Abstract
Post-cardiac arrest brain injury (PCABI), as the main cause of high mortality and long-term disability in patients, induces mitochondrial damage and cell apoptosis. Hypothermia is well-known as an effective neuroprotective therapy, but its underlying mechanisms deserve further exploration. Previous study has demonstrated that hypothermia provides neuroprotection via increasing PINK1/Parkin-mediated mitophagy. However, whether hypothermia can regulate both apoptosis and mitophagy through the PINK1/Parkin-VDAC3 signaling pathway or not. In this study, BV2 mouse microglial cells were cultured under oxygen-glucose deprivation for 6 h following reperfusion with or without hypothermia for 2–4 h. Cell viability was examined by trypan blue stain. Mitophagy was observed by transmission electron microscope. Mitochondrial membrane potential (MMP) and mitochondrial permeability transition pore (mPTP) opening were determined respectively by JC-1 staining and BBcellProbe M61 staining using a flow cytometer. Expression of mitophagy-related proteins (Cleaved PINK1, Parkin, SQSTM1/p62, Beclin-1, LC3B II/LC3B I), apoptosis-related proteins (Bcl-2, Cytochrome C, caspase-3, cleaved caspase3) and VDAC3 were assessed using western blot analysis and quantitative real-time PCR. The interaction between Parkin and VDAC3 was confirmed by immunofluorescence colocalization. The results showed that hypothermia alleviated MMP damage, inhibited mPTP opening, then decreased cell apoptosis and activated mitophagy at 2 h after temperature intervention, which might be mediated by the PINK1/Parkin-VDAC3 signaling pathway. Moreover, the effects of hypothermia were reduced or reversed at 4 h after temperature intervention. In conclusion, the potential mechanisms of hypothermia during oxygen-glucose deprivation/recovery could be summarized as follows:1) At 2 h after temperature intervention, hypothermia provided neuroprotective effects via promoting mitophagy and reducing apoptosis through activating the PINK1/Parkin-VDAC3 signaling pathway. 2) The curative effect of hypothermia was timeliness. At 4 h after temperature intervention, hypothermia aggravated apoptosis through inhibiting Parkin recruitment to mitochondria and aggravating the release of Cyt C through open mPTP.
Similar content being viewed by others
Introduction
Neurological injury resulting from cerebral ischemia-reperfusion (I/R) injury after the successful return of spontaneous circulation (ROSC) is well-known as the main cause of death or long-term disability in patients resuscitated from cardiac arrest (CA)1. CA induces cytotoxicity, apoptosis and excessive mitophagy, promoting post-cardiac arrest brain injury (PCABI)2,3. Currently, numerous studies have revealed that therapeutic hypothermia is the most effective therapy in improving the survival rate and ameliorating the neurological outcome in certain basic researches4and clinical settings5,6. Mechanically, the potential protective effects of hypothermia are attributed to reducing neuronal apoptosis7and regulating mitophagy to an appropriate extent. For instance, hypothermia protects neurocytes from I/R injury after CA via increasing mitophagy8or decreasing excessive mitophagy9, but its deeper mechanism remains incompletely understood.
Mitophagy is a selective autophagy, which eliminates impaired mitochondria with damaged mitochondrial membrane potential (MMP), recycles usable contents and maintains intracellular redox homeostasis10,11. However, persistent or excessive mitophagy causes mitochondrial dysfunction and cell death12. For instance, the apoptosis of neurons is gradually increased with the increase of mitophagy during brain I/R injury2, but the potential mechanisms remain mysterious. Recently, one study has demonstrated that the ubiquitination of voltage-dependent anion channel 1 (VDAC1) via E3 ubiquitin-protein ligase parkin (Parkin), regulates both mitophagy and apoptosis of neurons in parkinson disease13. Another study reveals that hypothermia protects neurocytes from PCABI via increasing PTEN-induced kinase 1 (PINK1) /Parkin-mediated mitophagy8. However, whether the regulation of voltage-dependent anion channel (VDAC) via Parkin is also involved in the neuroprotection of hypothermia has not been investigated.
Voltage-dependent anion channel 3 (VDAC3), as another isoform of VDAC1, plays a significant role in regulating the MMP and mediating metabolites exchange between mitochondria and the cytosol14. Our previous study showed that hypothermia decreased damage of MMP and inhibited cell apoptosis via promoting the ubiquitination of VDAC3 under the condition of oxygen and glucose deprivation-recovery (OGD/R, a model widely used to simulate cell I/R injury after CA)15. Furthermore, VDAC3 is also demonstrated to be ubiquitinated by Parkin16. However, whether the PINK1/Parkin-VDAC3 signaling pathway is involved in the neuroprotection of hypothermia through regulating mitophagy and attenuating apoptosis after PCABI has not been investigated in depth.
In this study, we hypothesized that hypothermia might activate the PINK1/Parkin-VDAC3 signaling pathway, then regulated mitophagy and attenuated apoptosis after PCABI. Based on this study, we proposed to reveal the underlying neuroprotective mechanism of hypothermia for treating PCABI and provided new biomarkers for examining the effects of hypothermia, in order to supply safer and more accurate hypothermia therapy.
Materials and methods
Materials
The materials used in this study were as follows: microglial mouse cell lines (catalog # CL-0493, Procell); high glucose Dulbecco’s modified Eagle’s medium (DMEM; catalog # C12430500BT, Gibco); 10% fetal bovine serum (FBS; catalog # 26010074, Gibco); DMEM without glucose (catalog # 11966025, Gibco); modular incubator chamber (catalog # MTC101, billups-rothenberg); single flowmeter (catalog # SFM3001, billups-rothenberg); dulbecco’s phosphate-buffered Saline (D-PBS, catalog # Lvn1023, livning); 0.4% trypan blue stain (catalog # C0040 solarbio); electron microscope fixing solution, (catalog #G1102, Servicebio); JC-1 MMP Assay (catalog # HY-K0601, medchemexpress); BBcellProbe M61 mPTP detection kit (catalog # BB-48122, BestBio); RIPA Lysis Buffer (catalog # C-1053, Applygen); Phenylmethanesulfonyl fluoride (PMSF; catalog # A1100, Applygen); Protease inhibitor cocktail (catalog # P1265-1, Applygen); NcmBlot blocking buffer (catalog # P30500, Ncmbio); rabbit anti-PINK1 antibody (catalog # 23274-1-AP, proteintech); rabbit anti-Parkin antibody (catalog # 32833, cell signaling technology); mouse anti-Parkin antibody (catalog # 4211, cell signaling technology); rabbit anti-VDAC3 antibody (catalog # 55260-1-AP, proteintech); rabbit anti-Bcl2 antibody (catalog # 26593-1-AP, proteintech); rabbit anti- Beclin-1 antibody (catalog # 3495 S, cell signaling technology); rabbit anti-Beta Actin antibody (catalog # 20536-1-AP, proteintech); rabbit anti-GAPDH antibody (catalog # 10494-1-AP, proteintech); rabbit anti-Cleaved Caspase 3 antibody (catalog # 9664, cell signaling technology); rabbit anti-caspase3 antibody (catalog # 14220, cell signaling technology); rabbit anti- SQSTM1/p62 antibody (catalog # 39749, cell signaling technology); rabbit anti-Cytochrom C antibody (catalog # 10993-1-AP, proteintech); rabbit anti-LC3B antibody (catalog # 43566, cell signaling technology); goat anti-rabbit HRP-conjugated IgG secondary antibody (catalog # ZB-2301, zsbio); Goat anti-Mouse IgG (H + L) Secondary Antibody, DyLight™ 488 (catalog # 35502, Thermo fisher scientific); Goat anti-Rabbit IgG (H + L) Secondary Antibody, DyLight™ 594(catalog # 35560, Thermo fisher scientific); HiScript III RT SuperMix for qPCR (+ gDNA wiper), (catalog # R323-01, Vazyme Biotech); FastPure Complex Cell/Tissue Total RNA Isolation Kit (catalog # RC113-C1, Vazyme Biotech); Taq Pro Universal SYBR qPCR Master Mix (catalog # Q712-03, Vazyme Biotech); QuickBlock™ Blocking Buffer for Immunol Staining (catalog # P0260, Beyotime).
