Abstract
Due to insufficient sensitivity of mammography in dense breasts, ultrasonography and MRI have been incorporated into breast screening, despite increasing false positive risks. While optical imaging has been suggested to improve specificity, its effectiveness in diagnosing breast lesions remains unclear. This study investigates the impact of clinical factors on the diagnostic performance of diffuse optical spectroscopic imaging (DOSI) for detecting breast malignancies. Between March and May 2022, 62 women with 62 breast lesions (37 malignant, 25 benign) were analyzed using DOSI to quantify the concentrations of tissue chromophores (HbO2, HHb, water, lipid), total hemoglobin concentration (THC), oxygen saturation (StO2) and tissue optical index (TOI). The ratio of each parameter was compared to those of contralateral normal breasts, and diagnostic performance was assessed using area under the curve (AUC) values. The TOI ratio demonstrated the highest AUC (0.904, 95% CI 0.831–0.977), followed by water (0.836, 95% CI 0.736–0.936) and THC (0.738, 95% CI 0.613–0.864). The TOI ratio showed no significant differences in diagnostic performance based on clinical factors, whereas the water and THC ratios varied according to breast thickness, nipple distance, and BMI. Overall, the TOI ratio demonstrated strong diagnostic performance, suggesting its potential for universal application in breast cancer diagnosis.
Similar content being viewed by others
Introduction
Breast cancer is the most prevalent cancer in women worldwide1. Fortunately, breast cancer screening programs have reduced breast cancer mortality by about 39% compared to non-attendance populations2. Currently, mammography is the gold standard for screening as it enables many women to be screened at low cost in short amounts of time, with several large-scale randomized controlled studies already confirming that it improves the survival rate of breast cancer patients3. However, the sensitivity of mammography decreases to 30–48% in women with dense breasts4. Among potential imaging methods, breast ultrasonography and breast magnetic resonance imaging (MRI) are the most commonly chosen auxiliary imaging tools for breast cancer diagnosis5. While their use increases sensitivity, it also increases false-positive rates, raising concerns about unnecessary biopsies.
Optic imaging has been implemented in breast cancer diagnosis and is thought to improve the specificity of breast imaging6,7,8,9,10,11,12,13,14. Diffuse optical spectroscopic imaging (DOSI) is a non-invasive technique using near-infrared (NIR) region wavelength to measure the concentrations of main tissue chromophores (i.e., deoxy-hemoglobin, oxy-hemoglobin, water, and lipid) and this data informs clinicians about tissue perfusion and metabolism15. Chromophores reflect biological states associated with cellular metabolism, angiogenesis, and the extracellular matrix, and can be used to produce an image indicative of biological states that can forecast lesion status. Using chromophore values, we can also attain a functional image that contains information that can assist in the diagnosis of malignant tumors.
However, it is necessary to establish the performance and decision-making impact of DOSI, particularly regarding clinical factors that can affect a patient’s hemodynamic condition, before its adoption as an adjunctive tool alongside mammography and conventional ultrasound for breast cancer diagnosis. While Leproux et al. recently reported that DOSI shows potential instrument stability by measuring three sources of error (operators, instruments, and calibration standards) for accuracy and precision7, no reports have evaluated the performance of DOSI according to patient characteristics and sonographic parameters.
In this study, DOSI was used to diagnose breast lesions in a total of 62 patients, including both premenopausal and postmenopausal Korean women. We determined how the diagnostic accuracy of each chromophore varied according to clinical variables.
Methods
Study design and participants
This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB No. 1-2022-0002). Signed informed consent was obtained from all participants. All experiments were performed in strict accordance with the relevant guidelines and regulations, including the Declaration of Helsinki. This study was conducted at Severance Hospital from March 2022 to May 2022. During this timeframe, 85 patients were initially recruited based on the following criteria. Inclusion criteria were patients who (a) were 20 years of age or older, (b) had lesions classified as BI-RADS categories 3–5, and (c) had provided consent for biopsy or had a previous biopsy result within the last 2 years. Exclusion criteria were patients who (a) were at risk of pregnancy, were pregnant, or were breastfeeding, (b) had a history of breast trauma or mastitis, (c) had undergone mastectomy or had breast implants, or (d) were photoallergic. Twenty-three patients were excluded from the study for reasons other than the original exclusion criteria: 2 patients declined participation after giving consent or did not have pathology results; 4 patients lacked raw and/or chromophore data; 15 patients had lesions obscured by the areola, leading to artifacts; and 2 patients either had unmarked areola areas during measurement or a lesion not centered in the 4 × 4 grid, making ROI definition impossible. As a result, a total of 62 patients were included in the final data analysis.
