Abstract
The hepatic steatosis index (HSI) has been demonstrated to have a significant correlation with prediabetes and diabetes; however, its potential association with reversion to normal glucose regulation (NGR) from prediabetes remains insufficiently investigated. The primary objective of this study was to elucidate the relationship between HSI levels and the probability of reversion to NGR among Chinese adults with prediabetes. This retrospective cohort study utilized health examination data from 11,241 Chinese adults with prediabetes. Cox proportional hazard models were employed to calculate hazard ratios (HR) and 95% confidence intervals (CI) to assess the association between HSI levels and reversion to NGR. Additionally, a Cox proportional hazards regression model incorporating cubic spline functions and smooth curve fitting was utilized to determine any nonlinear relationships between HSI levels and reversion to NGR. The results of the multivariate analyses demonstrated a significant association between reduced HSI levels and an increased likelihood of reversion to NGR (HR = 0.92, 95%CI: 0.89–0.95, P < 0.0001). Furthermore, a nonlinear relationship was identified between HSI levels and the reversion to NGR, with a critical threshold at an HSI value of 43.08. Below this threshold, a strong negative association was observed, markedly enhancing the probability of returning to normoglycemic status (HR = 0.91, 95% CI: 0.88–0.94, P < 0.0001). This study reveals a negative, nonlinear correlation between HSI levels and the reversion to NGR in individuals with prediabetes. These findings highlight the essential role of effectively managing HSI as part of comprehensive prediabetes treatment strategies, which may significantly enhance the likelihood of achieving normoglycemic status.
Similar content being viewed by others
Introduction
Prediabetes represents an intermediate stage between normal glucose regulation (NGR) and the onset of diabetes1. In 2019, approximately 7.5% of the world’s population, amounting to nearly 374 million individuals, were affected by prediabetes2. Projections suggest that by 2045, this prevalence will increase to 8.6%, affecting over 548 million people2. The situation is particularly concerning in China, where in 2017, the prevalence of prediabetes was estimated to be around 35.2%, impacting nearly 357 million adults3. Without appropriate intervention, prediabetes may progress to diabetes, leading to serious health complications. Nonetheless, a significant number of individuals with prediabetes can return to NGR, thus substantially lowering their risk of developing diabetes and its related complications4,5,6,7,8,9. Therefore, identifying predictive factors for reversion is essential for timely intervention and effective management of prediabetes.
Hepatic steatosis, often referred to as metabolic associated fatty liver disease (MAFLD), is currently the most prevalent cause of liver disease worldwide, with an estimated global prevalence of approximately 30%10. An increasing body of epidemiological evidence indicates that MAFLD is a significant independent risk factor for the development of diabetes, irrespective of the stage of hepatic steatosis11,12,13. Patients with MAFLD have more than twice the likelihood of developing diabetes compared to those without MAFLD14. This suggests that the presence of MAFLD, particularly in the prediabetic phase, may substantially influence the risk of progressing to diabetes. The hepatic steatosis index (HSI) is a non-invasive and validated tool for assessing hepatic steatosis, incorporating variables such as body mass index (BMI), aspartate aminotransferase (AST) and alanine aminotransferase (ALT)15,16. Several studies using a cohort design have revealed that elevated HSI levels correlate with a heightened risk of developing prediabetes and diabetes17,18,19. However, scant research has focused on the correlation between HSI levels and the reversion to NGR from prediabetes. Therefore, this investigation examines the relationship between the HSI and reversion to NGR in a Chinese population with prediabetes.
Methods
Data source
The dataset employed in this investigation was obtained from the DATADRYAD platform, a digital repository facilitating open access to raw research data. We accessed the dataset originally deposited by Chen et al.20. In accordance with Dryad’s terms of service, researchers are authorized to utilize these datasets for secondary analyses predicated on novel research hypotheses.
