Abstract
Modern oral nicotine delivery (MOND) offers potentially reduced harm nicotine delivery for adult smokers who do not wish to quit. Most clinical studies to date have characterised MOND with lower nicotine content, therefore, this study aimed to assess products with higher nicotine strengths. This randomised, open-label, cross-over, confinement study, conducted in Sweden, compared the nicotine pharmacokinetic and subjective effects of three MOND products, zoneX #5 slim (14 mg nicotine/pouch), zoneX #5 (16 mg) and zoneX #6 (20 mg), and a tobacco snus product (Skruf Slim Fresh #5 (16.6 mg)) in 27 adult snus or MOND users. The zoneX #6 product delivered the highest levels of nicotine; however, all products exhibited similar reductions in urge to use nicotine during and following a controlled product use session. Furthermore, the zoneX products generally scored more favourably than the snus on a 7-point Likert product evaluation scale for satisfaction, psychological reward, aversion and relief, assessed 8 h after the start of product use. There was some indication that pouch size may influence nicotine uptake, however, further characterisation is required. All study products demonstrated good short-term tolerability, with no serious adverse events observed. Overall, the findings support that MOND has tobacco harm reduction potential, providing a satisfactory alternative for adult smokers. Clinical Trial identifier: NCT05452278 https://clinicaltrials.gov/.
Similar content being viewed by others
Introduction
Smoking is a cause of serious diseases, including lung cancer, heart disease and emphysema. This is attributed to exposure to the smoke generated during tobacco combustion, which contains around 7000 chemicals, a number of which are classed as harmful and potentially harmful constituents (HPHCs)1,2. However, other forms of nicotine delivery are available, including next generation products (NGPs) like e-vapour products and heated tobacco products, which do not involve combustion in their use, and therefore are associated with decreases in exposures to the numbers and levels of toxicants present in tobacco smoke3,4. Providing adult smokers with such alternatives is the basis of tobacco harm reduction (THR).
Oral nicotine delivery (OND) does not involve tobacco combustion, and therefore exposure to the HPHCs associated with cigarette smoke is removed or substantially reduced5,6. OND categories include traditional products such as Scandinavian snus (hereafter, snus), and the more recent NGP innovation, modern oral nicotine delivery (MOND), which includes tobacco-free nicotine pouches (TFNPs). OND products are placed between the user’s upper lip and gum, and nicotine is absorbed into the bloodstream via the oral mucosa7. Whilst snus contain tobacco leaf as their base material, MOND typically contains a dry powder or plant fibre-based matrix along with pharmaceutical grade nicotine, flavourings, humectants, and additives to ensure stability. MOND is considered to have further THR potential due to the absence or reduced levels of tobacco leaf, which contains some HPHCs, including tobacco specific nitrosamines (TSNAs)5,8. Reductions in exposure to HPHCs has been demonstrated to translate to reductions in in vitro toxicological effects, as observed for a range of MOND products9,10,11,12,13. Furthermore, studies have reported that biomarkers of exposure to smoking-related toxicants and biomarkers of potential harm are reduced in MOND users compared to smokers14,15.
Further to reduction in smoking-related toxicant exposure, to act as an acceptable alternative and support THR strategies, products must deliver satisfactory nicotine levels and consumer experience. To maximise uptake by adult smokers, NGPs such as MOND are available in a range of nicotine strengths, flavours and formats (e.g., pouch size, moisture content) to offer alternatives to suit individual consumers’ preferences. It is therefore important to characterise these products in terms of nicotine delivery and the associated user satisfaction, to understand if these products can act as an acceptable THR alternative for adult smokers. A number of studies have reported the nicotine pharmacokinetics and subjective effects following the use of a range of MOND, of varying nicotine strengths, flavours and brands7,16,17,18,19,20. Whilst nicotine delivery profiles generally appear to vary between brands, including a range of nicotine strengths/ flavours, MOND offers slower time to maximum blood plasma nicotine concentration (Tmax) values compared to cigarettes7,18,19. Additionally, Tmax appears to be dependent on duration of product use for MOND.
Another important factor in providing potentially reduced harm alternative forms of nicotine delivery is that products must not offer increased abuse liability compared to cigarettes, which refers to the pharmacological potential to cause addictive effects, including reward/ relief behaviours, persistent use and/ or dependence21. Abuse liability is associated with rapid and effective nicotine delivery, for which cigarettes are recognised as possessing the highest degree, with nicotine replacement therapies such as patches, lozenges and gum the least21. The slower onset of nicotine delivery, as observed in previous studies on MOND7,18,19, and additionally lower overall levels of nicotine delivery, are associated with a lower abuse liability potential.
The majority of studies to date have focused on the clinical effects of MOND with lower nicotine content (~ 1.5-10 mg nicotine/ pouch), however, products of higher nicotine content have been less extensively characterised in the literature. This study, therefore, aimed to assess a selection of higher strength MOND product variants with regards to nicotine pharmacokinetics, subjective effects and short-term product tolerability. This study was a confined, randomised, cross-over, single-blind study which included three higher nicotine strength variants of the MOND product, zoneX (14, 16 and 20 mg nicotine/ pouch), and a snus product (Skruf slim, 16.6 mg nicotine/ pouch). The study was conducted in Sweden in adult snus or MOND users who were therefore familiar with these products and their use. The snus comparator was included to assess the outcomes for the zoneX products compared to a traditional tobacco product.
