Abstract
Rho GTPase-activating protein 4 (ARHGAP4) is implicated in poor prognosis across multiple malignancies. However, its role in driving stemness in colorectal cancer (CRC) remains unclear. In this study, we demonstrate that ARHGAP4 drives CRC stemness through a positive feedback loop involving MYH9/β-catenin/c-Jun, as validated by integrated bioinformatics analysis, in vitro and in vivo tumor stemness assays, co-immunoprecipitation, chromatin immunoprecipitation, and fluorescence recovery after photobleaching. These findings identify ARHGAP4 as a promising therapeutic target in CRC, particularly for addressing cancer stem cells (CSCs).
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) remains a leading global health challenge, ranking as the third most prevalent malignancy and a major cause of cancer-related mortality1. Despite therapeutic advances, tumor relapse and metastasis, driven by cancer stemness, remain key obstacles to improving patient outcomes2. A critical unmet need is the identification of molecular drivers that sustain stemness properties, which are tightly linked to tumor progression3. Thus, elucidating the mechanisms governing stemness regulation may provide novel strategies for CSC-targeted therapies.
The Rho GTPase-activating protein (RhoGAPs) family has emerged as a key regulator of oncogenic signaling, modulating cytoskeletal dynamics and cell motility4. However, compared to RhoGEFs, RhoGAPs remain poorly characterized, particularly in the context of CRC stemness5. Among these, ARHGAP4 as a member implicated in hematopoietic malignancies, has been proposed as a potential CRC biomarker6, yet its functional role and mechanisms of interplay with stemness regulators are unknown. Therefore, a study is urgently needed to elucidate the key molecules and the mechanism behind them.
Non-muscle myosin heavy chain 9 (MYH9) has emerged as a multifunctional player in mechanotransduction and cancer progression7. In hepatocellular carcinoma and gastric cancer, MYH9 has shown to enhances stemness8,9,10,11,12 and act as a protein scaffold, facilitating signal transduction13,14. Intriguingly, MYH9’s coiled-coil domain harbors intrinsically disordered regions (IDRs), suggesting a potential for liquid-liquid phase separation (LLPS)—a process increasingly recognized for organizing oncogenic condensates15. However, whether MYH9 undergoes LLPS, and how this is regulated by upstream partners remains unexplored.
Our study elucidates a novel mechanistic link between ARHGAP4 and CRC stemness through MYH9-mediated LLPS. By integrating multi-omics and functional analyses, we demonstrate that ARHGAP4 promotes CRC stemness by facilitating MYH9-dependent LLPS, which orchestrates GSK3β degradation complex assembly to activate β-catenin signaling. Furthermore, we reveal that this oncogenic axis is sustained through a c-Jun-mediated positive feedback loop that reinforces ARHGAP4/MYH9 expression. These findings not only establish ARHGAP4 as a promising therapeutic target but also provide the first evidence linking LLPS to stemness regulation in CRC, offering new mechanistic insights for precision oncology interventions.
Results
ARHGAP4 overexpression drives CRC progression
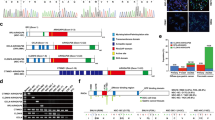
We performed data mining of CRC using the human cancer genome atlas (TCGA) database, from which we identified several differentially expressed genes (Fig. 1A). Among these differentially expressed genes, we intersected them with members of the RhoGAPs gene family (Fig. 1B). However, except for ARHGAP4, the other seven highly expressed genes had no significant effect on the overall survival (OS) of CRC patients (Fig. S1A). In contrast, CRC patients with high ARHGAP4 expression exhibited poorer OS, disease-specific survival, progression-free interval and relapse-free survival compared to those with low ARHGAP4 expression (Fig. 1C–F). Lasso regression analysis of these genes further confirmed that ARHGAP4 was the only gene within the selection criteria (Fig. 1G). To verify the authenticity of the results from the public database, we conducted a prognostic analysis on 78 CRC patients from the Affiliated Provincial Hospital of Fujian University. The results indicated that patients with relatively high ARHGAP4 expression had worse OS compared to those with low expression (Fig. 1H). Additionally, univariate and multivariate COX regression analyses on CRC samples from the TCGA database confirmed that ARHGAP4 is an independent risk factor for CRC (Supplementary Table 5, Fig. S1B).
A Gene difference analysis heat map of cancer and adjacent tissues of COAD and READ in TCGA database; B Venne diagram of the intersection of the gene set after the difference analysis of CRC data in TCGA and the RhoGAPs family gene members; C–E OS, DSS and PFI analysis were performed on patients with high and low expression of ARHGAP4 according to the median expression level by TCGA data; F RFS survival analysis of ARHGAP4 high and low expression groups was performed through the GEPIA database (including TCGA and GTEX data); G Lasso dimensionality reduction analysis of eight highly expressed genes in the intersection genes through TCGA data, and finally only ARHGAP4 was eligible; H KM overall survival curve analysis was performed on 78 patients with primary CRC from the Provincial Hospital Affiliated to Fuzhou University, and the patients were divided into high and low expression groups by median; I and J ARHGAP4 paired difference analysis in GSE44076 and TCGA; K Differential expression analysis of ARHGAP4 in pan-cancer; L The expression difference of ARGHGAP4 in CRC was verified by IHC staining of cancer and adjacent tissues in postoperative resection specimens of primary CRC patients in Fuzhou Provincial Hospital Affiliated to Fuzhou University; M–O The correlation between ARHGAP4 expression and T stage, N stage and clinical stage was analyzed in 78 patients with primary CRC from the Provincial Hospital Affiliated to Fuzhou University.
Furthermore, paired sample analysis of CRC data from TCGA and GSE44076 revealed a significant upregulation of ARHGAP4 expression in CRC (Fig. 1I, J). Pan-cancer analysis demonstrated conserved ARHGAP4 overexpression across multiple malignancies (Fig. 1K). Immunohistochemical (IHC) testing of cancerous and adjacent non-tumor tissues from primary CRC patients further confirmed this high expression status (Fig. 1L). Clinical data from the Fujian Provincial Hospital affiliated with Fuzhou University suggested that elevated ARHGAP4 expression is associated with higher T-stage, N-stage and clinical stage (Fig. 1M–O). These findings collectively suggest that ARHGAP4 is highly expressed in CRC and plays a role in its onset and progression.
ARHGAP4 promotes the malignant biological behavior of CRC cells
After determining the correlation between ARHGAP4 and CRC, we further systematically investigated its functional role through in vitro and in vivo experiments. Western blot analysis was performed on HCT116, SW48, RKO, SW620, and LoVo cell lines to assess the baseline protein expression levels of ARHGAP4 (Fig. S2A). HCT116 and RKO cell lines were selected for stable transfection to construct overexpression and knockdown models (Fig. S2B). Functional characterization demonstrated that ARHGAP4 overexpression significantly enhanced cellular proliferation in CCK-8 assays, while knockdown exerted opposite effect (Fig. 2A). This proliferative advantage was further confirmed by increased EdU incorporation rates in ARHGAP4-high cells (Fig. 2B, Fig. S2C) and augmented colony formation capacity (Fig. 2C, Fig. S2D). Consistent with these findings, the subcutaneous tumorigenesis experiment showed that ARHGAP4-overexpressing HCT116 cells significantly increased the tumor growth rate, size and weight compared to the control group (Fig. 2D). Furthermore, the Transwell invasion assay validated that ARHGAP4 promotes the in vitro invasive and metastatic capabilities of CRC cells (Fig. 2E, F). These experiments collectively demonstrate that ARHGAP4 enhances the malignant biological behaviors of CRC.
