Abstract
Environmental contamination by heavy metals is a growing concern due to their adverse impacts on plant health and ecological stability. In this study, the potential of Ramalina farinacea (L.) Ach. lichen extract to mitigate the toxic effects of nickel chloride (NiCl₂) on Allium cepa L. was investigated. The bulbs were exposed to various treatment groups, and a series of physiological, cytogenetic, biochemical, and anatomical analyses were performed. Throughout the experimental period, the control group was treated with tap water, while the treatment groups were treated with 0.5 g L−1 R. farinacea extract, 1.0 g L−1 R. farinacea extract, 1 mg L−1 NiCl2, 1 mg L−1 NiCl2 + 0.5 g L−1 R. farinacea extract, and 1 mg L−1 NiCl2 + 1.0 g L−1 R. farinacea extract, respectively. NiCl₂ exposure significantly impaired root development, disrupted mitotic activity, elevated oxidative stress markers, and caused DNA and chromosomal damage. Micronucleus (MN), sticky chromosome, vagrant chromosome, fragment, unequal chromatin distribution, bridge, vacuolated nucleus, and irregular mitosis were among the chromosomal aberrations (CAs) brought on by NiCl₂. However, co-treatment with R. farinacea extract effectively alleviated these adverse effects in a dose-dependent manner. The lichen extract restored mitotic index values, reduced CAs and MN formation, decreased malondialdehyde and proline accumulation, and helped preserve chlorophyll content and meristematic tissue integrity. LC–MS/MS analysis confirmed the presence of phenolic compounds with known antioxidant and metal-chelating activity, such as p-coumaric acid, ferulic acid, gentisic acid, vanillin, taxifolin, 4-hydroxybenzoic acid, and syringic acid. These findings highlight the protective role of R. farinacea extract against Ni-induced phytotoxicity and genotoxicity in A. cepa, suggesting its promising potential as a natural bioprotective agent for environmental and agricultural applications.
Similar content being viewed by others
Introduction
Metals and metalloids with an atomic density greater than 5 g cm−3 are classified as heavy metals and are naturally present in soil. The concentration of heavy metals in environmental systems, which has been increasing in response to geological events, is further elevated by anthropogenic activities1. Among the most prominent causes of heavy metal accumulation in the environment are rapid industrialization, the mining and smelting of metals, the production of batteries and other metal-based equipment, the intensive use of pesticides and fertilizers in agriculture, the burning of fossil fuels, and the disposal of municipal waste2. While macro-and micro-elements play a crucial role in physiological and biochemical processes, affecting growth and development in plants, excessive or prolonged exposure to elements in the heavy metal class can have detrimental effects on the health of both plants and other living organisms1.
Nickel (Ni), a naturally occurring element in water and soil, is classified as one of 23 metal pollutants. It was once called “devil’s copper” by miners who mistook it for copper ore. Subsequently, it was purified in 1951 by the Swedish chemist Axel Cronstedt and identified as the 28th element in the periodic table3. Various anthropogenic activities such as the use of fossil fuels, phosphate fertilizers, vehicle emissions, sludge water, smelting, and electroplating are the most common sources of Ni release to the environment. These emissions threaten not only the ecosystem but also human health4. While Ni is widely regarded as an essential element and micronutrient for plants, exhibiting numerous functions such as germination, growth, senescence, iron uptake, nitrogen assimilation, urease activity, and maintenance of photosynthetic pigments, it is toxic when present in elevated concentrations4,5. In humans, Ni toxicity through direct inhalation or ingestion can also occur as a result of lifelong dietary or occupational exposure. While Ni accumulation resulting from chronic exposure has been associated with various health concerns, including cardiovascular disease, kidney problems, and pulmonary fibrosis, the most significant consequences are observed in the development of tumors at the sites of Ni application, as evidenced in animal models. As a result, the International Agency for Research on Cancer (IARC) has classified all nickel compounds, with the exception of metallic Ni, as carcinogenic to humans6.
Lichens are defined as mutualistic life forms formed by a fungus with a photosynthetic organism, such as an alga or a cyanobacterium. These organisms are able to thrive in conditions where either partner would be unable to survive independently7. Indeed, they are considered to possess a high degree of tolerance to low temperatures, extreme drought, and UV radiation. This tolerance is attributed to the secondary metabolites that are produced as a result of the symbiotic integration of their constituent partners8. Furthermore, lichens have been described as a “treasure chest of bioactive natural products” due to the fact that even a single species of lichen has been reported to contain many metabolites simultaneously, each with distinct bioactivities9. At least 1000 different secondary compounds identified in various lichen species have been reported to have important activities such as analgesic, antioxidant, anticancer, antibiotic, antiviral, antifungal, antipyretic, and antioxidant7,10. Lichens are also excellent bioindicators that can accumulate excessive levels of heavy metals and other mineral elements due to their lack of a root system, stomata or protective cuticle11. This success may be due to the fact that a large proportion of metal ions are retained on the outer surface of the cell walls and not taken up into the cell, but it may also be due to the binding of metals with lichen secondary metabolites, organic acids, polysaccharides, oxalate crystals or melanin pigment12. The emergence of new pathogens, the evolution of existing diseases, the persistence of untreatable conditions, and increasing drug and vaccine resistance have raised interest in alternative therapies. Among these natural remedies with high bioactive potential, lichens are considered a promising resource9.
Ramalina farinacea (L.) Ach. is a member of the Ramalinaceae family and usually appears pendulous, greenish, and fruticose. The thallus grows in an epiphytic form. It has a central basal hapter that connects it to the growth surface. Many dusty patches called soralia, which release asexual reproductive particles called soredia, are seen on the lichen thallus. Due to its adaptability, this species can thrive in a diverse range of global environmental conditions13,14. Previous investigations have demonstrated that R. farinacea extracts have a high concentration of phenolic components and strong antibacterial and antioxidant properties15,16.
The investigation of heavy metal toxicity and the search for natural compounds that can be used to combat environmental pollutants are two different but linked goals for which A. cepa has gained importance as a plant model organism17,18. Yılmaz et al.19 suggested that A. cepa is an excellent plant for monitoring heavy metal toxicity because of its adventitious root system, which grows rapidly and comes into direct contact with the test chemical, and its large chromosomes (2n = 16), which can be easily observed under a research microscope. In addition, the Allium test does not require ethical approval and has a significant correlation (82%) with carcinogenicity in tests involving mammalian rodents.
This study aimed to explore whether the extract of R. farinacea could help reduce the toxic effects of nickel chloride (NiCl₂) exposure in A. cepa. A. cepa bulbs were treated with the prepared solutions, in order to achieve this purpose. A series of analyses were carried out, including physiological parameters (such as rooting percentage, weight increase, and root length), cytogenetic markers (mitotic index (MI), chromosomal aberrations (CAs), and micronucleus (MN) formation), and DNA damage assessment using the Comet assay. In addition, biochemical indicators like malondialdehyde (MDA), proline, and chlorophyll content were measured, along with the activities of superoxide dismutase (SOD) and catalase (CAT). Additionally, the integrity of the root meristem tissue was assessed under a microscope. Furthermore, the phenolic profile of the R. farinacea extract was characterized using LC–MS/MS analysis. To the best of our knowledge, there is no prior research exploring whether R. farinacea extract can counteract Ni-induced cytogenotoxicity in A. cepa. By presenting the first such evidence, this study has the potential to open a new avenue for linking the phenolic profile of a globally distributed lichen to the alleviation of Ni-driven physiological, cytogenetic, biochemical, and anatomical alterations.
