Abstract
Breast cancer remains the leading cause of cancer-related deaths worldwide, with the triple-negative breast cancer (TNBC) subtype exhibiting a particularly high mortality rate. Conventional immunotherapy treatments have proven ineffective for this subtype, highlighting the need for the identification of novel tumor antigens, such as Syntenin-1. This 32 kDa protein is linked to cellular proliferation, angiogenesis, and metastasis. Recent research has proposed both active (vaccines) and passive (antibodies) immunotherapy as potential complementary treatments for breast cancer. The primary objective of this study was to assess the efficacy of targeting Syntenin-1 through active and passive immunity as a strategy for developing new immunotherapies for TNBC. We conducted an in silico analysis to select a peptide derived from the amino acid sequence of Syntenin-1, which was synthesized chemically as MAP8. This peptide was administered to Balb/c mice to induce a humoral immune response. Immunized mice were then used to obtain polyclonal antibodies for evaluating active immunity. A total of twenty-eight Balb/c mice were divided into seven experimental groups. Tumor induction was achieved by administering the 4T1 cell line (5 × 104 cells) for 30 days in groups 3–7. Passive treatment was given at low (1 mg/kg) and high (1 mg/kg) doses on days 8, 15, and 22 following tumor induction. Mice were sacrificed to collect blood and organs for analyzing tumor growth, metastasis, and the humoral immune response. The KA-11-MAP8 peptide, derived from the PDZ-2 domain of Syntenin-1, successfully induced antibody production in Balb/c mice after administration. Purified antibodies were able to recognize the native protein in both the 4T1 cell line and the brain. Both passive and active treatments targeting Syntenin-1 resulted in reduced tumor size and fewer metastatic nodules in the lungs. This study provides evidence for the efficacy of the KA-11-MAP8 peptide derived from Syntenin-1 in eliciting a humoral immune response, which in turn impacts tumor development and metastasis in a murine model of TNBC.
Similar content being viewed by others
Introduction
Breast cancer (BC) is a disease with a high social, economic, and public health impact around the world, with around 2.3 million new cases, which correspond to 11.6% of the total cases of cancer. BC affects women mainly from sub-developing countries, and it is associated with genetic and non-genetic risk factors1,2. BC is classified according to the expression of the receptors to estrogens (ER), progesterone (PR), and human epidermal growth factor receptor 2 (HER2). The following molecular subtypes have been described: Luminal A (ER/PR+, HER2-), Luminal B (ER/PR+, HER2-), HER2 (ER/PR+), HER2 (ER/PR-), and basal type (ER-, PR-, HER2-) or triple-negative breast cancer (TNBC) due to the null expression of hormonal receptors3. A detailed analysis of the transcriptomic heterogenicity of this group has allowed the subclassification of basal-like-1 (BL1), basal-like 2 (BL2), mesenchymal (M), and luminal androgen receptor (LAR), sharing characteristics as a complex genome with a high number of activated and inactivated genes and a high mutation rate in comparison to other molecular types4,5.
TNBC corresponds to 15% of the total BC cases and is the most common type in young women; the main treatments are surgery and chemotherapy due to the shortage of molecular targets6; however, patients treated with chemotherapy have a high rate of recurrence and metastasis7,8,9. The TNBC is considered the most immunogenic type of BC and combines sessions of chemotherapy with immunotherapy to promote the activation of the immune system for the recognition, attack, and elimination of the tumoral cells through the empowerment of surveillance, antigen recognition, and generation of memory by immune cells10. Immunotherapy could be divided into passive and active treatments; passive therapies are based on the administration of Immune Checkpoint Inhibitors (ICI) such as Ipilimumab, Pembrolizumab, and Atezolizumab against CTLA-4, PD-1, and PD-L1, respectively11,12. Meanwhile, active therapies are focused on the analysis of potential molecular targets for the development of vaccines13.
Cancer vaccines have emerged as a promising therapeutic approach in recent years, particularly in preventing the recurrence and metastasis of tumors. These vaccines, often based on DNA and peptides, aim to induce an immune response in vivo against overexpressed molecules10. Tumor antigens can be classified as tumor-specific antigens (TSA) or tumor-associated antigens (TAA)14. TSA are typically associated with mutations in the DNA; making their identification is complex, and their expression is almost exclusive in each patient. TAA, on the other hand, are expressed at low levels in non-cancerous cells compared to the tumoral tissue15. The search and identification of tumoral antigens remain a significant challenge in the development of novel therapies against BC, and numerous studies have been conducted to identify new molecular targets for each BC subtype with immunological properties.
Syntenin-1 is a protein of 298 aa with a molecular weight of 32 kDa, codified by the SDCBP/MDA-9 gene (Syndecan Binding Protein/melanoma differentiation-associated gene-9) on chromosome 8. Syntenin-1 has two PDZ domains (PDZ-1 and PDZ-2) that interact with its target proteins by the C-terminal domain, suggesting a role as an adapter protein16. Syntenin-1 is involved in the biogenesis and uptake of exosomes by interacting with syndecans, which have an essential role in the cellular membrane for the interaction with growth factors, chemokines, extracellular matrix proteins, and receptors for adhesion, growth, and cellular differentiation17. In vivo and in vitro analysis of Syntenin-1 in breast cancer established a correlation between the expression of this protein and metastatic capacity, due to its overexpression in the cell lines MDA-MB-231 and MDA-MB-435. Also, it has been reported that Syntenin-1 activates the ERK1/2/MAPK pathway, and promotes motility and cellular invasion, leading to cancer progression18. Also, the inhibition of the PDZ1 domain of Syntenin-1 regulates the production of proinflammatory cytokines such as IL-10, IL-23, and IL-1β, which mediate the infiltration of MDSC and promote the tumoral immunosuppression. Also, it has been suggested that the inhibition of Syntenin-1 expression can decrease the number of tumoral cells and the number of metastatic nodes in the lungs19. Due to the critical role of Syntenin-1 in promoting cancer development, this research focuses on analyzing the active and passive immunity against the KA-11-MAP8 peptide derived from the PDZ-2 domain of Syntenin-1, aiming to induce a humoral immune response and impact tumoral development and metastasis in a murine TNBC model.
Methods
Ab initio modeling and identification of immunogenic and antigenic peptides in Syntenin-1
We download the FASTA amino acid sequence from NCBI’s protein database (https://www.ncbi.nlm.nih.gov/protein/), with access number NP_001007068.1. The ab initio modeling was performed in RaptorX (http://raptorx.uchicago.edu/), and the structure was evaluated in the server SAVESv6.1 of PROCHECK (https://saves.mbi.ucla.edu/) for the obtaining of data about the stereochemical structure of the protein. The identification of immunogenic peptides was performed in the database IEDB (Immune Epitope Database: http://tools.iedb.org/main/) and NetMHCII 2.3 (http://www.cbs.dtu.dk/services/NetMHCII/), while, antigenicity was performed using the Predicted Antigenic Peptides by the Kolaskar and Tongaonkar (http://imed.med.ucm.es/Tools/antigenic.pl).