A CCK-8 proliferation assay was performed on RKO and HCT116; B Statistical analysis of EdU proliferation fluorescence staining of four stable strains; C Statistical analysis of clone formation experiments of four stable strains; D Compare the tumor size, tumor growth curve and tumor weight of ARHGAP4 overexpressing HCT116 subcutaneously at a concentration of 2 × 106 cells/ mouse; E Transwell invasion assay of four stable strains.
ARHGAP4 promotes stemness in CRC
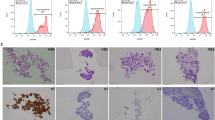
Given the established link between cancer stemness and tumor aggressiveness16.We investigate ARHGAP4 role in CRC stemness regulation. TCGA data analysis revealed significant positive correlations between ARHGAP4 expression and established stemness markers (Fig. S3). We further conducted spheroid culture experiments and observed that overexpression of ARHGAP4 enhanced the stemness of CRC cells, while knockdown of ARHGAP4 resulted in the opposite effect (Fig. 3A, B). Side population (SP) cell analysis also demonstrated that the ARHGAP4 overexpression group contained more cells with verapamil-sensitive signals, whereas the knockdown group showed the opposite pattern (Fig. 3C, D). Subsequently, we performed WB to assess the expression of tumor stemness markers, metastasis-related proteins, and cell cycle/proliferation proteins in the four stable transfectant groups. The results indicated that high ARHGAP4 expression enhanced the levels of CRC stemness markers, such as CD4417, metastasis-related proteins like N-cadherin and Vimentin18 as well as proliferation and oncogenic proteins, including c-Myc and Cyclin D119 (Fig. 3E). Additionally, protein immunoblotting from xenograft tumors in previous subcutaneous tumor models of nude mice also confirmed that in vivo tumorigenic models showed an increase in stemness markers as the expression of ARHGAP4 increased (Fig. 3F). Further, Limiting dilution assays using HCT116 cells in nude mice demonstrated that ARHGAP4 overexpression significantly enhanced tumor initiation capacity, as evidenced by increased tumor incidence, size, and weight at equivalent cell doses (Fig. 3G–I). Corresponding stemness assessments confirmed greater stem-like properties in ARHGAP4-high tumors (Fig. 3J). These comprehensive analyses establish ARHGAP4 as a critical regulator of CRC stemness that drives tumor malignancy.
A and B Suspension sphere formation experiments on HCT116 and RKO cell lines; C and D side population (SP) cell sorting experiment; E WB was used to analyze the relationship between the expression of ARHGAP4 and the expression of CD44, N-cardherin, Lgr5, EPCAM, c-Myc, Vimentin and Cyclin D1 in RKO and HCT116 cells; F WB determination of stemness index of xenograft tumors; G A schematic diagram of limited dilution tumor formation in BALB/ C nude mice to detect stemness; H Tumor formation under limited dilution; I 5 × 105 cell inoculation concentration per nude mouse, the weight of the tumor formed by the control group and the overexpression group was compared; J Statistical test of the frequency of stem cells displayed under limited dilution.
ARHGAP4 promotes CRC stemness through MYH9
To elucidate the molecular mechanism underlying ARHGAP4-mediated CRC progression and stemness, we performed immunoprecipitation (IP) of the ARHGAP4 protein followed by mass spectrometry analysis. The results revealed MYH9 as the top candidate interacting protein, with the highest numbers of binding peptides, characteristic peptides, and unique peptides in both cell lines (Fig. 4A). This interaction was confirmed endogenously through Co-IP (Fig. 4B) and demonstrated strong intracellular colocalization by immunofluorescence (Fig. 4C). To verify that the increased stemness in CRC cells induced by ARHGAP4 is mediated through MYH9, we performed WB analysis, which demonstrated that knockdown of MYH9 weakened the upregulation of proteins, such as CD44, Cyclin D1, and Vimentin caused by ARHGAP4 overexpression (Fig. 4D). Consistent with these findings, MYH9 depletion significantly impaired ARHGAP4-driven enhancements in colony formation, invasion capacity, and sphere formation (Fig. 4E–G, Fig. S4A–C). Additionally, in vivo subcutaneous tumorigenesis using a limiting dilution assay showed that MYH9 knockdown significantly reduced the tumor formation rate induced by ARHGAP4 overexpression (Fig. 4H, Fig. S4D). Through structural analysis, we identified key interaction sites between ARHGAP4 and MYH9. Site-directed mutagenesis of these residues (Fig. 4I, J) abolished the ARHGAP4-MYH9 interaction (Fig. 4K) and consequently impaired ARHGAP4’s ability to promote malignant phenotypes in vitro (Fig. 4L–N, Fig. S4E–G). These results collectively establish the ARHGAP4-MYH9 interaction as a critical molecular determinant of CRC stemness and progression.
A The top ten intersection proteins that can interact with ARHGAP4 in RKO and HCT116 cells obtained by LC-MS; B Co-IP analysis of RKO and HCT116 cell lines; C Immunofluorescence co-localization analysis of ARHGAP4 and MYH9 in cells, green fluorescence was MYH9, red fluorescence was ARHGAP4; D WB was performed on the constructed stable transgenic strains to show the changes of CD44, N-cardherin, Lgr5, EPCAM, c-Myc, Vimentin and Cyclin D1 under the changes of the two genes; E–G CLON assay, Transwell invasion assay and suspension culture assay were performed after simultaneous transfection of lentivirus with knockdown of MYH9 in ARHGAP4-overexpressing cells; H limited dilution of the revertant strain to form a tumor, the following picture is a statistical analysis of the frequency of stem cells; I Molecular docking prediction of the amino acid sites where ARHGAP4 interacts with MYH9. The blue represents MYH9, and the green represents ARHGAP4; J A schematic diagram of ARHGAP4 site mutation; K The interaction between ARHGAP4 and MYH9 was verified by Co-IP experiment; L–N Colony formation assay, statistical analysis of Transwell invasion assay and suspension sphere formation assay were performed on PCDNA, ARHGAP4-WT and ARHGAP4-MUT transfected cells.
The biological changes induced by ARHGAP4 are regulated by MYH9/GSK3β/β-catenin signal transduction
To delineate the signaling mechanisms underlying ARHGAP4-mediated CRC progression, which revealed significant enrichment of Wnt/β-catenin signaling pathways (Fig. 5A), a finding corroborated in the GSE44076 dataset (Fig. 5B). Given the established role of Wnt/β-catenin activation in promoting tumor stemness and EMT20. And prior reports linking GSK3β phosphorylation at Ser9 to β-catenin pathway activation in various cancers10,11,21,22, we investigated whether ARHGAP4 similarly modulates this pathway in CRC. WB results indicated that overexpression of ARHGAP4 led to increased levels of β-catenin and p-GSK3β (Ser9)/GSK3β, while knockdown of ARHGAP4 produced the opposite effect (Fig. 5C). Additionally, immunofluorescence analysis of β-catenin nuclear translocation and WB further demonstrated that the increased expression of ARHGAP4 promotes the accumulation of β-catenin in the nucleus (Fig. 5D, E). Further WB analysis revealed that overexpression of ARHGAP4 in the RKO cell line accelerated GSK3β protein degradation, whereas knockdown of ARHGAP4 in the HCT116 cell line slowed GSK3β degradation (Fig. 5F). Proteasome inhibition with MG132 rescued GSK3β levels in ARHGAP4-overexpressing cells but not in knockdown cells (Fig. 5G). Co-IP experiments further demonstrated that in ARHGAP4 high-expressing cells, GSK3β underwent increased ubiquitination (Fig. 5H).
