Abstract
To evaluate the effect of sulfinate salt on the bond performance of a two-step self-etch adhesive to an intracoronally bleached pulpal dentin surface. Intracoronally bleached bovine teeth were treated with or without sulfinate salt (sulfinate agent (SA): Clearfil DC activator) before 2-SEA (Clearfil SE Bond 2) application, while unbleached teeth served as the control (n = 5 teeth). Microtensile bond strength (µTBS) using the bonded surface area of 1 mm2 at the crosshead speed of 1 mm/min measurements after 24 h storage and thermocycles (TC), degree of conversion (DC) analyses by Raman spectroscopy (n = 3 teeth), ultrastructure of resin-dentin interface (n = 3 teeth), and intracoronally bleached pulp chamber dentin surface (n = 3 teeth) observations by scanning electron microscopy (SEM) were subsequently performed. Data were analyzed using the one-way ANOVA, Tukey’s post-hoc, and paired t-test. SA significantly increased the initial µTBS to bleached pulp chamber dentin surfaces (from 34.7 ± 4.5 to 50.6 ± 5.2 MPa, p < 0.001) and maintained post-TC bond durability (49.5 ± 8.8, p = 0.58). The application of SA also significantly increased DC on bleached pulp chamber dentin (p < 0.001). Interestingly, the highest DC was found in the SA group. SEM analyses revealed no obvious alteration in surface morphology; however, numerous and longer resin tags were observed at the resin-dentin interface in the bleached group, regardless of SA application. SA could improve bond performance together and enhance the polymerization of 2-SEA to intracoronally bleached pulp chamber dentin.
Similar content being viewed by others
Introduction
Endodontically treated teeth with coronal discoloration constitute a significant esthetic challenge, particularly in the anterior region. Although indirect restoration can mask discoloration, bleaching can sometimes improve the stump shade, making the indirect restoration blend more naturally with adjacent teeth1,2. In cases with minimal damage and a desire to preserve tooth structure or when cost and socioeconomic status are determinants, direct restorations are preferred as they are less invasive and cost-effective2,3. In such cases, bleaching is crucial in restoring the tooth’s color to look like that of the adjacent teeth4.
Intracoronal bleaching, a procedure in which a bleaching agent is placed into the pulp chamber for a week and the access to the chamber is sealed with temporary filling, is a treatment that whitens non-vital teeth from within the pulp chamber4. The treatment can be repeated at regular intervals until the whitening effect is satisfied5. Following endodontic treatment, access cavities should be restored with a bonded composite resin promptly to ensure coronal sealing and prevent re-infection6,7,8. However, several studies have suggested that if bleaching is required prior to restoration, it would be necessary to delay the bonding procedure of composite restoration for 1–2 weeks9,10,11,12. This delay is advised to avoid compromising the bond strength, as residual free-radical peroxide, which inhibits the polymerization of resin-based materials, remains on the tooth surface13. To overcome this issue, several authors have proposed the application of antioxidants such as sodium ascorbate in the pulp chamber14,15,16,17. Furthermore, various natural extracts (such as proanthocyanidin, propolis, quercetin) that have demonstrated satisfactory outcomes have been suggested for this purpose11,14,16,18,19,20,21.
However, these agents often require precise preparation and handling, which can be time-consuming and technique-sensitive, limiting their practical application in clinical settings. The availability of a ready-to-use commercial product designed specifically to address the challenge of compromised bond strength would significantly enhance convenience and simplify the clinical workflow. Despite the promising potential of these agents, to the best of our knowledge, there is currently no commercially available product specifically designed for this purpose. Identifying and utilizing an available product with suitable properties could offer a practical and convenient solution for clinicians by ensuring optimal bond strength without the need for delaying the restoration.
Sulfinate agents (SA) are utilized in dental commercial products primarily to act as a dual-cure activator. This product allows light-cure adhesives to be converted into dual-cure systems. This is particularly useful when bonding in areas where light penetration is limited or not possible, such as indirect restorations22,23. Additionally, studies have highlighted the reducing properties of SA, demonstrating their potential to restore bond strength in dentin pretreated with oxidizing agents24,25,26,27,28. When used as a pretreatment, sulfinate agents have been shown to enhance the microtensile bond strength and degree of conversion of adhesives applied to dentin surfaces, further improving adhesive performance23,24.