Study population
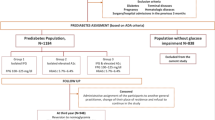
The study included adult participants (age range: 20–99 years, 54.8% male) from 32 medical check-up centers located in 11 cities (Shanghai, Beijing, Nanjing, Suzhou, Shenzhen, Changzhou, Chengdu, Guangzhou, Hefei, Wuhan, and Nantong) across different regions of China (Eastern: 45%, Central: 35%, Western: 20%)20. The age distribution demonstrated adequate representation across all age groups (20 to < 30 years: 13.6%; 30 to < 40 years: 38.8%; 40 to < 50 years: 20.4%; 50 to < 60 years: 15.0%; 60 to < 70 years: 8.3%; ≥ 70 years: 3.8%), with a balanced gender ratio observed within each age group20. Participants’ medical records were obtained from a database established by the Wealthy Medical Group, and all individuals underwent health check-ups between January 1, 2010, and December 31, 2016. The initial cohort comprised 685,277 adults aged 20 years and older who had participated in at least two health checkups. The following exclusion criteria were applied: (1) missing information on sex, weight, height, and fasting plasma glucose (FPG) at baseline (n = 135,317); (2) extreme BMI values (< 15 kg/m2 or > 55 kg/m2) (n = 152); (3) a short follow-up duration of less than 2 years (n = 324,233); (4) a pre-existing diagnosis of diabetes (n = 7,112); (5) unknown diabetes status at follow-up (n = 6,630); (6) not meeting prediabetes criteria at baseline (n = 185,815); and (7) missing information on AST FPG, or ALT (n = 14,777). Upon applying these exclusion criteria, the final study cohort consisted of 11,241 individuals with prediabetes at baseline (Fig. 1). Ethical approval for this study was granted by the Clinical Research Ethics Committee of Shenzhen Second People’s Hospital Dapeng New District Nan’ao Hospital. Moreover, the research adhered to the principles delineated in the Declaration of Helsinki, ensuring compliance with all relevant guidelines and regulatory standards.
Data collection
Demographic information, lifestyle factors, and family history of diabetes were collected using standardized questionnaires administered by trained personnel20. Height, weight, and blood pressure were measured by trained professionals using calibrated equipment and following uniform procedures to ensure consistency across all centers20. To further ensure consistency, all data collection and measurement staff underwent rigorous training before the original study began20. Data acquisition was performed by qualified personnel at the health examination facility. The comprehensive dataset included participant demographics (age, gender), anthropometric measurements (weight, height), lifestyle factors (alcohol consumption and smoking status), diastolic and systolic blood pressure (DBP and SBP), and family history of diabetes. Venous blood samples were collected following a minimum fasting period of 10 h. A range of biochemical markers were measured using a Beckman 5800 automated analyzer in a standardized laboratory setting. These markers included FPG, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), blood urea nitrogen (BUN), serum creatinine (Scr), ALT, and AST.
Hepatic steatosis index
The HSI formula, as developed by Jeong-Hoon Lee et al.15, was derived using a logistic regression model based on a large cohort of subjects. The HSI is a non-invasive tool designed to estimate the likelihood of hepatic steatosis using readily available clinical parameters. The formula includes a gender-specific adjustment, adding a constant of "2" for females to account for physiological differences in liver enzyme levels and body composition, thereby enhancing the accuracy of risk estimates across genders. The HSI was calculated using the following equations: For female subjects, the formula is HSI = 2 + (ALT/AST) × 8 + BMI15. For male subjects, the formula is HSI = (ALT/AST) × 8 + BMI15.
Diagnosis of prediabetes
Prediabetes was defined in accordance with the criteria established by the American Diabetes Association, which specifies FPG levels between 5.6 and 6.9 mmol/L21.
Definitions of outcome
The outcome was defined as a reversion to NGR, characterized by an FPG level below 5.6 mmol/L during follow-up and the absence of self-reported diabetes9,22.
Missing data processing
The participants with missing data were distributed as follows: SBP had 5 missing cases (0.04%), DBP had 5 missing cases (0.04%), LDL-C had 3,557 missing cases (31.64%), HDL-C had 4,136 missing cases (36.79%), TG had 89 missing cases (0.79%), TC had 89 missing cases (0.79%), Scr had 523 missing cases (4.65%), BUN had 1,265 missing cases (11.26%), drinking status had 7,817 missing cases (69.53%), and smoking status had 7,817 missing cases (69.53%). Multiple imputations were performed to address the issue of missing covariate data. The imputation model included LDL-C, drinking status, age, gender, HDL-C, smoking status, SBP, TG, BMI, TC, DBP, Scr, BUN, FPG, and family history of diabetes. The analysis of missing data was conducted under the assumption that the data were missing at random23,24.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) or median (interquartile range), and categorical variables are presented as frequencies and percentages (%). Participants were divided into four distinct groups according to the quartiles (Q1-Q4) of the HSI. Baseline characteristics across these quartile groups were also delineated. Group differences were evaluated using a one-way analysis of variance or the Kruskal–Wallis H test for continuous variables and the chi-square test for categorical variables.
