Abstract
Recombinant human interferon Alfa-2b vaginal suppository is a gynecological preparation mainly made of interferon, commonly used to treat diseases related to viral infections such as cervical erosion. As a recombinant protein drug, it is important to pay attention to the possibility of modifications that may lower the quality of the drug during the production process. The aim of this study is to evaluate the pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of this product in Chinese rhesus macaque after purification process changes, and to demonstrate that there is no difference in the biological activity of recombinant human interferon Alfa-2b vaginal suppository stock solution before and after process changes. There are 12 test animals: Chinese rhesus macaques who received a two-group crossover design and were subcutaneously injected with the same active dose of 500,000 IU/kg around the navel in the abdomen. According to maximum concentration (Cmax) and time of maximum concentration (Tmax) within non-parametric test (P > 0.05), geometric mean ratio of PK parameter Cmax for the drugs after purification process changes (sample S) compared to the before purification process changes one (sample R) was 97.09%, with a 90% confidence interval (CI) of 87.39–107.87%. The geometric mean ratio Cmax of serum Beta2-microglobulin (PDmax) for PD index is 100.07%, with a 90% CI of 97.16–103.07%; Geometric mean ratio of AUEC0 − t is 98.91%, with a 90% CI of 96.53–101.34%. The geometric mean of the PD index, neopterin PDmax, is 97.75%, with a 90% CI of 92.53–103.25%; Geometric mean of AUEC0 − t is 105.59%, with a 90% CI ranging from 97.22 to 114.68%. The important parameters of PK/PD meet the equivalence requirements, and biological activity of the recombinant human interferon Alfa-2b vaginal suppository stock solution after purification process change is no different from before the change. Under the same active dose administration conditions, the same biological effects were produced, achieving the same effect as before the change.
Similar content being viewed by others
Introduction
Interferon (IFN) is a group of cytokines with antiviral, immunomodulatory and antiproliferative activities, which was first described by Isaacs and Lindenmann in 19571. Interferon Alfa-2b can treat conjunctival melanoma2, pediatric herpetic pharyngitis3, polycythemia vera (PV)4, essential thrombocytosis (ET)5, myeloproliferative neoplasms6, chronic hepatitis B7, and chronic hepatitis C8. The production of natural human interferon Alfa-2b is rare, it is mainly produced by genetic engineering technology9. Recombinant human interferon α-2b vaginal suppository is a kind of gynecological finished pharmaceuticals mainly made of interferon α-2b, commonly used to treat viral cervical erosion related to human papilloma virus (HPV), herpes simplex virus (HSV), cytomegalovirus (CMV)10,11 etc.
During the production process gene-based recombinant human interferon Alfa-2b is prone to changes such as oxidation12, isomerization13, incomplete or mismatched disulfide bond pairing14, N-terminal acetylation15, etc., which may reduce the biological activity of the drug, enhance immunogenicity, bring side effects, etc., affecting the effectiveness and safety of the drug16,17,18,19. Therefore, the analysis and control of product-related proteins have gradually received attention in the quality control process of recombinant protein drug products20,21,22.
We used reporter gene assay to test the activity of the samples23. We constructed the cell line HEK293puro ISRE Luc, which was used as a biological activity assay cell. After adding cell lysate and luciferase substrate, the luminescence intensity is measured to determine the biological activity of recombinant human interferon Alfa-2b. The drugs have higher protein content and biological activity than before the process change.
In the present study, we administered the same active dose of human interferon Alfa-2b vaginal suppository stock solution to Chinese rhesus macaque, evaluated and analyzed the PK and PD profiles of the drug after purification process changes.
Purpose
Evaluate whether there is a difference in biological activity of recombinant human interferon Alfa-2b vaginal suppository stock solution before and after purification process changes. At the same active dose, Chinese rhesus macaques were selected for in vivo PK/PD experiments.
Table 1 shows the changes in biological activity of recombinant human interferon Alfa-2b vaginal suppository stock solution before purification process change (sample R) and the after process change one (sample S). In theory, when samples R and S are given the same biologically active dose to Chinese rhesus macaques, the drug effects that the animal body should produce should be equivalent.