Based on findings from previous studies, SA may offer significant benefits in enhancing bond strength and ensuring its durability to intracoronally bleached dentin, where bonding is compromised due to residual free-radical peroxide24. However, evidence supporting its effectiveness remains limited. Therefore, this study aimed to evaluate the effect of SA on bond performance after 24 h of storage and subsequent thermocycling in intracoronally bleached pulp chamber dentin. Additionally, its impact on adhesive polymerization, which refers to the degree of conversion, was assessed using Raman spectroscopy. The ultrastructure of the resin-dentin interface and the intracoronally bleached pulp chamber dentin surface was also observed using scanning electron microscopy (SEM). The null hypothesis was that SA would not affect the bond performance, degree of conversion or ultrastructure of the resin-dentin interface of self-etch adhesive applied to intracoronally bleached pulp chamber dentin surfaces.
Materials and methods
Experimental design
Bovine teeth were chosen due to their similar chemical composition to human teeth and the practical advantages they offer in experimental settings, addressing issues related to availability, tooth quality, and ethical concerns29,30. The primary variable in the study was the application of SA, with one group treated with SA and a control group without treatment. Additionally, specimens were stored for 24 h to stabilize the adhesive, and thermocycling was applied to simulate the temperature fluctuations typically encountered in the oral environment.
The response variables included bond strength, assessed through microtensile bond strength testing after 24-h storage and thermocycling, to evaluate the durability of the bond. The degree of polymerization was measured using Raman spectroscopy to assess the degree of conversion. Finally, scanning electron microscopy (SEM) was conducted to examine the ultrastructure of the resin-dentin interface and dentin morphology.
Materials
Two-step self-etch dental adhesives (2-SEA: Clearfil SE Bond 2, Kuraray Noritake Dental, Tokyo, Japan; SA: Clearfil DC activator, Kuraray Noritake Dental, Tokyo, Japan) and a resin composite (Clearfil AP-X flow; Kuraray Noritake Dental, Tokyo, Japan) were used in this study. The overview of these materials is presented in Table 1.
Sample preparation
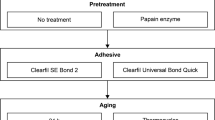
Thirty-three bovine teeth, collected from healthy slaughtered cow at a slaughterhouse, RoongRoj Farm, Bangkok, Thailand, were used in this study. These teeth were stored at − 20 °C and used within 3 months after extraction. The inclusion criteria for this study required extracted bovine incisors to have similar crown and root sizes and to be free of cracks and fractures. The teeth with any defect were excluded. The roots were cut perpendicularly to the tooth’s long axis 10 mm below CEJ and mounted with self-cure clear acrylic resin (Ortho-Jet, Lang, USA) in 16-mm-diameter PVC. The access opening was made using a long shank diamond round bur (Intensiv, Switzerland). The pulpal remnant was removed using a barbed broach. A long shank carbide round bur (Intensiv, Switzerland) was used to obtain straight-line access then applied the cavity to close the orifice of the root canal. The specimens were cleaned with deionized water under the ultrasonic cleaner for ten minutes. The root canal orifices were sealed with resin-modified glass ionomer cement (Vitrebond, 3 M ESPE, St Paul, MN, USA) using the Dycal carrier. The schematic of sample preparation is shown in Fig. 1.
Intracoronal bleaching procedure
Twenty-two bovine tooth specimens were treated with 35% hydrogen peroxide bleaching gel (Opalescence ENDO, Ultradent Products, South Jordan, UT, USA) according to the manufacturer’s instructions, while the other eleven specimens were used as unbleached controls. The bleaching agent was filled in the pulp chamber cavity leaving a 2-mm space for cotton pellets and zinc oxide temporary filling (Cavit G, 3 M ESPE, St. Paul, MN, USA; Fig. 1). The samples were stored in water at 37 °C for seven days/session. After two sessions, the zinc oxide temporary filling and cotton pellet were removed. The bleaching agent was washed out for 30 s using deionized water and the cavity was dried with oil-free air for 15 s using a triple syringe.
