Abstract
Prostaglandin D2 synthase (PTGDS), a newly identified anti-tumor target, shows promise in inhibiting various cancers and plays significant roles in the tumor microenvironment and immune regulation, yet a comprehensive pan-cancer analysis of its expression and prognostic value remains lacking. This study used multi-omics data from public databases to assess PTGDS’s expression, mutation, and modification in multiple cancers, integrated single-cell and spatial transcriptomic data to explore its relationship with immune cells, and conducted in vitro and in vivo experiments in breast cancer (BRCA). Results showed that PTGDS is significantly dysregulated in most cancers, with its expression associated with different outcomes depending on cancer type. It correlates with epigenetic and biological functions, and low expression in BRCA indicates poor prognosis. Overexpression of PTGDS can inhibit breast cancer cell proliferation and invasion, increase CD8+ T - cell activity, and enhance anti-tumor immunity. Combining it with anti-PD-L1 improves BRCA treatment. PTGDS is a potential prognostic biomarker and a novel immunotherapy target for BRCA.
Similar content being viewed by others
Introduction
Cancer continues to be a significant public health concern globally, with a yearly rise in both new cancer cases and deaths related to the disease1. The most prevalent cancer globally is BRCA in women (2.296 million cases, 11.5%)2. In recent years, immune checkpoint inhibitor-based immunotherapy has significantly transformed the treatment landscape for various types of tumors, becoming a frontline clinical therapy3,4. Not all patients experience benefits from immunotherapy, making it essential to discover and define new biomarkers to steer tumor immunotherapy and immune modulation. This effort will contribute to creating personalized treatment plans and achieving more lasting treatment outcomes.
Lipid transport protein prostaglandin D synthase (L-PGDS), or PTGDS, is found on chromosome 9 of humans (9q34.2 ~ 34.3) within the lipid transport protein family region5. It is a member of the superfamily of lipid transport proteins and plays a dual role in prostaglandin metabolism and lipid transport, catalyzing the production of PGD2 and transporting lipophilic substances6. Research indicates that PTGDS plays a role in several cellular functions, including the development of solid tumors, though its involvement in cancer formation is debated. Recent findings suggest that PTGDS is overexpressed in skin cutaneous melanoma (SKCM), ovarian cancer (OV), and hepatocellular adenoma7,8,9. Conversely, some studies have found that PTGDS is downregulated in prostate tumors, lung tumors, and gastric cancer, which may hinder tumor progression10,11,12. These findings suggest that PTGDS may play various regulatory roles in the progression of human cancers, potentially influencing tumor cell growth, migration, and the tumor immune microenvironment7,13,14. Nonetheless, comprehensive pan-cancer research exploring the prognostic significance and response to immunotherapy related to PTGDS has been insufficient. Due to the absence of a corresponding comprehensive perspective, studies focusing solely on single cancer types may overlook the mechanistic importance of specific target genes. Thus, it’s crucial to explore the critical role of PTGDS expression in different cancer types to guide future experimental and clinical research initiatives.
In this research, we utilized several public databases to investigate the expression levels of PTGDS in cancerous versus normal tissues. We also performed a multi-omics pan-cancer analysis to clarify the associations between this gene, clinical characteristics, and the variability observed across different omics layers. Moreover, we explored the potential roles of PTGDS and its influence on immune cell infiltration in various cancers. By analyzing survival data from TCGA and GEO databases, we assessed the prognostic significance of PTGDS concerning tumor prognosis and the response to immune checkpoint blockade therapy. Our analysis focused on DNA repair, DNA damage response, and the induction of cancer-related immune responses, confirming PTGDS as a biomarker for dendritic cell infiltration via the CIBERSORT algorithm. We conducted in vitro experiments to ascertain the effect of PTGDS on cell proliferation in BRCA cell lines. Our thorough analysis suggests that PTGDS could be a promising predictive biomarker for prognosis and response to tumor immunotherapy. We further investigated the role of PTGDS in BRCA progression. We found that PTGDS inhibited the tumor proliferation capacity of breast cancer cells by activating CD8+ T cell activity. PD-L1 blocking combined with PTGDS analogs is highly effective in treating BRCA. Therefore, this is a promising new BRCA combination treatment strategy.
Results
Expression patterns and prognostic analysis of PTGDS in human cancers
This study first systematically explored the correlation between PTGDS and various diseases, revealing that PTGDS is closely related to the occurrence and development of tumors, mainly showing a significant association with breast cancer (Fig. 1a). Given that these results are mainly based on statistical analysis of existing literature, and PTGDS may have different biological roles in different cancers, in order to further elucidate the role of PTGDS in cancer, we analyzed the expression levels of PTGDS mRNA in pan-cancer tissues and normal tissues. The results showed that in 19 types of cancer tissues, the expression level of PTGDS mRNA was significantly lower than that in corresponding normal tissues, suggesting that PTGDS may function as a tumor suppressor gene in most tumors, providing protective regulatory functions against tumor progression. Notably, among different pathological types of breast cancer, triple-negative breast cancer and Luminal B breast cancer exhibited the relatively low levels of PTGDS expression. Combined with clinical data, patients with these two pathological types of breast cancer have a poorer prognosis, further supporting the potential role of PTGDS as a protective factor in breast cancer (Fig. 1b). For cancer types lacking paired normal tissues, we compared the expression levels of PTGDS mRNA using the GEPIA2.0 database. The results showed that PTGDS mRNA was highly expressed in Diffuse Large B-Cell Lymphoma (DLBC), Ovarian Cancer (OV), and thymoma (THYM), while its expression was lower in adrenocortical carcinoma (ACC), low-grade glioma (LGG), acute myeloid leukemia (LAML), sarcoma (SARC), testicular germ cell tumor (TGCT), and uterine carcinosarcoma (UCS) (Fig. 1c). Additionally, the expression level of PTGDS mRNA was significantly correlated with tumor stage: in bladder urothelial carcinoma (BLCA) and stomach adenocarcinoma (STAD), PTGDS mRNA expression was positively correlated with tumor stage, while in kidney renal papillary cell carcinoma (KIRP), lung adenocarcinom (LUAD), and skin cutaneous melanoma (SKCM), its expression was negatively correlated with tumor stage (Fig. 1d). Further analysis of PTGDS protein expression levels using the UALCAN database revealed significant downregulation in glioblastoma multiforme (GBM), head and neck squamous cell carcinoma (HNSCC), (LUAD), renal cell carcinoma (RCC), and uterine corpus endometrial carcinoma (UCEC) (Fig. 1e). To evaluate the prognostic significance of PTGDS, we constructed Kaplan–Meier survival curves based on TCGA data. The analysis results showed that in breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), head and neck squamous cell carcinoma (HNSC), and low-grade glioma (LGG), patients with high PTGDS expression had significantly better overall survival (OS) and disease-free survival (DFS) compared to those with low expression, suggesting that PTGDS may have a protective role in these cancers. However, in KIRC, high PTGDS expression was associated with lower overall survival (OS) (Fig. 1f). In summary, the results of this study indicate that PTGDS may function as a protective factor in various cancers, with its expression levels closely related to tumor progression and patient prognosis.
