Abstract
Doxorubicin is an effective treatment for breast cancer, but drug resistance poses a significant challenge. Emerging regulators of drug resistance include microRNAs, small non-coding RNAs. Two miRNAs, miR-526b and miR-655, promote aggressive breast cancer phenotypes like cell proliferation, cancer stem cell upregulation, altered response to oxidative and metabolic stress and help tumor metastasis by activating COX-2/EP4/PI3K pathways. However, their impact on the effects of chemotherapeutic treatments remains unexplored. This study investigates the impact of overexpressing miR-526b and miR-655 on doxorubicin responses in breast cancer, focusing on pro-survival, apoptotic, and DNA-damage response pathways. We confirm the role of PI3K/Akt signalling in promoting cell survival and resistance in response to doxorubicin in vitro, though key differences exist between each cell line. High-throughput analysis of doxorubicin-exposed breast cancer cell lines, revealing novel mechanisms of miR-526b and miR-655. Overexpression of miR-526b and miR-655 may alter doxorubicin efficacy through mechanisms like DNA damage response changes, metabolic reprogramming, and immune pathway activation. Further investigation may uncover new therapeutic strategies to improve treatment efficacy and patient outcomes.
Similar content being viewed by others
Introduction
Breast cancer continues to be one of the most frequently diagnosed and deadliest forms of cancer in women, accounting for nearly a third of new cancer cases and a sixth of cancer-related deaths1. Despite the efficacy of chemotherapeutic agents, such as doxorubicin (DOX), drug resistance in breast cancer remains a major clinical challenge. Drug resistance is a multifaceted process involving various genetic and epigenetic alterations within cancer cells, influencing drug efflux, cellular responses to DNA damage and repair, apoptosis and cell cycle regulation2,3. The makeup of the tumor microenvironment (TME) has also been shown to be involved in drug resistance and treatment efficacy4. Understanding cellular and molecular interactions may reveal novel therapeutic targets and strategies to overcome resistance, improving treatment outcomes in breast cancer patients.
In recent years, microRNAs (miRNAs) have emerged as key regulators in cancer biology, influencing cell proliferation, apoptosis, metastasis, and drug resistance pathways5. These small, endogenous non-coding RNAs modulate gene expression post-transcriptionally, either by degrading target mRNAs or inhibiting their translation. Overexpression of two miRNAs, miR-526b and miR-655, in poorly metastatic breast tumor cell lines, MCF7 and SKBR3, enhanced aggressive cancer phenotypes, proliferation, migration, invasion, cancer stem cell population in vitro and tumorigenesis and metastasis in vivo through the COX-2/EP4/PI3K/Akt-signalling pathways. In addition, miR-526b and miR-655 expressions were significantly high in patient tumors compared to control tissue and associated with poor patient survival6,7. Shin et al.8 demonstrated that overexpression of either of these miRNAs in a poorly metastatic luminal breast cancer cell line, MCF7, increases ROS and superoxide production despite maintaining cell proliferation by regulating the expression of genes involved in redox regulation. As miR-526b and miR-655 overexpressing breast cancer cells show aggressive phenotypes under severe oxidative stress, this indicates that they may regulate cellular responses to stress, although the mechanism is unclear.
A study from Tordjman and Majumder et al.9 showed that both miR-526b and miR-655 target a common mRNA transcript, cytoplasmic polyadenylation element binding protein 2 (CPEB2), which has tumor suppressor properties, specifically isoform A (CPEB2A). CPEB2A is a polyadenylation protein shown to enhance p53 translation. MCF7-COX2 cells, which inherently express both miRNAs highly, show low CPEB2 expression, and interestingly, CPEB2 knockdown in normal mammary epithelial cells (MCF10A) showed downregulation of p53 and p21 protein expression, two major regulators of senescence and apoptotic signals10. Additionally, miR-655- and miR-526b-overexpression promote cell survival by activation of the PI3K/Akt pathway6,7,8,11,12,13. The PI3K/Akt pathway activates E3 ubiquitin-protein ligase MDM2, which precipitates the degradation of p5314,15. The roles of these miRNAs in cancer suggest a complex network of interactions with key pathways that govern cell survival, DNA repair mechanisms, and cellular responses to stress.
Doxorubicin (DOX) is often prescribed for the treatment of aggressive breast cancer, targeting rapidly dividing cells while disrupting DNA repair and generating free radicals and oxidative stress. DOX exposure increases inflammation and COX-2 overexpression. This, in turn, may activate the COX-2/EP4/PI3K/Akt pathway and promote the expression and function of oncogenic miR-526b and miR-655. In the current study, we aim to elucidate the effects of miR-526b and miR-655 on the responses to DOX treatment in breast cancer. By examining changes in cell viability, DNA damage response, and global alterations in gene expression following DOX treatment, we aim to discover the underlying mechanisms through which these miRNAs may influence DOX sensitivity.
Results
miR-526b and miR-655 expression in DOX responders vs. non-responders
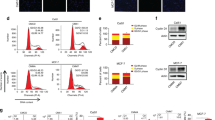
Analysis of all tumor types show that mean expression of miR-526b was marginally higher in non-responders (n = 92) to DOX (1.52) than in responders (n = 167) (1.38) (Fig. 1A). However, ROC analysis showed miR-526b is not a good predictor of DOX response while all tumors were considered, with an AUC of 0.503 (p = 0.47) (Fig. 1B). Analysis of breast cancer patients only showed that mean miR-526b expression was again higher in breast cancer patients that did not respond to DOX treatment (n = 21) (1.47), compared to those that did (n = 151) (1.27) (Fig. 1C). Here, ROC analysis indicated that predictive power of miR-526b was greater in distinguishing non-responders from responders (AUC = 0.604, p = 0.053) (Fig. 1D). There was not sufficient data available for analysis of miR-655 expression in breast cancer alone. However, including all cancer types, we found that mean miR-655 expression was higher in patients that were deemed non-responders to DOX (n = 27) (0.56), compared to patients who responded to DOX treatments (n = 28) (0.39) (Fig. 1E). The prognostic capacity of miR-655 was marginal, with an AUC value of 0.621 (p = 0.06) (Fig. 1F). However, these miR-655 result are limited by the sample size and exclusive breast cancer data was unavailable.
While neither miRNA expression was statistically significant in predicting patient response to DOX, the trends of high miRNA expression observed in DOX non-responders still piqued our interest in investigating whether either miRNA may alter how breast cancer cells respond to DOX.
ROC analysis of miR-526b and miR-655 in DOX responders and non-responders. ROC Plotter, an online database and analysis tool, was used to assess the association of miR-526b and miR-655 expression with DOX response in cancer patients. (A,B) Mean miR-526b expression and receiver operating characteristic (ROC) analysis based on DOX response in all cancer patients. (C,D) Mean miR-526b expression and ROC analysis based on DOX response in breast cancer patients. (E,F) Mean miR-655 expression and ROC analysis based on DOX response in all cancer patients. Samples without progression before the median follow up time were deemed responders and samples with progression before the median follow up time were deemed non-responders. AUC (Area Under the Curve), TPR (True Positive Rate) and TNR (True Negative Rate).
Cell viability in miRNA overexpressing breast cancer
The half-maximal inhibitory concentration (IC50) of DOX in MCF7 breast cancer cells was determined to be 0.80 µM (Fig. 2A). This concentration was utilized to assess the differential response of MCF7, MCF7-miR526b, MCF7-miR655 and MCF7-COX2 cell lines to DOX treatment. Cells were treated with DOX for 48 h and then allowed a recovery period of three days without treatment (DOX + Rest) (Fig. 2B).
Following 48 h of DOX treatment, a reduction in cell viability was observed across all cell lines (Fig. 2C). Notably, the parental MCF7 cells exhibited a more pronounced decrease in viability compared to the miR-526b, miR-655 and COX-2 overexpressing cell lines. Cell numbers did not increase following the rest period. Senescence-associated beta-galactosidase activity staining did not demonstrate any significant differences in senescence among the cell lines, regardless of treatment or rest period (Supplementary Fig. S1). These results indicate a potential modulation of DOX sensitivity by miR-526b and miR-655 in miRNA overexpressing cell lines.