Methods
Compliance with ethics guidelines
All the animals were acclimated under standard laboratory conditions (ventilated room, 25 ± 1 °C, 60 ± 5% humidity, 12 h light/dark cycle, single housing) and had free access to standard water and food. During the experiment, all Chinese rhesus macaques were fixed in the Monkey Fixed Chairs and no abnormalities were observed during blood collection (SYXK-2021-0003). All procedures were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China) and were approved by Institutional Animal Care and Use Committee (IACUC) and the Laboratory Animal Ethics Committee of Drug Safety Evaluation Research Center of Chongqing Medleader Bio-Pharm Co., Ltd (IACUC-2021-010). The study is reported in accordance with ARRIVE guidelines.
Experimental animal
Select 12 healthy Chinese rhesus macaques from Qingwei Macaque Farm in Chengkou County, with an equal number of males and females, weighing 3–5 kg (Production license number: SCXK (Chongqing) 2020-0001. Experimental Animal Use License: SYXK (Chongqing) 2021-0003). According to the concentration and biological activity of the samples before and after the purification process change (sample R: 1.94 mg/ml, 2.66e + 08IU/ml; sample S: 2.20 mg/ml, 5.10e + 08IU/ml), 12 Chinese rhesus macaques were divided into two groups A and B according to gender and weight, with 6 monkeys in each group. On the day of administration, each animal weighed 3–5 kg, and qualified animals were included in the experiment.
Purification method
We have developed a novel purification process that can effectively remove the product-related proteins of recombinant human interferon Alfa-2b. The existing engineering bacteria and fermentation process remain unchanged. We increased the feeding amount of each batch of engineering bacteria, optimized the initial extraction process, and replaced the original metal chelate chromatography, cation column chromatography and S-100 gel column chromatography with metal chelate affinity chromatography, reverse-phase column chromatography and ion-exchange chromatography. The quality of the product after purification process change with the product-related proteins being less than 3% has been significantly improved compared to the before process change one with about 40% of product-related proteins.
Administration method
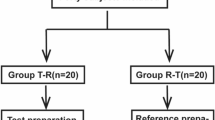
The study adopted a crossover design for drug administration, with Group A (three females and three males) receiving the test substance on an empty stomach in the first cycle and the control substance on an empty stomach in the second cycle; Group B (three females and three males) was administered in the order of using the control substance on an empty stomach in the first cycle and the test substance on an empty stomach in the second cycle; The cleaning period is 12 days (Fig. 1).
After random screening of the experimental animals, they were administered at a dose of 500,000 IU/kg. The first cycle is administered subcutaneously on the right side of the animal’s abdomen around the navel, and the second cycle is administered subcutaneously on the left side of the animal’s abdomen around the navel.
Accurate dosing was administered in two cycles without any abnormal dosing; During the experiment, no abnormal reactions were observed in the animals.
Blood sample collection
PK evaluation was conducted by detecting the content of human interferon Alfa-2b: blood samples were taken within 1 h before administration and at 0.5, 1.0, 2.0, 3.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0, 20.0, and 24 h after administration for the detection of human interferon Alfa-2b.
PD evaluation was performed by detecting Beta2-microglobulin and neopterin: blood samples were taken within 1 h before administration and at 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0, 24.0, 36.0, 48.0, and 72 h after administration for the detection of Beta2-microglobulin; Blood samples were taken within 1 h before administration and at 2.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0, 24.0, 36.0, 48.0, 72.0, 96.0, and 120 h after administration for the detection of neopterin.
Collect whole blood from the forelimb veins of experimental animals in a blood collection tube without any additives, let it stand at room temperature for 1 h, centrifuge at 2 ℃~8 ℃, 1,100 g for 10 min, and collect and package serum at room temperature. These samples were stored at ≤-70 °C until analysis.
PK analysis
The concentration of interferon Alfa-2b was determined using enzyme-linked immunosorbent assay (ELISA)24. The PK parameters of interferon Alfa-2b include area under the concentration versus time curve from time 0 to the last measurable concentration (AUC0–t), maximum serum concentration (Cmax), area under the concentration versus time curve from time 0 to infinity (AUC0−∞), the time when Cmax first appeared (Tmax), apparent end elimination rate constant (λz), elimination half-time (t1/2), apparent volume of distribution (Vz_F_obs), plasma clearance (Cl_F_obs), and apparent volume of distribution during the terminal phase (Vd/F).