a Diseases associated with PTGDS. The red dotted line indicates PTGDS-associated cancers. b Expression levels of PTGDS mRNA in pan-cancer and corresponding control tissues. c Box plots of log2 expression levels of PTGDS mRNA between tumor and normal tissues in 9 types of cancer. T and N represent tumor and normal tissues, respectively. d Expression levels of PTGDS across four different stages of 5 types of cancer. e Differences in protein expression between normal and primary tumor tissues in 5 types of cancer. f Kaplan–Meier curves are plotted to predict OS and DSS in TCGA patients. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
Copy number variation and single nucleotide variation analysis of PTGDS in pan-cancer
CNV (Copy Number Variation) and SNV (Single Nucleotide Variation) are two common types of genomic variations that hold significant importance in genetic and medical research. To investigate whether the PTGDS gene undergoes alterations at the genomic level, we systematically analyzed the CNV and SNV characteristics of PTGDS in pan-cancer. The results showed that PTGDS has a high mutation frequency in esophageal adenocarcinoma (EAC), OV, endometrial cancer, renal cancer, and cutaneous melanoma (Fig. 2a, b). Further analysis revealed that patients in the high PTGDS CNV group exhibited higher survival rates in CESC, pancreatic cancer (PAAD), STAD, and uveal melanoma (UVM), while in other cancer types, it was associated with lower survival rates (Fig. 2c). TMB (Tumor Mutational Burden) and MSI (Microsatellite Instability) are essential biomarkers in cancer research and clinical treatment, mainly guiding immunotherapy. Therefore, we explored the correlation between PTGDS TMB and MSI in pan-cancer. The results showed that PTGDS was significantly negatively correlated with TMB and MSI in various tumors such as ACC, GBM, HNSC, LGG, PAAD, prostate adenocarcinoma (PRAD), and STAD (Fig. 2d). Since high TMB is usually associated with greater genomic instability, which may promote tumor invasiveness and metastatic potential, this finding further supports the protective role of PTGDS in tumors. HRD (Homologous Recombination Deficiency) and Ploidy are two important genomic features in cancer research and clinical diagnosis, especially in breast and ovarian cancers. Our analysis indicated that PTGDS was significantly negatively correlated with HRD and Ploidy in various tumors such as ACC, GBM, BRCA, LAML, cholangiocarcinoma (CHOL), and SARC (Fig. 2e), further suggesting that PTGDS may play a protective role in most tumors. Tumor neoantigens are specific antigens produced by tumor cell mutations that can be recognized by the immune system and trigger anti-tumor immune responses, serving as key targets for immune checkpoint inhibitors, tumor vaccines, and T-cell therapies. To explore the relationship between PTGDS and tumor neoantigens, we retrieved unified and standardized pan-cancer datasets from the UCSC database and extracted PTGDS expression data from different samples. The results showed that PTGDS significantly correlated with tumor neoantigens in pheochromocytoma (PCPG). At the same time, it was significantly negatively correlated with tumor neoantigens in kidney chromophobe (KICH) (Fig. 2f). This finding indicates that PTGDS may have crucial guiding significance in patient immunotherapy.
a Genomic alterations of PTGDS in TCGA pan-cancer, including mutations, amplifications, and deep deletions. b Landscape of PTGDS SNVs in pan-cancer, including missense mutations, frameshift deletions, and splice site alterations. c Kaplan–Meier plots are drawn to show the prognostic significance of PTGDS CNVs in 6 types of cancer. d A radar plot showing the relationship between TMB, MSI, and PTGDS. e A bar chart showing the correlation coefficient between HRD or chromosomal ploidy and PTGDS expression. f Correlation scatter plots showing the association between PTGDS expression and neoantigen count in pan-cancer.
DNA damage repair, cell stemness, and methylation analysis of PTGDS in pan-cancer
The stability of the cancer genome relies on various DNA repair mechanisms, including DNA mismatch repair (MMR) and homologous recombination repair (HRR). These repair mechanisms are crucial in maintaining cancer cells’ stemness and genomic integrity. To comprehensively understand the function of PTGDS in cancer, we systematically analyzed the correlation between PTGDS and key functions such as DNA damage repair, cell stemness, and methylation in 12 human cancers. Comprehensive analysis showed that PTGDS expression was significantly negatively correlated with the primary functional states in 12 cancers, with only LUAD showing a positive correlation with various functions (Fig. 3a). Specifically, in the DNA damage repair pathway, PTGDS expression was significantly negatively correlated with DNA damage repair functions in glioma, high-grade glioma (HGG), retinoblastoma (RB), and uveal melanoma (UM) (Fig. 3b). We further overexpressed PTGDS in breast cancer cells and evaluated their impact on key DNA repair proteins. As shown in supplementary Fig. 1a, overexpression of PTGDS leads to significant changes in the expression levels of γ-H2AX and RAD51, indicating that PTGDS has a potential regulatory role in the DNA damage response of BRCA. This suggests that PTGDS may influence tumor genomic stability by regulating DNA repair mechanisms. In terms of cell stemness, PTGDS expression was significantly negatively correlated with most tumors such as hepatocellular carcinoma (LIHC), glioblastoma (GBML), LUAD, and BRCA. In contrast, it was positively correlated with thyroid cancer (THCA) (Fig. 3c). This finding indicates that PTGDS may be essential in inhibiting tumor stemness, thereby affecting tumor proliferation and metastatic potential. Using the TIDE network tool to analyze tumor data from the TCGA database, we found that PTGDS methylation levels significantly correlated with cytotoxic T lymphocyte (CTL) infiltration (Fig. 3d). CTLs typically express the CD8 molecule and are also known as CD8+ T cells, key effector cells in anti-tumor immune responses. Further analysis showed that in head and neck cancer, acute myeloid leukemia (AML), lung cancer, and gastric cancer, PTGDS promoter methylation levels were positively correlated with the expression of CTL marker proteins. Kaplan–Meier survival curves revealed that patients with higher PTGDS methylation levels had significantly better prognoses in glioblastoma, melanoma, and gastric cancer (Fig. 3e). These results suggest that PTGDS methylation may improve patient prognosis by enhancing CTL-mediated anti-tumor immune responses. Additionally, we explored the correlation between PTGDS and RNA methylation modifications such as m1A, m5C, and m6A. The results showed that increased PTGDS expression levels were significantly negatively correlated with RNA methylation modifications in most cancers (Fig. 3f), indicating that PTGDS may exert tumor-suppressive effects post-transcriptional levels by regulating RNA modifications.
