Abstract
Triple-negative breast cancer (TNBC) possesses high malignant and metastatic rates among all subtypes. Chemotherapy is a standard of care for TNBC but only a small moiety of patients achieved complete relief (CR) after chemotherapy. The recent concept of tumor ecosystem has provided new insights into solutions from an approach of enhancing anti-tumoral immunity of macrophages. We hereby observed a positive correlation of YTHDC2 abundance with anti-tumoral gene markers of macrophages. YTHDC2-high macrophages also exerted interactions with other immune cells such as T helper cells, cytotoxic T cells, and NK cells. Further investigation on the transcriptional regulatory network identified six transcriptional factors upregulated by YTHDC2, and they together influenced the expressions of TWISTNB and the oncogene MYC. Additionally, our survival analysis prompted that YTHDC2 is prognostic of higher chemo-therapeutic efficacy and better survival outcomes. We demonstrated that ample macrophage YTHDC2 indicates anti-tumoral phenotype polarization and propitious survival outcome in post-treatment TNBC patients (Clinical trial registry name: Chinese Clinical Trial Registry, Registration No.: ChiCTR2400084513, Registration Date: 2024-05-20).
Similar content being viewed by others
Introduction
The absence of estrogen/progesterone receptors and human epidermal receptor 2 (HER2) amplification renders triple-negative breast cancer (TNBC) highly resistant to endocrine- or HER2-targeted-therapy1. Chemotherapy has hence become a standard of care in clinic2. Metastasis and recurrence are common in TNBC patients who do not achieve complete relief (CR) after chemotherapy3; it is therefore impeding to improve the CR rate of the current treatment plans and overcome chemo-resistance in TNBC.
Recent studies have endorsed the evaluation of chemo-therapeutic efficacy from an approach focused on the anti-tumoral immune ecosystem, rather than solely on the cytotoxic effects of agents4,5. The concept of tumor immune microenvironment (TIME) prompts more attention towards exploring the interactions between tumor cells and other peripheral cells such as fibroblasts, stroma, and immune cells6, to explain the response to chemotherapy7,8,9.
Consensus had been reached that, as a vital cell type in the tumor microenvironment, macrophages generally differentiate into two distinct states, namely anti-tumoral and pro-tumoral phenotypes10. The anti-tumoral macrophages inhibit tumor growth through phagocytosis; they also work comprehensively with other immune cells such as NK cells, CD4+ and CD8+ T cells11. Pro-tumoral macrophages, on the other hand, promote tumor cell proliferation, vascularization, and metastasis12. Both categories have been proven to closely correlate with chemotherapy efficacy13,14,15.
One of the most common RNA modifications, N6-methyladenosine (m6A), has been demonstrated to extensively influence macrophage polarization16. For instance, m6A writer METTL317 and the m6A eraser FTO18 evidently promote pro-tumoral polarization of macrophages. However, when it comes to m6A readers, few substantial conclusions have been made19, and contradictory results are often reported, especially in the family of YTH proteins20. This is likely due to the overlapped binding sites among different YTH proteins21.
The m6A reader protein YTHDC2 has been shown to be involved in multiple physiological processes of various cancers22,23,24; it is also valuable as a diagnostic and prognostic biomarker25,26. Its mechanism in the regulation of macrophages, however, remains unclear. We hereby excogitated this study to explore the regulatory roles of YTHDC2 in macrophage polarization, its interactions with other immune cells, and its influence on clinical responses to chemotherapy.
Results
Sample collection and processing
A total of 19 TNBC patients (mean [SD] age, 45 [9] years) were included and the biopsied tumor samples were subjected to scRNA-seq (P01, P02, P03) along with the data referenced from the GSE169246 dataset27 of Gene Expression Omnibus (GEO) database in National Center for Biotechnology Information (NCBI). Please refer to the methods for detailed information (Fig. 1, Supplementary Table 1). We performed scRNA-seq of tumor-infiltrating CD45+ immune cells from all 19 patients. The combined tumor scRNA-seq dataset contained 23,369 high-quality cells from well-defined myeloid immune lineages (Supplementary Fig. 1a, b).
Identification of anti-tumoral and pro-tumoral macrophage populations in TNBC
Tumor-infiltrating macrophages were categorized into five sub-clusters (Fig. 2a, Supplementary Fig. 1c, d). PCA analysis showed that the macrophages from metastatic tumors tended to commingle and neighbor closely; but they were explicitly distinct from those of primary tumors (Fig. 2b). Cells in cluster 1 expressed hallmark genes SPP1 and APOE (Fig. 2a, c), which had been proven to be implicated in pro-tumoral macrophage immunity28,29. Similarly, cluster 2 macrophages expressed anti-tumoral genetic patterns (TNF+ and IL1B+) (Fig. 2a, c). We then delved into the differentially expressed (DE) genes across the five clusters to define subpopulation-specific transcriptional signatures (Fig. 2d). Cluster 3 was defined as the monocyte subset due to the co-expression of S100A8 and S100A9. Cluster 4 and 5 saliently expressed the genes encoding complement protein C1Q (C1QA, C1QB, and C1QC) (Fig. 2d). As a reference, scRNA-seq analysis was performed on peripheral blood mononuclear cells (PBMCs) to scan potential precursor cells of these tumor-infiltrating macrophages. The datasets from patients P02 and P03 were integrated with those from the GSE169246 dataset (patients P01 and GSEP03 were excluded because no peripheral blood sample was drawn or reserved). The resulting data revealed a diverse array of blood myeloid cells, including S100A9+ monocytes, CD16 (FCGR3A)+ monocytes, IL1B+ monocytes, CD14+ monocytes, HLA-DPB1+ monocytes, CD1C+ dendritic cells (DCs), CLEC9A+ DCs, plasmacytoid dendritic cells (pDCs), and NFKBIZ+ monocytes (Supplementary Fig. 2a–c). Among these cells, NFKBIZ+ monocytes are distinguished by their high expression levels of YTDHC2 (Supplementary Fig. 2d).
a Uniform Manifold Approximation and Projection (UMAP) embedding of tumor macrophages. The inset shows the normalized expression level of the CD68 gene (n = 19 patients, n = 42 tumor samples). b Principal component analysis (PCA) plot of cells in scRNA-seq. Single-cell level measurements for each tissue origin were visualized to obtain pseudo-bulk distribution for the PCA analysis, related to the UMAP plot in (a). c Single-cell transcription of selected markers defining tumor macrophage clusters. Transcription levels are color-coded: gray, not expressed; blue, expressed. d Dot plot representing the relative average expression of a subset of marker genes across all clusters. The marker genes in each cluster were identified by log fold change compared with the remaining clusters. e Dot plot showing the relative average expression of a subset of anti-tumoral and pro-tumoral marker genes (y-axis) across the clusters (x-axis). As indicated in the legend, dot size denotes the percentage of cells in a cluster expressing each gene. The dot color represents the relative average expression levels. f Differentially expressed (DE) genes between tumor anti-tumoral and pro-tumoral macrophages; averaged scaled gene expression of all cells in each group.
