Abstract
Oocytes play a crucial role in transmitting maternal mitochondrial DNA (mtDNA), essential for the continuation of species. However, the effects of mitochondrial reactive oxygen species (ROS) on mammalian oocyte maturation and mtDNA maintenance remain unclear. We investigated this by conditionally knocking out the Sod2 gene in primordial follicles, elevating mitochondrial matrix ROS levels from early oocyte stages. Our data indicates that reproductive aging in Sod2 conditional knockout females begins at 6 months, with oxidative stress impairing oocyte quality, particularly affecting OXPHOS complex II and mtDNA-encoded mRNA levels. Despite unchanged mtDNA mutation load, mtDNA copy numbers exhibited significant variations. Strikingly, reducing mtDNA copy numbers by reducing mtSSB protein, crucial for mtDNA replication, accelerated reproductive aging onset to three months, underscoring the critical role of mtDNA copy number maintenance under oxidative stress conditions. This research provides new insights into the relationship among mitochondrial ROS, mtDNA, and reproductive aging, offering potential strategies for delaying aging-related fertility decline.
Similar content being viewed by others
Introduction
The oocyte, the largest cell in the human body, plays a crucial role in transmitting maternal mitochondrial DNA (mtDNA) to offspring. Within a single oocyte, there can be up to 200,000 copies of mtDNA1,2. The maternal mtDNA within the oocyte is essential for mitochondrial energy production and significantly affects not only the oocyte itself but also subsequent generations, including embryonic development and the health of the offspring3,4. Currently, maternal age is recognized as a high-risk factor for diminished mtDNA quality and potentially reduced quantity, along with a decreased oocyte pool and lower oocyte quality5,6,7,8,9. The accumulation of reactive oxygen species (ROS), commonly known as free radicals, was proposed as the primary factor contributing to these consequences10,11. However, it has not been previously tested whether maternal mtDNA can be affected by accumulated ROS in oocytes. Furthermore, the reciprocal relationship between changes in mtDNA and the impact of ROS on oocytes has yet to be investigated.
The cellular ROS is mainly generated within the mitochondria through complex I and complex III in the electron transport chain (ETC). These ROS species mainly include superoxide and hydrogen peroxide (H2O2). Superoxide, the initial form of ROS, is predominantly produced within the mitochondrial matrix by complex I and III, as well as in the intermembrane space (IMS) by complex III12,13. Subsequently, these superoxide molecules can be converted to H2O2 by specific antioxidant enzymes, namely, SOD2 in the matrix and SOD1 in the IMS14,15,16. Other antioxidant enzymes further reduce H2O2 to water17,18. Due to the high permeability of the mitochondrial outer membrane to ROS, once generated in the IMS, they can be released into the cytosol. The intact mitochondrial inner membrane exhibits differential permeability for superoxide and H2O2, preventing superoxide release into the cytosol but allowing H2O2 to be released19. Based on their destination, ROS can be categorized as cytosolic or mitochondrial matrix ROS.
The Free Radical Theory of Aging, originally proposed by Denham Harman in the 1950s, suggests that ROS significantly contributes to the acceleration of aging in somatic cells10. According to this theory, during mitochondrial activity, the gradual release of mitochondrial matrix ROS in close proximity to mtDNA can lead to mtDNA damage, resulting in an accumulation of mtDNA mutations, reduced efficiency of electron transfer, and ultimately increased ROS generation. This theory highlights a vicious cycle involving mitochondrial ROS, mtDNA mutations, and the somatic aging process. However, recent deep sequencing findings from aged somatic tissues (e.g., C. elegans20, human brain21, colorectal epithelium22, and blood23), challenge this theory, as the leading mtDNA mutations are not G:C > T:A transversions, which are typically associated with oxidative stress. The following experimental tests on somatic tissues by several research groups, which deactivated the Sod2 gene in Drosophila24 and mouse hearts25, support that mitochondrial matrix ROS can impair organ function but do not provide evidence of an elevation in mtDNA mutations in somatic tissues.
Presently, studies investigating these specific processes in the mammalian female germline remain somewhat limited, leaving two key questions unresolved. First, to what extent can oocytes accumulate mitochondrial ROS during aging, and how sensitive is female oocyte maturation to this ROS stress? Second, does the presence of mitochondrial matrix ROS in oocytes affect the quantity or quality of mtDNA? If yes, can the change in mtDNA also sensitize the oocyte quality under oxidative stress? Prior research has not observed any detrimental effects on ovarian function after the deletion of the Sod2 gene26. Recent findings propose that mitochondrial complex I suppression in the early oocytes helps maintain a state of relative dormancy for several decades by reducing mitochondrial ROS27. These studies suggest that mitochondrial activity and the accumulation of ROS may be significantly lower in oocytes compared to somatic cells. However, considering the substantial increase in mtDNA during folliculogenesis1,2,28, which heavily depends on the mitochondrial membrane potential (MMP) contributed by the mitochondrial ETC, it is unlikely that mitochondria remain dormant during the whole maturation process. Additionally, even though complex I exhibits low expression in early oocytes27, alternative electron flow through complexes II-III-IV could generate MMP and mitochondrial ROS in these oocytes. In such a scenario, the impact of mitochondrial matrix ROS on female oocyte maturation and maternal mtDNA may follow a time-dependent accumulation pattern.