Kaplan–Meier curves based on HSI quartiles were plotted. We performed log-rank tests to statistically compare these curves, which confirmed significant differences among the groups. Cox proportional hazard models were used to estimate the crude and adjusted hazard ratios (HR) and their 95% confidence intervals (CI) to determine the association between HSI levels and reversion to NGR. To assess the relationship between the hepatic steatosis index (HSI) and reversion to normal glucose regulation, we performed z-score transformations of HSI before conducting Cox regression analysis. Three models were constructed. Model 1 was a univariate model. Model 2 was adjusted for age, SBP, drinking status, DBP, gender, family history of diabetes, and smoking status. Model 3 was further adjusted for baseline LDL-C, TG, FPG, HDL-C, BUN, and Scr. To evaluate multicollinearity among variables, the variance inflation factor (VIF) was calculated for the covariates. Covariates with a VIF of > 5 were considered collinear and excluded from the multivariate Cox proportional hazards regression models. Based on this criterion, TC was excluded from the final multivariate Cox regression model due to its high VIF value (Table S1). We evaluated the proportional hazards assumption by incorporating an interaction term between HSI and the logarithm of follow-up time in the model. A P-value of less than 0.05 for the interaction term was considered indicative of a violation of the proportional hazards assumption. Our analysis demonstrated no significant interaction (P > 0.05), thereby confirming that the proportional hazards assumption was satisfied (Table S2 and Figure S1).
In addition, a series of sensitivity analyses were conducted. Given the established associations of hypercholesterolemia with reversion to NGR5,25, we excluded subjects with TC > 5.2 mmol/L26 and TG > 1.7 mmol/L26 in these analyses to further elucidate the correlation between the HSI and reversion to NGR. Additionally, recognizing that HSI outliers may result from measurement errors and could potentially affect the stability of the observed relationship, HSI outliers (defined as values beyond the mean ± 3 standard deviations) were excluded to further investigate the association between HSI levels and reversion to NGR. Finally, the E-value was calculated to assess the potential impact of unmeasured confounders on the relationship between HSI levels and the reversion to NGR.
The cubic spline function and smooth curve fitting in the Cox regression framework were conducted to explore further the nonlinear correlation between HSI levels and reversion to NGR. When nonlinearity between HSI levels and reversion to NGR was detected, we calculated the inflection point using a two-piecewise Cox proportional hazards regression model. The log-likelihood ratio test was employed to select the most suitable model for elucidating the connection between HSI levels and reversion to NGR.
We further stratified the analyses by age (< 30, 30 to < 40, 40 to < 50, 50 to < 60, and ≥ 60 years), drinking status (current, ever, and never smoker), sex (male or female), smoking status (current, ever and never smoker), BMI (< 25, 25 to < 30, and ≥ 30 kg/m2), and family history of diabetes (no, yes), and tested the interaction between HSI levels and these variables by including an interaction term in the models. The statistical significance of the interaction term was assessed using a likelihood ratio test.
The data in this study was analyzed using Empower Stats software, version 2.2. A two-tailed P-value of < 0.05 was deemed to denote statistical significance.
Results
Characteristics of participants
The average age of the participants was 49.94 ± 14.02 years, with 7,512 (66.83%) being male (Table 1). Participants were stratified into four groups based on HSI quartiles: Q1 (HSI ≤ 29.84), Q2 (29.84 < HSI ≤ 33.06), Q3 (33.06 < HSI ≤ 36.76), and Q4 (HSI > 36.76). Individuals in the higher HSI quartiles (Q2, Q3, and Q4) tended to be predominantly male, and exhibited higher levels of Scr, TG, blood pressure, FPG, AST, LDL-C, BMI, BUN, ALT, and TC. Individuals in the higher HSI quartile (Q4) are younger compared to those in the lower quartiles (Q1, Q2, and Q3). Moreover, these groups exhibited lower HDL-C levels and a higher prevalence of a family history of diabetes, current smoking, and abstention from alcohol compared to those in the lower HSI groups.
The rate of the reversion to NGR
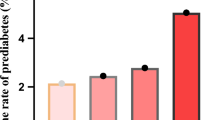
After an average follow-up period of 2.99 years, 4,768 patients with prediabetes (42.42%) reverted to NGR, as shown in Table 2. The cumulative reversion rates to NGR were 42.42% overall, 52.92% for the Q1 group, 43.06% for the Q2 group, 38.73% for the Q3 group, and 34.96% for the Q4 group. The annual reversion rates to NGR for the entire cohort and individuals in the HSI quartiles (Q1-Q4) were 14.19, 17.88, 14.16, 12.95, and 11.73 per 100 person-years, respectively. A significant negative trend was observed, with participants in higher HSI quartiles exhibiting lower reversion rates to NGR compared to those in lower HSI quartiles (P < 0.001 for trend).
