Abstract
This study aimed to examine the potential involvement of inflammatory imbalance and OFC subregion structure in the pathogenesis of agitation. In this study 119 schizophrenia patients were categorized into different subgroups of agitation using two-step cluster analysis. Peripheral cytokine and the OFC structure were examined in all subjects. Patients were assessed for immune-inflammatory response system and compensatory immunoregulatory reflex system (IRS/CIRS) reflecting the level of inflammatory imbalance. The immune biomarkers mainly include M1 (IL-6, IL-1β, IFN-α and TNF-α), T helper, Th-1 (IL-2, IL-12p70 and IFN-γ), Th-2 (IL-4 and IL-5), Th-17 (IL-17) and T regulatory cytokines (Treg) (IL-10). Compared with the low agitation group, the pro-inflammatory cytokine IL-6 was significantly higher in the high agitation group, as were the levels of the immune biomarkers Th-2, M1, IRS and IRS/CIRS. However, there was no significant difference in the OFC volume and cortical thickness between the two groups. In addition, left lateral OFC volume was negatively correlated with IRS/CIRS in the high agitation group. Moderation model showed that agitation significantly moderated the relationship between left lateral OFC volume and IRS/CIRS. Thus, the present study provides assistance in explaining the etiological mechanisms of agitation in schizophrenia.
Similar content being viewed by others
Introduction
Schizophrenia has a wide range of features including positive symptoms, negative symptoms, cognition symptoms, agitation and affective symptoms1. Agitation is one of the major causes of consultation and hospitalization of patients with schizophrenia2. Currently, agitation can be broadly defined as a state of motor restlessness accompanied by mental tension, characterized by excessive body movements, irritability, hostility, uncooperativeness, impulsivity and aggression3,4,5. It is estimated that approximately 20% of people with schizophrenia experience agitation during their lifetime, while approximately 39% of people with schizophrenia exhibit agitated behavior at the time of their first episode6,7. Agitation is a dynamic condition that may rapidly escalate from mild (distress, worry, fear, etc.) to out-of-control (violence, aggression, confusion, etc.)8. The development of agitation has a significant impact on the patient, aggravating the patient’s psychiatric symptoms and predicting a decline in the patient’s quality of life9. In addition, there is a familial clustering of agitation. Studies have shown that first-degree relatives of patients with schizophrenia have higher levels of agitation than healthy people without a family history of schizophrenia10,11. As one of the core symptoms of schizophrenia, research on the mechanisms of agitation may help to reveal some of the biological mechanisms of schizophrenia12.
The orbitofrontal cortex (OFC), located in the ventral part of the frontal lobe above the orbit, is one of the important brain structures for agitation regulation13,14. The OFC receives a large number of sensory and limbic system signal inputs, establishes a representation of the value of rewards and punishments, and then uploads the signals to decision-making brain regions (e.g., the prefrontal cortex, the anterior cingulate cortex, etc.), which modulate and correct the behaviors and emotions associated with rewards and punishments15. Patients with OFC damage has altered sensitivity to the results of reward and punishment, and reduced emotional regulation and impulse control. Thus, OFC damage is closely related to agitation. Reductions in OFC gray matter volume and cortical thickness have been repeatedly reported in patients with high agitation schizophrenia compared to patients with low agitation schizophrenia. A study of male schizophrenic patients found that the volume of OFC subregions was smaller in violent patients than in nonviolent patients16. Another study also found reduced OFC cortical thickness in schizophrenic patients who repeatedly exhibited impulsive aggression17. Similarly, in schizophrenic patients with antisocial personality disorder, violent patients had smaller OFC cortical thickness than nonviolent patients18. Correlation analyses showed a negative correlation between severity of agitation and OFC volume and cortical thickness in schizophrenic patients17,19. However, there are also some findings suggesting that there is no significant difference between OFC volume and cortical thickness in violent and nonviolent schizophrenic patients20. These inconsistent results may be related to factors such as small sample size, different sex of patients, and the presence of co-morbidities. In addition, since different subregions of the OFC have different functions, in which the medial OFC (mOFC) represents reward value, whereas the lateral OFC (lOFC) represents punishment and non-reward value and is involved in modifying emotional behavior15. Therefore, studies on the relationship between different subregions of the OFC and agitation may further substantiate the pathological changes in agitation in schizophrenia.
A growing number of studies have explored the relationship between peripheral cytokines and agitation. Cytokines are mainly categorized into pro-inflammatory and anti-inflammatory cytokines, which belong to the immune-inflammatory response system (IRS) and compensatory immune-regulatory reflex system (CIRS), respectively21. IRS activation refers to the elevated levels of pro-inflammatory cytokines produced by M1 (interleukin (IL)-6, IL-1β, interferon (IFN)-α and tumor necrosis factor (TNF)-α, T helper cell-1(Th-1) (IL-2, IL-12p70 and IFN-γ), and Th-17 cells (IL-17). In contrast, CIRS activation involves elevated levels of anti-inflammatory cytokines produced by Th-2 (IL-4 and IL-5) and T-regulatory cytokines (Treg) (IL-10). Macrophages exist in different functional states, including M1 and M2 macrophages. Of these, M1 macrophages produce pro-inflammatory cytokines and M2 macrophages exhibit immunomodulatory roles, producing Th-2 and Treg activity22,23. Th cells are polarized into Th-1, Th-2 and Th-17 phenotypes. Th-1 and Th-2 cytokine imbalances are associated with the pathophysiology of schizophrenia, while the Th-17 phenotype is involved in autoimmunity and pro-inflammatory properties24. While pro-inflammatory cytokines act primarily by sending signals to the brain to produce neurochemical, neuroendocrine, neuroimmune, and behavioral changes, CIRS increases levels of immunomodulatory products in order to downregulate the IRS and prevent excessive inflammation. Agitation suggests that deterioration in schizophrenia patients is accompanied by increased oxidative stress in the body, which may play a role in neurological progression through neuroinflammatory pathways25. Previous studies have found that inflammation may be related to agitation. A study of one-month unmedicated schizophrenia patients found that the severity of agitation was positively correlated with the levels of IL-17, IL-23, and transforming growth factor beta 1 (TGF-β1)26. Another study, conducted on patients with schizophrenia who were admitted to the emergency department, observed that the levels of TNF-α were higher in patients who displayed agitation compared to patients who did not. Furthermore, there was a tendency towards elevated levels of IFN-γ in schizophrenia patients with agitation. However, no significant differences were observed in the levels of IL-1β, IL-4, IL-6, IL-10, or TGF-β127. A recent study comparing male schizophrenia with different levels of agitation severity also found that violent schizophrenia had significantly higher C-reactive protein and C-reactive protein to albumin ratios than nonviolent patients28. Conversely, a separate study conducted on patients with first-episode schizophrenia found no discernible differences in the levels of IL-1β, IL-4, IL-6, IL-8, IL-10, or IL-12p70 between the two groups of patients with varying degrees of agitation29. Although these studies compared cytokine profiles in schizophrenia patients with different levels of agitation, the results were markedly inconsistent. This inconsistency may be due to the interaction between different cytokines. Therefore, the use of pro- and anti-inflammatory cytokine ratios reflecting the individual’s combined level of inflammation may help to obtain more consistent conclusions.