Cell culture and passage
The murine BV2 mouse microglial cells (catalog # CL-0493, Procell) were cultured in 10 cm dishes using high glucose DMEM supplemented with 10% FBS and cultured at 37℃ in a 5% CO2 incubator. The FBS was filtered through 0.22 μm filter unit (Millipore) and stored at −20 °C until use. The BV2 mouse microglial cells were passaged every 2–3 days, and their 3–6 passages were used for subsequent experiments. Cells were seeded into 6 cm dishes (8 × 10 6 cells/dish for trypan blue stain, transmission electron microscope, flow cytometry, western blotting and quantitative real-time PCR, qRT-PCR), 24-well plates (1 × 10 3 cells/well for immunofluorescence colocalization). The cell count, images capture, and quantitative analyses were performed via a double-blinded approach. Each experiment was repeated a total of four-six times.
Establishment of oxygen and glucose deprivation (OGD) and oxygen and glucose deprivation-recovery (OGD/R) models as well as intervention of hypothermia
OGD and OGD/R models of BV2 mouse microglial cells were established as follows. Cells were washed 2-3 times with D-PBS after they were seeded 20–24 h later, and were cultured in DMEM without both glucose and FBS. After that, the cells were placed in a modular incubator chamber, which was subsequently flushed with a 95% N2/5% CO2 gas mixture at 30 L/min for 3–5 min. Finally, the hypoxia chamber was placed in a 37 °C container for 6 h to produce the OGD model according to previous studies17,18. After OGD, the cells were incubated again in DMEM supplemented with glucose and 10% FBS. For normothermia treatment groups (OGD/R-NormoT), the cells were returned to the normoxic incubator (74% N2/21% O2/5% CO2) for 2–4 h at 37 °C. For hypothermia treatment groups (OGD/R-HypoT), the cells were returned to the normoxic incubator (74% N2/21% O2/5% CO2) for 2–4 h at 34 °C. For hypothermia groups (HypoT), cells without any treatment were incubated in the normoxic incubator (74% N2/21% O2/5% CO2) for 2–4 h at 37 °C. For sham group, cells without any treatment from beginning to end were incubated in the normoxic incubator (74% N2/21% O2/5% CO2) at 37 °C. (See Scheme 1.) Cells collected at the experimental termination were used for subsequent experiments.
Evaluation of cell viability assay
The cell viability of BV2 mouse microglia cells was examined by trypan blue stain19. At the experimental termination, cells were collected by digestion with 0.2% trypsin-EDTA and resuspended in 2 ml D-PBS. The suspension mixed with trypan blue stain was tested by cellular counter (Countstar BioLab, China). The values were expressed as the percentage of living cells.
Evaluation of mitophagy by transmission electron microscope (TEM)
BV2 mouse microglia cells were collected by digestion with 0.2% trypsin-EDTA at the experimental termination as above description. After washing once with D-PBS, the collection was fixed with electron microscope fixing solution and 1% osmium tetroxide, dehydrated with acetone, embedded with Ep812 and sliced with an ultramicrotome. Then the slices were stained with uranium acetate and lead citrate. Finally, the ultrastructure of mitophagy was observed by TEM (Hitachi, Japan)20.
Assessment of mitochondrial membrane potential (MMP) and mitochondrial permeability transition pore (mPTP) opening
The MMP was determined according to the instructions of JC-1 MMP Assay15. As described previously, BV2 mouse microglia cells were digested by 0.25% trypsin and resuspended at the experimental termination. Then every group’ s suspension containing 8 × 10 5 cells was volume to 1 ml using high glucose DMEM except for OGD (the suspension of OGD was volume with DMEM without glucose thoroughly) and was stained with 10 µg/ml JC-1 at 37 °C in the dark for 20 min. Subsequently, cells were washed twice with D-PBS, which were immediately measured by a flow cytometer (Beckman Coulter, USA): At the excitation wavelength of 488 nm, the fluorescence intensity of JC-1 at the emission wavelength at 588 nm (PE-Y588A) and that at the emission wavelength at 525 nm (FITC-B525A) were detected. The results were analyzed by CytExpert.
The mPTP opening was determined by BBcellProbe M61 mPTP detection kit21. According to the manufacturer’s instructions, the cell suspensions, same as what mentioned in MMP test, were incubated with BBcellProbe M61 as well as a quencher for 15 min at 37 °C in the dark, followed by centrifugation at 250 g at room temperature for 5 min. Subsequently, cells were washed twice with D-PBS, which were immediately measured by a flow cytometer (Beckman Coulter, USA): At the excitation wavelength of 488 nm, the fluorescence intensity of BBcellProbe M61 at the emission wavelength at 525 nm (FITC-B525A) were detected. The results were analyzed by FlowJo.
Western blotting
BV2 mouse microglia cells digested with 0.2% trypsin-EDTA at the experimental termination were lysed by RIPA lysis buffer containing PMSF and protease inhibitor at 4 ℃ for 30 min. After centrifugation at 15,000 rpm at 4℃ for 10 min, the supernate was collected, and its protein concentration was determined by the BCA protein assay kit and its mixture containing sample loading buffer was denatured by boiling at 100 °C for 15 min. Subsequently, the mixture from every group was subjected to SDS-PAGE gel electrophoresis with a total of 20 ug protein, and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, USA). After being blocked with 5% non-fat milk in TBST buffer for 4 h or blot blocking buffer for 30 min at room temperature, the PVDF membranes were incubated with primary antibody at 4 °C overnight. The primary antibodies used in this study were rabbit anti-PINK1 antibody (1:1,000), rabbit anti-Parkin antibody (1:1,000), rabbit anti-VDAC 3 antibody (1:750), rabbit anti-Bcl2 antibody (1:1,000) rabbit anti-Beta Actin antibody (1:1,000), rabbit anti-GAPDH antibody (1:1,000), rabbit anti-Cleaved Caspase3 antibody (1:500), rabbit anti-caspase3 antibody (1:1,000); rabbit anti- SQSTM1/p62 antibody (1:1,000), rabbit anti-Cytochrom C antibody (1:750), rabbit anti-LC3B antibody (1:1,000). After three washes with TBST buffer, the membranes were incubated with goat anti-rabbit HRP-conjugated IgG secondary antibodies (1:10,000–1:2,000) for 2 h each at room temperature. Protein bands were visualized by ChemiScope Capture (Clinx, China) with electrochemiluminescence. The density of the bands was quantified by using Image J software (version: lmageJ 1.54 g, URL: http://imagej.org).