Patient characteristics such as age, body mass index (BMI), bra cup size, menstrual state, menstrual phase, and last menstrual period (LMP) were surveyed before examinations were initiated.
Patients underwent mammography and breast parenchyma patterns were recorded. Patients underwent breast ultrasonography that was conducted and reviewed by a radiologist with 20 years of experience specializing in breast imaging. US BI-RADS category, tumor size, distance from skin to tumor, breast thickness, distance from nipple to tumor, and location details were obtained. Tumor diameter was measured along the X, Y, and Z axes, and the longest dimension was selected as the tumor size. The distance between the skin and tumor was measured from the top skin margin to the tumor’s most superficial site. Breast thickness was measured as the distance from the top skin margin to the superficial margin of the pectoralis major muscle. If there were any pathologic results for a benign lesion within 2 years of screening, no additional biopsy was required. Otherwise, patients underwent US-guided biopsy to confirm tumor pathology.
Instrumentation
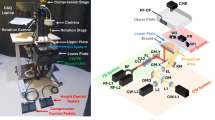
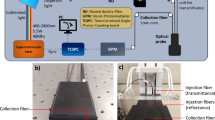
The DOSI device integrates frequency domain photon migration (FDPM) with broadband steady-state (SS) near-infrared (NIR) spectroscopy to perform quantitative, model-based broadband measurements of tissue absorption and reduced scattering spectra across a range from 650 to 1100 nm. Comprehensive details of this system are elaborated in previous literature16,17. In brief, the FDPM segment employs eight laser diodes to sequentially illuminate the breast tissue. Each laser diode, when activated, undergoes intensity modulation across 451 frequencies ranging from 50 to 500 MHz, over approximately 200 ms. The tissue’s diffusely scattered light is captured by a temperature- compensated, hand-held probe containing an avalanche photodiode (Hamamatsu S11519-10). The SS component utilizes a high-powered tungsten-halogen light source (Ocean Insight, HL-2000-HP-FHSA) for tissue illumination, and a grating-based spectrometer (Avantes, AvaSpec HS 2048 XL) to detect light scattering between 650 to 1100 nm. Both FDPM and SS illumination sources are connected to the probe through separate optical fibers, with a 30 mm source-detector separation, optimized for breast measurements and arranged in overlapping geometry.
For data analysis, FDPM and SS data are merged using a diffusion theory framework to yield broadband absorption (μa) and reduced scattering (μs′) spectra in the specified wavelength range. The FDPM analysis records the phase and amplitude of detected light relative to the source’s modulation frequency. These measurements are then fitted to a diffusive light transport model with semi-infinite boundary conditions to ascertain μa and μs′ values for each laser wavelength. The SS reflectance spectra are converted into absolute absorption spectra through a two-step process. Initially, the reduced scattering spectrum is assumed to follow a power law (μs′ = A · λ−b), with 'A' representing the scatter amplitude and 'b' the scatter power. This power-law fitting of FDPM data provides a scattering correction for the SS reflectance spectrum. Subsequently, SS reflectance intensity is scaled using the reflectance derived from FDPM-measured absorption and scattering values. The final step of attaining the absorption spectra involves extracting the absolute absorption spectrum by fitting the adjusted reflectance spectrum to a diffusion reflectance model.
