72.0563°W). PGPBs were isolated using the serial dilution method on Luria–Bertani (LB) agar medium (BD DIFCO, New Jersey, USA) supplemented with 1.8% agar. Two bacterial isolates, B. proteolyticus Cyn1 and B. safensis Cyn2, were identified using 16 S rRNA sequencing via the EZ-Taxon server, where Cyn1 demonstrated 100% nucleotide identity with B. proteolyticus TD42, and Cyn2 had 99.86% identity with B. safensis subsp. safensis. Their sequences were submitted to GenBank under accession numbers OM247622 (B. proteolyticus Cyn1) and OM247623 (B. safensis Cyn2). The isolates were previously characterized by our group Meza et al. (2022)35for their compatibility, plant growth promoting (PGP) activities, including phosphate solubilization, ammonia production, catalase activity, ACC deaminase production, and biofilm formation35. Additionally, their tolerance to abiotic stress was assessed, demonstrating that B. proteolyticus Cyn1 can withstand temperatures up to 80 °C, whereas B. safensis Cyn2 tolerates up to 70 °C. Scanning electron microscopy and confocal micrographs confirmed the viability of these strains under high-temperature conditions, with Cyn1 forming surface polysaccharide networks at 80°C35.
For experimental purposes, the bacterial samples B. proteolyticus Cyn1, B. safensis Cyn2, and a consortium of both (concentration: 108 CFU mL− 1) were cultured in Luria Bertani (LB) liquid medium (Difco™) according to the manufacturer’s instructions for 24 h in an incubator with an orbital shaker (YIHDER model LM-450D) at 30 °C and 150 rpm.
Qualitative and quantitative measurement of auxin production by bacteria
For auxin experiments, we followed the methodology of Gupta & Pandey (2019)90. Briefly, bacteria were inoculated in 50 mm each in LB medium (Difco™) supplemented with 0.1% tryptophan and incubated in a YIHDER model LM-450D orbital shaker for 7 days at 30 °C and 200 rpm. Auxin production was evaluated using Salkowski reagent (0.5 M FeCl3 + 70% perchloric acid), where 4 mL of bacterial culture was treated with 1 mL of reagent in test tubes. The tubes were left in darkness for 24 h, and colorimetric transformation to red indicated the presence of auxins. For quantitative measurement, the bacteria were cultured in LB medium (Difco™) supplemented with 0.1% tryptophan at 30 °C and 200 rpm for 7 days. Serial dilutions of 4 mL of the culture medium with sterile water were prepared from 10− 1 to 10− 9 with 1 mL of Salkowski reagent for 24 h. Absorbance was measured with 3 replicates in a MOBI (µ2 MicroDigital MOBI, Seoul, South Korea) spectrophotometer at 530 nm. A standard calibration curve for IAA was generated using IAA from Duchefa Biochemie (Herleem, Netherlands).
Seed bacterization and germination
Prior to inoculation, a rigorous seed disinfection protocol was applied to eliminate any pre-existing microorganisms. Initially, the seeds were rinsed thoroughly with running tap water and immersed in a solution of concentrated detergent diluted in distilled water until completely submerged. Following this, the seeds were rinsed again with distilled water. A sequential disinfection process was then conducted under sterile conditions. First, seeds were immersed in 0.5% sodium hypochlorite for 1 min, followed by three successive rinses with sterile distilled water. Subsequently, the seeds were transferred to a laminar flow cabinet. There, they were immersed in 50 mL of 10% (v/v) autoclaved detergent and agitated for 5 min. The seeds were then rinsed once more with sterile distilled water. Next, the seeds were submerged in 0.5% sodium hypochlorite solution, ensuring complete coverage, and rinsed three additional times with sterile water. Finally, a 2% sodium hypochlorite solution was added to cover the seeds, and a final set of three rinses with sterile distilled water was performed. This multi-step disinfection ensured the removal of any surface-borne microbial contaminants prior to bacterization. For bacterization, bacterial cultures of B. proteolyticus Cyn1, B. safensis Cyn2, and their 1:1 (v/v) consortium were grown separately in LB broth at 30 °C for 24 h. After incubation, cultures were centrifuged at 6,000 rpm for 10 min, and the resulting pellets were resuspended in sterile water. The final bacterial concentration was adjusted to approximately 10⁴ CFU mL−1 for B. proteolyticus Cyn1 and 102 CFU mL−1 for B. safensis Cyn2. For the consortium treatment, equal volumes of each suspension were mixed prior to seed inoculation. Each seed received 1 mL of the corresponding bacterial suspension directly on its surface (individual inoculation). The seeds were allowed to germinate in the dark at 24 °C for 3 days. After that, both sets with germinated seedlings (control and treated) were placed under a photoperiod of 16 h light and 8 h darkness for 2 additional days at 27 °C in a plant growth chamber. During the experiment, various germination and early growth parameters were recorded, including plumule length, radicle length, total root length, number of roots, germination rate, relative germination percentage, toxicity tolerance index, and vigor index, calculated according to the equations described below. In addition, antioxidant capacity (via ORAC assay) and total polyphenol content were analyzed in selected samples, as detailed in subsequent Sect.