qRT-PCR
BV2 mouse microglia cells digested with 0.2% trypsin-EDTA at the experimental termination were collected. After washing once with D-PBS, total RNA of each group was firstly extracted according to the instructions of the FastPure Complex Cell/Tissue Total RNA Isolation Kit22. Secondly, the cDNA was synthesized by reverse transcription with a final quality of 1000 ng of RNA according to the instructions of the HiScript III RT SuperMix for qPCR (+ gDNA wiper) kit. Finally, the levels of gene expression were tested by QuantStudio™ 5 (Thermo fisher scientific, USA) according to the instructions of the Taq Pro Universal SYBR qPCR Master Mix kit, and the expression of target genes was calculated by the 2-ΔΔCt method23,24.
The primers of mouse genes were as follows (from 5 ′−3 ′):
Microtubule-associated protein 1 light chain 3B (LC3B)
Forward Sequence TTATAGAGCGATACAAGGGGGAG
Reverse Sequence CGCCGTCTGATTATCTTGATGAG
VDAC3
Forward Sequence CAAAGGGTATGGGTTTGGCAT
Reverse Sequence TTGGTCTCTAGGTTGCCTGAT
PINK1
Forward Sequence CACACTGTTCCTCGTTATGAAGA
Reverse Sequence CTTGAGATCCCGATGGGCAAT
Parkin.
Forward Sequence TCTTCCAGTGTAACCACCGTC
Reverse Sequence GGCAGGGAGTAGCCAAGTT
Caspase3
Forward Sequence GGAGTCTGACTGGAAAGCCGAA
Reverse Sequence CTTCTGGCAAGCCATCTCCTCA
Coiled-coil, moesin-like BCL2-interacting protein (Beclin-1)
Forward Sequence CAGCCTCTGAAACTGGACACGA
Reverse Sequence CTCTCCTGAGTTAGCCTCTTCC
β-actin
Forward Sequence GTGACGTTGACATCCGTAAAGA
Reverse Sequence GCCGGACTCATCGTACTCC
Cytochrome C (Cyt C)
Forward Sequence GAGGCAAGCATAAGACTGGACC
Reverse Sequence ACTCCATCAGGGTATCCTCTCC
Sequestosome1 (SQSTM1/p62)
Forward Sequence GCTCTTCGGAAGTCAGCAAACC
Reverse Sequence GCAGTTTCCCGACTCCATCTGT
B-cell lymphoma-2 (Bcl-2)
Forward Sequence CCTGTGGATGACTGAGTACCTG
Reverse Sequence AGCCAGGAGAAATCAAACAGAGG
GAPDH:
Forward: 5 ′-ACAACTTTGGTATCGTGGAAGG-3,
Reverse: 5 ′-GCCATCACGCCACAGTTTC-3;
Immunofluorescence colocalization of Parkin and VDAC3
BV2 mouse microglia cells were subcultured with 1 × 10 3cells on coverslips in 24-well tissue culture plates. At the experimental termination, cells were fixed in 4% paraformaldehyde (PFA) for 20 min, permeabilized with 0.2% Triton-X-100 for 10 min and incubated in QuickBlock™ Blocking Buffer for Immunol Staining for 1 h at room temperature in turn. Subsequently, cells were incubated with primary antibodies containing mouse anti-Parkin antibody (1:200) and rabbit anti-VDAC3 antibody (1:200) overnight at 4 °C and secondary antibodies containing Goat anti-Mouse IgG (H + L) Secondary Antibody (DyLight™ 488) (1:500) and Goat anti-Rabbit IgG (H + L) Secondary Antibody (DyLight™ 594) (1:500) for 1 h at room temperature in the dark. Then cells were incubated with 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) solution for 10 min at room temperature in the dark. During this process, cells were washed thrice with D-PBS before the next operation. Finally, the prepared coverslips were covered on slides and were observed under LSM710 laser scanning confocal microscope (Carl Zeiss). The analysis of immunofluorescence colocalization was quantified by using the Image J Colocalization Coloc 2 plugin with Pearson’s coefficient25 (version: lmageJ 1.54 g, URL: http://imagej.org).
Statistical analysis
Data were presented as mean ± standard deviation (mean ± SD) from n ≥ 3 independent experiments and were analyzed by one-way analysis of variance (ANOVA) using GraphPad Prism software 9.3.1. P < 0.05 was defined as significant difference. Values of mean ± SD of all experimental tests for all study groups in this study were presented in the tables of supplementary materials 1.
Results
The effect of hypothermia on cell viability in model of OGD/R-induced BV2 mouse microglia cells damage
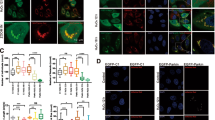
As shown in Fig. 1, the cell viability of both OGD and OGD/R groups was significantly decreased compared with the sham group (P < 0.05), and there were no significant differences between the sham group and the HypoT groups. Compared with the OGD group, the cell viability of the OGD/R groups was further significantly decreased (P < 0.05). Meanwhile, the cell viability of the OGD/R-HypoT group was significantly increased compared with the OGD/R-NormoT group at 2 h after hypothermia intervention (P < 0.05), but there were no significant differences between the OGD/R-HypoT group and the OGD/R-NormoT group at 4 h after hypothermia intervention. Hence, these data indicated that hypothermia protected cell viability from OGD/R injury compared with normothermia at 2 h after temperature intervention. However, both hypothermia and normothermia further impaired cell viability at 4 h after temperature intervention under the condition of OGD/R.
Effects of hypothermia on cell viability in model of OGD/R-induced BV2 mouse microglia cells damage. Cell viability was determined by trypan blue stain. (A) The histogram of every group’ s cell viability at 2 h after temperature intervention. (B) The histogram of every group’s cell viability at 4 h after temperature intervention. (C) The line chart of cell viability. Data was presented as the mean ± SD ( n ≥ 3). * P < 0.05, vs. Sham group; # P < 0.05, vs. OGD group; & P < 0.05, vs. OGD/R-NormoT-2 h groups; $ P < 0.05, vs. OGD/R-NormoT-4 h group.