Materials and methods
Preparation of materials, test solutions and experimental design
A. cepa bulbs were purchased from a local market in Giresun, Türkiye. Firstly, the bulbs were selected based on similar size and weight (9.21–9.32 g). Old roots and the outermost brown scales of the bulbs were then removed. R. farinacea samples were collected from Doğankent, Çatalağaç Village (Giresun, Türkiye) at an altitude of ca. 704 m meters in November 2022. The lichen species were identified by Prof. Dr. Kadir Kınalıoğlu (Giresun University, Department of Biology, Botany Division) according to the study of Brodo et al.20.
All procedures involving plants and lichen samples (including the collection of A. cepa bulbs and R. farinacea thalli) were conducted in accordance with national, international, and institutional guidelines and legislation. The lichens were meticulously cleaned to remove debris and other plant material and then dried at room temperature in a shaded environment. The dried samples were powdered using a mechanical grinder, and aqueous extracts were prepared. The A. cepa bulbs were initially exposed to the lichen extracts in a pre-trial phase. Bulbs were placed in glass tubes with their basal plates in direct contact with the extract solutions. The impact of the extracts on adventitious root growth was monitored over three consecutive mitotic cycles. Two concentrations (0.5 g L−1 and 1.0 g L−1) were selected for the main study. The appropriate concentration of NiCl₂ (CAS No: 0007718549/Sigma) was determined based on the study by Yalçın et al.21, which identified 50 mg L⁻1 as a suitable concentration for use in A. cepa applications.
For the main experiment, bulbs were randomly assigned into six groups (n = 50). Each group was incubated in sterile beakers containing the respective treatment solutions (tap water, 0.5 g L−1 R. farinacea extract, 1.0 g L−1 R. farinacea extract, 1 mg L−1 NiCl2, 1 mg L−1 NiCl2 + 0.5 g L−1 R. farinacea extract, and 1 mg L−1 NiCl2 + 1.0 g L−1 R. farinacea extract) for 72 h at room temperature in complete darkness. For chlorophyll analysis, some of the bulbs from each group remained in treatment for 144 h, as this measurement required a longer exposure period than the standard 72-h setup. During the application period, solutions were refreshed daily to maintain consistency (Table 1).
Physiological parameter assessment
The rooting percentage was calculated using Eq. 1, based on the number of bulbs that developed roots relative to the total number in each group (n = 50):
(NRB: Number of rooted bulbs, TNB: Total number of bulbs).
Root elongation was assessed by directly measuring the length of adventitious roots using a ruler (n = 10). Additionally, the weight increase was determined by subtracting the initial bulb weight from the final weight recorded at the end of the experiment (n = 10)22.
Evaluation of cytogenetic markers
Root tips were initially rinsed to eliminate surface residues and then fixed using Clarke’s solution (3:1 glacial acetic acid:ethanol), followed by hydrolysis in 1 N HCl at 60 °C for 13 min. Post-hydrolysis, roots were stained in 1% acetocarmine for 14 h and squashed onto slides. A final drop of 45% acetic acid was used to complete the slide preparation. Random fields were imaged using a research microscope (IRMECO IM-450 TI) at 500 × magnification. Ten slides per group were analyzed. MI, MN, and CAs were evaluated following the method described by Macar et al.23. MN scoring followed the criteria established by Fenech et al.24.
MI was calculated using Eq. 2 based on the ratio of mitotic cells to total cells (n = 10):
(NCIM: Number of cells in mitosis, TCC: Total cell count).
In evaluating CAs-including MN-a total of 1,000 cells were examined per group. For the calculation of the MI, observations were expanded to cover 10,000 cells in total.
To assess DNA damage, particularly tail formation, we employed the Comet assay protocol developed by Chakraborty et al.25, with slight modifications, including the addition of a lysis step. DNA isolation was performed following the protocol outlined by Sharma et al.26. The slides used for the assay were first immersed in ethanol for 24 h and then dried in an oven. A layer of 1% normal melting point agarose was applied to each slide, after which a coverslip was gently placed on top. Once the agarose solidified, a second layer was formed by mixing freshly isolated cells with 1% low melting point agarose in a 1:7 ratio. This mixture was added on top of the first layer, again covered with a coverslip. Prior to electrophoresis, slides were immersed in freshly prepared lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH 10, containing 1% Triton X-100 and 10% DMSO) for 1 h at 4 °C. The alkaline version of the assay (pH > 13) was applied to detect single-strand breaks, alkali-labile sites, and incomplete excision repair sites. Slides were briefly frozen (5 min) and then subjected to electrophoresis for 40 min to promote DNA unwinding and migration.
To neutralize the alkaline conditions post-electrophoresis, slides were rinsed three times with Tris–HCl buffer (0.4 M, pH 7.5) at 25 °C, each rinse lasting five minutes. After staining the slides with 80–100 µL of ethidium bromide, they were visualized under a fluorescence microscope. Comet images were analyzed using the TriTek 2.0.0.38 Automatic Comet Assay Software, which calculated the DNA content in both the head and tail regions. The degree of DNA damage was inferred from the percentage of tail DNA, interpreted according to the scale of Pereira et al.27: ≤ 5% as negligible, 5–20% weak, 20–40% moderate, 40–75% severe, and ≥ 75% as extensive damage. All measurements were conducted in ten replicates.
Biochemical parameter evaluation
Root tissues treated with NiCl2, R. farinacea extract, and their combinations were used for enzyme and oxidative stress analyses. Leaf samples were used for chlorophyll (chlorophyll a and b) measurements. SOD and CAT activities were determined following Zou et al.28’s protocol. Briefly, 0.5 g of root tissue was homogenized in 5 mL of 50 mM phosphate buffer (pH 7.8) and centrifuged at 10,000 rpm for 25 min. The resulting supernatant was stored at + 4 °C for further analysis.
For SOD, a reaction mixture containing supernatant, nitroblue tetrazolium (NBT), methionine, riboflavin, EDTA, PVP, and buffer (0.05 M sodium phosphate buffer with pH 7.8) was exposed to fluorescent light for 15 min. Absorbance was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme required to inhibit 50% of NBT reduction (n = 10) (Beauchamp and Fridovich, 1971)29.
CAT activity was assessed spectrophotometrically at 240 nm, measuring the decomposition of hydrogen peroxide. The reaction mixture included distilled water, hydrogen peroxide, and phosphate buffer, with absorbance recorded after enzyme addition (n = 10) (Beers and Sizer, 1952)30.
Lipid peroxidation was evaluated by measuring MDA content using the thiobarbituric acid (TBA) assay (Ünyayar et al., 2006)31. Root tissue (0.5 g) was homogenized in 5% trichloroacetic acid. After reacting with TBA at 95 °C and rapid cooling, the mixture was centrifuged at 10,000 g. The absorbance was then measured at 532 nm (n = 10).
Chlorophyll a and b content in leaves was determined via the method proposed by Witham et al.32, using 100% acetone for pigment extraction and measuring absorbance at 645 nm and 663 nm after centrifugation (n = 10).
Proline levels of the groups were determined according to the procedure of Bates et al.33. For this purpose, 0.2 g of root sample was homogenized in 10 mL of 3% aqueous sulfosalicylic acid, and 2 mL of the homogenate was mixed with the same volumes of glacial acetic acid and acid-ninhydrin. The mixture was boiled in a water bath (100 °C) for 60 min to allow the mixture to react, and the reaction was terminated using an ice bath. Then, 4 mL of toluene was added to the mixture and vortexed for 10 s to ensure mixing. At a wavelength of 520 nm, the chromophore phase’s absorbance was measured to calculate proline concentration. A freshly prepared proline standard was used for the calculation.
Meristematic tissue observations
Longitudinal sections from adventitious roots were stained with 1% methylene blue and analyzed microscopically. A total of 10 sections per group were assessed. Meristematic damage was categorized as: undamaged (–), mild (*), moderate (**), or severe (***).