3D structure and peptide selection
We identified candidate peptides from the selected model using the Protean 3D software from DNAStar Lasergene Protein (https://www.dnastar.com/software/protein/). The 3D structure was visualized using PyMOL (https://pymol.org/2/) and Protter (http://wlab.ethz.ch/protter/start/). We ultimately chose one peptide based on its cellular localization, immunogenicity, physicochemical properties, and 3D structure, which we named KA-11-MAP8. The selected peptide was chemically synthesized by Pepmic (http://www.pepmic.com/) in a multi-antigenic peptide format with eight ramifications (MAP8). Following the synthesis of the peptide in MAP8 format, high-performance liquid chromatography (HPLC) and mass spectrometry (MS) were conducted (Supplementary Material 1).
Mice immunization
Fifteen eight-week-old female Balb/c mice were immunized subcutaneously with 10 µg of KA-11-MAP8, mixed with incomplete Freund’s adjuvant (Sigma Aldrich Cat# F5506). The mice were monitored for 120 days during which we evaluated their immune response by detecting antibodies against the inoculated peptide.
Cell culture
The murine triple-negative breast cancer (TNBC) cell line 4T1, which is derived from a spontaneous breast tumor in mice, was thawed in a water bath at 37 °C. The cells were cultured in 70 mm2 plates using DMEM (Dulbecco’s Modified Eagle Medium, Gibco LOT2661717) supplemented with 8% fetal bovine serum (FBS). The plates were then incubated at 37 °C with 5% CO2 for 24 h or until the cells reached approximately 70% confluence. To recover the cells, we added 4 mL of Trypsin (Gibco Trypsin–EDTA Cat# 253000-54) and incubated them for 5 min at 37 °C. The cells were then detached by pipetting and transferred to a 15 mL tube, which was centrifuged at 2500 rpm for 10 min. Finally, the cells were counted using a digital cell counter (Life Technologies, Countess® II FL Cat# AMQAX1000) and resuspended in filtered PBS to develop the tumoral model.
Indirect and Cell-ELISA
For the Indirect ELISA, the selected peptide was diluted to a final concentration of 1 µg/mL in a carbonate buffer with a pH of 9.6. A volume of 100 µL of this solution was used to coat high-binding 96-well plates (Costar Cat#3590). The plates were incubated for two hours at 37 °C and subsequently blocked with skimmed milk (Difco LOT2339387) diluted in PBS-T (Phosphate-Buffered Saline with Tween 20) at a concentration of 0.05%. For the Cell-ELISA, the 4T1 cell line was seeded in 96-well culture plates until they reached a final confluence of 80%. The cells were fixed with 4% paraformaldehyde for 15 min at room temperature (RT), washed with sterile PBS for 1 min, and then permeabilized with Triton 100X diluted 1:100 in sterile PBS for 30 min with gentle agitation. The plates were blocked for two hours with skimmed milk.
In both techniques, the primary antibodies used were serum samples diluted 1:500 in PBS with a pH of 7.2, which were incubated overnight at 4 °C. The following secondary antibodies were then applied: monoclonal antibodies anti-IgG (H + L whole molecule at 1:8,000), anti-Mu (IgM at 1:6,000; Jackson ImmunoResearch Cat#AB2340064), anti-Gamma (IgG at 1:8,000; Thermo Fisher Scientific Cat#A16084), and isotype-specific antibodies anti-IgG1 (Abcam Cat#AB98693), anti-IgG2a (Abcam Cat#AB98698), anti-IgG2b (Abcam Cat#AB98703), and anti-IgG3 (Abcam Cat#AB98708), all diluted to 1:4,000. These secondary antibodies, which were coupled to horseradish peroxidase (HRP), were incubated for two hours at 37 °C. After each step, the plates were washed three times with PBS-T 0.05% for 5 min. The enzymatic reaction was developed using O-phenylenediamine dihydrochloride (Sigma-Aldrich LOT#SLBW6100) and terminated with 2 N sulfuric acid (H2SO4). The optical density was measured at 492 nm using a microplate reader.
Dot blot and Western blot
The nitrocellulose membranes were fixed with total protein extract derived from the brain (positive control) using RIPA buffer, which consists of 50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40, protease inhibitors, and PMSF. The KA-11-MAP8 peptide was added to the mixture, and it was incubated overnight at 4 °C. Blocking was performed using 5% skimmed milk diluted in PBS-Tween 0.005% for one hour on an orbital shaker at room temperature. The membranes were then incubated with antibodies against Syntenin-1. A commercial antibody from Santa Cruz Biotechnology (Cat#sc-515538, diluted 1:1500) was used in combination with a polyclonal antibody derived from Balb/C mice inoculated with the KA-11-MAP8 peptide (dilution 1:1000). This incubation lasted for three hours at room temperature. For detection, a secondary antibody, HRP-goat anti-mouse IgG (dilution 1:8000), was applied. The reaction was developed using the Western Blotting Luminol Reagent from Santa Cruz Biotechnology (Cat#sc-2048). In the Western blot analysis, total protein was extracted from 4T1 cells and the brain. Samples were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were tested with the anti-Syntenin-1 antibody (dilution 1:1500; Santa Cruz Biotechnology, Cat#sc-515538), as described earlier. The secondary antibody, HRP-goat anti-mouse IgG (dilution 1:8000), was used for detection.
Breast cancer murine model
4T1 cells were resuspended in sterile PBS to achieve a final concentration of 50,000 cells per 100 µL prior to immunization. Surgical instruments were sterilized using a 70% ethanol solution. Before inoculation, the animals were sedated with intraperitoneal Pentobarbital, and the abdominal area was disinfected with 70% ethanol. Using dissecting forceps, the nipple was clamped, and the cells were inoculated subcutaneously at a 30° angle in the inguinal breast using 1 mL syringes with 25 G needles. After the procedure, the animals were returned to their cages and monitored without manual intervention for the following days to avoid complications. On the fifth day, we assessed the tumor for inflammation and growth. For tumor volume calculation, we utilized the following formula: \(V=0.5 x L x W\)2.
Polyclonal antibody production and purification
Serum samples were collected from seven Balb/c mice that were immunized with the KA-11-MAP8 peptide for antibody purification. For quantitative analysis, the polyclonal antibodies (pAb) were purified using protein A Sepharose CL-4B (GE Life Sciences, Cat # GE17-0963-03) according to the manufacturer’s instructions. The concentration of purified IgG (measured in mg/mL) was determined by assessing the optical density (OD) at 280 nm using a microplate reader. Purity was evaluated through SDS-PAGE and visualized with Coomassie blue staining (BioTek Epoch).