A Mountain map of sample pathway enrichment in ARHGAP4 high expression group after GSEA analysis; B Visual enrichment analysis of EMT pathway and WNT/β-catenin pathway in ARHGAP4 high expression group in TCGA and GSE44376 by GSEA enrichment analysis. NES > 0 indicates that ARHGAP4 has an up-regulated effect on this pathway; C WB was used to verify the β-catenin pathway in RKO and HCT116 cell lines; D WB was used to verify the nuclear and cytoplasmic changes of β-catenin protein in HCT116 cell line with ARHGAP4 knockdown and RKO cell line with ARHGAP4 overexpression; E Immunofluorescence staining was used to verify the nuclear translocation of β-catenin protein in HCT116 cell line with ARHGAP4 knockdown and RKO cell line with ARHGAP4 overexpression; F WB analysis of RKO and HCT116 cells treated with CHX; G WB analysis of RKO and HCT116 cells treated with MG132; H Co-IP detected the ubiquitination of GSK3β in RKO and HCT116 cells; I After knocking down MYH9, the changes of β-catenin pathway proteins in ARHGAP4 overexpression strains were observed; J Analysis of nuclear and cytoplasmic expression of β-catenin after knockdown of MYH9; K CHX was used to treat the complemented strain at different times and then WB was used to detect the change of GSK3β protein; L The recovery strain was treated with MG132 and then the changes of GSK3β protein were detected by WB; M Co-IP was used to detect the ubiquitination of GSK3β in the revertants.
To determine the key role of MYH9 in this axis, we performed MYH9 knockdown experiments based on ARHGAP4 overexpression to study the upstream and downstream responses. WB results confirmed that MYH9 knockdown effectively reduced the activation of the β-catenin pathway induced by ARHGAP4 overexpression (Fig. 5I). Furthermore, nuclear translocation WB results also confirmed that MYH9 knockdown significantly restored the levels of β-catenin in both the nucleus and the cytoplasm (Fig. 5J). Consistently, MYH9 knockdown also effectively reversed the reduction in GSK3β protein degradation caused by ARHGAP4 overexpression in the HCT116 and RKO cell lines (Fig. 5F, G). Similarly, MYH9 knockdown significantly reversed the increase in ubiquitination of GSK3β associated with ARHGAP4 overexpression (Fig. 5H). Subsequently, we treated control cell lines and ARHGAP4-OE + MYH9-Sh cell lines with Laduviglusib (WNT/β-catenin pathway activator) and treated ARHGAP4-overexpressing cell lines with KYA1797K (WNT/β-catenin pathway inhibitor) (Fig. S5A). We then performed colony formation assays, Transwell invasion assays, and spheroid formation assays. The results indicated that after treatment with pathway modulators, the proliferation, migration, and stemness characteristics of both the control and recovery groups in the HCT116 and RKO cell lines were significantly increased. Meanwhile, the biological changes induced by ARHGAP4 overexpression were reversed by KYA1797K (Figs. S5B–G). In conclusion, our results suggest that ARHGAP4 promotes the progression and stemness of CRC cells through the MYH9/GSK3β axis, which activates the β-catenin pathway.
ARHGAP4-mediated increased MYH9 phase separation promotes GSK3β ubiquitination complex formation
After confirming the signal axis, we were curious about how ARHGAP4 regulates the ubiquitination of GSK3β through MYH9. We observed that under conditions where the expression of ARHGAP4 changed, the E3 ubiquitin ligase for GSK3β, tumor necrosis factor receptor-associated factor 6 (TRAF6), did not show significant changes (Fig. 6A)23,24. However, when the corresponding ubiquitin ligase did not increase, the enhanced ubiquitination could potentially be attributed to an increase in the assembly of the overall degradation complex25,26. Therefore, we hypothesized that ARHGAP4 may form a complex with GSK3β, TRAF6 and MYH9 to promote GSK3β ubiquitination. Co-IP experiments revealed that MYH9, GSK3β and TRAF6 form a complex in CRC cells, though ARHGAP4 showed no direct interaction with GSK3β or TRAF6 (Figs. 6B, S6A). We further speculated that ARHGAP4 might influence the interactions among the other three proteins. The Co-IP results indicated that overexpression of ARHGAP4 leads to a relative enhancement of their binding, while downregulation of ARHGAP4 results in the opposite effect (Fig. 6C–E). Although MYH9 is known to play a role as a scaffold protein in multiple signaling pathways, it is not clear whether its activity involves phase separation27. Structural analysis shows that the Coiled-coil domain of MYH9 contains IDRs, which is a sign of phase separation drive (Fig. S6B)28. The prediction analysis of multiple databases (POUNDR, catGRANULE, PSPHunter) consistently shows that the domain (Fig. 6F, G, Supplementary Table 8) has a strong phase separation tendency. The formation of cytoplasmic droplets of MYH9-GFP was verified by experiments, and the dissolution of condensate after FRAP (Fig. 6H, I) and 1,6-hexanediol treatment confirmed its dynamic liquid-like properties (Fig. 6J). Furthermore, we constructed a truncated form of MYH9 to determine whether the CC domain drives the phase separation of MYH9 (Fig. 6K). Coagulation experiments in vitro and in vivo showed that MYH9-GFP-CCΔ (coiled-coil deletion) lost phase separation ability, while MYH9-GFP-NTΔ (N-terminal deletion) retained phase separation ability (Fig. 6L).
A WB of TRAF6 and ARHGAP4 was performed on the stably transfected strains; B The interaction of four proteins in HCT116 cells was detected by Co-IP analysis. C–E The protein purification and pull-down experiments of MYH9, GSK3β and TRAF6 were performed under the condition of ARHGAP4 expression changes, and the assembly of MYH9 with TRAF6, ARHGAP4 and GSK3β was analyzed by Co-IP; F and G The phase separation ability of MYH9 was predicted by POUNDR database and catGRAUNLE database; H Fusion of MYH9 aggregates in vivo, uptake at a scale of 10 um; I FRAP experiments of MYH9 aggregates in vivo and in vitro, uptake at a scale of 10 um; J Uptake imaging before and after adding 1,6-hexanediol; K A schematic diagram of the construction of MYH9 truncations; L Coagulation formation of different truncated MYH9 in vitro and in vivo.