Methods
Study design
This randomised, open-label, cross-over, confinement study in adult snus and MOND consumers aimed to evaluate nicotine pharmacokinetics (PK), subjective effects and short-term safety and tolerability, following use of three MOND products and a comparator snus product.
The primary objectives of the study were to compare the maximum observed plasma concentration (Cmax) and the area under the nicotine plasma concentration–time curve (AUCt) after the use of each product. The secondary objectives reported here were to evaluate other PK parameters (time to Cmax (Tmax); AUC from timepoint 0–90 min (AUC0-90); AUC from timepoint 0 to infinity (AUC0-inf); observed plasma concentration at the last sampling timepoint (Clast), terminal elimination half-life (T1/2(z))), to evaluate product subjective effects and to evaluate the safety and tolerability of each of the study products used.
The study was approved by the Swedish Ethical Review Authority (SERA), carried out in accordance with the ethical principles outlined in the Declaration of Helsinki, International Conference of Harmonisation (ICH)/ Good Clinical Practice (GCP), European Union Clinical Trials Directive and applicable local regulatory requirements. Twenty-seven adult snus and MOND users participated in the study, which was conducted at a single clinical site. Subjects attended two clinic visits, (i) screening and (ii) a 4-day confinement period; follow-up telephone calls with subjects were also carried out a week after the last study product use. All subjects provided written informed consent prior to the study commencing. Clinical Trial identifier: NCT05452278 (clinicaltrials.gov, 06-07-2022). The first study subject was screened on 28-09-2021, the first subject used a study product on 04-10-2021 and the last subject completed the study on 02-11-2021.
Subjects
Thirty-four subjects were screened, and following this, 27 were randomised to the treatment groups (Fig. 1). The sample size was comparable to those used in similar studies (for example, 30 participants in Liu et al.18 and 24 participants in Chapman et al.7), and was considered sufficient to achieve the study objectives. From the 34 subjects screened, three did not meet the eligibility criteria, three withdrew consent prior to randomisation and one was a reserve not included in the study. Twenty-seven subjects commenced the study, completed all study visits and received an end of study phone call within seven days of completion. The participant population included 16 males and 11 females, all of whom were of non-Hispanic or Latin ethnicity. Twenty-two subjects were white, three were Asian and two were Black or African American origin. The mean age was 31.1 years (standard deviation (SD) = 11.8), and the mean body mass index (BMI) was 23.5 kg/m2 (SD = 3.0). Full tabulated details or baseline demographics and clinical characteristics can be found in Table S1 ( supplementary information).
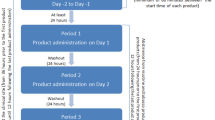
Study design overview: three tobacco-free nicotine pouch products (zoneX #5 (16 mg nicotine/ pouch), zoneX #5 slim (14 mg) and zoneX #6 (20 mg)) and one Skruf tobacco-containing snus product (16.6 mg nicotine/ pouch) were randomised to 27 subjects for assessments across four days. h: hours; PK: pharmacokinetic.
The following study inclusion criteria were applied: subjects were willing and able to provide written informed consent to take part in the study; subjects were a male or female aged ≥ 19 years at the time of screening; BMI of ≥ 18.0 and ≤ 30 kg/m2; clinically normal medical history, physical findings, vital signs, ECG and laboratory values at the time of screening (evaluated by the Investigator); user of snus and/ or MOND for more than or equal to one year with a minimum weekly consumption of two or more snus cans. Importantly, given the higher strength products on trial, the subjects had to confirm a willingness and be considered able by the Investigator to use MOND with a nicotine content in the range of 14 mg to 20 mg per pouch.
Study exclusion criteria included: history of any clinically significant disease or disorder which the Investigator deemed could put the subject at risk because of participation in the study, influence the study outcomes or the subject’s ability to participate; any clinically significant illness, medical or surgical procedure or trauma within four weeks of first use of the study products; any planned major surgery within the study duration; any positive screening result for serum hepatitis B surface antigen, hepatitis C antibody or human immunodeficiency virus (HIV); during screening, any vital signs values outside of the following ranges after 10 min supine rest: systolic blood pressure < 90 or > 140 mmHg, diastolic blood pressure < 50 or > 90 mmHg, or pulse < 40 or > 90 beats per minute; females who were pregnant or currently breast feeding; history of severe allergy/ hypersensitivity or ongoing allergy/ hypersensitivity, or historical hypersensitivity to drugs with a similar chemical structure or class to nicotine; planned treatment or treatment with another product (within one month) or investigational drug (within three months) prior to Day -1 of the study; a positive result for drugs of abuse or alcohol at screening or admission to the research unit; a history of alcohol abuse or excessive intake of alcohol, presence or history of drug abuse, assessed by the Investigator; a history, or current use, of anabolic steroids, assessed by the Investigator; excessive consumption of caffeine (daily intake of more than five cups of caffeine-containing beverages); plasma donation within one month of screening or blood donation (or corresponding blood loss) within three months prior to screening; intention to change smoking habit or make a quit attempt within 3 months from the screening visit; the Investigator thought the subject was unlikely to comply with study procedures, restrictions and requirements.