Next, we measured the transcriptional levels of genes to distinguish anti-tumoral and pro-tumoral macrophage populations (Fig. 2e). Notably, cluster 1 exhibited upregulation of pro-tumoral transcripts such as PPARA, CD163, and MRC1 (CD206). In contrast, anti-tumoral gene expressions (IL1A, NOS1, IFNG, and NFKB1) were more enriched in cluster 230. DE genes confirmed this finding and identified CTSD as the most upregulated gene in pro-tumoral macrophages, whereas IL1B was the most upregulated in anti-tumoral macrophages (Fig. 2f).
YTHDC2 positively correlates with the expressions of anti-tumoral marker genes in intra-tumoral macrophages of TNBC
We then aimed to investigate how YTHDC2 corresponds to the abundance of gene transcription in the two macrophage phenotypes. The macrophage population was divided into two categories according to the expression level of YTHDC2, the YTHDC2-high and YTHDC2-low groups (Supplementary Fig. 3a–c). It was observed that YTHDC2 was expressed at the highest amplitude of average impression in cluster 2 (anti-tumoral macrophage) (Fig. 3a, b). Meanwhile, YTHDC2-high macrophages had higher activity scores for anti-tumoral (M1) macrophage marker genes (Fig. 3c). Consistent with this, the activities of some representative anti-tumoral marker genes including SOCS3, REL, TNF, CXCL10, and LGALS1 were relatively upregulated in the YTHDC2-high macrophages (Fig. 3d). YTHDC2-high macrophage also notably exhibited transcriptional upregulation of anti-tumoral genes such as CD80, CD86, IL1B, TNF, and FCGR3A (Supplementary Fig. 3d, e). On the other hand, the expressions of pro-tumoral marker genes (IL10, CCL2, EGR2, and MAFB)31 were more enriched in YTHDC2-low macrophages (Fig. 3e, f). These findings demonstrated that the m6A reader protein YTHDC2 positively correlates with the expression of anti-tumoral marker genes in TNBC macrophages.
a Dot plot representing the relative average expression of m6A-related genes across all clusters. The marker genes in each cluster were identified by log fold change compared with the remaining clusters. b Normalized expression (log-transformed) of key m6A-related genes comparing tumor anti-tumoral and pro-tumoral macrophages aggregated by patients in a pseudo-bulk approach (n = 19 patients, p-values calculated by non-parametric test). c Comparison of averaged activity scores for anti-tumoral gene signatures between anti-tumoral and pro-tumoral macrophages (n = 19 patients, n = 42 tumor samples, two-sided t-test, *P < 0.05). d DE genes between tumor YTHDC2-high and YTHDC2-low macrophages; averaged scaled gene expression of all cells in each group. e Ranking of differentially expressed genes between tumor YTHDC2-high and YTHDC2-low macrophages, colored by P-value (Wilcoxon rank-sum test). Selected genes are highlighted by indicated categories. f Dot plot representing the relative average expression of anti-tumoral and pro-tumoral marker genes across YTHDC2-high and YTHDC2-low clusters. The marker genes in each cluster were identified by log fold change compared with the other cluster.
YTHDC2 modulates macrophage interaction with natural killer/CD4+ T/CD8+ T cells in TNBC
Diverse immune cells regulate macrophages mainly through receptor-ligand interactions32. Investigation of cellular communication revealed that, compared with YTHDC2-low macrophages, YTHDC2-high macrophages interacted more extensively with CD4+ and CD8+ effector memory T cells (CD4+ TEM and CD8+ TEM), CD8+ resident memory and naïve T cells (CD8+ TRM and CD8+ TN) and natural killer cells (NK) in both the Chemo cohort and the Chemo + Anti-PDL1 cohort. One exception was CD4+ regulatory T cells (CD4+ TREG), where their mutual interaction showed a tendency to decline as the level of YTHDC2 increased (Fig. 4a, Supplementary Fig. 4a). The enriched signaling pathways included MHC-I, MHC-II, IFN-γ, CCL, and CXCL pathways (Fig. 4b). We then scrutinized the cognate receptor-ligand pairs between NK/CD4+ T/CD8+ T cell populations and macrophages33. The major histocompatibility complex (MHC) class II molecules (HLA-DMA, HLA-DMB, HLA-DOA, HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DQA1, HLA-DQB1, HLA-DRA, HLA-DRB1 and HLA-DRB5) pairing with CD4 receptors are preferentially expressed on the surface of YTHDC2-high macrophage34. Similar trends could be observed in the MHC class I molecules (HLA-A, HLA-B, HLA-C, HLA-E, and HLA-F) pairing with CD8 receptors (Fig. 4c, Supplementary Fig. 4b). Given the principal role of MHC molecules in processing and presenting antigens to T lymphocytes35, high activity of HLA-CD4/CD8 interactions implied intense involvement of macrophages as the antigen-presenting cells (APC) to other immune cells. Considering TREG cells as a subpopulation to suppress immunity36, a decrease of HLA-CD4 pairing activity in YTHDC2-high macrophages indicated that YTHDC2 reduced the antigen-presenting process of macrophages to CD4+ TREG cells.
a Circle plot visualizing the communication strength between YTHDC2-low (upper panel)/YTHDC2-high (lower panel) macrophages and other immune cells in all samples composed of both the Chemo cohort and the Chemo + Anti-PDL1 cohort. The thickness of the line indicates the strength of intracellular communications. b Violin plots showing the normalized scRNA-seq expression distribution of selected ligand-receptor pairs for YTHDC2-low and YTHDC2-high tumor macrophage samples. c Expression of receptor-ligand pairs that can mediate interaction between tumor macrophage clusters YTHDC2-high/YTHDC2-low and other immune cell clusters, ligand from macrophages, and the receptor on other immune cells (CD4+ TEM: CD4+ effector memory T cell; CD4+ TREG: CD4+ regulatory T cell; CD8+ TEM: CD8+ effector memory T cell; CD8+ TRM: CD8+ resident memory T cell; CD8+ TN: CD8+ naive T cell; NK: natural killer cell) in the Chemo cohort (upper panel) and the Chemo + Anti-PDL1 cohort (lower panel). Dot color reflects communication probabilities and dot size represents computed p-values (one-sided permutation test) for interactions that increased in YTHDC2-high or YTHDC2-low macrophages; empty space indicates no difference between YTHDC2-high and YTHDC2-low macrophages. Red boxes indicate the YTHDC2-high/YTHDC2-low change in the signals derived from MHC class I molecules and MHC class II molecules.