Phenolic composition of the lichen extract
The phenolic profile of R. farinacea extract was analyzed at Hitit University’s HUBTUAM using LC–MS/MS. Dried R. farinacea powder (1 g) was extracted with a methanol-dichloromethane (4:1) solution. After filtration through a 0.45 µm syringe filter, the filtrate was analyzed using an ODS Hypersil column (4.6 × 250 mm) at 30 °C with a flow rate of 0.7 mL/min over 34 min.
Statistical analysis
All data were processed using IBM SPSS Statistics v23. Results were expressed as mean ± standard deviation (SD). Differences among groups were assessed using one-way ANOVA followed by Duncan’s multiple comparison test. Significance was accepted at p < 0.05.
Results and discussion
The effects of R. farinacea extract, NiCl₂, and mixtures of the two materials on physiological parameters in A. cepa bulbs are presented in Table 2. While the rooting percentages of the control, RF 1, and RF 2 groups were 99%, 98%, and 100%, respectively, the rooting percentage of the Ni group exposed to NiCl₂ decreased to 60% (Table 2). On the other hand, the rooting percentages of the NiRF 1 and NiRF 2 groups treated with a mixture of NiCl2 and varying doses of R. farinacea extract were 71% and 79%, respectively. In addition, both root length and weight gain of the RF 1 and RF 2 groups treated with only R. farinacea extracts were statistically indistinguishable from the control group values. Meanwhile, root length and weight gain of the Ni group decreased by approximately 76.0% and 74.5%, respectively, compared to the control group. Similar to the rooting percentage, the root length and weight gain of the NiRF 1 and NiRF 2 groups were higher than those of the Ni group but lower than the control values (Table 2). Compared to the NiRF 1 group, all physiological parameter results of the NiRF 2 group exposed to the mixture containing NiCl₂ and a higher dose of R. farinacea extract were closest to the control level. Indeed, the root length and weight increase of this group were approximately 2.7 and 2.5 times that of the Ni group, respectively.
The groups treated with pure lichen extract showed no statistical difference with the control group in terms of rooting percentage, root length, and weight gain, suggesting that the preferred doses of R. farinacea extract were not toxic to A. cepa. Our results confirmed the study showing that all doses slowed root growth in A. cepa treated with 0.25–2.00 mM Ni nitrate34. In addition, the EC50 value in the aforementioned study was determined to be only 0.25 mM Ni2⁺. Similarly, Kalefetoğlu Macar et al.35 and Yılmaz et al.19 previously showed that 1 mg L−1 NiCl2 restricted rooting, root elongation, and bulb weight gain in A. cepa. The underlying mechanisms responsible for the substantial suppression of growth in the Ni group may involve several different processes. For instance, the presence of Ni2⁺ ions in the root medium may disrupt the balance of ions such as sodium (Na⁺), potassium (K⁺), and hydrogen (H+) that are transported into the plant. This imbalance may lead to a disruption of osmotic regulation, a process that plays a critical role in plant growth and water uptake34. In addition, Ni2+ disrupts chromosome structure and causes a slowdown in mitotic division, reducing cell proliferation35. Furthermore, Demchenko et al.36 showed that Ni suppressed cell division and differentiation in Triticum aestivum roots, especially in the pericycle and endodermis by inhibiting cell elongation and causing necrosis. Through a study in Arabidopsis thaliana, Ni has been shown to cause gravity defects, suppress the distribution of auxin hormone towards the shoot, induce the accumulation of reactive oxygen species (ROS), disrupt the orientation and integrity of microtubules, and reduce cell elongation37.
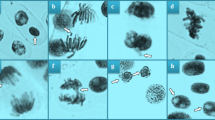
The cytogenetic effect of NiCl2, R. farinacea extract and R. farinacea extract applied in a mixture with NiCl2 on A. cepa roots was investigated by using MI, MN, CAs and DNA damage parameters. When MI, MN, and CAs were considered, no significant difference was observed between the mean values of the C, RF 1, and RF 2 groups (p > 0.05) (Table 3). Therefore, it was determined that the doses of lichen extract selected for this study did not demonstrate any adverse effect on the cytogenetic parameters, similar to the physiological measurements. On the other hand, compared to the control group, the MI value of the Ni group decreased significantly, while the frequency of MN (Fig. 1a) and CAs increased significantly. The MI value in this group decreased by approximately 43.7 compared to the control group. In A. cepa root meristem cells treated with NiCl2, the observed reduction in root elongation was accompanied by a decrease in MI, indicating that growth retardation may be due, at least in part, to the suppression of mitosis (Tables 2 and 3). The CAs observed in the Ni group were listed as sticky chromosome (Fig. 1b), vagrant chromosome (Figs. 1c, f), fragment (Fig. 1d), unequal distribution of chromatin (Fig. 1e), bridge (Fig. 1f), vacuolated nucleus (Fig. 1g), and irregular mitosis (Fig. 1h) (Table 3). In the NiRF 1 and NiRF 2 groups, MI increased while MN and CAs decreased compared to the Ni group. Among them, the NiRF 2 group, containing the higher dose of the lichen extract, was closest to the control group in all parameters (Table 3). Our results were consistent with the study showing that nickel nitrate decreased MI in A. cepa var. aggregatum and induced the accumulation of MN and CAs such as fragmented chromosome, sticky chromosome, laggard chromosome, bridge, and binucleus38. Similarly, da Cunha Neto et al.39 revealed that high doses of Ni decreased MI but increased MN and CAs in A. cepa. In addition, Yılmaz et al.19 reported that exposure to 1 mg L−1 NiCl2 led to a variety of CAs, including fragmentation, bridge, vagrant and sticky chromosomes, unevenly distributed chromatins, reverse polarization and budded nuclei. Furthermore, Gantayat et al.40 showed that Ni exposure induced spindle abnormalities such as adhesive bridges in anaphase, multipolarity, early segregation, clustering of chromosomes, and late segregation, as well as direct chromosomal damage including chromosome breakage, chromosome erosion, and laggard chromosomes in A. cepa. The reduction in MI, indicative of decreased cell division, serves as a marker of cytotoxicity and genotoxicity38. According to Abubacker and Sathya41, meristematic cells of A. cepa roots exposed to different concentrations of heavy metals were unable to initiate the stages of cell division due to interactions of heavy metals with DNA, resulting in a dose-dependent decrease in MI. da Cunha Neto et al.39 proposed that the reduction of MI is due to abnormalities in the cell cycle or chromatin dysfunction caused by the interaction of DNA with metals. According to Singh et al.42, heavy metals lead to spindle-associated chromosomal abnormalities during mitosis. The reduction in MI may be due to N2+-dependent disturbances in the spindle apparatus, which can lead to metaphase arrest and consequently reduce the transition to anaphase and telophase38. Slowing or complete arrest of the cell cycle may also be attributable to the CAs and DNA damage induced by excessive Ni2+ concentrations. MI reduction in A. cepa caused by water sources contaminated with heavy metals, including Ni, has been reported. This reduction is associated with loss of membrane integrity, electrolyte leakage, and decreased cell viability due to damage to the root epidermis43. In another study with nickel oxide nanoparticles, the decrease in MI in A. cepa was explained by the deterioration of cellular levels of reduced glutathione and oxidized glutathione, components of the Asada-Halliwell cycle, which play an important role in the suppression of oxidative stress and consequently increased ROS accumulation44.