Study products
This study included four test products: three were MOND products of different nicotine contents, zoneX #5 slim (14 mg nicotine per pouch), zoneX #5 regular (16 mg) and zoneX #6 regular (20 mg); the fourth was a snus comparator, Skruf Fresh slim (16.6 mg nicotine per pouch). The regular pouches were 18 × 32 mm in size and the slim pouches 14 × 36 mm. All products were manufactured by Skruf Snus, A.B., Sweden, a wholly owned subsidiary of Imperial Brands PLC, and the study was conducted in Sweden. At the time of the study, the Skruf snus was on the Swedish market, zoneX #5 slim product was on the market in Norway, the zoneX #5 product was on the market in Austria, Denmark and Finland and the zoneX #6 pouch was not present on any market. All products were provided directly to the research clinic from the manufacturing plant in labelled cans. The MOND contained pharmaceutical-grade nicotine combined with a food-grade plant fibre-based substrate, flavourings and humectants to retain moisture and additives to maintain product stability. The zoneX #5 slim product additionally contained a small amount of tobacco, due to the requirements for sale on the Norwegian market. The MOND products were the Cold Blast variant, which has a mint flavour profile. Products were provided along with instructions for use: a pouch was to be placed between the upper lip and gum for 20 min; pouches were not to be chewed or swallowed (normal swallowing of saliva was allowed).
Study procedure
Study visit 1 took place Days -28 to -1 and consisted of an eligibility check, health status review and assessment of tobacco and nicotine consumption. Subjects were provided with nicotine cessation advice and contact information for a cessation support service if requested.
Visit 2 consisted of a 4-day confinement period; subjects were admitted to the clinic on the evening of Day -1 and remained there until Day 4. On the evening of Day -1, subjects received baseline assessments for clinical laboratory profile, vital signs and electrocardiograms (ECG); subjects also attended a familiarisation session with regards to the study products and questionnaire format. Here, the clinical team explained how the study products were to be used, and subjects were able to view the products and their packaging. The session did not include a product trial, and the products used were not part of the clinical study but were retained as demonstration samples for accountability purposes. Subjects were allowed to use their own tobacco/ nicotine products until 10 pm on the evening of Day -1. At 10 pm, the subjects’ own products were collected by a clinical team member and returned upon completion (Day 4). On the morning of Day 1, the pre-use assessments were carried out and eligibility was confirmed, then subjects were randomised and administered a single pouch according to their product randomisation sequence. Blood sampling for the PK assessment was carried out pre-product use and at 5, 10, 15, 20, 25, 30, 45, 60, 90 min, and 2, 4, 6, 8 h post product use start. Questionnaires were completed by the subjects at defined intervals throughout the day. Subjects’ product tolerability was also monitored throughout the day. After the 8 h timepoint, subjects were dispensed and allowed to use their own nicotine-containing products ad lib until 10 pm if they requested. Breakfast was served approximately 1 h prior to product use; lunch 4 h after the start of each product use; a snack, dinner and an evening snack were served approximately 7, 9 and 11 h post- start of product use, respectively. Water was allowed ad lib except 30 min before the start of product use until 1 h after the start of product use.
Days 2, 3 and 4 had the same schedule (except for eligibility checks and randomisation). On Day 4, subjects left the clinic following completion of the 8 h assessment. On Day 7 (± 1 day) (visit 3), follow-up phone calls were made to subjects to record any adverse events (AEs). A study procedure overview can be found in Fig. 1.
Randomisation to one of four treatment sequences was carried out to minimise bias and the study was single-blind, i.e., the subjects did not know the identity not the strength/ nicotine content of each product, but the Investigator and study staff knew the identities of each study product. The products did differ in size but were otherwise identical in appearance. Randomisation was carried out by CTC computationally using SAS Proc Plan (SAS Version 9.4). The randomisation list contained subject number, sequence and treatment and was kept by the randomiser until database lock. A copy of the randomisation list was provided to the research clinic. The randomisation sequence is detailed in Fig. 1.
With regards to prior and concomitant therapies, medications (prescribed or non-prescribed, including antacids, analgesics, herbal remedies, vitamin supplements and minerals) deemed necessary for the subject’s safety and wellbeing could be given at the discretion of the Investigator during the residential period. The Sponsor was then consulted to determine whether or not the subject could continue in the study. All concomitant medications were recorded in the electronic case report form.
Study assessments
Pharmacokinetic assessment: Blood samples (approx. 4 ml/sample) were collected using an indwelling venous catheter at the timepoints described. Pre-product use samples were taken within 5 min prior to the start of product use. Nicotine concentrations in the blood plasma was analysed by Lablytica AB using a validated LC–MS/MS method.
Urge to use nicotine: Subjects were asked to evaluate their urge to use nicotine on a 100 mm visual analogue scale (VAS) with the anchor points of 0 mm = not at all/ no urge and 100 mm = extremely/ extreme urge. Evaluations were taken pre-product use and at 5, 10, 15, 20, 25, 30, 45, 60, 90 min, and 2, 4, 6, 8 h post-product use start.
Product evaluation: Subjects were asked to self-assess their experience in relation to using the products with regards to the endpoints of satisfaction, psychological reward, aversion and relief using a 7-point Likert (1 = not at all and 7 = extremely) Product Evaluation Scale (PES)22. Responses were recorded 8 h post-start of the study product use.
Short-term product safety and tolerability: AEs (including serious AEs (SAEs)) were recorded from the start of the first product used until visit 3. Severity/ intensity was graded by the Investigator as mild, moderate or severe and assessed as unlikely, possibly or probably related to the study product. Clinically significant changes in laboratory assessments (clinical chemistry, haematology, urinalysis, pregnancy, SARS-COV-2 detection), vital signs and ECGs were also assessed throughout.
