Abstract
The “Life’s Essential 8” (LE8) is a comprehensive lifestyle assessment tool by the American Heart Association, designed to mitigate cardiovascular disease risk by optimizing lifestyle factors including diet, physical activity, and other health metrics. While individual components of LE8 have been linked to reduced cancer risks, comprehensive studies on LE8 score trajectories over time and their relation to cancer risk are lacking. This study employed the Kailuan cohort, involving 48,330 participants who underwent three health examinations from 2006 to 2010 to determine their LE8 scores. LE8 score trajectories were analyzed using latent mixture modeling, and their association with incident cancer risks was assessed through Cox proportional hazard models, adjusting for confounders such as age, biological sex, and lifestyle behaviors. Three distinct LE8 trajectory patterns were identified: low-stable (21.2%), moderate-stable (49.4%), and elevated-stable (29.4%). Participants with elevated-stable trajectories showed a 21% (HR = 0.79; 95% CI: 0.70–0.90), 27% (HR = 0.73, 95% CI: 0.57–0.92), 51% (HR = 0.49, 95% CI: 0.32–0.77), 31% (HR = 0.69, 95% CI: 0.50–0.97) and 39% (HR = 0.61, 95% CI: 0.39–0.95) reduction in overall, lung, breast, colorectal and liver cancer risk compared to those with low-stable scores. Notably, the protective effect of elevated-stable LE8 scores against breast cancer was pronounced in participants with elevated CRP levels, indicating an interaction between inflammation and LE8 trajectories. Maintaining high and stable LE8 scores significantly associated with reduced cancer risk, underscoring the importance of integrating LE8 into public health and clinical strategies for cancer prevention. Kailuan study, ChiCTR–TNRC–11,001,489. Registered August 24, 2011-Retrospectively registered, http://www.chictr.org.cn/showprojen.aspx?proj=8050.
Similar content being viewed by others
Introduction
The “Life’s Essential 8” (LE8) is a comprehensive lifestyle assessment tool created by the American Heart Association (AHA) to help individuals reduce their risk of cardiovascular diseases (CVD) by evaluating and improving their lifestyle habits1. It encompasses eight key areas: diet quality, physical activity, nicotine exposure, sleep health, body weight, blood glucose, cholesterol levels, and blood pressure. By optimizing these lifestyle factors, individuals can significantly lower their risk of chronic conditions such as heart disease1, stroke2, and non-alcoholic fatty liver disease (NAFLD)3. Good dietary habits, regular physical activity, and high-quality sleep are crucial for managing health metrics like body weight4, blood sugar5, and cholesterol levels6, and maintaining overall metabolic and emotional balance7. Thus, LE8 not only acts as a preventive measure but also as an initiative for promoting comprehensive lifestyle changes, enhancing life quality and longevity by mitigating the incidence of chronic diseases.
Trajectory modeling, which track changes in variables or behaviors over time, offer a dynamic and more accurate alternative to single measurements that only capture data at a specific point8,9. This method is particularly advantageous for lifestyle assessments such as the LE8, given that health-related behaviors and risk factors are subject to fluctuations due to life transitions, health conditions, or aging. By tracking how an individual’s lifestyle and health metrics develop over time, trajectory studies facilitate tailored interventions and circumvent the limitations inherent in basing clinical decisions or lifestyle recommendations on static data10. This approach not only enhances the accuracy and efficacy of health interventions but also contributes to more favorable long-term health outcomes.
While specific research linking the LE8 directly to cancer risk reduction is yet to be established, there is considerable evidence suggesting that each component of LE8 has a profound impact on factors associated with the onset and progression of various cancers11,12. Given these associations, it is reasonable to hypothesize that adherence to LE8 guidelines, which promote overall wellness, could potentially lower cancer risk. More importantly, maintaining an ideal trajectory of LE8 over a long period—emphasizing sustained, high-quality dietary habits, consistent physical activity, and management of body weight and metabolic health—might provide more significant protective benefits against cancer compared to shorter periods of adherence or suboptimal compliance.
Currently, no prospective studies have been conducted to examine the direct relationship between trajectories of the LE8 scores over time and subsequent cancer risks. This study seeks to fill this void by analyzing the association between four-year LE8 score trajectories and the risk of incident cancer in a comprehensive, prospective cohort study.