The effects of hypothermia on mitophagy and apoptosis in model of OGD/R-induced BV2 mouse microglia cells damage
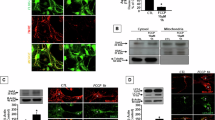
TEM was used to monitor whether mitophagy was involved in the neuroprotection of hypothermia during OGD/R. As shown in Fig. 2A, the results suggested that the mitochondrial membrane was intact and the mitochondrial cristae was clear in the sham group. Compared with the sham group, the mitochondria of the HypoT groups were unbroken, but the mitochondria of the OGD-related groups were broken. The broken mitochondria manifested as mitochondrial swollen, mitochondrial matrix electron density decreased, mitochondrial cristae fractured and mitochondrial membrane rifted. Compared with the OGD group, the damage of mitochondria was increased and the mitophagy was decreased in the OGD/R-NormoT groups and the OGD/R-HypoT-4 h group. Nevertheless, the OGD/R-HypoT-2 h group increased the level of mitophagy and alleviated the damage of mitochondria compared with the OGD/R-NormoT-2 h group. Furthermore, western blotting and qRT-PCR were used to determine whether mitophagy and/or apoptosis induced by hypothermia are involved in the neuroprotection during OGD/R. Compared with the OGD/R-NormoT-2 h group, hypothermia significantly promoted the ratio of LC3B II/ LC3B I and the expression of LC3B, and reduced the ratio of cleaved caspase3/caspase3 as well as the expression of cleaved caspase3 at the protein level at 2 h after temperature intervention, which suggested hypothermia promoted mitophagy26,27,28and reduced apoptosis29,30 according to previous studies. However, the ratios of LC3B II/ LC3B I and LC3B at the protein level between the OGD/R-HypoT-4 h group and the sham group were insignificant. The ratios of cleaved caspase3/caspase3 and cleaved caspase3 in the OGD/R- HypoT-4 h group were significantly higher than the sham group and the OGD/R-NormoT-4 h group at the protein level (Fig. 2B-J). Hence, these data suggested that hypothermia did not improve mitophagy but aggravated apoptosis at 4 h after temperature intervention. At the mRNA level, hypothermia also significantly promoted the expression of LC3B compared with normothermia at 2 h after temperature intervention (Fig. 2K-L). Thus, these data proposed that hypothermia promoted mitophagy and alleviated apoptosis compared with normothermia at 2 h after temperature intervention under the condition of OGD/R, but aggravated apoptosis and did not improve mitophagy at 4 h after temperature intervention.
Effects of hypothermia on mitophagy and apoptosis in model of OGD/R-induced BV2 mouse microglia cells damage. (A) The representative electron micrographs of mitochondria were observed by TEM. Red arrow indicated mitophagy-like event, double yellow arrow represented severe broken mitochondria, yellow arrow represented severe broken mitochondria, blue arrow represented normal mitochondria. Scale bar = 500 nm. (B-J) The western bands of LC3B I, LC3B II, cleaved caspase 3, caspase3, β-actin and GAPDH, as well as the quantitative analysis. (K-L) The mRNA expression of LC3B and the quantitative analysis. Data was presented as the mean ± SD (n ≥ 3). * P < 0.05, vs. Sham group; # P < 0.05, vs. OGD group; & P < 0.05, vs. OGD/R-NormoT-2 h groups; $ P < 0.05, vs. OGD/R-NormoT-4 h group.
The effects of hypothermia on MMP and mPTP opening in model of OGD/R-induced BV2 mouse microglia cells damage
Flow cytometry was performed to investigate whether MMP and mPTP opening were involved in the neuroprotection of hypothermia during OGD/R. As shown in Fig. 3, the MMP of the OGD/R groups detected by JC-1 assay was significantly decreased compared with the sham group. Nonetheless, hypothermia significantly alleviated the damage of MMP at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group, but the protective effect of hypothermia was reversed at 4 h after temperature intervention compared with the OGD/R-NormoT-4 h group. Moreover, compared with the sham group, the opening of mPTP tested by BBcellProbe M61 assay was significantly excessively activated during OGD/R groups except the OGD/R-HypoT-2 h group and the HypoT-2 h group. Hypothermia significantly inhibited the opening of mPTP at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group, but the protective effect of hypothermia was reversed at 4 h after temperature intervention compared with the OGD/R-NormoT-4 h group. Similarly, the expression of VDAC3, as an important component of mPTP, also was significantly increased during the condition of OGD/R. Hypothermia also significantly reduced the expression of VDAC3 at both protein and mRNA levels at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group. However, the expression of VDAC3 between the OGD/R-HypoT group (2 h: 0.9619 ± 0.1595, 4 h: 0.961 ± 0.1402) and the OGD/R-NormoT-4 h group (1.029 ± 0.1809) was insignificant, and was significantly higher than the sham group. Thus, these data suggested that hypothermia might reduce mPTP opening via decreasing the expression of VDAC3, alleviating MMP damage at 2 h after temperature intervention under the condition of OGD/R. At 4 h after temperature intervention, hypothermia could not maintain the inhibition of VDAC3 expression under the condition of OGD/R. Conversely, hypothermia aggravated mPTP opening via the high expression of VDAC3, magnifying MMP damage at 4 h after temperature intervention under the condition of OGD/R.
Effects of hypothermia on MMP and mPTP opening in model of OGD/R-induced BV2 mouse microglia cells damage. (A) The scatter diagram of MMP detected by flow cytometry. Under normal conditions, JC-1 aggregated in the mitochondrial matrix to multimer. When MMP was reduced, JC-1 could not aggregate in the mitochondrial matrix, but existed as a monomer. (B-C) The quantitative analysis of damaged MMP percentage. (D, F) The peak superposition diagram of mPTP opening tested by flow cytometry. Under normal conditions, the opening of mPTP was not excessively activated, and the fluorescence of BbcellProbe M61 was relatively higher. When mitochondria were damaged, the opening of mPTP was excessively activated, and the fluorescence of BbcellProbe M61 was relatively lower as well as the representative diagram shifts left compared with the base line. (E, G) The quantitative analysis of the mean fluorescence of BbcellProbe M61, which represented the opening of mPTP. (H-J) The western bands of VDAC3 and the quantitative analysis. (K-L) The mRNA expression of VDAC3 and the quantitative analysis. Data was presented as the mean ± SD (n ≥ 3). * P < 0.05, vs. Sham group; # P < 0.05, vs. OGD group; & P < 0.05, vs. OGD/R-NormoT-2 h groups; $ P < 0.05, vs. OGD/R-NormoT-4 h group.
The effects of hypothermia on mitophagy-related and apoptosis-related proteins in model of OGD/R-induced BV2 mouse microglia cells damage
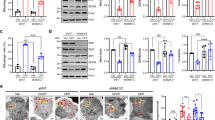
The PINK1/Parkin pathway plays a vital role in mitophagy activation31. Specifically, PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and SQSTM1/p6232,33. In this study, we used western blotting and qRT-PCR to explore whether these mitophagy-related proteins (Cleaved PINK1 (cPINK1), Parkin, SQSTM1/p62, Beclin-1) and apoptosis-related proteins (Bcl-2, Cyt C) were involved in the neuroprotection of hypothermia during OGD/R. As shown in Fig. 4, at protein levels, hypothermia significantly promoted the expression of cPINK1, Parkin, Beclin-1 and Bcl-2, as well as reduced the expression of SQSTM1/p62 and Cyt C at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group, which suggested that hypothermia activated mitophagy and reduced apoptosis during OGD/R. Compared with the OGD/R-NormoT-4 h group, the OGD/R-HypoT-4 h group significantly promoted the expression of cPINK1, Parkin, Bcl-2 and Cyt C. Compared with the sham group, the HypoT group significantly promoted the expression of cPINK1, Parkin, and Bcl-2.