Data analyses
Nicotine pharmacokinetics: Data from all subjects who had used at least one of the study products, provided an evaluable plasma nicotine concentration profile, and had no AEs or protocol deviations deemed to affect the PK output (e.g., vomiting, subject not following restrictions, wrong product given, etc.) were used for the PK analyses.
Nicotine PK parameters for each product (Cmax (ng/ml), AUCt (AUC0-t) (h*ng/ml), T1/2(z) (minutes), AUC0-90 (h*ng/ml), AUC0-inf (h*ng/ml), Clast (ng/ml)) were calculated by Non-Compartmental Analysis (NCA) using Phoenix WinNonlin® software version 8.1 (Certara, USA). Tmax (minutes) values were calculated using R software23 and the PKNCA package24. Calculations were based on the actual sampling times recorded during the study.
The elimination rate constant, Lambdaz (with acceptance criteria; R2, extrapolated AUC) was calculated to support baseline adjustment for the PK parameters. Cmax and Tmax were derived from the observed plasma nicotine concentration data. AUC was calculated using log-linear trapezoidal interpolation (linear—up, log—down). Concentrations below the lower limit of quantification (LLOQ) occurring before Cmax were treated as zero and concentrations below LLOQ occurring after Cmax were omitted from the analysis. AUCt and Cmax were corrected for and calculated with and without nicotine baseline corrections. Where plasma nicotine concentrations were above the lower limit of quantification immediately prior to product use (− 5 min), PK parameters were calculated from baseline adjusted concentrations using subjects’ elimination rate constant.
Statistical comparisons between products were carried out using a linear mixed-effects repeated measurements analysis of variance model. Least Square Means (LSM) and 95% Confidence Interval (CI) were derived for the products’ Cmax, AUC0-t and AUC0-inf outcomes. LSM ratios, 95% CIs of the LSM ratios, and adjusted p values using Tukey method are provided for the product comparisons. For Tmax, an additional analysis was carried out using a Kruskal–Wallis test, as this data is not usually normally distributed and often includes tied values, therefore the parametric methods used for Cmax and AUC were not appropriate.
Urge to use nicotine: Emax (the maximum change from baseline VAS score) and AUCt (the area under the change from baseline VAS score versus time curve from time 0 to 480 min; calculated using the linear trapezoidal method with linear interpolation using actual sample times) were calculated for each product for statistical comparison. Statistical analyses were carried out between products using a linear mixed-effects repeated measurements analysis of variance model. From this model, the LSM and 95% CI were derived for the products’ Emax and AUCt outcomes. LSM ratios, 95% CIs of the LSM ratios, and adjusted p values using Tukey method are provided for the product comparisons. To assess changes in urge to use nicotine across time between the products, a repeated measures analysis using a linear mixed-effects model was conducted. The model included timepoint, product, and their interaction as fixed effects, with subject as a random effect to account for within-subject variability.
Product evaluation: Outcomes relating to questions in each sub-category were combined and summarised descriptively for each study product. PES-differences between products on sub-category PES scores, at each time point, were analysed using Wilcoxon Rank Sum tests.
Results
Nicotine pharmacokinetic assessment
Nicotine pharmacokinetic parameters (detailed in Table 1) were recorded up to 8h following the start of a 20-min controlled product use period with one of the four respective study products. Figure 2 indicates the baseline-corrected plasma nicotine concentrations measured at the timepoints detailed. Use of the zoneX #6 20 mg pouch resulted in significantly higher levels of blood plasma nicotine (assessed using comparison of Cmax, AUCt and AUC0-inf (detailed in Table 2)) compared to the other three study products, which did not demonstrate significantly different blood plasma nicotine profiles to one another. The zoneX #5 slim (14 mg) product did appear to achieve a slightly higher average Cmax when compared to the zoneX #5 regular (16 mg) product, however, this was not a significant effect.
Nicotine pharmacokinetic profiles (baseline adjusted blood plasma nicotine concentrations) of the study products following the start of a 20-min controlled use period, assessed at timepoints between 0 and 480 min. Values in milligrams (mg) refer to pouch nicotine content. Number of subjects per product is detailed in Table 1. Data plotted with error bars can be found in supplementary Fig. S1.
For Tmax, although the comparator product exhibited a numerically higher mean value, nonparametric analysis using the Kruskal–Wallis test revealed no statistically significant differences among the products (H = 1.43, p = 0.70). Due to the non-normal distribution and presence of tied values inherent to Tmax, and the corresponding use of nonparametric methods, this parameter was not included in Table 2, which is limited to parameters analysed using parametric statistical approaches.
Subjective assessment
The subjects’ urge to use nicotine was evaluated using a 100mm VAS, with the anchor points 0mm (no urge) and 100mm (extreme urge), pre-administration of each study product and up to 8h, at the timepoints detailed (Fig. 3a). For all four study products, the maximum reduction in urge to use nicotine compared to baseline (pre-administration) (Emax) was recorded 15 min post-administration. Upon statistical comparison of the Emax values (Table 3), none of the products induced significantly different outcomes from one another, in contrast to the higher nicotine delivery achieved by the zoneX #6 (20 mg) product. The only significant difference observed for the AUCt was between zoneX #5 Slim (14 mg) and the Skruf snus product (Fig. 3a).