Reconstruction of transcriptional modules related to macrophage YTHDC2 expression in TNBC
Transcription factors (TFs) work as a perplexing network to control the expression of the genome37; their comprehensive effects guide intra- and intercellular metabolism and responses to stimuli38. A deeper understanding of those regulatory mechanisms may provide new insights for potential targets in cancer39. Here our implementation of SCENIC (single-cell regulatory network inference and clustering) analysis constructed a transcriptional regulatory network, aiming to locate the dominant upregulated TFs in the YTHDC2-high macrophages40. A total of 148 significant TFs regulating 3038 genes were identified (Fig. 5a, Supplementary Data 1). The size of each TF varied from 10 to 223 genes, with a median size of 21 genes. Among those with higher activities in the YTHDC2-high macrophages, STAT1, IRF7, FOSL2, REL, ATF3, and CREM regulated the largest number of genes (Fig. 5a, Supplementary Fig. 5) and their regulating genes were highly overlapped (Supplementary Table 2). GO enrichment analysis presented terms specific to lymphocyte migration, lymphocyte chemotaxis, cellular response to biotic responses, type I interferon (IFN), and T cell activation (Supplementary Fig. 6). We also performed a gene interaction network analysis showing ATF3 lying in the center of the six TFs, indicating the principal role of ATF3 among them (Fig. 5b). To further refine the downstream targets, we focused on the collective genes related to YTHDC2 and ATF3, yielding 144 genes (Fig. 5c). Some notorious oncogenes, like MYC, were included, connoting that YTHDC2 may impact ATF3 expression and likely collaborate to alter the function of macrophages.
a Identification of TFs for YTHDC2-high/YTHDC2-low macrophages. Blue boxes highlight the TFs associated with YTHDC2-high macrophages. Red color indicates the TFs that regulate the largest number of genes. Brackets indicate the number of genes regulated by each TF. b Interaction network of the six critical TFs upregulated by YTHDC2 in macrophage. c Overlap between genes related to YTHDC2 and genes related to ATF3 in the GeneCards database.
MYC and TWISTNB are potential downstream targets in the YTHDC2-ATF3 axis of TNBC macrophages
Closer scrutiny was given to the 144 overlapped genes related to both YTHDC2 and ATF3. Using the Degree algorithm in the Cytoscape software, we identified several hub genes, including TP53, AKT1, MYC, GAPDH, ACTB, etc. (Fig. 6a, Supplementary Fig. 7a, c, e, g). STRING analysis unraveled MYC as a crucial member in the interaction network (Fig. 6b, Supplementary Fig. 7b, d, f, h), indicating its significant role in the downstream of the YTHDC2-ATF3 axis. GSEA analyses including KEGG, Rectome, and GO pathways revealed the enrichment of multiple cancer-related signaling pathways in MYC regulation such as PI3K-AKT, TP53 signaling, and SLITs/ROBOs (Fig. 6c–e). We also appraised whether any common gene was regulated by all or most of the six YTHDC2-upregulated TFs, and found out that four of them (FOSL2, REL, ATF3, and CREM) modulated the expression of TWISTNB (Supplementary Fig. 8). Further GSEA analysis revealed the functional involvement of TWISTNB in various stages of rRNA transcription, translation, and ribosome synthesis (Fig. 6f–h).
a Hubba analysis using the Degree algorithm in Cytoscape to identify hub genes using 144 genes from the Venn diagram intersection. b STRING analysis of the hub genes identified by the Degree algorithm in Cytoscape using 144 genes from Venn diagram intersection. c–e GSEA (hypeR) on genes regulated by MYC, with enrichment analysis for KEGG (c), Reactome (d), and GO (e) pathways. f–h GSEA (hypeR) on genes regulated by TWISTNB, with enrichment analysis for KEGG (f), Reactome (g) and GO (h) pathways. Red color indicates protumoral signal pathways and critical biological functions.
YTHDC2 as a reflector of treatment response to chemotherapy with/without anti-PDL1 in TNBC patients
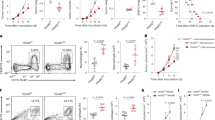
We assessed the expression levels of YTHDC2 in TNBC patients who achieved various treatment responses. Four objective responses were set up as endpoints for evaluation: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Please refer to the methods for more detail. Our results presented that the levels of YTHDC2 in tumor-infiltrating macrophages climbed up as the response to treatments improved (Fig. 7a–c). Next, patients were grouped based on the treatment plan: chemotherapy vs chemotherapy + anti-PD-L1. Again, the expression levels of YTHDC2 escalated to the utmost as the therapeutic endpoint reached complete response; the upward trends in YTHDC2 abundance were evident in both groups (Fig. 7d–i). These results connoted that YTHDC2 serves as a marker of treatment response in TNBC patients receiving chemotherapy with or without immunotherapy.
a, d, g UMAP plots for macrophages from samples with different responses to chemotherapy with/without anti-PDL1 (a), chemotherapy (d), and chemotherapy + anti-PDL1 (g) derived from the combined plot in Fig. 2a (excluding monocyte cluster). Each dot represents an individual cell. Color coding is shown in Fig. 2a. b, e, h Comparison of averaged YTHDC2 expression levels between macrophages in samples receiving chemotherapy with/without anti-PDL1 (b), chemotherapy alone (e), or chemotherapy + anti-PDL1 (h) with different clinical responses, related to the UMAP plot in Fig. 2a. c, f, i Box plots showing expression level of YTHDC2 in tumor macrophages from patient samples receiving chemotherapy/chemotherapy + anti-PDL1 (c), chemotherapy alone (f), or chemotherapy + anti-PDL1 (i) with different clinical responses. Each dot represents an individual cell (median test, *P < 0.05).
YTHDC2 predicts the survival rate of breast cancer samples in the TCGA dataset
By subjecting 142 samples with TNBC in The Cancer Genome Atlas (TCGA) dataset41,42 to survival analysis (Supplementary Fig. 9a), we observed the YTHDC2-high macrophage geneset positively correlates with the overall survival (OS) of the entire cohort (Supplementary Fig. 9b). Similar trends were observed in various subgroups, including age >30 years (Supplementary Fig. 9c), age ≤55 years (Supplementary Fig. 9d), pathological M0 (Supplementary Fig. 9e), pathological N0/1 (Supplementary Fig. 9f), pathological N0/1/2 (Supplementary Fig. 9g), pathological N0/1/2/3a/3b (Supplementary Fig. 9h), stage I/II (Supplementary Fig. 9i), and stage I/II/III/X (Supplementary Fig. 9j).