Given the significant importance of maternal mtDNA for species maintenance and the distinct differences in germ cell development compared to somatic cells, we aim to understand the relationship between mitochondrial matrix ROS and maternal mtDNA, as well as the oocyte quality in females. To achieve this, we conditionally knocked out the mouse Sod2 gene in the early oocytes within primordial follicles, to increase mitochondrial matrix ROS in the initial oocyte pools, which have the longest lifespans among all the oocyte maturation stages29. Our observations revealed that conditional knockout females displayed signs of reproductive aging at approximately 6 months of age, suggesting that oxidative stress from mitochondrial matrix ROS may induce oocyte defects in a time-accumulated manner. Interestingly, we did not observe any differences in mtDNA mutations between the knockout and control oocytes, but there were variations in mtDNA copy numbers. Specifically, the knockout oocytes initially displayed an increase in mtDNA copy numbers, followed by a subsequent decrease. Notably, once the basal level of mtDNA copy number in the oocytes decreased by deactivating half of the mtSSB, an important factor in mtDNA replication30,31, the reproductive aging phenomena in the knockouts intensified. This underscores the protective role of maintaining mtDNA copy number in keeping oocytes healthy under oxidative stress. In summary, our study provides a comprehensive analysis of the influence of mitochondrial matrix ROS on oocyte quality and maternal mtDNA, which holds significant implications for developing treatments to delay female reproductive aging.
Results
Sod2 depletion in oocytes leads to reproductive aging
To investigate whether the accumulation of endogenous mitochondrial ROS could result in decreased oocyte quality, we generated an oocyte-specific knockout mouse model of the Sod2 gene using the Cre-loxP system (Fig. 1a). We bred Sod2loxp/loxp mice32 with Gdf9-Cre transgenic mice33, in which Cre recombinase is activated in oocytes from primordial follicle stages shortly after birth. The conditional knockout mice (Sod2loxp/loxp, +/Gdf9 Cre; abbreviated as L/L, Cre) displayed no significant differences in ovary weight or body size (Fig. S1a). Immunohistochemistry of various follicle stages (Fig. S1b) confirmed the successful depletion of SOD2 protein in oocytes from primordial follicle stages, and western blotting of MII oocytes further validated the depletion of SOD2 protein (Fig. 1b). We observed increased mitochondrial superoxide in L/L, Cre oocytes, starting from the age of 3 months (20% higher above L/L), without significant difference at 3-week-old compared to controls. Such increased mitochondrial superoxide remained when we detected at the later age of 6 months with 24% higher compared to control. (Fig. 1c, d). Furthermore, we assessed their fertility through the 6-month mating test and found that most L/L, Cre and even +/L, Cre females can only give birth to 2 to 3 litters (Fig. 1e, f), significantly lower than the control group (4.5 litters per female). Consequently, the reduced total litter numbers lead to a lower total pup number born to L/L, Cre and +/L, Cre females (13.4 ± 2.3 and 24.2 ± 4.0 pups per female, respectively), compared to control mice (31.6 ± 3.4 pups per female) (Fig. 1g). Interestingly, the pup number from each litter did not show significant differences between control and L/L, Cre groups, with a slight increase in +/L, Cre females (Fig. 1h). We also conducted additional analysis to examine the increased total number of pups from each tested female as they age. Our findings revealed almost no increase in the number of pups produced by L/L, Cre females at around 6 months of age. In contrast, control females showed a continuous increase in pup numbers as they aged throughout the fertility test. The increased pup numbers in +/L, Cre females were found to be intermediate between the control and L/L, Cre females (Fig. S1c). All these mating test data showed that the accumulation of mitochondrial matrix ROS, specifically in oocytes, led to a decline in reproductive capacity in females as ages, as early as 6 months old, indicating the phenomenon of reproductive aging. By further evaluating the number of ovulated mature (MII) oocytes at multiple age points, we found that there was no significant difference between L/L, Cre and control mice from 3 weeks to 9 months of age (Fig. S1d). These data suggested that the declined reproduction in L/L, Cre females was not caused by a reduced quantity of MII oocytes.