MN (Fig. 1a), which is considered direct evidence of structural CA accumulation associated with the presence of mutagens, occurs when acentric chromosome fragments or entire chromosomes left behind in anaphase migration are surrounded by a nuclear membrane different from that of the main nucleus45. Polo-Ceron46 reported that Ni2+ ions interact with the DNA molecule. Ni genotoxicity, which can be monitored by the formation of CAs and MNs, is caused not only by the direct binding of this heavy metal to DNA but also by ROS induced by the presence of Ni, which attack DNA, proteins, and lipids and increase chromosome instability. Failure in correct segregation of chromosomes due to inhibition of microtubule polymerization, disruption of spindle structure, and loss of function of enzymes involved in DNA repair by Ni may also be considered among the mechanisms underlying Ni-induced MN and CA formation47. Stickiness (Table 3, Fig. 1b), the most common damage in the Ni group, is a signal of high, irreversible, and fatal damage40. It may be caused by the dysfunction of certain non-histone proteins involved in both the segregation and organization of chromatids48. Stickiness can lead to the formation of acentric fragments (Table 3, Fig. 1d) or bridges (Table 3, Fig. 1f) during mitosis, depending on the presence of mutagenic agents, as a result of abnormal adhesions between chromosomes lacking telomeric regions49. The presence of fragments indicates chromosome breakage, and such alterations may occur concurrently with spindle fiber disturbances, potentially affecting their functionality. Stickiness, the reunion of broken ends, and chromosomal breaks may all be linked to the induction of bridges involving one or multiple chromosomes50. Vagrant, caused by a defect in the organization of the spindle apparatus, is one of the most common CAs in the Ni group (Table 3, Fig. 1c). This phenomenon occurs when a chromosome starts migrating towards the poles before its own set, causing an uneven distribution of genetic material in daughter cells (Table 3, Fig. 1e)51. Vacuolated nucleus (Table 3, Fig. 1g), one of the most common CAs observed in Ni-exposed cells, is a nuclear alteration characterized by the presence of vacuoles devoid of genetic material within the nucleus, and may be associated with genotoxic stress and mitotic disturbances, including aneuploidy52. Irregular mitosis is a chromosomal rearrangement frequently observed in Ni-treated cells (Table 3, Fig. 1h). This finding serves as an additional marker of Ni-induced spindle dysfunction.
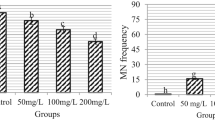
Ostling and Johanson53 first monitored the migration of DNA fragments to the anode in neutral media. This movement was later tested in alkaline conditions by Singh et al.54 (1988), who developed a more specific, sensitive, and reproducible second version of the assay. Since then, it has been used to detect single-strand breaks and alkali-resistant regions in DNA as well as cross-linked DNA55. The mechanism of the Comet assay56, which has been used since 1996 to detect induced genotoxicity in plants, is based on the migration of DNA from the nucleus of cells with damaged DNA in electrophoresis. This migration creates the image of a comet with a “head” and a “tail.” The DNA of the head moves more slowly from cathode to anode in the electrical field and lags behind the faster migrating tail57. In this study, the tail DNA observed in the Comet assay was taken into account to determine the DNA damage induced by Ni, R. farinacea extract, and R. farinacea extract applied simultaneously with Ni (Table 4, Fig. 2). According to the grading scale, the DNA damage in the control, RF 1, and RF 2 groups was “negligible.”. On the other hand, in the Ni group, the percentage of tail DNA increased to 42.5, and the damage was now classified as “severe”. The damage levels in the NiRF 1 and NiRF 2 groups were significantly reduced compared to the Ni group and were measured as “moderate” in the NiRF 1 group and “weak” in the NiRF 2 group. Our findings are in agreement with Yılmaz et al.19 who showed that Ni causes DNA damage in A. cepa. Faisal et al.58 also demonstrated that NiO nanoparticles led to DNA damage in Solanum lycopersicum using alkaline Comet assay. In addition, research involving welders in India has indicated that exposure to Ni results in an elevated presence of comet tail DNA59. Moreover, Comet assay investigations revealed that single-strand fractures were seen in the kidneys and lungs of rats following an acute injection of NiCl260. Ni can produce ROS that attack DNA or attach directly to genetic material. Direct reversal, base excision repair, nucleotide repair, mismatch repair, homologous-recombination repair, and non-homologous end-joining repair pathways are among the DNA damage repair processes that Ni may also inhibit47. Furthermore, heavy metals, including Ni, was found to increase the level of 8-hydroxy-2-deoxyguanosine, which indicates oxidative lesions of the guanine base in DNA61.
Comet tail DNA percentage. Control: Tap water, RF 1: 0.5 g L−1 R. farinacea extract, RF 2: 1.0 g L−1 R. farinacea extract, Ni: 1 mg L−1 NiCl2, NiRF 1: 1 mg L−1 NiCl2 + 0.5 g L−1 R. farinacea extract, NiRF 2: 1 mg L−1 NiCl2 + 1.0 g L−1 R. farinacea extract. Different letters above the means indicate significant differences at p < 0.05.
In order to analyze the biochemical changes induced in A. cepa root meristem cells by NiCl2, lichen extract, and lichen extract applied in a mixture with NiCl2, SOD and CAT enzyme activities as well as MDA, proline, chlorophyll a, and chlorophyll b levels were investigated (Fig. 3). The RF 1 and RF 2 groups, which were treated with 0.5 g L−1 and 1.0 g L−1 R. farinacea extract, respectively, did not differ statistically from the control group with respect to biochemical parameters (p > 0.05). Therefore, the doses of lichen extract selected for the treatments did not result in biochemical stress. In the Ni group, proline and MDA levels as well as the enzyme activities were increased compared to the control group. The mean SOD and CAT activities of this group were 2.3 and 2.5 times those of the control group, respectively. Furthermore, the mean MDA concentration of the Ni group was 2.7 times that of the control, while the proline concentration was 2.3 times that of the control group. The NiRF 1 and NiRF 2 groups exhibited a significant decrease in both MDA and proline levels, in addition to SOD and CAT activities, when compared to the Ni group (p < 0.05) (Fig. 3). The degree of reduction in both proline and MDA and enzyme activities was higher in the NiRF 2 group than in the NiRF 1 group. The changes in MDA, SOD, and CAT are key indicators used to monitor oxidative stress. Indeed, in response to stress, plants produce an excessive amount of ROS, which causes oxidative damage to the lipids in their membranes62. Altaf et al.63 reported that elevated Ni levels resulted in substantial accumulation of ROS and MDA, a marker of lipid peroxidation, in the cell membranes of Capsicum annuum. Baccouch et al.64 proposed that lipid peroxidation might represent a pivotal phytotoxic effect of Ni on membrane integrity, which could potentially result in growth inhibition. In the present study, Ni-induced growth suppression was concomitant with MDA accumulation (Table 2 and Fig. 3). The enzymes SOD and CAT are two important members of the team that fights against oxidative damage in the cell. The former converts the superoxide radical into hydrogen peroxide, while the latter catalyzes the breakdown of hydrogen peroxide into water and oxygen65. Our findings supported the study of Baccouch et al.64, which showed that MDA accumulation was induced in Zea mays seedlings exposed to NiCl2, while SOD and CAT activities were altered. However, in the aforementioned study, SOD activity remained constant while CAT activity exhibited an increase. Alternatively, Ni treatment has been observed to enhance the activity of all isoforms of SOD in Oryza sativa seedlings, while exhibiting no significant impact on CAT activity66. In another study, Ni-induced oxidative damage in roots of Alyssum bertolonii and Nicotiana tabacum plants was compared, and it was suggested that damage could be minimized by high endogenous CAT and, to a lesser extent, SOD activities in A. bertolonii, a nickel hyperaccumulator67. Treatment with Ni results in elevated levels of superoxide and hydrogen peroxide68. Hydrogen peroxide, which is not eliminated by CAT or other members of the antioxidant apparatus, generates the hydroxyl radical, especially through the Fenton reaction. This radical directly disrupts DNA bases, strand structure, and chromosome integrity. The resultant consequences manifest as nuclear and chromosomal abnormalities, MN, and genotoxic effects69. The present study also proposes that Ni-induced and insurmountable ROS production is one of the primary causes of DNA and chromosomal damage. In addition to its role as a nonenzymatic antioxidant, proline is also a potent osmoprotectant, increasing to significant levels in plants under biotic and abiotic stress. In addition to its importance as an osmoprotectant, proline exhibits a robust nonenzymatic antioxidant profile, particularly through the scavenging of hydroxyl radicals and the quenching of singlet oxygen. The presence of proline in stress-fighting cells has been demonstrated to stabilize DNA, proteins, enzymes, and membranes. Furthermore, proline can act as a source of nitrogen and carbon during the post-stress growth period70,71. Atta et al.71 suggested that exogenously applied proline increased growth, antioxidant capacity, and photosynthetic performance, which is consistent with our findings. Subsequent to treatment with nickel sulfate, an accumulation of free proline was observed in the roots and leaves of pea seedlings72. When assessing the protective effect of proline against nickel toxicity, it is noteworthy to consider its dual function as both a metal chelator and a signaling molecule that facilitates adaptive responses in plants under conditions of heavy metal stress73.