Materials and methods
Study population
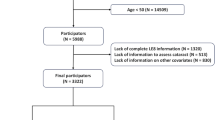
The Kailuan study, which has been detailed in a previous publication13,14, is a prospective cohort study located in Tangshan, southeast of Beijing. Launched in June 2006, the study included 101,510 participants from the Kailuan Group, comprising both active employees and retirees, with the demographic spread consisting of 81,110 men and 20,400 women. The participants were recruited from 11 hospitals linked with the Kailuan community and participated in initial assessments that included questionnaires, clinical exams, and laboratory tests. The study was structured with biennial follow-ups after the initial examination. While the broader study began follow-ups right after the initial 2006 examination, our specific research-initiated follow-up after the 2010 third health examination, using three LE8 measurements from 2006 to 2010 to analyze LE8 score’s trajectories. In the present study, 57,790 individuals underwent health assessments on three health examinations between 2006 and 2010. Participants with a prior cancer diagnosis before the commencement of follow-up in 2010 (n = 779), identified using ICD-10 codes, as well as those with incomplete data on LE8 components (n = 7136) or missing information on other vital covariates (n = 1545), were excluded. Consequently, a total of 48,330 participants were included in the final analysis (see Supplementary Fig. 1). The research protocol received approval from the Ethics Committees of Kailuan General Hospital and Aerospace Center Hospital. All participants gave their written informed consent and did not receive any financial incentive.
Measurement and quantification of LE8 score
The LE8 scores were determined using the algorithm proposed by the AHA for LE8. Assessment included the evaluation of eight metrics—four behavioral (diet, physical activity, smoking, sleep) and four physiological (Body Mass Index [BMI], non-high-density lipoprotein cholesterol [non-HDL-C], blood glucose, and blood pressure)—each scored on a scale from 0 to 100. The overall LE8 score was computed as the arithmetic average of these individual scores. Based on their composite scores, participants were categorized into three levels: high (80–100), moderate (50–79), and low (0–49). Detailed descriptions of the measurements, definitions, and scoring methods are provided in Supplementary Table 1. Data on diet, physical activity, smoking habits, and sleep duration were collected through standardized questionnaires during interviews conducted by trained and certified interviewers. Body measurements for BMI calculations were taken with participants in light attire and without shoes, computed as weight (kg) over squared height (m²). BMI scoring adhered to the World Health Organization standards: 100 for BMI between 18.5 and 22.9, 75 for 23.0 to 24.9, 50 for 25.0 to 29.9, 25 for 30.0 to 34.9, and 0 for a BMI of 35.0 or higher. Blood pressure measurements were taken twice following a five-minute rest with a calibrated mercury sphygmomanometer, and the mean of these measurements was recorded. Fasting for a minimum of eight hours was required before drawing blood for glucose and lipid profiling. Non-HDL-C was calculated by subtracting HDL-C from total cholesterol (TC).
Outcome ascertainment
Cancer cases were identified using multiple methods through December 31, 2022. These included biennial self-reports from participants regarding symptoms or prior diagnoses, medical record examinations from the Tangshan Medical Insurance System, Kailuan Social Security System, and Provincial Vital Statistics, as well as analyses of hospital discharge summaries from 11 affiliated hospitals. All cancer diagnoses were verified by specialists through distinct clinical indicators or histopathological findings from the respective medical facilities. Cancers were categorized according to the International Classification of Diseases, Tenth Revision (ICD-10). Specific categories included head and neck cancers (C00-C14, C30–C32, C71, C73), esophageal (C15), stomach (C16), small intestine (C17), colorectal (C18–C21), liver (C22), gallbladder and extrahepatic bile duct (C23–C24), pancreatic (C25), lung (C34), bone and soft tissue (C40–C41, C49), skin (C43–C44), breast (C50), cervical (C53), uterine (C54–C55), ovarian (C56), prostate (C61), kidney (C64–C65), bladder (C67), lymphomas (C81–C89), leukemia and multiple myeloma (C90–C96). Deaths were also confirmed via the Kailuan Social Security System.