(a) Mean reported urge to use nicotine at timepoints up to 480 min following the start of a 20-min product use session with each of the four study products. Responses were recorded according to a 100mm visual analogue scale (VAS). Values in milligrams (mg) refer to pouch nicotine content. Number of subjects per product can be found in Table. 1. Data plotted with error bars can be found in supplementary Fig. S2. (b) Average product evaluation scale (PES) scores (7-point Likert scale, detailed in the legend) in each of four categories, satisfaction, psychological reward, aversion and relief, for each of the four study products, eight hours following the start of a 20-min controlled product use session. Values in milligrams (mg) refer to pouch nicotine content. Statistical comparisons to Skruf Slim Fresh #5 were carried out using Wilcoxon signed rank tests; *p < 0.05, **p < 0.01.
To assess whether urge to use nicotine changed consistently over time across product conditions, we conducted a repeated measures analysis using a linear mixed-effects model. The model included timepoint, product, and their interaction as fixed effects, with subject as a random effect to account for within-subject variability. The analysis revealed a significant main effect of timepoint (p < 0.001), indicating that urge to use nicotine varied over time. However, the interaction between timepoint and product was not statistically significant (all p > 0.47), suggesting that no product condition demonstrated a consistently greater reduction in urge over time relative to others. These findings indicate that while urge to use nicotine generally decreased over time, the pattern of reduction was not significantly different across product conditions.
A seven-point Likert scale was used to evaluate subjects’ responses to questions within the four sub-categories of satisfaction, psychological reward, aversion and relief, 8h following the start of the 20min controlled use session for the four study products (Fig. 3b). Full outcomes for the PES can be found in the supplementary information (Table S2). In the PES subcategory ‘satisfaction’, the zoneX #5 slim product had the highest average score, which was found to be significantly higher than that achieved for the Skruf product. The mean scores ranged between 4.24 and 4.76 for the zoneX products, whilst the mean score for Skruf Slim Fresh #5 was 4.02. For the ‘psychological reward’ subcategory, the mean scores ranged between 4.17 and 4.41 for the zoneX products, while the mean score for the Skruf reference product was 3.90. The mean score for zoneX #6 was significantly higher compared to the Skruf product, whereas the two other zoneX products did not differ from the Skruf product nor each other significantly. For the ‘aversion’ subcategory, zoneX #6 exhibited a significantly higher score compared to Skruf, although overall, aversion scores were lower than the other endpoints for all four study products. The mean scores for the aversion subcategory ranged between 2.25 and 2.66 for the zoneX products, whilst the mean score for the Skruf product was 1.86. In the subcategory ‘relief’, average scores ranged between 4.72 and 4.90 for the zoneX products, and the mean average score was 4.53 for the Skruf reference product. All three zoneX products exhibited significantly higher scores compared to the Skruf product.
Short-term tolerability of the study products
For the duration of the study, up to the end of follow-up (visit 3), adverse effects and clinical tolerability endpoints were monitored. In total, there were 24 AEs reported by 10 of the 27 subjects who completed the study. All the observed AEs were mild to moderate in intensity, and most were assessed as unlikely to be due to the use of the study product/s. Three AEs (headache and flatulence (× 2 events)) were assessed as at least possibly related to the use of the study product/s, with the most commonly reported AE being headache, which occurred in five subjects on six occasions. Five of these events were assessed as mild in intensity, whilst the remaining event was assessed as moderate. There were no relevant differences amongst the four study products with regards to the types of AEs reported, their frequency, intensity, or causality. No deaths, SAEs, or withdrawals due to the AEs were observed in this study.
Furthermore, there were no clinically significant changes from baseline (i.e., prior to each study product administration) to 8-h post administration in mean systolic blood pressure, diastolic blood pressure or pulse rate overall, for any of the study products used. Additionally, there were no clinically significant changes from baseline (i.e., Day-1) to the end of Day 4 in mean values, for any of the ECG parameters assessed, overall or in any of the treatment sequences. In the laboratory evaluations, there were no clinically significant changes from baseline to the end of Day 4 in mean clinical chemistry or haematology, overall or in any of the treatment sequences. There were also no individual values assessed as clinically significant. Occasional post-dose, abnormal values assessed as non-clinically significant were observed for several parameters and subjects.
Overall, there were no concerns based on the evaluations undertaken. A single use of any of the four study products for the 20-min controlled use and ad lib use periods, was well tolerated in the study population as assessed by AEs, clinical laboratory parameters, vital signs, and ECGs.
Discussion
This study aimed to assess the nicotine PK and subjective effects of three MOND products and compare these outcomes to a traditional tobacco snus product. Higher strength MOND products were selected for the study as there is currently limited data on these in the literature. During the study, the short-term tolerability of the study products was also assessed. Overall, the MOND products delivered satisfactory levels of nicotine to adult snus and MOND users and demonstrated good short-term tolerability profiles.
Blood plasma nicotine PK was reflected by subjective effects and pouches demonstrated good short-term tolerability
Upon comparison of the blood plasma nicotine delivery achieved by the three MOND products, the highest strength product (zoneX #6 (20 mg)) delivered significantly higher levels, measured by Cmax and AUC. This was expected due to the higher nicotine content within the pouch, leading to greater nicotine availability for release. However, when the zoneX #5 (16 mg) and zoneX #5 Slim (14 mg) products were compared, although not significant, the 16 mg product delivered slightly lower levels of nicotine than the 14 mg product. This may potentially be due to the ‘slim’ format of the 14 mg pouch, where more of the pouch content may be in contact with the user’s saliva/ gum and there may be increased time taken for saliva to permeate a larger pouch, and therefore less nicotine release possible within the controlled use time. However, this has not been experimentally validated with regards to these products. Compared to the Skruf snus (16.6 mg) product, which was of similar nicotine content to the zoneX #5 (16 mg) product, both #5 products delivered similar nicotine levels, and a general dose-proportionality was observed when compared to the zoneX #6 (20 mg) product.