However, we also observed that the addition of ARHGAP4 further promotes the formation of the GSK3β complex. Based on this, we hypothesize that the spontaneous phase separation ability of MYH9 may be enhanced through interaction with ARHGAP4. Molecular dynamics simulations confirmed that the interaction between ARHGAP4 and MYH9 is mediated by ionic interactions and presents a relatively stable interaction state (Fig. 7A). Furthermore, phase separation mediated by the Coiled-coil domain is further enhanced by interactions, such as charge and ionic interactions. To verify this, we co-transfected ARHGAP4-mCherry and MYH9-GFP plasmids into 293 T cells and observed that the presence of ARHGAP4 induced the formation of droplets with MYH9, with more aggregates observed in cells expressing ARHGAP4 (Fig. 7B). FRAP analysis confirmed that these droplets were formed by phase separation (Fig. 7C). The 1,6-hexanediol experiment also demonstrated the fluidity of these droplets (Fig. 7D). Subsequently, purified the two proteins in vitro and performed the corresponding experiments, observing that the phase separation ability of MYH9 was enhanced with increasing ARHGAP4 protein concentration, and these droplets retained fluidity (Fig. 7E, F). Further experiments with the previously constructed ARHGAP4-WT and ARHGAP4-MUT plasmids revealed that only ARHGAP4 capable of interacting with MYH9 enhanced the formation of aggregates (Fig. 7G). Co-IP experiments showed that only the supplementation of ARHGAP4-WT in both cell types promoted the formation of the GSK3β complex (Fig. 7H, Fig. S6C, D). Recruitment imaging of the aggregates also demonstrated that GSK3β was recruited into the aggregates only when MYH9 phase separation ability was enhanced, while MYH9 lacking phase separation did not form aggregates nor recruit GSK3β (Fig. 7I). Consistent with these findings, ARHGAP4-enhanced MYH9 phase separation was identified as a key factor in promoting the ubiquitination of GSK3β and the subsequent activation of the β-catenin pathway (Fig. S6E, Fig. 7J, K). These molecular biology results align with the analysis from GEPIA, which similarly indicates that groups with higher expression of both ARHGAP4 and MYH9 exhibit poorer OS (Fig. S6F). These data collectively represent the first characterization of MYH9 phase separation ability and its enhancement through interaction with ARHGAP4. This interaction serves as the direct cause for the increased formation of the GSK3β complex, facilitated by ARHGAP4 in MYH9 scaffolding role.
A Molecular dynamics analysis of the interaction strength between ARHGAP4 and MYH9; B The formation of MYH9 and ARHGAP4 aggregates in vivo when ARHGAP4 was overexpressed and not overexpressed, and the uptake was at a scale of 10 um; C The co-localization of MYH9 (GFP) and ARHGAP4 (mcherry) in vivo and the FRAP imaging of the co-formed aggregates were taken at a scale of 10 um; D In vivo and in vitro imaging uptake of the aggregates formed by ARHGAP4 and MYH9 before and after the addition of 1.6-hexanediol at a scale of 10 um; E MYH9 protein uptake in vivo and in vitro at a scale of 10 um; F The co-localization of MYH9 (GFP) and ARHGAP4 (mcherry) in vitro and the FRAP imaging of the co-formed aggregates were taken at a scale of 10 um; G the formation of MYH9 aggregates after different ARHGAP4 was transferred, and the uptake was at a scale of 10 um; H Co-IP analysis of GSK3β complex assembly in HCT116 cells and RKO cells transfected with PCDNA, ARHGAP4-WT and ARHGAP4-MUT plasmids after knockdown of ARHGAP4; I In vivo fluorescence analysis of the recruitment of GSK3β by MYH9 aggregates was taken at a scale of 10 um; J WB was used to detect the changes of β-catenin pathway after transfection of PCDNA, ARHGAP4-WT and ARHGAP4-MUT plasmids; K WB was used to detect the distribution of β-catenin in PCDNA, ARHGAP4-WT and ARHGAP4-MUT transfected cells.
ARHGAP4 upregulates c-Jun in CRC to form a positive feedback loop
Previous studies have shown that the up-regulation of Wnt/β-catenin pathway activates the increase of downstream target gene c-Jun, and MYH9 is one of the transcriptional regulatory targets of c-Jun29. Our data also show that ARHGAP4-mediated MYH9 phase separation activates the β-catenin pathway, but whether it will increase the expression level of MYH9 based on this signal axis needs further verification. we performed a correlation analysis between ARHGAP4 and MYH9 using CRC data from TCGA. The results indicated a strong positive correlation between ARHGAP4 and MYH9 (Fig. S7A). Previous studies have shown that c-Jun acts as an upstream positive regulator of MYH9 expression, serving as a transcription factor for MYH9 regulation30. To investigate this, we also analyzed the correlation between c-Jun and MYH9 using CRC data from TCGA. The results indicated a positive correlation between the two (Fig. S7B). Furthermore, to verify the accuracy and authenticity of the bioinformatics analysis, we performed RT-qPCR on cell lines, which also confirmed this positive correlation (Fig. 8A, B). Notably, the upregulation of the WNT/β-catenin pathway has been shown to promote the increase of c-Jun expression and our subsequent protein immunoblotting experiments confirmed this finding (Fig. 8C, D). Additionally, after treating the corresponding cells with pathway regulators, we further confirmed that the overexpression of c-Jun and the subsequent changes in MYH9 are mediated through ARHGAP4 regulation of the β-catenin pathway (Fig. 8E, F).
A and B The mRNA levels of c-Jun and MYH9 in RKO and HCT116 cells; C and D The protein changes of MYH9, β-catenin and c-Jun; E and F WB was used to detect the changes of c-Jun and MYH9 protein levels in the case of pathway recovery; G Venn diagram of the intersection of ARHGAP4 upstream transcription factors in GTRD database and Knock TF database; H JASPR database showed the sites with the highest binding score in the c-Jun and ARHGAP4 promoter regions; I The correlation between ARHGAP4 and c-Jun was analyzed by TCGA database; J ChIP analysis verified that c-Jun could interact with the promoter of ARHGAP4; K c-Jun transcriptional regulation of ARHGAP4 promoter activity report; L the mRNA levels of ARHGAP4 and MYH9 under the change of c-Jun expression; M The protein levels of ARHGAP4 and MYH9 were changed under the change of c-Jun expression.
Next, we explored the upstream transcription factors of ARHGAP4. Using the Knock TF and GTRD databases, we analyzed the transcription factors upstream of ARHGAP4. Surprisingly, we discovered that c-Jun is also a transcription factor for ARHGAP4 (Fig. 8G). External validation of this data was conducted using the JASPAR database (Fig. 8H). Furthermore, the correlation analysis using TCGA data showed a significant positive correlation between c-Jun and ARHGAP4 expression (Fig. 8I). Subsequently, ChIP assays on the c-Jun protein confirmed that c-Jun binds to the promoter region of ARHGAP4 DNA (Fig. 8J). Additionally, promoter activity reporter assays further confirmed the transcriptional activity of c-Jun on the ARHGAP4 promoter region (Fig. 8K). Moreover, after inhibiting or overexpressing c-Jun, we measured the protein and RNA levels of ARHGAP4 and MYH9. The results indicated a significant positive correlation between c-Jun and both at the protein and RNA levels (Fig. 8L, M). In conclusion, our data suggest that ARHGAP4 not only upregulates the β-catenin pathway by indirectly mediating the assembly of the GSK3β ubiquitination complex but also promotes the upregulation of MYH9 and the transcription factor c-Jun in the context of pathway activation. This signaling ultimately forms a positive feedback loop.