Bonding procedure
After the bleaching process, the lingual surface was cut using a carborundum disc (Carborundum Abrasives, Sao Paulo, SP, Brazil) to retrieve 4 × 5 mm2 of relatively flat dentin specimens. Then the specimens were air-dried and treated with or without SA (Clearfil DC activator, Kuraray Noritake Dental, Tokyo, Japan) for five seconds, followed by air-drying for five seconds as shown in Fig. 1. The unbleached pulp chamber dentin served as a control (n = 5 teeth). A 2-SEA (Clearfil SE Bond 2, Kuraray Noritake Dental, Tokyo, Japan) was applied according to the manufacturer’s instructions (Table 1) and light-cured for 10 s (1000 mW/cm2, Valo Grand, Ultradent, South Jordan, UT, USA). Each 2-mm-thick resin composite (Clearfil AP-X Flow, Kuraray Noritake Dental, Tokyo, Japan) increment was placed on the bonded surface and light-cured for 20 s. The teeth were stored in water at 37 °C 24 h before microtensile bond strength tests.
Microtensile bond strength (µTBS) test
Each bonded specimen was sectioned perpendicularly to the bond interface into stick-shaped specimens (bonded surface area of 1.0 ± 0.1 mm2) using a low-speed diamond saw with water cooling (Isomet, Buehler; Lake Bluff, IL, USA) as shown in Fig. 2. Twelve sticks from the central part of each bonded specimen were employed. The µTBS test was performed immediately or after 10,000 cycles of thermocycling (TC) according to the Academy of Dental Materials guidance31, i.e., between 5 °C and 55 °C, with a dwell time of 30 s in each bath and a transfer time of 5 s. After the designated aging procedure, the sticks were attached to a universal testing machine (EZ-SX Test, Shimadzu, Kyoto, Japan) and subjected to the µTBS test at a crosshead speed of 1 mm/min.
IBM SPSS version 29.0 was used for all data analyses. The sticks were considered statistical units (n = 30 sticks). The Shapiro–Wilk and Levene’s test indicated that the µTBS data were normally distributed and had homogeneous variance, respectively. The data were analyzed using the one-way ANOVA followed by Turkey’s multiple comparison tests. The bonding durability was analyzed by comparing µTBS after 24 h and TC in each group using t-tests. The threshold for statistical significance was set at p < 0.05.
Failure mode analysis
After the µTBS test, the dentin and composite sides of the fractured specimens were both desiccated, sputter-coated with gold, and observed using a scanning electron microscope (Quanta250, FEI, USA). Failure modes were classified as follows: adhesive failure (> 80% of the fractures occurred between the adhesive and dentin); cohesive failure in the dentin (> 80% of fractures occurred in the dentin); cohesive failure in the resin (> 80% of the fractures occurred in the adhesive and/or the overlying resin composite); mixed failure (combination of adhesive and cohesive failure, each < 80% of the fracture). The percentage surface area was estimated by superimposing a 10 × 10 table on the SEM photomicrographs. The failure mode percentages were statistically analyzed using the non-parametric Pearson chi-squared test.
Degree of conversion (DC) at the adhesive-dentin interface
Three bonded specimens per group were prepared according to the protocol described above. Each specimen was cut perpendicularly to the bonded interface into 1.5-mm-thick slices as shown in Fig. 1. Three central slices from each specimen were selected and polished with 800-grit, 1000-grit, 1200-grit, and 2000-grit SiC paper. Raman spectra were collected at the adhesive-dentin interface using a Raman microscope (Renishaw InVia, Renishaw plc, Gloucheshire, UK) with a 785 nm laser wavelength, 10 s of exposure time, 5× magnification, and spectral ranges from 620 to 1720 cm− 1. Spectra were obtained from five selected sites that were 100 μm apart at the center of each resin-dentin slice. The degree of conversion (DC) was calculated as follows:
where “R” is the ratio of aliphatic to aromatic peak intensities at 1640 cm−1 and 1610 cm−1 for cured and uncured adhesives, respectively32. The mean DC of each resin-dentin slice was calculated and considered the statistical units (n = 9). The Shapiro–Wilk test and Levene’s test were applied to verify the normality of data distribution and homogeneity of variances, respectively. The DC values were analyzed using the one-way ANOVA followed by the Dunnett T3 test.