We then broadened our analysis to encompass all breast cancer patients within the TCGA dataset41,42 (Supplementary Figs. 10a and 11a). A positive correlation emerged between the anti-tumoral macrophage geneset30 and the overall survival (OS) of the entire cohort (Supplementary Fig. 10b). Detailed examination of various subgroups (Supplementary Fig. 10c. age >40 years; d. age ≤50 years; e. left lower quadrant; f. left upper quadrant; g. ER-positive; h. PR positive; i. infiltrating ductal carcinoma; j. no adjuvant treatment history; k. pathological T1/2/3; l. pathological N0/1; m. pathological M0; n. pathological Stage I/II/III) consistently demonstrated positive correlations. Conversely, the pro-tumoral macrophage geneset28,29,31 exhibited an inverse relationship with the OS in the entire cohort (Supplementary Fig. 11b), with analogous patterns observed across all the subgroups (Supplementary Fig. 11c. ER-positive; d. PR positive; e. HER2 positive; f. no neoadjuvant therapy history; g. right lateral; h. post-menopause; i. age ≥50).
Overexpression of ex vivo YTHDC2 promotes anti-tumoral macrophage polarization and their phagocytosis to tumor cells
We embarked on an ex vivo study to ascertain the impact of YTHDC2 on macrophage polarization using the THP.1 cell line. In our experimental design, cells were efficiently transfected with either a YTHDC2-overexpressed plasmid (experimental group) or a blank plasmid (comparison group), while the control group cells remained unaltered (Supplementary Fig. 12). Remarkably, the mean fluorescence intensity (MFI) of THP-1 cells in the experimental group was significantly higher than those in both the comparison and control groups (Fig. 8a, b). Correspondingly, the mRNA expression levels of YTHDC2 were markedly elevated in the YTHDC2-transfected cells (Fig. 8c). Subsequently, we employed flow cytometry to validate the establishment of pro-tumoral (CD80−/CD206+) and anti-tumoral (CD80+/CD206−) macrophage subpopulations within the THP.1 cell line28,29,30,31 (Supplementary Fig. 13). The findings demonstrated a reduced ratio of CD206+/CD80+ cells in the YTHDC2-overexpressed group in contrast to the comparison and control groups (Fig. 8d). This compelling evidence suggests that YTHDC2 overexpression promotes the expression of anti-tumoral phenotype markers, indicating its significant role in driving the differentiation of anti-tumoral macrophages. Further, we assessed the phagocytic activity of macrophages against tumor cells in vitro within this experimental framework. The results were persuasive in showing that YTHDC2-overexpressed macrophages exhibited a pronounced enhancement in their phagocytic capabilities toward tumor cells (Fig. 8e, f).
a, b Peak graph (a) and histogram (b) showing the mean fluorescence intensity (MFI) of green fluorescence protein (GFP) in THP.1 cells from the experimental group (YTHDC2-overexpressed plasmid), the comparison group (blank plasmid), and the control group (unpaired t-test, ****P < 0.0001). c RT-PCR analyses showing the levels of YTHDC2 mRNA in THP.1 cells from the experimental, comparison, and control groups (unpaired single-tailed t-test, *P < 0.05). d Representative plots and quantitative analyses showing the ratios of CD206+/CD80+ THP.1 cells in the experimental, comparison, and control groups (unpaired single-tailed t-test, *P < 0.05). e Morphology of co-cultured MDA-MB-231 tumor cells and THP.1 cells under an optical and inverted fluorescence microscope. f Representative plots (left) and quantitative analyses of macrophage phagocytosis (right) in the experimental, comparison, and control groups (unpaired t-test, *P < 0.05).
Discussion
Understanding the mechanisms driving anti-tumoral phenotype polarization can pave the way for developing new therapeutic strategies to enhance the tumor-suppressive roles of macrophages in TNBC. YTHDC2, as the largest YTH domain-containing protein, has been shown to inhibit lung adenocarcinoma tumorigenesis22,43 and be negatively correlated with clinical stages of colonic adenocarcinoma44, constituting the rationale of selecting YTHDC2 for further research. Despite previous studies of YTHDC2 downregulation in TNBC25, its collaborative role in the performance of immune cells, especially macrophages, has not been fully elucidated. Using single-cell sequencing, we demonstrated that YTHDC2 promotes the expression of the anti-tumoral genome in macrophages. This finding was further substantiated by ex vivo experiments, which revealed that YTHDC2 overexpression in the THP-1 cell line significantly favored the differentiation of macrophages into an anti-tumoral phenotype, as well as enhanced their phagocytic activity against tumor cells. Thus, YTHDC2 emerges as a key determinant of anti-tumoral macrophage performance in both chemo-responsive and chemo-resistant TNBC patients. It was noted that although some other m6A reader proteins, such as HNRNPC, were shown to be expressed at higher percentages in anti-tumoral macrophages than YTHDC2, yet their average expressions were lower than that of YTHDC2 (Fig. 3a). Moreover, HNRNPC has been widely proven to promote carcinoma carcinogenesis and metastasis45,46; its expressing significance was likely at lower priority when deliberating their roles of regulating anti-tumoral capacities of macrophages. The scRNA-seq analyses of PBMC revealed a series of characteristic monocyte clusters. Among these, NFKBIZ+ monocytes notably exhibited a distinct expression level of YTHDC2. Previous studies have demonstrated that NFKBIZ plays a pivotal role in activating a cascade of inflammatory response genes, including IL6 and IL12b47,48, which are well-established anti-tumoral markers. Based on these findings, we speculated that the peripheral blood NFKBIZ+ monocytes are likely to serve as the precursors of anti-tumoral macrophages, contributing significantly to the body’s immune response against tumors.
Our results were also evident that YTHDC2-high macrophages exhibited a pronounced antigen-presenting capacity to NK/CD4+ T/CD8+ T cells, while their interactions with TREG cells, a representative immunosuppressing cell type, were substantially diminished. This observation aligns with a previous study that reported YTHDC2’s correlation with the infiltrating levels of B cells, CD8+ T cells, CD4+ T cells, neutrophils, and dendritic cells in head and neck squamous cell carcinoma23. This implied the enhancement of overall immunity within the tumor microenvironment, highlighting the critical role of YTHDC2 in this regulatory chain.