Assessment of confounders
Data on covariates such as age, biological sex, income level (> 685 $ in 2010), educational attainment (≥ high school), daily alcohol intake (100 ml or more), and sedentary behavior (≥ 8 h per day) were gathered through questionnaires at the initial examination. Hypertension was defined by either a systolic blood pressure (SBP) of 140 mmHg or more, a diastolic blood pressure (DBP) of 90 mmHg or more, a prior diagnosis, or the use of antihypertensive drugs. Serum levels of C-reactive protein (CRP), alanine aminotransferase (ALT), and hepatitis B surface antigen (HBsAg) were quantified using a Hitachi 747 autoanalyzer at the central laboratory of Kailuan Hospital. Diabetes was determined based on a fasting blood glucose (FBG) level of 7 mmol/L or higher, previous diabetes diagnosis, or the use of diabetes medications. Other conditions like liver cirrhosis, fatty liver, gallstones, and gallbladder polyps were identified through abdominal ultrasound (PHILIPS HD-15) or via medical records from Tangshan.
Statistical analysis
The primary exposure in this investigation was defined as the changes in LE8 score trajectories from 2006 to 2010. To classify distinct developmental trajectory groups within the study population, latent mixture modeling was utilized via the PROC TRAJ procedure in SAS. The analysis began with a model featuring five trajectory patterns and was progressively compared against models containing four, three, two, and one trajectory pattern. The Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC) were employed to determine the optimal trajectory pattern, with a preference for the model displaying the least negative value. Moreover, models with varying functional forms were assessed, starting from the highest degree polynomial. The process included evaluating the significance of cubic, quadratic, and linear terms to ascertain the most suitable functional form for each trajectory pattern.
Continuous variables were summarized as mean ± standard deviation or medians (interquartile range [IQR]) and analyzed using ANOVA or the Kruskal-Wallis Test, depending on their distribution. Categorical variables were reported as frequencies (percentages) and examined using the Chi-square test. Person-years were calculated from the initial examination (2010) to the end of 2021, or until death or cancer diagnosis, whichever occurred first. The dose-response relationship between LE8 and the overall cancer risk was assessed using restricted cubic splines (RCS) for the years 2006, 2008, and 2010. The hazard ratios (HRs) and 95% confidence intervals (CIs) were computed using Cox proportional hazards models for overall and specific-site cancer risks. Only the first type of cancer diagnosed was considered in the pooled analysis, with site-specific analyses making necessary adjustments for relevant risk factors. Adjustments varied by cancer type, with stratification by sex for sex-specific cancers.
Inflammation and LE8 might synergistically enhance cancer risk15. To explore this relationship, a subgroup analysis was performed based on participants’ CRP levels (< 3 mg/L vs. ≥3 mg/L). Multiple sensitivity analyses were conducted to validate the robustness of the results. Initially, events occurring within the first year of follow-up were omitted to reduce the likelihood of reverse causation. Additionally, individuals taking antihypertensive, hypoglycemic, or lipid-lowering medications were excluded to eliminate potential confounding effects of these treatments on the relationship between LE8 score trajectories and cancer risk. Given the possibility of death preceding a cancer diagnosis, competing risks analysis was implemented, utilizing both a cause-specific hazard (CS) model and a sub-distribution proportional hazard (SD) model16.
Results
LE8 score trajectory patterns
In the ultimate analysis, three distinct trajectories were identified from 2006 (the year of the initial physical assessment) through 2010. We constructed trajectory models with 5, 4, and 3 groups, and evaluated the BIC and AIC values for each model. The BIC values ranged from − 458106.7 to − 435761.2, and the AIC values ranged from − 458047.6 to − 435682.5. After assessing the results, we determined that the three-trajectory model was the best fit, as it provided the most accurate representation of the data (Fig. 1). The low-stable trajectory comprised 10,273 individuals (21.2%) who continuously recorded low LE8 scores. The moderate-stable trajectory was observed in 23,880 participants (49.4%), who consistently showed moderate LE8 scores. The elevated-stable trajectory accounted for 14,257 participants (29.4%), who consistently exhibited high LE8 scores.
Baseline characteristics of the study population by LE8 trajectories
This investigation involved 48,330 participants, comprising 37,372 men (77.33%) and 10,958 women (22.67%), with an average age of 49.21 ± 11.76 years (range 18 to 96 years). Baseline demographic and clinical features, categorized by LE8 score trajectories, are detailed in Table 1. There were significant statistical differences in several baseline variables among the groups, including age, BMI, blood pressure, FBG, TC, HDL-C, TG, CRP, ALT, male sex, family income, educational levels, levels of physical activity, alcohol consumption patterns, and the prevalence of diabetes, hypertension, fatty liver, and gallstone disease, as well as HBsAg seropositivity, with LE8 scores in 2006, 2008, and 2010 (all with p-values < 0.05). Conversely, the occurrence of gallbladder polyps did not show significant variations across the three predefined groups.