Measurements and spectral analysis
After the mammography and sonography images were evaluated, all patients were measured with DOSI. The instruments and measurement procedures used to obtain images via DOSI are described in detail in a previous study18. In sonography, the location of a tumor was marked with a landmark sticker, and after confirming that there was no lesion by evaluating the symmetrical location of the contralateral breast with sonography, that corresponding site was also marked with a landmark sticker. For DOSI measurements, patients were positioned supine on the examination bed. The measurement grid size was set to 4 cm in width and length based on the lesion location as determined by the sonography examination. Within the measuring range, the probe was moved at 1 cm intervals and measurements were taken. If the areola or dot was within the measurement range, its position was recorded along the horizontal X-axis (line) and vertical Y-axis (channel). A grid was drawn on the normal (contralateral) breast mirroring the grid previously drawn on the breast with the lesion.
To select the ROI (region of interest), in circumstances where the lesion was clearly visible, the ROI was selected as is. In cases including the areola, the areola region was eliminated before selecting the ROI from the remaining location. In cases where the areola was excluded and it was difficult to identify a lesion, the central five areas were also selected as ROIs19.
By fitting a linear combination of known molar extinction coefficient spectra to the measured tissue absorption spectrum, the concentrations of oxy-hemoglobin (HbO2), deoxy-hemoglobin (HHb), water, and lipid were computed. Total hemoglobin concentration (THC = [HbO2] + [HHb]), tissue oxygen saturation (StO2 = [HbO2]/THC), and tissue optical index (TOI = log10(THC· [water]/[lipid])) were determined from these computed concentrations. Previous studies have demonstrated that TOI exhibits an excellent capability for enhancing the contrast and sensitivity of imaging for detecting malignant lesions13,14,20. For lesion visualization, quantitative functional images were created using the biodistribution of chromophores in the tissues of interest.
The lesion-to-normal (L/N) ratio of chromophores was computed as follows. The L/N ratio was calculated by dividing the average measurement at the lesion site by the average measurement at the normal (contralateral) site. Each average was obtained by summing the values of all n spots and dividing by n.
Data collection and statistical analysis
To identify clinical factors that might influence the diagnosis of DOSI, subgroup analyses according to 10 categories were conducted. The subgroups were arbitrarily divided as follows: age (under 50 vs 50 or older), BMI (normal or underweight vs overweight or obese), bra cup size (A vs B-E), menopause status (premenopausal vs perimenopausal or postmenopausal), LMP in premenopausal and perimenopausal subjects (early menstruation phase vs late menstruation phase), maximal diameter of tumor (2 cm or less vs over 2 cm), distance from skin to tumor (6 mm or less vs over 6 mm), breast thickness (2 cm or less vs over 2 cm), distance from nipple to tumor (3 cm or less vsover 3 cm), and BI-RADS category (3 and 4A vs 4B, 4C and 5). The early menstrual phase refers to a period of 15 days or less from the LMP to the date of DOSI measurement, while the late menstrual phase indicates a period exceeding 15 days. The median breast thickness was 2.0 cm (minimum: 1.1 cm, maximum: 3.8 cm) and the median distance from nipple to tumor was 4 cm (minimum: 1 cm, maximum: 8 cm). For categorical clinical factors, the Chi-square test or Fisher’s exact test were used when differentiating benign and malignant groups, while the two-sample t-test or Wilcoxon rank-sum test were used to evaluate the distribution of continuous clinical factors between the benign and malignant groups of each subgroup. The BI-RADS density from mammography was also reviewed.
For the chromophoresL/N values, the number of study subjects, mean, standard deviation, median, minimum, and maximum values for each malignant/benign group were calculated. The Shapiro–Wilk test was performed to validate the normality of distribution for all variables. A two-sample t-test was used for normally distributed variables, while the Wilcoxon rank sum test was performed for non-normally distributed variables to determine whether there were significant differences between groups. In addition, a box plot was provided to demonstrate the distribution of each chromophore type (Fig. 1). The area under the curve (AUC), 95% confidence interval (CI), and p-value from the likelihood ratio test were calculated for differentiating benign/malignant groups within each subgroup. Chi-square statistics with one degree of freedom were calculated using each AUC and standard error was used to compare the difference in AUC between subgroups. The two-sided test was applied in all data analyses, and the statistical significance threshold was set at 0.05. All statistical analyses were performed with SAS (version 9.4, North Carolina, USA).