As illustrated by the Kaplan–Meier Survival Probability curves in Fig. 2, participants in the lowest quartile of the HSI demonstrated a significantly higher cumulative incidence of reversion to NGR compared to those in the highest quartile. The differences in cumulative incidence across the HSI quartiles were statistically significant, as confirmed by the log-rank test (P < 0.001).
The relationship between HSI levels and reversion to NGR
The association between the HSI and the likelihood of reversion to NGR is presented in Table 3. The initial univariate analysis (Model 1) identified a significant inverse relationship between HSI and reversion to NGR (HR = 0.85, 95% CI: 0.82–0.87, P < 0.0001). This negative association remained significant in Model 2, which adjusted for age, SBP, drinking status, DBP, gender, family history of diabetes, and smoking status, resulting in an HR of 0.86 (95% CI: 0.84–0.89, P < 0.001). Upon further comprehensive adjustment for all potential confounding variables in Model 3 (including the covariates from Model 2, as well as LDL-C, TG, FPG, HDL-C, BUN, and Scr), the analysis demonstrated that each 1-SD increase in HSI was associated with an 8% decrease in the probability of reversion to NGR (HR = 0.92, 95% CI: 0.89–0.95, P < 0.0001). Additionally, using the first quartile (Q1) as the reference, the HR and 95% CI for the second (Q2), third (Q3), and fourth (Q4) quartiles were 0.88 (0.82–0.95), 0.88 (0.81–0.96), and 0.80 (0.73–0.88) in Model 3, respectively.
Sensitivity analysis
Table 4 presents a sensitivity analysis for individuals with TC ≤ 5.2 mmol/L and TG ≤ 1.7 mmol/L. The HSI exhibited a significant negative correlation with NGR in participants with TC ≤ 5.2 mmol/L (Model 4; HR: 0.89, 95% CI: 0.86–0.93, P < 0.0001) and TG ≤ 1.7 mmol/L (Model 5; HR: 0.91, 95% CI: 0.88–0.95, P < 0.0001). Similarly, in participants without HSI outliers, a consistent negative association between HSI and reversion to NGR was observed (Model 6; HR: 0.92, 95% CI: 0.89–0.95, P < 0.0001). Additionally, we found that the E-value (1.31) exceeded the relative risk associated with HSI and potential unmeasured confounders (1.13). This suggests that unknown or unmeasured confounders are unlikely to impact the relationship between HSI and reversion to NGR significantly. These supplementary analyses further affirm the robustness of our findings, reinforcing the validity of the observed relationship between HSI and the likelihood of NGR reversion under varying lipid profiles.
Nonlinear relationship between HSI and reversion to NGR
The Cox proportional hazards regression model, utilizing cubic spline functions and smooth curve fitting (as presented in Table 5 and Fig. 3), identified a nonlinear relationship between the HSI and reversion to NGR, with a P-value for nonlinearity of 0.030. An inflection point was detected at an HSI level of 43.08. Below this threshold, the likelihood of reversion to NGR decreased as HSI levels increased (HR: 0.91, 95%CI: 0.88–0.94, P < 0.0001). Conversely, when the HSI level exceeded this inflection point, no significant association was observed between HSI and reversion to NGR (HR: 1.13, 95% CI: 0.95–1.34, P = 0.1794).
The nonlinear relationship between HSI levels and reversion to normoglycemia from prediabetes. A nonlinear relationship between HSI levels and reversion to normoglycemia from prediabetes was detected after adjustment for age, gender, SBP, DBP, drinking status, family history of diabetes, smoking status, FPG, HDL-C, BUN, LDL-C, Scr, and TG.
The results of subgroup analyses
Table 6 details the results of the subgroup analyses. No significant interactions were found in all subgroups, including family history of diabetes, BMI, smoking status, age, drinking status, and gender.
Discussion
In this extensive retrospective cohort study involving Chinese adults with prediabetes, we identified a significant association between lower levels of the HSI and an enhanced likelihood of reversion to NGR. A nonlinear relationship was also observed, demonstrating a stronger correlation at HSI levels ≤ 43.08. Sensitivity analyses and subgroup analyses consistently demonstrated a robust association between HSI and reversion to NGR.