Analysis of cell viability after DOX exposure. (A) Dose-response curve for DOX-treated MCF7. (B) Timeline of treatment and recovery (created with BioRender.com). (C) Viability of MCF7, MCF7-miR526b, MCF7-miR655 and MCF7-COX2 cells after treatment. Data represented as mean +/- SD, (n = 3, ****p < 0.0001, one-way ANOVA, Dunnett’s post-hoc). (D) p53, (E) p21 and (F) PUMA gene expression. Data shown as mean +/- SD (n = 4 for MCF7 and n = 3 for MCF7-miR526b, MCF7-miR655 and MCF7-COX2. DOX: MCF7 vs. MCF7-miR655 *p = 0.0229 in (D), in DMSO: MCF7 vs. MCF7-miR526b ***p = 0.0006, MCF7 vs. MCF7-COX2 ****p < 0.0001; in DOX: MCF7 vs. MCF7-COX2 *p = 0.0328; in DOX + Rest: MCF7 vs. MCF7-COX2 *p = 0.0490 in (E), in DMSO: MCF7 vs. MCF7-COX2 *p = 0.0251; in DOX: MCF7 vs. MCF7-miR526b *p = 0.0443, MCF7 vs. MCF7-miR655 ***p = 0.0012; in DOX + Rest: MCF7 vs. MCF7-miR655 *p = 0.0323, MCF7 vs. MCF7-COX2 **p = 0.0012 in (F), one-way ANOVA, Dunnett’s post-hoc). (G) Western blot images and quantification for (H) p53, (I) p21, (J) p-AKT(Ser473) and (K) PARP cleavage. For full blot images, see Supplementary Figs. S2-S6. Data presented as mean +/- SD (For p53, n = 4 for MCF7, MCF7-miR526b, n = 3 for MCF7-miR655 and MCF7-COX2. MCF7: DMSO vs. DOX **p = 0.0044, MCF7-miR526b: in DMSO vs. DOX **p = 0.0025, MCF7-miR655: DMSO vs. DOX *p = 0.0142, DMSO vs. DOX + Rest *p = 0.0367, MCF7-COX2: DMSO vs. DOX ***p = 0.0003, DOX vs. DOX + Rest ****p < 0.0001 in (H). For p21, n = 5 for MCF7, MCF7-miR526b, n = 4 for MCF7-miR655 and n = 3 for MCF7-COX2. MCF7: DMSO vs. DOX *p = 0.0369, MCF7-miR526b: DMSO vs. DOX **p = 0.0024, DOX vs. DOX + Rest *p = 0.0316, MCF7-miR655: DMSO vs. DOX **p = 0.0023, DOX vs. DOX + Rest *p = 0.0167, MCF7-COX2: DMSO vs. DOX **p = 0.0035, DOX vs. DOX + Rest *p = 0.0273 in (I), one-way ANOVA with Tukey’s post-hoc. For p-AKT/AKT, n = 3 for all cell lines. DOX: MCF7 vs. MCF7-COX2 *p = 0.0420 and MCF7-COX2: DMSO vs. DOX *p = 0.0182 in (J). For the fraction of cleaved PARP measurement, n = 3 for all cell lines. MCF7-miR526b: DMSO vs. DOX + Rest *p = 0.0318 and DOX vs. DOX + Rest *p = 0.0491 in (K), two-way ANOVA with Tukey’s post-hoc). Non-significant comparisons (p > 0.05) not labelled.
Since our model cell line, MCF7, is wild-type for p53, we analyzed TP53 gene expression, as well as the gene expression of two transcriptional targets of p53, CDKN1A (p21) and BBC3 (PUMA) (Fig. 2D and F).
Analyses revealed that gene expression levels of p53 were elevated in the MCF7-miR526b and MCF7-miR655 cell lines compared to parental MCF7 cells across control (DMSO) and initial DOX treatment conditions (Fig. 2D). After the rest period, p53 gene expression levels rebounded in these cell lines, and were not significantly altered compared to MCF7. In the case of MCF7-COX2, p53 gene expression is lower in comparison to the parental cell line in all cases, though this was not statistically significant (Fig. 2D).
Gene expression of p21 was significantly lower in MCF7-miR526b and MCF7-COX cells and marginally higher in MCF7-miR655, relative to MCF7, in DMSO (Fig. 2E). After DOX exposure and following the recovery period, p21 expression was lower in all experimental cell lines relative to MCF7, significantly so in the case of MCF7-COX2. PUMA gene expression was significantly lower in MCF7-COX2 cells and marginally lower in MCF7-miR526b and MCF7-miR655 cells, relative to MCF7, in DMSO (Fig. 2F). Following DOX exposure, PUMA gene expression was significantly greater in both MCF7-miR526b and MCF7-miR655 cells but unchanged in MCF7-COX2 cells. After the recovery period, PUMA gene expression was unchanged in MCF7-miR526b and significantly reduced in the case of MCF7-miR655 and MCF7-COX2.
Additionally, p53 and p21 protein expression were assessed via western blot (Fig. 2G). In all cell lines, p53 protein expression was significantly elevated in response to DOX treatment (Fig. 2H). This expression remained significantly elevated relative to the control condition in the MCF7-miR655 cell line. In all other cell lines, this level was reduced following the three-day recovery period, significantly so in the MCF7-COX2 cell lines. Similarly, p21 protein expression was elevated in all cell lines following treatment (Fig. 2I). After the three-day rest period, expression reduced significantly in MCF7-miR526b, MCF7-miR655 and MCF7-COX2, but not in MCF7.
AKT phosphorylation (Ser473) was analyzed following DOX treatment as a measure of pro-survival cell signalling (Fig. 2G). All cell lines displayed a relative increase in the ratio of AKT phosphorylation (p-AKT/total-AKT) after treatment and the rest period (Fig. 2H). This increase was slight in the MCF7 and MCF7-miR655 cell lines, more prominent in the MCF7-miR526b cell line and significant in the case of MCF7-COX2 following initial DOX treatment relative to both its DMSO counterpart and DOX treated MCF7 cells. This indicates that the pro-survival pathway is activated in all MCF7 cell lines, with miRNA-overexpressing cells most effective in surviving stress.
Poly (ADP-ribose) polymerase (PARP) expression and cleavage were analyzed to investigate pro-apoptotic signalling after DOX treatment by comparing the relative abundance of full-length PARP (~ 116 kDa) to a cleaved fragment (~ 89 kDa) found during apoptosis (Fig. 2G). Results show a modest increase in PARP cleavage following DOX exposure in all cell lines (Fig. 2K). This trend increased further following the rest period, significantly so in MCF7-miR526b.
Assessing DNA damage and DDR in miRNA overexpressing breast cancer
Comet assay results show increase levels of DNA damage after DOX exposure for all cells lines (Fig. 3A). Analysis of the comet assays (Fig. 3A), including tail DNA percent (Fig. 3B), tail moment (Fig. 3C) and olive moment (Fig. 3D), indicate that DNA damage increased in all cell lines following DOX treatment, although these changes were not significant within the same cell line. In MCF7 and MCF7-miR655 cells, each comet metric was reduced following the rest period, indicating DNA repair. On the other hand, the level of DNA damage, measured by tail DNA percentage, in MCF7-miR526b cells remains elevated, though tail moment and olive moment were marginally reduced, indicating that DNA damage was not repaired. On the other hand, MCF7-COX2 cells remain mostly unaltered by DOX treatment, indicated by the marginal increase in DNA tail percentage, tail moment, and comet moment following rest. While DNA damage continued to rise in MCF7-COX2 over the course of treatment and recovery period, relative to the other cell lines, it scored the lowest metrics in each condition, significantly so when compared to MCF7 exposed to DOX (Fig. 3B and D). Of all the luminal A breast cancer cell lines, MCF7-COX2 is the most aggressive cell line; the results above are indicative of altered DOX response due to COX-2 interference.
DOX induced DNA damage assessed via the comet assay. (A) Comet assay images from each cell lines in DMSO, DOX and rest conditions. Images taken at 4X magnification, and the scale bar is 1,000 μm. Insets were imaged at 20X magnification. Quantification of (B) tail DNA percentage, (C) tail moment and (D) olive moment. Data shown as mean +/- SD (n = 4 bioreplicates, DOX: MCF7 vs. MCF7-COX2 *p = 0.0372 in (B), **p = 0.0079 in (C) and **p = 0.0048 in (D), two-way ANOVA with Tukey’s post-hoc). Non-significant comparisons (p > 0.05) not labelled.