(A-M) The western bands of cPINK1, Parkin, Beclin-1, Bcl-2, SQSTM1/p62 and Cyt C, as well as the quantitative analysis. Data was presented as the mean ± SD (n ≥ 3). * P < 0.05, vs. Sham group; # P < 0.05, vs. OGD group; & P < 0.05, vs. OGD/R-NormoT-2 h groups; $ P < 0.05, vs. OGD/R-NormoT-4 h group.
As shown in Fig. 5, at mRNA levels, hypothermia also significantly promoted the expression of Parkin and Bcl-2, as well as reduced the expression of Cyt C at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group. Compared with the OGD/R-NormoT-4 h group, the OGD/R-HypoT-4 h group significantly promoted the expression of Parkin. Compared with the sham group, the HypoT group significantly promoted the expression of Parkin and reduced the expression of Cyt C.
(A-L) The mRNA expression of PINK1, Parkin, Beclin-1, Bcl-2, SQSTM1/p62 and Cyt C, as well as the quantitative analysis. Data was presented as the mean ± SD (n ≥ 3). * P < 0.05, vs. Sham group; # P < 0.05, vs. OGD group; & P < 0.05, vs. OGD/R-NormoT-2 h groups; $ P < 0.05, vs. OGD/R-NormoT-4 h group.
The effects of hypothermia on Parkin-VDAC3 colocalization in model of OGD/R-induced BV2 mouse microglia cells damage
Immunofluorescence colocalization was performed to investigate whether the interaction between Parkin and VDAC3 was involved in the neuroprotection of hypothermia during OGD/R. As shown in Fig. 6, there was visible positive colocalization between Parkin and VDAC3 in each group according to Pearson’s correlation coefficient25,34. Obviously, as shown in merge, hypothermia promoted Parkin recruitment to mitochondria and colocalization with mitochondrial membrane protein VDAC3 at 2 h after temperature intervention compared with the OGD/R-NormoT-2 h group. However, at 4 h after temperature intervention, the recruitment of Parkin was evidently decreased compared to 2 h after temperature intervention.
Effects of hypothermia on Parkin-VDAC3 colocalization in model of OGD/R-induced BV2 mouse microglia cells damage. Subcellular localizations of Parkin (red), VDAC3 (green), and DAPI (blue) were observed. (Scale bars, 20 μm.) Colocalization analysis results: the 2D intensity histogram of colocalization and Pearson’s correlation coefficient (Pearson’s R value).
Discussion
PCABI is recognized as a primary clinical complication contributing to a high mortality rate and long-term disability1. Up to now, there are lack of effective therapies to provide neuroprotection in clinic. Hypothermia was once proved to be neuroprotective, but its implementation was restricted for lack of convincing basic evidence. This study demonstrated that OGD/R reduced MMP and excessively activated the opening of mPTP, inducing cell apoptosis. Hypothermia alleviated MMP damage, inhibited mPTP opening and decreased cell apoptosis at an early stage after temperature intervention, which might be related to the activation of mitophagy via stimulating PINK1/Parkin-VDAC3 signaling pathway. However, with the increase of hypothermia intervention time, the protective effects were lowered, which might be relevant to apoptosis caused by excessive mPTP opening. Thus, an appropriate intervention of hypothermia contributes to better prognoses for patients who suffer from PCABI, and the monitoring of mitophagy/apoptosis-related proteins may provide a both safer and effective hypothermia therapy in clinic.
Accumulating evidence has revealed that upregulation of mitophagy provides intrinsic neuroprotection in I/R-related brain injury35. At 2 h after intervention during OGD/R, this study also observed that hypothermia promoted mitophagy, reduced cell apoptosis and improved cell viability. Differently, hypothermia did not promote mitophagy, and increased cell apoptosis and impaired cell viability at 4 h after intervention during OGD/R. Mechanically, the potential mechanism might be connected with the PINK1/Parkin-VDAC3 signaling pathway.
In recent years, increasing studies have discovered that PINK1/Parkin-mediated mitophagy is activated and provides neuroprotection during cerebral I/R injury8,36. Under the condition of OGD/R, cPINK1, as a mature form of PINK1, launched mitophagy37via recruiting Parkin onto the outer mitochondrial membrane to eliminate damaged mitochondria with MMP loss38,39. Furthermore, the mitophagy mediated by PINK1/Parkin was found to depend on VDAC40and SQSTM/p6232,41. In detail, mitochondrial proteins like VDAC3 were ubiquitinated by Parkin, and then mediated the engulfment of damaged mitochondria by mitophagy via combining with an autophagy receptor SQSTM/p6232,42. Furthermore, SQSTM/p62 bound with microtubule-associated protein 1 light chain 3 (LC3) on the phagophore, and the conversion of LC3B-I to LC3B II induced mitophagy43. Briefly, the decrease of SQSTM/p62 protein expression and the increase of LC3B II/LC3B I ratio represent mitophagy activation during OGD/R44,45. At 2 h after intervention during OGD/R, this study discovered that hypothermia significantly increased the expression of cPINK1 and Parkin, enhanced the ratio of LC3B II/LC3B I, and decreased the expression of VDAC3 and SQSTM/p62. Thus, hypothermia might activate mitophagy via PINK1/Parkin at an early stage. Additionally, this study revealed that hypothermia also significantly increased the recruitment of Parkin to mitochondria. Mitochondrial Parkin then colocalized with mitochondrial membrane protein VDAC3 at 2 h after intervention during OGD/R. Our previous study also proved that hypothermia reduced apoptosis through promoting VDAC3 ubiquitination during OGD/R injury. Consequently, hypothermia might activate mitophagy via stimulating the PINK1/Parkin-VDAC3 signaling pathway at an early stage. Therefore, the PINK1/Parkin-VDAC3 signaling pathway might be a new therapeutic target of hypothermia for PCABI.
As an essential component of mPTP, VDAC3 not only contributed to MMP damage and mitochondria swelling15, but also related to the release of Cyt C (an initial trigger of apoptosis activated the apoptotic executor molecules like caspase 346). During brain ischemia, the release of Cyt C through mPTP opening induced apoptosis47,48. At 2 h after temperature intervention during OGD/R, this study found that hypothermia significantly inhibited mPTP opening and alleviated MMP damage through decreasing the expression of VDAC3. Meanwhile, the inhibition of mPTP opening reduced the release of Cyt C and then restrained apoptosis initiation. Overall, hypothermia reduced apoptosis and promoted mitophagy through activating the PINK1/Parkin-VDAC3 signaling pathway at an early stage, reducing cell death during OGD/R (see Fig. 7).