Plant-bacteria association through root colonization was observed 5 days after bacterization using confocal microscopy. Prior to observation, bacterial strains were stained using the LIVE/DEAD BacLight™ kit, which differentially labels viable and non-viable cells. SYTO 9, which binds to nucleic acids in live cells, was used at an excitation/emission of 485 nm/498 nm, while propidium iodide (PI), which penetrates only damaged membranes, was used at 535 nm/617 nm. Fluorescent cell quantification was performed using ImageJ software (National Institutes of Health, USA). Fluorescence images were processed using the “Threshold” plugin to segment fluorescent cells from the background and subsequently analyzed using the “Analyze Particles” plugin to determine the number of fluorescent cells. Threshold and particle analysis parameters were optimized to ensure accurate quantification. Results were expressed as the number of fluorescent cells per field of view91.
Temperature tolerance screening
A pot experiment was conducted using the three best-performing bean landraces ‘Tórtola’, ‘Sapito’, and ‘Mantequilla’ subjected to a range of temperature treatments (25 °C, 30 °C, 35 °C, and 40 °C). Germinated seedlings were transplanted into pots containing a sterilized mixture of 50% sand and 50% organic humus (1:1 v/v). The pots were placed in a growth chamber under controlled environmental conditions. Light was maintained at a 16-hour light / 8-hour dark photoperiod, with a light intensity of approximately 340 µmol m⁻2 s−1 and relative humidity adjusted to 50–70%, depending on the treatment. Temperature was regulated according to the assigned heat stress condition for each experimental group. Seedlings were maintained at 25 °C, 30 °C, 35 °C, or 40 °C for 10 days to evaluate plant responses under increasing thermal stress. Plants were irrigated with 50 mL of distilled water every two days under 25–35 °C conditions, whereas under 40 °C, irrigation was applied daily (50 mL) to ensure adequate water availability and reduce additional stress from drought.
Physiological traits determination
Photosynthetic pigments
Photosynthetic pigments were measured by collecting 0.1 g of leaves from each plant, adding 5 mL of methanol, macerating, and filtering with a 0.45 μm polyvinylidene fluoride (PVDF) filter. The mixture was centrifuged at 4000 rpm for 1 min, and the supernatant was transferred to a 96-well microplate. Absorbance was measured at 662, 646, and 470 nm for chlorophyll a (Ca), b (Cb), and carotenoids92. Concentrations were calculated using the following equations and presented in a Heatmap through the Heatmapper server93:
Total phenolic content
Total phenol content of the leaves and roots for each treatment were separately determined using the Folin–Ciocalteu method as described in Plaza et al. (2023)94. Briefly, 1 g of dried and ground leaf or root sample was extracted using 40 mL of 80% (v/v) ethanol in a conical tube. The mixture was placed on an orbital shaker for 1 h and then filtered through 40-micron nylon filters. The resulting supernatant (extract) was transferred to a transparent 96-well plate, and absorbance was measured at 750 nm using a microplate reader. (Synergy HTX Multi-Mode Reader, Biotek, Santa Clara, CA, USA) with transparent plate, and results were expressed as gallic acid equivalents.
ORAC assay
The antioxidant capacity of the leaves and roots for each treatment was separately measured using a microplate reader (Synergy HTX Multi-Mode Reader, Biotek, Santa Clara, CA, USA) with black plates of 96 wells. Briefly, 0.5 g of dried and ground leaf or root sample was extracted using 20 mL of a 50% acetone hydroalcoholic solution in a conical tube. The mixture was shaken on an orbital shaker for 1 h and then filtered through 40-micron nylon filters. Sodium fluorescein, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH radical solution) and Trolox standard solution were used. Fluorescence was recorded every 5 min over 60 min, and results were expressed as µM Trolox Equivalents (TE) as described by Plaza et al. (2023)94.