Results
There were 37 malignant lesions and 25 benign lesions out of 62 total lesions, with the benign lesions including atypical ductal hyperplasia (n = 1), fibrocystic change (n = 5), fibroadenomatoid hyperplasia (n = 4), fibroadenoma (n = 12), fibroepithelial tumor (n = 2), and stromal fibrosis (n = 1). The atypical ductal hyperplasia was categorized as a benign lesion because it was not upgraded after surgical biopsy. Average (SD, range) patient age was 50 years old (11, ranging from 29 to 80). Patients with malignant lesions were on average older than patients with benign lesions; 53 years old (10, ranging from 33 to 80) versus 45 years old (10, ranging from 29 to 71), respectively (p = 0.003). Table 1 shows the distribution of malignant and benign lesions by subgroups divided according to clinical factors. Malignant lesions were more prevalent in older (> 50 years old) and perimenopausal or postmenopausal patients, tended to have larger tumor diameters (> 2 cm), were located further from the nipple (> 3 cm), and were interpreted with higher BI-RADS categories (4B, 4C, and 5) (p < 0.05). In comparison, subgroups categorized by BMI, bra cup size, menstrual phase in premenopausal and perimenopausal patients, distance from skin to tumor, and breast thickness did not significantly differ between malignant and benign lesions. Mammographic density was available in 56 subjects: BI-RADS B in 7, C in 41 and D in 8. The majority of cases (87.5%) were BI-RADS C or D; however, a subgroup analysis according to mammographic density was not done.
Overall, the malignant group had higher average L/N values for THC, HHb, HbO2, water, and TOI compared to the benign group, but a lower average L/N value for lipid (Table 2; Figs. 1, 2 and 3). There was no significant difference in the distribution of StO2 between the malignant and benign groups. In subgroup analyses, the malignant group exhibited greater average TOIL/N values than the benign group for all subgroups. Also, the malignant group exhibited greater average waterL/N values than the benign group for all subgroups except for patients in the late menopausal phase. The StO2_L/N value was not significantly different between the malignant and benign groups for all subgroups. In the subgroups with vascularity, none of the chromophores were statistically different between the malignant and benign groups.
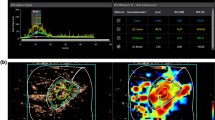
Ultrasonography and DOSI images of a 53-year-old woman with a new lesion detected on screening ultrasonography in the right breast at 9 o’clock, 2 cm from the nipple. The 12 mm hypoechoic lesion with a peripheral echogenic halo was parallel and categorized as BI-RADS 4A. Biopsy confirmed the lesion as a fibrocystic change.
Overall, TOI and water were the top-performing chromophores, with AUCs of 0.904 (95% CI 0.831–0.977) and 0.836 (95% CI 0.736–0.936), respectively (Table 3). Overall, all chromophores except StO2_L∕N showed statistically significant predictive capability for malignancy, with AUCs greater than 0.681. TOIL/N and waterL/N were the two best chromophores to exhibit statistically significant predictions of malignancy in most of the subgroups (17 and 16 out of 18 subgroups, respectively), followed by THCL/N (9 out of 18 subgroups). As for differences in the diagnostic performances of chromophores between the subgroups categorized by clinical factors, TOIL/N did not show any significant difference between any of the subgroups. However, waterL/N showed a statistically significant difference in diagnostic performance according to breast thickness and distance from the nipple. Higher performance was observed in patients whose breasts were thicker than 2 cm compared to those with breasts less than or equal to 2 cm (AUC 0.948 vs 0.738, p = 0.033) and patients in whom the distance between the lesion and nipple was further than 3 cm compared to patients with distances closer or equal to 3 cm (AUC 0.957 vs 0.692, p = 0.011). THCL/N and HbO2_L/N better predicted malignancy in the normal or underweight subgroup (AUC of THCL/N 0.920 vs 0.631, p = 0.012) (AUC of HbO2_L/N 0.890 vs 0.545, p = 0.006) and the late menstruation phase subgroup (AUC of THCL/N 0.920 vs 0.529, p = 0.011) (AUC of HbO2_L/N 0.920 vs 0.543, p = 0.006). The diagnostic performance of lipidL/N was significantly different between patients whose lesions were bigger than 2 cm and those whose lesions were smaller or equal to 2 cm (AUC 0.868 vs 0.506, p = 0.013).