SEM observation of resin-dentin interface
After testing for DC, the resin-dentin slices were treated with 37% phosphoric acid for 30 s and immersed in 5.25% sodium hypochlorite for 10 min. After being cleaned with deionized water ultrasonically, the resin-dentin specimens were desiccated, sputter-coated with gold, and observed using SEM (Quanta250, FEI, USA) at 3000× magnification.
SEM observation of bleached pulp chamber dentin surfaces
To observe the morphology of treated dentin, the bleached pulp chamber dentin surfaces were treated with or without SA, while the untreated pulp chamber dentins were used as the control (n = 3 teeth). The specimens were fixed using 2.5% glutaraldehyde in phosphate-buffered saline for two hours at 4 °C and serially dehydrated in an ascending series of ethanol as follows: 50%, 70%, and 80% ethanol for 25 min each at 4 °C, then 90% and 95% ethanol for 25 min each at room temperature, 100% ethanol twice for 25 min, after which it was immersed in hexamethyldisilane (HMDS) for 10 min and dried in a desiccator at room temperature for 24 h. After being sputter-coated with gold, the dentin surfaces were observed using SEM (Quanta250, FEI, USA) at 10,000× magnification.
Results
µTBS
Table 2 presents the µTBS of the experimental groups after storage for 24 h and TC. Intracoronal bleaching significantly decreased the initial µTBS (p < 0.001) and also the bond strength after TC (p < 0.001) of the pulp chamber dentin, while the application of SA could reverse the bond strength of bleached pulp chamber dentin (p < 0.001), as the same level of the unbleached group (p = 0.99). In addition, SA stabilized the µTBS after TC (p = 0.58).
Failure mode
The failure mode distributions in each group are presented in Table 3. The failure mode distributions differed significantly among the groups (p = 0.01). The majority of failures were mixed, except those in the group where SA was applied after TC in which most failures were cohesive failures in the dentin. Moreover, the rate of adhesive failure was reduced in groups in which SA was applied.
Degree of conversion
The representative Raman peaks are shown in Fig. 2. The aromatic C=C peak intensity at 1610 cm−1 remained consistent across all groups. However, an increase in the aliphatic C=C peak at 1640 cm−1 was clearly observed after bleaching. In contrast, SA application slightly decreased the aliphatic C=C peak intensity. As presented in Table 4, intracoronal bleaching significantly reduced the DC of the adhesive compared with the unbleached groups (p < 0.001). The DC of the SA-treated group significantly increased (p < 0.001) and was significantly higher than that of the unbleached control group (p = 0.004).
Representative Raman spectrum acquired on the normal pulp chamber dentin, bleached with and without SA application. A slight increase in the aliphatic C=C peak at 1640 cm−1 can be observed in bleaching group (green) compared with a normal pulp chamber dentin (blue). While the aromatic C=C peak at 1610 cm−1 is similar in each group. A decrease in the aliphatic C=C peak at 1640 cm−1 can be clearly observed after SA application (red). SA sulfinate agent.
SEM observation of the resin–dentin interface
The representatives of SEM micrographs of the resin-dentin interface are presented in Fig. 3. The numerous longer resin tags were observed in the groups of bleached pulp chamber dentin, regardless of SA application. However, the shortly uniformed resin tags were observed in the unbleached control group.
Representative SEM micrographs of the resin tag system of the resin-dentin interface formed by 2-SEA Clearfil SE Bond 2 (×3000 magnification). In the control group (A), uniform resin tags within a thin hybrid layer were observed. Numerous and longer resin tags (white arrow) were found in the bleached group (B) and bleached with SA application (C). SA sulfinate agent, AD adhesive, D dentin.
SEM observation of the bleached pulp chamber dentin surface
The representatives of SEM micrographs of the resin-dentin interface are presented in Fig. 4. After bleaching, demineralization was promoted and could be observed partially at the intertubular dentin (Fig. 4B). The bleached dentin surfaces were not obviously changed after SA application; however, homogenous surfaces can be slightly observed (Fig. 4C).