The HSI reflects hepatic fat accumulation, which is closely linked to metabolic dysfunction and insulin resistance. As such, interventions aimed at reducing hepatic steatosis may help lower HSI and improve the likelihood of NGR reversion. Dietary modifications, such as caloric restriction and adherence to a Mediterranean diet rich in unsaturated fats and dietary fiber, have been shown to reduce hepatic fat content and improve insulin sensitivity27. Physical activity, including both aerobic exercise and resistance training, has demonstrated efficacy in reducing hepatic steatosis independent of weight loss28. Pharmacological options, such as insulin sensitizers (e.g., metformin, pioglitazone) and emerging therapies like GLP-1 receptor agonists (e.g., liraglutide), have also shown promise in reducing hepatic fat and improving metabolic outcomes29. Weight loss, achieved through lifestyle interventions or bariatric surgery, remains one of the most effective strategies for reducing hepatic steatosis, with even modest weight loss (5–10% of body weight) leading to significant improvements in liver health and metabolic parameters30. These interventions highlight the clinical relevance of HSI as a modifiable target for improving metabolic health and promoting NGR reversion.
The rate of reversion to NGR identified in our research aligns with prior findings in the literature. Prior research, employing FPG as a reversion to NGR, documented that the rate of reversion to NGR was 42.977% of Chinese individuals with prediabetes within 2.883 years6. Furthermore, a recent systematic review, which encompassed 47 studies focusing on the reversion to NGR, were reported to vary between 33 and 59% over a follow-up period of one to five years31.
In our study, we found that individuals in higher HSI quartiles exhibited a lower prevalence of current alcohol consumption, which contradicts conventional expectations regarding the relationship between alcohol and hepatic steatosis. This observation can be attributed to several factors. First, individuals with higher HSI scores have previously consumed alcohol at higher rates. However, upon recognizing the potential risks associated with their liver health, they may have become more health-conscious, leading to a reduction or cessation of alcohol intake. Second, it is essential to consider the role of obesity and dyslipidemia in the development of hepatic steatosis. Individuals in higher HSI quartiles often present with greater levels of obesity and elevated lipid profiles, which may contribute more significantly to the progression of hepatic steatosis than alcohol consumption alone. Consequently, the effects of alcohol may be overshadowed by these metabolic factors, emphasizing the complexity of the relationship between HSI, alcohol consumption, and liver health.
The accumulation of hepatic lipids is widely recognized as a significant risk factor for the onset of diabetes11,13. The HSI, a crucial indicator of liver fat accumulation, serves as an effective screening tool for MAFLD15. Recent research indicates that HSI is not only associated with liver steatosis but also correlates with prediabetes, diabetes, and insulin resistance17,18,19,32,33. Therefore, we hypothesize that reductions in HSI may increase the likelihood of reverting to NGR in individuals with prediabetes. However, literature exploring this relationship remains limited. A retrospective study involving 14,443 normoglycemic individuals demonstrated that each 1-SD increase in HSI was associated with an 81% higher risk of developing diabetes (HR: 1.81, 95%CI: 1.61–2.03)18. Additionally, a Japanese retrospective study employing propensity score matching, which included 14,280 participants, revealed that individuals diagnosed with MAFLD had a 98% higher risk of diabetes onset (HR: 1.98, 95%CI: 1.41–2.80, P < 0.0001)11. Similarly, a South Korean study involving 13,218 individuals found that those with worsening MAFLD severity faced a substantially higher risk of diabetes compared to those whose MAFLD was in remission (Odds Ratio: 6.13, 95%CI: 2.56–14.68)34. The Diabetes Remission Clinical Trial conducted by Lean et al.35 further demonstrated that early-onset diabetes is characterized by significantly elevated hepatic fat levels, which critically contribute to the persistence of diabetic symptoms. In contrast, achieving diabetes remission is closely linked to substantial reductions in liver and pancreatic fat content, particularly following weight loss interventions35,36. Despite these findings, few studies have specifically investigated the relationship between HSI levels and the transition from prediabetes to NGR. This study provides evidence that lower HSI levels enhance the likelihood of reverting to NGR in Chinese adults with prediabetes. These findings contribute to better public health outcomes and improved management of prediabetes.
The mechanisms precisely linking the HSI to the probability of reverting to NGR are not yet fully elucidated. Excessive accumulation of fat in the liver, as indicated by a high HSI, is associated with increased fatty deposits in β-cells. This accumulation leads to cellular damage and impairs the functional capacity of β-cells, which are crucial for insulin production and glucose regulation37. As hyperglycemia becomes chronic, it exacerbates stress on β-cells, further impairing their function38. Empirical studies indicate that weight loss and caloric restriction contribute to a reduction in ectopic fat. This reduction is associated with restoring β-cell function and improving hepatic insulin sensitivity, potentially facilitating a transition back to NGR36,39,40.