γ-H2AX staining (Fig. 4A–D) increased in all DOX-treated cells (Fig. 4E). After rest, staining intensity significantly decreased in all cell lines, except for MCF7. Unlike the comet assay, γ-H2AX staining suggests that DNA-damage is greatest in the MCF7-COX2 cells after DOX treatment (Fig. 4E), significantly greater than both MCF7 and MCF7-miR526b cell lines.
γH2AX (S139) staining and DNA damage response protein expression. Fluorescent images of γ-H2AX staining for (A) MCF7, (B) MCF7-miR526b, (C) MCF7-miR655 and (D) MCF7-COX2 cells following DOX treatment and rest. Images taken at 40X magnification, and the scale bar is 100 μm. Red, γ-H2AX; blue, DAPI. (E) Quantification of γ-H2AX staining. Data shown as mean +/- SD (n = 3 bioreplicates, MCF7: DMSO vs. DOX **p = 0.0046; MCF7-miR526b: DMSO vs. DOX **p = 0.0014, DOX vs. DOX + Rest *p = 0.0316; MCF7-miR655: DMSO vs. DOX ***p = 0.0005, DOX vs. DOX + Rest *p = 0.0325; MCF7-COX2: DMSO vs. DOX ****p < 0.0001, DOX vs. DOX + Rest ****p < 0.0001; DOX: MCF7 vs. MCF7-COX2 *p = 0.0339, MCF7-miR526b vs. MCF7-COX2 *p = 0.0209, two-way ANOVA with Tukey’s post-hoc). (F) Western blot images of ATM, Chk2 and p-Chk2 following DOX treatment and rest. For full blot images, see Supplementary Fig. S7-S9. Quantification of western blot analysis for (G) ATM (n = 2 bioreplicates), (H) Chk2 and (I) p-Chk2. Data in (H) and (I) shown as mean +/- SD (For Chk2, n = 4 bioreplicates, MCF7-miR655: DMSO vs. DOX ***p = 0.0007, DMSO vs. DOX + Rest ***p = 0.0004, one-way ANOVA with Tukey’s post-hoc. For p-CHK2, n = 3 for MCF7, MCF7-miR526b and MCF7-miR655 and n = 4 for MCF7-COX2). Non-significant comparisons not labelled.
After DOX treatment, ATM protein expression is elevated in all cell lines, most noticeably in MCF7-miR526b and MCF7-miR655, and marginally in MCF7 (Fig. 4F and G). After the rest, ATM remained elevated in both MCF7-miR526b and MCF7-miR655, with a maximum increase in MCF7 and nominal changes observed in MCF7-COX2 cells.
After DOX treatment, total Chk2 protein expression was reduced in MCF7-miR526b, MCF7-miR655 and MCF7-COX2, significantly so in MCF7-miR655 (Fig. 4F and H). Chk2 remained significantly reduced in the MCF7-miR655 and MCF7-COX2 cell lines after the recovery period and marginally reduced in MCF7-miR526b and MCF7.
While total Chk2 decreased over the treatment and recovery period for all cell lines, p-Chk2 increased after treatment for MCF7, MCF7-miR655 and MCF7-COX2, though not significantly, and remained unchanged in MCF7-miR526b (Fig. 4F and I). After the three-day recovery period, p-Chk2 expression decreased on average, though it remained marginally elevated relative to pre-treatment levels, except for MCF7-miR655, where p-Chk2 levels increased further.
Identification of DEGs associated with miRNA-overexpression via RNA-sequencing
To better understand the potential effects of miR-526b, miR-655, and COX-2 overexpression, RNA-seq analysis was performed. Only the control condition and the 48-hour DOX treatments were sequenced. We analyzed differentially expressed genes (DEGs) for each miRNA-overexpressing aggressive cell line MCF7-miR526b, MCF7-miR655 and MCF7-COX2 relative to the miRNA-low MCF7 cell (Fig. 5A and C). Genes with a fold change above or below 1.5 and − 1.5, as well as an FDR-adjusted p-value less than 0.05, were considered significant DEGs. We also identified the ten most upregulated and ten most downregulated genes for each experimental cell line relative to MCF7 following DOX treatment (Supplementary Fig. S10).
Using Gene Set Enrichment Analysis (GSEA), performed with MSigDB Hallmark gene sets, we identified the top enriched hallmark gene sets, as well as the hallmark gene sets of apoptosis and the p53 pathway, for each miRNA-overexpressed cell line relative to MCF7 (Fig. 5D and O). In the MCF7-miR526b cell line, the most significant upregulated enrichment was the hallmark oxidative phosphorylation (OXPHOS) gene set (NES = 1.71, FDR = 0.0148) (Fig. 5D), possibly indicating enhanced metabolic or mitochondrial activity. Conversely, the most significant downregulated enrichment was the hallmark G2M checkpoint gene set (NES= -2.36, FDR = 0.0) (Fig. 5E), suggesting a possible suppression of cell cycle progression. The hallmark apoptosis (NES= -1.74, FDR = 0.0006) (Fig. 5F) and the p53 pathway (NES= -1.26, FDR = 0.1394) (Fig. 5G) gene sets were enriched amongst the downregulated genes in MCF7-miR526b compared to MCF7, suggesting a weaker apoptotic response and impaired p53 signalling in the MCF7-miR526b cell line.
In the MCF7-miR655 cell line, the most significant upregulated gene set was the hallmark interferon alpha response (NES = 2.43, FDR = 0.0) (Fig. 5H), suggesting immune response involvement or inflammatory signalling. The hallmark E2F targets gene set was the most significantly downregulated enrichment (NES = -2.28, FDR = 0.0) (Fig. 5I), indicating reduced transcription of genes involved in cell cycle progression. Unlike MCF7-miR526b, the hallmark apoptosis gene set was not significantly enriched in either the MCF7-miR655 or in MCF7 cells (NES = 1.01, FDR = 0.7070) (Fig. 5J), indicating apoptotic signalling remained unaltered in both cell lines. As with the MCF7-miR526b cell line, the hallmark p53 pathway gene set was enriched in the downregulated MCF7-miR655 genes, although this was not significant (NES = -1.05, FDR = 0.5521) (Fig. 5K).
For MCF7-COX2, the most significant upregulated enrichment is the hallmark MYC targets V2 gene set (NES = 1.83, FDR = 0.002) (Fig. 5L), indicating an increase in MYC-driven transcription. COX-2 overexpression in MCF7 cells stimulates cancer stem cells, and MYC-target gene upregulation supports that finding. Just like MCF7-miR655, the most significant downregulated enrichment is the hallmark E2F target gene set (NES = − 2.38, FDR = 0.0) (Fig. 5M), indicating a reduction in cell cycle regulation. However, there was a negative enrichment in both the hallmark apoptosis (NES = − 1.16, FDR = 0.18) (Fig. 5N) and p53 pathway (NES = -1.56, FDR = 0.0071) (Fig. 5O) gene sets in miRNA-overexpressed cells compared to the MCF7 cell line, apoptotic response due to alterations in apoptotic signalling or responses and cellular stress responses due to COX-2 overexpression.
RNA-sequencing and enriched hallmark gene sets and biological processes. Volcano plots assessing differentially expressed genes in either (A) MCF7-miR526b, (B) MCF7-miR655 or (C) MCF7-COX2 cell lines, relative to MCF7 after DOX treatment. Log2 fold change cutoffs are under − 1.5 (blue) or above 1.5 (red) and adjusted p-values below 0.05. GSEA enrichment plots show the most significant upregulated and downregulated enrichments as well as hallmark apoptosis and p53 pathway gene set enrichments for (D–G) MCF7-miR526b, H-K) MCF7-miR655 and (L–O) MCF7-COX2 relative to MCF7 after DOX treatment. Dot plots of the top ten most significantly enriched biological processes for the upregulated DEGs of (P) MCF7-miR526b, (Q) MCF7-miR655 and (R) MCF7-COX2 and downregulated DEGs of (S) MCF7-miR526b, (T) MCF7-miR655 and (U) MCF7-COX2 relative to MCF7 after DOX treatment.