When assessed with regards to subjective effects, the blood plasma nicotine delivery from all four study products was sufficient to reduce urge to use nicotine to a similar extent. In fact, none of the outcomes showed significant differences from one another, except for the AUC between the #5 Slim and Skruf snus products. Although not significant, least reduction in urge to use nicotine was observed for the Skruf snus product, indicating that the zoneX products were able to deliver satisfactory levels of nicotine above or equal to those of the traditional tobacco product. Additionally, when the subjective measures of satisfaction, psychological reward, aversion and relief were examined, there were some significant differences between the zoneX and Skruf products. As observed with the urge to use nicotine measure, the #5 Slim product was able to deliver greater satisfaction than the Skruf snus product, and although the zoneX #6 product demonstrated greatest aversion, perhaps due to the higher nicotine delivery, this product also delivered greater psychological reward compared to the Skruf snus. Furthermore, all three zoneX products elicited significantly greater relief than the Skruf snus product. This suggests that MOND may act as an acceptable tobacco-free alternative, with a positive user experience, to traditional tobacco products. Although the zoneX #6 product achieved higher nicotine delivery, the two #5 products were still able to provide similar subjective effects.
When compared to the nicotine pharmacokinetic outcomes for other products of lower nicotine contents in the literature, the 14 mg, 16 mg and 16.6 mg products delivered comparable Cmax levels. For example, Lunell et al.16 reported a non-baseline adjusted Cmax level of 14.7ng/ml for the Zyn 6 mg product following a 60-min product use session, and an AUC0-inf value of 57.7ng/ml*h, and Liu et al. (2022) reported for an 8 mg nicotine on! MOND product a baseline adjusted Cmax value of 15.4ng/ml. Across the literature, inherent differences between product offerings, e.g., pouch filler substrate, nicotine form, pouch size, moisture content, etc., appear to influence nicotine pharmacokinetics7,16,17,18,19,20. This indicates that other factors, rather than just higher nicotine content, can also contribute to nicotine delivery.
The short-term tolerability of the study products was additionally assessed. During the study period, including both the controlled use sessions and the daily ad lib use periods, there were a limited number of mild to moderate AEs deemed to be related to the use of the study products, and the clinical parameters assessed did not deviate significantly from baseline levels. This was observed for all study products, which is as expected, due to the toxicological assessments carried out on these products and their ingredients at the levels included, prior to becoming available to consumers.
zoneX products offer THR potential to adult smokers
Key to THR is offering adult smokers potentially reduced harm nicotine delivery alternatives, which are acceptable in terms of user experience and satisfaction. To this end, NGP are available with optionality to suit consumer preference; MOND offerings include different nicotine strengths, pouch formats (e.g., regular, slim, etc.), flavours and moisture contents. This study investigated the effects of higher strength zoneX products than previously tested7, and demonstrated that, compared to a tobacco snus product, the products were able to deliver nicotine to satisfactory levels and with positive subjective effects. The zoneX #6 (20 mg) product delivered higher levels of nicotine than the other products tested in this study, which was coupled with slightly greater aversion scores compared to the other products. This product, however, still has a place in the market for those users who prefer a greater level of nicotine delivery.
Furthermore, although the zoneX #6 20 mg product delivered higher levels of nicotine than the lower strength zoneX products and the Skruf snus product tested, when considering abuse liability, nicotine delivery was lower than levels observed for usual brand cigarettes under controlled use25. Although Cmax was greater than some reported cigarette values (11.6ng/ml)7, as with other oral nicotine products, Tmax was much later than for cigarettes7,17,18. Increased Tmax suggests a potentially reduced abuse liability compared to cigarettes, through slower nicotine delivery. Further to this, when compared to subjective outcomes for cigarettes, the study products demonstrated potentially greater or similar reductions in urge to smoke/ use nicotine products7,17, indicating sufficient levels of satisfaction to act as an alternative to traditional tobacco products.
Coupled with a satisfactory user experience compared to traditional tobacco products, MOND has also been associated with reductions in the numbers and levels of toxicants in comparison to traditional tobacco products6,8,26, which appear to translate into significant reductions in biomarkers of exposure/ potential harm14,15. Although not measured in this study, it is expected that this would be the case based on the extract chemistry of zoneX products (data not shown).
Overall, the zoneX products delivered satisfactory levels of nicotine, with positive subjective effects, but with potential substantial reductions in the numbers and levels of toxicants associated with traditional tobacco product use, particularly smoking. Therefore, the MOND products used in this study demonstrate THR potential.
Limitations and future directions
Although this study provides novel information on the nicotine pharmacokinetic, subjective and short-term tolerability profiles of higher strength zoneX MOND products, the data must be viewed within the context of its limitations. The data suggests that pouch size may have an effect on nicotine pharmacokinetics, as the #5 slim pouch of lower nicotine content than the regular #5 product, achieved slightly higher, but not significantly different, blood plasma nicotine levels. However, as only one slim pouch was included in the study, it would be of future interest to assess a greater variety of pouch size formats to elucidate whether any trend exists. Whilst the #5 slim pouch additionally contained a small amount of tobacco, this was not expected to have a significant effect on the nicotine pharmacokinetic outcomes, although further validation of this would be required. Additionally, whilst some comparison has been made to the outcomes in the literature for other MOND, no direct comparison was made to other manufacturers’ products in this study.