After adjusting for confounding variables, we identified a nonlinear relationship between the HSI and reversion to NGR. Our analysis revealed that when HSI levels were below 43.08, there was a 9% decrease in the likelihood of reversion to NGR for each 1-standard deviation increase in HSI. Conversely, at HSI levels above 43.08, no significant association between HSI and NGR reversion was detected. These findings suggest that lower HSI levels may serve as a predictive indicator for reversion in individuals with higher NGR, highlighting the importance of early lifestyle interventions to enhance long-term outcomes. The nonlinear association between HSI and reversion to NGR, with an inflection point at 43.08, may reflect distinct pathophysiological mechanisms at different levels of hepatic steatosis. Below the threshold, HSI likely captures the degree of hepatic fat accumulation, which is closely linked to insulin resistance and glucose dysregulation37. As hepatic steatosis improves, insulin sensitivity may recover, facilitating NGR reversion41. However, beyond the threshold, the association becomes non-significant, potentially due to the presence of more advanced metabolic dysfunction. At higher HSI levels, additional factors such as chronic inflammation, hepatic fibrosis, and systemic insulin resistance may dominate the metabolic landscape, diminishing the relative contribution of hepatic fat to glucose regulation42. Furthermore, a ceiling effect in the predictive capacity of HSI may occur, as it may no longer capture incremental changes in hepatic fat or metabolic dysfunction relevant to glucose regulation. Individuals with very high HSI values may also represent a distinct population with more severe metabolic comorbidities, such as advanced non-alcoholic steatohepatitis or hypertriglyceridemia43,44, where interventions targeting hepatic steatosis alone may be insufficient to restore normal glucose regulation45. These findings highlight the complex interplay between hepatic steatosis and systemic metabolic health, particularly in advanced disease states.
Our study possesses several notable strengths. Firstly, it is based on a large-scale, multicenter cohort from China, providing comprehensive and robust data. Secondly, our analyses account for a wide array of potential confounders, including lifestyle factors, biochemical markers, and demographic characteristics. Additionally, our investigation identified a nonlinear relationship between the HSI and reversion to NGR. Lastly, we employed some sensitivity analyses to enhance the rigor and validity of our results.
However, there are limitations to our study using secondary data. First, despite our efforts to adjust for known confounders, unmeasured variables such as diet, weight loss, physical activity, medications for prediabetes, or inflammatory markers may have influenced the observed association between HSI and NGR reversion. However, we calculated the E-value of 1.31, exceeding the relative risk of HSI and unmeasured confounders, which was 1.13. This suggests a potential independent relationship between HSI and NGR regression. In the future, we will conduct our own studies and incorporate comprehensive data, including relevant medication history, lifestyle factors, or inflammatory markers, to further validate our findings. Second, HSI measurements were conducted only at baseline, without capturing long-term variations. Future studies incorporating repeated HSI assessments are necessary to validate our findings. Third, reversion to NGR was determined based on FPG levels alone, without considering hemoglobin A1c or oral glucose tolerance tests, which might have led to misclassification. Fourth, the timing and frequency of measurements were predetermined, potentially missing important temporal variations. Fifth, the absence of detailed geographic and socioeconomic data limits our understanding of potential regional influences. Additionally, as our study population consisted exclusively of Chinese adults, the generalizability of our results to other ethnic groups or populations may be limited. Future studies involving diverse populations are needed to collect comprehensive data to confirm our findings. Sixth, the lack of detailed geographic and socioeconomic data in the original dataset limits our ability to explore how regional differences may have influenced our findings. To address this, we plan to design future studies that will collect more granular data, including participants’ specific locations and socioeconomic status. This will allow us to understand better the potential impact of geographic and socioeconomic factors on the relationship between HSI and reversion to NGR. Seventh, given the limited generalizability of our findings to populations with higher baseline HSI levels, future randomized controlled trials in cohorts with more severe hepatic steatosis, such as those with higher obesity rates, are warranted to investigate further the relationship between HSI and reversion to normal glucose regulation and to validate the observed nonlinear association. Lastly, the original study excluded participants with visit intervals of less than two years and did not report cases of reversion to normal glucose regulation during the initial two years of follow-up. In the present study, reversion to normal glucose regulation within the first two years was unavoidable among patients with prediabetes. Therefore, we preferred to exclude participants with less than two years of follow-up, which could have led to selection bias. In future studies, we plan to design a new study that includes participants with follow-up durations of less than two years. This will allow us to evaluate the impact of short-term follow-up on changes in glucose metabolism and provide a more comprehensive understanding of the study population.