Discussions
The emergence of CSCs is one of the key factors contributing to CRC progression and poor treatment outcomes31. Increasing evidence suggests that targeting CSCs could be a promising therapeutic strategy for CRC in the future32. However, the high heterogeneity of CSCs presents significant challenges to such targeted therapies33. Therefore, identifying and targeting the critical signaling pathways that drive CSCs transformation in CRC represents a relatively feasible approach for targeted treatment34. In CRC, ARHGAP4 emerges as a key regulator of stemness, distinct from other RhoGAP family members, such as ARHGAP29, which governs signaling in melanoma35, unlike its counterparts, ARHGAP4 predominantly exerts its oncogenic effects in CRC through non-canonical pathways. Our findings confirm the significance of ARHGAP4 as a therapeutic target for CRC and further support recent reports on members of the RhoGAPs family, which play a diverse key role in a wide range of signal transduction.
Protein-protein interactions, especially the formation of complexes, play a crucial role in signal transduction pathways36,37. For instance, mutations and aberrant formation of subunits in the SWI/SNF complex can significantly affect chromatin remodeling, leading to diseases, particularly cancer38. Additionally, in the past five years, the discovery of PANoptosis bodies, which are also formed by protein-protein interactions, has garnered attention39. Abnormal assembly of these complexes induces an inflammatory form of cell death, characterized by apoptosis, necrosis, and pyroptosis, which contributes to disease development40. Studying how to target these abnormally increased complexes or exploring the mechanisms behind their aberrant formation holds great potential for the treatment of diseases, particularly cancer41,42. Reports have shown that MYH9 plays a role in promoting cancer progression across various tumor types43,44,45. Recent studies indicate that multiple upstream proteins facilitate tumor progression through their interaction with MYH914,46,47. MYH9 acts as a scaffolding protein that regulates the ubiquitination of GSK3β and activates the β-catenin pathway, thereby promoting malignant tumor progression10. Current research has confirmed that the N-terminal of MYH9 interacts with GSK3β, which could be a functional site responsible for altering the ubiquitination levels of GSK3β11. Furthermore, the involvement of chaperone proteins that interact with MYH9 might promote the formation of this protein complex, thereby disrupting GSK3β, a key negative regulatory molecule in the β-catenin pathway. Our study is the first to confirm the presence of this protein complex in cCRC and provides evidence that ARHGAP4 acts as an important regulatory molecule in this disruptive complex. Moreover, we demonstrate that reducing the interaction between ARHGAP4 and MYH9 significantly diminishes the abnormal pro-cancer signaling cascade.
Recently, the introduction of LLPS has provided researchers with a more comprehensive understanding of the molecular mechanisms underlying diseases, such as cancer48. However, there remains a significant gap in our understanding of the fundamental forces driving phase separation. Recent studies have confirmed that Coiled-coil regions exhibit phase separation capabilities, further solidifying the foundation of research in this field49. In our study, we found that MYH9 also possesses phase separation ability. An increasing number of studies suggest that proteins with phase separation capabilities promote the formation of condensates and downstream signaling pathways with the assistance of chaperone proteins. As an uncharacterized phase-separating molecule, we verified the phase separation ability of MYH9 and its dependence on interacting partners for enhanced phase separation. Moreover, combining this with our finding that MYH9, upon losing its interactions, failed to promote the formation of the GSK3β ubiquitination complex, we were able to confirm the central role of enhanced phase separation in driving abnormal signaling. This further highlights the significance of targeting aberrant phase separation as a strategy to delay or even treat tumors. Overall, we have effectively broadened the scope of phase separation research and provided a clearer explanation of the mechanisms behind MYH9-mediated complex formation.
It is important to note that targeting positive feedback loops in tumor stem cells is a critical direction in cancer therapy50. As mentioned earlier, the disruption of GSK3β is achieved by increasing the destruction of complex assembly. This disruption in complex formation promotes the activation of the β-catenin pathway, which in turn upregulates the expression of c-Jun, an upstream transcription factor of MYH929. Furthermore, we have identified that c-Jun is also a transcription factor for ARHGAP4. Its upregulation leads to increased expression of both ARHGAP4 and MYH9, thereby facilitating the formation of the positive feedback loop. Notably, the elevation of ARHGAP4, especially its interaction with MYH9, plays a crucial role in the assembly of downstream complexes, which serves as the key initiator in the formation of this feedback loop. Future targeted therapies aimed at ARHGAP4 or its interaction sites with MYH9 could effectively weaken the positive feedback activation that regulates the malignant progression of CRC.
However, this study has several limitations. First, the selection of ARHGAP4 in this study was based on high-expression genes within the RhoGAPs family. Future research should focus on investigating the roles of low-expression RhoGAPs family genes in CRC. Second, our pathway exploration primarily concentrated on the WNT/β-catenin pathway. We did not further analyze whether ARHGAP4 could mediate biological regulation through molecular mechanisms beyond stemness. This aspect also warrants further investigation in future studies. Third, whether the enhanced phase separation of MYH9 by ARHGAP4 regulates complex assembly beyond GSK3β remains to be further elucidated. Lastly, the lack of additional preclinical models to assess the potential of targeting the ARHGAP4-MYH9 axis in clinical translational therapies remains a limitation.
Overall, this study reveals that the stable interaction between ARHGAP4 and MYH9 enhances the phase separation ability of MYH9. The increased phase separation of MYH9 subsequently recruits and mediates the formation of the ubiquitination complex of GSK3β, which directly activates the Wnt/β-catenin pathway, thereby promoting stemness and malignant biological behavior in CRC. Furthermore, the activation of this pathway induces the upregulation of the downstream target gene c-Jun, which in turn transcriptionally activates ARHGAP4 and MYH9, establishing a positive feedback loop (Fig. 9). This complex mechanism crosstalk underscores the potential therapeutic significance of targeting the ARHGAP4-MYH9 interaction.
ARHGAP4 is highly expressed in CRC. This makes ARHGAP4 and MYH9 interact more frequently and promotes the phase separation ability of MYH9. The increased phase separation ability further recruits GSK3β and increases the assembly of GSK3β ubiquitinated complexes. Subsequently, GSK3β was degraded by ubiquitination and the activation of the β-catenin pathway promoted the malignant biological behavior of CRC. At the same time, the downstream target gene c-Jun of the pathway is up-regulated and in turn transcriptionally regulates MYH9 and ARHGAP4 to form a positive feedback loop.
Methods
Bioinformatics analysis
The data used for analysis were obtained from two datasets: TCGA and GSE44076 (from the GEO database), focusing on CRC. The data were processed, analyzed, and visualized using R Studio (R Version 4.4.0). The specific R packages used include “tidyverse”, “ggplot2”, “dplyr” and “TCGAbiolinks.”
Cell lines and clinical samples
Cell lines HCT116, RKO, SW48, SW620, Lovo and HEK293T were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM/F12 medium containing 10% fetal bovine serum (cat # AUS-01S-02, Cell-Box, Australia) (Shanghai BasaMedia Biological Technology, China). Clinical specimens were collected from 78 patients after surgery and ten pairs of paraffin sections of colorectal tumor biopsy in Fujian Provincial Hospital. The specific information of clinical data is shown in Supplementary Table 1, and the baseline information of clinical patients is shown in Supplementary Table 2.