Whilst there is evidence in the literature that MOND products contain reduced levels and numbers of the harmful chemicals found in tobacco leaf/ cigarette smoke, and this translates to reductions in biomarkers of exposure/ potential harm6,14,15, this study did not assess pouch extract chemistry of the products used or clinical biomarker analysis. Extract chemistry studies may provide an indication of substantial reductions in tobacco-related toxicants, and therefore it could be inferred that related biomarkers would not be present in the blood following product use, if not present in the product.
Whilst the study provides evidence that the MOND products tested may be an acceptable alternative to traditional tobacco products, how this translates to actual switching rate and displacement of use has not yet been defined. Therefore, it would be beneficial to explore this in a real-world setting to further define the THR potential of the zoneX MOND products.
Conclusions
Overall, this study has demonstrated that the three zoneX MOND products tested provide satisfactory levels of nicotine delivery for adult smokers with no intention to quit smoking, reducing urge to use nicotine following use, and with positive subjective outcomes. The products also exhibited good short-term tolerability profiles, indicated by the low number of product-related AEs, and absence of SAEs. This study demonstrates that zoneX MOND offers THR potential and an acceptable tobacco-free alternative to traditional tobacco product use.
Data availability
The datasets generated in the study are available from the corresponding author upon reasonable request.
Abbreviations
- AE:
-
Adverse event
- AUC:
-
Area under the plasma concentration–time curve
- AUCt :
-
AUC from 0 to the time of the last sampling timepoint, i.e. time 0-last
- AUC0 -inf :
-
AUC from timepoint 0 to infinity
- AUC0 -90 :
-
AUC from timepoint 0–90 min
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- Clast :
-
Observed plasma concentration at the last sampling timepoint
- Cmax :
-
Maximum observed plasma concentration
- ECG:
-
Electrocardiogram
- GCP:
-
Good clinical practice
- h:
-
Hours
- HIV:
-
Human immunodeficiency virus
- HPHC:
-
Harmful and potentially harmful constituent
- ICH:
-
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- LLOQ:
-
Lower limit of quantification
- LS:
-
Least squares (mean)
- Max:
-
Maximum value
- mg:
-
Milligrams
- Min:
-
Minimum value
- mmHg:
-
Millimetre mercury
- MOND:
-
Modern oral nicotine delivery
- NCA:
-
Non-compartmental analysis
- NGP:
-
Next generation (nicotine) product
- OND:
-
Oral nicotine delivery
- PES:
-
Product evaluation scale
- PK:
-
Pharmacokinetic
- TFNP:
-
Tobacco-free nicotine pouch
- THR:
-
Tobacco harm reduction
- Tmax :
-
Time to Cmax
- TSNA:
-
Tobacco-specific nitrosamine
- T1 /2(z) :
-
Terminal elimination half-life
- SERA:
-
Swedish Ethical Review Authority
- SAE:
-
Serious adverse event
- SAS:
-
Statistical Analysis System (software)
- SD:
-
Standard deviation
- VAS:
-
Visual analogue scale
References
Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US). Available from: https://www.ncbi.nlm.nih.gov/books/NBK53017/ (2010).
FDA. Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Federal Register (2012).
National Academies of Sciences Engineering and Medicine (NASEM). Public Health Consequences of E-Cigarettes. (National Academies Press, 2018)
McNeill, A., Brose, L. S., Calder, R., Bauld, L. & Robson, D. Evidence review of e-cigarettes and heated tobacco products 2018. A report commissioned by Public Health England. London: Public Health England (2018).
Grandolfo, E. et al. Tobacco-Free Nicotine Pouches and Their Potential Contribution to Tobacco Harm Reduction: A Scoping Review. Cureus. 16(2), e54228. https://doi.org/10.7759/cureus.54228 (2024).
Azzopardi, D., Liu, C. & Murphy, J. Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem. Toxicol. 45(5), 2246–2254. https://doi.org/10.1080/01480545.2021.1925691 (2022).
Chapman, F. et al. A randomised, open-label, cross-over clinical study to evaluate the pharmacokinetic, pharmacodynamic and safety and tolerability profiles of tobacco-free oral nicotine pouches relative to cigarettes. Psychopharmacology 239(9), 2931–2943. https://doi.org/10.1007/s00213-022-06178-6 (2022).
Back, S., Masser, A. E., Rutqvist, L. E. & Lindholm, J. Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs). BMC Chem. 17(1), 9. https://doi.org/10.1186/s13065-023-00918-1 (2023).
Bishop, E. et al. An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches. Food Chem. Toxicol. 145, 111713. https://doi.org/10.1016/j.fct.2020.111713 (2020).
East, N., Bishop, E., Breheny, D., Gaca, M. & Thorne, D. A screening approach for the evaluation of tobacco-free ‘modern oral’ nicotine products using Real Time Cell Analysis. Toxicol. Rep. 25(8), 481–488. https://doi.org/10.1016/j.toxrep.2021.02.014 (2021).
Miller-Holt, J. et al. In vitro evaluation of mutagenic, cytotoxic, genotoxic and oral irritation potential of nicotine pouch products. Toxicol. Rep. 15(9), 1316–1324. https://doi.org/10.1016/j.toxrep.2022.06.008 (2022).