a Correlation between PTGDS expression and 12 cancer functional states. b Scatter plots showing the interrelationship between cancer DNA repair and PTGDS expression. c Bar charts showing the interrelationship between cancer stemness and PTGDS expression. d A table showing the correlation between PTGDS methylation levels and CTL-related factors. The third column represents CTL correlation, and the fourth column represents the CTL dysfunction z-score of the interaction term. e Scatter plots and Kaplan–Meier survival analyses show the association between PTGDS methylation levels and CTL markers, and survival analyses are grouped by high and low PTGDS methylation. f A heatmap showing the correlation between PTGDS expression and RNA regulation in pan-cancer. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
Functional mechanisms of PTGDS in cancer and interaction analysis of co-expressed genes
We conducted a series of functional enrichment analyses to deeply explore the specific mechanisms by which PTGDS functions in cancer and its interactions with co-expressed genes. First, using the String web tool, we identified the top 10 proteins interacting with PTGDS (Fig. 4a). Further analysis revealed that the five proteins most correlated with PTGDS were GPRC5B, LCNL1, NKX6-2, PAQR6, and PLEKHB1. These proteins were positively associated with PTGDS in most tumors, with Lipocalin-like 1 (LCNL1) showing the strongest correlation with PTGDS (Fig. 4c). LCNL1 is a protein related to the Lipocalin family, potentially involved in lipid binding, immune regulation, antioxidant activity, and cell signaling. Studies suggest that LCNL1 may influence tumor occurrence and development by regulating cell proliferation, apoptosis, metastasis, or immune responses, indicating that PTGDS may exert its tumor-suppressive functions through interactions with LCNL1. Additionally, we investigated the impact of high PTGDS expression on key signaling pathways in tumors. The WNT pathway, mTOR pathway, and p53/RB pathway are core signaling pathways regulating cell proliferation, metabolism, apoptosis, and the cell cycle, whose abnormal activation or inactivation is closely related to the occurrence and development of various cancers. Analysis showed that high PTGDS expression was significantly negatively correlated with abnormal changes (such as activation or inactivation) in the WNT, mTOR, and p53/RB pathways (Fig. 4b). This finding further supports the potential role of PTGDS in inhibiting tumor occurrence or progression, suggesting it may serve as a potential biomarker or therapeutic target. To further reveal the functional network of PTGDS, we enriched the top 100 genes co-expressed with PTGDS and analyzed their functions. The results showed that these genes were mainly enriched in biological processes such as lipid binding, transmembrane transporter activity, and cell surface receptor signaling pathways (Fig. 4d). Additionally, GSEA enrichment analysis indicated that PTGDS was most closely associated with specific signaling pathways in the liver, colorectal, renal clear cell carcinoma, and gastric cancer (Fig. 4e). These results reveal the multifunctional regulatory network of PTGDS in cancer, providing new insights into its potential mechanisms in tumor biology.
a Experiments verified a protein-protein interaction network of PTGDS binding partners. b Box plots showing the expression of PTGDS between pathway-level expression change groups and non-change groups in 6 types of cancer. c Correlation between PTGDS and the top 5 PTGDS co-expressed genes in each cancer type (left) and all cancer samples (right). d A circular plot of GO pathways enriched by the top 100 PTGDS co-expressed genes, with the top genes of each path on the left side of the corresponding color band. e GSEA analysis of the enrichment of KEGG and HALLMARK items in PANCancer, grouped by the median expression of PTGDS.
Predictive potential of PTGDS in cancer immunotherapy response
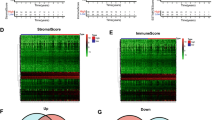
Stromal Score, Immune Score, and ESTIMATE Score are calculation methods based on gene expression data used to quantify the proportion of stromal cells and immune cells in the tumor microenvironment. These scoring systems are critical in tumor research and clinical applications, especially in assessing the tumor microenvironment, predicting prognosis, and guiding immunotherapy. To explore the immunological role of PTGDS in the tumor microenvironment, we evaluated the ESTIMATE scores of PTGDS in different cancer types. The results showed that in most cancers, PTGDS was significantly positively correlated with ESTIMATE Score and Immune Score, including lung squamous cell carcinoma (LUSC), BLCA, TGCT, UVM, colon adenocarcinoma (COAD), BRCA, and neuroblastoma (NB) (Fig. 5a). Notably, tumors with higher ESTIMATE Scores are usually more sensitive to immunotherapy and may be associated with better prognoses. Tumors with more immune cell infiltration are typically more sensitive to immune checkpoint inhibitors (such as PD-1/PD-L1 inhibitors), so the positive correlation of PTGDS suggests it may be associated with good responses to immunotherapy, indicating strong anti-tumor immune responses or immunotherapy sensitivity. However, in GBMLGG, PTGDS was negatively correlated with ESTIMATE Score. To further analyze the potential role of PTGDS in tumor immunotherapy, we examined the correlation between PTGDS and immune checkpoints. The results showed that in most tumors, PTGDS was positively correlated with both inhibitory and stimulatory immune checkpoints (Fig. 5b). This finding indicates that PTGDS may have a profound impact on tumor immunotherapy. Tumor immune subtypes (Immune Subtypes) classify tumors based on the composition of immune cells, gene expression characteristics, and immune response states in the tumor microenvironment, helping to understand tumor immune characteristics and provide a basis for personalized immunotherapy. Therefore, we studied the differential expression of PTGDS in different cancer immune subtypes. Histograms showed that PTGDS expression varied most significantly among different immune subtypes in BRCA, consistent with the high heterogeneity of BRCA and its diverse responses to immunotherapy (Fig. 5c). Notably, PTGDS expression was highest in the C2 subtype (IFN-y Dominant) of BRCA (Fig. 5d). The C2 subtype is dominated by Th1 cells, cytotoxic T cells (CTLs), and NK cells, which are usually more sensitive to immune checkpoint inhibitors. This result suggests that combining PTGDS with PD-L1 inhibitors may yield better therapeutic effects for breast cancer patients. Additionally, we explored the correlation between PTGDS and various chemokines, receptors, stimulators, and methylation immunostimulators. Heatmaps showed that in pan-cancer analysis, PTGDS was positively correlated with chemokines, receptors, and stimulators, while it was negatively correlated with methylation immunostimulators (Fig. 5e). Finally, using the TISMO network tool, we compared the changes in PTGDS expression in cancer cell lines before and after cytokine treatment. The results showed that in breast cancer cell lines, PTGDS expression was downregulated after TGF-β1 treatment. In contrast, PTGDS expression was upregulated in melanoma cell lines after IFN-β, IFN-y, and TGF-β1 treatment (Fig. 5f). These results indicate that PTGDS may play an essential role in the activation of immune stimulators and immune checkpoints, thereby influencing the immunosuppressive state of the tumor microenvironment.
a Bar charts showing the correlation between PTGDS and ESTIMATEScore, ImmuneScore, and StromalScore (left), and scatter plots of the top 6 correlations for each Score (right). b Correlation between PTGDS expression and immune checkpoints in pan-cancer. c Correlation between PTGDS and immune subtypes. d Expression of PTGDS in 6 immune subtypes across six types of cancer. e Heatmaps showing the correlation between PTGDS expression and chemokines (top left), receptors (bottom left), and immune stimulants (top right), and a heatmap of PTGDS promoter methylation levels with immune stimulants (bottom right). f Box plots of PTGDS expression before and after cytokine therapy. ESTIMATEScore is a score that estimates the presence of stromal and immune cells in malignant tumor tissues using expression data. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
Correlation analysis of PTGDS with immune cell infiltration and spatial expression patterns
To deeply explore the role of PTGDS in tumor immunity, we systematically analyzed its correlation with immune cell infiltration. Using the CIBERSORT algorithm, we identified the correlation between 22 types of immune cells and PTGDS. The results showed that in most cancers, PTGDS was significantly positively correlated with B cells and CD8+ T cells, while it was negatively correlated with dendritic cells, neutrophils, and eosinophils (Fig. 6a). This finding suggests that PTGDS may enhance anti-tumor immune responses by regulating the infiltration of B and CD8+ T cells. We focused on myeloid dendritic cells to further analyze the relationship between PTGDS and dendritic cells. We used various algorithms to examine the correlation between PTGDS and dendritic cells in different states. The results showed that PTGDS was significantly negatively correlated with activated dendritic cells in multiple tumors and positively correlated with resting dendritic cells (Fig. 6b). This result suggests that PTGDS may affect immune regulation in the tumor microenvironment by inhibiting the activation of dendritic cells. We obtained spatial transcriptome data from the Spatial DB database to explore the spatial distribution relationship between PTGDS and immune cells. We analyzed the spatial expression patterns of PTGDS and dendritic cell markers FCER1A and CD40 in breast ductal carcinoma and triple-negative breast cancer tissues. The results showed that in breast ductal carcinoma, the spatial distribution of PTGDS and FCER1A was highly similar, suggesting they may be co-expressed, while in triple-negative breast cancer, the spatial distribution of PTGDS, FCER1A, and CD40 showed significant differences, indicating they may function independently (Fig. 6c). Additionally, to identify the cell types expressing PTGDS in tumor tissues, we analyzed the single-cell expression profiles of PTGDS from the TISCH database. Heatmaps showed that PTGDS was widely expressed in 33 cell types, with relatively high expression levels in dendritic cells and fibroblasts (Fig. 6d). Violin plots further confirmed that in most tumor tissues, dendritic cells highly expressed PTGDS (Fig. 6e). These results suggest that PTGDS may play an important immune regulatory role in dendritic cells and fibroblasts.