Biochemical changes induced by NiCl₂, R. farinacea extract and NiCl₂ + R. farinacea extract. Control: Tap water, RF 1: 0.5 g L−1 R. farinacea extract, RF 2: 1.0 g L−1 R. farinacea extract, Ni: 1 mg L−1 NiCl2, NiRF 1: 1 mg L−1 NiCl2 + 0.5 g L−1 R. farinacea extract, NiRF 2: 1 mg L−1 NiCl2 + 1.0 g L−1 R. farinacea extract. Different letters above the means indicate significant differences at p < 0.05. MDA: malondialdehyde, SOD: superoxide dismutase, CAT: catalase.
Chlorophyll is the photosynthetic pigment that is primarily responsible for light absorption and participation in photochemical reactions. It is found in plants predominantly as chlorophyll a, which has a methyl group on pyrrole ring II, and chlorophyll b, which has a formyl group74. A substantial decline in chlorophyll a and chlorophyll b levels was evident in A. cepa leaves subjected to Ni stress (Fig. 3). The Ni group demonstrated a 62.3% decrease in chlorophyll a level and a 77.1% decrease in chlorophyll b level when compared to the control group. On the other hand, a significant increase in chlorophyll levels was observed in the NiRF 1 and NiRF 2 groups compared to the Ni group (p < 0.05) (Fig. 3). In accordance with the present findings, there is evidence that nickel reduces chlorophyll levels in plants such as Pisum sativum75, Zea mays76, Mentha arvensis77, and A. cepa44. The decline in chlorophyll content observed in plants experiencing stress may be attributed to the inhibition of chlorophyll biosynthesis or the elevated activity of the chlorophyllase enzyme, which catalyzes chlorophyll degradation. Furthermore, the inhibition of enzymes responsible for chlorophyll biosynthesis, such as δ-aminolevulinic acid dehydratase and protochlorophyllide reductase, may also contribute to this decrease77. Ni has been reported to damage the photosynthetic apparatus at all levels by destroying mesophyll cells, disrupting the structure and size of the chloroplast grana, and abnormalizing the structure of the thylakoid membrane78. In addition, Ni exposure-induced intracellular ROS may also reduce photosynthetic pigment levels by directly oxidizing the chlorophyll molecule68.
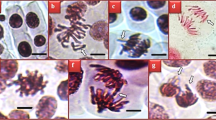
The protective effects of R. farinacea extract against NiCl₂-induced meristematic tissue damage are presented in Table 5 and Fig. 4. Microscopic examination of the meristematic region in root tips revealed no meristematic tissue damage in the control, RF 1 and RF 2 in groups (Fig. 4a, c, g, k). These findings confirm that the selected doses of R. farinacea extract (0.5 g L−1 and 1 g L−1) are non-toxic and do not induce damage in meristematic tissue. In contrast, exposure to NiCl₂ led to pronounced structural disruptions in the meristematic tissue in the Ni group, indicating its phytotoxic and cytotoxic potential. Specifically, NiCl₂ exposure led to epidermal cell damage (Fig. 4b) and cortical cell damage (Fig. 4h), flattened nucleus (Fig. 4d), and thickened cortical cell walls (Fig. 4i) at the severe level, in addition to the presence of cells with MN (Fig. 4f), binuclear cells (Fig. 4e), thickened conduction tissue (Fig. 4l), and substance accumulation within cortical cells (Fig. 4j) at the moderate level. These alterations collectively indicate significant meristematic tissue injury induced by NiCl₂ treatment. However, co-treatment of R. farinacea extract with NiCl₂ resulted in a dose-dependent ameliorative effect in all meristematic tissue damage types. The NiRF 2 group, which received the higher dose of R. farinacea extract noticeably reduced meristematic tissue damages compared to the Ni-only group. This suggests that R. farinacea extract, particularly at 1 g L−1, provides substantial protection against Ni-induced cellular injury by preserving cell integrity and reducing cytological disruptions in root meristems. In the present study, epidermal cell deformation, nuclear flattening, and cortical cell disruption, were observed in NiCl₂-treated groups, collectively indicating severe membrane damage. These abnormalities observed in this study are associated with oxidative stress damage triggered by Ni-derived ROS, as indicated by elevated SOD, CAT, and MDA levels19. In addition, Kalefetoğlu Macar et al.35 noted that degradation of genetic material can alter the structural integrity of the nucleus, and thus, flattened nucleus, binuclear cells, and cells with MN observed in this study may represent a combined outcome of genotoxic and cytotoxic stress. This supports the idea that Ni-induced damage is not limited to membrane systems but also affects the nuclear architecture and possibly cell division processes. The observed protective effects of R. farinacea extract are likely attributable to its rich content of bioactive compounds with known antioxidant properties. Potential mechanisms underlying the protective effects of these compounds include suppression of oxidative stress and lipid peroxidation, as well as preservation of genetic material as evidenced by the observed reduction in MN frequency and maintenance of meristem tissue integrity. This aligns with previous studies demonstrating the capacity of plant-derived antioxidants to alleviate heavy metal-induced genotoxicity and anatomical damage in A. cepa18,79,80.
Meristematic tissue damage induced by NiCl2 exposure. Normal appearance of epidermal cell (a), epidermal cell damage (b), nucleus normal appearance-oval (c), flattened nucleus (d), binuclear cell (e), cell with MN (f), normal appearance cortical cell (g), cortical cell damage (h), thickened cortical cell wall (i), substance accumulation in cortical cell (j), conduction tissue normal appearance (k), thickened conduction tissue (l).