Peripheral cytokines may contribute to structural changes in the OFC. A recent postmortem study showed an association between gene expression of proinflammatory cytokines and reduced OFC gray matter volume in patients with schizophrenia30. Results of an in vivo study showed that elevated levels of TNF-α in patients with schizophrenia spectrum disorders were associated with increased thickness of the right mOFC31. A study including 44 patients with chronic schizophrenia found that IL-8 and IL-10 levels were associated with OFC volume, whereas IL-2 and IL6 levels were not32. Specifically, IL-8 levels were positively correlated with left mOFC gray matter volume, whereas IL-10 levels were negatively correlated with left lOFC and left mOFC gray matter volume. However, another study of patients with first-episode schizophrenia did not find a relationship between peripheral cytokines (including IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p70, IFN-γ, and TNF-α) and OFC structure33. These inconsistent results suggest that it is necessary to explore the relationship between peripheral cytokines and the structure of OFC subregions using integrated levels of inflammation.
The aim of this study was to investigate the role of peripheral cytokines and OFC subregion structure in schizophrenia agitation. We selected newly hospitalized schizophrenic patients and divided them into high and low agitation groups based on cluster analysis of patient agitation scores. Differences in OFC subregion structure and peripheral cytokine levels were compared between the two groups. In addition, to clarify the role of proinflammatory/anti-inflammatory imbalance in agitation, the relationship between cytokine imbalance and OFC structure in schizophrenia patients with different agitation severity was analyzed using the z-unit weighted composite score method. We hypothesized that agitation is associated with OFC structural damage and inflammatory imbalance, and in particular, that more severe OFC damage and inflammatory imbalance exist in highly agitated schizophrenic patients.
Methods
Participants
A total of 119 inpatients with schizophrenia who were hospitalized in the psychiatric department of the Affiliated Brain Hospital of Nanjing Medical University were included in this study. The study was approved by the Affiliated Brain Hospital of Nanjing Medical University and conducted in accordance with the Declaration of Helsinki, with all participants providing written informed consent34.The patients were selected from the Affiliated Brain Hospital of Nanjing Medical University, and the diagnosis of schizophrenia was determined by two attending-level psychiatrists based on the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)35. The inclusion criteria were: (1) aged 18 to 65 years; (2) meeting the diagnostic criteria for schizophrenia according to the DSM-5; (3) a score of no less than 35 on the Brief Psychiatric Rating Scale (BPRS); (4) participants of Han ethnicity and right-handedness. The exclusion criteria included: (1) subjects with a history of head injury, seizures, cerebrovascular disease, neurological disorders, or thyroid dysfunction; (2) participants meeting the diagnostic criteria for other mental disorders according to the DSM-5, such as depression and other disorders belonging to the schizophrenia spectrum (bipolar disorder, schizoaffective disorder, schizotypal personality disorder), or other neurodevelopmental disorders that could affect brain structure or produce agitation symptoms (autism, attention deficit hyperactivity disorder, oppositional defiant disorder, conduct disorder); (3) participants meeting criteria for alcohol or drug abuse or dependence; (4) those with severe hearing or visual impairment; (5) individuals with severe physical illnesses or serious medication side effects, such as severe extrapyramidal syndrome or delayed onset movement disorders; (6) pregnant or breastfeeding participants; (7) those who had used immunomodulators, neurotrophics, or antioxidants for ≥ 8 weeks prior to enrollment.
Peripheral marker assaying and processing
The concentration of plasma cytokines, including 10 selected pro-inflammatory cytokines (IL-2, IL-5, IL-6, IL-8, IL-1β, IL-12p70, IL-17, IFN-α, IFN-γ, and TNF-α) and 2 anti-inflammatory cytokines (IL-4 and IL-10), was quantified using the enzyme-linked immunosorbent assay (ELISA) method. On the second day of hospital admission, 5 ml of fasting venous blood samples were collected from all subjects and transferred to EDTA anticoagulant tubes. The samples were then centrifuged (1000 g/min, 4 °C, 10 min) to obtain the supernatant, which was stored at −80 °C for further analysis. For each cytokine, a 96-well ELISA plate was set up with blank wells, standard wells, and sample wells, as well as duplicate wells. The optical density of the samples was determined using a standard curve based on the measurement of standard samples. All samples were analyzed by two experienced technicians who were blinded to the patients’ identities and clinical information. The within-run and between-run coefficients of variation for all analyses were found to be less than 10%.
Calculation of the cytokine imbalance ratio
To calculate the cytokine imbalance ratio using the Statistical Package for the Social Sciences (SPSS) version 26 (IBM), the following steps were taken: (1) Convert the plasma levels of each cytokine into standard z-scores using the formula z = (x - µ)/σ, where x is the measurement value of the subject, µ and σ are the mean and standard deviation (SD) of the group level. (2) Sum up the z-scores of the major cytokines produced by different subgroups, including M1 (zIL-6 + zIL-1β + zIFN-α + zTNF-α), Th-1 (zIL-2 + zIL-12p70 + zIFN-γ), Treg (zIL-10), Th-2 (zIL-4 + zIL-5), Th-17 (zIL-17), M1pTh-1pTh-17 (the sum of z-scores of all cytokines in M1, Th-1, and Th-17 subgroups), Th-2pTreg (the sum of z-scores of all cytokines in Th-2 and Treg subgroups), IRS (the sum of z-scores of zIL-2 + zIL-5 + zIL-6 + zIL-8 + zIL-17 + zIL-1β + zIL-12p70 + zIFN-α + zIFN-γ + zTNF-α), CIRS (the sum of z-scores of zIL-4 + zIL-10). (3) Use the z-unit weighted composite scoring ratio described in previous studies to estimate cytokine imbalance, including Th-1/Th-2 ratio, M1pTh-1pTh-17/Th-2pTreg ratio, and IRS/CIRS ratio21,36. For example, IRS/CIRS ratio = (zIL-2 + zIL-5 + zIL-6 + zIL-8 + zIL-17 + zIL-1β + zIL-12p70 + zIFN-α + zIFN-γ + zTNF-α) - (zIL-4 + zIL-10)21,24,37.
Structural neuroimaging acquisition and processing
The patient underwent magnetic resonance imaging (MRI) on the second day of admission. The MRI data was acquired using a 3.0-T Siemens Verio scanner (Siemens Healthcare, Erlangen, Germany). To reduce head movement and scanning noise, foam pads and soft earplugs were utilized. The brain volume sequence with the following parameters was used to obtain sagittal three-dimensional T1-weighted images: repetition time (TR) = 2000 ms; echo time (TE) = 2.3 ms; inversion time = 900 ms; flip angle (FA) = 7°; field of view (FOV) = 256 mm × 256 mm; matrix size = 256 × 256; slice thickness = 1 mm with a gap of 0.5 mm; 192 sagittal slices; acquisition time = 353 s. All participants were instructed to close their eyes, lie still, relax, minimize movement, avoid thinking about anything, and particularly, refrain from falling asleep during data acquisition. All participants in the study are required to undergo MRI scans using the exact same scanner.
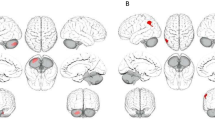
The FreeSurfer v7.1.1 software (http://surfer.nmr.mgh.harvard.edu) was used to obtain measurements of the subcortical volume and cortical thickness in the OFC. Scans were evaluated for artifacts, followed by first-level automated reconstruction, registering the scans to a standard space, and skull stripping. The images were then edited to remove the dura mater, nasal sinuses, and blood vessels. Second-level and third-level automated reconstruction were performed to extract measurements of cortical thickness and subcortical volume of OFC subregions. Ultimately, the OFC was divided into four subregions: mOFC, lOFC, right mOFC, and right lOFC. The rostral boundary of the lateral division of the OFC was the rostral extent of the lateral orbital gyrus (where it appears with the frontomarginal sulcus), and the caudal boundary was the caudal portion of the lateral orbital gyrus. The medial and lateral boundaries were the midpoint of the olfactory sulcus and the lateral bank of the lateral orbital sulcus and/or the circular insular sulcus, respectively. The rostral boundary of the medial division of the OFC was the rostral extent of the medial orbital gyrus, and the caudal boundary was the caudal portion of the medial orbital gyrus or gyrus rectus. The medial and lateral boundaries were the cingulate cortex on the inflated surface and the medial bank of the superior frontal gyrus (or the cingulate gyrus when visible), respectively38. For volume measurements, the total intracranial volume (ICV) was also extracted for covariate purposes. Visual inspection of the segmented cortical surface results did not reveal any segmentation defects. Additionally, to ensure comparability and standardization of the image data, the SPSS is used for data normalization.