a CIBERSORT calculates immune cell infiltration in pan-cancer. b Correlations between PTGDS and immune cells in various tumors. Red represents a positive correlation, and purple represents a negative correlation. c Spatial transcriptomics shows the spatial expression of PTGDS, FCER1A, and CD40 markers. The dot color indicates the expression level of the marker. d Expression of PTGDS in pan-cancer single-cell clusters. e Violin plots of PTGDS expression in GEO pan-cancer single-cell clusters. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
Correlation analysis of PTGDS with T cells in pan-cancer
To further explore the regulatory role of PTGDS in tumor immunity, we analyzed its relationship with dendritic cells. We systematically studied its correlation with NK cells, regulatory T cells (Treg cells), and cancer-associated fibroblasts (CAFs) infiltration. The results showed that in breast cancer, PTGDS was significantly positively correlated with T cells and CAFs, a phenomenon also observed in other tumors (Fig. 7a). Purity-adjusted correlation analysis further indicated that in most tumors (such as breast cancer), PTGDS was positively correlated with CRFs, NK cells, and T cells (Fig. 7b). Cytotoxic T lymphocytes (CTLs) are important effector cells in the immune system, playing a key role in anti-tumor and anti-viral immunity by directly killing target cells. We further examined the correlation between PTGDS and CTLs to analyze the correlation between PTGDS and CD8+ T cells. The results showed that in breast cancer, PTGDS had the most substantial and most significant positive correlation with CD8+ T cells (Fig. 7c). This finding suggests that PTGDS may exert tumor-suppressive effects in breast cancer by regulating the infiltration of CD8+ T cells. Additionally, we used the TIDE network tool to explore the relationship between PTGDS promoter methylation and cancer subtypes, CTL infiltration, and risk. The results showed that PTGDS promoter methylation significantly correlated with CTL infiltration in BRCA, leukemia, and neuroblastoma (Fig. 7d). Correlation scatter plots and Kaplan–Meier survival curves further indicated that PTGDS promoter methylation was significantly associated with increased CTL infiltration and predicted more prolonged survival in BRCA, leukemia, and neuroblastoma patients. These results suggest that PTGDS may play an essential role in tumor immune suppression by regulating CTL-mediated anti-tumor immune responses.
a Heatmap of the association between PTGDS levels and NK cell, Treg, and CAF infiltration. b Correlations between PTGDS and NK, Treg, and CAF in 6 types of cancer with purity and purity-adjusted correlations. c Multiple algorithms calculate the correlation between PTGDS and CD8+ T cell infiltration. d Correlations between PTGDS expression and CTL, T cell dysfunction, and risk.
PTGDS as a predictive biomarker for cancer treatment response and screening of potential targeted drugs
To explore whether PTGDS can predict cancer treatment responses, we used the ROCplotter database to analyze the relationship between PTGDS expression and treatment outcomes in three cancers: BRCA, OV, and COAD. The results showed that in OV and COAD, PTGDS expression levels were relatively higher in non-responders to Docetaxel, Avastin, or Fluoropyrimidines monotherapy. In BRCA, PTGDS expression was significantly higher in responders to Tamoxifen treatment. It showed an upward trend in responders to Anthracycline and Lxabepilone treatment, with pathological complete response (pCR) AUC values of 0.7, 0.605, and 0.706, respectively (Fig. 8a). These results suggest that PTGDS may serve as a potential biomarker for drug sensitivity in breast cancer patients. Given that high PTGDS expression is associated with drug treatment resistance in OV and COADREAD patients, we further screened potentially more effective anti-PTGDS drugs. Using the cMap tool, we screened compounds that could induce transcriptional changes opposite to increased PTGDS expression in 9 different tumor cell lines, ultimately identifying 30 compounds with potential targeting capabilities against PTGDS (Fig. 8b). Additionally, through the RNAactDrug database we also screened drugs related to PTGDS mRNA expression. We found that PTGDS inhibitors mainly include isotretinoin, p-fuchsin, and quizartinib, while its analogs include azaserin, 2-deoxy-2-fluorocytidine, and CUDC-305 byproducts (Fig. 8c). To analyze the interaction between PTGDS and compounds, we used AlphaFold2 software for homology modeling of the PTGDS protein and validated its overall quality factor (OQF) as 93.9394 using SAVESv6.0. Subsequently, molecular docking analysis using Discovery Studio showed successful docking of irinotecan and nitrocaramiphen with PTGDS, with LibDockScores of 97.41 and 93.18, respectively. Figure 8d displays the three-dimensional structure of the docking pocket and two-dimensional images of interaction forces and distances, indicating that irinotecan and nitrocaramiphen bind to PTGDS residues through multiple interaction forces.
a Box plots show the difference in PTGDS expression between responders and non-responders, and ROC shows the predictive accuracy of PTGDS levels for patient treatment response. b A heatmap showing the top 30 compounds, with experimentally induced transcriptional changes opposite to those affected by intermediate PTGDS expression grouping. Color bars and block colors indicate similarity scores. c Bar charts showing Spearman correlations between PTGDS grouping and mRNA changes induced by RNAactDrug drugs. d Homology modeling of PTGDS with drugs, with 2D images on the left representing drug 2D structures, interacting amino acid residues, and molecular forces. On the right are 3D models of PTGDS docking with drugs.