Discussion and conclusions
Malignant lesions show high proliferation and metabolism accompanied by enriched blood supply due to angiogenesis. Theoretically, as water and THC indirectly reflect cellularity and blood supply, they should be elevated in malignant lesions compared to benign lesions. In tumor tissue, not only was an increase in water content found, but also a spectrum shift indicating a change in water-binding states15,20. The expansion of breast tumors leads to the displacement of adjacent adipose tissue, which lowers the lipid content of lesions. Our study demonstrated that malignant lesions had higher lesion-to-normal tissue ratios for THC, water, HbO2 and HHb compared to benign lesions. On the other hand, malignant lesions exhibited lower average lesion-to-normal tissue ratios for lipids compared to benign lesions. TOI, which added water and lipid contrast to enhance lesion detection, was also significantly increased in malignant lesions compared to benign lesions. StO2 was not significantly different between benign and malignant lesions, which is consistent with previous literatures14,15,18. StO2, which is a measure of the balance between oxygen delivery and consumption, may show less pronounced variations in blood oxygenation compared to the changes in tissue oxygenation driven by tumor hypermetabolism. Overall, the majority of chromophores except for StO2 were statistically significant predictions of malignancy, which is consistent with previous literature14. However, Leproux et al.18 found no significant differences in the lipid levels of benign and malignant lesions, and while THC had a higher AUC value than TOI in their study, our study found TOI to have the highest diagnostic performance for breast cancer. These differences may be due to different study demographics and population size. TOI is a calculated value that incorporates not only THC but also information on water and lipids which could have helped differentiate benign and malignant lesions in this study. Therefore, TOI seems to show a higher diagnostic performance for distinguishing benign and malignant lesions compared to THC in this study, a finding seen in both premenopausal and postmenopausal women.
Several researches have suggested that malignancy index and specific tumor component (STC) index can differentiate malignant tumor from benign tumor18,21,22. Leproux et al. demonstrated that the malignancy index exhibited the greatest predictive performance, with an AUC of 0.99 (95% CI 0.97–1.00)18. Further validation is required to confirm its role as a biomarker.
The novelty of our study lies in the subgroup analysis that included clinical factors that could influence the diagnostic performance of chromophores. When comparing chromophores according to patient characteristics and sonographic factors, TOI exhibited statistically significant differences in the mean value of chromophores between benign and malignant groups regardless of patient characteristics and sonographic parameters. Furthermore, it showed no statistically significant difference in diagnostic performance among all subgroups, which suggests that TOI shows excellent potential as a diagnostic parameter that can be applied to the general population for breast cancer. Water was also another reliable factor for differentiating benign and malignant groups in most of the subgroups, except for the two above. As for within subgroup comparisons, water exhibited diverse diagnostic performances for malignancy in patients who showed thin breast thickness (2 cm or less than 2 cm of breast thickness) and lesions that were in close proximity to the nipple (3 cm or less than 3 cm) compared to their respective counter-subgroups (AUC 0.692–0.738, vs 0.948–0.957, respectively). Those conditions may result in a bias from adjacent overlying vasculature within thin breasts or in the subareolar area. However, the impact may be confounded by influences from BMI or breast density and further studies with larger study populations are necessary before these findings can be readily accepted and applied in clinical practice.
The diagnostic performances of THC, HbO2 and HHbwere significantly higher in the late menstrual phase subgroup (AUCs and 95% CIs of THC: 0.920, 0.777–1.000; HbO2: 0.920, 0.777–1.000; and HHb: 0.860, 0.665–1.000, respectively) than the early subgroup (AUCs and 95% CI of THC: 0.529, 0.263–0.794; HbO2: 0.543, 0.374–0.767; and HHb: 0.737, 0.552–0.922, respectively). Even though there was no significant difference in TOI, TOI in the late menstrual phase subgroup still show higher AUC, indicating that the menstrual phase influences the vascularity of breast tissue. A previous study suggested that breast hemoglobin concentrations are elevated in the late menstrual (secretory) phase23, and this may increase the contrast between malignant and benign lesions. Therefore, we recommend that breast cancer screening incorporating the DOSI system be done in the late menstrual phase. The diagnostic performance of TOI was maintained even in the early menstrual phase subgroup (0.857, 0.699–1.000) and overall premenopausal subgroup (0.907, 0.808–1.000). BMI also seemed to have influenced the diagnostic performance of THC. However, bra cup size did not affect the diagnostic performance of any of the seven chromophores. The discrepancy observed between BMI and bra cup size, which were expected to be correlated, may be partly due to inaccurate bra cup size. Breast size was not directly measured but surveyed, which means that patient preferences for certain sizes could have influenced bra cup size. Additionally, the dense breast composition of Asian women may have also contributed to the widening of such differences.