Lentiviral and plasmid transfection vectors
ARHGAP4 overexpression and no-load and nonsense sequence and knockdown sequence lentivirus design and coating, MYH9 nonsense sequence and knockdown sequence lentivirus, MYH9-GFP, GFP, ARHGAP4-mcherry, MYH9-GFP-NTΔ, YH9-GFP-CCΔ and GSK3β-mcherry were provided by Wuhan Puyun Biotechnology Co., Ltd.Technical support. The PCDNA, wild-type and mutant plasmids of ARHGAP4 were provided by Shanghai Hanheng Biotechnology Co., Ltd. See Supplementary Table 3 for the specific sequence.
Western-blotting (WB)
Cells were washed with PBS after removing the culture medium. Protein extraction was performed using NP40 lysis buffer (Herui Biotechnology, China) with phosphatase (100x, MCE, China) and protease inhibitors (100x, MCE, China) at 4 °C, followed by centrifugation. The supernatant was mixed with 5X loading buffer (Herui Biotechnology, China) and boiled at 100 °C for 10 min. SDS-PAGE gels (Biyuntian Biotechnology, China, P0012A) with 12% concentration were used for Western blotting. Protein transfer was conducted on 0.45 μm PVDF membranes (Millipore, USA, IPFL00010). The membranes were blocked with BSA solution (Merck Biotech, Germany, V900933) and incubated with primary antibodies overnight. After washing with TBST (3 × 15 min), secondary antibody incubation was performed for 1 h. Membranes were then washed and developed using ECL detection reagent (GLPBIO, USA, GK10008) on a BIO-RAD system. Antibody details are listed in Supplementary Table 4.
IP Assay (Co-IP)
The Co-IP kit was purchased from Thermo Fisher, China. Antibody details are in Supplementary Table 3. Briefly, cells were washed with PBS and lysed on ice for 15 min in 1 mL of pre-chilled lysis/wash buffer supplemented with phosphatase and protease inhibitors (100×; MCE, China). Lysates were centrifuged at 12,500 g for 15 min at 4 °C. For input samples, the supernatant was mixed with 5× loading buffer and denatured at 100 °C for 10 min. For IP, 100 µL of supernatant was incubated with 2 µL of IgG or IgG1 antibody (Wuhan Sanjing Biotech, China) overnight at 4 °C. The following day, protein complexes were processed as per the kit instructions. Co-IP samples were then subjected to electrophoresis and transferred for WB using standard protocols.
Immunohistochemical staining (IHC)
Tissue sections from Fujian Provincial Hospital were deparaffinized, antigen retrieved, and incubated with ARHGAP4 antibody (1:200) at 4 °C for 8 h. DAB chromogen staining was applied, followed by hematoxylin counterstaining. Slides were dehydrated, mounted with neutral gum, and air-dried before observation.
EdU cell proliferation assay
Adherent cells were treated with EdU solution for 2 h, fixed with paraformaldehyde, and permeabilized with Triton-X100. EdU staining was applied for 30 min, followed by DAPI nuclear staining. The cells were washed and analyzed by microscopy.
Immunofluorescence Staining (IF)
Adherent cells were washed with PBS, fixed with paraformaldehyde, and permeabilized with Triton-X100. After blocking with goat serum, cells were incubated with primary antibodies (ARHGAP4, MYH9, β-catenin) overnight. After washing, fluorescent secondary antibody was applied, followed by DAPI staining. Images were captured using a Leica DMI8 microscope.
CCK-8 proliferation assay and clone assay
For the CCK-8 assay, cells were seeded in 96-well plates and incubated with CCK-8 solution for 2 h, with absorbance measured. Data were analyzed using GraphPad Prism. For the clonogenic assay, cells were seeded in 6-well plates, stained with crystal violet after two weeks, and quantified using ImageJ, followed by statistical analysis with GraphPad Prism.
Suspension spheroid formation assay
After cell digestion, cells were resuspended and counted. Cell suspension containing 400 cells per well was prepared using spheroid culture medium (Sicogee Biotechnology Co., Ltd., China). 1 mL of the cell suspension was added to each well, and cells were gently mixed. After 1–2 weeks, spheroid formation was analyzed by counting the spheroids in each well and taking images. Data were processed using GraphPad Prism (GraphPad Prism 10.1.2), with three independent replicates per group for statistical analysis.
SP cell sorting
Reagents used for SP cell sorting included Hoechst 33342 (Sicogee Biotechnology Co., Ltd., China) and verapamil (Sicogee Biotechnology Co., Ltd., China). The procedure followed that described by Wei Rao et al. 51. The final result measured was the proportion of cells with Hoechst 33342 efflux. The final concentration of verapamil used in the experiment was unified at 40 uM/ml.
Transwell invasion assay
Matrigel was diluted in serum-free DMEM/F12 medium and applied to the Transwell chamber membrane to polymerize at 37 °C. Cells were prepared, resuspended in serum-free medium, and seeded into the upper chamber. After incubation, cells were fixed, stained with crystal violet, and images captured. The number of invasive cells was counted and data analyzed using GraphPad Prism.
Subcutaneous tumor formation and tumor experiment in nude mice
The experimental animals used were male BALB/C nude mice devoid of a thymus. Tumor formation experiments were conducted by subcutaneously injecting cells at three different concentrations: 1 × 105, 5 × 105, and 5 × 104 cells per mouse. In the subcutaneous tumor formation experiment, 2 × 106 cells per mouse were used for injection. Mice were euthanized when the tumor size was less than 1500 mm³, and the tumors were subjected to imaging, weighing, and growth curve plotting for xenograft evaluation. Tumor sizes were recorded every two days from day 7 post-inoculation until euthanasia, and tumor growth curves were drawn during subsequent analysis. All statistical analysis and graphical representations were performed using GraphPad Prism version 10.1.2.
Protein mass spectrometry analysis
Protein mass spectrometry was performed using an LC-MS/MS method for enzyme digestion analysis. Following the purification of ARHGAP4 from RKO and HCT116 cells via magnetic bead pull-down, the purified proteins underwent subsequent analysis. After the cleaning steps, the magnetic beads were processed using a C18 membrane-packed column for peptide desalting, followed by mass spectrometry analysis. This entire process was managed by Luming Biotechnology Co., Ltd., Shanghai, China.
Detection of protein degradation and recovery
Specifically, this experiment is to detect proteasome pathway-mediated protein determination52. HCT116 and RKO stable cell lines were treated with 100 µM of cycloheximide for 1, 2, and 4 h. Protein extraction was followed by Western blotting to detect changes in GSK3β protein levels. Further analysis was performed by treating HCT116 and RKO stable cell lines with 20 µM of the proteasome inhibitor MG132 for 12 h, followed by protein extraction and Western blotting to examine changes in GSK3β protein levels.
Phase separation prediction
The IDRs of MYH9 were analyzed using the PONDR database (https://www.pondr.com/). The phase separation potential of MYH9 was further analyzed using the PSPHunter database (http://psphunter.stemcellding.org/) and the catGRAUNLE database (https://tools.tartaglialab.com/catgranule). The amino acid sequence of MYH9 was obtained from the Uniprot database (https://www.uniprot.org/).