PD analysis
Serum Beta2-microglobulin and neopterin was used as the biomarker to analyze the PD profiles of interferon Alfa-2b25,26,27,28. The concentration of Beta2-microglobulin was determined using turbidimetric inhibition immuno assay and neopterin was determined using mass spectrometry (UPLC-MS-MS)23。The PD parameters of Beta2-microglobulin and neopterin included the area under the serum biomarker effect–time curve from time zero to the last quantifiable time point (AUEC0–t), geometric mean ratio Cmax of serum Beta2-microglobulin and neopterin (PDmax), time of PDmax (Tmax), apparent end elimination rate constant (λz), elimination half-time (t1/2), apparent volume of distribution (Vz_F_obs), plasma clearance (Cl_F_obs), and maximum tolerated dose (MTD). Whether there is no significant difference (P ≥ 0.05) or bioequivalence is achieved as the basis for determining whether PD is equivalent.
Statistical methods
PK/PD parameters were calculated using Phoenix WinNonlin v.8.2. SAS v.9.4 was used for other statistical analyses. A non-compartment model based on the Kolmogorov-Smirnov test was used. The arithmetic mean, standard deviation, median, maximum, minimum, CI, and geometric mean were assessed. Tmax were analyzed using a non-parametric testing method based on the Kolmogorov-Smirnov test. Based on the bioequivalence set (BES) of Cmax, AUC0 − t, and AUC0−∞, a mixed effects model analysis was conducted with drug, period, and sequence as fixed effects and nested subjects in the sequence as random effects. Double one-sided t-test statistics and P-values were provided to determine whether sample S and sample R met the bioequivalence criteria.
The PD parameters PDmax and AUEC0 − t were analyzed using a difference test and the same evaluation method as the PK parameters (Cmax, AUC0 − t). The arithmetic mean, standard deviation, median, maximum, minimum, CI, and geometric mean of PD parameters (PDmax, AUEC0–t) of Beta2-microglobulin and neopterin were assessed.
Results
Quality control
PK determination of recombinant human interferon Alfa-2b vaginal suppository stock solution was conducted, with 13 blood collection points and 2 administration cycles set for each Chinese rhesus macaque per week, totaling 312 samples. Among them, 48 samples were subjected to incurred sample reanalysis (ISR), accounting for 15.38% of the total sample size.
The accuracy deviation of low concentration positive quality control (QL), medium concentration positive quality control (QM), high concentration positive quality control (QH) during measurement ranges from − 10.99 to 19.65%, with 97.44% of the quality control samples under control; the accuracy deviation of the QL, QM, and QH for sample reanalysis is between − 7.31% and − 1.15% (Table 2), and 100% of the quality control samples are under control. The quality control results are acceptable.
The one of PD indices Beta2-microglobulin measured unknown samples after the validation of the biological sample analysis method is completed. Each week, 12 blood collection points and 2 dosing cycles were set up for each Chinese rhesus macaque, with a total of 288 samples. Among them, 48 samples were subjected to incurred sample reanalysis (ISR), accounting for 16.67% of the total sample size. Set 2 concentration levels for quality control samples, QL: 1.07e-03 mg/ml, QH: 2.93e-03 mg/ml. Two standard curves were established for biological sample detection, and quality control samples with QL and QH concentrations were simultaneously measured. The accuracy of the quality control QL and QH of the samples ranged from 90.65 to 104.67%. After reanalysis, the accuracy of the quality control QL and QH ranged from 92.52 to 100% (Table 3). The quality control samples were 100% under control, and the quality control results were acceptable.