There were some limitations to this study. First, the number of patients was limited and from a single institution. Second, an inhomogeneous distribution of patient characteristics between malignant and benign lesions was noted in our study. Malignant lesions were more prevalent in older (> 50y-o) and perimenopausal or postmenopausal patients than in younger or premenopausal patients. Also, malignant lesions tended to have larger tumor diameters (> 2 cm), be further from the nipple (> 3 cm), and show vascularity on Doppler images. Because of the partial volume effect, larger lesions may have shown a higher contrast relative to normal tissue compared to smaller lesions. Additionally, for lesions located at the same depth, a larger lesion is more likely to be intersected by the diffuse light field than a smaller lesion. While DOSI effectively detects lesions and provides significant physiological contrast with background tissue, its spatial resolution for tumor localization is lower than that of ultrasound24. Consequently, tumor locations were marked following ultrasound evaluation to ensure accuracy. Moreover, because of the inherent characteristics of DOSI, there are difficulties when measuring subareolar lesions and fifteen cases were excluded from data analysis, indicating that our study may not apply to this type of lesion. Lastly, although DOSI has been under research for over two decades and is now studied as a means of tumor assessment, it still remains a laboratory-based technique. Limitations in size and cost pose challenges for the commercialization of DOSI. In the future, cost-effective and miniaturization of essential components with similar performance could lead to its regular use in routine screening or routine clinical practices.
Given the limitations discussed above, advances in artificial intelligence (AI) present promising opportunities for enhancing the clinical application of DOSI. Machine learning algorithms, when applied to combine multiple chromophore data, can uncover intricate, multi-dimensional patterns and can enable more sophisticated analyses, offering a holistic view of the data to improve diagnostic accuracy. Additionally, integrating DOSI-derived chromophore metrics with AI-driven multi-modal analysis has the potential to refine diagnostic outcomes by leveraging complementary imaging modalities. AI algorithms trained on multi-institutional datasets can further overcome variations in DOSI metrics caused by demographic or technological differences, significantly increasing its generalizability across diverse clinical settings.
In this study, we compared the diagnostic performance of DOSI for breast malignancy according to patient characteristics and sonographic factors in premenopausal and postmenopausal women. TOI showed the strongest performance for diagnosing breast malignancy among the 7 chromophores with a high AUC value (0.904, 95% CI 0.831–0.977), with no significant difference in diagnostic performance regarding patient characteristics and sonographic parameters. This result suggests that TOI evaluated with DOSI has the potential to be universally applied to the general population. Future research should encompass a broader range of lesion types, especially benign lesions with vascularity and focus on aligning patient characteristics, such as age, lesion size, and lesion depth. This approach is crucial to gain a deeper understanding of the underlying factors that contribute to contrast in benign and malignant breast tumors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Massat, N. J. et al. Impact of screening on breast cancer mortality: The UK program 20 years on. Cancer Epidemiol. Biomark. Prev. 25, 455–462 (2016).
Gøtzsche, P. C. & Jørgensen, K. J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, Cd001877 (2013).
Freer, P. E. Mammographic breast density: Impact on breast cancer risk and implications for screening. Radiographics 35, 302–315 (2015).
Nounou, M. I. et al. Breast cancer: Conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer (Auckl) 9, 17–34 (2015).
Kukreti, S. et al. Characterization of metabolic differences between benign and malignant tumors: High-spectral-resolution diffuse optical spectroscopy. Radiology 254, 277–284 (2010).