PTGDS overexpression inhibits cell viability in BRCA cells
To further validate the effect of PTGDS on the proliferation capacity of tumor cells, we also performed colony formation assays (Fig. 9a). The results showed that after overexpressing the PTGDS gene, both cancer cell line types exhibited a decrease in the number and volume of colonies formed (Fig. 9b). The efficiency of PTGDS overexpression was confirmed through PCR experiments. In wound healing assays, we observed that the migration of PTGDS-overexpressing breast cancer cell lines, MDA-MB-231 and HCC1806, was significantly reduced compared to control cells (Fig. 9c). Column chart analyses indicated that the differences between the two groups were statistically significant (Fig. 9d). Next, we conducted transwell assays to analyze the effects of PTGDS on the migration and invasion capabilities of the breast cancer cell line types (Fig. 9e). The results indicated that following PTGDS overexpression, the migration and invasion abilities of the MDA-MB-231 and HCC1806 cell lines were significantly diminished (Fig. 9f). As shown in supplementary Fig. 1b and c, the overexpression of PTGDS in the JIMT-1 and SK-BR-3 cell lines also inhibited the migration of tumor cells. To investigate the distribution of PTGDS with other immune cells, we conducted multiplex immunofluorescence staining. We found that patients with high PTGDS expression had more significant infiltration of CD8+ T cells and CD20+ B cells (Fig. 9g, h). In contrast, patients with low PTGDS expression exhibited reduced immune cell infiltration. This further demonstrates the relationship between PTGDS and immune cells.
a, b Colony formation assays. After PTGDS overexpression, the proliferation ability of breast cancer cell lines MDA-MB-231 and HCC1806 is significantly decreased. c, d Wound healing assays. After PTGDS overexpression, the migration ability of breast cancer cell lines MDA-MB-231 and HCC1806 is significantly decreased. e, f Transwell assays. After PTGDS overexpression, the migration and invasion abilities of breast cancer cell lines MDA-MB-231 and HCC1806 are significantly reduced. g, h Multiple immunohistochemical staining was performed to verify the co-localization of PTGDS CD8+ T cells and CD20+ B cells in clinical samples. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
PTGDS overexpression enhances CD8+ T cell infiltration and improves the efficacy of anti-PD-L1 antibody (αPD-L1 Ab) treatment
To examine the tumor suppressive effect of PTGDS in vivo, we established a stable PTGDS-overexpressing murine breast cancer cell line, 4T1. We inoculated the stable cell line into immunocompetent BALB/c mice to generate subcutaneous xenografts and treated them with either αPD-L1 Ab or IgG isotype control (Fig. 10a). The results showed that PTGDS overexpression significantly inhibited tumor growth compared to the control group. Furthermore, the combination of PTGDS overexpression and αPD-L1 Ab treatment resulted in the most significant reduction in tumor burden (Fig. 10b–d). At the end of the treatment, tumor samples were collected for further analysis. Flow cytometric analysis indicated that both αPD-L1 Ab and PTGDS overexpression enhanced the proliferation and activation of CD8+ T cells. Notably, when αPD-L1 Ab was combined with PTGDS overexpression, there was a marked enrichment and activation of CD8+ T cells in the tumor area (Fig. 10e–g). These findings suggest that PTGDS overexpression enhances CD8+ T cell proliferation, infiltration, and functional activity, thereby significantly improving the efficacy of αPD-L1 Ab treatment. Overall, these results indicate that PTGDS promotes tumor immune evasion by facilitating CD8+ T cell infiltration and that PTGDS overexpression can enhance the effectiveness of αPD-L1 Ab treatment.
a Schematic flowchart of the in vivo experimental procedure in BALB/c mice. b Images of subcutaneous xenograft tumors harvested from euthanized mice in different groups. c, d Tumor growth curves and tumor weights in different groups. e–g Flow cytometric analysis and quantitative assessment of CD3+ cells within CD45+ cells and CD8+ cells within CD3+ T cells in the tumors. (Z-score test: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.).
Discussion
Aberrantly expressed products can develop sequentially into cancer, with these oncogenic products arising from genomic alterations, transcriptional abnormalities, post-translational modifications, or epigenetic regulation of specific genes. These changes can influence patient prognosis through various mechanisms15,16,17,18. Here, we provide a comprehensive overview of a recently discovered cancer gene, PTGDS; describe its clinical significance, multi-omics characterization, and role in tumor immunity; and screen for potential targeted anticancer drugs. There are studies suggesting that PTGDS affects cancer progression, but the evidence is limited, and the results are inconsistent5,7,19. Our study showed that PTGDS was highly expressed in KIRC and predicted shorter survival, whereas, in CESC, DLBC, HNSC, and LGG, it indicated a better prognosis, suggesting a tumor type-dependent factor.
Previous studies have less frequently revealed the role of PTGDS in cancer immunity. Intriguingly, we found that PTGDS exhibits both pro-tumorigenic and anti-tumorigenic roles, and this functional duality may arise from its spatiotemporal-specific responses to the tumor microenvironment and cell types. In pro-cancer mechanisms, PTGDS drives cell proliferation and induces chemotherapy resistance (such as enhancing doxorubicin resistance) via the MYH9-mediated Wnt-β-catenin-STAT3 signaling axis in diffuse large B-cell lymphoma (DLBCL)20, while in Peripheral T cell lymphoma (PTCL), where the PTGDS inhibitor AT56 suppresses tumor growth by inducing ferroptosis (characterized by lipid reactive oxygen species accumulation and upregulation of FTH1/FTL) and enhances sorafenib sensitivity21. Conversely, its tumor-suppressive effects are exemplified in renal cell carcinoma, where PTGDS facilitates plasma cell infiltration into tertiary lymphoid structures (TLS) and potentiates anti-tumor immunity through the CXCL12-CXCR4 axis22. In our findings, we observed that higher PTGDS expression was linked to low immune infiltration, immunosuppressive cancer subtypes, and an overall reduction in cytokine receptors. Also, in some cancer cells treated with anticancer cytokines, we observed reduced expression of PTGDS, suggesting an immunosuppressive role of PTGDS in some cancers. More specifically, dendritic cells (DC) play a key role in environmental remodeling, as supported by extensive or single-cell transcriptional sequencing data and the co-expression of PTGDS and DC biomarkers. Considering the DNA repair potential of PTGDS, we speculate that the high expression of PTGDS correlates with the relevant pathways of DNA repair and the polarisation of DC. However, as we have revealed, PTGDS appears to be a reliable biomarker of DC infiltration. In addition, PTGDS was associated with high infiltration of NK, Treg and CAFs and CD8+ T-cell dysfunction, suggesting a broad immunosuppressive effect.
We discovered a surprisingly strong correlation between IL4, a Th2 cytokine, and PTGDS across 40 types of cancer. Research indicates that dendritic cells boost tumor antigen uptake by producing IL12, while IL4 suppresses IL12 upregulation, impacting DCs’ ability to bind antigens23,24,25. We suggest that PTGDS may attenuate DC immunity by promoting IL4 upregulation and thereby attenuating DC immunity.
Our research has deepened the understanding of PTGDS, not only revealing its pivotal role in maintaining genetic stability but also uncovering its potential in predicting the prognosis of various cancers and their response to immunotherapy. Through functional experiments in BRCA, we have confirmed the significant role of PTGDS, which may provide a direction for the development of new oncological treatment strategies. Nevertheless, our research has certain limitations and requires further exploration and validation through additional mechanism experiments and clinical trials to elucidate the precise molecular mechanisms of PTGDS mediated tumorigenesis and immune therapy functions through different DC subtypes. Although PTGDS holds potential as both a prognostic biomarker and therapeutic target in cancer, its clinical translation faces three major challenges: (1) Spatiotemporal heterogeneity of PTGDS expression requires systematic resolution through spatial transcriptomics to delineate tissue-specific regulatory networks, alongside the establishment of cross-cancer expression atlases; (2) Insufficient cancer adaptation of targeted delivery systems: Due to the poor barrier penetration ability of inhibitors such as AT56 in solid tumors (such as glioma and renal cancer), their efficacy is limited, and they face issues of protease degradation and rapid clearance in hematological tumors. Therefore, there is an urgent need to develop nanocarriers to achieve transmembrane delivery and tumor microenvironment specific release; (3) Lack of dynamic monitoring standards based on PTGDS: for example, lymphoma requires monitoring of PTGDS mutations in circulating tumor DNA (ctDNA) in the peripheral blood, while solid tumors require assessment of PTGDS expression heterogeneity within the tumor in conjunction with radiomics. But we believe that the future endeavors will enhance our comprehension of the role of PTGDS and its potential as a target for cancer therapy.