Control and experimental groups
Twenty-eight mice obtained from the bioterium of the Hospital Infantil de México Federico Gómez were divided into seven groups, with four mice in each group (n = 4). The groups included three control groups (Groups 1–3) and four experimental groups (Groups 4–7). Group 1: Non-tumoral induction without treatment. Group 2: Non-tumoral induction with the administration of passive treatment. Group 3: Tumor development without treatment. Group 4: Subcutaneous inoculation with KA-11-MAP8 prior to tumor induction. Group 5: Tumor induction with passive treatment using a polyclonal antibody at a dosage of 1 mg/kg. Group 6: Tumor induction with passive treatment using a polyclonal antibody at a dosage of 5 mg/kg. Group 7: Tumor induction with a combination of active and passive therapy using a polyclonal antibody at a dosage of 3 mg/kg. All animals were monitored for 30 days following the initial inoculation with the 4T1 cell line.
Passive treatment with the polyclonal antibody against Syntenin-1
The polyclonal antibody against Syntenin-1 was administered at doses of 1 (group 5), 3 (group 7), and 5 (group 6) mg/kg and diluted in sterile PBS to a final volume of 100 µL. The first treatment was administered intratumorally on the 8th, 15th, and 22nd days after inoculation with the 4T1 cell line. During the experiment, the weights of the mice were registered.
Tumoral growth and metastasis
After the 30th day of tumor induction, mice were euthanized for the extraction of the primary tumor and internal organs to evaluate metastasis. Euthanasia was performed using cervical dislocation, followed by asepsis with 70% ethanol. First, the mammary gland was extracted, and the number, size, and weight of the tumor masses were recorded. Next, the liver, ribs (bone), kidneys, and spleen were extracted to note the weight and number of metastatic foci. The organs were then preserved in 10% formalin.
Syntenin-1 expression in 4T1 cells and tissues
Immunocytochemistry (ICC): The streptavidin–biotin peroxidase-based immunocytochemical method was conducted using the biotin-streptavidin peroxidase detection system. The 4T1 cells and tissues underwent antigen retrieval using the ImmunoDNA Retriever Citrate (Bio SB, Santa Barbara, CA, USA) for 3 min and 9 min, respectively, at 120 °C. The samples were then incubated with the primary antibody, anti-Syntenin-1 (Santa Cruz Biotechnology, Cat# sc-515538), for one hour, followed by treatment with biotin and streptavidin peroxidase. The reaction was developed using the chromogen 3,3′-diaminobenzidine (DAB) and subsequently counterstained with Mayer’s hematoxylin. Immunohistochemistry (IHC): For the immunohistochemistry of tissue sections, 3-mm thick paraffin sections were cut from a paraffin block, deparaffinized with xylene, and IHC-stained following a procedure similar to that used for ICC. As negative controls, both cytological and tissue samples were processed without the primary antibody.
Statistical analysis and ethical statement
GraphPad Prism v.8 was used to perform all the graphs, and by using a one-way ANOVA test, we tested for significant statistical differences among groups.
Results
Selection of the KA-11-MAP8 peptide
Syntenin-1 is a 298 amino acid protein identified as a tumor antigen that promotes tumor proliferation, migration, and metastasis. Initially, we performed ab initio modeling of the 3D structure of Syntenin-1 using its FASTA sequence. From the RaptorX server, we obtained five structural models and selected the one with the highest number of amino acids in allowed regions according to the Ramachandran plot. The final model showed 86.1% of amino acids in the core region, 12.4% in allowed regions, and only 1.5% in generous regions. We visualized the model using PyMOL, identifying the N-terminal, C-terminal, and PDZ domains (Fig. 1, panel A). Using the FASTA sequence of the protein, we conducted an in-silico analysis to identify immunogenic and antigenic peptides. A total of 69 peptides were identified (Supplementary Material 2), including 42 T cell epitopes that are naturally processed by the proteasome and demonstrate binding activity to MHC class I and II molecules (Fig. 1, panel B). Additionally, we identified 27 B-cell epitopes that were accessible and antigenic (Fig. 1, panel C). We filtered these peptides based on their immunogenic and antigenic properties, resulting in a preselected list of 12 peptides (Fig. 1, panel D). The final peptide was chosen by considering its localization within the 3D structure, its biophysical characteristics, and its subcellular localization (Fig. 1, panel E). The selected peptide, found in the PDZ2 domain of Syntenin-1, has been named KA-11-MAP8.
In silico analysis and identification of the KA-11-MAP8 peptide derived from Syntenin-1. (A) Ab initio modeling of the 3D structure of Syntenin-1. This structure illustrates the four functional domains of Syntenin-1: the N-terminal (amino acids 1–109), PDZ1 (amino acids 110–193), PDZ2 (amino acids 194–274), and the C-terminal (amino acids 275–298). Ramachandran plots were utilized to assess the quality of the model. (B,C) In silico analysis for the identification of immunogenic and antigenic sites. B) The number of peptides with the capacity to bind to MHC I/II for T cell activation. (C) The number of peptides exhibiting physical and antigenic properties for B cell activation. Bepipred was used for linear epitope prediction, Emini for surface accessibility prediction, and K&T (Kolaskar & Tongaonkar) for antigenicity. (D) Localization of the preselected peptides. This section provides a schematic representation of the preselected peptides. The image was created in Protter (https://wlab.ethz.ch/protter/start/).
Immunogenicity of KA-11-MAP8 peptide and polyclonal antibody production
To provide in vivo evidence of the immunogenicity of the KA-11-MAP8 peptide, we inoculated the peptide mixed with Freund’s incomplete adjuvant. A total of fifteen Balb/C mice were immunized on days 2, 15, 45, and 95. Blood samples were collected from the mice on days 30, 70, and 120 to test for the presence of antibodies against the peptide (Supplementary Material 3). We detected antibodies against the KA-11-MAP8 peptide in all immunized mice (Fig. 2, panel A). Notably, on day 120, we confirmed the presence of both IgM and IgG antibodies, with slight variations in the antibody response observed among the immunized mice (Fig. 2, panel B). Following the evaluation of the immunogenic properties of the peptide, we designed an experimental procedure to induce active immunity. The mice were divided into three groups for either polyclonal antibody purification or use in experimental groups four and seven (Supplementary Material 4). From the fifteen previously immunized mice, we bled seven individuals, and the collected serum was utilized to purify an anti-Syntenin-1 polyclonal antibody that specifically recognizes the amino acid sequence of the KA-11-MAP8 peptide. The purity of the antibody was analyzed using a 12% SDS-PAGE, which allowed us to visualize the heavy (50 kDa) and light (25 kDa) chains of the antibody (Fig. 2, panel C). Using indirect ELISA, we verified that the purified antibody belongs to the IgG isotype (Fig. 2, panel D), consisting primarily of the sub-isotypes IgG2b and IgG3, with smaller amounts of IgG1 and IgG2a (Fig. 2, panel E).