To further explore the possible biological functions altered by miR-526b, miR-655 or COX-2 expression, gene ontology (GO) biological process enrichment analysis was conducted on the significantly upregulated and downregulated DEGs (Fig. 5P and U). In MCF7-miR526b, upregulated biological processes (BPs) include those associated with cardiac conduction, cell-cell adhesion, synapse assembly and regulation of cell migration (Fig. 5P). This suggests that miR-526b affects metastatic capabilities, cell mobility, and migration following DOX exposure. These observations support known oncogenic miR-526b functions, which are further stimulated in the presence of DOX. Downregulated BPs include those involved in positive regulation of leukocyte apoptosis, protein oxidation, mitotic spindle attachment, prostaglandin metabolism and inflammatory response (Fig. 5S), which indicates reduced or suppressed stress and inflammatory responses during DOX exposure.
In MCF7-miR655, upregulated BPs were associated with antiviral immune responses, which agrees with the previous enrichment of the hallmark interferon alpha response gene set (Fig. 5Q). Downregulated BPs include neurotransmitter transport and metabolism, as well as synapse assembly (Fig. 5T). These BPs indicate that miR-655 promotes inflammatory responses and downregulates neurotransmitter responses when exposed to DOX. In MCF7-COX2, upregulated BPs were involved in skeletal muscle satellite cell proliferation, neurogenesis, chemotaxis and interleukin-1 production (Fig. 5R), which are known COX-2-regulated functions. Downregulated BPs include those involved in cell adhesion and negative regulation of epithelial cell proliferation and differentiation but also contained an overlap of seemingly related BPs from the upregulated DEGs, such as those involved in neurogenesis, tissue development and immune response. These conflicting results may indicate dysfunction or dysregulation of these processes in MCF7-COX2 cells when exposed to DOX (Fig. 5U).
Protein-protein interaction (PPI) network construction and hub gene analysis
Differential gene expression was also assessed for each cell line after DOX treatment (DMSO vs. DOX) (Supplementary Fig. S11) to determine the overall response for each cell line. Interestingly, GSEA and BP enrichment analysis indicate that each cell line responded similarly to DOX treatment (Supplementary Fig. S12 and Supplementary Fig. S13, respectively).
The DEGs for each cell line following DOX treatment (Supplementary Fig. S11) were compared for commonalities. We identified 473 upregulated (Fig. 6A) and 324 downregulated (Fig. 6B) genes, which were common across each of the four cell lines. Functional enrichment analysis, using Enrichr, was performed to identify the top 10 significant BPs associated with each gene list (Fig. 6C and D). The upregulated gene list was significantly enriched in BPs associated with extracellular matrix remodeling (GO:0030198, GO:0045229, GO:0043062), calcium ion transport (GO:0070588, GO:0098659, GO:0097553, GO:0098703) and synaptic regulation (GO:0098815, GO:0051968, GO:2000463) (Fig. 6C). The downregulated gene list was enriched in BPs associated with DNA damage response and repair (GO:0006259, GO:0006259, GO:0006261) as well as cell cycle regulation and mitosis (GO:0090068, GO:0007052, GO:0000070, GO:1902850, GO:0000819, GO:0071173, GO:0045841) (Fig. 6D). Functional enrichment shows genes involved in ECM remodeling are upregulated, and genes which can repair DNA damage induced by DOX are downregulated. Interestingly, none of these BPs directly suggest apoptosis, DNA damage, senescence or cell stress. Instead, they are linked via alterations to cell adhesion and intracellular signalling in response to DOX, indicative of cellular processes that might be associated with the apoptotic process.
Protein-protein interaction (PPI) networks generated with STRING show functional and physical interactions between DEGs (Fig. 6E and F). Topological analysis was performed using degree, betweenness, and closeness centrality measures to rank the genes within each network. Based on combined centrality scores, the top five genes were identified as “hub genes” for each network (Fig. 6G). These hub genes were CALML5, IL6, GNG2, GRIN2B, and IGF1 within the upregulated PPI network, and CDK1, CCNA2, PLK1, CDC45, and EXO1 within the downregulated PPI network. Of the ten hub genes, CALML5 and IL6 were validated in vitro via qRT-PCR (Supplementary Fig. S14). We show that after doxorubicin exposure, the expression of CALML5 and IL6 increase, in agreement with the sequencing results.
Cell line comparison of DOX response and identification of network hub genes. Venn diagrams comparing (A) upregulated and (B) downregulated DEGs between each cell line after DOX treatment. Dot plots depicting the top ten enriched biological processes associated with the (C) 473 common upregulated DEGs and (D) 324 common downregulated DEGs. Interaction networks of (E) upregulated and (F) downregulated DEGs. (G) The top 5 hub genes from each network. Heat maps display the expression of each identified hub gene in each experimental cell line relative to MCF7 in (H) control (DMSO) and (I) DOX treatment conditions.
After establishing that these hub genes are shared between all cell lines in response to DOX, we wanted to assess the expression of each hub gene within each experimental cell line (MCF7-miR526b, MCF7-miR655 and MCF7-COX2) relative to MCF7 in both control (Fig. 6H) and treatment conditions (Fig. 6I). In the control condition, there was no significant difference in hub gene expression between MCF7 and either MCF7-miR526b or MCF7-miR655. However, in the MCF7-COX2 cell line, CALML5 was significantly downregulated, and CCNA2, CDC45, EXO1 and PLK1 were significantly upregulated. In the DOX condition, we found that CDK1, CCNA2, EXO1 and PLK1 were significantly downregulated in MCF7-miR526b cells, and CALML5, CDK1 and PLK1 were significantly downregulated in MCF7-miR655. While CALML5 remained significantly downregulated in MCF7-COX2 relative to MCF7 cell line after DOX treatment, CDK1, CCNA2, CDC45, EXO1, and PLK1 were now significantly downregulated as well.
Hub gene expression in TCGA-BRCA tissue samples
Data from The Cancer Genome Atlas (TCGA) was accessed to analyze the expression of each of the identified hub genes (Fig. 7). Pathologically normal tissue samples were compared to luminal A, luminal B, HER2-enriched and basal breast cancer subtypes. Four of the five upregulated hub genes (IL6, GNG2, GRIN2B, IGF1) have markedly lower expression in all tumor subtypes compared to normal tissue – CALML5 being the exception, where expression was greatest in HER2 and basal samples and low in luminal subtypes (Fig. 7A–E). Between subtypes, there is less discrepancy in gene expression. All five of the identified downregulated hub genes showed greater expression in breast cancer tissue than in normal tissue (Fig. 7F–J), indicative of these genes’ roles in cancer initiation and progression. Additionally, the expression of each downregulated hub gene (CDK1, CCNA2, CDC45, EXO1 and PLK1) tends to be highest in more aggressive breast cancer subtypes, lowest in luminal A samples and more significant in luminal B, HER2-enriched and basal samples, indicative of the role of these genes in aggressive cancer phenotypes.
Analysis of hub gene expression in breast cancer subtypes. (A–E) log2(TPM + 1) expression of the 5 upregulated hub genes. (F–J) log2(TPM + 1) expression of the top 5 downregulated hub genes. Tissue types include normal patient tissue (n = 113), as well as luminal A (lumA, n = 571), luminal B (lumB, n = 209), HER2-enriched (Her2, n = 82) and basal (n = 197) subtypes. Data sourced from TCGA, shown as violin plots, medians denoted with solid lines, first quartiles and third quartiles denoted with dotted lines (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, Kruskal-Wallis with Dunn’s post-hoc).
Survival analysis of hub genes in breast cancer patients
Overall survival (OS) for breast cancer patients was analyzed with KM plotter, based on the expression of each hub gene (Fig. 8). Low CALML5 (HR = 0.72, p = 0.0066) and IL6 (HR = 0.79, p = 0.043) expression were associated with poor survival in all breast cancer patients. However, neither gene was significant in chemotherapy-treated patients (Fig. 8A and B). GNG2 and IGF1 showed strong associations, with GNG2 (HR = 0.62, p = 2e-5) and IGF1 (HR = 0.43, p = 4e-14) linked to better overall survival in all breast cancer patients, as well as chemotherapy-treated patients (HR = 0.42, p = 6.1e-5) and (HR = 0.22, p = 1.4e-8), respectively (Fig. 8C and E). In contrast, GRIN2B was not significantly associated with survival in all patients (HR = 0.88, p = 0.31) but was linked to poor outcomes in chemotherapy-treated patients (HR = 1.73, p = 0.021) (Fig. 8D).