After the validation of the biological sample analysis method, the determination of unknown samples begins for the other PD indices, neopterin. Fourteen blood collection points and two dosing cycles were set up for each rhesus monkey per week, with a total of 336 samples. Among them, 48 samples were subjected to incurred sample reanalysis (ISR), accounting for 14.29% of the total sample size. Unknown samples will be measured after the validation of the biological sample analysis method is completed. Measure each unknown sample once. Four concentration levels were set for the quality control samples: QL: 4.000e-03 mg/ml, QGM: 15.000e-03 mg/ml, QM: 50.000e-03 mg/ml, QH: 75.000e-03 mg/ml. Establish a new standard curve for each analysis batch of biological samples, and simultaneously measure quality control samples at four concentrations: QL, QGM, QM, and QH. The accuracy of the quality control samples QL, QM, QGM, and QH ranges from 101.92 to 104.08%, with 88.33% of the quality control samples under control. The accuracy of quality control QL, QM, QGM, and QH for reanalysis ranged from 96.13 to 99.44% (Table 4). 91.67% of the reanalysis quality control samples are under control.
Pharmacokinetics (PK)
Samples R and samples S were given the same biologically active dose to the experimental animal Chinese rhesus macaque. Due to the higher biological activity of sample S at the same volume, the content of human interferon Alfa-2b determined by PK was slightly lower than that of sample R. The difference in Tmax may affect the half-life of sample R and sample S, and have an impact on AUC0 − t, and AUC0−∞.
The Tmax of sample R is 3–8 h, and sample S is 3–6 h. From the median comparison, the Tmax of sample R and sample S are both 4 h, can be considered equivalent. The Vz_f_obs of sample R is 539.00 ± 0.28IU/pg, and the sample S is 574.88 ± 124.98IU/pg. Compared with sample R, sample S has a larger apparent distribution volume, indicating that the recombinant human interferon Alfa-2b vaginal suppository stock solution has better performance after the process change. In terms of drug elimination half-life (t1/2), λz, Cl_f_obs, and sample exposure (Cmax, AUC0 − t, and AUC0−∞), sample R was slightly higher than sample S. This may be due to the higher biological activity of sample S than sample R, resulting in experimental concentrations lower than sample R and affecting PK parameters (Fig. 2; Table 5).
Based on the geometric mean of Cmax, the PK equivalence between sample R and sample S was analyzed. The 90% CI was 87.39%~107.87%, which meets the equivalence requirements. This proves that the biological equivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution after the process change is established in the main PK parameters compared to before the change (Table 6).
According to the non-parametric Tmax test, the results showed no statistical difference (P > 0.05) between sample R and sample S, further proving the bioequivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution after the process change compared to before the change (Table 7).
Pharmacodynamics (PD)
Evaluate serum Beta2-microglobulin and neopterin as biomarkers for PD.
For the PD parameter of serum Beta2-microglobulin, under the conditions of administering the same biologically active dose of sample R and sample S to the experimental animal Chinese rhesus macaque, the median Tmax of sample R and sample S were both 16 h; The λz of sample R is the same as that of sample S; On AUEC0 − t, sample R is almost identical to sample S, indicating that the Tmax, λz, and AUEC0 − t of Beta2-microglobulin before and after the process change can be considered equivalent. The PDmax of sample R is 1.638 ± 0.143 mg/L, while the PDmax of sample S is even higher at 1.647 ± 0.230 mg/L. The drug elimination half-life (t1/2) of sample R is 78.33 ± 12 h, and the t1/2 of sample S is 101.68 ± 36.8 h. The half-life of the modified sample has been extended; The Vz_F_obs of sample R is 1058.59 ± 132.77IU/ng, and sample S is 1103.07 ± 206.20IU/ng. After the change, the apparent distribution volume of the sample is larger; Compared with sample R, sample S has better results in Vz_F_obs (Fig. 3; Table 8).
The geometric mean of Beta2-microglobulin PDmax is 100.07%, with a 90% CI of 97.16% ~103.07%. The geometric mean of AUEC0 − t is 98.91%, with a 90% CI of 96.53%~101.34%. Analyze the PD equivalence of Beta2-microglobulin between sample R and sample S, and determine the bioequivalence based on whether the upper and lower limits of the geometric mean ratio’s 90% CI fall between 80% and 125%. PDmax and AUEC0 − t meet the requirements. Prove that the bioequivalence of the main PD parameter Beta2-microglobulin in the recombinant human interferon Alfa-2b vaginal suppository stock solution after process changes is established compared to before the changes (Table 9).