Immunogenicity of the KA-11-MAP8 and purification of polyclonal antibodies. (A) Antibody levels in mice inoculated with the KA-11-MAP8 peptide were evaluated using an indirect ELISA, with serum samples serving as the primary antibody. (B) Antibody sub-isotype evaluation was conducted using serum samples collected on day 120. The levels of IgM (anti-Mu, 1:6,000; Jackson ImmunoResearch Cat#AB2340064) and IgG (anti-Gamma, 1:8,000; Thermo Fisher Scientific Cat#A16084) were assessed via indirect ELISA. (C) Antibody purity was tested using SDS-PAGE (12%) stained with Coomassie blue. The SDS-PAGE results displayed two lanes: one for the molecular weight marker (BioRad Cat#1610373EDU) and another showing the denatured purified polyclonal antibody with both heavy chain (HC) and light chain (LC) bands. (D) Antibody isotype analysis. (E) IgG sub-isotypes in the polyclonal antibody were evaluated through an indirect ELISA, using the purified polyclonal antibody as the primary antibody. For the evaluation of antibody subtypes, anti-Mu (IgM, 1:6,000; Jackson ImmunoResearch Cat#AB2340064) and anti-Gamma (IgG, 1:8,000; Thermo Fisher Scientific Cat#A16084) were utilized as secondary antibodies. Additionally, for IgG sub-isotypes, anti-IgG1 (Abcam Cat#AB98693), anti-IgG2a (Abcam Cat#AB98698), anti-IgG2b (Abcam Cat#AB98703), and anti-IgG3 (Abcam Cat#AB98708) coupled to HRP were diluted 1:4,000.
To test the recognition of the native protein, peptide, and antibodies for subsequent experiments, we performed a Dot blot using protein extracts derived from the brains of Balb/C mice that constitutively express Syntenin-1. The KA-11-MAP8 peptide served as the antigen, while the primary antibodies included a purified polyclonal antibody and a commercial anti-Syntenin-1 antibody. The polyclonal antibody successfully recognized both the native protein and the KA-11-MAP8 peptide, whereas the commercial antibody only detected the native protein. We also used Western blotting to confirm that the commercial antibody recognized the denatured protein at the expected molecular weight (Fig. 3, panel A). To analyze the subcellular localization of the protein, we conducted immunocytochemistry, which revealed membranal, cytoplasmic, and nuclear localization of Syntenin-1 (Fig. 3, panel B). After confirming the recognition of the native protein in the 4T1 cell line, we designed a cell-ELISA experiment. In this setup, 4T1 cells were seeded and fixed in 96-well plates. We used the purified polyclonal antibody as the primary antibody and a secondary antibody consisting of anti-mouse whole molecule (H + L), anti-Mu, anti-Gamma, anti-IgG1, anti-IgG2a, anti-IgG2b, or IgG3 coupled to HRP (Fig. 3, panel C). Our observations indicated that the polyclonal antibody contained all the IgG sub-isotypes, primarily IgG2a and IgG2b (Fig. 3, panel D). These results provide evidence of the recognition of the complete protein in the cell line, which will be used for tumor induction in Balb/C mice via the polyclonal antibody for passive treatment.
Recognition of the KA-11-MAP8 peptide and the native form of Syntenin-1 by the polyclonal antibody. (A) Dot blot and Western blot analysis were performed. The positive control consisted of total protein extracts derived from the brain, while the conjugate control (CC) was used to assess the biological activity of the secondary antibody. A commercial antibody, anti-Syntenin-1 C-3 (Santa Cruz Biotechnology, Cat#sc-515538), served as an additional positive control. The Western blot included the 4T1 cell line and total protein extracts from the brain. (B) Immunocytochemistry was conducted using the 4T1 cell line, which is derived from a spontaneous breast tumor in mice. Staining was performed using the streptavidin–biotin peroxidase-based immunocytochemical method. The primary antibodies used were the polyclonal antibody and the anti-Syntenin-1 C3 commercial antibody. In the negative control, the primary antibody was omitted. (C) Schematic representation of the cell-ELISA method used to assess antibody isotypes and sub-isotypes. This image was created using Biorender (https://www.biorender.com/). (D) Antibody subtypes and sub-isotypes present in the polyclonal antibody. A cell-ELISA was performed using 4T1 cell cultures grown in 96-well plates. The primary antibody employed was the polyclonal antibody, while the secondary antibodies included monoclonal antibodies: anti-IgG (H + L whole molecule 1:8,000), anti-Mu (IgM 1:6,000; Jackson ImmunoResearch, Cat#AB2340064), anti-Gamma (IgG 1:8,000; Thermo Fisher Scientific, Cat#A16084), as well as sub-isotypes anti-IgG1 (Abcam, Cat#AB98693), anti-IgG2a (Abcam, Cat#AB98698), anti-IgG2b (Abcam, Cat#AB98703), and anti-IgG3 (Abcam, Cat#AB98708) conjugated to HRP, and diluted to 1:4,000.
The administration of passive and active immunity against the PDZ2 domain of Syntenin-1 decreases the tumoral growth and metastasis in vivo
After confirming that the immunized mice had developed active immunity (antibodies) against Syntenin-1 and that the polyclonal antibody for passive treatment recognized the 4T1 cell line, we tested the effects of both active and passive immunity against Syntenin-1 on tumor growth and metastasis in a murine model of TNBC. For this study, twenty-eight Balb/C mice were divided into seven groups: three control groups and four experimental groups. Groups 1 and 2 did not undergo tumor induction; however, group 2 received passive treatment (3 mg/kg). In group 3, tumor induction was performed without any treatment. Group 4 consisted of mice that had been previously inoculated with the KA-11-MAP8 peptide (active immunity). Groups 5 and 6 were treated with the polyclonal antibody on days 8, 15, and 22 after inoculation with the 4T1 cell line, at low (1 mg/kg) and high (5 mg/kg) doses, respectively. Finally, group 7 included mice that had both active immunity and passive treatment. On day 30, the mice were sacrificed to extract the primary tumor, lungs, spleen, bone, and liver for histology, immunocomplex, immunohistochemistry, and assessment of humoral immune response (Supplementary Material 5).