Higher expression of CCNA2 (HR = 1.84, p = 4.2e-7) and CDC45 (HR = 1.54, p = 1.9e-4) and lower expression of PLK1 (HR = 0.67, p = 4.6e-4) were significantly associated with poor overall survival in all patients (Fig. 8G, H and J). This trend was also observed in chemotherapy-treated patients, where CCNA2 (HR = 1.81, p = 0.0062) and CDC45 (HR = 1.76, p = 0.01) were predictive of poor survival, and PLK1 (HR = 0.43, p = 0.0026) was associated with better outcomes. These three hub genes were downregulated in all three miRNA-high cell lines following DOX exposure, relative to MCF7. CDK1 expression was associated with favorable patient outcome in all patients (HR = 0.78, p = 0.038). However, this trend was not significant for chemotherapy treated patients (HR = 0.81, p = 0.36) (Fig. 8F). EXO1 expression trended towards poor survival outcomes in all patients (HR = 1.18, p = 0.19) and chemotherapy-treated patients (HR = 1.29, p = 0.24); neither case was significant (Fig. 8I).
Discussion
The aim of this study was to determine the effects of miR-526b, miR-655 and COX-2 overexpression on DOX sensitivity in breast cancer. Given prior findings that miR-526b and miR-655 target CPEB2A, a tumor suppressor involved in p53 translation, we hypothesized that miRNA overexpression would reduce p53 and p21 levels and promote PI3K/Akt activation, leading to apoptotic resistance and enhanced cell survival. Although each of the MCF7-miR526b, MCF7-miR655, and MCF7-COX2 cell lines showed reduced sensitivity to DOX treatment compared to MCF7, the main mechanism behind this response is probably not related to the interaction of these miRNAs with CPEB2A or their effect on p53 expression. Instead, each cell line shows distinct mechanisms that promote cell survival.
MCF7-miR526b cells maintained elevated p53, p21 and PUMA levels despite no initial significant alteration in PARP cleavage, reduced enrichment in apoptosis and enhanced viability relative to MCF7. These results indicate that p53 signalling may be disrupted downstream of p53 activation, in a way that impairs apoptosis or cell cycle arrest in comparison to the parental cell line. The observed enrichment in OXPHOS was an interesting finding, as a recent study has shown that miR-526b overexpression facilitates breast cancer’s adaptation to metabolic stress16. These results indicate possible metabolic adaptations promoting cellular energy production and survival under stressful conditions, which have been shown with doxorubicin and other anthracyclines in breast cancer17.
In MCF7-miR655, although p53 levels remained comparable to MCF7, p21 expression was consistently lower, and PUMA expression was transient – spiking after treatment then declining following recovery, akin to the cleavage of PARP. These results explain the enhanced cell viability relative to MCF7, suggesting an activation of apoptosis that is not sustained, enabling cell survival after the removal of DOX. Notably, the enrichment of interferon alpha (INF-α) responses, as well as the enrichment of immune-associated biological processes, suggests alternative mechanisms of stress adaptation. INF-α signalling has been shown to play a dual role in cancer, contributing to both tumor suppression and disease progression, depending on context. INF-α typically behaves as a tumor suppressor by promoting apoptosis, immune activation and cell cycle arrest18. However, chronic INF-α signalling may enhance adaptive cellular stress responses, DNA repair pathways, and cell survival, while promoting immune evasion19. Like MCF7-miR526b, MCF7-miR655 cells displayed reduced DNA damage, as well as increased ATM and p-Chk2 expression, supporting the idea of enhanced DNA damage repair.
MCF7-COX2 cells displayed the least sensitivity to DOX, the lowest p53 activity, and the most significant spike in AKT phosphorylation. PI3K/Akt activation in response to DOX treatment is not a new observation and is considered a potential factor in the development of resistance, making it a promising therapeutic target20. In the case of cancer driven by COX-2, direct COX-2 inhibition poses limitations, including a shared limitation of anthracycline therapies, namely cardiotoxicity. Therefore, targeting PI3K/Akt downstream of COX-2 would be a more effective option21,22,23. Downregulation of p53 activity, PI3K/Akt activation, as well as an enrichment in MYC target genes, support a survival mechanism associated with pro-inflammatory and mitogenic signalling. Prior studies have reported that MCF7-COX2 cells display increased expression of WNT transcription factors, including Cyc-D1, AXIN1 and AXIN2, but MYC gene expression remained unaltered13although observations of altered MYC target gene expression in DOX treatment, as we report here, are new. Although MYC gene expression itself was unchanged, MYC expression is often regulated post-transcriptionally24. It was shown that MYC regulates the expression of several stress-regulatory genes in miRNA-high cell secretions25. The enrichment of MYC targets in DOX-treated MCF7-COX2 cells indicates that COX-2 overexpression may promote enhanced cell survival and mechanisms of drug resistance through WNT-based MYC signalling26.
GSEA analysis identified that each cell line responds similarly to DOX exposure relative to control conditions. For all cell lines used in this study, the hallmark p53 pathway gene set was enriched in the upregulated genes, while the hallmark E2F targets gene set was enriched in the downregulated genes. Both findings suggest that DOX exposure perturbs cell cycle progression via p53 signalling and E2F suppression. We identified CALML5, IL6, GNG2, GRINB2 and IGF1 as upregulated hub genes and CDK1, CCNA2, PLK1, CDC45 and EXO1 as downregulated hub genes, shared between all cell lines in response to DOX. We chose to prioritize the validation of CALML5 and IL6, as the roles of CALML5 in cancer are not well documented, and IL6 has a connection to COX-2 via inflammatory signalling, as well as the enriched immune response observed in the sequencing data of MCF7-miR655.
CALML5 is a calcium-binding protein linked to epidermal differentiation27but poorly characterized in cancer28,29,30. While upregulated in response to DOX for all cell lines, CALM5 was significantly downregulated in MCF7-COX2 compared to MCF7, warranting further investigation due to its variable role in cancer, regardless of the therapeutic context.
GNG2, a G-protein subunit involved in cell proliferation, migration, and survival31was particularly interesting to us. Studies suggest that GNG2 can act as a tumor suppressor in certain cancers by inhibiting cell migration and metastasis32as well as Akt and ERK activity33. Low GNG2 expression was significantly associated with poor patient survival and more aggressive breast cancer subtypes. Furthermore, GNG2 expression was downregulated in MCF7-miR526b, MCF7-miR655 and MCF7-COX2 relative to MCF7. These results suggest that miRNA and COX-2-associated repression of GNG2 may combine synergistically with pre-existing PI3K/Akt activation to promote cell survival.
GRIN2B is an NMDA (N-methyl-D-aspartate) receptor subunit linked with neurodegenerative diseases34. The roles of NMDA receptors in tumor progression is explored35specifically with respect to PI3K/Akt signalling36,37as well as promoting cancer stemness38. It is more highly expressed in triple-negative breast cancer35consistent with our findings. GRIN2B is linked with poor breast cancer survival in chemotherapy-exposed tumors and is most highly expressed in the aggressive MCF7-COX2 cell line following DOX exposure. Given that COX-2 is a known regulator of Akt signalling and has been linked to NMDA receptor activity39future studies should investigate the possible relationship between COX-2 and NMDA receptors and receptor subunits in breast cancer progression.
Conversely, the downregulation of CDK1, CCNA2, PLK1, CDC45, and EXO1 suggests a disruption of both cell cycle progression and DNA replication. CDK1 and CCNA2 are essential regulators of the G2/M transition, and their suppression indicates cell cycle arrest40. PLK1 is crucial for mitotic cell division, and its overexpression has been linked to chemoresistance in TNBC, suggesting that its downregulation may enhance DOX sensitivity41. The downregulation of CDC45, a DNA replication licensing factor, and EXO1, a key exonuclease involved in DNA repair, further suggests the suppression of cell division and increased levels of DNA damage42,43.