Clinical assessment
On the day of the MRI, the BPRS is used to assess the patients’ psychiatric symptoms, while the Brief Agitation Rating Scale (BARS) is used to evaluate the severity of agitation. The BARS scale is derived from the Cohen-Mansfield Agitation Inventory (CMAI) and is a shorter version of it. It consists of 10 selected items out of the original 29 items in CMAI, using a 7-point scoring system to measure the frequency of aggression and agitation. These selected items cover three dimensions included in CMAI, namely physical aggression, verbal aggression, and non-aggressive agitation39. The BARS scale accounts for around 90% of the variance in CMAI scores40. The equivalent chlorpromazine (CPZ) dose is calculated using the current medication dosing41.
Statistical analysis
Demographic variables
The values of demographic variables such as age, sex, education level, illness duration, age of onset, body mass index (BMI), CPZ dose equivalents, BPRS, BARS, ICV and clinical variables were assessed for statistical analysis using SPSS. Box plots and scatter plots were generated using GraphPad Prism 9.5. The significance level was set at P < 0.05, and all analyses were two-tailed. In order to determine different subgroups based on varying degrees of agitation, two-step cluster analysis was conducted using BARS score on all participants, which automatically determined the optimal number of categories. The overall model quality, measured within the range of 0–1.0, needs to exceed 0.5. To evaluate normality, the Shapiro-Wilk test and Kolmogorov–Smirnov test were used. Between-group differences for normally distributed variables were analyzed using independent t-tests for continuous variables or chi-square analysis for categorical variables. In cases where the variables did not follow a normal distribution, the Mann-Whitney U test was applied for testing differences. Normally distributed data were presented as mean ± SD, while skewed data were represented using the median and interquartile range (IQR).
Between-group differences in peripheral cytokines and brain structure
The present study employed covariance analysis to compare the differences in z scores of cytokines, cytokine imbalance, and cortical thickness in the OFC subregions between the high-agitation and low-agitation groups of patients with schizophrenia, while controlling for illness duration as a covariate. Covariance analysis was employed to test the hypothesis that the volumes of OFC subregions were equal between the two groups, with adjustments made for ICV and illness duration. To account for multiple comparisons, the Benjamini-Hochberg procedure was employed to control the false discovery rate (FDR) of the twelve cytokines and four OFC subregion structures tested in each analysis (P < 0.05).
Correlation between cytokine imbalance and brain structure
A partial correlation analysis was conducted to examine the correlation between cytokine imbalance and the volume of the OFC subregions, with illness duration and ICV included as additional covariates. Additionally, a partial correlation analysis was employed to investigate the relationship between cytokine imbalance and the thickness of the OFC subregions, with illness duration as a covariate. The Benjamini-Hochberg procedure was employed to control the FDR of the analysis of the four OFC subregions’ structures (P < 0.05). Subsequently, variables of interest were further assessed based on the significant correlation results.
Moderation models
The moderation model was employed to ascertain whether agitation acts as a significant moderator in the relationship between cytokine imbalance and brain structure. For each model predicting the volume of the OFC subregion (Y), the predictive effect of each cytokine (X) was evaluated while controlling for covariates such as illness duration and ICV. Agitation grouping (W) was then included as the moderating variable. To determine if agitation grouping was a significant moderator in the relationship between cytokine imbalances and OFC subregion volume, we performed tests referencing the highest-order unconditional interactions. Additionally, we examined the conditional effects of each predicting factor at each group level to determine if the relationship between cytokine imbalances and OFC subregion volume differed between the high agitation subgroup and low agitation subgroup. In total, 3 models were constructed and multiple comparisons were corrected for using the FDR (with a significance threshold of P= 0.05). The analysis was performed using the PROCESS module in SPSS42.
Results
Demographics
In this study, a total of 119 patients with schizophrenia (46 males and 73 females) are included. All patients provide plasma samples, and 75 patients undergo MRI scans. These 119 patients with schizophrenia are subjected to two-step clustering analysis, and based on BARS scores, all participants are divided into a high agitation group and a low agitation group. The quality of the model is 0.7. It is noteworthy that 58.8% of the patients (n = 70) are classified into the low agitation group, while 41.2% of the patients (n = 49) are classified into the high agitation group. There are no statistically significant differences between the two groups of participants in terms of age, sex, BMI, education level, and CPZ dosage equivalent (all P’s > 0.05). In comparison to the low agitation schizophrenia, the high agitation schizophrenia exhibits more severe psychiatric symptoms and higher BPRS scores (P < 0.01). Nevertheless, there is a tendency for longer illness duration in the high agitation schizophrenia compared to the low agitation schizophrenia (P < 0.1). Consequently, illness duration is regarded as a confounding variable and included in the analyses of both between-group and within-group comparisons. The demographic and clinical characteristics of the participants are summarized in Table 1.
Comparisons of peripheral cytokine and brain structure
The study finds the expression levels of pro-inflammatory factors, including zIL-2 (P = 0.043), zIL-5 (P = 0.010), zIL-6 (P = 0.001), zIL-8 (P = 0.026), and zIFN-γ (P = 0.044), are higher in the high agitation schizophrenia compared to the low agitation schizophrenia. Moreover, the expression level of the anti-inflammatory factor zIL-4 (P = 0.025) is also higher in the high agitation schizophrenia. However, only zIL-6 reaches the level of significance after correcting for FDR. The levels of other cytokines, including zIL-10 (P = 0.439), zIL-17 (P = 0.584), zIL-1β (P = 0.069), zIL-12p70 (P = 0.915), zIFN-α (P = 0.318), and zTNF-α (P = 0.270) do not demonstrate significant differences between the two groups (See Fig. 1). In terms of the comparison of the structure of the OFC between the two groups, the volume of the right lOFC (P = 0.013) is found to be larger in the high agitation schizophrenia compared to the low agitation schizophrenia. However, after correcting for FDR, the difference does not reach statistical significance. No significant differences are observed in the volume of the other OFC subregions, including the right mOFC (P = 0.320), left lOFC (P = 0.163), and left mOFC (P = 0.139), in comparison to the low agitation schizophrenia. The cortical thickness of the OFC subregions does not differ between the two groups, including the right lOFC (P = 0.287), right mOFC (P = 0.562), left lOFC (P = 0.436), and left mOFC (P = 0.583) (see Supplementary Material, Figure S1).
Differences in cytokine levels between high and low agitation schizophrenia patients. IL-6 levels were higher in the high agitation group compared to the low agitation group, and other cytokine levels were not significantly different between the two groups, adjusted for illness duration. Abbreviations: IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; ns, no significance; CI: confidence interval. Cytokine levels were standardized. Solid line indicates 95% CI, dashed line indicates median. **P < 0.01.