The transcriptional regulatory network analysis of YTHDC2 discovered six highly active TFs in YTHDC2-high macrophages. Among these, ATF3 (Activating Transcription Factor 3) emerged as the most interactive one promoting anti-tumoral macrophage polarization49 (Supplementary Fig. 6a). CREM (Cyclic AMP-Responsive Element Modulator), known for its role in regulating anti-bacterial responses, also participated in genomic transcription and macrophage differentiation50 (Supplementary Fig. 6b). IRF7 (Interferon Regulatory Factor 7) profoundly facilitates cellular response to interferons and performs a versatile role in multiple biological processes, including macrophage polarization51 (Supplementary Fig. 6c). REL (REL protein, an NF-κB subunit) controls critical inflammatory metabolism in macrophages52, aside from its broad involvements of T cell differentiation, cellular responses to interleukin-1 and cytokine productions (Supplementary Fig. 6d). STAT1 (Signal Transducers and Activators of Transcription 1) has been previously identified to be essentially involved in immune system stimulation and macrophage reprogramming53 (Supplementary Fig. 6e). FOSL2 (FOS-Like 2 transcription factor) regulates cellular etiology of lymphocytes and closely correlates with macrophage reprogramming as well54 (Supplementary Fig. 6f). While their engagements of macrophage differentiation or polarization have been previously reported, it is exceptional to demonstrate the key role of ATF3 and their regulatory pathways through YTHDC2. Collectively, they significantly contribute to maintaining the anti-tumoral phenotype and functionality of macrophages.
Our exploration of downstream components indicated MYC being a major target regulated by both YTHDC2 and ATF3, among others, leading to a series of perplexing oncogenic responses. Numerous studies have reported that the m6A modification affects the stability of MYC transcripts55, and other m6A readers like YTHDF2 alter MYC transcriptional processes56. MYC has been identified as a marker of pro-tumoral macrophages57. Given that ATF3 primarily participates in immune cell-activating regulations, it is reasonable to infer that ATF3 suppresses the expression of MYC. We further discovered that TWISTNB is upregulated by four of the six active TFs in YTHDC2-high macrophages. TWISTNB is deeply involved in the transcription of rDNA and ribosome generation; although its genetic alteration in multiple cancers was previously witnessed58, its influence on the anti-tumoral performance of macrophages remained unreported. We have created a graphic abstract to illustrate the potential YTHDC2-ATF3-MYC/TWISTNB regulatory pathway in anti-tumoral macrophages (Fig. 9).
Our results from the survival analyses of the TCGA and GSE databases are consistent with prior conclusions that the affluence of YTHDC2 correlates with a favorable prognosis for cancer patients24,26. In non-small cell lung carcinoma, patients with low levels of YTHDC2 had a significantly poor prognosis26; similar outcomes were reported in another study on head and neck squamous cell carcinoma26. This may connote its prognostic relevance in a pan-cancer setting.
There are, nevertheless, some limitations to be addressed in future research. First, in vivo models were lacking and the effect of YTHDC2 analogs was not tested. A larger-scaled sample pool, along with the verification of animal models, would render our findings more persuasive. Second, given the intricacy of the whole intracellular transcriptional regulatory network, it cannot be excluded that other signaling pathways, apart from our conclusions, may predominate to determine macrophage polarization. A comprehensive analysis taking all the transcriptional components into account is necessary to verify such a statement.
In this study, we conclusively demonstrated that YTHDC2 is a determinant in inducing the anti-tumoral phenotype polarization of macrophages and has prognostic value for better survival outcomes in TNBC patients. A scrutiny of its regulatory network revealed its role in enhancing macrophage interactions with other immune cells (CD4+, CD8+, and NK cells). Its intracellular modulation went through the upregulation of ATF3 and TWISTNB, alongside the suppression of MYC. To our best knowledge, this is a novel study that expounds on potential regulatory mechanisms of tumor-infiltrating macrophage YTHDC2 in influencing its phenotype polarization, immune capacity, and therapeutic outcomes in TNBC. We sincerely hope this study will be enlightening to further exploration of regulatory pathways and the clinical implementation of YTHDC2 analogs.
Methods
Materials
Trypsin-EDTA, High Glucose, Penicillin-streptomycin, Hank’s Balanced Salt Solution (HBSS), collagenase, neutral protease, DNase I, Red Blood Cell Removal Solution, and Wash buffer were procured from Invitrogen, Thermo Fisher Scientific Inc. (MA, USA). Roswell Park Memorial Institute (RPMI) -1640 and fetal bovine serum were purchased from Gibco, Thermo Fisher Scientific Inc. (MA, USA). The transfection kit, lentiviral constructs over-expressing YTHDC2, and negative control lentiviral constructs were sourced from Shanghai Genechem Co., Ltd. (Shanghai, China). 2-Acetoxy-1-methoxypropane (PMA) was purchased from Yongjin Biotech Co., Ltd. (Guangzhou, China). Primers for qRT-PCR were from Jinweizhi Co., Ltd. (Suzhou, China). The RNA Quick Purification kit was procured from Yishan Co., Ltd (Shanghai, China). TB Green@Premix Ex TaqTMII(Tli RNaseH Plus) and PrimeScriptT RT Master Mix (Perfect Real Time) were purchased from TaKaRa Bio(Tokyo, Japan). Flow cytometry antibodies BV785-anti-CD11b, APC-anti-Cd80, and PE-Cy7-anti-Cd206 were purchased from BD Bioscience Co., Ltd (New Jersey, USA). 384 well-white PCR plates were purchased from LABTIDE (Shanghai, China). PrimeSurface® 96U plates were purchased from Sumitomo (Ōsaka, Japan). The Eclipse Ti2-E Fluorescent Inverted microscope was purchased from NIKON Shanghai Co., Ltd (Shanghai, China). The LightCycler® 480 thermal cycler was obtained from Roche (Basel, Switzerland). The LSRFortessa Flow cytometer was purchased from BD Bioscience Co., Ltd (New Jersey, USA).