Cellular homeostasis assay
MDA assay
MDA levels were determined by the thiobarbituric acid (TBA) reaction method95. Tissue samples were pulverized in liquid nitrogen, mixed with trichloroacetic acid (TCA), and centrifuged. Supernatants were incubated with TCA and TBA at 100 °C for 30 min, and absorbance was measured at 532 and 600 nm.
Proline concentration determination
Free proline concentration was determined following Bates et al. (1973)96. Tissue samples were homogenized with sulfosalicylic acid, centrifuged, and reacted with ninhydrin reagent. Chromophore was separated with toluene, and absorbance was read at 525 nm. Proline content was calculated using a standard proline curve.
Correlation and PCA analysis
The statistical analysis was performed using R software version 4.1.297. Various approaches were applied to assess the relationship between plant growth promoting bacteria (PGPB) in common beans under thermal stress. First, a multivariate analysis using Principal Component Analysis (PCA) was conducted with the package “stats”, aiming to reduce the dimensionality of the data and explore the relationships between the key variables influencing bacterial treatment across the nine landraces. To assess pairwise correlations between variables, a correlation matrix was constructed using the package “corr”, based on Spearman’s rank correlation coefficient. A Spearman’s coefficient (rs) equal to or less than 0.3 is interpreted as a weak effect size; rs = 0.6, moderate effect size; and rs = 0.6 onwards, strong effect size. Pairwise significance was also reported and determined by Student’s t-test. This allowed the identification of the most significant relationships between plant growth parameters.
Subsequently, GLMM models were constructed to evaluate the effect of each bean landrace subjected to the treatments (B. proteolyticus Cyn1, B. safensis Cyn2, and their consortium), and the control without bacterization (fixed effects) and their interaction on plant growth parameters (response variables): plumule length, radicle length, root length, number of roots, root length, germination rate, relative germination percentage, toxicity tolerance index, and vigor index. A model was built for each response variable; the plant individual was included as a random variable in all models. All GLMM models were constructed using the glmmTMB function in the glmmTMB package98. Three plant individuals of each bean landrace were tested for each growth-promoting bacterial treatment.
To determine the best models, the GLMM with the lowest Akaike information criterion (AIC) was selected. The fit of all models will be assessed using diagnostic plots from the DHARMa package99. The model with uniformity of residuals (Kolmogorov-Smirnov test) was selected by comparing the plot of standardised residuals with the predicted values. The estimated marginal means for each fixed effect were tabulated using the R emmeans package and the significance of pairwise differences were tested using Tukey’s method. Wald type II chi-square (x2) tests were performed using the Anova function of the CAR package100.
Data availability
The datasets analysed during the current study are available in the https://www.ncbi.nlm.nih.gov/repository, accession numbers OM247622 and OM247623.
References
Ammar, G. Plant beneficial symbionts: fashionable charming members in the phytomicrobiome community. Future Perspect. Med. Pharm. Environ. Biotechnol. 1(1), 19–30. https://doi.org/10.21608/fpmpeb.2024.266342.1007 (2024).
Dirección Meteorológica de Chile. Servicios climáticos. https://www.meteochile.gob.cl/PortalDMC-web/index.xhtml.
Saleem, A. et al. Securing a sustainable future: the climate change threat to agriculture, food security, and sustainable development goals. J. Umm Al-Qura Univ. Appl. Sci.. https://doi.org/10.1007/s43994-024-00177-3 (2024).
Maity, A. et al. Climate change impacts on seed production and quality: current knowledge, implications, and mitigation strategies. Seed Sci. Technol. 51(1), 65–96. https://doi.org/10.15258/sst.2023.51.1.07 (2023).
Arriagada, O. et al. A past genetic bottleneck from Argentine beans and a selective sweep led to the race Chile of the common bean (Phaseolus vulgaris L). Int. J. Mol. Sci. 25(7), 4081. https://doi.org/10.3390/ijms25074081 (2024).
Blair, M. W., Li, H., Nekkalapudi, L., Becerra, V. & Paredes, M. Nutritional traits of beans (Phaseolus vulgaris): nutraceutical characterization and genomics. In Compendium of Crop Genome Designing for Nutraceuticals. 611–638. https://doi.org/10.1007/978-981-19-4169-6_23 (Springer Nature Singapore, 2023).
Márquez, K. et al. Nutritional characterization of Chilean landraces of common bean. Plants 13(6), 817. https://doi.org/10.3390/plants13060817 (2024).