Representative SEM micrographs of pulp chamber dentin surfaces (×10,000 magnification). Intracoronal bleaching (B) alters the surface morphology of pulp chamber dentin, showing partial demineralization at the intertubular dentin (white arrow) compared with the normal pulp chamber dentin (A). The bleached dentin surfaces were not obviously changed after SA application (C); however, homogenous surfaces can be observed slightly. SA sulfinate agent.
Discussion
According to the findings of this study, SA application significantly increases the initial µTBS to the bleached pulp chamber dentin surfaces and stabilizes the post-TC bond durability. Additionally, an increasing degree of polymerization was also observed in this study. However, there was no significant difference in the resin-dentin interface’s ultrastructure after pretreating the bleached dentin surfaces with a sulfinate agent. Thus, the null hypothesis was partially rejected.
Several studies have demonstrated the relationship between dentin surface alteration after bleaching and adhesive bond strength reduction, especially when the adhesive step was performed immediately after bleaching4,10,33. These changes included alterations in ultrastructure and reductions in the mineral composition of the dentin surface, which hinder resin infiltration and chemical interactions of the adhesive monomer and hydroxyapatite4,33. Additionally, the increase in pH value of the bleached dentin surface seems to impair the bond strength10. Moreover, the bleaching agents release free oxygen radicals, breaking down larger chromogenic pigments into smaller ones, thereby lightening tooth color34. These oxygen radicals remaining within the tooth structures are thought to prevent resin infiltration and impair the polymerization of the adhesive, causing a reduction in the bond strength of the adhesive10,11.
The present study confirmed that intracoronal bleaching significantly impaired the bond strength of SEA and jeopardized the bond durability. To reverse this effect, it is recommended to delay the procedure for 1–2 weeks9,10,11,12 which increases the number of dental visits and delays the restoration of endodontic access sealing. The application of antioxidants such as sodium ascorbate, proanthocyanidin, propolis, quercetin, etc. has been recommended11,14,16,17,18,19,20,35. These antioxidants can reverse the negative effect of oxygen radicals by oxidant/antioxidant reaction kinetics14. Additionally, the antioxidants could improve bond durability by suppressing the activity of the endogenous proteolytic enzyme36,37. However, practical considerations such as differences in preparation techniques, concentrations, application times, and rapid decomposition may limit the practicality of antioxidant use14,17,35.
In this study, we demonstrated that the application of commercially available SA improved the initial dentin bond strength and durability of SEA bonded immediately after intracoronal bleaching, which can be recommended for clinical application. SA enhances bond performance by two potential mechanisms. First, SA exhibits a reducing ability that neutralizes residual oxidizing molecules on the dentin surface, leading to the improvement of the bond performance compromised by oxidizing agents, such as eugenol, sodium hypochlorite, and hypochlorous acid24,25,27,28,38. The bleaching agent used in this study behaves similarly to these oxidizing agents, also leaving residual oxygen radicals in the dentin structure which impair the polymerization of the adhesive by premature chain termination25,38. The present study proved that SA application could reverse the negative impact of bleaching on the degree of polymerization of the adhesive. The results from Raman spectroscopy demonstrated a significant increase in the conversion of aliphatic C=C bonds after the application of SA on bleached pulp chamber dentin. Surprisingly, the DC of the SA group was significantly higher than that of the unbleached control group.
Another potential mechanism of action of the SA is to initiate and accelerate the adhesive’s polymerization, which may explain the unexpected degree of conversion. Therefore, its application has been proposed for enhancing dentin adhesion under conditions of insufficient light irradiation; for example, bonding to root canal dentin or the luting of indirect restorations24,39,40. A recent study demonstrated that pretreatment of the dentin surface with SA improved the DC of SEA even under sufficient light irradiation23. In addition, SA was also recommended as a dual-cure activator, initiating polymerization upon contact with the chemical cure initiator in self-cure or dual-cure resin composites41,42. However, in this experimental design, we primarily focused on the reducing ability of the sulfinate agent and its potential benefits for bonding to intracoronally bleached dentin. Since the two-step self-etch adhesive system, which including a hydrophobic resin, was applied to the sulfinate-treated surface, we consider that the sulfinate agent does not interact significantly with the resin composite. Nonetheless, the exact interaction of sulfinate agents with the polymerization system of light-cured adhesives or composite materials remains unclear, and different application methods, such as mixing with universal bond products, may indeed affect the conversion of the composite resin at the interface between the bonding resin and the composite.