Leproux, A. et al. Performance assessment of diffuse optical spectroscopic imaging instruments in a 2-year multicenter breast cancer trial. J. Biomed. Opt. 22, 121604 (2017).
Tromberg, B. J. et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2, 26–40 (2000).
Franceschini, M. A. et al. Frequency-domain techniques enhance optical mammography: Initial clinical results. Proc. Natl. Acad. Sci. U. S. A. 94, 6468–6473 (1997).
Pogue, B. W. et al. Quantitative hemoglobin tomography with diffuse near-infrared spectroscopy: Pilot results in the breast. Radiology 218, 261–266 (2001).
Taroni, P. et al. Clinical trial of time-resolved scanning optical mammography at 4 wavelengths between 683 and 975 nm. J. Biomed. Opt. 9, 464–473 (2004).
Chance, B. et al. Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: A six-year, two-site study. Acad. Radiol. 12, 925–933 (2005).
Leproux, A. et al. Assessing tumor contrast in radiographically dense breast tissue using Diffuse Optical Spectroscopic Imaging (DOSI). Breast Cancer Res. 15, R89 (2013).
Cochran, J. M. et al. Breast cancer differential diagnosis using diffuse optical spectroscopic imaging and regression with z-score normalized data. J. Biomed. Opt. 26 (2021).
Cerussi, A. et al. In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J. Biomed. Opt. 11, 044005 (2006).
Bevilacqua, F., Berger, A. J., Cerussi, A. E., Jakubowski, D. & Tromberg, B. J. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl. Opt. 39, 6498–6507 (2000).
O’Sullivan, T. D., Cerussi, A. E., Cuccia, D. J. & Tromberg, B. J. Diffuse optical imaging using spatially and temporally modulated light. J. Biomed. Opt. 17, 071311 (2012).
Leproux, A. et al.Differential diagnosis of breast masses in South Korean premenopausal women using diffuse optical spectroscopic imaging. J. Biomed. Opt. 21, 74001 (2016).
Tromberg, B. J. et al. Predicting responses to neoadjuvant chemotherapy in breast cancer: ACRIN 6691 trial of diffuse optical spectroscopic imaging. Cancer Res. 76, 5933–5944 (2016).
Chung, S. H. et al. In vivo water state measurements in breast cancer using broadband diffuse optical spectroscopy. Phys. Med. Biol. 53, 6713–6727 (2008).
Kukreti, S., Cerussi, A., Tromberg, B. & Gratton, E. Intrinsic tumor biomarkers revealed by novel double-differential spectroscopic analysis of near-infrared spectra. J. Biomed. Opt. 12, 020509 (2007).
Pogue, B. W. et al. Characterization of hemoglobin, water, and NIR scattering in breast tissue: Analysis of intersubject variability and menstrual cycle changes. J. Biomed. Opt. 9, 541–552 (2004).
Wang, J. et al. In vivo quantitative imaging of normal and cancerous breast tissue using broadband diffuse optical tomography. Med. Phys. 37, 3715–3724 (2010).
Zhu, Q. et al. Early-stage invasive breast cancers: Potential role of optical tomography with US localization in assisting diagnosis. Radiology 256, 367–378 (2010).
Acknowledgements
MID (Medical Illustration & Design), as a member of the Medical Research Support Services of Yonsei University College of Medicine, providing excellent support with medical illustration.
Funding
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, Republic of Korea, the Ministry of Food and Drug Safety) (Project Number: KMDF202011A01-04).
Author information
Authors and Affiliations
Contributions
M.J.K. conceptualized the study, curated the data, acquired funding, led the investigation, and supervised the project. Y.K., C.L., and M.J.K. performed formal analysis, while Y.K., C.L., H.H., and S.-H.H. contributed to methodology. Y.K. wrote the original draft, with J.Y. and M.J.K. handling review and editing. Validation was conducted by Y.K., J.Y., C.L., and M.J.K. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kwon, Y., Yoon, J., Lim, C. et al. Impact of clinical factors on the diagnostic performance of diffuse optical spectroscopic imaging for breast cancer. Sci Rep 15, 14508 (2025). https://doi.org/10.1038/s41598-025-98519-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98519-6