The association of LE8 trajectories with overall and specific-site cancer risk
Over a mean follow-up period of 10.39 ± 2.02 years (502,042.57 person-years in total), 2306 new cancer cases were identified. The mean follow-up times for each subgroup were 10.21 ± 2.24 years (104,846.01 person-years), 10.39 ± 2.00 years (247,320.68 person-years), and 10.51 ± 1.87 years (149,875.88 person-years) for the low-stable, moderate-stable, and elevated-stable trajectory groups, respectively. The absolute-count of cancer cases by site can be found in Supplementary Table 2. Figure 2 demonstrates the dose-response curve relating LE8 scores from 2006, 2008, or 2010 to the overall risk of cancer, showing a negative relationship; as LE8 scores increased, the risk of cancer declined. Table 2 outlines how different LE8 score trajectories correlate with cancer risk. In comparison to the low-stable LE8 score trajectory, the moderate-stable and elevated-stable trajectories were linked to a 15% (HR = 0.85; 95% CI 0.77–0.95) and 21% (HR = 0.79; 95% CI 0.70–0.90) reduction in overall cancer risk, respectively, when adjustments were made for confounding factors including age, biological sex, CRP, WC, marital status, family income, sedentary behavior, and alcohol intake. All Cox models, including those for overall cancer, lung cancer, breast cancer, colorectal cancer, and liver cancer, did not violate the proportional hazards (PH) assumption, as confirmed by the Schoenfeld residual global test (all P > 0.05).
Table 3 illustrates the association between LE8 score trajectories and the incidence of specific cancers. When compared with the low-stable LE8 score group, the moderate-stable group had a reduced risk of developing breast and liver cancers by 49% (HR = 0.51, 95% CI: 0.35–0.77) and 34% (HR = 0.66, 95% CI: 0.46–0.95) respectively. Additionally, the elevated-stable group showed lower incidences of lung, breast, colorectal, and liver cancers by 27% (HR = 0.73, 95% CI: 0.57–0.92), 51% (HR = 0.49, 95% CI: 0.32–0.77), 31% (HR = 0.69, 95% CI: 0.50–0.97), and 39% (HR = 0.61, 95% CI: 0.39–0.95), respectively.
In the subgroup analysis, we further explored the impact of CRP levels on the association between LE8 trajectories and the risk of overall and specific-site cancer incidence (Fig. 3). In the group with CRP levels below 3 mg/L, either the moderate or elevated-stable patterns significantly reduced the risk of overall, lung, and colorectal cancers. In the group with CRP levels of 3 mg/L or higher, these patterns significantly lowered the risk of overall, breast, and liver cancers. Importantly, CRP levels significantly modified the relationship between LE8 trajectories and cancer risk (p for interaction < 0.05).
Sensitivity analysis and competing risk analysis
In the sensitivity analysis, we sequentially excluded participants taking antihypertensive, antidiabetic, and lipid-lowering medications to mitigate the potential influence of oral medications on the trajectories of LE8 scores and cancer incidence risks. We also excluded individuals diagnosed with cancer within the first year to address the possibility of reverse causation (Table 4). Our findings indicate that, across all sensitivity analysis models, either moderate-stable or elevated-stable LE8 trajectories significantly reduced the risk of overall, lung, breast, and colorectal cancer. However, the protective effect of LE8 trajectories on liver cancer risk disappeared after excluding those taking antihypertensive drugs or removing individuals diagnosed with cancer in the first year. During the follow-up, a total of 3,463 non-cancer-related deaths occurred, which may act as competing risks, potentially affecting our observations of new cancer cases. We employed the competitive risk models, specifically the CS and SD models, to account for non-cancer-related deaths as competing events. Both in the CS and SD models, the moderate-stable and elevated-stable LE8 trajectories demonstrated significant protective effects against overall, lung, breast, colorectal, and liver cancers (Supplementary Table 3).