Comparisons of cytokine imbalance
Significant differences in cytokine imbalance are observed between the two groups (See Fig. 2). The high agitation schizophrenia exhibits significantly elevated levels of M1 (P = 0.004), Th-2 (P = 0.002), IRS (P = 0.011), CIRS (P = 0.036), and IRS/CIRS ratio (P = 0.015) in comparison to the low agitation schizophrenia. Following the application of the FDR correction, the significant differences in M1, Th-2, IRS, and IRS/CIRS ratio remain. No significant differences are observed between the two groups in Th-1 (P = 0.220), Th-17 (P = 0.584), Treg (P = 0.439), Th-1/Th-2 ratio (P = 0.441), and M1 + Th-1 + Th-17/Th-2 + Treg ratio (P = 0.316).
Differences in inflammatory imbalance between high and low agitation schizophrenia patients. The levels of cytokine composite scores Th-2, M1, IRS, and IRS/CIRS ratio were significantly higher in the high agitation group compared with the low agitation group, adjusted for illness duration. Abbreviations: Th, helper T cell; Treg, regulatory T cells; IRS, the immune-inflammatory response system; CIRS, the compensatory immune-regulatory reflex system; ns, no significance; CI, confidence interval. Solid line indicates 95% CI, dashed line indicates median. *P < 0.05.
Relationships among cytokines and brain structure
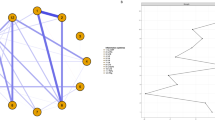
This study analyzed the relationship between cytokine levels and OFC subregion structure in schizophrenia patients with different severity of agitation. In patients with low agitation schizophrenia, there was a negative correlation between zIL-6 levels and right mOFC volume using illness duration and ICV as covariates (r = −0.299, P = 0.049), and a positive correlation between zIL-8 levels and the cortical thickness of the left mOFC using illness duration as covariate (r = 0.320, P = 0.032) (see Fig. 3A). In patients with high agitation schizophrenia, there was a negative correlation between zIL-17 levels and left lOFC volume (r = −0.428, P = 0.026), a negative correlation between zIFN-γ levels and left mOFC volume using illness duration and ICV as covariates (r = −0.424, P = 0.028), and a positive correlation between zIL-4 levels and left mOFC cortical thickness using illness duration as covariate (r = 0.424, P = 0.025) (see Fig. 3B).
Correlation analysis between cytokines and OFC subregion structure in high and low agitation schizophrenia patients. (A) Correlation analysis of indicators of cytokines with OFC subregion volume (adjusted for illness duration and ICV) and OFC subregion cortical thickness (adjusted for illness duration) in patients with low agitation schizophrenia. The numbers shown in the volcano plots are correlation coefficients. The results showed that there was a negative correlation between zIL-6 levels and right mOFC volume using illness duration and ICV as covariates (r = −0.299, P = 0.049), and a positive correlation between zIL-8 levels and the cortical thickness of the left mOFC using illness duration as covariate (r = 0.320, P = 0.032) in the low agitation group. (B) Correlation analysis of indicators of cytokines with OFC subregion volume (adjusted for illness duration and ICV) and OFC subregion cortical thickness (adjusted for illness duration) in patients with high agitation schizophrenia. The numbers shown in the volcano plots are correlation coefficients. The results showed that there was a negative correlation between zIL-17 levels and left lOFC volume (r = −0.428, P = 0.026), a negative correlation between zIFN-γ levels and left mOFC volume using illness duration and ICV as covariates (r = −0.424, P = 0.028), and a positive correlation between zIL-4 levels and left mOFC cortical thickness using illness duration as covariate (r = 0.424, P = 0.025) in the high agitation group. Abbreviations: lOFC, the lateral orbitofrontal cortex; mOFC, the medial orbitofrontal cortex, ICV, intracranial volume. *P < 0.05.
Relationships among cytokine imbalance and brain structure
The IRS/CIRS ratio is negatively correlated with left lOFC volume in high agitation schizophrenic patients using illness duration and ICV as covariates (r = −0.448, P = 0.019). There is no significant correlation between other cytokine imbalances and OFC subregion structure (See Fig. 4). Following this, a moderation model is used to verify whether agitation moderates the relationship between IRS/CIRS ratio and left lOFC volume. The moderation model error control FDR is 5%. Result shows that agitation is a significant moderator of the relationship between IRS/CIRS ratio and left lOFC volume (F = 4.744, P = 0.033). The simple slope test for the moderation model l is shown in Fig. 5.
Correlation analysis between inflammatory imbalance and OFC subregion structure in high and low agitation schizophrenia patients. (A) Correlation analysis of indicators of inflammatory imbalance such as Th-1/Th2, M1 + Th-1 + Th-17/Th-2 + Treg and IRS/CIRS with OFC subregion volume (adjusted for illness duration and ICV) and OFC subregion cortical thickness (adjusted for illness duration) in patients with low agitation schizophrenia. The numbers shown in the volcano plots are correlation coefficients. The results showed that no significant correlation was found between indicators of inflammatory imbalance and both OFC subregion volume and cortical thickness in the low agitation group. (B) Correlation analysis of indicators of inflammatory imbalance such as Th-1/Th2, M1 + Th-1 + Th-17/Th-2 + Treg and IRS/CIRS with OFC subregion volume (adjusted for illness duration and ICV) and OFC subregion cortical thickness (adjusted for illness duration) in patients with high agitation schizophrenia. The numbers shown in the volcano plots are correlation coefficients. The results showed that the IRS/CIRS ratio was only found to be negatively correlated with left lOFC volume in high agitation schizophrenia (r = −0.448, P = 0.019). Abbreviations: Th, helper T cell; Treg, regulatory T cells; IRS, the immune-inflammatory response system; CIRS, the compensatory immune-regulatory reflex system; lOFC, the lateral orbitofrontal cortex; mOFC, the medial orbitofrontal cortex, ICV, intracranial volume. Summary of z-scores for inflammatory imbalances in different subgroups of patients, including Th-1/Th-2 = (zIL-2 + zIL-12p70 + zIFN-γ) - (zIL-4 + zIL-5), M1pTh-1pTh-17/Th-2pTreg = (zIL-1β + zIL-2 + zIL-6 + zIL-12p70 + zIL-17 + zIFN-α + zTNF-α + zIFN-γ) - (zIL-4 + zIL-5 + zIL-10), IRS/CIRS = (zIL-2 + zIL-5 + zIL-6 + zIL-8 + zIL-17 + zIL-1β + zIL-12p70 + zIFN-α + zIFN-γ + zTNF-α)- (zIL-4 + zIL-10). z = (x - µ)/σ, where x is the subject’s measurement, and µ and σ are the mean and standard deviation at the group level. *P < 0.05.
Simple slope test for moderation model. Moderation model was used to validate the moderating effect of agitation on the IRS/CIRS ratio and left lOFC volume. The results showed that agitation was a significant moderator of the relationship between IRS/CIRS ratio and left lOFC volume (F = 4.744, P = 0.033). Abbreviations: IRS, the immune-inflammatory response system; CIRS, the compensatory immune-regulatory reflex system; lOFC, the lateral orbitofrontal cortex. IRS/CIRS = (zIL-2 + zIL-5 + zIL-6 + zIL-8 + zIL-17 + zIL-1β + zIL-12p70 + zIFN-α + zIFN-γ + zTNF-α)- (zIL-4 + zIL-10). z = (x - µ)/σ, where x is the subject’s measurement, and µ and σ are the mean and standard deviation at the group level.
Discussion
This study provided evidence for the role of peripheral cytokines and OFC subregion structure in the development of schizophrenic agitation. Compared to patients with low-agitation schizophrenia, patients with high-agitation schizophrenia had significantly higher levels of the peripheral cytokine IL-6, and significantly higher levels of the cytokine composite scores Th-2, M1, IRS, and IRS/CIRS ratio. In addition, the IRS/CIRS ratio was negatively correlated with the left lOFC grey matter volume in the high-agitation group, whereas this phenomenon was not found in the low-agitation group. However, there was no significant difference in gray matter volume and cortical thickness in the OFC subregion between the two groups. Thus, the moderation model showed that the severity of agitation significantly moderated the relationship between left lOFC grey matter volume and IRS/CIRS ratio. These findings suggested that inflammatory imbalance may be involved in the development of agitation in schizophrenia and that inflammatory imbalance may be correlated with altered OFC structure.