During OGD/R, the Bcl-2/Beclin-1 complex also plays a vital role in both apoptosis and mitophagy. Studies proved that Beclin-1 dissociated from the Bcl-2/Beclin1 complex activated mitophagy49, and Bcl-2 dissociated from the Bcl-2/Beclin1 complex reduced apoptosis50, alleviating OGD/R injury. At 2 h after intervention during OGD/R, this study also observed that hypothermia promoted mitophagy and reduced apoptosis through increasing the expression of Bcl-2 and Beclin1. Thus, hypothermia might provide neuroprotection through dissociating the Bcl-2/Beclin1 complex, and then reducing apoptosis and promoting mitophagy (see Fig. 7).
Cellular signal pathway diagram of hypothermia at 2 h after temperature intervention. On the one hand, hypothermia promoted the expression of Cleaved PINK1 at first, and then recruited Parkin onto the outer mitochondrial membrane, thereby decreasing the expression of VDAC3 as well as SQSTM/p62, and then increased the ratio of LC3B II/LC3B I and reduced the release of Cyt C via decreasing mPTP opening, subsequently reduced the expression of downstream proapoptotic proteins such as cleaved caspase3 and caspase3 during OGD/R. Finally, hypothermia provided neuroprotective effects via alleviating apoptosis and promoting mitophagy. On the other hand, hypothermia increased the expression of Bcl-2 and Beclin-1 through disrupting the interaction between Bcl-2 and Beclin-1 during OGD/R, alleviating apoptosis and promoting mitophagy in the end. Thus, hypothermia provided neuroprotective effects via activating the PINK1/Parkin-VDAC3 signaling pathway.
In contrast to the neuroprotective effects of hypothermia at 2 h after temperature intervention, this study found that hypothermia exacerbated MMP damage, increased the release of Cyt C through magnifying mPTP opening, and aggravated apoptosis at 4 h after temperature intervention during OGD/R. At 4 h after temperature intervention, hypothermia did not promote mitophagy compared with the sham group, but induced the similar impairment of cell viability compared with normothermia. We hypothesized that the adverse effects of hypothermia might be attributed to the unsustainable inhibition of VDAC3 expression under the condition of OGD/R. The hypothesis was confirmed with these results in this study: at 4 h after temperature intervention, hypothermia increased the expression of cPINK1, Parkin, Beclin-1 and SQSTM/p62, and did not change the expression of VDAC3 compared with normothermia. Hypothermia also did not regulate the ratio of LC3B II/LC3B I compared with the sham group. Meanwhile, the recruitment of Parkin from cytoplasm to mitochondria and the colocalization with mitochondrial membrane protein VDAC3 also were inhibited. As for apoptosis, although hypothermia improved the expression of Bcl-2, it also increased the expression of cleaved caspase3 and caspase3, inducing apoptosis at 4 h after temperature intervention during OGD/R. The cause of apoptosis was discovered that hypothermia magnified mPTP opening via the high expression of VDAC3, and then aggravated the release of Cyt C at 4 h after temperature intervention during OGD/R. Based on the above results and current studies, the reasons why the neuroprotection of hypothermia was attenuated at 4 h after temperature intervention might be associated with the following facts: (1) the recruitment of Parkin from cytoplasm to mitochondria and the colocalization with mitochondrial membrane protein VDAC3 were inhibited; (2) the inhibition of VDAC3 expression was unsustainable; (3) the opening of mPTP via the high expression of VDAC3 was magnified; (4) the release of Cyt C through mPTP opening was aggravated; 4) the expression of SQSTM/p62 was increased and the ratio of LC3B II/LC3B I returned to normal level (see Fig. 8).
Additionally, why the recruitment of Parkin from cytoplasm to mitochondria and the colocalization with mitochondrial membrane protein VDAC3 were inhibited at 4 h after temperature intervention during OGD/R? Numerous studies have found that overexpression of Bcl-2 regulates mPTP opening via preventing the ubiquitination of VDAC during apoptosis. Moreover, overexpression of Bcl-2 has also been shown to inhibit Parkin translocation to the outer mitochondrial membrane51,52. In conclusion, the recruitment of Parkin from cytoplasm to mitochondria was inhibited might be connected to the overexpression of Bcl-2. (see Fig. 8).
Cellular signal pathway diagram of hypothermia at 4 h after temperature intervention. Under the condition of OGD/R, hypothermia inhibited Parkin recruitment to mitochondria and magnified mPTP opening via the high expression of VDAC3, and then aggravated the release of Cyt C through mPTP. Hypothermia also increased the expression of SQSTM/p62 and Beclin-1, but did not change the ratio of LC3B II/LC3B I. Finally, hypothermia induced apoptosis but did not promote mitophagy. The recruitment of Parkin from cytoplasm to mitochondria might be relevant to the overexpression of Bcl-2. (Cleaved PINK1, cPINK1; OMM, outer mitochondrial membrane; IMS, inner mitochondrial stroma; IMM, inner mitochondrial membrane). Red represents the different effects between 2 h and 4 h after hypothermia intervention.
The advantages and disadvantages of our study as follows. Advantages: (1) Proposing a new signaling pathway, which might provide novel biomarkers for creating a both safer and effective hypothermia therapy. (2) Providing novel biomarkers (like VDAC3 or Cyt C) for monitoring and evaluating the therapeutic effects of hypothermia therapy. (3) discovering the adverse effects of hypothermia, in favor of exploring the safest and most effective parameters for hypothermia clinical application. Disadvantages: (1) This study was conducted in vitro instead of in vivo, and there was a difference between species. Further studies on humans/animals are required to identify whether hypothermia provides neuroprotective effects via the PINK1/Parkin-VDAC3 signaling pathway or not. (2) There are many other signaling pathways that are involved in mitophagy and apoptosis, and the deeper mechanisms of mitophagy and apoptosis deserve further exploration. (3) Lack of drug-assisted hypothermia treatments: Drugs assisted with hypothermia might improve outcomes and minimize hypothermia’s adverse effects over extended durations, and the PINK1/Parkin-VDAC3 signaling pathway might be a new target for drug intervention.
Conclusion
The basic mechanisms of hypothermia during OGD/R could be summarized as follows: (1) hypothermia could regulate both apoptosis and mitophagy through the PINK1/Parkin-VDAC3 signaling pathway. (2) At 2 h after temperature intervention, hypothermia provided neuroprotective effects via activating the PINK1/Parkin-VDAC3 signaling pathway. (3) The curative effect of hypothermia was timeliness. At 4 h after temperature intervention, hypothermia aggravated apoptosis through inhibiting Parkin recruitment to mitochondria and aggravating the release of Cyt C through open mPTP. Thus, this study might provide novel biomarkers (like VDAC3 or Cyt C) for monitoring and evaluating the therapeutic effects of hypothermia therapy, in order to supply a both safer and more effective hypothermia therapy.
Data availability
The raw data supporting the conclusions of this article will be available upon request to the corresponding author, without undue reservation, to any qualified researcher.