Louwaars, N. P. & Manicad, G. Seed systems resilience—An overview. Seeds 1(4), 340–356. https://doi.org/10.3390/seeds1040028 (2022).
Martínez-Barradas, V. et al. Drought tolerance evaluation of ‘zorzal,’ the most cultivated common bean in chile, a country facing desertification. Agricultural Res. 13(1), 41–52. https://doi.org/10.1007/s40003-023-00679-2 (2024).
Egamberdieva, D., Wirth, S. J., Shurigin, V. V., Hashem, A. & Abd_Allah, E. F. Endophytic bacteria improve plant growth, symbiotic performance of Chickpea (Cicer arietinum L.) and induce suppression of root rot caused by fusarium Solani under salt stress. Front. Microbiol. 8 https://doi.org/10.3389/fmicb.2017.01887 (2017).
Fanai, A. et al. Plant growth promoting bacteria (PGPB)-induced plant adaptations to stresses: an updated review. PeerJ 12, e17882. https://doi.org/10.7717/peerj.17882 (2024).
Zahra, S. T. et al. Salt-tolerant plant growth-promoting bacteria (ST-PGPB): an effective strategy for sustainable food production. Curr. Microbiol. 81(10), 304. https://doi.org/10.1007/s00284-024-03830-6 (2024).
Cao, M. et al. Optimistic contributions of plant growth-promoting bacteria for sustainable agriculture and climate stress alleviation. Environ. Res. 217, 114924. https://doi.org/10.1016/j.envres.2022.114924 (2023).
Waday, Y. A., Girma Aklilu, E., Bultum, M. S., Ramayya Ancha, V. & Beyene, D. Isolation and characterization of plant growth-promoting rhizobacteria from coffee plantation soils and its influence on maize growth. Appl. Environ. Soil. Sci. 2022, 1–8. https://doi.org/10.1155/2022/5115875 (2022).
Jana, S. K., Islam, M. M., Hore, S. & Mandal, S. Rice seed endophytes transmit into the plant seedling, promote plant growth and inhibit fungal phytopathogens. Plant. Growth Regul. 99(2), 373–388. https://doi.org/10.1007/s10725-022-00914-w (2023).
Chandran, H., Meena, M. & Swapnil, P. Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 13(19), 10986. https://doi.org/10.3390/su131910986 (2021).
Khatoon, Z. et al. Role of plant growth-promoting bacteria (PGPB) in abiotic stress management. In Mitigation of Plant Abiotic Stress by Microorganisms, 257–272 https://doi.org/10.1016/B978-0-323-90568-8.00012-2 (Elsevier, 2022).
Gupta, A. et al. Plant growth promoting rhizobacteria (PGPR): A sustainable agriculture to rescue the vegetation from the effect of biotic stress: a review. Lett. Appl. Nanobiosci. 10(3), 2459–2465. https://doi.org/10.33263/LIANBS103.24592465 (2021).
Basu, A. et al. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability 13(3), 1140. https://doi.org/10.3390/su13031140 (2021).
Shaffique, S. et al. A review on the role of endophytes and plant growth promoting rhizobacteria in mitigating heat stress in plants. Microorganisms 10(7), 1286. https://doi.org/10.3390/microorganisms10071286 (2022).
Zulfiqar, S., Sharif, S., Saeed, M. & Tahir, A. Role of carotenoids in photosynthesis. In Carotenoids: Structure and Function in the Human Body, 147–187 https://doi.org/10.1007/978-3-030-46459-2_5 (Springer International Publishing, 2021).
Rácz, A. et al. Fight against cold: photosynthetic and antioxidant responses of different bell pepper cultivars (Capsicum annuum L.) to cold stress. Biol. Futur. 74(3), 327–335. https://doi.org/10.1007/s42977-023-00182-3 (2023).
Sharma, P., Jha, A. B., Dubey, R. S. & Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26. https://doi.org/10.1155/2012/217037 (2012).
Suárez, J. C. et al. Bioactive compounds and antioxidant activity in seeds of bred lines of common bean developed from interspecific crosses. Foods 12(15), 2849. https://doi.org/10.3390/foods12152849 (2023).
Nabizadeh, E., Dolatmand, N., Haghshenas, M. & Ahmadi, K. Physiological and biochemical changes of maize (Zea mays ‘MV500’) in response to heat stress under levels of Salicylic acid. Eur. J. Biology. 0(0), 0–0. https://doi.org/10.26650/EurJBiol.2023.1216574 (2023).