TNBC chemotherapy clinical trial and study design
The study was approved by the Institutional Review Board of Sun Yat-sen Memorial Hospital (Reference No.: SYSKY-2023-1210-01) and informed consents were obtained from all participants. The participants gave consent for their personal and clinical details to be published in this paper. Research involving human research participants, material, and data has been performed in accordance with the Declaration of Helsinki. Patients in the chemotherapy cohort (n = 10) received either a paclitaxel (90 mg/m2) on Days 1, 8, and 15 of every 28-day cycle or doxorubicin (90 mg/m2) plus cyclophosphamide (600 mg/m2) on Day 1 of every 21-day cycle. Patients in the chemotherapy plus anti-PDL1 cohort (n = 9) received atezolizumab (840 mg) on Days 1 and 15 of every 28-day cycle besides chemotherapy. Paired biopsied samples of metastatic and primary tumor tissues were collected at a baseline of < 2 weeks prior to treatment, <12 weeks after treatment initiation, as well as disease progression. One patient had tumor samples after disease progression (Fig. 1, Supplementary Table 1). Clinical responses were evaluated based on imaging assessments of tumor sizes at the 8th week since the treatment started (two treatment cycles). Ultrasound was used to evaluate the breast tumors and enhanced computed tomography (CT) was used to evaluate the metastatic tumors in the chest and abdomen. Patients in both cohorts received 6~8 cycles of therapy as the standard guideline instructed. We classified all participants according to the volume reduction rate (VRR) of the tumor as below: complete response (CR): VRR > 90%; partial response (PR): 30% < VRR < 90%; stable disease (SD): −30% < VRR < 30%; progressive disease (PD): VRR < −30% (Fig. 1, Supplementary Table 1). The clinical trial was registered at the Chinese Clinical Trial Registry (Registration Number: ChiCTR2400084513, Registration Date: 2024-05-20).
Single-cell suspension preparation
We manually excised and enzymatically digested tumor or tumor-draining lymph node (TdLN) tissues with 0.1% (1 mg/ml) collagenase I, 0.1% (1 mg/ml) neural protease, and 0.02% (0.2 mg/ml) DNase I at 37 °C. Digested tissues then went through 100 μm filters. We harvested the cells at the surface and re-diluted them with Hank’s Balanced Salt Solution (HBSS) until a concentration of 700-1200 cells/μL was reached. The cell suspension was subjected to scRNA-seq within 30 min.
Dimension reduction and unsupervised clustering
The expression matrix was imported to the Seurat R package (v4.3.0.1)59 for dimension reduction. We first normalized the matrix using the ‘NormalizeData’ function, during which the individual UMI counts for each gene were divided by the total UMI counts in each cell and multiplied by the scale factor of 1000, followed by natural-log transformation. Using the ‘FindVariableFeatures’ function with the ‘vst’ method, the 2000 most variable genes for each sample were identified according to the normalized expression matrix. Subsequently, the unique lists of highly variable genes were subjected to principal component analysis (PCA). The gene expression matrices from each sample were merged into a whole gene expression matrix of all cells before PCA. Then we applied the ‘ScaleData’ function to scale and center the gene expression matrix. PCA was performed on the selected variable genes to identify the top principal components using the ‘RunPCA’ function of Seurat. We observed substantial variability between cells from different samples, caused by a combination of biological and technical variations, which was called batch effects, leading to cluster cells firstly by sample heterogeneity rather than cell types. Harmony (v0.1.1) was next applied to integrate the 42 datasets into a shared space for unsupervised clustering. In brief, the obtained PCA matrix was fed into the ‘RunHarmony’ function implemented in Seurat. Uniform Manifold Approximation and Projection (UMAP) was visualized after Harmony integration, indicating all cells were well mixed into the shared space. Harmony reduction was applied as input for building the Shared Nearest Neighbor (SNN) graph using Principal components and nearest neighbors to perform the clustering analysis. We applied the Louvain clustering algorithm to group the cells into different clusters, and SNN graph construction adopted these parameters to avoid grouping cells from distinct compartments.
Cell interaction analysis
To evaluate mutual interactions between T cells and myeloid cell clusters in tumors, CellChat (v1.6.1, R package)33 was applied to infer and visualize intercellular communication based on the expression of known ligand-receptor pairs. In this study, the interactions between CD8+T cells, CD4+T cells, NK cells, and macrophages were mainly studied, with a focus on changes in predicted cell-cell communication between YTHDC2-high and YTHDC2-low samples. Firstly, other immune cell - macrophage interactions in YTHDC2-high & YTHDC2-low groups were inferred by following CellChat’s recommendation of individual datasets with default parameters (except that thresh.p = 0.05 in ‘identifyOverExpressedGenes’function, raw.use = TRUE and population.size = TRUE in ‘computeCommunProb’ function, min.cells = 10 in ‘filterCommunication’function). All categories of ligand-receptor interactions in the database were used in the analysis. Subsequently, YTHDC2-high and YTHDC2-low objects were merged by the ‘mergeCellChat’ function for comparison. Finally, the ‘netVisual_bubble’ function was applied to visualize differences of communication probabilities by ligand-receptor pairs in both directions, other immune cells to macrophages as well as macrophages to other immune cells.
Transcriptional regulation network analysis
To identify the shared transcriptional regulation network in different groups, we subjected the gene expression matrix and the transcription factors (TFs) database to calculate the correlations between TFs and genes40. Then the activity scores of different TFs were evaluated according to the area under curve (AUC). The higher the AUC score, the more active the TF. Subsequently, the AUC scores of different TFs were scaled to identify active TFs in each group.
Survival analysis
To evaluate the correlation between YTHDC2 and disease prognosis, we obtained YTHDC2-related geneset from the GENECARD database. We subjected the geneset to OS analysis. The OS data of patients with breast cancer were referenced from the TCGA database. Based on the average expression value of each gene in the geneset, we classified the patients into two groups (YTHDC2-high and YTHDC2-low groups) and examined their prognoses. The prognosis of each group of patients was examined by Kaplan–Meier survival estimators and the survival outcomes of the two groups were compared by log-rank tests. The survival outcomes with significant differences were examined and we selected the value that yields the lowest log-rank P-value to be the best cutoff value of the two groups.
Cell lines
THP.1 and MDA-MB-231 breast cancer cells were obtained from the American Type Culture Collection (ATCC, USA).
Cell culture and macrophage polarization
THP.1 cells were cultured in RPMI-1640 containing 10% fetal bovine serum (FBS, heat-inactivated) and penicillin-streptomycin antibiotics (both 50 μg/ml). The medium was incubated at 37 °C with 5% CO2. The cells were then seeded into 6-well plates (1 × 106/well) and treated with 100 ng/ml PMA for 48 h to generate M0-like macrophages.