According to the non-parametric Tmax test of Beta2-microglobulin, the results showed that there was no statistical difference between sample R and sample S (P > 0.05), further proving that the bioequivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution after the process change was established on the PD index Beta2-microglobulin compared to before the change (Table 10).
For the PD parameters of neopterin, under the same biologically active dose of sample R and sample S given to the experimental animal Chinese rhesus macaque, the median Tmax of sample R and sample S were both 24 h; The λz, PDmax, and Cl_f_obs of sample R and sample S are almost identical which indicates that these PD parameters of neopterin can be considered equivalent before and after the process change. In addition, the parameters t1/2, Vz_f_obs, and AUEC0 − t are not significantly different, indicating that there is no significant difference in the PD parameters of the sample neopterin before and after the change (Fig. 4; Table 11).
The geometric mean of PDmax for neopterin is 97.75%, with a 90% CI from 92.53 to 103.25%. The geometric mean of AUEC0 − t is 105.59%, with a 90% CI from 97.22 to 114.68%. The bioequivalence of the PD parameter neopterin for sample R and sample S was determined based on whether the upper and lower limits of the geometric mean ratio of 90% CI fell between 80% and 125%. PDmax and AUEC0 − t meet the requirements. Prove that the bioequivalence of important PD parameters on the PD indicator neopterin in the recombinant human interferon Alfa-2b vaginal suppository stock solution after process changes is established compared to before the changes (Table 12).
According to the non-parametric Tmax test of neopterin, the results showed that there was no statistical difference (P > 0.05) between sample R and sample S. This further proves that the biological equivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution after the process change is established on the PD index neopterin compared to before the change (Table 13).
Discussion
With the increasing awareness of the harm of Human Papilloma Virus (HPV), the use of the recombinant human interferon Alfa-2b vaginal suppository is also becoming commonplace. As an immunomodulatory agent, recombinant human interferon Alfa-2b vaginal suppository has multiple medical indications, including the treatment of HPV clearance, chronic hepatitis B/C, and multiple sclerosis. It is one of the first-line treatment drugs for cervical intraepithelial neoplasia (CIN)29. The wide clinical application and high demand are the characteristics of the recombinant human interferon Alfa-2b vaginal suppository, which are also the difficulties that major manufacturers need to overcome. It is reliable to obtain high-purity and highly active human interferon Alfa-2b by optimizing the purification process. However, changing the process may have an impact on the product, such as changes in its structure and biological activity. Therefore, it is necessary to conduct comparative studies on animal PK/PD to demonstrate the bioequivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution before and after the process changes.
The study selected Chinese rhesus macaques as experimental animals and used serum human interferon Alfa-2b as the PK detection index, Beta2-microglobulin and neopterin as biomarkers to analyze the PK/PD characteristics of interferon Alfa-2b18,19,20. Measure the concentration of Beta2-microglobulin using immunoturbidimetry; Mass spectrometry (UPLC-MS-MS) was used to determine the concentration of neopterin and evaluate the differences in biological effects of the recombinant human interferon Alfa-2b vaginal suppository stock solution in Chinese rhesus macaques before and after process changes. Before sample analysis, unknown samples were detected using random methods. During PK serum sample determination, the accuracy deviation of accompanying quality control QL/QM/QH ranged from − 10.99 to 19.65%, with 97.44% of the quality control samples under control; The accuracy of the accompanying quality control QL, QM, QGM, and QH for reanalysis ranged from 96.13 to 99.44%, with 91.67% of the samples under control, meeting the PK quality control requirements. The serum sample determination of PD index Beta2-microglobulin was accompanied by quality control QL, and the accuracy of QH ranged from 90.65 to 104.67%. After reanalysis of accompanying quality control QL, the accuracy of QH ranged from 92.52 to 100%, and the quality control sample was 100% under control; The accuracy of the accompanying quality control QL, QM, QGM, and QH for serum samples of the indicator neopterin ranged from 101.92 to 104.08%, with 88.33% under control. The accuracy of the accompanying quality control QL, QM, QGM, and QH for reanalysis ranges from 96.13 to 99.44%, with 91.67% of the samples under control, meeting the PD quality control requirements.