It was found that there was no significant difference in gray matter volume and cortical thickness of the OFC subregion in the high agitation group compared to the low agitation group, with a trend toward greater gray matter volume in the right lOFC only. Previous studies have found no significant differences in OFC gray matter volume and cortical thickness in schizophrenic patients with different states of agitation20,29. But it has also been shown that OFC gray matter volume and cortical thickness were smaller in patients with highly agitated schizophrenia than in patients with low agitated schizophrenia16,17. Our findings were partially consistent with previous studies. Although some studies have found a positive correlation between OFC gray matter volume and agitation severity in schizophrenia16. However, greater volume did not necessarily reflect better neurological function. Increased volume may reflect decreased nerve density, increased neuron size, or may reflect edema or other pathophysiological processes43. Therefore, one possible explanation for the trend toward increased gray matter volume in the OFC of the high agitation patients in this study was that excessive increases in proinflammatory cytokines adversely affect neuronal function, blood-brain barrier permeability, and brain structure, resulting in edema of brain structures. See below for a detailed elaboration. The study results of the OFC structure did not indicate whether there was impairment of its function, and future studies should further investigate OFC function in patients with schizophrenic agitation. In addition, previous studies have shown that patients with unilateral damage to the right OFC have impaired social behavior, decision making, emotional processing, and personality, whereas patients with left OFC damage have normal social and interpersonal behavior44. The above studies may suggest a relationship between altered right-sided OFC function and agitation, but confirmation of the findings needs to await studies of OFC function.
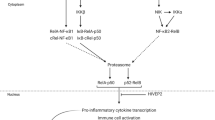
A large body of evidence supported the role of cytokines in the pathology of agitation45. However, fewer studies have analyzed the differences in cytokines in patients with schizophrenia according to the severity of agitation. We compared peripheral cytokine levels in two groups of patients and found that the levels of the proinflammatory cytokine IL-6 were significantly higher in patients with high agitation schizophrenia than in patients with low agitation schizophrenia, whereas no significant differences were seen in the levels of other proinflammatory and anti-inflammatory cytokines between the two groups. IL-6 was the cytokine most consistently elevated in the brains of schizophrenia patients found in the brain, especially in the OFC46. High levels of the pro-inflammatory cytokine IL-6 in agitated patients with schizophrenia have detrimental effects on neurotransmitter activity, neuroplasticity, apoptosis, and neurogenesis, damaging brain structures, which may be related to agitation47. It has been reported that intermittent burst disorder, an impulsive aggression disorder patients have elevated levels of IL-6, although there is no evidence yet to support elevated IL-6 in patients with agitated schizophrenia48. IL-6 can be communicated between the circulatory system and the central nervous system through an incomplete or disrupted blood-brain barrier49. Specifically, the pro-inflammatory cytokine IL-6 adversely affects the body by influencing tryptophan (TRP) metabolism. In general, TRP can be metabolized through two major pathways: one was the retention of the indole ring to synthesize biologically active indole compounds including serotonin as a neurotransmitter and melatonin as a neuromodulator, while on the other hand, oxidative cleavage of the indole ring initiates the kynurenine metabolism pathway, which is ultimately broken down to quinolinic acid or kynurenine50,51. Elevated IL-6 induces an upregulation of indoleamine 2,3-dioxygenase (IDO) activity, a rate-limiting enzyme that converts TRP to kynurenine52. Increased IDO activity may lead to a decrease in TRP, a decrease in 5-HT synthesis and an increase in quinolinic acid. Elevated levels of quinolinic acid led to potential disruption of blood-brain barrier integrity53. Also decreased 5-HT levels and/or function are associated with aggressive behavior54,55. In addition, previous studies have explored the levels of other cytokines and found no differences in IL-1β, IL-4, IL-8, IL-10,IL-12p70, and IFN-γ levels in patients with schizophrenic agitation compared to those with non-agitated schizophrenia27. However, it has also been found that TNF-α, IFN-γ, and IL-10 are greater in impulsive than in non-impulsive psychosis, and that the inflammatory imbalance represented by IFN-γ/IL-10 was also higher in patients with impulsive psychosis29,56.
In this study, in addition to measuring the traditional Th-1/Th-2 and Th-17/Treg ratios, we also used the IRS/CIRS ratio as a measure of cytokine imbalance, which was capable of revealing the combined effects of pro- and anti-inflammatory cytokines more comprehensively21,57. The results of the study showed that IRS/CIRS ratio was higher in high agitated schizophrenia patients compared to low agitated schizophrenia patients, while no significant differences were seen in other cytokine imbalance measures. The higher cytokine imbalance IRS/CIRS ratio may be due to overactivation of pro-inflammatory cytokines (IRS, M1) and under-activation of anti-inflammatory cytokines (Th-2). Our findings were consistent with prior findings. Previous studies have shown that the proinflammatory/anti-inflammatory cytokine imbalance represented by IFN-γ/IL-10 was greater in patients with high agitation psychiatric patients than in patients with low agitation56. Also, a positive correlation between the inflammatory imbalance represented by hypersensitive C-reactive protein/IL-10 and the severity of agitation was confirmed in patients with schizophrenia58. Although these studies used only single cytokine to reflect pro/anti-inflammatory cytokine imbalances, the present results supported the possibility of an inflammatory imbalance in highly agitated schizophrenic patients. And increased inflammatory imbalance may correlated with the occurrence of agitation in schizophrenic patients. As previously discussed, increased pro-inflammatory levels activated the kynurenine pathway, causing quinolinic acid levels to rise53. In addition to acting as a direct the N-methyl-D-aspartate (NMDA) receptor agonist, quinolinic acid increased neuronal glutamate release and decreased glutamate uptake and recycling by astrocytes59. Studies in animals and humans have shown that elevated glutamate levels increased aggressive behavior. Microinjections of glutamate into the periaqueductal gray matter of cats elicited defensive rage60. Similarly, mice lacking glutamate receptors showed reduced aggressive behavior compared to otherwise normal mice61,62. Consistent findings have been found in human studies, where the risk of aggressive behavior in subjects increased as glutamate levels increased63. In addition, the large release and accumulation of glutamate in patients led to overstimulation of neurons, causing excitotoxicity. The excitotoxicity of glutamate occurred primarily through binding to receptors on the cell membrane of neurons, causing an increase in intracellular Na+ and Ca2+. Increased intracellular Ca2+ concentration would cause abnormal mitochondrial function, protease activation, increased reactive oxygen species and nitric oxide (NO) release, thus causing neuronal death; increased intracellular Na+would cause excess water to enter the cell, resulting in neuronal cytotoxic edema and cell death64,65. The cytotoxic edema of glutamate may explain the presence of a trend toward increased volume of the OFC subregion found in this study, whereas cell death due to increased glutamate levels may be responsible for the structural damage to the OFC observed in previous studies. Therefore, we further analyzed the relationship between inflammatory imbalance and OFC subregion structure.