Together, these findings highlight the novel regulatory roles of miR-526b and miR-655 in the cellular response to DOX, providing insight into potential pathways involved in drug sensitivity or resistance in breast cancer. Future directions should focus on the effects of targeting miR-526b, miR-655, and COX-2 expression, as well as their downstream signalling, in combination with DOX treatment. It is also evident that DOX responses are altered by miRNA and COX2 and each tumor subtype shows various expression of hub genes. Hence, the validation of these hub genes in vitro and the investigation of their functional role in response to DOX and other chemotherapeutics in vivo will also be required.
Conclusion
This study demonstrates novel roles of miR-526b, miR-655, and COX-2 in DOX response in breast cancer through distinct mechanisms, such as alterations in DDR, metabolic reprogramming, and immune pathway activation. Understanding these interactions may enable the development of new therapeutic strategies to improve the efficacy of DOX and related treatments therapeutics.
Materials and methods
Cell culture
Human breast cancer cell line MCF7 was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Stable breast cancer cell lines were generated by transfecting cells with gene and miRNA expression plasmids as previously described6,7,13. MCF7 cells were transfected with either pCMV-IRES2-EGFP Mock (empty) vector or pCMV-IRES2-EGFP-COX-2 expression plasmids13. Similarly, MCF7 cells were transfected with either pCMV-MIR Mock (empty) vector, or pCMV-MIR miR-526b or pCMV-MIR miR-655 expression plasmids to obtain stable miRNA overexpressing cell lines MCF7-526b7 and MCF7-miR6556.
We used MCF7-COX2 cells as a positive control for both MCF7-miR526b and MCF7-miR655. Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 media (Gibco, ON, Canada), supplemented with 10% fetal bovine serum (VWR, ON, Canada) and 1% Pen-Strep (Gibco, ON, Canada). Cells were incubated at 37 °C with 5% CO2. Transfected cells were maintained with 200ng/mL Geneticin (Cat. No: G418, Bio Basic).
Drug treatment
MCF7 cells were seeded into 96-well plates at 5,000 cells per well and allowed to attach overnight. Cells were treated with DOX (Cat. No: D5220, Sigma-Aldrich) at concentrations of 0, 0.25, 0.5, 1.0 and 2.0µM for 48 h. Dimethyl sulfoxide (DMSO) was used as the control. Cell viability was measured with the CyQUANT NF Cell Proliferation Assay (Cat. No: C35007, ThermoFisher Scientific). Briefly, old cell growth media was removed, washed with PBS and cells were incubated in the supplied fluorescent DNA dye for 30 min at 37 °C. Fluorescent intensity (Ex. 485 nm and Em. 530) was measured in a Tecan Spark Cyto Plate Reader. Experiments were performed in duplicates and replicated for at least three biological triplicates. Intensity values were compared relative to the control group. The quadratic interpolation function in GraphPad Prism (v10.0.2) (GraphPad Software, MA, USA) was used to determine the IC50 concentration to be 0.80µM. For subsequent experiments, DOX was added to cells in culture, once 70% confluent, at a final concentration of 0.80µM for 48 h either with or without a 3-day recovery (rest) period. For the rest period, media with DOX was removed after 48 h of treatment; cells were then washed with PBS and given fresh media. The media was then changed again on the second day of the rest period.
Cell viability
Cells were seeded into 6-well cell culture plates at 100,000 cells/well, and treatment started 12 h later. Following treatment, the cells were trypsinized and counted using the automated Corning Cell Counter. The average of duplicate counts was taken for each biological replicate. Viability was assessed as cell number relative to the day of treatment.
Senescence-associated beta-galactosidase (SA-ß-Gal) activity staining
Senescence was assessed by staining for ß-galactosidase activity using the CellEvent Senescence Green Detection Kit (Cat. No: C10850, ThermoFisher Scientific) per the manufacturer’s protocol. Fluorescent images were taken on the Nikon Eclipse Ti-2 microscope.
RNA extraction, cDNA synthesis and real-time qPCR
RNA was extracted using Qiazol and RNeasy Mini Kits (Cat. No: 79306 and 74104, Qiagen) and quality was assessed by spectrophotometry. 1 µg of RNA was used for cDNA synthesis with the High-Capacity cDNA Reverse Transcription Kit (Cat. No: 4368813, ThermoFisher Scientific). For quantitative real-time PCR (qRT-PCR), the Luna Universal PCR Master Mix (Cat. No: M3004, New England Biolabs), and gene specific probes (ThermoFisher Scientific) were used. Probes include those for TP53 (Hs01034249_m1), CDKN1A (p21, Hs00355782_m1), PUMA (BBC3, Hs00248075_m1), CALML5 (Hs00249968_s1) and IL6 (Hs00174131_m1). For ΔCT calculations, RPL5 (Hs03044958_g1) was used as the housekeeping gene. Fold Changes were calculated using the 2−ΔΔCT method44.
SDS-PAGE and western blotting
Cells were lysed with cell lysis buffer supplemented with protease and phosphatase inhibitors (Cat. No: 9803 and 5872, New England Biolabs). Protein concentrations were assessed with bicinchoninic acid (BCA) (Cat. No: SK3021, Bio Basic). 20–30 µg of protein was loaded for SDS-PAGE electrophoresis using 10% (p53, Chk2/p-Chk2, PARP, AKT/p-AKT), 12% (p21), and 4–15% gradient (ATM) SDS-polyacrylamide gels. Transfers were done onto a nitrocellulose membrane (0.2 μm pore size) (Cat. No: 10600007, Cytiva). Primary antibodies for proteins of interest include p21 (Cat. No: 2947, Cell Signaling Technology), p53 (Cat. No: sc-126, Santa Cruz Biotechnology), ATM (Cat. No: 2873, Cell Signaling Technology), Chk2 (Cat. No: 13954-1-AP, Proteintech), p-Chk2 (Thr68) (Cat. No: 29012-1-AP, Proteintech), PARP (Cat. No: 9542 S, Cell Signaling Technology), AKT (Cat. No: 9272 S, Cell Signaling Technology) and p-AKT (Ser473) (Cat. No: 9271 S, Cell Signaling Technology). Primary antibodies were incubated in dilutions of 1:500 for p21, p53, AKT and p-AKT, and 1:200 for ATM, Chk2, p-Chk2 and PARP at 4 °C overnight with agitation. Blots were incubated with either anti-ACTB (Cat. No: sc-47778, Santa Cruz Biotechnology), anti-Tuba1 (Cat. No: Y054861, Applied Biomedical Materials) or anti-vinculin (Cat. No: sc-73614, Santa Cruz Biotechnology) primary antibodies at 1:1,000 dilutions for housekeeping proteins. Blots were washed and incubated with Azurespectra 700 and Azurespectra 800 (Cat. No: AC2129 and AC2134, Azure Biosystems) NIR-fluorescent secondary antibodies at 1:10,000 dilution for 1 h at room temperature. Dry membranes were imaged using an Azure Sapphire Biomolecular Imager (Azure Biosystems) and analyzed using the AzureSpot software with rolling ball background noise elimination and housekeeping gene normalization. Graphs illustrate fold changes of normalized fluorescent band volume (normalized volume) relative to untreated samples. AKT phosphorylation was analyzed as the ratio of phosphorylated AKT to total AKT. The cleavage of PARP was analyzed as the ratio of cleaved PARP (~ 88 kDa fragment) to total PARP.
Single-cell gel electrophoresis (comet assay)
Comet assays were performed with the Abcam Comet Assay Kit (Cat. No: ab238544, Abcam) according to the manufacturer’s protocol. Electrophoresis was performed in an alkaline running buffer at 26 V (1 V/cm) and 300 mA for 25 min. Fluorescent images were taken on the Nikon Eclipse Ti-2 microscope at 4X and 10X magnification. Comet metrics (tail DNA %, tail moment and olive moment) were analyzed via the ImageJ plugin OpenComet (v1.3.1)45. 20 images were analyzed per sample. Means for each statistic were calculated over three biological replicates.