Raza, A. et al. Assessment of proline function in higher plants under extreme temperatures. Plant. Biol. 25(3), 379–395. https://doi.org/10.1111/plb.13510 (2023).
Ullah, A., Sun, H., Yang, X. & Zhang, X. Drought coping strategies in cotton: increased crop per drop. Plant. Biotechnol. J. 15(3), 271–284. https://doi.org/10.1111/pbi.12688 (2017).
Azeem, M., Javed, S. & Zahoor, A. F. Bacillus species as potential plant growth promoting rhizobacteria for drought stress resilience. Russ. J. Plant Physiol. 70(4), 59. https://doi.org/10.1134/S1021443723600538 (2023).
AlAli, H. A., Khalifa, A. & Almalki, M. Plant growth-promoting rhizobacteria from ocimum Basilicum improve growth of phaseolus vulgaris and Abelmoschus esculentus. South. Afr. J. Bot. 139, 200–209. https://doi.org/10.1016/j.sajb.2021.02.019 (2021).
Khan, M. H. U. et al. Bacillus safensis with plant-derived smoke stimulates rice growth under saline conditions. Environ. Sci. Pollut. Res. 24(30), 23850–23863. https://doi.org/10.1007/s11356-017-0026-y (2017).
Yang, P. et al. Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant. Sci. 14 https://doi.org/10.3389/fpls.2023.1078100 (2023).
Abdelsalam, S., Abu Hujier, N., Sharif, F. A. & Fahd, M. I. Improvement of phaseolus vulgaris growth by inoculation with multifunctional native rhizobacteria isolated from rhizospheric soils in Gaza strip- Palestine. J. Sci. Res. Sci. 37(2), 1–29. https://doi.org/10.21608/jsrs.2020.129928 (2020).
Etesami, H., Jeong, B. R. & Glick, B. R. Potential use of Bacillus spp. As an effective biostimulant against abiotic stresses in crops—A review. Curr. Res. Biotechnol. 5, 100128. https://doi.org/10.1016/j.crbiot.2023.100128 (2023).
Yang, N. et al. Emergent bacterial community properties induce enhanced drought tolerance in Arabidopsis. NPJ Biofilms Microbiomes. 7(1), 82. https://doi.org/10.1038/s41522-021-00253-0 (2021).
Meza, C. et al. Plant-growth-promoting bacteria from rhizosphere of Chilean common bean ecotype (Phaseolus vulgaris L.) supporting seed germination and growth against salinity stress. Front. Plant. Sci. 13 https://doi.org/10.3389/fpls.2022.1052263 (2022).
Oficina de Programa sobre Evaluación Mundial de los Recursos Hídricos. Informe Mundial de Las Naciones Unidas Sobre El Desarrollo de Los Recursos Hídricos 2020 Datos y Cifras.
Hawkins Martínez, J. J., Ortiz Aragón, A. N. & Larios González, R. C. Siembra a doble Surco y Surco Sencillo y Su efecto En El rendimiento de semilla de Frijol Común (Phaseolus vulgaris L). La. Calera. 22(39). https://doi.org/10.5377/calera.v22i39.15165 (2022).
Kapoor, D., Sharma, P., Sharma, M. M. M., Yadav, S. & Husen, A. Exploring soil microbiota and their role in plant growth, stress tolerance, disease control and nutrient immobilizer. Biocatal. Agric. Biotechnol. 61, 103358. https://doi.org/10.1016/j.bcab.2024.103358 (2024).
Das, P. P. et al. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 214, 113821. https://doi.org/10.1016/j.envres.2022.113821 (2022).
El-Saadony, M. T. et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: mechanisms, challenges and future perspectives. Front. Plant. Sci. 13 https://doi.org/10.3389/fpls.2022.923880 (2022).
Liu, A., Contador, C. A., Fan, K. & Lam, H. M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front. Plant. Sci. 9 https://doi.org/10.3389/fpls.2018.01860 (2018).
Hungria, M., Nogueira, M. A. & Araujo, R. S. Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol. Fertil. Soils. 49(7), 791–801. https://doi.org/10.1007/s00374-012-0771-5 (2013).
de Souza, R., Ambrosini, A. & Passaglia, L. M. P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 38(4), 401–419. https://doi.org/10.1590/S1415-475738420150053 (2015).
Khoso, M. A. et al. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: current perspective. Plant. Stress. 11, 100341. https://doi.org/10.1016/j.stress.2023.100341 (2024).