Previous studies have revealed the complexity of the relationship between cytokines and OFC subregions. A positive correlation was found between the levels of pro-inflammatory cytokines IL-8 and TNF-α and mOFC volume in schizophrenia patients, whereas a significant negative correlation was found between the levels of anti-inflammatory cytokine IL-10 and mOFC volume, which may correspond to the neurobiological basis of the different agitation subtypes32. Similar results were also found in patients with bipolar disorder with high impulsivity, with a significant negative correlation between the proinflammatory factor IFN-γ and mOFC volume66. Combined with the assessment dimensions of the BARS, we hypothesized that physical agitation (e.g., aggression) may be related to motor regulatory functions in the lOFC; whereas verbal agitation (e.g., verbal abuse) and emotional agitation (e.g., irritability) may be closely related to dysfunction of reward appraisal in the mOFC, and that volume damage to the region may weaken the ability to regulate emotions, which may in turn trigger overactivation of the anti-inflammatory system. However, increased levels of anti-inflammatory cytokines in OFC brain regions were found in autopsy results of patients with depressive disorders who died by suicide, and the alterations in cytokines differed between genders67. Specifically, IL-4 level was increased in the OFC of female suicide victims, and IL-13 level was increased in the OFC of male suicide victims. There may be potential interactions between cytokines and OFC structures, but given the differences between the different cytokines, we suggested a combined role of pro- and anti-inflammatory cytokines by calculating an index of inflammatory imbalance. Correlation analysis showed that IRS/CIRS ratio was negatively correlated with left lOFC volume. Also, further modulation modeling showed that agitation was a significant modulator of inflammatory imbalance and OFC structural damage, providing evidence for a relationship between inflammatory imbalance and OFC in agitated schizophrenic patients. At the level of neural mechanisms, previous findings have shown increased levels of astrocytes and microglia correlated with neuroinflammation in the central nervous system of patients with schizophrenia68,69,70. Microglia were the primary recipients of peripheral inflammatory signals reaching the brain, and activated microglia initiate an inflammatory cascade response that resulted in the release of associated cytokines, chemokines, inflammatory mediators, as well as reactive nitrogen and reactive oxygen species that induce reciprocal activation of astrocytes, thereby amplifying inflammatory signals within the central nervous system47. Cytokines, including IL-1, IL-6, and TNF-α, as well as IFN-α and IFN-γ, induced IDO to catabolize tryptophan into quinolinic acid, a potent NMDA agonist and stimulator of glutamate release. Because quinolinic acid had a glutamate-like structure, it was a competitive agonist of the NMDA receptor, competitively inhibiting glutamate release and increasing its content in the synaptic gap71. And dopamine function may be secondary to altered glutamatergic function, so glutamate levels were elevated and dopaminergic neurons were activated72. Therefore, elevated levels of glutamate and dopamine in agitation schizophrenia patients may be another cause of structural damage in the OFC. One study found a negative relationship between dopamine levels and left OFC volume in schizophrenia patients73. Dopamine was associated with reward processing, reward-related instrumental behaviors, and could modulate the function of the OFC74. Dysregulation of the reward circuitry when dopamine levels were abnormal may lead to abnormal OFC functioning and thus agitation75. A link between elevated glutamate levels and agitation has also been demonstrated63. The cytotoxicity produced by elevated glutamate levels following inflammatory imbalance may mediate the oxidative stress response and neuronal Ca2+in-flow, allowing reactive oxidants and nitrosative substances to accumulate intracellularly, disrupting synaptic transmission in the brain resulting in cell death, and ultimately, may lead to structural alterations in OFC subregions25,76. In conclusion, inflammatory imbalance may be impairing the structure of the OFC subregion by regulating canine uric acid metabolism increasing quinolinic acid, glutamate, and dopamine levels.
Several limitations of the current study should be mentioned here. Firstly, it would have been more beneficial to conduct tests for relevant inflammatory factors in the cerebrospinal fluid to demonstrate altered levels of inflammation within the central nervous system. Secondly, although no significant difference in CPZ equivalents was observed between the two groups in this study, the findings do not exclude the influence of antipsychotic drugs. Thirdly, we employed the BARS to assess the severity of schizophrenic agitation; however, a multidimensional assessment may prove more effective in responding to agitation. Fourth, agitation is a heterogeneous phenomenon with different subtypes such as physical, verbal and emotional agitation. There is a lack of research on OFC structure and inflammation levels in patients with schizophrenia with different subtypes of agitation, which may be one of the reasons for the inconsistent results of previous studies. Future studies should include a larger sample of agitation schizophrenia patients with different subtypes in order to gain a clearer understanding of the specific effects of the inflammatory imbalance and the changes in OFC structure on the behavioral outcomes. Fifth, this study was only able to indicate a possible association between inflammatory imbalance and agitation, but the causal relationship between the two is not yet clear, and potential longitudinal or interventional studies are recommended to elucidate directionality. Finally, future studies involving a more comprehensive range of cytokines and related metabolites may provide a more adequate understanding of the mechanisms of schizophrenic agitation.
Conclusion
In conclusion our findings suggest that high agitation patients exhibit a trend towards larger right lOFC volume and higher IL-6 levels compared to low agitation schizophrenia patients. There was a significant negative correlation between IRS/CIRS levels and left lOFC volume in high agitation schizophrenia patients. Further moderating models suggested a moderating effect of different severity of agitation on IRS/CIRS levels with left lOFC volume. Thus, the present study provides assistance in explaining the etiological mechanisms of agitation in schizophrenia and offers potential directions for the future development of targeted treatments.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Wallwork, R. S., Fortgang, R., Hashimoto, R., Weinberger, D. R. & Dickinson, D. Searching for a consensus five-factor model of the positive and negative syndrome scale for schizophrenia. Schizophr Res. 137, 246–250. https://doi.org/10.1016/j.schres.2012.01.031 (2012).
Piechniczek-Buczek, J. Psychiatric emergencies in the elderly population. Emerg Med Clin North Am 24, 467–490, viii, (2006). https://doi.org/10.1016/j.emc.2006.01.008
Battaglia, J. Pharmacological management of acute agitation. Drugs 65, 1207–1222. https://doi.org/10.2165/00003495-200565090-00003 (2005).
Mohr, P., Pecenák, J., Svestka, J., Swingler, D. & Treuer, T. Treatment of acute agitation in psychotic disorders. Neuro Endocrinol. Lett. 26, 327–335 (2005).
Vieta, E. et al. Protocol for the management of psychiatric patients with psychomotor agitation. BMC Psychiatry. 17 https://doi.org/10.1186/s12888-017-1490-0 (2017).
Pilowsky, L. S., Ring, H., Shine, P. J., Battersby, M. & Lader, M. Rapid tranquillisation. A survey of emergency prescribing in a general psychiatric hospital. Br. J. Psychiatry. 160, 831–835. https://doi.org/10.1192/bjp.160.6.831 (1992).
Dean, K. et al. Aggressive behaviour at first contact with services: findings from the AESOP first episode psychosis study. Psychol. Med. 37, 547–557. https://doi.org/10.1017/s0033291706008920 (2007).
Martinez-Raga, J. et al. 1st international experts’ meeting on agitation: conclusions regarding the current and ideal management paradigm of agitation. Front. Psychiatry. 9, 54. https://doi.org/10.3389/fpsyt.2018.00054 (2018).
Sagayadevan, V. et al. Mediating effect of symptom severity on the relationship between aggression, impulsivity and quality of life outcomes among patients with schizophrenia and related psychoses. Front. Psychiatry. 14, 1154083. https://doi.org/10.3389/fpsyt.2023.1154083 (2023).
Ho, B. C., Barry, A. B. & Koeppel, J. A. Impulsivity in unaffected adolescent biological relatives of schizophrenia patients. J. Psychiatr Res. 97, 47–53. https://doi.org/10.1016/j.jpsychires.2017.11.008 (2018).