Immunocytochemistry (ICC)
Immunocytochemistry was performed for phosphorylated H2A histone family member X (γ-H2AX (S139)). Cells were washed twice with ice-cold PBS and fixed with methanol for 10 min at -20 °C. Following fixation, cells were washed and blocked with 1% BSA in PBS for 1 h at room temperature. Fixed samples were incubated in primary γ-H2AX (S139) antibody (Cat. No: 9718, Cell Signaling Technology) at a 1:800 dilution in 0.1% BSA in PBS, overnight at 4 °C with agitation. Samples were washed and incubated with TRITC-conjugated secondary antibody (Cat. No: T-2769, ThermoFisher Scientific) at a dilution of 1:1,000 in 0.1% BSA in PBS for 1 h at room temperature. Cells were washed three times with PBST (0.05% Tween) and mounted onto coverslips with DAPI-supplemented mounting medium (Cat. No: Ab104139, Abcam). Slides were sealed, stored at 4 °C and imaged the following day. Fluorescent imaging was done on the Nikon Eclipse Ti-2 at 40X magnification. Staining was analyzed using the NIS Elements AR software (v5.42.01), where the region of interest for quantification was limited to the nucleus of each cell within a given image, resulting in an average fluorescent intensity for each image. At least 10 images were analyzed per sample.
RNA-sequencing and high throughput data pipeline
RNA-sequencing was outsourced and performed at the Centre d’expertise et de services Génome Québec (CES) (McGill University, Montréal, Canada). Samples were treated with either DMSO or DOX for 48 h. Following treatment, cells were resuspended in Qiazol (Qiagen, MD, USA) and frozen before shipment on dry ice. mRNA library preparation with polyA enrichment was performed before sequencing with the NovaSeq 6000 sequencing system (PE100–25 M reads). Sequence quality was assessed with FastQC (v 0.11.9)46 and trimmed with Trimmomatic (v0.39)47. Sequences were aligned and counted with the Spliced Transcripts Alignment to a Reference (STAR) (v2.7.11b)48 and High-throughput Sequence (HTSeq) packages (v2.0.8)49 against the GRCh38.p14 reference genome. Count files were analyzed for differential gene expression with the Bioconductor package, DESeq2 (v1.46.0)50in RStudio (v2024.12.0.467, R v4.4.2). Log2 fold changes were computed, and multiple testing correction was performed using the Benjamini-Hochberg method to control for false discovery. This data is represented graphically in volcano plots and heatmaps generated in RStudio using the ggplot2 (v3.5.1)51 package and the ComplexHeatmap (v2.24.0)52 package, respectively. Genes with an adjusted p-value less than 0.05 and log2 fold changes above 1.5 or below − 1.5 were considered differentially expressed genes (DEGs) and included in downstream analysis. This data can be found in the Gene Expression Omnibus (GEO) database53 under the series record GSE295502.
Gene set enrichment analysis (GSEA)
Gene set enrichments were assessed with the Gene Set Enrichment Analysis (GSEA) software (v4.3.3)54. Hallmark (H) Molecular Signature Database (MSigDB) gene sets were analyzed. Differentially expressed genes were ranked by the signal-to-noise metric, and normalized enrichment scores (NES) were calculated after 1,000 gene set permutations. Gene sets with an FDR under 0.25 were considered significantly enriched.
Analysis of enriched biological processes
DEGs were categorized into upregulated and downregulated groups. These lists were submitted to the Enrichr web server55 to assess enriched biological processes (BPs). The raw data for these enrichments were downloaded from Enrichr and replotted to display the top 10 biological processes, ranked by adjusted p-value, along with associated gene ratios and counts.
Network and hub gene analysis
Up and downregulated DEGs were submitted to STRING56 to generate protein interaction networks. The full network type, where edges indicated both functional and physical protein interactions, was chosen with a high confidence interaction score of 0.7. The network was exported to Cytoscape (v3.10.3)57 and the cytoHubba plugin (v0.1)58 was used to assess network topology. The Degree, Betweenness and Closeness topological analysis methods were used to identify the most connected nodes (genes) within each network. The top 5 genes based on combined topological scores from each of the three methods applied were considered hub genes for a particular network.
Receiver operating characteristic (ROC) analysis
The publicly available online tool ROC plotter59 was used to perform receiver operating characteristic analysis and determine the classifier performance of each miR-526b and miR-655 expression in breast cancer patients who did and did not respond to DOX treatment. miRNA expression is presented as normalized (log2(x + 1)) sequence counts obtained from the pan-cancer analysis of The Cancer Genome Atlas. Samples from patients without progression before the median follow-up time were considered responders, while patients with progression before the median follow-up time were considered non-responders.
Kaplan-Meier (KM) survival analysis
Overall survival analysis based on gene expression was performed using the online survival analysis tool, KM plotter60. The RNA-seq mRNA data set was used, and analysis was performed for all breast cancer patients and those who received chemotherapy (+ chemotherapy). Patient groups were split by the optimal cutoff value determined by the KM plotter algorithm.
Accession of the cancer genome atlas (TCGA)-breast cancer (BRCA) patient data
Transcriptomic breast cancer data were obtained from the Cancer Genome Atlas (TCGA)61 via the TCGAbiolinks R package62. Patients were stratified by normal tissue and the four basic breast cancer subtypes based on their PAM50 classification63. Expression values in transcripts per million (TPM) were transformed (log2(TPM + 1)) and assessed for each gene of interest.
Statistical analysis
Statistical analysis and graphing were performed using GraphPad Prism Software (v10.4.0). One-way analysis of variance (ANOVA), followed by Dunnett’s post hoc test for multiple comparisons, was used to assess cell viability (Fig. 2C) and cell line differences in gene expression (Fig. 2C and F). One-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons, was used to assess treatment differences in protein expression (Figs. 2H and I and 4G, H and I). Two-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons, was used to analyze cell line and treatment differences in AKT phosphorylation (Fig. 2J), PARP cleavage (Fig. 2K), tail DNA percentage, tail moment and olive moment (Fig. 3B and D), as well as differences in immunofluorescent cell staining (Fig. 4E). Differential gene expression analysis was performed using the default parameters of the DESeq2 package (v1.38.3) (Figs. 5A and C and 6H and I). Count values were normalized using the median-of-ratios method, which applies a size factor to the counts of each sample. This size factor is the median calculated from the ratios of the raw count of each gene within a sample to the geometric mean of each gene across all samples. Gene-wise dispersion estimates were modeled under a negative binomial distribution, with empirical Bayes shrinkage. Log2 fold changes were calculated from the fitted model to quantify differences in expression between either conditions or cell types, and the Wald test was used to determine statistical significance. The Benjamini-Hochberg method was used to calculate adjusted p-values, controlling for the false discovery rate. Adjusted p-values below 0.05 were considered significant. For gene expression data from TCGA, normality was assumed due to large sample sizes. Differences in gene expression between sample types were assessed via non-parametric Kruskal-Wallis test, followed by Dunn’s multiple comparisons test (Fig. 7). Based on the a priori assumption of upregulation after doxorubicin exposure, as observed from the sequencing results, one-tailed t-tests were used to statistically assess the qPCR validation of the identified hub genes CALML5 and IL6. Statistical significance was defined as p < 0.05.
Data availability
The data presented in this study are available on request from the corresponding author. Sequencing data has been deposited in the GEO database under the series record GSE295502.
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Bukowski, K., Kciuk, M. & Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J. Mol. Sci 21 (2020).
Gu, Y. et al. Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer. Mol. Biomed. 6, 2 (2025).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Peng, Y. & Croce, C. M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. Ther. 1, 15004 (2016).
Majumder, M. et al. COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Sci. Rep. 8, 327 (2018).
Majumder, M., Landman, E., Liu, L., Hess, D. & Lala, P. K. COX-2 elevates oncogenic miR-526b in breast cancer by EP4 activation. Mol. Cancer Res. 13, 1022–1033 (2015).
Shin, B. et al. miR526b and miR655 induce oxidative stress in breast cancer. Int J. Mol. Sci 20 (2019).
Tordjman, J. et al. Tumor suppressor role of cytoplasmic polyadenylation element binding protein 2 (CPEB2) in human mammary epithelial cells. BMC Cancer. 19, 561 (2019).
Chen, Q. et al. PIK3R3 inhibits cell senescence through p53/p21 signaling. Cell. Death Dis. 11, 798 (2020).