After euthanizing the mice, we conducted a macroscopic analysis of each organ. We recorded the weights, particularly focusing on the primary tumor and the lungs, where we searched for macro metastasis (Fig. 4, panel A). The average tumor volume in group 3 was 2.1 cm3, while in the treatment groups, the average volume was approximately 0.8 cm3. A significant reduction in tumor volume was observed in groups four to seven compared to group 3, which did not receive any treatment (Fig. 4, panel B). A similar tendency was noted regarding tumor weight, with a mean of 1.6 g in group 3 and 0.9 g in group 6 (Fig. 4, panel C). We did not observe significant changes in lungs weight; however, through our macroscopic analysis, we found more macro metastasis in the lungs of the mice in group 3 compared to those in groups four to seven (those receiving active and passive treatment) (Fig. 4, panel D). In the spleen, we noted an increase of about 4 to 10 times in weight in the groups that received 4T1 cells. No significant changes were observed in the weights of the liver, bone, or kidneys (Supplementary Material 6).
The effect of active and passive treatment with the KA-11-MAP8 peptide and polyclonal antibody on tumor growth and lung metastasis in Balb/c mice. (A) Representative images of breast tissue and lungs from the control and experimental groups. (B) Volume of the primary tumor, with comparisons made between Group 3 and the other groups. The statistical significance is noted as follows: *p = 0.0104 (Group 4), **p = 0.0082 (Group 5), and ***p = 0.0077 (Group 6). (C) Tumor weight measurements. (D) Weight of the lungs, expressed in grams. Groups 1 to 7 (G1-G7) are indicated. The plus sign (+) in the passive treatment denotes the dose of polyclonal antibody administered: + : 1 mg/kg; ++: 3 mg/kg; +++: 5 mg/kg.
The extracted organs were paraffin-embedded, and tissue slices were stained with hematoxylin and eosin (H&E). In the healthy breast tissue, we observed adipocytes, interlobular ducts, and lobules. However, the morphology of the breast tissue changed significantly in the mice with primary tumors (groups 3–7). Tumor cells invaded the normal breast tissue, and we also noted the infiltration of polymorphonuclear cells, primarily neutrophils. Another noteworthy observation was the loss of breast ducts and lobules; approximately 60% necrosis of the primary tumor was evident across all experimental groups, regardless of differences in tumor size. To investigate the presence of immunocomplexes in the breast tissue and lungs, we added biotin and streptavidin-HRP to one slice of each mouse, but the results were negative in both control and experimental groups (Supplementary material 7).
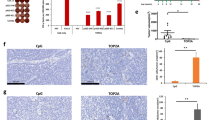
We conducted immunohistochemistry to examine changes in the expression of Syntenin-1 in both control and experimental groups. Group 1 displayed negative expression, while groups 2, 4, and 7 showed mild expression. Groups 3, 5, and 6 exhibited high expression levels. In the lungs, we observed metastatic foci in all groups with induced tumors. The number and size of these foci varied between groups. The lowest counts of metastatic foci were found in groups 5 and 6, with medians of 11.5 and 14.25, respectively, compared to group 3, which had a median of 33.5 metastatic foci. Regarding Syntenin-1 expression, group 1 had negative expression, while in the experimental groups, expression was observed only in the metastatic nodules in the lungs (Fig. 5).
Morphological Evaluation and Syntenin-1 Expression in Control and Experimental Groups. (A) This panel shows representative images of H&E staining and immunohistochemistry (IHC) for the primary tumor and lungs in both control and experimental groups. For the IHC, we used the commercial antibody anti-Syntenine-1 C-3 (Santa Cruz Biotechnology, Cat#sc-515538), which was stained using a streptavidin–biotin peroxidase-based immunohistochemical method. (B) The panel highlights the anatomopathological characteristics of the analyzed tissues. The left panel displays the percentage of necrosis, the middle panel shows the percentage of mitosis per area, and the right panel illustrates the number of metastatic nodules. Statistical analysis was performed using one-way ANOVA, revealing significant results: *p = 0.0245 (between Groups 5 and 6) and *p = 0.0456 (between Groups 6 and 7). Groups 1 to 7 are denoted as G1-G7. The symbol "+" in the passive treatment indicates the dose of the administered polyclonal antibody: +denotes 1 mg/kg; ++ denotes 3 mg/kg; and +++ denotes 5 mg/kg.
Due to the impact of the administered peptide on tumor size and the number of metastatic foci, we analyzed the potential humoral immune response mechanisms involved. Serum samples collected during euthanasia were used for a Cell-ELISA to detect the presence of total antibodies, IgM, total IgG, and its sub-isotypes. All groups tested negative for IgM, while the highest antibody levels were observed in groups 6 and 7. Circulating IgG antibodies were present in all experimental groups (groups 3–7) as well as in group 2, which received passive treatment without tumor induction. Notably, IgG1 was the predominant sub-isotype detected in groups 2, 5, 6, and 7. In addition, IgG2a and IgG3 were found in all experimental groups; however, low levels of IgG2a antibodies were observed in nearly all of the groups (Fig. 6).
Detection of antibody isotypes and sub-isotypes in serum collected from both control and experimental groups. The measured antibody levels include total IgG (heavy + light chains), IgM, and specific IgG sub-isotypes: IgG1, IgG2a, IgG2b, and IgG3. Groups 1 to 7 are labeled as G1 through G7. In the passive treatment section, the '+' symbol indicates the dose of the administered polyclonal antibody: + indicates 1 mg/kg, ++ indicates 3 mg/kg, and +++ indicates 5 mg/kg.
Discussion
In recent decades, there has been a growing focus on improving and developing complementary therapies for cancer due to the various economic, psychological, and health challenges associated with conventional treatments. Among these therapies, immunotherapy has emerged as a promising approach, often referred to as third-generation therapy. It is primarily administered in advanced stages of cancer and includes personalized treatments such as T-cell therapy, antibodies, and vaccines, tailored to the specific antigens expressed by different types of cancer20,21,22,23. Syntenin-1 is recognized as a tumor-associated antigen linked to the development of several types of tumors, including melanoma, glioma, small cell lung cancer, liver cancer, and breast cancer. It is considered a potential alternative treatment for HER2 in cases of triple-negative breast cancer16,24,25.
Recent reports indicate that the overexpression of Syntenin-1 in breast cancer enhances cell migration and invasion of tumor cells, primarily due to its PDZ domains, particularly the PDZ2 domain, which has strong stimulatory effects26,27. Given the importance of the PDZ domains, we conducted in silico and structural analyses of Syntenin-1 and selected a peptide derived from the PDZ2 domain to be synthesized as a multi-antigenic peptide with eight branches (MAP8). The structure of MAP8 is a molecular construct with branched poly-lysine nuclei, featuring α and ε-amino groups in the lateral chains. This design is ideal for creating flexible branching points for peptide binding. Additionally, MAP8 has a molecular weight of 10.56 kDa, which makes it suitable as an immunogen without the need for conjugation to a carrier protein28.