Discussion
In this comprehensive, prospective cohort study, we observed that compared to a low-stable LE8 score trajectory over four years, the following associations emerged: (1) a moderate-stable LE8 score trajectory was associated with a reduced risk of overall cancer, as well as specific reductions in breast and liver cancer risk; (2) an elevated-stable LE8 score trajectory was linked to a decreased risk of overall cancer, and more specifically, lung, breast, colorectal, and liver cancers. Furthermore, we found that CRP significantly modified the protective effect of LE8 on breast cancer risk. In individuals with a CRP level greater than 3 mg/L, both moderate-stable and elevated-stable LE8 score trajectories significantly reduced the incidence of breast cancer.
Research concerning the direct implications of LE8 on cancer risk remains limited; however, there is a substantial body of literature examining the relationship between LE8 and various other chronic diseases. Studies like those conducted by Zhang et al. have explored how LE8 correlates with biological aging, a factor indirectly related to cancer risks17. López-Bueno et al. have identified significant associations between optimal cardiovascular health, as defined by LE8, and reduced mortality in cancer survivors, highlighting its potential influence on long-term cancer outcomes18. Moreover, Wang et al. have shown that LE8 metrics are inversely related to the development of non-alcoholic fatty liver disease, another chronic condition that shares common risk factors with various cancers3. Additionally, Li et al. provided insights into how LE8, coupled with genetic susceptibility, affects the incidence of CVD19, while Wu et al. documented the impact of LE8 on reducing stroke risks, emphasizing the broad spectrum of chronic diseases influenced by cardiovascular health2.
While specific studies on the relationship between LE8 and cancer risk are sparse, each element within the LE8 framework has been substantiated as significantly associated with the risk of cancer initiation and development. Dietary patterns rich in nutrients are linked to lowered risks of cancers such as colorectal and breast cancer20,21. Regular physical activity reduces risks notably for colorectal and breast cancer22,23. Nicotine exposure from smoking is robustly associated with cancers of the lung, throat, nasal cavities, paranasal sinuses, nasopharynx, stomach, liver, kidney (renal cell carcinoma), and uterine cervix24. Adequate sleep, maintaining a healthy weight, and managing blood lipids are crucial, as poor management of these is associated with increased risks of breast, kidney, and other cancers25. Additionally, elevated blood glucose levels and diabetes are linked to higher risks of breast, colorectal, endometrial, and gallbladder cancer26, while hypertension is associated with risks for kidney and bladder cancers27. The significant impact of lifestyle and physiological factors on cancer risk underscores the need for a comprehensive approach to health management that integrates both behavioral and medical strategies.
In our study, we found that adherence to LE8 significantly contributes to the prevention of breast cancer onset, particularly among individuals with high levels of inflammation. Chronic inflammation is widely recognized as a catalyst for cancer development, as it promotes genomic instability, alters gene expressions, and fosters a pro-tumorigenic environment28. Our research as shown in Table 1 supports the notion that lifestyle adjustments consistent with LE8—such as maintaining a balanced diet and engaging in regular physical activity—significantly reduce systemic inflammation29. These reductions are crucial, as they correlate with a decreased risk of cancer. Additionally, LE8 components like sufficient sleep and effective stress management also play vital roles in diminishing inflammation, thereby potentially lowering the incidence of cancer-related cellular transformations30,31. This comprehensive approach aligns with existing literature emphasizing the importance of lifestyle changes to mitigate inflammation and reduce the risk of breast cancer, especially in high-risk populations.
The superiority of trajectory research in predicting and managing health outcomes is well-documented across numerous studies. Trajectory analysis, which evaluates the progression of biomarkers over time, provides significant insights beyond static measurements at single time points. For instance, Huang et al. demonstrated that the trajectory of the triglyceride-glucose index significantly predicts stroke incidence among hypertensive patients32. Similarly, Wang et al. showed that changes in waist circumference over time are associated with the incidence of cardiovascular diseases in a Chinese cohort, suggesting that the dynamic measurement provides a more accurate risk assessment than single measurements33. Additionally, Wu et al. highlighted the importance of monitoring the trajectory of cardiovascular health scores to predict future cardiovascular events effectively34. Moreover, Feng et al. explored the trajectory of fasting blood glucose and its link to cancer risk, providing evidence that longitudinal data could reveal risk patterns obscured in cross-sectional analyses35. Lastly, Zhou et al. investigated the combined trajectories of triglyceride-glucose index and lifestyle factors, further substantiating the holistic benefit of considering multiple longitudinal data streams to predict cardiovascular disease outcomes36. These studies collectively underline trajectory research’s enhanced capability to forecast health risks and outcomes, enabling more tailored and effective interventions.