Niza-Costa, M. et al. Geographically disperse, culturable seed-associated microbiota in forage plants of alfalfa (Medicago sativa L.) and pitch clover (Bituminaria Bituminosa L.): characterization of beneficial inherited strains as plant Stress-Tolerance enhancers. Biology (Basel). 11(12), 1838. https://doi.org/10.3390/biology11121838 (2022).
Li, Y. et al. Biofilms formation in plant growth-promoting bacteria for alleviating agro-environmental stress. Sci. Total Environ. 907, 167774. https://doi.org/10.1016/j.scitotenv.2023.167774 (2024).
Kunkel, B. N. & Johnson, J. M. B. Auxin plays multiple roles during plant–pathogen interactions. Cold Spring Harb Perspect. Biol. 13(9), a040022. https://doi.org/10.1101/cshperspect.a040022 (2021).
Yu, Z., Zhang, F., Friml, J. & Ding, Z. Auxin signaling: research advances over the past 30 years. J. Integr. Plant. Biol. 64(2), 371–392. https://doi.org/10.1111/jipb.13225 (2022).
Pandey, C., Dheeman, S., Prabha, D., Negi, Y. K. & Maheshwari, D. K. Plant growth-promoting bacteria: effective tools for increasing nutrient use efficiency and yield of crops. 293–313 https://doi.org/10.1007/978-3-030-65447-4_13 (2021).
Kameswara Rao, N., Dulloo, M. E. & Engels, J. M. M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 64(5), 1061–1074. https://doi.org/10.1007/s10722-016-0425-9 (2017).
Jung, J. K., Lee, S. U., Kozukue, N., Levin, C. E. & Friedman, M. Distribution of phenolic compounds and antioxidative activities in parts of sweet potato (Ipomoea batata L.) plants and in home processed roots. J. Food Compos. Anal. 24(1), 29–37. https://doi.org/10.1016/j.jfca.2010.03.025 (2011).
Ganesan, K. & Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 18(11), 2331. https://doi.org/10.3390/ijms18112331 (2017).
Flieger, J., Flieger, W., Baj, J., Maciejewski, R. & Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 14(15), 4135. https://doi.org/10.3390/ma14154135 (2021).
Tang, J. et al. Biosynthetic pathways and functions of Indole-3-Acetic acid in microorganisms. Microorganisms 11(8), 2077. https://doi.org/10.3390/microorganisms11082077 (2023).
Mondal, S., Halder, S. K., Yadav, A. N. & Mondal, K. C. Microbial consortium with multifunctional plant growth-promoting attributes: future perspective in agriculture. I219–258. https://doi.org/10.1007/978-981-15-3204-7_10 (2020).
Sofi, P. A. et al. Integrating root architecture and physiological approaches for improving drought tolerance in common bean (Phaseolus vulgaris L). Plant. Physiol. Rep. 26(1), 4–22. https://doi.org/10.1007/s40502-021-00570-8 (2021).
Romanowicz, K. J., Freedman, Z. B., Upchurch, R. A., Argiroff, W. A. & Zak, D. R. Active microorganisms in forest soils differ from the total community yet are shaped by the same environmental factors: the influence of pH and soil moisture. FEMS Microbiol. Ecol. 92(10), fiw149. https://doi.org/10.1093/femsec/fiw149 (2016).
Glick, B. R. Beneficial Plant-Bacterial Interactions https://doi.org/10.1007/978-3-319-13921-0 (Springer International Publishing, 2015).
Martínez-Ballesta, M., del Egea-Gilabert, C. & Conesa, C. The importance of ion homeostasis and nutrient status in seed development and germination. Agronomy 10(4), 504. https://doi.org/10.3390/agronomy10040504 (2020).
Racioppo, A. et al. Potential use of plant growth-promoting bacteria to enhance growth and soil fertility in marginal areas: focus on the Apulia region, Italy. Agronomy 13(12), 2983. https://doi.org/10.3390/agronomy13122983 (2023).
Carr, K., Ozowara, X. & Sloey, T. M. Effects of climate change on seed germination may contribute to habitat homogenization in freshwater forested wetlands. Plant. Ecol. 225(10), 1023–1033. https://doi.org/10.1007/s11258-024-01451-4 (2024).
Sita, K. et al. Food legumes and rising temperatures: effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant. Sci. 8 https://doi.org/10.3389/fpls.2017.01658 (2017).
Silva, D. A. et al. AF. Influence of high temperature on the reproductive biology of dry edible bean (Phaseolus vulgaris L.). Sci Agric. 77(3). https://doi.org/10.1590/1678-992x-2018-0233 (2020).