The immunization of the animal model with the synthetic peptide was conducted using Balb/c mice, which are commonly utilized in research related to immunology, physiology, and cancer. These mice are known for their Th2 immune response, characterized by the release of cytokines such as IL-4, IL-6, and IL-10, which promote a stronger humoral response29,30. The peptide was administered in conjunction with Freund’s incomplete adjuvant, a water–oil emulsion often used to stimulate long-lasting antibody production in subunit vaccines due to its ability to provide slow antigen release31,32. The results from the immune response evaluation following the administration of the MAP8 peptide derived from Syntenin-1 indicated the presence of IgG antibodies up to 120 days following the initial inoculation. However, IgM production was absent at day 120. IgM antibodies are typically produced during the first two weeks after antigen administration as part of the adaptive immune system, while IgG antibodies are associated with both adaptive and memory immunity, remaining in circulation for a more extended period33.
The high levels of IgG detected in the immunized mice were advantageous for antibody purification through protein A affinity chromatography, a leading method for antibody purification. Protein A, located in the cell wall of Staphylococcus aureus, has a strong affinity for the Fc domain of IgG antibodies, allowing them to be immobilized in the column34,35,36,37,38. This purification process resulted in the generation of a polyclonal antibody that included all four sub-isotypes of IgG: IgG1, IgG2a, IgG2b, and IgG3. We evaluated the ability of both the polyclonal and commercial antibodies to recognize the KA-11-MAP8 peptide and the native protein in the 4T1 tumor cell line and brain tissue through various immunoassays. Notably, only the polyclonal antibody was able to recognize the KA-11-MAP8 peptide. In the total protein extract derived from the brain, both antibodies tested positive. In the brain, Syntenin-1 plays a role in regulating axonal growth through its interactions with Unc51.1 and Rab5, and it may also be involved in memory and learning due to its participation in the signaling pathway with Rheb39,40. Additionally, both antibodies recognized the native protein in the 4T1 cell line through immunohistochemistry (IHC) and cell-ELISA. The cell-ELISA assay is particularly valuable as it allows for the direct attachment of antigens (tumor cells), enhancing recognition and signal interaction41,42.
The 4T1 cell line is commonly used to establish syngeneic murine models of breast cancer. It is derived from a spontaneous breast tumor in Balb/c mice that test positive for the mouse mammary tumor virus (MMTV). One of the key features of the 4T1 cell line is its focus on research related to triple-negative breast cancer, characterized by the absence of estrogen, progesterone, and HER2 receptors43,44. Previous studies have shown that inhibiting Syntenin-1, either by silencing it or using specific antibodies, can have a significant impact on migration, proliferation, and growth in both human and mouse cell lines. This effect is linked to Syntenin-1's interaction with syndecans, which disrupts cellular communication and affects the subcellular localization of β1 integrin, a protein that promotes cellular adhesion and migration45. In the human cell lines MDA-MB-231 and MCF7, Syntenin-1 plays a role in the transition from the G1 phase to the S phase of the cell cycle. This function is facilitated through its interaction with FGF-2 and FGFR1; without Syntenin-1, these proteins are degraded46. Additionally, during metastasis, Syntenin-1 interacts with Scr, which reduces the activation of NFκB and inhibits the expression of adhesion molecules such as MMP-2 and MMP-947.
Finally, we correlated the results obtained with observations of the different sub-isotypes and types of immunoglobulins. We found the presence of IgG antibodies in all experimental groups, with particular focus on the IgG sub-isotypes, which exhibit variations in their biological functions. The sub-isotypes IgG3 and IgG2b are known to be associated with complement activation and Fcγ receptors in non-activated T cells, while in activated T cells, IgG2a enhances the continuity of the active immune response48. Following the passive treatment, we identified the subclasses of IgG antibodies present in the samples collected at euthanasia among the experimental groups, noting significant differences between them. In the experimental group that received low-dose passive treatment, we detected low levels of IgG2a and IgG2b. These sub-isotypes are reported to have various biological activities, including the release of inflammatory mediators, opsonization, complement activation, and cytotoxic activity49. Additionally, the activation of FcγRIV receptors by IgG2b promotes inflammation and the recruitment of effector innate immune cells50. These biological functions of antibodies in the tumor microenvironment may be linked to their effects on the size of the primary tumor and the number of metastatic nodules. However, further experiments using monoclonal antibodies targeting the selected peptide are needed to assess the potential passive therapeutic utility of the PDZ2 domain of Syntenin-1.
Research has shown that the overexpression of Syntenin-1 can promotes tumor growth and increase metastasis. However, there is a lack of studies exploring the potential of Syntenin-1 as a therapeutic target for triple-negative breast cancer. This research provides evidence for using Syntenin-1 as a molecular target in the development of new immunotherapies for breast cancer. Additionally, it highlights the benefits of utilizing bioinformatics tools for identifying immunogenic peptides and emphasizes the importance of animal models in cancer research.
Conclusion
In this study, we provided evidence about the regions in Syntenin-1 with antigenic and immunogenic properties that promote the activation of the immune system, specifically, antibodies that can recognize the overexpressed protein in cells and tissues. Administrated active and passive therapies against Syntenin-1 showed a high effectivity in the reduction of the size of the tumor and number of metastatic nodules in the murine breast cancer model, which corroborated the potential utility of the PDZ2 of Syntenin-1 as a potential therapeutic target for the development and improvement of complementary treatments focused in the different types of immunotherapies in breast cancer.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BC:
-
Breast cancer
- TNBC:
-
Triple negative breast cancer
- TSA:
-
Tumor specific antigens
- TAA:
-
Tumor associated antigens
- SDCBP:
-
Syndecan Binding Protein
- MDA-9:
-
Melanoma differentiation associated gene-9
- MDSC:
-
Myeloid derived suppressor cells
- MAP8:
-
Multiantigenic peptide with eight ramifications
- HPLC:
-
High performance liquid chromatography
- MS:
-
Mass spectrum
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74(3), 229–263. https://doi.org/10.3322/caac.21834 (2024).
Łukasiewicz, S. et al. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an. Cancers (Basel). 13(17), 4287. https://doi.org/10.3390/cancers13174287 (2021).
Yin, L. et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 22, 61. https://doi.org/10.1186/s13058-020-01296-5 (2020).
Asleh, K., Riaz, N. & Nielsen, T. O. Heterogeneity of triple negative breast cancer: Current advances in subtyping and treatment implications. J. Exp. Clin. Cancer Res. 41(1), 265. https://doi.org/10.1186/s13046-022-02476-1 (2022).