In conclusion, we conducted a multi-omics analysis of PTGDS across various cancers and recognized it as a potential prognostic biomarker. High expression of PTGDS is associated with activation of immune cells, especially CD8+ T cells. Overexpression of PTGDS can enhance the infiltration of CD8+ T cells and improve the efficacy of anti-PD-L1 therapy, suggesting its potential as a therapeutic target.
Methods
Data access and procession
The UCSC Xena platform was utilized to access databases from TCGA (which includes 33 types of cancer) and the Genotype-Tissue Expression (GTEx) project, comprising 54 standard tissue samples. Any cancer type that had fewer than three samples was excluded from the analysis. A log2 transformation of (transcripts per million (TPM) + 1) was applied to normalize the transcriptional data. PTGDS protein sequences in FASTA format were sourced from the NCBI protein database.
The single-cell sequencing datasets of BRCA (GSE114725; GSE114724), lung adenocarcinoma (LUAD) (GSE97168; GSE131907), colorectal cancer (CRC) (GSE146771; GSE139555; GSE144735; GSE132465), chronic lymphocytic leukemia (CLL) (GSE111014), hepatocellular carcinoma (HCC) (GSE112271; GSE98638; GSE140228), glioblastoma multiforme (GBM) (GSE141946) were collected from GEO.
Analyses of PTGDS concerning cancer-associated genomic alterations and antigen correlations
The Cancer Types Summary module on cBioPortal was used to evaluate the frequencies of three types of genomic alterations—mutations, amplifications, and deep deletions—across different cancers26. We integrated processed pan-cancer SNV data with structural protein domains obtained from the R package “maftools” to generate a mutation map for PTGDS. Kaplan–Meier curves were produced using the Copy Number module of the Tumor Immune Dysfunction and Exclusion (TIDE) tool, which explores the relationships between genomic or transcriptional changes in cancer and responses to immunotherapy, to investigate the prognostic implications of PTGDS copy number variants (CNVs). Additionally, tumor mutation load (TMB), microsatellite instability (MSI), homologous recombination defects (HRD), and neoantigen data from previous studies were calculated via the online tool Sangerbox, focusing on TMB, MSI, neoantigens, and their association with PTGDS expression.
The examination of PTGDS’s relationship with DNA mismatch repair, cancer stemness, and epigenetic modifications
The correlation between PTGDS expression in different cancers and the functional status of 12 cancers was visualized. To further show the correlation between DNA repair and PTGDS expression in cancer, we selected four cancer types to be visualized by scatter plots. Use of Sangerbox to express the correlation between cancer stem cells and PTGDS expression. The connection between PTGDS promoter methylation and cytotoxic T-lymphocytes (CTL), as well as patient survival, is outlined in the TIDE methylation module. Additionally, heat maps were created to illustrate the relationship between PTGDS and 44 modifier genes, including N1-methyladenosine (m1A), 5-methylcytosine (m5C), and N6-methyladenosine (m6A)27,28,29.
The PTGDS interaction network and functional enrichment analyses
The current protein-protein interaction networks for PTGDS that have been experimentally validated were explored using the online tool String30. Somatic alterations across various key pathways at the pan-cancer level were examined on UALCAN, while the relationship between pathway-related signaling and PTGDS expression was assessed using GEPIA 2.0. The top 100 co-expressed genes associated with PTGDS across pan-cancer were identified through the similar gene detection feature in GEPIA 2.0. Functional analysis of these top 100 genes was conducted using the R package “clusterProfiler,” with a false discovery rate (FDR) set at <0.05, and Gene Ontology (GO) annotations were obtained from the R package “org.Hs.eg.db.” The correlation between PTGDS and the top five co-expressed genes was visualized using heatmaps generated by TIMER 2.0 and scatterplots created with GEPIA 2.0. Furthermore, the functional enrichment results for PTGDS were analyzed for gene set enrichment using GSEA software31,32. Based on the median expression of PTGDS, cancer samples were classified into low and high PTGDS groups (p < 0.05)33.
Microenvironment analysis of PTGDS in pan-cancer
This analysis was accomplished using Expression Data (ESTIMATE) to calculate stromal and immune scores, utilizing the R package “ESTIMATE” (version 1.0.13). We obtained immune checkpoint markers from previous studies, analyzed their correlation with PTGDS, analyzed the expression correlation of PTGDS with immune isoforms, and compared the expression levels of these isoforms in pan-cancers in the isoforms module of the tumor-immune system interaction portal TISDB34,35. We also used Sangerbox to generate heat maps showing the relationship between PTGDS expression and different chemokines, chemokine receptors, and immunostimulatory factors across their respective modules. To assess the effect of cytokine treatment on PTGDS expression, we utilized the Tumor Immune Syngeneic Mouse online tool to compare gene expression levels in cell lines before and after cytokine treatment36.
The CIBERSORT algorithm calculated the immune cell infiltration correlation of PTGDS. Ten different algorithms were used to correlate dendritic cells with PTGDS using TIMER 2.0. In addition, spatial expression levels and overlap of PTGDS and dendritic cell markers CD86 and CD40 in colorectal and prostate cancers were analyzed using the SpatialDB online tool, a spatially disaggregated transcriptome database. To achieve single-cell resolution, we analyzed PTGDS expression across different cell subtypes in pan-cancer using single-cell datasets sourced from GEO and the Tumor Immune Single-cell Hub (TISCH)37.
Immune infiltration analysis of PTGDS in pan-cancer environment
TIMER2.0 effectively showcased the interrelationships between PTGDS expression and various immune cell types, including regulatory T cells (Tregs), cancer-associated fibroblasts (CAFs), NK cells, and CD8+ T cells, utilizing diverse immunological algorithms38. In addition, we investigated the role of PTGDS in T cell dysfunction and CTL-associated prognosis in pan-cancer subtypes by TIDE.
Single-cell transcriptome analysis
The computational pipeline was implemented in the R statistical environment (v4.1.1). Single-cell transcriptomic profiles were processed through the Seurat analytical framework (v4.2) with the following optimizations: Cellular entries exhibiting either insufficient gene representation (<300 detected genes) or excessive transcriptional activity (>7500 genes) were excluded, along with cells displaying mitochondrial gene contribution exceeding 25% of total UMIs. Prior to dimensional reduction, we systematically filtered mitochondrial-encoded genes, ribosomal protein transcripts, and hemoglobin subunits from the expression matrix39.