Preparation of relevant systems for phase separation experiments
In vivo phase separation experiments were performed by transfecting the appropriate plasmids into HEK293T cells. For in vitro studies, proteins were initially obtained through protein purification. All proteins were expressed in Escherichia coli BL21 (DE3) cells (Herrui Biotechnology Co., Ltd., China) with the addition of 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG), followed by incubation at 25 °C for 16 h. The cell pellet from 500 mL of cultured cells was resuspended in a binding buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 10 mM imidazole) containing a protease inhibitor mixture (1 mM PMSF) and subjected to sonication. The resulting supernatant was purified using Ni-NTA agarose (Invitrogen, R901-15), followed by elution with an elution buffer (50 mM Tris-HCl, pH 7.4, 1 M KCl, 5% glycerol, and 200 mM imidazole). The protein was further concentrated using a Millipore filter and dialyzed against a storage buffer (50 mM Tris-HCl, 300 mM KCl, 1 mM DTT, and 5% glycerol). The purified protein was stored at -80 °C. For the experiment, the protein was resuspended in LLPS buffer (25 mM Tris-HCl, 150 mM KCl, 0.5 mM DTT, 8% PEG8000, and 2.5% glycerol) and adjusted to the required concentrations (The concentration of MYH9 protein in vitro was fixed at 20 uM).
Live cell imaging technology
Cells were seeded in confocal culture dishes (NEST, 801002) and transfected with 2 μg of plasmid for 48 h. Live cell imaging was conducted using a Leica TCS SP8 STED microscope (Leica Microsystems) at 37 °C and 5% CO2. All procedures adhered to the manufacturer’s guidelines, and images were captured in confocal mode with a 100× oil immersion objective.
Fluorescence recovery after photobleaching (FRAP) experiment
FRAP analysis of in vitro or cellular biomolecular condensates was carried out at room temperature using the FRAP module of a Leica TCS SP8 STED confocal microscope system (Leica Microsystems). The droplet to be bleached was located using the 100× oil immersion objective, and a circular region was photobleached with full laser power. Time-lapse images were then captured. Fluorescence recovery was measured against the initial fluorescence intensity using ImageJ, and the data were plotted using GraphPad Prism.
Uptake experiment after treatment with 1,6-Hexanediol
Following the same protocol as live cell imaging, 48 h after transfection, 2% 1,6-hexanediol was introduced into the culture medium. Time-lapse imaging was then performed using the Leica TCS SP8 STED confocal microscope system with a 100× oil immersion objective.
Prediction and correlation analysis of upstream transcription factors
The prediction of upstream transcription factors was performed using the Knock TF database and the GTRD database (https://gtrd18-06.biouml.org/). The binding sites of transcription factors were predicted using the JASPAR database (https://jaspar.elixir.no/), and the promoter sequence of ARHGAP4 was obtained from the NCBI website (https://www.ncbi.nlm.nih.gov/). Correlation analysis between genes was conducted using Pearson correlation analysis on CRC data from the TCGA database.
Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation (ChIP) assays were conducted using the SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) (#9003) from CST Biotechnology. The procedure was carried out according to the instructions provided in the kit’s manual. The antibody for c-Jun (JUN) was purchased from CST.
Promoter Activity Assay
The promoter region of ARHGAP4 was retrieved from the NCBI website (https://www.ncbi.nlm.nih.gov/). A ‘promoter-reporter gene-terminator’ vector construction method was used, with the luciferase (LUC) gene as the reporter. The reporter gene vector and c-Jun plasmid were co-transfected into HEK293T cells. Luciferase activity was measured with a microplate reader to evaluate the transcriptional regulatory function.
Statistical methods
Bioinformatics analysis was performed using RStudio, with the necessary R packages installed for the analysis. Statistical analysis of the experimental data was done using SPSS 27.0 and GraphPad Prism 10.1.2. All experiments were repeated at least three times. Survival analysis was conducted with the Kaplan-Meier method, followed by the Log-rank test. Data are presented as mean ± standard deviation (mean ± SD). The independent sample t-test was used for comparing two groups, while one-way analysis of variance (ANOVA) was used for comparisons among multiple groups. A p value of <0.05 was considered statistically significant. nsP represents > 0.05, *P represents < 0.05, **P represents < 0.01, ***P represents < 0.001.
Data availability
The data sets used in this study have all been shown in the method. The original data of WB has been stored in the supplementary materials. The original data can be provided according to reasonable requirements.
References
Fernandez-Rozadilla, C. et al. Deciphering colorectal cancer genetics through multi-omic analysis of 100,204 cases and 154,587 controls of European and east Asian ancestries. Nat. Genet 55, 89–99 (2023).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Rathinam, R., Berrier, A. & Alahari, S. K. Role of Rho GTPases and their regulators in cancer progression. Front. Biosci.16, 2561–2571 (2011).
Kreider-Letterman, G., Carr, N. M. & Garcia-Mata, R. Fixing the GAP: the role of RhoGAPs in cancer. Eur. J. Cell Biol. 101, 151209 (2022).
Frey, Y., Lungu, C. & Olayioye, M. A. Regulation and functions of the DLC family of RhoGAP proteins: implications for development and cancer. Cell Signal 125, 111505 (2025).
Fu, M. S. et al. Analysis of ARHGAP4 expression with colorectal cancer clinical characteristics and prognosis. Front. Oncol. 12, 899837 (2022).
Liu, Q. et al. MYH9: a key protein involved in tumor progression and virus-related diseases. Biomed. Pharmacother. 171, 116118 (2024).
Ye, G. et al. Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis. Theranostics 10, 7545–7560 (2020).
Xia, Z. K. et al. Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer. Dis. Esophagus 25, 427–436 (2012).
Lin, X. et al. Silencing MYH9 blocks HBx-induced GSK3β ubiquitination and degradation to inhibit tumor stemness in hepatocellular carcinoma. Signal Transduct. Target Ther. 5, 13 (2020).
Gao, S. et al. TUBB4A interacts with MYH9 to protect the nucleus during cell migration and promotes prostate cancer via GSK3β/β-catenin signalling. Nat. Commun. 13, 2792 (2022).
Zhong, Y. et al. MYH9-dependent polarization of ATG9B promotes colorectal cancer metastasis by accelerating focal adhesion assembly. Cell Death Differ. 28, 3251–3269 (2021).
Liu, L. et al. MYH10 combines with MYH9 to recruit USP45 by deubiquitinating snail and promotes serous ovarian cancer carcinogenesis, progression, and cisplatin resistance. Adv. Sci.10, e2203423 (2023).
Hu, R. et al. TMEM120B strengthens breast cancer cell stemness and accelerates chemotherapy resistance via β1-integrin/FAK-TAZ-mTOR signaling axis by binding to MYH9. Breast Cancer Res. 26, 48 (2024).
Liu, Z. et al. Liquid‒liquid phase separation: roles and implications in future cancer treatment. Int J. Biol. Sci. 19, 4139–4156 (2023).
Yang, L. et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target Ther. 5, 8 (2020).
Ma, Y. S. et al. Targeting colorectal cancer stem cells as an effective treatment for colorectal cancer. Technol. Cancer Res. Treat. 19, 1533033819892261 (2020).
Liu, F. et al. LINC00586 represses ASXL1 expression thus inducing epithelial-to-mesenchymal transition of colorectal cancer cells through LSD1-mediated H3K4me2 demethylation. Front. Pharm. 13, 887822 (2022).