Research on the mechanisms by which LE8 reduces cancer risk remains unclear, but several potential pathways have been suggested. LE8, which encompasses essential lifestyle and health factors such as diet, physical activity, and tobacco use, is believed to modulate cancer risk through multiple biological processes. Firstly, improved dietary habits and increased physical activity can reduce inflammation and oxidative stress, both of which are known contributors to carcinogenesis37,38. Secondly, maintaining a healthy body weight through adherence to LE8 can lower the levels of circulating insulin and insulin-like growth factors, which have been implicated in the development of various cancers39,40. Additionally, smoking cessation, a critical component of LE8, reduces exposure to carcinogens and allows for the recovery of normal cellular processes, thereby decreasing cancer risk41,42. Lastly, controlling blood pressure, cholesterol, and glucose levels, as advocated by LE8, can mitigate the metabolic and hormonal imbalances that facilitate tumor growth43,44. Collectively, these factors illustrate the multifaceted approach through which LE8 may contribute to cancer prevention.
The primary strength of this study lies in its innovative examination of the relationship between LE8 score trajectories and cancer risk, effectively addressing potential confounders including lifestyle factors and histories of cancer-related diseases. The robustness of the findings is further enhanced by the study’s prospective design and large scale. However, several limitations need to be considered. First, the absence of comprehensive data on specific risk factors like hepatitis C virus for liver cancer and Helicobacter pylori for stomach cancer constrains our ability to adjust fully for all confounding variables. Second, the data, sourced solely from the Kailuan community, may not accurately represent the broader Chinese population, limiting the generalizability of the results. Third, due to incomplete dietary data, we relied on indirect measures such as salt intake and tea consumption, which may not fully capture all dietary factors related to the LE8 score2,45. Although the method used to calculate the diet score is unconventional, it was chosen due to insufficient dietary information, rather than using the DASH score. However, there is limited research on the correlation between this method and the DASH score. Future studies should incorporate more detailed dietary data to better assess dietary patterns in relation to LE8. Forth, the impact of LE8 on breast cancer risk may be limited by the exclusion of factors such as age at menarche, breastfeeding history, and parity, which were not considered in this study. These factors are known to influence breast cancer risk, and their omission restricts the ability to fully assess the relationship between LE8 and breast cancer. Future research should incorporate these variables to provide a more comprehensive understanding of how LE8 influences breast cancer risk. Additionally, one limitation of this study is the reliance on self-reported data for key lifestyle factors such as diet, smoking, and alcohol consumption. These factors are known to be subject to recall bias and social desirability bias, potentially leading to inaccuracies in the reported information. Lastly, the observed flat trajectory patterns over the four-year study period, suggesting minimal changes in LE8 scores, could be influenced by the relatively healthier profile of the cohort compared to the general population.
Conclusions
This study emphasizes that a consistent high-stable trajectory in LE8 scores can substantially lower the incidence of various types of cancer, such as lung, breast, colorectal, and liver cancer. The results advocate for the integration of LE8 into public health and clinical cancer prevention frameworks, highlighting the health benefits of long-term commitment to LE8-guided lifestyle changes. This reinforces the value of adopting LE8 as a foundational element in preventive healthcare strategies, aiming to achieve significant improvements in public health outcomes.
Data availability
Data will be made available upon reasonable request (Zhao Li, E-mail: goodlizhao@sina.com).
References
Lloyd-Jones, D. M. et al. Life’s essential 8: updating and enhancing the American heart association’s construct of cardiovascular health: A presidential advisory from the American heart association. Circulation 146 (5), e18–e43 (2022).
Wu, S. et al. Life’s essential 8 and risk of stroke: a prospective Community-Based study. Stroke 54 (9), 2369–2379 (2023).
Wang, L., Yi, J., Guo, X. & Ren, X. Associations between life’s essential 8 and non-alcoholic fatty liver disease among US adults. J. Translational Med. 20 (1), 616 (2022).
de Hoogh, I. M. et al. A novel personalized systems nutrition program improves dietary patterns, lifestyle behaviors and Health-Related outcomes: results from the habit study. Nutrients 13(6), 1763 (2021).