Derakhshan, F. & Reis-Filho, J. S. Pathogenesis of triple-negative breast cancer. Annu. Rev. Pathol. 17, 181–204. https://doi.org/10.1146/annurev-pathol-042420-093238 (2022).
Zhu, M., Liang, C., Zhang, F., Zhu, L. & Chen, D. A Nomogram to predict disease-free survival following neoadjuvant chemotherapy for triple negative breast cancer. Front. Oncol. 11, 690336. https://doi.org/10.3389/fonc.2021.690336 (2021).
Van den Ende, N. S. Triple-negative breast cancer and predictive markers of response to neoadjuvant chemotherapy: A systematic review. Int. J. Mol. Sci. 24(3), 2969. https://doi.org/10.3390/ijms24032969 (2023).
Ganesan, K. et al. Ononin inhibits tumor bone metastasis and osteoclastogenesis by targeting mitogen-activated protein kinase pathway in breast cancer. Res. (Wash D C) 7, 0553. https://doi.org/10.34133/research.0553 (2024).
Xie, Y. et al. Isoliquiritigenin reduces brain metastasis by circNAV3-ST6GALNAC5-EGFR axis in triple-negative breast cancer. Cancer Lett. 624, 217734. https://doi.org/10.1016/j.canlet.2025.217734 (2025).
Agostinetto, E. et al. Immunotherapy for HER2-positive breast cancer: Clinical evidence and future perspectives. Cancers 14(9), 2136. https://doi.org/10.3390/cancers14092136 (2022).
Thomas, R., Al-Khadairi, G. & Decock, J. Immune checkpoint inhibitors in triple negative breast cancer treatment: Promising future prospects. Front. Oncol. 10, 600573. https://doi.org/10.3389/fonc.2020.600573 (2021).
Geurts, V. & Kok, M. Immunotherapy for metastatic triple negative breast cancer: Current paradigm and future approaches. Curr. Treat. Options. Oncol. 6, 628–643. https://doi.org/10.1007/s11864-023-01069-0 (2023).
Kudelova, E. et al. Genetic heterogeneity, tumor microenvironment and immunotherapy in triple-negative breast cancer. Int. J. Mol. Sci. 23(23), 14937. https://doi.org/10.3390/ijms232314937 (2022).
Abbott, R. C., Cross, R. S. & Jenkins, M. R. Finding the keys to the CAR: Identifying novel target antigens for T cell redirection immunotherapies. Int. J. Mol. Sci. 21, 515. https://doi.org/10.3390/ijms21020515 (2020).
Haen, S. P., Löffler, M. W., Rammensee, H.-G. & Brossart, P. Towards new horizons: Characterization, classification and implications of the tumour antigenic repertoire. Nat. Rev. Clin. Oncol. 17, 595–610. https://doi.org/10.1038/s41571-020-0387-x (2020).
Shimada, T., Yasuda, S., Sugiura, H. & Yamagata, K. Syntenin: PDZ protein regulating signaling pathways and cellular functions. Int. J. Mol. Sci. 20, 4171. https://doi.org/10.3390/ijms20174171 (2019).
Kashyap, R. et al. Syntenin-knock out reduces exosome turnover and viral transduction. Sci. Rep. 11, 4083. https://doi.org/10.1038/s41598-021-81697-4 (2021).
Yang, Y. et al. Elevated expression of syntenin in breast cancer is correlated with lymph node metastasis and poor patient survival. Breast Cancer Res. BCR 15, R50. https://doi.org/10.1186/bcr3442 (2013).
Pradhan, A. K. et al. Pharmacological inhibition of MDA-9/Syntenin blocks breast cancer metastasis through suppression of IL-1β. Proc. Natl. Acad. Sci. 118, e2103180118. https://doi.org/10.1073/pnas.2103180118 (2021).
Routh, E. D. et al. Evaluation of tumor antigen-specific antibody responses in patients with metastatic triple negative breast cancer treated with cyclophosphamide and pembrolizumab. J. Immunother Cancer 11(3), e005848. https://doi.org/10.1136/jitc-2022-005848 (2023).
Kyte, J. A. et al. ICON: A randomized phase IIb study evaluating immunogenic chemotherapy combined with ipilimumab and nivolumab in patients with metastatic hormone receptor positive breast cancer. J. Transl. Med. 18, 269. https://doi.org/10.1186/s12967-020-02421-w (2020).
Wagner, J. et al. A single-cell atlas of the tumor and immune ecosystem of human breast cancer. Cell 177, 1330-1345.e18. https://doi.org/10.1016/j.cell.2019.03.005 (2019).
Gupta, S. et al. Screening of oncogenic proteins and development of a multiepitope peptide vaccine targeting AKT1 and PARP1 for breast cancer by integrating reverse vaccinology and immune-informatics approaches. Asian Pac. J. Cancer Prev. 26(1), 327–338. https://doi.org/10.31557/APJCP.2025.26.1.327 (2025).
Das, S. K. et al. MDA-9/Syntenin (SDCBP): Novel gene and therapeutic target for cancer metastasis. Pharmacol. Res. 155, 104695. https://doi.org/10.1016/j.phrs.2020.104695 (2020).
Imjeti, N. S. et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. 114, 12495–12500. https://doi.org/10.1073/pnas.1713433114 (2019).
Koo, T. H. et al. Syntenin is overexpressed and promotes cell migration in metastatic human breast and gastric cancer cell lines. Oncogene 21, 4080–4088. https://doi.org/10.1038/sj.onc.1205514 (2002).
Christensen, N. R. et al. PDZ domains as drug targets. Adv. Ther. 2, 1800143. https://doi.org/10.1002/adtp.201800143 (2019).
Zannella, C. et al. SARS-CoV-2 fusion peptide conjugated to a tetravalent dendrimer selectively inhibits viral infection. Pharmaceutics 15(12), 2791. https://doi.org/10.3390/pharmaceutics15122791 (2023).
Li, J., Wu, H., Liu, Y. & Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6 BALB/c and ICR. Exp. Anim. 69, 326–335. https://doi.org/10.1538/expanim.19-0148 (2020).
Busch, R. A., Jonker, M. A., Pierre, J. F., Heneghan, A. F. & Kudsk, K. A. Innate mucosal immune system response of BALB/c vs C57BL/6 mice to injury in the setting of enteral and parenteral feeding. JPEN J. Parenter. Enteral. Nutr. 40(2), 256–263. https://doi.org/10.1177/0148607114558489 (2016).
Melssen, M. M., Fisher, C. T., Slingluff, C. L. & Melief, C. J. M. Peptide emulsions in incomplete Freund’s adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer. J. Immunother Cancer 10(9), e004709. https://doi.org/10.1136/jitc-2022-004709 (2022).