Kasprzak, A. Prognostic biomarkers of cell proliferation in colorectal cancer (CRC): from immunohistochemistry to molecular biology techniques.Cancers 15, 4570 (2023).
He, K. & Gan, W. J. Wnt/β-catenin signaling pathway in the development and progression of colorectal cancer. Cancer Manag Res. 15, 435–448 (2023).
Li, Q. et al. SAMD9 promotes postoperative recurrence of esophageal squamous cell carcinoma by stimulating MYH9-mediated GSK3β/β-catenin signaling. Adv. Sci.10, e2203573 (2023).
Hu, S. et al. Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of Wnt-β-catenin-STAT3 signaling. Cell Death Differ. 29, 642–656 (2022).
Ko, R., Park, J. H., Ha, H., Choi, Y. & Lee, S. Y. Glycogen synthase kinase 3β ubiquitination by TRAF6 regulates TLR3-mediated pro-inflammatory cytokine production. Nat. Commun. 6, 6765 (2015).
Que, T. et al. HMGA1 stimulates MYH9-dependent ubiquitination of GSK-3β via PI3K/Akt/c-Jun signaling to promote malignant progression and chemoresistance in gliomas. Cell Death Dis. 12, 1147 (2021).
Cockram, P. E. et al. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 28, 591–605 (2021).
Cervia, L. D. et al. A ubiquitination cascade regulating the integrated stress response and survival in carcinomas. Cancer Discov. 13, 766–795 (2023).
Liang, M. et al. Oligomerized liprin-α promotes phase separation of ELKS for compartmentalization of presynaptic active zone proteins. Cell Rep. 34, 108901 (2021).
Ahn, J. H. et al. Phase separation drives aberrant chromatin looping and cancer development. Nature 595, 591–595 (2021).
Hou, R. et al. ENKUR expression induced by chemically synthesized cinobufotalin suppresses malignant activities of hepatocellular carcinoma by modulating β-catenin/c-Jun/MYH9/USP7/c-Myc axis. Int J. Biol. Sci. 18, 2553–2567 (2022).
Hou, R. et al. Chemically synthesized cinobufagin suppresses nasopharyngeal carcinoma metastasis by inducing ENKUR to stabilize p53 expression. Cancer Lett. 531, 57–70 (2022).
Radu, P. et al. The impact of cancer stem cells in colorectal cancer. Int. J. Mol. Sci. 25, 4140 (2024).
Garza Treviño, E. N. et al. Cell therapy as target therapy against colon cancer stem cells. Int. J. Mol. Sci 24, 8163 (2023).
Zheng, H. et al. Characterization of stem cell landscape and identification of stemness-relevant prognostic gene signature to aid immunotherapy in colorectal cancer. Stem Cell Res. Ther. 13, 244 (2022).
Wang, M. et al. ZNF217-activated Notch signaling mediates sulforaphane-suppressed stem cell properties in colorectal cancer. J. Nutr. Biochem 125, 109551 (2024).
Li, Y. et al. EHMT2 promotes tumorigenesis in GNAQ/11-mutant uveal melanoma via ARHGAP29-mediated RhoA pathway. Acta Pharm. Sin. B 14, 1187–1203 (2024).
Saibu, O. A. et al. Protein-protein interaction and interference of carcinogenesis by supramolecular modifications. Bioorg. Med. Chem. 81, 117211 (2023).
Dalbanjan, N. P. & Praveen Kumar, S. K. A chronicle review of in-silico approaches for discovering novel antimicrobial agents to combat antimicrobial resistance. Indian J. Microbiol. 64, 879–893 (2024).
Mittal, P. & Roberts, C. W. M. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 17, 435–448 (2020).
Lee, S. et al. AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 597, 415–419 (2021).
Samir, P., Malireddi, R. K. S. & Kanneganti, T. D. The PANoptosome: a deadly protein complex driving pyroptosis, apoptosis, and necroptosis (PANoptosis). Front. Cell Infect. Microbiol 10, 238 (2020).
Dalbanjan, N. P., Eelager, M. P. & Narasagoudr, S. S. Microbial protein sources: a comprehensive review on the potential usage of fungi and cyanobacteria in sustainable food systems. Food Humanit. 3, 100366 (2024).
Dalbanjan, N. P. et al. Descriptive review on conversion of waste residues into valuable bionanocomposites for a circular bioeconomy. Nano-Struct. Nano-Objects 39, 101265 (2024).
Liu, L. et al. MYH9 overexpression correlates with clinicopathological parameters and poor prognosis of epithelial ovarian cancer. Oncol. Lett. 18, 1049–1056 (2019).
Chen, Z. et al. Targeting MYH9 represses USP14-mediated NAP1L1 deubiquitination and cell proliferation in glioma. Cancer Cell Int. 23, 220 (2023).
Wang, B. et al. MYH9 promotes growth and metastasis via activation of MAPK/AKT signaling in colorectal cancer. J. Cancer 10, 874–884 (2019).
Xie, C. et al. Mitochondrial micropeptide STMP1 enhances mitochondrial fission to promote tumor metastasis. Cancer Res. 82, 2431–2443 (2022).
Garlapati, C. et al. A novel role for KIFC1-MYH9 interaction in triple-negative breast cancer aggressiveness and racial disparity. Cell Commun. Signal 22, 312 (2024).
Peng, P. H., Hsu, K. W. & Wu, K. J. Liquid-liquid phase separation (LLPS) in cellular physiology and tumor biology. Am. J. Cancer Res. 11, 3766–3776 (2021).
He, Z., Sommer, J. U. & Harmon, T. S. Impact of coiled-coil domains on the phase behavior of biomolecular condensates. ACS Macro Lett 14, 413–419 (2025).
Taniguchi, S. et al. Tumor-initiating cells establish an IL-33-TGF-á niche signaling loop to promote cancer progression. Science 369, eaay1813 (2020).
Rao, W. et al. Enhanced enrichment of prostate cancer stem-like cells with miniaturized 3D culture in liquid core-hydrogel shell microcapsules. Biomaterials 35, 7762–7773 (2014).
Zhang, L. et al. FBXO22 promotes the development of hepatocellular carcinoma by regulating the ubiquitination and degradation of p21. J. Exp. Clin. Cancer Res. 38, 101 (2019).
Acknowledgements
Acknowledgments: This work was supported by financial grants from the Natural Science Foundation of Fujian Province (grant number: 2022J011024) to Weihua Li.
Author information
Authors and Affiliations
Contributions
All authors agree to be responsible for all aspects of the work, and ultimately approve the version to be published. L.-S.L., M.-Y.L., and C.-Y.Z.: material preparation, retrieval, and data collection. T.-S., L.-S.L., and C.-Y.F.: Visualization. L.-S.L. and C.-Y.Z.: Write the original. L.-S.T., Y.-C.S., and L.-W.H.: Supervision and conceptualization. L.-S.T. and L.-W.H.: Revised draft. L.-W.H.: Funding supports.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Chen, Y., Tan, S. et al. ARHGAP4/MYH9/β-catenin/c-Jun positive feedback loop promotes colorectal cancer stemness. npj Precis. Onc. 9, 285 (2025). https://doi.org/10.1038/s41698-025-01022-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41698-025-01022-4