Under the same active dosage administration conditions, the sample R has a slightly larger apparent distribution volume compared to the sample S. The geometric mean ratio of PK parameter Cmax is 97.09%, and the 90% CI is 87.39%~107.87%, which meets the equivalence requirements. The geometric mean ratio of serum Beta2-microglobulin PDmax for PD index is 100.07%, with a 90% CI of 97.16–103.07%. The geometric mean ratio of AUEC0 − t is 98.91%, with a 90% CI of 96.53–101.34%, indicating that the bioequivalence of the recombinant human interferon Alfa-2b vaginal suppository stock solution after process modification is established in terms of PD index Beta2-microglobulin compared to before the modification. The geometric mean of the PD index, neopterin PDmax, is 97.75%, with a 90% CI of 92.53–103.25%. The geometric mean of AUEC0 − t is 105.59%, with a 90% CI of 97.22–114.68%. The bioequivalence of the PD indicator neopterin before and after the process change is established.
Conclusion
The PK/PD data were subjected to Tmax non-parametric testing, and there was no significant difference (P > 0.05) between sample S after the process change and sample R before the change. Therefore, it is believed that when the process of the recombinant human interferon Alfa-2b vaginal suppository stock solution was changed, the same biological effects were produced at the same active dose, achieving the same effect as before the change.
Data availability
The data utilized in this study are available from the corresponding author upon reasonable request.
References
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R Soc. Lond. B Biol. Sci. 147 (927), 258–267 (1957).
Cid⁃Bertomeu, P. & Huerva, V. Use of interferon alpha 2b to manage conjunctival primary acquired melanosis and conjunctival melanoma. Surv. Ophthalmol. 67 (5), 1391–1404 (2022).
Ye, Y. Z. et al. Efficacy and safety of interferon α⁃2b spray for herpangina in children: a randomized, controlled trial. Int. J. Infect. Dis. 107, 62–68 (2021).
Gisslinger, H. et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol. 7 (3), e196–e208 (2020).
Okikiolu, J. et al. Real world experience with ropeginterferon alpha-2b (Besremi) in essential thrombocythaemia and polycythaemia vera following exposure to pegylated interferon alfa-2a (Pegasys). Leuk. Res. Rep. 19, 100360 (2022).
Vachhani, P. et al. Interferons in the treatment of myeloproliferative neoplasms. Ther. Adv. Hematol. 15, 20406207241229588 (2024).
Huang, Y. W. et al. Ropeginterferon alfa-2b every 2 weeks as a novel pegylated interferon for patients with chronic hepatitis B. Hepatol. Int. 14 (6), 997–1008 (2020).
Hsu, S. J. et al. Ropeginterferon Alfa-2b administered every two weeks for patients with genotype 2 chronic hepatitis C. J. Formos. Med. Assoc. 120 (3), 956–964 (2021).
Fazeli, M. R. & Hezarjaribi, N. A simplified process for purification and refolding of Recombinant human Interferon-α2b. Iran. Biomed. J. 26 (1), 85–90 (2022).
Sun, Y., Xu, J., Zhou, H., You, L. & Zhu, Y. Influence of Lacidophilin Vaginal Capsules plus rh-IFN-α2b on Efficacy, Vaginal Microecology, and Safety of Patients with HPV Infection. Evid Based Complement Alternat Med. ; 2022:3632053. (2022).
Ivanina, A. et al. The topical novel formulations of interferon α-2в effectively inhibit HSV-1 keratitis in the rabbit eye model and HSV-2 genital herpes in mice. Viruses 16 (6), 989 (2024).
Bandi, S., Singh, S. M., Shah, D. D., Upadhyay, V. & Mallela, K. M. G. 2D NMR analysis of the effect of asparagine deamidation versus methionine oxidation on the structure, stability, aggregation, and function of a therapeutic protein. Mol. Pharm. 16 (11), 4621–4635 (2019).
Shaldzhyan, A. et al. Clean and folded: production of active, high quality Recombinant human interferon-λ1. Process. Biochem. 111, 32–39 (2021).
Wilkinson, C. et al. Improved yield of Recombinant human IFN-α2b from mammalian cells using heterologous signal peptide approach. Protein Expr Purif. 198, 106125 (2022).