Pollack, K. E. et al. Incomplete Freund’s adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment. J. Immunother. Cancer. https://doi.org/10.1136/jitc-2020-000544 (2020).
Samsudin, F., Yi Yeo, J., Ken-En Gan, S. & Bond, J. Not all therapeutic antibody isotypes are equal: the case of IgM versus IgG in Pertuzumab and Trastuzumab. Chem. Sci. 11, 2843–2854. https://doi.org/10.1039/C9SC04722K (2020).
Hober, S., Nord, K. & Linhult, M. Protein A chromatography for antibody purification. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 848(1), 40–47. https://doi.org/10.1016/j.jchromb.2006.09.030 (2007).
Mahshid, Z., Ilnaz, S. M., Mohsen, F. & Alireza, G. Mechanism of antibodies purification by protein A. Anal. Biochem. 609, 113909. https://doi.org/10.1016/j.ab.2020.113909 (2020).
Assaat, L. D. et al. Production of a polyclonal antibody against acrylamide for immunochromatographic detection of acrylamide using strip tests. J. Adv. Vet. Anim. Res. 6(3), 366–375. https://doi.org/10.5455/javar.2019.f356 (2019).
Imura, Y. et al. El lavado con soluciones alcalinas en la purificación de la proteína A mejora las propiedades fisicoquímicas de los anticuerpos monoclonales. Sci. Rep. 11, 1827. https://doi.org/10.1038/s41598-021-81366-6 (2021).
Ramos, A. M., González, J. & Aguilar, O. Protein A chromatography: Challenges and progress in the purification of monoclonal antibodies. J. Sep. Sci. 42(9), 1816–1827. https://doi.org/10.1002/jssc.201800963 (2019).
Sarkar, D., Boukerche, H., Su, Z. Z. & Fisher, P. B. mda-9/Syntenin: More than just a simple adapter protein when it comes to cancer metastasis. Cancer Res. 68(9), 3087–3093. https://doi.org/10.1158/0008-5472.CAN-07-6210 (2008).
Sugiura, H. et al. Rheb activation disrupts spine synapse formation through accumulation of syntenin in tuberous sclerosis complex. Nat. Commun. 6, 6842. https://doi.org/10.1038/ncomms7842 (2015).
Chen, Y. J. et al. Development of a highly sensitive enzyme-linked immunosorbent assay (ELISA) through use of poly-protein G-expressing cell-based microplates. Sci. Rep. 8, 17868. https://doi.org/10.1038/s41598-018-36192-8 (2018).
Molnár, E. Cell-based enzyme-linked immunosorbent assay (Cell-ELISA) analysis of native and recombinant glutamate receptors. Methods Mol. Biol. 47–54, 2019. https://doi.org/10.1007/978-1-4939-9077-1_4 (1941).
Liu, Y. et al. Anticancer effects of ACT001 via NF-κB suppression in murine triple-negative breast cancer cell line 4T1. Cancer Manag. Res. 12, 5131–5139. https://doi.org/10.2147/CMAR.S244748 (2020).
Paschall, A. V. & Liu, K. An orthotopic mouse model of spontaneous breast cancer metastasis. J. Vis. Exp. 114, 54040. https://doi.org/10.3791/54040 (2016).
Humphries, J. D., Paul, N. R., Humphries, M. J. & Morgan, M. R. Emerging properties of adhesion complexes: What are they and what do they do?. Trends Cell Biol. 25(7), 388–397. https://doi.org/10.1016/j.tcb.2015.02.008 (2015).
Zimmermann, P. et al. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell. 9(3), 377–388. https://doi.org/10.1016/j.devcel.2005.07.011 (2005).
Pradhan, A. K. et al. MDA-9/Syntenin/SDCBP: New insights into a unique multifunctional scaffold protein. Cancer Metastasis Rev. 39(3), 769–781. https://doi.org/10.1007/s10555-020-09886-7 (2020).
Collins, A. M. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol. Cell Biol. 94(10), 949–954. https://doi.org/10.1038/icb.2016.65 (2016).
Nimmerjahn, F., Bruhns, P., Horiuchi, K. & Ravetch, J. V. Fcgamma RIV: A novel FcR with distinct IgG subclass specificity. Immunity 23(1), 41–51. https://doi.org/10.1016/j.immuni.2005.05.010 (2005).
Nimmerjahn, F. et al. FcγRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc. Natl. Acad. Sci. 107(45), 19396–19401. https://doi.org/10.1073/pnas.1014515107 (2010).
Acknowledgements
We are very grateful with Leonel Martínez-Cristóbal and Raúl Castro-Luna members of the Bioterium of the Hospital Infantil de México Federico Gómez for all their help during the development of all the experimental procedures. Also, we want to thanks Yolanda Medina-Flores, Olga Mata-Ruíz and Lourdes Lloret Sánchez from the InDRE for their help during the purification of the polyclonal antibody. Finally, we appreciate all the technical help of the members from the LIDM: Ana María Méndez-Vera, María Guadalupe Beltrán-Palacios, Fernanda Donaji-Memije Soto, Ariel Eduardo-Manzo Rodríguez, Ximena Aurora Hernández-Cuenca, and Andrea Michelle Madera-Contreras. During this work, María Lilia Nicolás-Morales (CVU 1230221) received a master scholarship from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, M. L. N.-M., K.C.-S and A. V.-V.; methodology, M. L. N.-M., K.C.-S.; resources, V. M. L.-P., I. P.-R., L. C. A.-R., E. F.-A.; data analysis, M. D. G.-M., C. R.-N. C. A. S.-B; writing original draft preparation, M. L. N.-M., K.C.-S.; writing—review and editing, M. E.-R., C. A. S.-B. K. C.-S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This project has the approval of the Internal Committee for the Care and Use of Laboratory Animals (CICUAL) of the Universidad Autónoma de Guerrero (CICUAL-02/2021). All the used Balb/c mice were obtained from the bioterium of the Universidad Autónoma de Guerrero (polyclonal antibody production) and the Hospital Infantil de México Federico Gómez (triple negative breast cancer murine model). For animal treatment, we consider the ARRIVE guidelines (https://arriveguidelines.org). Also, all procedures involving animal manipulation were performed in accordance with NOM-062-ZOO-1999.
Consent for publication
All listed authors approved the submitted version of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nicolás-Morales, M.L., Luna-Pineda, V.M., Serrano-Bello, C.A. et al. The administration of passive and active immunotherapy against Syntenin-1 decreased the tumoral growth and pulmonary metastasis in a murine model of triple-negative breast cancer. Sci Rep 15, 32134 (2025). https://doi.org/10.1038/s41598-025-17914-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-17914-1