Plasmid construction, transfection, and expression level analyses
Lentiviral constructs over-expressing YTHDC2 and negative control lentiviral constructs were purchased from Shanghai Genechem Co., Ltd. (Shanghai, China). All lentiviral vectors co-encode green fluorescent protein (GFP). THP. 1 cells (5 × 104/well) were seeded into 6-well plates with 2 ml RPMI-1640 and cultured for 24 h at 37 °C and 5% CO2. THP. 1 cells of the experimental or comparison group were transfected with YTHDC2-overexpressed lentiviral constructs or negative control lentiviral constructs packaging plasmids, respectively, using the transfection kit following the manufacturer’s instructions. Lentivirally transduced THP. 1 cells were examined for transfection efficiency (GFP positivity rate) under a fluorescence microscope before use as targets ex vivo.
Quantitative PCR (qPCR)
RNA of YTHDC2 was isolated using an RNA purification kit, and reverse transcribed using PrimeScriptT RT Master Mix (Perfect Real Time) for RT-qPCR. For quantitative PCR, template cDNA, primers, and TB Green@Premix Ex TaqTMII(Tli RNaseH Plus) were used according to the manufacturer’s instructions. The expression levels of mRNA were analyzed with LightCycler®96 (Roche). The strand sequences of YTHDC2 primers were as follows: forward: ′-CCAGCATTACACCCACCTCAGAAG-3′; reverse: ′-TGGAGGAGAAGGACTAGCACAAGG-3′.
Flow cytometry and polarization detection
After adding the desired combination of fluorochrome-conjugated antibodies, the THP.1 cells to be tested were incubated on ice for 30 min in the dark. Thereafter, the M0-like THP.1 cells were subjected to flow cytometry analysis. Anti-tumoral (M1) and pro-tumoral (M2) markers on THP.1 cells were detected with the following panel: APC-anti-CD80 and PE-Cy7-anti-CD206. Flow cytometry data were acquired on a BD LSRFortessa and analyzed with FlowJo X10 (FlowJo).
Assessment of phagocytosis to tumor cells in the YTHDC2-overexpressed cells
1 × 104 MDA-MB-231 breast cancer cells were cultured in a PrimeSurface® 96U plate for 72 h. Then M0-like THP.1 cells of the experimental or comparison group were added to the 96-well cell culture plate containing MDA-MB-231 cells at a ratio of 1:2, respectively. After co-culture for 72 h, an inverted fluorescence microscopy was applied for white light and fluorescence image capture. Then cells were harvested with trypsin digestion solution, stained with BV785-anti-CD11b, and analyzed by flow cytometry using a BD LSRFortessa. The percent of GFP+ events within the CD11b+ population was plotted as a percentage of phagocytosis. Data are represented as mean ± standard error of triplicate wells. Statistical significance between the experimental and comparison groups was calculated using an unpaired single-tailed t-test; *P < 0.05.
Statistics
Statistical analysis was performed in GraphPad Prism 6.0 (GraphPad). Each figure legend denotes the statistical test used. All central tendencies indicate the mean and all error bars indicate s.e.m. unless otherwise indicated. All t-tests were two-sided unless otherwise indicated. For all figures, * indicates P < 0.05, ** indicates P < 0.01, *** indicates P < 0.001 and **** indicates P < 0.0001.
Data availability
This work is part of a clinical trial (clinical registry number ChiCTR2400084513, associated links https://www.chictr.org.cn/bin/project/edit?pid=227290). Raw data and processed, de-identified scRNA-seq gene expression datasets generated during this study is deposited and publically available at the Gene Expression Omnibus (GEO) with the accession number GSE263995. This paper also analyzes existing, publicly available data. The accession number for this dataset is listed in the “Methods” section. This paper does not report the original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
Harbeck, N. & Gnant, M. Breast cancer. Lancet 389, 1134–1150 (2017).
Cortes, J. et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396, 1817–1828 (2020).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Grünwald, B. T. et al. Spatially confined sub-tumor microenvironments in pancreatic cancer. Cell 184, 5577–5592.e18 (2021).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147 (2022).
Müerköster, S. et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 64, 1331–1337 (2004).
Sherman, M. H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014).
Banerjee, S. et al. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin. Cancer Res. 22, 415–425 (2016).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Bill, R. et al. CXCL9:SPP1 macrophage polarity identifies a network of cellular programs that control human cancers. Science 381, 515–524 (2023).
Satoh, T. et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature 495, 524–528 (2013).
Mitchem, J. B. et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 73, 1128–1141 (2013).
De Palma, M. & Lewis, C. E. Cancer: macrophages limit chemotherapy. Nature 472, 303–304 (2011).
Zheng, Y. et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 114, 3625–3628 (2009).
Yin, H. et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 12, 1394 (2021).
Han, X. et al. RNA m6A methylation modulates airway inflammation in allergic asthma via PTX3-dependent macrophage homeostasis. Nat. Commun. 14, 7328 (2023).
Yao, J. et al. Macrophage IRX3 promotes diet-induced obesity and metabolic inflammation. Nat. Immunol. 22, 1268–1279 (2021).
He, L. et al. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 18, 176 (2019).
Patil, D. P., Pickering, B. F. & Jaffrey, S. R. Reading m6A in the transcriptome: m6A-binding proteins. Trends Cell Biol. 28, 113–127 (2018).
Lee, Y., Choe, J., Park, O. H. & Kim, Y. K. Molecular mechanisms driving mRNA degradation by m6A modification. Trends Genet. 36, 177–188 (2020).
Ma, L. et al. The m6A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11-dependent antioxidant function. Redox Biol. 38, 101801 (2021).
Li, Y. et al. The m6A reader protein YTHDC2 is a potential biomarker and associated with immune infiltration in head and neck squamous cell carcinoma. PeerJ 8, e10385 (2020).
Sun, S. et al. Downregulation of m6 A reader YTHDC2 promotes tumor progression and predicts poor prognosis in non-small cell lung cancer. Thorac. Cancer 11, 3269–3279 (2020).
Wang, S., Zou, X., Chen, Y., Cho, W. C. & Zhou, X. Effect of N6-methyladenosine regulators on progression and prognosis of triple-negative breast cancer. Front. Genet. 11, 580036 (2021).
Zhang, Y. et al. Identification of m6A methyltransferase-related genes predicts prognosis and immune infiltrates in head and neck squamous cell carcinoma. Ann. Transl. Med. 9, 1554 (2021).
Zhang, Y. et al. Single-cell analyses reveal key immune cell subsets associated with response to PD-L1 blockade in triple-negative breast cancer. Cancer Cell 39, 1578–1593.e8 (2021).
Qi, J. et al. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat. Commun. 13, 1742 (2022).
Zheng, N. et al. M2 macrophage-derived exosomes suppress tumor intrinsic immunogenicity to confer immunotherapy resistance. Oncoimmunology 12, 2210959 (2023).