Fekih-Romdhane, F., Maktouf, H. & Cheour, M. Aggressive behaviour in antipsychotic-naive first-episode schizophrenia patients, their unaffected siblings and healthy controls. Early Interv Psychiatry. 17, 299–310. https://doi.org/10.1111/eip.13329 (2023).
van Os, J., Kapur, S. & Schizophrenia Lancet 374, 635–645, doi:https://doi.org/10.1016/s0140-6736(09)60995-8 (2009).
Wallis, J. D. Orbitofrontal cortex and its contribution to Decision-Making. Annu. Rev. Neurosci. 30, 31–56. https://doi.org/10.1146/annurev.neuro.30.051606.094334 (2007).
Weiss, E. M. Neuroimaging and neurocognitive correlates of aggression and violence in schizophrenia. Scientifica (Cairo) 158646, (2012). https://doi.org/10.6064/2012/158646 (2012).
Rolls, E. T. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct. Function. 228, 1201–1257. https://doi.org/10.1007/s00429-023-02644-9 (2023).
Lu, J. et al. Brain structural alterations associated with impulsiveness in male violent patients with schizophrenia. BMC Psychiatry. 24 https://doi.org/10.1186/s12888-024-05721-3 (2024).
Hoptman, M. J., Antonius, D., Mauro, C. J., Parker, E. M. & Javitt, D. C. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am. J. Psychiatry. 171, 939–948. https://doi.org/10.1176/appi.ajp.2014.13111553 (2014).
Narayan, V. M. et al. Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am. J. Psychiatry. 164, 1418–1427. https://doi.org/10.1176/appi.ajp.2007.06101631 (2007).
Kumari, V. et al. Dysfunctional, but not functional, impulsivity is associated with a history of seriously violent behaviour and reduced orbitofrontal and hippocampal volumes in schizophrenia. Psychiatry Res. 173, 39–44. https://doi.org/10.1016/j.pscychresns.2008.09.003 (2009).
Yu, T., Pei, W., Xu, C., Zhang, X. & Deng, C. Prediction of violence in male schizophrenia using sMRI, based on machine learning algorithms. BMC Psychiatry. 22, 676. https://doi.org/10.1186/s12888-022-04331-1 (2022).
Roomruangwong, C. et al. The role of aberrations in the Immune-Inflammatory response system (IRS) and the compensatory Immune-Regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol. Neurobiol. 57, 778–797. https://doi.org/10.1007/s12035-019-01737-z (2019).
Maes, M., Song, C. & Yirmiya, R. Targeting IL-1 in depression. Expert Opin. Ther. Targets. 16, 1097–1112. https://doi.org/10.1517/14728222.2012.718331 (2012).
Martinez, F. O. & Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13. https://doi.org/10.12703/p6-13 (2014).
Maes, M. & Carvalho, A. F. The compensatory Immune-Regulatory reflex system (CIRS) in depression and bipolar disorder. Mol. Neurobiol. 55, 8885–8903. https://doi.org/10.1007/s12035-018-1016-x (2018).
Korkmaz, Ş., Kaymak, A., Neşelioğlu, S. U., Erel, Ö. & S. & Thiol-disulphide homeostasis in patients with schizophrenia: the potential biomarkers of oxidative stress in acute exacerbation of schizophrenia. Clin. Psychopharmacol. Neurosci. 22, 139–150. https://doi.org/10.9758/cpn.23.1084 (2024).
Li, H. et al. Plasma levels of Th17-related cytokines and complement C3 correlated with aggressive behavior in patients with schizophrenia. Psychiatry Res. 246, 700–706. https://doi.org/10.1016/j.psychres.2016.10.061 (2016).
Hashimoto, K., Larsen, J. B., Stunes, A. K., Vaaler, A. & Reitan, S. K. Cytokines in agitated and non-agitated patients admitted to an acute psychiatric department: A cross-sectional study. Plos One. 14 https://doi.org/10.1371/journal.pone.0222242 (2019).
Balcioglu, Y. H., Balcioglu, K., Oncu, S. S., Turkcan, F., Yorulmaz, A. C. & A. & Impulsive and aggressive traits and increased peripheral inflammatory status as Psychobiological substrates of homicide behavior in schizophrenia. Eur. J. Psychiatry. 36, 207–214. https://doi.org/10.1016/j.ejpsy.2022.01.004 (2022).
Fan, Y. et al. Grey matter volume and its association with cognitive impairment and peripheral cytokines in excited individuals with schizophrenia. Brain Imaging Behav. 16, 2618–2626. https://doi.org/10.1007/s11682-022-00717-7 (2022).
Zhang, Y. et al. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Translational Psychiatry. 6, e982–e982. https://doi.org/10.1038/tp.2016.238 (2016).
Lizano, P. et al. Multivariate relationships between peripheral inflammatory marker subtypes and cognitive and brain structural measures in psychosis. Mol. Psychiatry. 26, 3430–3443. https://doi.org/10.1038/s41380-020-00914-0 (2021).
Wu, D. et al. Association of peripheral cytokine levels with cerebral structural abnormalities in schizophrenia. Brain Res. 1724 https://doi.org/10.1016/j.brainres.2019.146463 (2019).
Lesh, T. A. et al. Cytokine alterations in first-episode schizophrenia and bipolar disorder: relationships to brain structure and symptoms. J. Neuroinflamm. 15 https://doi.org/10.1186/s12974-018-1197-2 (2018).
World Medical, A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. Jama 310, 2191–2194 (2013).
Regier, D. A., Kuhl, E. A. & Kupfer, D. J. The DSM-5: classification and criteria changes. World Psychiatry. 12, 92–98. https://doi.org/10.1002/wps.20050 (2013).
Chen, W. et al. Inflammatory disequilibrium and lateral ventricular enlargement in treatment-resistant schizophrenia. Eur. Neuropsychopharmacol. 72, 18–29. https://doi.org/10.1016/j.euroneuro.2023.03.014 (2023).
Maes, M. et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur. Neuropsychopharmacol. 10, 119–124. https://doi.org/10.1016/s0924-977x(99)00062-0 (2000).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31, 968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021 (2006).
Sommer, O. H. & Engedal, K. Reliability and validity of the Norwegian version of the brief agitation rating scale (BARS) in dementia. Aging Ment Health. 15, 252–258. https://doi.org/10.1080/13607863.2010.519318 (2011).
Sommer, O. H., Kirkevold, Ø., Cvancarova, M. & Engedal, K. Factor analysis of the brief agitation rating scale in a large sample of Norwegian nursing home patients. Dement. Geriatr. Cogn. Disord. 29, 55–60. https://doi.org/10.1159/000265542 (2010).
Woods, S. W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 64, 663–667. https://doi.org/10.4088/jcp.v64n0607 (2003).
Hayes A. F.
Hoptman, M. J. et al. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Res. 140, 133–145. https://doi.org/10.1016/j.pscychresns.2005.07.004 (2005).
Tranel, D., Bechara, A. & Denburg, N. L. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, Decision-Making, and emotional processing. Cortex 38, 589–612. https://doi.org/10.1016/s0010-9452(08)70024-8 (2002).
Chang, H. B. et al. The role of substance use, smoking, and inflammation in risk for suicidal behavior. J. Affect. Disord. 243, 33–41. https://doi.org/10.1016/j.jad.2018.09.005 (2019).
Alexandros Lalousis, P. et al. Inflammatory subgroups of schizophrenia and their association with brain structure: A semi-supervised machine learning examination of heterogeneity. Brain. Behav. Immun. 113, 166–175. https://doi.org/10.1016/j.bbi.2023.06.023 (2023).
Miller, A. H., Maletic, V. & Raison, C. L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 65, 732–741. https://doi.org/10.1016/j.biopsych.2008.11.029 (2009).