We used canonical correlation analysis (CCA) to correct for batch effects across datasets40. The 2000 most dynamically expressed transcripts were selected based on variability scores calculated by Seurat’s feature selection algorithm. Expression matrices were standardized through Z-score transformation before conducting principal component decomposition, with subsequent nonlinear projection into 2D space using UMAP (visualization parameters: spread = 1, min.dist = 0.3) guided by the top 30 principal components. Graph-based clustering at a granularity setting of 0.5 resolution partitioned cells into distinct populations. Differentially expressed signature molecules for each cluster were identified by comparative analysis against all other groups, applying a minimum log2-fold change threshold of 1 (equivalent to 2-fold expression difference) through Seurat’s differential testing module. Cellular identities were systematically annotated by cross-referencing these discriminative markers against established references in the CellMarker 2.0 repository and the PanglaoDB compendium41,42,43.
Spatial transcriptome analysis
ST data were analyzed with Seurat package (version 4.2) using similar method as scRNA-seq39. The single cell data were predicted on the ST data with the FindTransferAnchors and TransferData functions from the Seurat package with default settings.
Assessment of therapeutic responses targeting PTGDS, along with compounds and molecular docking studies
This online tool (ROC Plotter) analyses transcriptome data from four cancer types to relate gene expression to treatment response. The “Query” tool from cMAP was utilized to identify compounds that target PTGDS, resulting in a heatmap showcasing the top 30 compounds linked to differentially expressed gene markers associated with anti-PTGDS activity. Furthermore, the relationship between PTGDS and drug sensitivity was confirmed using RNAactDrug, which serves as a comprehensive resource for drug sensitivity and RNA molecular correlations, selecting drugs with an FDR < 0.0544. PTGDS proteins were modeled using AlphaFold2 software. Docking was carried out with Discovery Studio (version 4.5). After preparing PTGDS and the ligands, the binding sites and conformations of the compounds were identified, with LibDock chosen for the docking process. The site with the highest LibDockScore and its molecular conformation was selected for further analysis. The findings feature a three-dimensional representation of the binding pocket along with a two-dimensional illustration of the distances between intermolecular forces.
Cell transfection and RT-PCR
The breast cancer cell lines MDA-MB-231, HCC1806, JIMT-1, SK-BR-3, and 4T1 were acquired from the Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences in Shanghai, China. These cells were grown in a DMEM medium with the addition of 10% FBS and 1% penicillin/streptomycin. Cells were seeded at a density of 50% in 6-well plates and transfected with negative control (Vector) and overexpression plasmids. Lipofectamine 3000 was used for all transfections. RT-PCR was performed using SYBR Green Master Mix (Q111-02, Vazyme), with mRNA expression levels normalized to GAPDH mRNA levels. The 2−ΔΔCT method was employed for quantitative expression analysis. The PTGDS overexpression sequence and all primers were provided by Qingdao Biotechnology, with sequences detailed in Supplementary Table 1.
Multiplex immunohistochemical (mIHC) and Western blot (WB) analysis
In this study, mIHC was performed using a multiple immunofluorescence kit (Aifang, Hunan, China), following the manufacturer’s guidelines. Total proteins were isolated using RIPA buffer, separated by 10% SDS-PAGE, and transferred to PVDF membranes. After blocking with 5% milk, the membranes were incubated overnight with primary antibodies at 4 °C, followed by HRP-conjugated secondary antibodies. Details about the antibodies used can be found in Supplementary Table 2, with clinical information. The collection and analysis of human samples and clinical data received approval from the Ethics Committees of The Cancer Hospital, Chinese Academy of Medical Sciences, China (NCC2024C-1095). Uncropped scans of the most important blots were seen in Supplementary Fig. 2.
Animal model
In the xenograft model, we used 4-week-old female BALB/c mice, which were divided into four groups. A total of 2 × 106 treated 4T1 cells were resuspended in 100 μl PBS and injected into the right and left groin regions of the mice for tumorigenicity assessment. Two weeks later, each group received treatment as follows: (1) IgG (HY-P990682, MedChemExpress, USA, 100 μg, i.p. every three days); (2) anti-PD-L1 antibody (αPD-L1 Ab, HY-P99145, MedChemExpress, USA, 100 μg, i.p. every three days). Tumor weight and volume were recorded every 4 days. Tumor volume was measured using calipers and calculated using the formula: (length × width²)/2. The mice were euthanized by cervical dislocation, and the target tissues were promptly collected for histological and molecular biological analyses. The Animal Experiment Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences approved all animal experiments (NCC2024A630).
Flow cytometry analysis of tumor-infiltrating immune cells in BALB/c mice
Post-treatment, tumors were harvested from the mice in each treatment group. The tissues were mechanically dissociated into single-cell suspensions using PBS, followed by enzymatic digestion with 5 mg ml–1 collagenase IV at 37 °C for 1.5 h with shaking. The resulting cell suspensions were filtered through a 70-μm cell strainer, treated with resuspended in RPMI 1640 medium supplemented with 10% FBS. The isolated immune cells were activated with phorbol 12-myristate 13-acetate (PMA) and incubated for 4 h at 37 °C with shaking. Subsequently, the cells were stained with fluorescently labeled antibodies against CD45, CD3, and CD8 for 30 min at room temperature in the dark. To assess cytotoxic T-cell function and proliferation, additional stains with antibodies against IFN-γ, and Ki67 were performed. The stained cell suspensions were analyzed using a Beckman Coulter Cytoflex S flow cytometer. Details about the antibodies used can be found in Supplementary Table 3.
Statistical analysis
All data processing, statistical evaluations, and graphical representations were conducted using R software (version 4.2.0). To assess and compare overall survival (OS) across different subtypes, Kaplan–Meier survival analysis combined with log-rank tests was utilized. For continuous variables, differences between two groups were analyzed using either the Wilcoxon rank-sum test or Student’s t test, depending on data distribution. Categorical variables were compared using χ2 tests or Fisher’s exact tests, as appropriate. To account for multiple comparisons, p-values were adjusted using the false discovery rate (FDR) correction. Associations between variables were examined using Pearson correlation analysis. All statistical tests were two-tailed, and a significance threshold of p < 0.05 was applied.
Data availability
The datasets used in this paper are available online, as described in the Methods section.No new algorithms were developed for this article. All code generated for analysis is available from the authors upon request.
Code availability
No new algorithms were developed for this article. All code generated for analysis is available from the authors upon request.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Cunha, A. R. D. et al. The global, regional, and national burden of adult lip, oral, and pharyngeal cancer in 204 countries and territories: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 9, 1401–1416 (2023).
Sax, A. et al. Defects in the necroptosis machinery are a cancer resistance mechanism to checkpoint inhibitor immunotherapy. J. Immunother. Cancer 13, https://doi.org/10.1136/jitc-2024-010433 (2025).
Xia, L., Zhu, X., Wang, Y. & Lu, S. The gut microbiota improves the efficacy of immune-checkpoint inhibitor immunotherapy against tumors: From association to cause and effect. Cancer Lett. 598, 217123 (2024).
Omori, K. et al. Lipocalin-type prostaglandin D synthase-derived PGD(2) attenuates malignant properties of tumor endothelial cells. J. Pathol. 244, 84–96 (2018).
Hu, S. et al. Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of Wnt-β-catenin-STAT3 signaling. Cell Death Differ. 29, 642–656 (2022).
Shimanuki, M., Takeda, K., Kawaguchi, M., Suzuki, T. & Shibahara, S. Lipocalin-type prostaglandin D synthase as a marker for the proliferative potential of melanocyte-lineage cells in the human skin. J. Dermatol 39, 699–704 (2012).