Chaves-Barrantes, N. F. & Gutiérrez-Soto, M. V. Respuestas al estrés Por Calor En Los cultivos. I. Aspectos moleculares, bioquímicos y siológicos. Agronomía Mesoamericana. 28(1), 237. https://doi.org/10.15517/am.v28i1.21903 (2016).
Li, Y. et al. Indigenous PGPB inoculant from Qinghai-Tibetan plateau soil confer drought-stress tolerance to local grass Poa annua. Int. J. Environ. Res. 16(5), 85. https://doi.org/10.1007/s41742-022-00470-1 (2022).
Balliu, A. et al. Environmental and cultivation factors affect the morphology, architecture and performance of root systems in soilless grown plants. Horticulturae 7(8), 243. https://doi.org/10.3390/horticulturae7080243 (2021).
Xiong, R. et al. Root system architecture, physiological and transcriptional traits of soybean (< scp > Glycine max L.) in response to water deficit: A review. Physiol. Plant. 172(2), 405–418. https://doi.org/10.1111/ppl.13201 (2021).
González-García, M. P. et al. Temperature changes in the root ecosystem affect plant functionality. Plant. Commun. 4(3), 100514. https://doi.org/10.1016/j.xplc.2022.100514 (2023).
Barbosa, J. Z. et al. Meta-analysis reveals benefits of co-inoculation of soybean with azospirillum Brasilense and Bradyrhizobium spp. In Brazil. Appl. Soil. Ecol. 163, 103913. https://doi.org/10.1016/j.apsoil.2021.103913 (2021).
Beebe, S. E., Rao, I. M., Blair, M. W. & Acosta-Gallegos, J. A. Phenotyping common beans for adaptation to drought. Front. Physiol. 4 https://doi.org/10.3389/fphys.2013.00035 (2013).
Liu, X., Quan, W. & Bartels, D. Stress memory responses and seed priming correlate with drought tolerance in plants: an overview. Planta 255(2), 45. https://doi.org/10.1007/s00425-022-03828-z (2022).
Khaeim, H. et al. Impact of temperature and water on seed germination and seedling growth of maize (Zea mays L). Agronomy 12(2), 397. https://doi.org/10.3390/agronomy12020397 (2022).
Giri, A., Heckathorn, S., Mishra, S. & Krause, C. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 6(1), 6. https://doi.org/10.3390/plants6010006 (2017).
Parthasarathi, T., Firdous, S., Mariya David, E., Lesharadevi, K. & Djanaguiraman, M. Effects of high temperature on crops. In Advances in Plant Defense Mechanisms https://doi.org/10.5772/intechopen.105945 (IntechOpen, 2022).
Camejo, D. et al. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant. Physiol. 162(3), 281–289. https://doi.org/10.1016/j.jplph.2004.07.014 (2005).
Elnaggar, A. et al. Citrullus colocynthis regulates photosynthetic and biochemical processes to develop stress resilience and sustain growth under sub-optimal temperatures. Plant. Stress. 12, 100502. https://doi.org/10.1016/j.stress.2024.100502 (2024).
Bode, S. et al. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. 106(30), 12311–12316. https://doi.org/10.1073/pnas.0903536106 (2009).
Mathur, S., Agrawal, D. & Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol B. 137, 116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010 (2014).
Martins, M. Q. et al. Protective response mechanisms to heat stress in interaction with high [CO2] conditions in coffea spp. Front. Plant. Sci. 7 https://doi.org/10.3389/fpls.2016.00947 (2016).
Uarrota, V. G., Stefen, D. L. V., Leolato, L. S., Gindri, D. M. & Nerling, D. Revisiting carotenoids and their role in plant stress responses: from biosynthesis to plant signaling mechanisms during stress. In Antioxidants and Antioxidant Enzymes in Higher Plants, 207–232 https://doi.org/10.1007/978-3-319-75088-0_10 (Springer International Publishing, 2018).
Kavi Kishor, P. B. & Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant. Cell. Environ. 37(2), 300–311. https://doi.org/10.1111/pce.12157 (2014).
Bhaskara, G. B., Yang, T. H. & Verslues, P. E. Dynamic proline metabolism: importance and regulation in water limited environments. Front. Plant. Sci. 6 https://doi.org/10.3389/fpls.2015.00484 (2015).