The damage induced by Ni in A. cepa was partially ameliorated by R. farinacea extract applied simultaneously with Ni. While the recovery level did not attain control levels, the recovery rate exhibited a notable increase with increasing lichen extract dosage. Lichens have emerged as a promising biological source of metabolites exhibiting diverse bioactivities81. The protective effect of R. farinacea lichen extract against Ni-induced toxicity is attributable to its phenolic compounds, which are known for their antioxidant and metal-chelating properties. Consequently, the phenolic substances in the extract were analyzed (Fig. 5). LC–MS/MS analysis revealed that R. farinacea extract is rich in p-coumaric acid, ferulic acid, gentisic acid, vanillin, taxifolin, 4-hydroxybenzoic acid, and syringic acid, in descending order. Şahin et al.15 previously demonstrated the high antioxidant capacity of different lichen species belonging to the genus Ramalina, including R. farinacea, by ABTS and the related total phenolic content by Folin-Ciocalteu methods. The ROS scavenging capacity of Ramalina lichens was also demonstrated by DPPH and reducing power assays82. Additionally, Luo et al.83 investigated the phenolics contained in the lichen extract by thin layer chromatography and HPLC in a study with Ramalina conduplicans. These studies have shown that the phenolic content of lichen extracts may vary depending on species differences, the environmental conditions under which they were collected, the extraction method used, and the type of solvent. The findings of this study are in line with the existing literature showing the protective effect of lichens against various degradative chemicals. For instance, Cladonia foliacea extract has been shown to reduce MI reduction induced by hydrogen peroxide, as well as CAs accumulation and DNA damage in A. cepa root cells84. Furthermore, mitomycin C-mediated oxidative stress, CAs and MN formation and DNA damage in human peripheral lymphocytes were reduced in parallel with increasing doses of Xanthoria elegans extract85. In another study, Kotan et al.86 found that Cetraria islandica lichen extract reduced the levels of MDA and MN in human lymphocytes, which increased due to aflatoxin B1 treatment. The most prevalent phenolic component in R. farinacea extract was identified as p-coumaric acid (Fig. 5), which has been demonstrated to be effective in the chelation of metals implicated in ROS formation and the direct neutralization of ROS87. Under zinc and cadmium stress, coumaric acid in Kandelia obovata was found to be effective by chelating metal ions rather than by eliminating hydroxyl radicals88. p-Coumaric acid has also been shown to increase enzymatic and non-enzymatic antioxidant capacity in sweet cherry fruit, providing defense against fungi, an organic stressor89. Furthermore, p-coumaric acid was found to significantly prevent the decrease in SOD and CAT activities induced by cadmium and reduce lipid peroxidation90. According to Zhang et al.91, p-coumaric acid suppresses the activation of enzymes involved in postharvest chlorophyll catabolism in broccoli, preventing pigment loss and thus yellowing. In the same study, it was determined that MDA and superoxide radical, which are normally formed during the yellowing of broccoli, remained stable in p-coumaric acid-treated plants. The protective activity of ferulic acid, the second dominant phenolic component in R. farinacea extract (Fig. 5), on DNA molecules against hydrogen peroxide and UV was described as “excellent.”92. In addition to being a free radical scavenger, ferulic acid also inhibits the enzymes that cause free radicals to form and increases the activity of scavenging enzymes93. It was reported that gentisic acid, which was observed to be present in R. farinacea extract (Fig. 5), significantly reduced MN levels when applied simultaneously with the mutagen benzo[a]pyrene to HTC cells, and this effect increased over time94. Although p-coumaric acid was identified as the main phenolic compound in R. farinacea extract, the presence of other bioactive phenolics indicates a possible synergistic effect that enhances the total antioxidant capacity of the extract and its protective efficacy against nickel-induced stress.
Conclusion
Heavy metal contamination poses a significant threat to plant, wildlife, and human health, making the development of eco-friendly, natural protection strategies a research priority. In this study, the protective potential of R. farinacea extract against NiCl₂-induced toxicity was investigated using the A. cepa model. The results demonstrated that R. farinacea extract significantly alleviated NiCl₂-induced toxic impact on A. cepa. These protective effects are likely associated with its high phenolic content and strong antioxidant properties. In conclusion, R. farinacea extract emerges as a promising natural bioprotective agent against heavy metal-induced stress, offering valuable potential for applications in agricultural biotechnology, and human health applications. The rich bioactive profile of R. farinacea makes it a promising low-cost natural resource for use in herbal remedies or supplements designed to protect against oxidative damage and toxic environmental agents such as heavy metals. Further investigations are warranted to elucidate the specific bioactive constituents of R. farinacea and to comprehensively characterize their underlying mechanisms of action.
Data availability
This article provides all the data supporting the findings of the study. For further queries, the corresponding author was contacted.
References
Ghuge, S. A., Nikalje, G. C., Kadam, U. S., Suprasanna, P. & Hong, J. C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard. Mater. 450, 131039 (2023).
Sperdouli, I. Heavy metal toxicity effects on plants. Toxics 10(12), 715 (2022).
Das, K. K. et al. Primary concept of nickel toxicity-an overview. J. Basic Clin. Physiol. Pharmacol. 30(2), 141–152 (2019).
Hassan, M. U. et al. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ. Sci. Pollut. Res. 26, 12673–12688 (2019).
Ejaz, U. et al. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 14, 1154571 (2023).
Begum, W. et al. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 12(15), 9139–9153 (2022).
Kosanić, M. et al. Phytochemical composition, biological activity and anti-inflammatory potential of acetone extract from the lichen Platismatia glauca (L.) WL Culb. & CF Culb. Nat. Prod. Res. 39(5), 1111–1121 (2025).
Prado, T., Degrave, W. M. S. & Duarte, G. F. Lichens and health-trends and perspectives for the study of biodiversity in the Antarctic Ecosystem. J. Fungi. 11(3), 198 (2025).
Singh, G., Dal Grande, F., Martin, F. M. & Medema, M. H. Breaking into nature’s secret medicine cabinet: Lichens-a biochemical goldmine ready for discovery. New Phytol. 246(2), 437–449 (2025).
Shrestha, G., El-Naggar, A. M., St. Clair, L. L. & O’Neill, K. L. Anticancer activities of selected species of North American lichen extracts. Phytother. Res. 29, 100–107 (2015).
Das, K., Goel, N., Baweja, P., Rani, A. & Uniyal, P. L. Lichens as bioindicators and biomonitoring agents. Environ. We Int. J. Sci. Tech. 15, 131–144 (2020).
Rola, K. Insight into the pattern of heavy-metal accumulation in lichen thalli. J. Trace Elem. Med. Biol. 61, 126512 (2020).
Álvarez, R. et al. Different strategies to achieve Pb-tolerance by the two Trebouxia algae coexisting in the lichen Ramalina farinacea. J. Plant Physiol. 169(18), 1797–1806 (2012).
Moya, P., Molins, A., Martínez-Alberola, F., Muggia, L. & Barreno, E. Unexpected associated microalgal diversity in the lichen Ramalina farinacea is uncovered by pyrosequencing analyses. PLoS ONE 12(4), e0175091 (2017).
Şahin, S., Oran, S., Şahintürk, P., Demir, C. & Öztürk, Ş. Ramalina lichens and their major metabolites as possible natural antioxidant and antimicrobial agents. J. Food Biochem. 39(4), 471–477 (2015).
Aoussar, N. et al. Phytochemical constituents, antioxidant and antistaphylococcal activities of Evernia prunastri (L.) Ach., Pseudevernia furfuracea (L.) Zopf. and Ramalina farinacea (L.) Ach. from Morocco. Arch. Microbiol. 203, 2887–2894 (2021).
Yalçın, E., Macar, O., Kalefetoğlu Macar, T., Çavuşoğlu, D. & Çavuşoğlu, K. Multi-protective role of Echinacea purpurea L. water extract in Allium cepa L. against mercury (II) chloride. Environ. Sci. Pollut. Res. 28, 62868–62876 (2021).
Üstündağ, Ü., Macar, O., Kalefetoğlu Macar, T., Yalçın, E. & Çavuşoğlu, K. Effect of Melissa officinalis L. leaf extract on manganese-induced cyto-genotoxicity on Allium cepa L. Sci. Rep. 13, 22110 (2023).
Yılmaz, H., Kalefetoğlu Macar, T., Macar, O., Çavuşoğlu, K. & Yalçın, E. DNA fragmentation, chromosomal aberrations, and multi-toxic effects induced by nickel and the modulation of Ni-induced damage by pomegranate seed extract in Allium cepa L. Environ. Sci. Pollut. Res. 30(51), 110826–110840 (2023).