Coccaro, E. F., Lee, R. & Coussons-Read, M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 71, 158–165. https://doi.org/10.1001/jamapsychiatry.2013.3297 (2014).
Leonard, B. & Maes, M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav Rev. 36, 764–785. https://doi.org/10.1016/j.neubiorev.2011.12.005 (2012).
Fiore, A. & Murray, P. J. Tryptophan and Indole metabolism in immune regulation. Curr. Opin. Immunol. 70, 7–14. https://doi.org/10.1016/j.coi.2020.12.001 (2021).
Davidson, M., Rashidi, N., Nurgali, K. & Apostolopoulos, V. The role of Tryptophan metabolites in neuropsychiatric disorders. Int. J. Mol. Sci. 23 https://doi.org/10.3390/ijms23179968 (2022).
Baharikhoob, P. & Kolla, N. J. Microglial dysregulation and suicidality: A Stress-Diathesis perspective. Front. Psychiatry. 11, 781. https://doi.org/10.3389/fpsyt.2020.00781 (2020).
Osuch, B., Misztal, T., Pałatyńska, K. & Tomaszewska-Zaremba, D. Implications of kynurenine pathway metabolism for the immune system, Hypothalamic-Pituitary-Adrenal axis, and neurotransmission in alcohol use disorder. Int. J. Mol. Sci. 25 https://doi.org/10.3390/ijms25094845 (2024).
Coccaro, E. F., Fanning, J. R., Phan, K. L. & Lee, R. Serotonin and impulsive aggression. CNS Spectr. 20, 295–302. https://doi.org/10.1017/s1092852915000310 (2015).
Coccaro, E. F., Lee, R. & Coussons-Read, M. Cerebrospinal fluid inflammatory cytokines and aggression in personality disordered subjects. Int. J. Neuropsychopharmacol. 18, pyv001. https://doi.org/10.1093/ijnp/pyv001 (2015).
Das, S. et al. Aggression as an independent entity even in psychosis- the role of inflammatory cytokines. J. Neuroimmunol. 292, 45–51. https://doi.org/10.1016/j.jneuroim.2016.01.012 (2016).
Chiang, S. S., Riedel, M., Schwarz, M. & Mueller, N. Is T-helper type 2 shift schizophrenia-specific? Primary results from a comparison of related psychiatric disorders and healthy controls. Psychiatry Clin. Neurosci. 67, 228–236. https://doi.org/10.1111/pcn.12040 (2013).
Zhang, Q. et al. Increased ratio of high sensitivity C-reactive protein to interleukin-10 as a potential peripheral biomarker of schizophrenia and aggression. Int. J. Psychophysiol. 114, 9–15. https://doi.org/10.1016/j.ijpsycho.2017.02.001 (2017).
Guillemin, G. J. Quinolinic acid, the inescapable neurotoxin. Febs J. 279, 1356–1365. https://doi.org/10.1111/j.1742-4658.2012.08485.x (2012).
Bandler, R. Identification of hypothalamic and midbrain periaqueductal Gray and neurons mediating aggressive and defensive behavior by intracerebral microinjections of excitatory amino acids. Neurol. Neurobiol. 12 (1984).
Adamczyk, A. et al. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav. Brain Res. 229, 265–272. https://doi.org/10.1016/j.bbr.2012.01.007 (2012).
Vekovischeva, O. Y., Aitta-aho, T., Verbitskaya, E., Sandnabba, K. & Korpi, E. R. Acute effects of AMPA-type glutamate receptor antagonists on intermale social behavior in two mouse lines bidirectionally selected for offensive aggression. Pharmacol. Biochem. Behav. 87, 241–249. https://doi.org/10.1016/j.pbb.2007.04.020 (2007).
Coccaro, E. F., Lee, R. & Vezina, P. Cerebrospinal fluid glutamate concentration correlates with impulsive aggression in human subjects. J. Psychiatr Res. 47, 1247–1253. https://doi.org/10.1016/j.jpsychires.2013.05.001 (2013).
Zhang, J. et al. Intracellular ion and protein nanoparticle-induced osmotic pressure modify astrocyte swelling and brain edema in response to glutamate stimuli. Redox Biol. 21, 101112. https://doi.org/10.1016/j.redox.2019.101112 (2019).
Zheng, Z. et al. Co-activation of NMDAR and mGluRs controls protein nanoparticle-induced osmotic pressure in neurotoxic edema. Biomed. Pharmacother. 169, 115917. https://doi.org/10.1016/j.biopha.2023.115917 (2023).
Mohite, S. et al. Correlations between peripheral levels of inflammatory mediators and frontolimbic structures in bipolar disorder: an exploratory analysis. CNS Spectr. 27, 639–644. https://doi.org/10.1017/s1092852921000596 (2022).
Tonelli, L. H. et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. 117, 198–206. https://doi.org/10.1111/j.1600-0447.2007.01128.x (2007).
Catts, V. S., Wong, J., Fillman, S. G., Fung, S. J. & Shannon Weickert, C. Increased expression of astrocyte markers in schizophrenia: association with neuroinflammation. Aust N Z. J. Psychiatry. 48, 722–734. https://doi.org/10.1177/0004867414531078 (2014).
Doorduin, J. et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J. Nucl. Med. 50, 1801–1807. https://doi.org/10.2967/jnumed.109.066647 (2009).
van Berckel, B. N. et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11 C]PK11195 positron emission tomography study. Biol. Psychiatry. 64, 820–822. https://doi.org/10.1016/j.biopsych.2008.04.025 (2008).
Bryleva, E. Y. & Brundin, L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 112, 324–330. https://doi.org/10.1016/j.neuropharm.2016.01.034 (2017).
McGuire, P., Howes, O. D., Stone, J. & Fusar-Poli, P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol. Sci. 29, 91–98. https://doi.org/10.1016/j.tips.2007.11.005 (2008).
Rogeau, A. et al. The relationship between glutamate, dopamine, and cortical Gray matter: A simultaneous PET-MR study. Mol. Psychiatry. 27, 3493–3500. https://doi.org/10.1038/s41380-022-01596-6 (2022).
Kahnt, T. & Tobler, P. N. Dopamine modulates the functional organization of the orbitofrontal cortex. J. Neurosci. 37, 1493–1504. https://doi.org/10.1523/jneurosci.2827-16.2016 (2017).
Winstanley, C. A. et al. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav. Brain Res. 210, 263–272. https://doi.org/10.1016/j.bbr.2010.02.044 (2010).
Clausen, M. V., Hilbers, F. & Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 8, 371. https://doi.org/10.3389/fphys.2017.00371 (2017).
Acknowledgements
We are deeply grateful to the participants for their generous dedication. Equally important were the contributions of the psychiatrists involved with recruiting and diagnosing patients.
Funding
This work was supported by the Scientific Research Foundation of Nanjing Medical University (grant number 201715048 and ZKX17030).
Author information
Authors and Affiliations
Contributions
Conceptualization, YS; investigation, YS, YD. and TY; methodology, YS, YD, TY, LY and CP; resources, SY; supervision, SY; validation, SY; visualization, SY, DY, XG, ZR and LR; writing, SY, DY, TY, LY, CP, XG, ZR, and LR. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests.
Ethics approval
This study was approved by the Ethics Committee of The Affiliated Brain Hospital of Nanjing Medical University, Jiangsu, China (Ethic approval numbers:2023-KY144-01).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, Y., Tang, Y., Li, Y. et al. Role of peripheral cytokines and orbitofrontal cortex subregion structure in schizophrenia agitation. Sci Rep 15, 14125 (2025). https://doi.org/10.1038/s41598-025-99033-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-99033-5