Conclusion
Our study demonstrates that lower HSI levels are significantly associated with an increased likelihood of reverting to NGR among Chinese adults with prediabetes when HSI levels fall below 43.08. This finding underscores the importance of managing HSI levels as a critical component of prediabetes management, as it may enhance the probability of achieving NGR.
Data availability
The datasets generated and/or analysed during the current study are available in the DataDryad repository, https://datadryad.org/stash/dataset/doi:10.5061%2Fdryad.ft8750v.
Abbreviations
- NGR:
-
Normal glucose regulation
- MAFLD:
-
Metabolic ssociated fatty liver disease
- HIS:
-
Hepatic steatosis index
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- FPG:
-
Fasting plasma glucose
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipid cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- BUN:
-
Blood urea nitrogen
- Scr:
-
Serum creatinine
- SD:
-
Standard deviation
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- Ref:
-
Reference
References
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes-2023. Diabetes Care 46(Suppl 1), S19–S40 (2023).
Cho, N. H. et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018).
Li, Y. et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 369, m997 (2020).
Liu, X., Wu, S., Song, Q. & Wang, X. Reversion from pre-diabetes mellitus to normoglycemia and risk of cardiovascular disease and all-cause mortality in a Chinese population: A prospective cohort study. J. Am. Heart Assoc. 10(3), e19045 (2021).
Chen, W., Hu, H., Cao, C., Liu, D. & Han, Y. Link between remnant cholesterol and the reversion to normoglycemia in Chinese adults with prediabetes: A 5-year cohort study. Sci. Rep. 14(1), 18098 (2024).
Mo, Z., Hu, H., Han, Y., Cao, C. & Zheng, X. Association between high-density lipoprotein cholesterol and reversion to normoglycemia from prediabetes: An analysis based on data from a retrospective cohort study. Sci. Rep. 14(1), 35 (2024).
Mo, Z. et al. Association between non-high-density lipoprotein to high-density lipoprotein ratio and reversion to normoglycemia in people with impaired fasting glucose: A 5-year retrospective cohort study. Diabetol. Metab Syndr. 15(1), 259 (2023).
Bodicoat, D. H. et al. Incident Type 2 diabetes and the effect of early regression to normoglycaemia in a population with impaired glucose regulation. Diabet. Med. 34(3), 396–404 (2017).
Lazo-Porras, M. et al. Regression from prediabetes to normal glucose levels is more frequent than progression towards diabetes: The CRONICAS Cohort Study. Diabet. Res. Clin. Pract. 163, 107829 (2020).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 77(4), 1335–1347 (2023).
Zheng, X. et al. Association between nonalcoholic fatty liver disease and incident diabetes mellitus among Japanese: A retrospective cohort study using propensity score matching. Lipids Health Dis. 20(1), 59 (2021).
Saponaro, C., Gaggini, M. & Gastaldelli, A. Nonalcoholic fatty liver disease and type 2 diabetes: Common pathophysiologic mechanisms. Curr. Diab. Rep. 15(6), 607 (2015).
Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 70(5), 962–969 (2021).
Movahedian, M. et al. Fatty liver index and risk of diabetes incidence: A systematic review and dose-response meta-analysis of cohort studies. Prim. Care Diabet. 14(6), 577–583 (2020).
Lee, J. H. et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42(7), 503–508 (2010).
Meffert, P. J. et al. Development, external validation, and comparative assessment of a new diagnostic score for hepatic steatosis. Am. J. Gastroenterol. 109(9), 1404–1414 (2014).
Cai, X. et al. Hepatic steatosis index and the risk of type 2 diabetes mellitus in china: Insights from a general population-based cohort study. Dis. Markers 2022, 3150380 (2022).
Si, R., Xiao, J., Zheng, K., Yin, Y. & Li, Y. Association between the hepatic steatosis index and risk of incident type 2 diabetes mellitus in the normoglycemic population: A longitudinal prospective study in Japan. Diabet. Metab. Syndr. Obes. 17, 2317–2326 (2024).
Aizawa, T. et al. Hepatic steatosis and high-normal fasting glucose as risk factors for incident prediabetes. J. Endocr. Soc. 6(9), c110 (2022).
Chen, Y. et al. Association of body mass index and age with incident diabetes in Chinese adults: A population-based cohort study. BMJ Open 8(9), e21768 (2018).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabet. Care 37(1Suppl_1), S81–S90 (2014).
Classification and Diagnosis of Diabetes. Standards of medical care in diabetes-2022. Diabet. Care 45(Suppl 1), S17–S38 (2022).