Brodo, I. M., Sharnoff, S. D. & Sharnoff, S. Lichens of North America (Yale University Press, 2001).
Yalçın, E., Yapar, K., Çavuşoğlu, K., Demirtaş, G. & Çavuşoğlu, K. Nickel-induced oxidative stress in Allium cepa (Amaryllidaceae). In Congress Book of 1st International Congress on Plant Biology 10–12 May 2018, Konya-Turkey (2018).
Macar, O., Kalefetoğlu Macar, T., Çavuşoğlu, K., Yalçın, E. & Yapar, K. Lycopene: An antioxidant product reducing dithane toxicity in Allium cepa L. Sci. Rep. 13, 2290 (2023).
Macar, O., Kalefetoğlu Macar, T., Yalçın, E. & Çavuşoğlu, K. Acute multiple toxic effects of trifloxystrobin fungicide on Allium cepa L. Sci. Rep. 12, 15216 (2022).
Fenech, M. et al. HUMN Project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. 534, 65–75 (2003).
Chakraborty, R., Mukherjee, A. K. & Mukherjee, A. Evaluation of genotoxicity of coal fly ash in Allium cepa root cells by combining comet assay with the Allium test. Environ. Monit. Assess. 153, 351–357 (2009).
Sharma, A. D., Gill, P. K. & Singh, P. DNA isolation from dry and fresh samples of polysaccharide-rich plants. Plant. Mol. Biol. Rep. 20, 415–415 (2002).
Pereira, C. S. A. et al. Evaluation of DNA damage induced by environmental exposure to mercury in Liza aurata using the comet assay. Arch. Environ. Contam. Toxicol. 58, 112–122 (2010).
Zou, J., Yue, J., Jiang, W. & Liu, D. Effects of cadmium stress on root tip cells and some physiological indexes in Allium cepa var. agrogarum L. Acta. Biol. Crac. Ser. Bot. 54, 129–141 (2012).
Beauchamp, C. & Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44, 276–287 (1971).
Beers, R. F. & Sizer, I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952).
Ünyayar, S., Celik, A., Çekiç, F. Ö. & Gözel, A. Cadmium-induced genotoxicity, cytotoxicity and lipid peroxidation in Allium sativum and Vicia faba. Mutagenesis 21, 77–81 (2006).
Witham, F. H., Blaydes, D. R. & Devlin, R. M. Experiments in plant physiology (ed. Witham, F. H.) 167–200 (Van Nostrand Reinhold, 1971).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water stress studies. Plant Soil. 39, 205–207 (1973).
Akbaş, H., Dane, F. & Meriç, Ç. Effect of nickel on root growth and the kinetics of metal ions transport in onion (Allium cepa) root. Indian J. Biochem. Biophys. 46(4), 332–336 (2009).
Kalefetoğlu Macar, T., Macar, O., Çavuşoğlu, K., Yalçin, E. & Yapar, K. Turmeric (Curcuma longa L.) tends to reduce the toxic effects of nickel (II) chloride in Allium cepa L. roots. Environ. Sci. Pollut. Res. 29(40), 60508–60518 (2022).
Demchenko, N. P., Kalimova, I. B. & Demchenko, K. N. Effect of nickel on growth, proliferation, and differentiation of root cells in Triticum aestivum seedlings. Russ. J. Plant Physiol. 52, 220–228 (2005).
Lešková, A., Zvarík, M., Araya, T. & Giehl, R. F. Nickel toxicity targets cell wall-related processes and PIN2-mediated auxin transport to inhibit root elongation and gravitropic responses in Arabidopsis. Plant Cell Physiol. 61(3), 519–535 (2020).
Pharmawati, M., Wirasiti, N. N. & Wrasiati, L. P. Genotoxic and antigenotoxic potential of encapsulated Enhalus acoroides (L. f.) Royle leaves extract against nickel nitrate. Caryologia 75(2), 89–99 (2022).
da Cunha Neto, A. R. et al. Toxicity of heavy metals that affect germination, development and cell cycle of Allium cepa L. Bull. Environ. Contam. Toxicol. 111(2), 22 (2023).
Gantayat, S., Mania, S., Pradhan, C. & Das, A. B. Ionic stress induced cytotoxic effect of cadmium and nickel ions on roots of Allium cepa L. Cytologia 83(2), 143–148 (2017).
Abubacker, M. N. & Sathya, C. Genotoxic effect of heavy metals Cr, Cu, Pb and Zn using Allium cepa L. biosciences. Biotechnol. Res. Asia. 14(3), 1181–1186 (2017).
Singh, S. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 137, 177–193 (2017).
Bianchi, J., Espindola, E. L. G. & Marin-Morales, M. A. Genotoxicity and mutagenicity of water samples from the Monjolinho River (Brazil) after receiving untreated effluents. Ecotoxicol. Environ. Saf. 74(4), 826–833 (2011).
Manna, I. & Bandyopadhyay, M. The impact of engineered nickel oxide nanoparticles on ascorbate glutathione cycle in Allium cepa L. Physiol. Mol. Biol. Plants. 29(5), 663–678 (2023).
Onoja, A. O. et al. Micronuclei formation: small nuclear packages with big genomic consequences. Nucleus 1–21 (2025).
Polo-Ceron, D. Cu (II) and Ni (II) complexes with new tridentate NNS thiosemicarbazones: Synthesis, characterisation, DNA interaction, and antibacterial activity. Bioinorg. Chem. Appl. 2019, 3520837 (2019).
Guo, H. et al. Nickel carcinogenesis mechanism: DNA damage. Int. J. Mol. Sci. 20(19), 4690 (2019).
Tang, K. H. D. Genotoxicity of microplastics on living organisms: Effects on chromosomes, DNA and gene expression. Environments 12, 10 (2025).
Gupta, K., Kumar, A. & Singh, K. Cyto-genotoxic assay of dimethoate-30 through chromosomal events in Allium cepa L. roots. Acta Physiol. Plant. 47(5), 1–7 (2025).
Sadıç, E. & Çelik, T. A. Environmental hazards of light cigarette butts and filters: Physiological, cytotoxic, and genotoxic assessment using the Allium cepa L. assay. Environ. Sci. Pollut. Res. 1–11 (2025).
Sabeen, M. et al. Allium cepa assay based comparative study of selected vegetables and the chromosomal aberrations due to heavy metal accumulation. Saudi J. Biol. Sci. 27(5), 1368–1374 (2020).
Sarsar, O. et al. Multifaceted investigation of esfenvalerate-induced toxicity on Allium cepa L. Sci. Rep. 15, 1–17 (2025).
Ostling, O. & Johanson, K. J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 123, 291–298 (1984).
Singh, N. P., McCoy, M. T., Tice, R. R. & Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191 (1988).
Costea, M. A. et al. The comet assay as a sustainable method for evaluating the genotoxicity caused by the soluble fraction derived from sewage sludge on diverse cell types, including lymphocytes, coelomocytes and Allium cepa L. cells. Sustainability 16, 457 (2024).
Koppen, G. & Verschaeve, L. The alkaline comet test on plant cells: A new genotoxicity test for DNA strand breaks in Vicia faba root cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 360, 193–200 (1996).
Tyutereva, E. V., Strizhenok, A. D., Kiseleva, E. I. & Voitsekhovskaja, O. V. Comet assay: Multifaceted options for studies of plant stress response. Horticulturae 10(2), 174 (2024).
Faisal, M. et al. Phytotoxic hazards of NiO-nanoparticles in tomato: a study on mechanism of cell death. J. Hazard. Mater. 250–251, 318–332 (2013).