Gervin, E. et al. Chemically induced hypoxia enhances MiRNA functions in breast cancer. Cancers (Basel) 12 (2020).
Hunter, S., Nault, B., Ugwuagbo, K. C., Maiti, S. & Majumder, M. Mir526b and Mir655 promote tumour associated angiogenesis and lymphangiogenesis in breast cancer. Cancers (Basel) 11 (2019).
Majumder, M. et al. COX-2 induces breast cancer stem cells via EP4/PI3K/AKT/NOTCH/WNT axis. Stem Cells. 34, 2290–2305 (2016).
Mayo, L. D. & Donner, D. B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. U S A. 98, 11598–11603 (2001).
Chen, J. M., Bai, J. Y. & Yang, K. X. Effect of Resveratrol on doxorubicin resistance in breast neoplasm cells by modulating PI3K/Akt signaling pathway. IUBMB Life. 70, 491–500 (2018).
Nault, B. D. & Majumder, M. miR-526b enhances glucose metabolism in breast cancer cells, an effect reversed by targeting the COX-2/EP4 pathway. Mol. Biol. Rep. 52, 351 (2025).
McGuirk, S. et al. Resistance to different anthracycline chemotherapeutics elicits distinct and actionable primary metabolic dependencies in breast cancer. Elife 10 (2021).
Fenton, S. E., Saleiro, D. & Platanias, L. C. Type I and II interferons in the Anti-Tumor immune response. Cancers (Basel) 13 (2021).
Vidal, P. Interferon alpha in cancer immunoediting: from elimination to escape. Scand. J. Immunol. 91, e12863 (2020).
He, Y. et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal. Transduct. Target. Ther. 6, 425 (2021).
De Paz Linares, G. A., Opperman, R. M., Majumder, M. & Lala, P. K. Prostaglandin E2 receptor 4 (EP4) as a therapeutic target to impede breast Cancer-Associated angiogenesis and lymphangiogenesis. Cancers (Basel) 13 (2021).
Gopaul, V. L. et al. A prospective tumour marker for breast cancer: YWHAB and its role in promoting oncogenic phenotypes. Breast Cancer (Dove Med. Press. 16, 935–956 (2024).
Castel, P. et al. Rationale-based therapeutic combinations with PI3K inhibitors in cancer treatment. Mol. Cell. Oncol. 1, e963447 (2014).
Scholz, B. A. et al. WNT signaling and AHCTF1 promote oncogenic MYC expression through super-enhancer-mediated gene gating. Nat. Genet. 51, 1723–1731 (2019).
Feser, R. et al. Breast cancer cell secretome analysis to Decipher MiRNA regulating the tumor microenvironment and discover potential biomarkers. Heliyon 9, e15421 (2023).
You, Z. et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J. Cell. Biol. 157, 429–440 (2002).
Sun, B. K. et al. CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation. Genes Dev. 29, 2225–2230 (2015).
Kitazawa, S., Takaoka, Y., Ueda, Y. & Kitazawa, R. Identification of calmodulin-like protein 5 as tumor-suppressor gene silenced during early stage of carcinogenesis in squamous cell carcinoma of uterine cervix. Int. J. Cancer. 149, 1358–1368 (2021).
Kanamori, K. et al. CALML5 is a novel diagnostic marker for differentiating thymic squamous cell carcinoma from type B3 thymoma. Thorac. Cancer. 14, 1089–1097 (2023).
Debald, M. et al. Specific expression of k63-linked ubiquitination of calmodulin-like protein 5 in breast cancer of premenopausal patients. J. Cancer Res. Clin. Oncol. 139, 2125–2132 (2013).
Yajima, I. et al. GNG2 inhibits invasion of human malignant melanoma cells with decreased FAK activity. Am. J. Cancer Res. 4, 182–188 (2014).
Luo, C., Xiao, Z. & Yang, W. GNG2 inhibits brain metastases from colorectal cancer via PI3K/AKT/mTOR signaling pathway. Sci. Rep. 15, 1787 (2025).
Zhao, A. et al. GNG2 acts as a tumor suppressor in breast cancer through stimulating MRAS signaling. Cell. Death Dis. 13, 260 (2022).
Li, D. & He, L. Association study between the NMDA receptor 2B subunit gene (GRIN2B) and schizophrenia: a huge review and meta-analysis. Genet. Med. 9, 4–8 (2007).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Dong, W., Sun, S. J., Qin, J. J. & Liu, G. M. Fyn stimulates the progression of pancreatic cancer via Fyn-GluN2b-AKT axis. Eur. Rev. Med. Pharmacol. Sci. 24, 109–121 (2020).
Li, L. et al. GKAP acts as a genetic modulator of NMDAR signaling to govern invasive tumor growth. Cancer Cell. 33, 736–751e735 (2018).
She, X., Gao, Y., Zhao, Y., Yin, Y. & Dong, Z. A high-throughput screen identifies inhibitors of lung cancer stem cells. Biomed. Pharmacother. 140, 111748 (2021).
Strauss, K. I. & Marini, A. M. Cyclooxygenase-2 Inhibition protects cultured cerebellar granule neurons from glutamate-mediated cell death. J. Neurotrauma. 19, 627–638 (2002).
Ghafouri-Fard, S. et al. A review on the role of Cyclin dependent kinases in cancers. Cancer Cell. Int. 22, 325 (2022).
Maire, V. et al. Polo-like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 73, 813–823 (2013).
Chen, C. C. et al. EXO1 suppresses double-strand break induced homologous recombination between diverged sequences in mammalian cells. DNA Repair. (Amst). 57, 98–106 (2017).
Srinivasan, S. V., Dominguez-Sola, D., Wang, L. C., Hyrien, O. & Gautier, J. Cdc45 is a critical effector of myc-dependent DNA replication stress. Cell. Rep. 3, 1629–1639 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods 25, 402–408 (2001).
Gyori, B. M., Venkatachalam, G., Thiagarajan, P. S., Hsu, D. & Clement, M. V. OpenComet: an automated tool for comet assay image analysis. Redox Biol. 2, 457–465 (2014).
Andrews, S. FastQC: A Quality Control Tool For High Throughput Sequence Data (2010). http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Villanueva, R. A. M. & Chen, Z. J. ggplot2: Elegant Graphics for Data Analysis, 2nd edition. Meas-Interdiscip. Res. 17, 160–167 (2019).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Barrett, T. et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res. 41, D991–995 (2013).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U S A. 102, 15545–15550 (2005).
Chen, E. Y. et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 14, 128 (2013).
Szklarczyk, D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Chin, C. H. et al. CytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 8 (Suppl 4), S11 (2014).
Fekete, J. T. & Gyorffy, B. ROCplot.org: validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3,104 breast cancer patients. Int. J. Cancer. 145, 3140–3151 (2019).
Gyorffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innov. (Camb). 5, 100625 (2024).
Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Colaprico, A. et al. TCGAbiolinks: an r/bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 44, e71 (2016).
Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 27, 1160–1167 (2009).
Acknowledgements
We would like to acknowledge these various funding agencies for their support. This work is supported by the Lotte and John Hecht Memorial Foundation, National Science and Engineering Research Council of Canada (NSERC) Discovery Grants to M.M. This study is also supported by the Canada Research Chair Program (CRCP), the Canada Foundation for Innovation (CFI), and Research Manitoba Matching fundings to M.M. R.M.O. received a NSERC Undergraduate Student Research Award, a Research Manitoba Master’s Studentship Award and a Canadian Institute of Health Research (CIHR) Frederick Banting and Charles Best Canada Graduate Scholarship-Master’s (CGS M) award.
Author information
Authors and Affiliations
Contributions
R.M.O. and M.M. conceived and designed the project. R.M.O. performed in vitro experiments and analyzed data. R.M.O. and S.M. analyzed RNA-sequence data and created figures. R.M.O. and M.M. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
This study is approved by the Brandon University Ethical Approval Committee (approval # 23056) and the Brandon University Biosafety Committee (approval # 2020-BIO-02).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Opperman, R.M., Maiti, S. & Majumder, M. Investigating the effects of miR-526b and miR-655 on doxorubicin sensitivity in breast cancer. Sci Rep 15, 33584 (2025). https://doi.org/10.1038/s41598-025-18719-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-18719-y