The present study demonstrated the alteration of dentin surface morphology after intracoronal bleaching (Fig. 4). Longer resin tags could be clearly observed in the bleached dentin groups, regardless of SA application. The increase in dentin permeability after bleaching could be the reason43. As we also know the resin tag’s length does not influence the bond performance44 but the DC does45. The results of this study support DC’s positive correlation with bond durability.
SA application exhibits a potential for improving the adhesion of 2-SEA to intracoronally bleached dentin which can reduce dental visits and reduce the waiting time of patients seeking restoration after endodontic treatment. Dentists can enhance bond performance on intracoronally bleached dentin by applying the SA, known as dual-cure activator, directly to the surface for 5 s, followed by air drying before applying the adhesive. This protocol facilitates improved bonding outcomes without the need for a delay period following the bleaching process. The immediate application of the final restoration to the access cavity for endodontic treatment, the tooth is mechanically strengthened reducing the risk of fracture. Also by the tight sealing of the cavity, the coronal leakage is prevented, which is reported to be the cause of the periapical lesions6,7,8.
In addition, the application of SA would not affect the color of final restoration. This is because the SA is colorless solution, and it does not form any film at the interface following the application of the self-etching and bonding agents. The color stability of the bond interface with sulfinate treatment is considered superior to that of groups without sulfinate agent, due to the improved conversion of the bonding resin46.
Conclusion
Within the limitations of the present study, it can be concluded that pretreatment with commercially available SA facilitated polymerization and improved the bond performance of self-etch adhesives to intracoronally bleached pulp chamber dentin. A 5-s application of sulfinate agents after bleaching reduces patient wait times and enhances treatment efficiency. Immediate restoration of the access cavity during endodontic treatment also strengthens the tooth, reduces fracture risk, and prevents coronal leakage, which can lead to periapical lesions.
Data availability
The data that support the findings of this study are available from the corresponding author, [Kittisak Sanon], upon reasonable request.
References
Tronstad, L. et al. Influence of coronal restorations on the periapical health of endodontically treated teeth. Endod Dent. Traumatol. 16 (5), 218–221 (2000).
Dias, P. C. & Franco F. B. M. J., Palma-Dibb, R. G., Faraoni, J. J. Different approaches for aesthetic rehabilitation of discolored nonvital anterior teeth. RGO-Revista Gaúcha De Odontologia. 69, e20210039 (2021).
Singh, N., Chaturvedi, T., Baranwal, H. C. & Wang, C. K. Management of discolored nonvital tooth by walking bleach technique: a conservative approach. J. Int. Clin. Dent. Res. Organ. 12 (1), 67–71 (2020).
Timpawat, S., Nipattamanon, C., Kijsamanmith, K. & Messer, H. H. Effect of bleaching agents on bonding to pulp chamber dentine. Int. Endod. J. 38 (4), 211–217 (2005).
Carrasco, L. D. et al. Efficacy of intracoronal bleaching techniques with different light activation sources. Int. Endod. J. 40 (3), 204–208 (2007).
Ray ha, T. R. O. P. E. M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int. Endod. J. 28 (1), 12–18 (1995).
Heling, I. et al. Endodontic failure caused by inadequate restorative procedures: review and treatment recommendations. J. Prosthet. Dent. 87 (6), 674–678 (2002).
Safavi, K. E., Dowden, W. E. & Langeland, K. Influence of delayed coronal permanent restoration on endodontic prognosis. Dent. Traumatol. 3 (4), 187–191 (1987).
Shinohara, M. S., Peris, A. R., Pimenta, L. A. & Ambrosano, G. M. Shear bond strength evaluation of composite resin on enamel and dentin after nonvital bleaching. J. Esthet. Restor. Dent. 17 (1), 22–29 (2005). discussion 29.