Danadevi, K., Rozati, R., Banu, B. S. & Grover, P. Genotoxic evaluation of welders occupationally exposed to chromium and nickel using the Comet and micronucleus assays. Mutagenesis 19, 35–41 (2004).
Şaplakoğlu, U., İşcan, M. & İşcan, M. DNA single-strand breakage in rat lung, liver and kidney after single and combined treatments of nickel and cadmium. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 394(1–3), 133–140 (1997).
Li, C. et al. Exogenous melatonin enhances the tolerance of tiger nut (Cyperus esculentus L.) via DNA damage repair pathway under heavy metal stress (Cd2+) at the sprout stage. Ecotoxicol. Environ. Saf. 265, 115519 (2023).
Cao, Y. Y. et al. Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in cucumber. Plant Cell Physiol. 60(3), 562–574 (2019).
Altaf, M. A. et al. Physiological and biochemical responses of pepper (Capsicum annuum L.) seedlings to nickel toxicity. Front. Plant Sci. 13, 950392 (2022).
Baccouch, S., Chaoui, A. & El Ferjani, E. Nickel toxicity induces oxidative damage in Zea mays roots. J. Plant Nutr. 24(7), 1085–1097 (2001).
Sipahi Kuloğlu, S., Yalçin, E., Çavuşoğlu, K. & Acar, A. Dose-dependent toxicity profile and genotoxicity mechanism of lithium carbonate. Sci. Rep. 12, 13504 (2022).
Maheshwari, R. & Dubey, R. S. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul. 59, 37–49 (2009).
Boominathan, R. & Doran, P. M. Ni-induced oxidative stress in roots of the Ni hyperaccumulator, Alyssum bertolonii. New Phytol. 156(2), 205–215 (2002).
Gajewska, E. & Skłodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20, 27–36 (2007).
Asadi, N. S., Heidari, M. M. & Khatami, M. Protective effect of Berberis vulgaris on Fenton reaction-induced DNA cleavage. Avicenna J. Phytomed. 9(3), 213 (2019).
Rejeb, K. B., Abdelly, C. & Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284 (2014).
Atta, N. et al. Proline-mediated redox regulation in wheat for mitigating nickel-induced stress and soil decontamination. Sci. Rep. 14, 456 (2024).
Gajewska, E. & Skłodowska, M. Antioxidative responses and proline level in leaves and roots of pea plants subjected to nickel stress. Acta Physiol. Plant. 27(3), 329–340 (2005).
Hayat, S. et al. Role of proline under changing environments: A review. Plant Signal. Behav. 7(11), 1456–1466 (2012).
Li, X., Zhang, W., Niu, D. & Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 342, 112030 (2024).
Singh, S., Mishra, S., Kumari, R. & Agrawal, S. B. Response of ultraviolet-B and nickel on pigments, metabolites and antioxidants of Pisum sativum L. J. Environ. Biol. 30(5), 677 (2009).
Drążkiewicz, M. & Baszyński, T. Interference of nickel with the photosynthetic apparatus of Zea mays. Ecotoxicol. Environ. Saf. 73(5), 982–986 (2010).
Nabi, A., Naeem, M., Aftab, T. & Khan, M. M. A. Alterations in photosynthetic pigments, antioxidant machinery, essential oil constituents and growth of menthol mint (Mentha arvensis L.) upon nickel exposure. Braz. J. Bot. 43(4), 721–731 (2020).
Chen, C., Huang, D. & Liu, J. Functions and toxicity of nickel in plants: Recent advances and future prospects. Clean-Soil Air Water. 37(4–5), 304–313 (2009).
Macar, O., Kalefetoğlu Macar, T., Çavuşoğlu, K. & Yalçın, E. Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against copper (II) chloride toxicity. Environ. Sci. Pollut. Res. 27, 1428–1435 (2020).
Çavuşoğlu, D., Macar, O., Kalefetoğlu Macar, T., Çavuşoğlu, K. & Yalçın, E. Mitigative effect of green tea extract against mercury (II) chloride toxicity in Allium cepa L. model. Environ. Sci. Pollut. Res. 29(19), 27862–27874 (2022).
Moreira, A. S. N., Braz-Filho, R., Mussi-Dias, V. & Vieira, I. J. C. Chemistry and biological activity of Ramalina lichenized fungi. Molecules 20(5), 8952–8987 (2015).
Ristic, S., Rankovic, B. & Stamenkovic, S. Biopharmaceutical potential of two Ramalina lichens and their metabolites. Curr. Pharm. Biotechnol. 17(7), 651–658 (2016).
Luo, H. et al. Antioxidant activities of edible lichen Ramalina conduplicans and its free radical-scavenging constituents. Mycoscience 51, 391–395 (2010).
Pandır, D., Hilooglu, M. & Kocakaya, M. Assessment of anticytotoxic effect of lichen Cladonia foliacae extract on Allium cepa root tips. Environ. Sci. Pollut. Res. 25, 32478–32490 (2018).
Turkez, H., Aydin, E. & Aslan, A. Xanthoria elegans (Link)(lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 64, 679–686 (2012).
Kotan, E., Alpsoy, L., Anar, M., Aslan, A. & Agar, G. Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB1 in human lymphocytes in vitro. Toxicol. Ind. Health. 27(7), 599–605 (2011).
Shen, Y. et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 111, 579–587 (2019).
Chen, S. et al. Effects of phenolic acids on free radical scavenging and heavy metal bioavailability in Kandelia obovata under cadmium and zinc stress. Chemosphere 249, 126341 (2020).
Liu, X. et al. p-Coumaric acid induces antioxidant capacity and defense responses of sweet cherry fruit to fungal pathogens. Postharvest Biol. Technol. 169, 111297 (2020).
Navaneethan, D. & Rasool, M. p-Coumaric acid, a common dietary polyphenol, protects cadmium chloride-induced nephrotoxicity in rats. Ren. Fail. 36(2), 244–251 (2014).
Zhang, X. et al. Insights into profiling of p-coumaric acid treatment on delaying the yellowing of broccoli. Postharvest Biol. Technol. 201, 112371 (2023).
Sevgi, K., Tepe, B. & Sarikurkcu, C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 77, 12–21 (2015).
Zduńska, K., Dana, A., Kolodziejczak, A. & Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 31(6), 332–336 (2018).
Cavalcante, F. M. L., Almeida, I. V., Düsman, E., Mantovani, M. S. & Vicentini, V. E. P. Cytotoxicity, mutagenicity, and antimutagenicity of the gentisic acid on HTC cells. Drug Chem. Toxicol. 41(2), 155–161 (2018).
Acknowledgements
The authors would like to thank Giresun University Scientific Research Projects Office for its support of this study (FEN-BAP-A-240222-51).
Statement regarding experimental research on plants
Experimental research and field studies on plant/lichen and plant/lichen parts (A. cepa bulbs, R. farinacea), including the collection of plant material, comply with relevant institutional, national, and international guidelines and legislation.
Funding
The consumables of this study were supplied by the Scientific Research Projects Office (FEN-BAP-A-240222-51), Giresun University.
Author information
Authors and Affiliations
Contributions
T.K.M., O.M., E.Y. and K.Ç. designed the experiments and performed the analyses; K.K. carried out the collection of lichen samples and their identification; E.Y. and K.Ç. analyzed the biochemical parameters and the damage to meristematic cells; K.Ç. carried out the statistical analysis; T.K.M. and O.M. wrote the manuscript with the help of K.K., E.Y. and K.Ç.; T.K.M. edited the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kalefetoğlu Macar, T., Macar, O., Kınalıoğlu, K. et al. Ramalina farinacea mitigates cytogenotoxicity and physiological, biochemical, and anatomical alterations induced by nickel in Allium cepa. Sci Rep 15, 35527 (2025). https://doi.org/10.1038/s41598-025-19674-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-19674-4