Giorgetti, S. I. et al. Development of highly stable and de-immunized versions of Recombinant alpha interferon: promising candidates for the treatment of chronic and emerging viral diseases. Clin. Immunol. 233, 108888 (2021).
Carvalho, S. F., Pereiro, A. B. & Araújo, J. M. M. Simultaneous purification of human interferon Alpha-2b and serum albumin using bioprivileged fluorinated ionic Liquid-Based aqueous biphasic systems. Int. J. Mol. Sci. 25 (5), 2751 (2024).
Li, M. et al. Comprehensive characterization of higher order structure changes in methionine oxidized monoclonal antibodies via NMR chemometric analysis and biophysical approaches. MAbs 16 (1), 2292688 (2024).
Yin, H. R. et al. Study of methionine oxidization in human interferon α 2b using HPLC and UPLC⁃Q⁃TOF⁃MS. Chin. J. Pharm. Anal. 41 (7), 1203–1208 (2021).
Grune, T. Oxidized protein aggregates: formation and biological effects. Free Radic Biol. Med. 150, 120–124 (2020).
Wen, Y. & Jawa, V. The impact of product and process related critical quality attributes on immunogenicity and adverse immunological effects of biotherapeutics. J. Pharm. Sci. 110 (3), 1025–1041 (2021).
Liu, Y. et al. Simultaneous monitoring and comparison of multiple product quality attributes for cell culture processes at different scales using a LC/MS/MS based Multi-Attribute method. J. Pharm. Sci. 109 (11), 3319–3329 (2020).
Nunavath, R. S. et al. Quality by design in pharmaceuticals: A review of its impact on regulatory compliance and product quality. Drug Res. (Stuttg). 74 (1), 18–23 (2024).
Committe National Pharmacopoeia. Pharmacopoeia of People’s Republic of China[M]. Part 1 (Beijing; China Medical Science Press., 2020).
Miyachi, N., Zagrijtschuk, O., Kang, L., Yonezu, K. & Qin, A. Pharmacokinetics and pharmacodynamics of ropeginterferon alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin. Drug Investig. 41, 391–404 (2021).
Wagner, S. M., Melchardt, T. & Greil, R. Ropeginterferon alfa-2b for the treatment of patients with polycythemia vera. Drugs Today (Barc). 56 (3), 195–202 (2020).
Huang, Y. W., Qin, A., Tsai, C. Y. & Chen, P. J. Novel pegylated interferon for the treatment of chronic viral hepatitis. Viruses 14 (6), 1128 (2022).
Sumpio, B. E., Ernstoff, M. S. & Kirkwood, J. M. Urinary excretion of interferon, albumin, and beta 2-mi-croglobulin during interferon treatment. Cancer Res. 44, 3599–3603 (1984).
Miyachi, N., Zagrijtschuk, O., Kang, L., Yonezu, K. & Qin, A. Pharmacokinetics and pharmacodynamics of ropeginterferon Alfa-2b in healthy Japanese and Caucasian subjects after single subcutaneous administration. Clin. Drug Investig. 41 (4), 391–404 (2021).
Li, R. et al. Guttate psoriasis induced by interferon alfa-2b suppository treatment for high-grade cervical intraepithelial neoplasia. Dermatol. Ther. ;): e11 ): e15834. (2022).
Funding
This work was supported by Science and Technology Development Plan Program of Jilin Province(20210204092YY).
Author information
Authors and Affiliations
Contributions
XZ: Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing- Original Draft, Writing - Review & Editing; BZ: Methodology, Validation, Software, Formal Analysis, Writing - Review & Editing; YG: Formal analysis, Writing - Review & Editing; YL: Conceptualization, Funding Acquisition, Resources, Project administration, Supervision, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Role of the funder
The funding sources had no role in the design and conduct of the study; collection,
management, analysis, and interpretation of the data; preparation, review, or approval of the.
manuscript; and decision to submit the manuscript for publication.
Disclosures
No potential conflict of interest relevant to this article was reported.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, X., Zhou, B., Gong, Y. et al. Investigation into the pharmacodynamics and pharmacokinetics of recombinant human interferon alfa-2b vaginal suppository following process optimization in chinese rhesus macaque. Sci Rep 15, 15932 (2025). https://doi.org/10.1038/s41598-025-98813-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-98813-3