White, I. R., Royston, P. & Wood, A. M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 30(4), 377–399 (2011).
Groenwold, R. H. et al. Missing covariate data in clinical research: When and when not to use the missing-indicator method for analysis. CMAJ 184(11), 1265–1269 (2012).
Huang, Z. et al. Triglyceride to high-density lipoprotein cholesterol ratio is associated with regression to normoglycemia from prediabetes in adults: A 5-year cohort study in China. J. Transl. Med. 21(1), 868 (2023).
Li, Y. et al. Association between triglyceride glucose-body mass index and non-alcoholic fatty liver disease in the non-obese Chinese population with normal blood lipid levels: A secondary analysis based on a prospective cohort study. Lipids Health Dis. 19(1), 229 (2020).
Zelber-Sagi, S., Salomone, F. & Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 37(7), 936–949 (2017).
Hallsworth, K. et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60(9), 1278–1283 (2011).
Cegla, J. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomized, placebo-controlled phase 2 study. Ann. Clin. Biochem. 53(4), 518 (2016).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149(2), 367-378.e5 (2015).
Richter, B., Hemmingsen, B., Metzendorf, M. I. & Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 10(10), D12661 (2018).
Kahl, S. et al. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS ONE 9(4), e94059 (2014).
Chen, C. et al. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011–2014). Ecotoxicol. Environ. Saf. 218, 112295 (2021).
Sung, K. C., Wild, S. H. & Byrne, C. D. Resolution of fatty liver and risk of incident diabetes. J. Clin. Endocrinol. Metab. 98(9), 3637–3643 (2013).
Lean, M. E. et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 391(10120), 541–551 (2018).
Taylor, R. et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab. 28(4), 547–556 (2018).
London, A., Lundsgaard, A. M., Kiens, B. & Bojsen-Moller, K. N. The role of hepatic fat accumulation in glucose and insulin homeostasis-dysregulation by the liver. J. Clin. Med. 10(3), 390 (2021).
Ferrannini, E. et al. beta-cell function in obesity: Effects of weight loss. Diabetes 53(Suppl 3), S26–S33 (2004).
Romero-Gomez, M., Zelber-Sagi, S. & Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 67(4), 829–846 (2017).
Lim, E. L. et al. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54(10), 2506–2514 (2011).
Petersen, K. F. et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54(3), 603–608 (2005).
Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184(10), 2537–2564 (2021).
Khani, V., Momeni, M. A. & Hatami, B. Comparison of hepatic steatosis index as noninvasive diagnostic tool and liver ultrasound for non-alcoholic steatosis in the adult population. Gastroenterol. Hepatol. Bed. Bench. 15(4), 360–365 (2022).
Fennoun, H. et al. Interest of hepatic steatosis index (HSI) in screening for metabolic steatopathy in patients with type 2 diabetes. Pan. Afr. Med. J. 37, 270 (2020).
Genua, I. & Cusi, K. Pharmacological approaches to nonalcoholic fatty liver disease: Current and future therapies. Diabet. Spectr. 37(1), 48–58 (2024).
Author information
Authors and Affiliations
Contributions
Haiyong Zeng, Fei Yu, Haofei Hu, and Yong Han contributed to the concept and design of the study and drafted the manuscript. Changchun Cao and Ruixue Ye analyzed the data and reviewed the manuscript. Haiyong Zeng, Fei Yu, Haofei Hu, and Yong Han oversaw the project’s progress, contributed to the discussion, and reviewed the manuscript. Changchun Cao and Ruixue Ye are the guarantors of this work and, as such, had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The original study received approval from the Rich Healthcare Group Review Board. Furthermore, the Review Board waived the requirement for informed consent for this retrospective study. Additionally, our study received approval from the Clinical Research Ethics Committee of Shenzhen Second People’s Hospital Dapeng New District Nan’ao Hospital. We ensured full compliance with the principles of the Declaration of Helsinki, as well as all relevant guidelines and regulatory standards.
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, H., Yu, F., Hu, H. et al. Nonlinear relationship between hepatic steatosis index and reversion to normal glucose regulation in Chinese adults with prediabetes. Sci Rep 15, 4269 (2025). https://doi.org/10.1038/s41598-025-88314-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88314-8
Keywords
This article is cited by
-
Non-linear relationship between the hepatic steatosis index and glycemic progression: evidence from a large-scale retrospective cohort study in a Chinese population
BMC Gastroenterology (2025)
-
Spatially-informed interpolation for reconstructing lake area time series using semantic neighborhood correlation
Scientific Reports (2025)