Abbreviations
- Bcl-2:
-
B-cell lymphoma-2
- Beclin-1:
-
Coiled-coil, moesin-like BCL2-interacting protein
- CA:
-
Cardiac arrest
- cPINK1:
-
Cleaved PINK1
- Cyt C:
-
Cytochrome C
- I/R:
-
Cerebral ischemia-reperfusion
- LC3B:
-
Microtubule-associated protein 1 light chain 3B
- MMP:
-
Mitochondrial membrane potential
- mPTP:
-
Mitochondrial permeability transition pore
- OGD/R:
-
Oxygen and glucose deprivation-recovery
- OGD:
-
Oxygen and glucose deprivation
- Parkin:
-
E3 ubiquitin-protein ligase parkin
- PCABI:
-
Post-cardiac arrest brain injury
- PINK1:
-
PTEN-induced kinase 1
- qRT-PCR:
-
Quantitative real-time PCR
- ROSC:
-
Return of spontaneous circulation
- SQSTM1/p62:
-
Sequestosome1
- VDAC:
-
Voltage-dependent anion channel
- VDAC1:
-
Voltage-dependent anion channel 1
- VDAC3:
-
Voltage-dependent anion channel 3
References
Sandroni, C., Cronberg, T. & Sekhon, M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 47, 1393–1414. https://doi.org/10.1007/s00134-021-06548-2 (2021).
Huang, Y. et al. Mitophagy in the Hippocampus is excessive activated after cardiac arrest and cardiopulmonary resuscitation. Neurochem. Res. 45, 322–330. https://doi.org/10.1007/s11064-019-02916-z (2020).
Xie, B., Gao, X., Huang, Y., Zhang, Y. & Zhu, S. Remote ischemic postconditioning inhibits hippocampal neuronal apoptosis and Mitophagy after cardiopulmonary resuscitation in rats. Shock (Augusta Ga). 55, 74–82. https://doi.org/10.1097/shk.0000000000001596 (2021).
Ye, S. et al. Comparison of the durations of mild therapeutic hypothermia on outcome after cardiopulmonary resuscitation in the rat. Circulation 125, 123–129. https://doi.org/10.1161/circulationaha.111.062257 (2012).
Lascarrou, J. et al. Targeted temperature management for Cardiac arrest with Nonshockable Rhythm. N. Engl. J. Med. 381, 2327–2337. https://doi.org/10.1056/NEJMoa1906661 (2019).
Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 346, 549–556, doi:https://doi.org/10.1056/NEJMoa012689 (2002).
Zhang, B. et al. Temperature variability does not attenuate the Beneficial effects of Therapeutic Hypothermia on Cellular apoptosis and endoplasmic reticulum stress in the cerebral cortex of a Swine Cardiac arrest Model. Neurocrit. Care. 34, 769–780. https://doi.org/10.1007/s12028-020-01083-2 (2021).
Hu, Y. et al. Increased PINK1/Parkin-mediated mitophagy explains the improved brain protective effects of slow rewarming following hypothermia after cardiac arrest in rats. Exp. Neurol. 330, 113326. https://doi.org/10.1016/j.expneurol.2020.113326 (2020).
Yu, S. et al. Inhibition of mitochondrial calcium uniporter protects neurocytes from ischemia/reperfusion injury via the inhibition of excessive mitophagy. Neurosci. Lett. 628, 24–29. https://doi.org/10.1016/j.neulet.2016.06.012 (2016).
Liu, K. et al. Mitophagy in ischaemia/reperfusion induced cerebral injury. Neurochem. Res. 38, 1295–1300. https://doi.org/10.1007/s11064-013-1033-0 (2013).
Konstantinos, P. & Nektarios, T. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 56 https://doi.org/10.1016/j.exger.2014.01.021 (2014).
Jian, Y. et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol. Vitro. 51 https://doi.org/10.1016/j.tiv.2018.05.001 (2018).
Jin, S. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl. Acad. Sci. U S A. 117 https://doi.org/10.1073/pnas.1909814117 (2020).
Lemeshko, V. V. VDAC as a voltage-dependent mitochondrial gatekeeper under physiological conditions. Biochim. Biophys. Acta Biomembr. 1865, 184175. https://doi.org/10.1016/j.bbamem.2023.184175 (2023).
Zhao, S. et al. Hypothermia-Induced Ubiquitination of Voltage-Dependent Anion Channel 3 protects BV2 microglia cells from cytotoxicity following oxygen-glucose Deprivation/Recovery. Front. Mol. Neurosci. 13, 100. https://doi.org/10.3389/fnmol.2020.00100 (2020).
Reina, S., Guarino, F., Magrì, A. & De Pinto, V. VDAC3 as a potential marker of mitochondrial status is involved in Cancer and Pathology. Front. Oncol. 6, 264. https://doi.org/10.3389/fonc.2016.00264 (2016).
Jun, X. et al. Melatonin-induced ApoE expression in mouse astrocytes protects endothelial cells from OGD-R induced injuries. Transl Psychiatry. 10 https://doi.org/10.1038/s41398-020-00864-9 (2020).
Bei, S. et al. Propofol inhibited autophagy through ca(2+)/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol. Med. 24 https://doi.org/10.1186/s10020-018-0054-1 (2018).
Zhao, H. et al. In vitroCryptotanshinone attenuates oxygen-glucose Deprivation/ Recovery-Induced Injury in an model of neurovascular unit. Front. Neurol. 10 https://doi.org/10.3389/fneur.2019.00381 (2019).
Zang, J. et al. Inhibition of PDE1-B by Vinpocetine regulates Microglial exosomes and polarization through enhancing Autophagic Flux for Neuroprotection against ischemic stroke. Front. cell. Dev. Biology. 8, 616590. https://doi.org/10.3389/fcell.2020.616590 (2020).
Hu, F. et al. Berberine inhibits excessive autophagy and protects myocardium against ischemia/reperfusion injury via the RhoE/AMPK pathway. Int. J. Mol. Med. 53 https://doi.org/10.3892/ijmm.2024.5373 (2024).
Gufeng, G. et al. Comprehensive analyses of m6A RNA methylation patterns and related immune microenvironment in idiopathic pulmonary arterial hypertension. Front. Genet. 14 https://doi.org/10.3389/fgene.2023.1222368 (2023).
, J. K, L., T,, D. &, S. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25 https://doi.org/10.1006/meth.2001.1262 (2002).
Alejandro, H. et al. An Optimized Single Nucleotide Polymorphism-Based Detection Method Suggests That Allelic Variants in the 3’ Untranslated Region of RRAS2 Correlate with Treatment Response in Chronic Lymphocytic Leukemia Patients. Cancers (Basel). 15. https://doi.org/10.3390/cancers15030644 (2023).
French, A., Mills, S., Swarup, R., Bennett, M. & Pridmore, T. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 3, 619–628. https://doi.org/10.1038/nprot.2008.31 (2008).
Yun, S. et al. Protection of Taohong Siwu Decoction on PC12 cells injured by oxygen glucose deprivation/reperfusion via mitophagy-NLRP3 inflammasome pathway in vitro. J. Ethnopharmacol. 301 https://doi.org/10.1016/j.jep.2022.115784 (2022).
David, R. Ceramide targets autophagosomes to mitochondria and induces lethal mitophagy. Nat. Chem. Biol. 8 https://doi.org/10.1038/nchembio.1059 (2012).