Sampath Kumar, A. et al. Exercise and insulin resistance in type 2 diabetes mellitus: a systematic review and meta-analysis. Annals Phys. Rehabilitation Med. 62 (2), 98–103 (2019).
Mann, S., Beedie, C. & Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. (Auckland NZ). 44 (2), 211–221 (2014).
Wickham, S. R., Amarasekara, N. A., Bartonicek, A. & Conner, T. S. The big three health behaviors and mental health and Well-Being among young adults: A Cross-Sectional investigation of sleep, exercise, and diet. Front. Psychol. 11, 579205 (2020).
Liu, T. et al. C-reactive protein trajectories and the risk of all cancer types: a prospective cohort study. Int. J. Cancer. 151 (2), 297–307 (2022).
Deng, L. et al. The association of metabolic syndrome scores trajectory patterns with risk of all cancer types. Cancer 130 (12), 2150–2159 (2024).
Mésidor, M., Rousseau, M. C., O’Loughlin, J. & Sylvestre, M. P. Does group-based trajectory modeling estimate spurious trajectories? BMC Med. Res. Methodol. 22 (1), 194 (2022).
Samet, J. M. Tobacco smoking: the leading cause of preventable disease worldwide. Torac. Surg. Clin. 23 (2), 103–112 (2013).
Soerjomataram, I. & Bray, F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat. Reviews Clin. Oncol. 18 (10), 663–672 (2021).
Liu, C. et al. New-Onset age of nonalcoholic fatty liver disease and cancer risk. JAMA Netw. Open. 6 (9), e2335511 (2023).
Liu, C. et al. Predicted lean body mass trajectories, and cancer risk and cancer-specific and all-cause mortality: a prospective cohort study. J. Cachexia Sarcopenia Muscle. 14 (6), 2916–2924 (2023).
Liu, T. et al. The combination of metabolic syndrome and inflammation increased the risk of colorectal cancer. Inflamm. Research: Official J. Eur. Histamine Res. Soc. [et al]. 71 (7–8), 899–909 (2022).
Wolbers, M., Koller, M. T., Witteman, J. C. & Steyerberg, E. W. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiol. (Cambridge Mass). 20 (4), 555–561 (2009).
Zhang, R. et al. Association between life’s essential 8 and biological ageing among US adults. J. Translational Med. 21 (1), 622 (2023).
López-Bueno, R. et al. Global prevalence of cardiovascular risk factors based on the life’s essential 8 score: an overview of systematic reviews and meta-analysis. Cardiovascular. Res. 120 (1), 13–33 (2024).
Li, X., Ma, H., Wang, X., Feng, H. & Qi, L. Life’s essential 8, genetic susceptibility, and incident cardiovascular disease: a prospective study. Arterioscler. Thromb. Vasc. Biol. 43 (7), 1324–1333 (2023).
Grosso, G. et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 75 (6), 405–419 (2017).
Newman, T. M., Vitolins, M. Z. & Cook, K. L. From the table to the tumor: the role of mediterranean and Western dietary patterns in shifting Microbial-Mediated signaling to impact breast cancer risk. Nutrients 11(11), 2565 (2019).
Jurdana, M. Physical activity and cancer risk. Actual knowledge and possible biological mechanisms. Radiol. Oncol. 55 (1), 7–17 (2021).
Oruç, Z. & Kaplan, M. A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 11 (5), 348–366 (2019).
Sasco, A. J., Secretan, M. B. & Straif, K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung cancer (Amsterdam Netherlands). 45 (Suppl 2), S3–9 (2004).
Li, W. et al. Self-reported sleep disorders and the risk of all cancer types: evidence from the Kailuan cohort study. Public. Health. 223, 209–216 (2023).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E. & Ioannidis, J. P. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ (Clinical Res. ed). 350, g7607 (2015).
Connaughton, M. & Dabagh, M. Association of hypertension and Organ-Specific cancer: A Meta-Analysis. Healthc. (Basel Switzerland) 10(6), 1074 (2022).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420 (6917), 860–867 (2002).
Yang, Y. C., Johnson, M. P., Schorpp, K. M., Boen, C. E. & Harris, K. M. Young adult risk factors for cancer: obesity, inflammation, and sociobehavioral mechanisms. Am. J. Prev. Med. 53 (3s1), S21–s29 (2017).