Elkhatib, H. et al. Surface pH and bond strength of a self-etching primer/adhesive system to intracoronal dentin after application of hydrogen peroxide bleach with sodium perborate. Oper. Dent. 28 (5), 591–597 (2003).
Karadas, M. & Demirbuga, S. Influence of a short-time antioxidant application on the dentin bond strength after intracoronal bleaching. Microsc. Res. Tech. 82 (10), 1720–1727 (2019).
Souza-Gabriel, A. E. et al. Effect of bleaching protocols with 38% hydrogen peroxide and post-bleaching times on dentin bond strength. Braz. Dent. J. 22 (4), 317–321 (2011).
Lai, S. C. et al. Reversal of compromised bonding to oxidized etched dentin. J. Dent. Res. 80 (10), 1919–1924 (2001).
Freire, A. et al. Reaction kinetics of sodium ascorbate and dental bleaching gel. J. Dent. 37 (12), 932–936 (2009).
Türkün, M. & Kaya, A. D. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J. Oral Rehabil. 31 (12), 1184–1191 (2004).
Ghaleb, M. et al. The Effect of different bleaching protocols, used with and without sodium ascorbate, on bond strength between composite and enamel. Mater. (Basel) ;13(12). (2020).
Thapa, A., Vivekananda, P. A. & Thomas, M. S. Evaluation and comparison of bond strength to 10% carbamide peroxide bleached enamel following the application of 10% and 25% sodium ascorbate and alpha-tocopherol solutions: an in vitro study. J. Conserv. Dent. 16 (2), 111–115 (2013).
Lin, X. J., Hong, D. W., Lu, Z. C. & Yu, H. Effect of quercetin pretreatment on the immediate and aged bond strength of bleached dentin. J. Mech. Behav. Biomed. Mater. 135, 105476 (2022).
Arslan, S., Balkaya, H., Durukan, S. M. & Silici, S. The effect of propolis on the bond strength of composite resin to enamel after intracoronal bleaching with different bleaching agents. Aust. Endod. J. 49 (Suppl 1), 366–373 (2023).
Vidhya, S., Srinivasulu, S., Sujatha, M. & Mahalaxmi, S. Effect of grape seed extract on the bond strength of bleached enamel. Oper. Dent. 36 (4), 433–438 (2011).
Safinaz, D. et al. Effect of Green Tea Extract Antioxidant on Dentin Shear Bond Strength and Resin-tag Penetration Depth After Non-vital Bleaching (F1000Res, 2023).
Arrais, C. A., Giannini, M. & Rueggeberg, F. A. Effect of sodium sulfinate salts on the polymerization characteristics of dual-cured resin cement systems exposed to attenuated light-activation. J. Dent. 37 (3), 219–227 (2009).
Hasegawa, M. et al. Degree of conversion and dentin bond strength of light-cured multi-mode adhesives pretreated or mixed with sulfinate agents. Dent. Mater. J. 40 (4), 877–884 (2021).
Nakatani, H. et al. Effectiveness of pretreatment with phosphoric acid, sodium hypochlorite and sulfinic acid sodium salt on root canal dentin resin bonding. J. Prosthodont. Res. 64 (3), 272–280 (2020).
Sanon, K. et al. Smear layer deproteinization with NaOCl and HOCl: do application/wash-out times affect dentin bonding of one-step self-etch adhesives? Dent. Mater. J. 41 (3), 353–362 (2022).
Sanon, K. et al. Application of Sulfinate Agent in Conjunction with HOCl Smear-Layer Deproteinization improves dentin bonding durability of one-step self-etch adhesives. J. Adhes. Dent. 24, 223–232 (2022).
Thanatvarakorn, O. et al. Smear layer-deproteinizing improves bonding of one-step self-etch adhesives to dentin. Dent. Mater. 34 (3), 434–441 (2018).
Wongsorachai, R. N. et al. Effect of polymerization accelerator on bond strength to Eugenol-Contaminated Dentin. J. Adhes. Dent. 20 (6), 541–547 (2018).
DeWald, J. P. The use of extracted teeth for in vitro bonding studies: a review of infection control considerations. Dent. Mater. 13 (2), 74–81 (1997).