Anthony, R. Mitochondrial fission and mitophagy are independent mechanisms regulating ischemia/reperfusion injury in primary neurons. Cell. Death Dis. 12 https://doi.org/10.1038/s41419-021-03752-2 (2021).
Shanshan, Q. et al. Silencing PAQR3 protects against oxygen-glucose deprivation/reperfusion-induced neuronal apoptosis via activation of PI3K/AKT signaling in PC12 cells. Life Sci. 265 https://doi.org/10.1016/j.lfs.2020.118806 (2020).
Yang, G. et al. Lysine-specific demethylase 1 inhibition enhances autophagy and attenuates early-stage post-spinal cord injury apoptosis. Cell. Death Discov. 7 https://doi.org/10.1038/s41420-021-00455-7 (2021).
Wade, J., Alban, H., Jin-Mi, H. & O. & Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell. Biol. 19 https://doi.org/10.1038/nrm.2017.129 (2018).
Sven, G. et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell. Biol. 12 https://doi.org/10.1038/ncb2012 (2010).
Liu, Y. et al. Hypoxia-induced GPCPD1 depalmitoylation triggers mitophagy via regulating PRKN-mediated ubiquitination of VDAC1. Autophagy 19, 2443–2463. https://doi.org/10.1080/15548627.2023.2182482 (2023).
Aaron, J. S., Taylor, A. B. & Chew, T. L. Image co-localization – co-occurrence versus correlation. J. Cell Sci. 131 https://doi.org/10.1242/jcs.211847 (2018).
Lingzhi, W., Enqiang, C., Hailin, Z. & Daqing, M. Regulated cell death in hypoxic-ischaemic encephalopathy: recent development and mechanistic overview. Cell. Death Discov. 10 https://doi.org/10.1038/s41420-024-02014-2 (2024).
Zhang, X. et al. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9, 1321–1333. https://doi.org/10.4161/auto.25132 (2013).
Ravanidis, S. & Doxakis, E. RNA-Binding proteins implicated in mitochondrial damage and Mitophagy. Front. cell. Dev. Biology. 8, 372. https://doi.org/10.3389/fcell.2020.00372 (2020).
Jun, L. et al. Targeting neuronal mitophagy in ischemic stroke: an update. Burns Trauma. 11 https://doi.org/10.1093/burnst/tkad018 (2023).
Wu, X. et al. Hydrogen exerts neuroprotective effects on OGD/R damaged neurons in rat hippocampal by protecting mitochondrial function via regulating mitophagy mediated by PINK1/Parkin signaling pathway. Brain Res. 1698, 89–98. https://doi.org/10.1016/j.brainres.2018.06.028 (2018).
Sun, Y., Vashisht, A., Tchieu, J., Wohlschlegel, J. & Dreier, L. Voltage-dependent anion channels (VDACs) recruit Parkin to defective mitochondria to promote mitochondrial autophagy. J. Biol. Chem. 287, 40652–40660. https://doi.org/10.1074/jbc.M112.419721 (2012).
Garcia-Garcia, J. et al. TRIM27 is an autophagy substrate facilitating mitochondria clustering and mitophagy via phosphorylated TBK1. Febs j. 290, 1096–1116. https://doi.org/10.1111/febs.16628 (2023).
Mazure, N. VDAC in cancer. Biochim. et Biophys. Acta Bioenergetics. 1858, 665–673. https://doi.org/10.1016/j.bbabio.2017.03.002 (2017).
Supansa, P. et al. Direct Interaction of ATP7B and LC3B proteins suggests a Cooperative Role of Copper Transportation and Autophagy. Cells 10 https://doi.org/10.3390/cells10113118 (2021).
Mao, Z. et al. Ligustilide ameliorates hippocampal neuronal injury after cerebral ischemia reperfusion through activating PINK1/Parkin-dependent mitophagy. Phytomedicine: Int. J. Phytotherapy Phytopharmacology. 101, 154111. https://doi.org/10.1016/j.phymed.2022.154111 (2022).
Dong, W. et al. IKKα contributes to ischemia-induced autophagy after acute cerebral ischemic injury. Annals Translational Med. 10, 160. https://doi.org/10.21037/atm-22-517 (2022).
Li, J. et al. PGAM5 exacerbates acute renal injury by initiating mitochondria-dependent apoptosis by facilitating mitochondrial cytochrome c release. Acta Pharmacol. Sin. 45, 125–136. https://doi.org/10.1038/s41401-023-01151-1 (2024).
Kadam, A., Jadiya, P. & Tomar, D. Post-translational modifications and protein quality control of mitochondrial channels and transporters. Front. cell. Dev. Biology. 11, 1196466. https://doi.org/10.3389/fcell.2023.1196466 (2023).
Rahi, V. & Kaundal, R. Exploring the intricacies of calcium dysregulation in ischemic stroke: insights into neuronal cell death and therapeutic strategies. Life Sci. 347, 122651. https://doi.org/10.1016/j.lfs.2024.122651 (2024).
Zexiang, D. et al. LncRNA SNHG14 promotes OGD/R-induced neuron injury by inducing excessive mitophagy via miR-182-5p/BINP3 axis in HT22 mouse hippocampal neuronal cells. Biol. Res. 53 https://doi.org/10.1186/s40659-020-00304-4 (2020).
Jin, F. et al. Oxygen-Glucose-Deprivation/Reoxygenation-Induced Autophagic Cell Death depends on JNK-Mediated phosphorylation of Bcl-2. Cell. Physiol. Biochem. 38 https://doi.org/10.1159/000443057 (2016).
Simone, W., Tabitha, J., Ramona, H., Frank, K., Angelika, B. & E. & Kill one or kill the many: interplay between mitophagy and apoptosis. Biol. Chem. 402 https://doi.org/10.1515/hsz-2020-0231 (2021).
Emilie, H., Richard, G., Sean, C., Seamus, J. & P, C. & Bcl-2 family proteins participate in mitochondrial quality control by regulating Parkin/PINK1-dependent mitophagy. Mol. Cell. 55 https://doi.org/10.1016/j.molcel.2014.06.001 (2014).
Funding
This study was supported by the High-Level Public Health Technical Talent Building Program (Discipline Leader-01-01), the Capital’s Funds for Health Improvement and Research (CFH 2022-1-2032), the National Natural Science Foundation of China (820721), and the Beijing Hospitals Authority’s Ascent Plan (DFL20240302).
Author information
Authors and Affiliations
Contributions
L.Z., S.Y., and Z.T. designed this study. L.Z., H.C., C.H. and X.W. conducted this experiment. L.Z., L.A., Z.S. and Z.L. organized the database and performed the sta-tistical analysis. L.Z. wrote the first draft of the manuscript. R.S. and Z.T. revised the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, L., Yang, S., Cui, H. et al. Hypothermia regulates mitophagy and apoptosis via PINK1/Parkin-VDAC 3 signaling pathway during oxygen-glucose deprivation/recovery injury. Sci Rep 15, 4607 (2025). https://doi.org/10.1038/s41598-025-89176-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-89176-w