Yu, F. et al. Preclinical Assessment of tobacco-free nicotine pouches demonstrates reduced in vitro toxicity compared with tobacco snus and combustible cigarette smoke. Appl. Vitro Toxicol. https://doi.org/10.1089/aivt.2021.0020 (2022).
Yu, F. et al. Multi-endpoint in vitro toxicological assessment of snus and tobacco-free nicotine pouch extracts. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 895, 503738. https://doi.org/10.1016/j.mrgentox.2024.503738 (2024).
Azzopardi, D. et al. Assessment of biomarkers of exposure and potential harm, and physiological and subjective health measures in exclusive users of nicotine pouches and current, former and never smokers. Biomarkers 28(1), 118–129. https://doi.org/10.1080/1354750X.2022.2148747 (2023).
Rensch, J., Edmiston, J., Wang, J., Jin, X. & Sarkar, M. A randomized, controlled study to assess changes in biomarkers of exposures among adults who smoke that switch to oral nicotine pouch products relative to continuing smoking or stopping all tobacco use. J. Clin. Pharmacol. 63(10), 1108–1118. https://doi.org/10.1002/jcph.2293 (2023).
Lunell, E., Fagerström, K., Hughes, J. & Pendrill, R. Pharmacokinetic comparison of a novel non-tobacco-based nicotine pouch (ZYN) With conventional, tobacco-based swedish snus and american moist snuff. Nicotine Tob. Res. 22(10), 1757–1763. https://doi.org/10.1093/ntr/ntaa068 (2020).
Rensch, J. et al. Nicotine pharmacokinetics and subjective response among adult smokers using different flavors of on!® nicotine pouches compared to combustible cigarettes. Psychopharmacology 238(11), 3325–3334. https://doi.org/10.1007/s00213-021-05948-y (2021).
Liu, J. et al. Nicotine pharmacokinetics and subjective responses after using nicotine pouches with different nicotine levels compared to combustible cigarettes and moist smokeless tobacco in adult tobacco users. Psychopharmacology 239(9), 2863–2873. https://doi.org/10.1007/s00213-022-06172-y (2022).
McEwan, M. et al. A randomised study to investigate the nicotine pharmacokinetics of oral nicotine pouches and a combustible cigarette. Eur. J. Drug Metab. Pharmacokinet. 47(2), 211–221. https://doi.org/10.1007/s13318-021-00742-9 (2022).
Azzopardi, D. et al. A randomised study to assess the nicotine pharmacokinetics of an oral nicotine pouch and two nicotine replacement therapy products. Sci. Rep. 12(1), 6949. https://doi.org/10.1038/s41598-022-10544-x (2022).
Fearon, I. Human abuse liability assessment of e-cigarettes: Why, what and how?. Drug Test Anal. 15(10), 1211–1221. https://doi.org/10.1002/dta.3251 (2023).
Hatsukami, D. K., Zhang, Y., O’Connor, R. J. & Severson, H. H. Subjective responses to oral tobacco products: scale validation. Nicotine Tob. Res. 15(7), 1259–1264. https://doi.org/10.1093/ntr/nts265 (2013).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2024).
Denney, W., Duvvuri, S. & Buckeridge, C. Simple, automatic noncompartmental analysis: the PKNCA R package. J. Pharmacokinet. Pharmacodyn. 42(1), 11–107 (2015).
McDermott, S. et al. An assessment of nicotine pharmacokinetics and subjective effects of the pulze heated tobacco system compared with cigarettes. Sci. Rep. 13(1), 9037. https://doi.org/10.1038/s41598-023-36259-1 (2023).
Mallock, N. et al. Levels of nicotine and tobacco-specific nitrosamines in oral nicotine pouches. Tob. Control. 33(2), 193–199. https://doi.org/10.1136/tc-2022-057280 (2024).
Acknowledgements
The authors would like to thank Clinical Trials Consultants (CTC) AB for carrying out the clinical trial, the study subjects for their participation, and the Imperial Brands internal Reading Committee for their critical review of the manuscript. Simon McDermott—Former employee of Imperial Brands PLC, 121 Winterstoke Road, BS3 2LL, Bristol, UK.
Funding
This study was funded by Imperial Brands PLC, manufacturers of the oral nicotine products used in this study.
Author information
Authors and Affiliations
Contributions
FC and RM drafted the manuscript, SM designed the study, XC and TV carried out statistical analyses on the data, VT sourced the study samples, MS and TN contributed to the writing of the manuscript, all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
This study was funded by Imperial Brands PLC, manufacturers of the oral nicotine products used in this study. At the time of study, FC, RM, SM, XC, TV, VT, MS and TN were employees of Imperial Brands PLC or its subsidiaries, and XC, TV, MS, TN hold stocks/ shares in the company.
Ethical approval
The study was approved by the Swedish Ethical Review Authority (SERA), carried out in accordance with the ethical principles outlined in the Declaration of Helsinki, International Conference of Harmonisation (ICH)/ Good Clinical Practice (GCP), European Union Clinical Trials Directive and applicable local regulatory requirements.
Informed consent
All subjects provided written informed consent prior to the study commencing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chapman, F., Morrissey, R., McDermott, S. et al. Evaluation of high-nicotine oral products shows potential to reduce tobacco-related harm by offering satisfying alternatives. Sci Rep 15, 34636 (2025). https://doi.org/10.1038/s41598-025-21812-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-21812-x