Soares, F. Z. et al. Bovine tooth is a substitute for human tooth on bond strength studies: a systematic review and meta-analysis of in vitro studies. Dent. Mater. 32 (11), 1385–1393 (2016).
Armstrong, S. et al. Academy of dental materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (µTBS) approach. Dent. Mater. 33 (2), 133–143 (2017).
Hass, V. et al. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 32 (6), 732–741 (2016).
Chng, H. K., Yap, A. U., Wattanapayungkul, P. & Sim, C. P. Effect of traditional and alternative intracoronal bleaching agents on microhardness of human dentine. J. Oral Rehabil.. 31 (8), 811–816 (2004).
Bernardon, J. K. et al. Clinical performance of vital bleaching techniques. Oper. Dent. 35 (1), 3–10 (2010).
Jung, K-H. et al. Time of application of sodium ascorbate on bonding to bleached dentin. Scanning 2017 (1), 6074253 (2017).
Sanon, K., Sanchavanakit, N. & Srisawasdi, S. Grape seed extract reduces active gelatinases using an etch-and-rinse mode universal adhesive. J. Adhes. Dent. 21 (2), 159–165 (2019).
Perdigão, J., Reis, A. & Loguercio, A. D. Dentin adhesion and MMPs: a comprehensive review. J. Esthet Restor. Dent. 25 (4), 219–241 (2013).
Sanon, K. et al. Application of sulfinate agent in conjunction with HOCl Smear-layer deproteinization improves dentin bonding durability of one-step self-etch adhesives. J. Adhes. Dent. 24 (1), 223–232 (2022).
Kadowaki, Y. et al. Bond performance of touch and cure adhesives on resin core systems. Dent. Mater. J. 35 (3), 386–391 (2016).
Yoshihara, K. et al. Touch-cure polymerization at the composite cement-dentin interface. J. Dent. Res. 220345211001020 (2021).
Tay, F. R. et al. Factors contributing to the incompatibility between simplified-step adhesives and self-cured or dual-cured composites. Part II. Single-bottle, total-etch adhesive. J. Adhes. Dent. 5 (2), 91–105 (2003).
Michaud, P. L. & MacKenzie, A. Compatibility between dental adhesive systems and dual-polymerizing composite resins. J. Prosthet. Dent. 116 (4), 597–602 (2016).
Carrasco, L. D., Fröner, I. C., Corona, S. A. M. & Pécora, J. D. Effect of internal bleaching agents on dentinal permeability of non-vital teeth: quantitative assessment. Dent. Traumatol. 19 (2), 85–89 (2003).
Kharouf, N. et al. Does adhesive layer thickness and tag length influence short/long-term bond strength of universal adhesive systems? An in-vitro study. Appl. Sci. 11 (6), 2635 (2021).
Sato, K. et al. Dentin bonding durability of two-step self-etch adhesives with improved of degree of conversion of adhesive resins. J. Adhes. Dent. 19 (1), 31–37 (2017).
Benavides-Reyes, C. et al. Color stability and degree of conversion of gingiva-colored resin-based composites. J. Esthet. Restor. Dent. 35 (6), 896–903 (2023).
Author information
Authors and Affiliations
Contributions
N.P. Principal investigator, data collection, performed the analysis, visualization, wrote the main manuscript text: K.S. Conceived and designed the analysis, data collection, contributed data/analysis tools, performed the analysis, resource, wrote the paper. P.T. Conceived and designed the analysis, visualization, wrote the paper. P.S. Conceived and designed the analysis, data collection, SEM observation. R.B. Contributed data/analysis tools (raman microscopy), wrote the paper. T.N. Conceived and designed the analysis (raman microscopy), wrote the paper. V.S. Contributed data/analysis tools (raman microscopy), wrote the paper. C.K. and D.N. Conceived and designed the analysis, wrote the paper. J.T. Conceived and designed the analysis, wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Piyanuluk, N., Sanon, K., Thongthai, P. et al. Effect of sulfinate salt on bonding and polymerization of adhesive to intracoronally bleached dentin. Sci Rep 15, 2501 (2025). https://doi.org/10.1038/s41598-025-86659-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-86659-8