Kim, H. J. et al. Blood monocyte-derived CD169(+) macrophages contribute to antitumor immunity against glioblastoma. Nat. Commun. 13, 6211 (2022).
Liu, S. et al. S100A4 enhances protumor macrophage polarization by control of PPAR-gamma-dependent induction of fatty acid oxidation. J. Immunother. Cancer 9, e002548 (2021).
Cohen, M. et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175, 1031–1044.e18 (2018).
Jin, S. et al. Inference and analysis of cell-cell communication using CellChat. Nat. Commun. 12, 1088 (2021).
Camilli, G. et al. Regulation and trafficking of the HLA-E molecules during monocyte-macrophage differentiation. J. Leukoc. Biol. 99, 121–130 (2016).
Klein, J. & Sato, A. The HLA system. First of two parts. N. Engl. J. Med. 343, 702–709 (2000).
Ohkura, N. & Sakaguchi, S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 30, 465–474 (2020).
Lambert, S. A. et al. The human transcription factors. Cell 172, 650–665 (2018).
Vaquerizas, J. M., Kummerfeld, S. K., Teichmann, S. A. & Luscombe, N. M. A census of human transcription factors: function, expression and evolution. Nat. Rev. Genet. 10, 252–263 (2009).
Kant, R. et al. Deregulated transcription factors in cancer cell metabolisms and reprogramming. Semin. Cancer Biol. 86, 1158–1174 (2022).
Bravo González-Blas, C. et al. SCENIC+: single-cell multiomic inference of enhancers and gene regulatory networks. Nat. Methods 20, 1355–1367 (2023).
Wang, Z., Jensen, M. A. & Zenklusen, J. C. A practical guide to The Cancer Genome Atlas (TCGA). Methods Mol. Biol. 1418, 111–141 (2016).
Tomczak, K., Czerwińska, P. & Wiznerowicz, M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp. Oncol. 19, A68–A77 (2015).
Zhai, D. et al. LIM-domain binding protein 2 regulated by m6A modification inhibits lung adenocarcinoma cell proliferation in vitro. Nan Fang. Yi Ke Da Xue Xue Bao 41, 329–335 (2021).
Xu, D., Shao, J., Song, H. & Wang, J. The YTH domain family of N6-methyladenosine “readers” in the diagnosis and prognosis of colonic adenocarcinoma. Biomed. Res. Int. 2020, 9502560 (2020).
Zheng, J. et al. USP39 promotes hepatocellular carcinogenesis through regulating alternative splicing in cooperation with SRSF6/HNRNPC. Cell Death Dis. 14, 670 (2023).
Zheng, Z. et al. CircPPAP2B controls metastasis of clear cell renal cell carcinoma via HNRNPC-dependent alternative splicing and targeting the miR-182-5p/CYP1B1 axis. Mol. Cancer 23, 4 (2024).
Green, I. D. et al. Macrophage development and activation involve coordinated intron retention in key inflammatory regulators. Nucleic Acids Res. 48, 6513–6529 (2020).
Muñoz-Rojas, A. R., Kelsey, I., Pappalardo, J. L., Chen, M. & Miller-Jensen, K. Co-stimulation with opposing macrophage polarization cues leads to orthogonal secretion programs in individual cells. Nat. Commun. 12, 301 (2021).
Lu, H. et al. MCPIP1 restrains mucosal inflammation by orchestrating the intestinal monocyte to macrophage maturation via an ATF3-AP1S2 axis. Gut 72, 882–895 (2023).
Sawka-Verhelle, D. et al. PE-1/METS, an antiproliferative Ets repressor factor, is induced by CREB-1/CREM-1 during macrophage differentiation. J. Biol. Chem. 279, 17772–17784 (2004).
Chen, L. et al. NF-kappaB p65 and SETDB1 expedite lipopolysaccharide-induced intestinal inflammation in mice by inducing IRF7/NLR-dependent macrophage M1 polarization. Int. Immunopharmacol. 115, 109554 (2023).
Leslie, J. et al. c-Rel orchestrates energy-dependent epithelial and macrophage reprogramming in fibrosis. Nat. Metab. 2, 1350–1367 (2020).
Ma, S. et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells. Nat. Immunol. 24, 255–266 (2023).
Sarode, P. et al. Reprogramming of tumor-associated macrophages by targeting beta-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci. Adv. 6, eaaz6105 (2020).
Su, R. et al. R-2HG exhibits anti-tumor activity by targeting FTO/m6A/MYC/CEBPA signaling. Cell 172, 90–105.e23 (2018).
Dixit, D. et al. The RNA m6A reader YTHDF2 maintains oncogene expression and is a targetable dependency in glioblastoma stem cells. Cancer Discov. 11, 480–499 (2021).
Pello, O. M. Macrophages and c-Myc cross paths. Oncoimmunology 5, e1151991 (2016).
Rossetti, S., Wierzbicki, A. J. & Sacchi, N. Mammary epithelial morphogenesis and early breast cancer. Evidence of involvement of basal components of the RNA polymerase I transcription machinery. Cell Cycle 15, 2515–2526 (2016).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Acknowledgements
This work was supported by grants from Guangdong Innovation and Entrepreneurship Team Projects (2019BT02Y198), the Science and Technology Foundation of the Guangdong Province (2021B1515230001), the National Science Foundation of China (82071859, 82071860, 82371856), Guangdong Natural Science Foundation (2020B1515120007), Guangzhou Innovation and Entrepreneurship Team Projects (2024A03J1055) and Shanwei Innovation and Entrepreneurship Team Projects (2021A005, 2023A012, 2023A010).
Author information
Authors and Affiliations
Contributions
H.J., Y.M., L.M., H.S., and J.X. performed and analyzed experiments. D.Z. revised the results and wrote the paper. Y.Y. and S.H. conceived ideas and supervised the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jin, H., Zhang, D., Ma, Y. et al. YTHDC2 manipulates anti-tumoral macrophage polarization and predicts favorable outcomes in triple negative breast cancer. npj Precis. Onc. 9, 119 (2025). https://doi.org/10.1038/s41698-025-00880-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-00880-2
This article is cited by
-
Enhancer regulation in cancer: from epigenetics to m6A RNA modification
Archives of Pharmacal Research (2025)











 represents inhibitory or down-regulations on the targets downstream. The critical regulatory proteins (YTHDC2, ATF3, MYC and TWISTNB) are highlighted.
represents inhibitory or down-regulations on the targets downstream. The critical regulatory proteins (YTHDC2, ATF3, MYC and TWISTNB) are highlighted.