Lim, W. et al. Prostaglandin D₂ synthase related to estrogen in the female reproductive tract. Biochem. Biophys. Res. Commun. 456, 355–360 (2015).
Nault, J. C. et al. Argininosuccinate synthase 1 and periportal gene expression in sonic hedgehog hepatocellular adenomas. Hepatology 68, 964–976 (2018).
Kim, J. et al. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 65, 6189–6198 (2005).
Ragolia, L., Palaia, T., Hall, C. E., Klein, J. & Büyük, A. Diminished lipocalin-type prostaglandin D(2) synthase expression in human lung tumors. Lung Cancer 70, 103–109 (2010).
Zhang, B. et al. PGD2/PTGDR2 signaling restricts the self-renewal and tumorigenesis of gastric cancer. Stem Cells 36, 990–1003 (2018).
Fukuoka, T. et al. Prostaglandin D synthase is a potential novel therapeutic agent for the treatment of gastric carcinomas expressing PPARγ. Int J. Cancer 137, 1235–1244 (2015).
Alves, M. R. et al. Prostaglandin D2 expression is prognostic in high‑grade serous ovarian cancer. Oncol. Rep, https://doi.org/10.3892/or.2019.6984 (2019).
Calabrese, C. et al. Genomic basis for RNA alterations in cancer. Nature 578, 129–136 (2020).
Compe, E. & Egly, J. M. Nucleotide excision repair and transcriptional regulation: TFIIH and beyond. Annu. Rev. Biochem. 85, 265–290 (2016).
Chen, L., Liu, S. & Tao, Y. Regulating tumor suppressor genes: post-translational modifications. Sig. Transduct. Target Ther. 5, 90 (2020).
Luo, Y. et al. RNA modification gene WDR4 facilitates tumor progression and immunotherapy resistance in breast cancer. J. Adv. Res, https://doi.org/10.1016/j.jare.2024.06.029 (2024).
Sun, C. et al. Gene expression profiles analysis identifies a novel two-gene signature to predict overall survival in diffuse large B-cell lymphoma. Biosci. Rep. 39, https://doi.org/10.1042/bsr20181293 (2019).
Hu, S. et al. Glycoprotein PTGDS promotes tumorigenesis of diffuse large B-cell lymphoma by MYH9-mediated regulation of Wnt-beta-catenin-STAT3 signaling. Cell Death Differ. 29, 642–656 (2022).
Hu, S. et al. Targeting PTGDS Promotes ferroptosis in peripheral T cell lymphoma through regulating HMOX1-mediated iron metabolism. Br. J. Cancer 132, 384–400 (2025).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541 e525 (2022).
Taylor, P. R., Paustian, C. C., Koski, G. K., Zimmerman, D. H. & Rosenthal, K. S. Maturation of dendritic cell precursors into IL12-producing DCs by J-LEAPS immunogens. Cell Immunol. 262, 1–5 (2010).
Trinchieri, G. & Gerosa, F. Immunoregulation by interleukin-12. J. Leukoc. Biol. 59, 505–511 (1996).
Theoharides, T. C. & Conti, P. Mast cells to dendritic cells: Let IL-13 shut your IL-12 down. J. Allergy Clin. Immunol. 147, 2073–2074 (2021).
Venetis, K. et al. Mismatch repair (MMR) and microsatellite instability (MSI) phenotypes across solid tumors: a comprehensive cBioPortal study on prevalence and prognostic impact. Eur. J. Cancer 217, 115233 (2025).
Xu, C., Xu, X., Huang, Y., Shang, S. & Ma, L. RNA methylation: a new promising biomaker in cancer liquid biopsy. Biochim. Biophys. Acta. Rev. cancer 1880, 189337 (2025).
Ding, Y. P., Liu, C. C. & Yu, K. D. RNA modifications in the tumor microenvironment: insights into the cancer-immunity cycle and beyond. Exp. Hematol. Oncol. 14, 48 (2025).
Amort, T. et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biol. 18, 1(2017).
Szklarczyk, D. et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–d612 (2021).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Hu, X. et al. Single-Cell and Single-Nuclei transcriptomics profiling reveals dynamic cellular features in tumor-related adipose microenvironment of breast cancer patients with high BMI. Transl. Oncol. 57, 102408 (2025).
Zeng, C., Xu, C., Wei, Y., Ma, F. & Wang, Y. Training and experimental validation a novel anoikis- and epithelial‒mesenchymal transition-related signature for evaluating prognosis and predicting immunotherapy efficacy in gastric cancer. J. Cancer 16, 1078–1100 (2025).
Ru, B. et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics35, 4200–4202 (2019).
Li, Y. et al. Decoding tumor heterogeneity in uveal melanoma: basement membrane genes as novel biomarkers and therapeutic targets revealed by multi-omics approaches for cancer immunotherapy. Front. Pharmacol. 14, 1264345 (2023).
Zeng, Z. et al. TISMO: syngeneic mouse tumor database to model tumor immunity and immunotherapy response. Nucleic Acids Res. 50, D1391–d1397 (2022).
Sun, D. et al. TISCH: a comprehensive web resource enabling interactive single-cell transcriptome visualization of tumor microenvironment. Nucleic Acids Res. 49, D1420–d1430 (2021).
Chen, W. et al. A new 4-gene-based prognostic model accurately predicts breast cancer prognosis and immunotherapy response by integrating WGCNA and bioinformatics analysis. Front. Immunol. 15, 1331841(2024).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e3529 (2021).
Petegrosso, R., Li, Z. & Kuang, R. Machine learning and statistical methods for clustering single-cell RNA-sequencing data. Brief. Bioinforma. 21, 1209–1223 (2020).
Hu, C. et al. CellMarker 2.0: an updated database of manually curated cell markers in human/mouse and web tools based on scRNA-seq data. Nucleic Acids Res. 51, D870–d876 (2023).
Franzén, O., Gan, L. M. & Björkegren, J. L. M. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database 2019, https://doi.org/10.1093/database/baz046 (2019).
Zeng, C. et al. Integrated bulk and single-cell transcriptomic analysis unveiled a novel cuproptosis-related lipid metabolism gene molecular pattern and a risk index for predicting prognosis and antitumor drug sensitivity in breast cancer. Discov. Oncol. 16, 318 (2025).
Dong, Q. et al. RNAactDrug: a comprehensive database of RNAs associated with drug sensitivity from multi-omics data. Brief. Bioinforma. 21, 2167–2174 (2020).
Acknowledgements
This research was supported by the Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (PY202429) and Shenzhen Medical Research Fund (D2402001).
Author information
Authors and Affiliations
Contributions
J.L. and Z.Q. Performed bioinformatics analysis and wrote the manuscript; D.Z. and Y.L. performed molecular biology experiments and wrote the manuscript; Z.W., J.L., and L.Z. collated and analyzed the data and typeset figures; Y.X. revised the manuscript; S.P. and P.Z. conceived and designed the study, supervised the conduct of the study, revised the manuscript and provided financial support. All authors reviewed the manuscript and approved the submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Qu, Z., Zhu, D. et al. Pan cancer research reveals the role of PTGDS in tumor suppression and immune regulation. npj Precis. Onc. 9, 319 (2025). https://doi.org/10.1038/s41698-025-01097-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-025-01097-z