Fukui, S., Shimbo, T. & Kobayashi, D. Both increased and decreased sleep duration over time are associated with subsequent cancer development. Sleep. Breath. = Schlaf Atmung. 26 (4), 2035–2043 (2022).
Suh, H. W. et al. The mindfulness-based stress reduction program for improving sleep quality in cancer survivors: a systematic review and meta-analysis. Complement. Ther. Med. 57, 102667 (2021).
Huang, Z. et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: a prospective cohort study. Cardiovasc. Diabetol. 21 (1), 141 (2022).
Wang, L. et al. A prospective study of waist circumference trajectories and incident cardiovascular disease in China: the Kailuan cohort study. Am. J. Clin. Nutr. 113 (2), 338–347 (2021).
Wu, S. et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw. Open. 2 (5), e194758 (2019).
Feng, X. et al. The association between fasting blood glucose trajectory and cancer risk in Chinese population without diabetes. Int. J. Cancer. 147 (4), 958–966 (2020).
Zhou, H. et al. Multi-trajectories of triglyceride-glucose index and lifestyle with cardiovascular disease: a cohort study. Cardiovasc. Diabetol. 22 (1), 341 (2023).
Lee, I. M. et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet (London England). 380 (9838), 219–229 (2012).
Jelic, M. D., Mandic, A. D., Maricic, S. M. & Srdjenovic, B. U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 17 (1), 22–28 (2021).
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London England). 371 (9612), 569–578 (2008).
Vigneri, R., Goldfine, I. D. & Frittitta, L. Insulin, insulin receptors, and cancer. J. Endocrinol. Investig. 39 (12), 1365–1376 (2016).
Alberg, A. J., Brock, M. V. & Samet, J. M. Epidemiology of lung cancer: looking to the future. J. Clin. Oncology: Official J. Am. Soc. Clin. Oncol. 23 (14), 3175–3185 (2005).
Matulewicz, R. S., Sherman, S. & Bjurlin, M. A. Smoking cessation and cancer survivorship. Jama 324 (14), 1475 (2020).
Morrison, F., Shubina, M. & Turchin, A. Encounter frequency and serum glucose level, blood pressure, and cholesterol level control in patients with diabetes mellitus. Arch. Intern. Med. 171 (17), 1542–1550 (2011).
Gutiérrez-Salmerón, M. et al. Metabolic and hormonal remodeling of colorectal cancer cell signalling by diabetes. Endocr. Relat. Cancer. 28 (6), R191–r206 (2021).
Huo, Z. et al. Life’s essential 8 and heart failure among patients with chronic kidney disease: the Kailuan cohort study. Eur. J. Prev. Cardiol. 31 (7), 824–831 (2024).
Acknowledgements
The authors would like to express their gratitude to all the survey teams of the Kailuan study group for their valuable contributions, as well as to the study participants who generously provided their information.
Funding
This work was supported by the Capital Health Research and Development of Special Fund (2022-2-4084), Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0502003), and National Natural Science Foundation of China (82472662) to Zhao Li, and Peking University International Hospital Research Grant (YN2023QN07) to Wenzai Shi.
Author information
Authors and Affiliations
Contributions
All the work reported in the paper has been performed by the authors unless clearly specified in the text. WZS: Methodology, Software, Writing- Original draft preparation; YMW: Writing- Reviewing and Editing; SHC: Supervision, Validation; PCW: Methodology; DM: Data curation, Software; JYZ: Data curation, Investigation; QSZ: Conceptualization, Supervision; ZL: Conceptualization, Supervision, Validation, Resources. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding authors
Ethics declarations
Ethics statement
This study obtained ethical approval from the ethics committees of Kailuan General Hospital and Peking University People’s Hospital, and it adhered to the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from participants or their legal representatives. The Kailuan Study was retrospectively registered in the Chinese Clinical Trial Register on February 12, 2020 (ChiCTR2000029767; https://www.chictr.org.cn/showprojEN.html?proj=48316).
Transparency statement
The manuscript’s guarantor affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shi, W., Wang, Y., Chen, S. et al. The association of life’s essential 8 scores trajectory patterns with the risk of all cancer types. Sci Rep 15, 9600 (2025). https://doi.org/10.1038/s41598-025-94009-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-94009-x