Siddique, A., Kandpal, G. & Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: an overview. J. Pure Appl. Microbiol. 12(3), 1655–1659. https://doi.org/10.22207/JPAM.12.3.73 (2018).
Davey, M. W., Stals, E., Panis, B., Keulemans, J. & Swennen, R. L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 347(2), 201–207. https://doi.org/10.1016/j.ab.2005.09.041 (2005).
Mihaljević, I. et al. Comparative study of drought stress effects on traditional and modern apple cultivars. Plants 10(3), 561. https://doi.org/10.3390/plants10030561 (2021).
Zhang, Y., Luan, Q., Jiang, J. & Li, Y. Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy. Front. Plant. Sci. 12 https://doi.org/10.3389/fpls.2021.735275 (2021).
Hong, E. et al. Effects of high temperature stress on the physiological and biochemical characteristics of paeonia ostii. Int. J. Mol. Sci. 24(13), 11180. https://doi.org/10.3390/ijms241311180 (2023).
Tiwari, Y. K. Proline as a key player in heat stress tolerance: insights from maize. Discover Agric. 2(1), 121. https://doi.org/10.1007/s44279-024-00084-5 (2024).
Martínez-Barradas, V. et al. PvMYB60 gene, a candidate for drought tolerance improvement in common bean in a climate change context. Biol. Res. 57(1), 52. https://doi.org/10.1186/s40659-024-00528-8 (2024).
Gupta, S. & Pandey, S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front. Microbiol. 10 https://doi.org/10.3389/fmicb.2019.01506 (2019).
O’Brien, J., Hayder, H. & Peng, C. Automated quantification and analysis of cell counting procedures using ImageJ plugins. J. Visualized Experiments. https://doi.org/10.3791/54719 (2016).
Saini, A., Singh, J. & Kant, R. Comparative study of estimation methods for efficient extraction of chlorophyll a and carotenoids using different solvents. Bull. Pure Appl. Sciences- Bot. 41b(2), 79–86. https://doi.org/10.5958/2320-3196.2022.00009.X (2022).
Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44(W1), W147–W153. https://doi.org/10.1093/nar/gkw419 (2016).
Plaza, A. et al. Effects of extraction methods on phenolic content, antioxidant and antiplatelet activities of tomato pomace extracts. Plants 12(5), 1188. https://doi.org/10.3390/plants12051188 (2023).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 125(1), 189–198. https://doi.org/10.1016/0003-9861(68)90654-1 (1968).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant. Soil. 39(1), 205–207. https://doi.org/10.1007/BF00018060 (1973).
R Core Team. R: A Language and Environment for Statistical Computing. (2017).
Magnusson, K., Nilsson, A., Andersson, G., Hellner, C. & Carlbring, P. Level of agreement between problem gamblers’ and collaterals’ reports: A Bayesian Random-Effects Two-Part model. J. Gambl. Stud. 35(4), 1127–1145. https://doi.org/10.1007/s10899-019-09847-y (2019).
Hartig, F. & DHARMa Residual diagnostics for hierarchical (Multi-Level / Mixed) regression models. CRAN: contributed packages. https://doi.org/10.32614/CRAN.package.DHARMa (2016).
Fox, J. & Weisberg, S. An R companion to applied regression. (2018).
Acknowledgements
The research team thanks the Regional Centers Scientific Development Strengthening program 2020-2025 (R20F0001). They are also thanksful to Ramón Amigo, Amanda Morales and Ariel Hormazabal for help in the pot experiments. In addition, the support of the facilities manager Isabel Vidal Beltrán of Confocal Microscopy at the Universidad Católica del Maule of the FONDEQUIP-ANID EQM200122 project is appreciated. Finally, the authors acknowledge the Proyecto Anillos de Investigación ANID-2024. Code: ATE230007.
Funding
This study was funded by the Scientific Strengthening Program of Regional Centers ANID-R20F0001 and Proyecto Anillos de Investigación ANID-2024. Code: ATE230007.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by C.M., F.V., M.Y., and A.P. N.F., and J.M-N performed the statistical analysis. All authors read and approved the final manuscript. C.M. prepared the first draft of the manuscript. R.C., P.R., B.C., P.A-J., and A.B. revised the manuscript. A.B. conceived and designed the study. B.C., and P.A-J. obtained the funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Meza, C., Valenzuela, F., Yáñez, M. et al. Auxin producing plant growth promoting bacteria enhance temperature stress tolerance in Chilean common bean landraces. Sci Rep 15, 30359 (2025). https://doi.org/10.1038/s41598-025-15645-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-15645-x