a Mouse breeding strategy to generate oocyte-specific Sod2-deficient mice. Sod2L/L (L/L) male transgenic mice that expressed Cre recombinase under the control of the Gdf9 promoter were intercrossed with Sod2L/L female mice to generate Sod2L/L, Gdf9-Cre (L/L, Cre) female mice. Created with BioRender.com. b Western blot detection of SOD2 expression in the MII oocytes of 3-week-old L/L, Cre and L/L female mice. Each lane contains 50 MII oocytes. ACTIN was used as a loading control. The experiments were repeated 3 times, and a representative image is shown. c Representative image of HKSOX-2m staining (green) of MII oocytes from L/L and L/L, Cre mice at different ages. Scale bar: 50 µm. d Quantification of HKSOX-2m signals of MII oocytes from L/L and L/L, Cre mice at different ages. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, **p < 0.01, ***p < 0.001. e Total pup number of each female during the 6-month fertility test, wild-type (WT); L/L; +/L, Cre and L/L, Cre female mice, with each column representing all pups from one female mouse. f Number of total litters. The control group consists of mice from both the WT and L/L groups. Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, ****p < 0.0001. g Total pup number from each group. The control group consists of mice from both the WT and L/L groups. Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, ****p < 0.0001. h Average litter sizes from each group. The control group consists of mice from both the WT and L/L groups. Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01. i The representative morphology of two-cell stage embryos and blastocysts after in vitro fertilization. MII oocytes were obtained from 20 to 22-week-old L/L, Cre and L/L mice. After fertilization, the in vitro cultures were performed under 5% O2 (5% O2/5% CO2/90% N2). The sperm were obtained from 12 to 14-week-old wild-type C57BL/6 male mice. Scale bar: 100 μm. j The fertilization rate and blastocyst rate of L/L, Cre and L/L mice oocytes after in vitro fertilization. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, ****p < 0.0001.
Since the declined reproduction appeared in L/L, Cre females at approximately 6 months old, we then assessed their oocyte fertilization capacity and subsequent embryonic development at that age using in vitro fertilization (IVF) technique. To minimize extra oxidative stress from the culture environment, we opted for a 5% oxygen concentration during in vitro culture, which closely resembles the actual in vivo embryo development environment34. Our results showed that only 24% of L/L, Cre oocytes after fertilization could develop into the 2-cell stage, with a mere 3.5% of these 2-cell embryos reaching the blastocyst stage, which was significantly lower than that of the control group (64% fertilization rate and 78% of 2-cell embryos developing into blastocysts) (Fig. 1i, j). Moreover, even reducing the oxygen concentration to 1% during in vitro culture did not rescue the developmentally arrested phenotype of L/L, Cre oocytes from 6-month-old females (Fig. S1e). In conclusion, female mice with a conditional knockout of Sod2 gene displayed signs of reproductive aging, with their oocytes exhibiting reduced fertilization rate and reduced capacity for early embryonic development.
Sod2 depletion in oocytes results in aberrant spindle structure and accumulation of lipid droplets
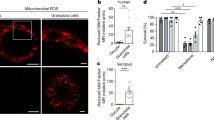
Chromosome segregation aberrations commonly manifest in aging oocytes, engendering aneuploid embryos35,36,37,38. We thus assessed the oocyte’s nuclear structure, specifically the spindle structure (inclusive of microtubule and chromosomal alignment), in females of varying ages using immunofluorescence. We found that by the age of 6 months, L/L, Cre females produced a substantial proportion (44%) of oocytes with abnormal spindle structures, specifically characterized by chromosome misalignment, compared to 14% in control oocytes. By 9 months, this proportion slightly increased to 46% in L/L, Cre oocytes and 24% in control oocytes (Fig. 2a, b). However, we did not observe any significant differences at 3 weeks and 3 months of age (Fig. 2b). Concurrently, ATP levels in MII oocytes derived from 6-month-old L/L, Cre females were markedly decreased in comparison to the control group (35.3% reduction to L/L levels) (Fig. 2c).
a Representative image of spindle morphology (red: tubulin; blue: chromosomes) in oocytes. Circles delineate oocyte boundaries. Up panel: normal homologous chromosome alignment on metaphase II spindles in L/L oocytes. Low: spindle abnormalities in L/L, Cre oocytes. b Proportion of oocytes with abnormal spindles from (a). Data from 3-5 independent experiments, 3-4 mice per group. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, *p < 0.05, ***p < 0.001. c ATP levels in individual oocytes from L/L and L/L, Cre mice across various ages. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, *p < 0.05. d Schematic diagram of preparation procedure for MII oocyte TEM from superovulated 6-month-old L/L and L/L, Cre mice. Created with BioRender.com. e TEM images of MII oocytes from 6-month-old L/L (left) and L/L, Cre (right) mice. Mitochondria (white arrows); lipid droplets (LD, yellow dashed lines). f Representative image of BODIPY staining (green) of MII oocytes from 6-month-old L/L and L/L, Cre female mice. g Quantification of BODIPY signals from (f). M.F.I: mean fluorescence intensity. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, ****p < 0.0001. h Mitochondrial area analysis of MII oocytes from (e). Violin plots show median and quartiles (dashed black line). L/L: 238 mitochondria, L/L, Cre: 161 mitochondria. Kruskal-Wallis test: **p < 0.01. i Mitochondrial longer diameter (major axis) and shorter diameter (minor axis) of MII oocytes from (e). Violin plots indicate median and quartiles (dashed black line). L/L: 238 mitochondria, L/L, Cre: 161 mitochondria. Kruskal-Wallis test: ***p < 0.001, ****p < 0.0001. j Mitochondrial circularity in MII oocytes from (e), indicating shape uniformity. A circularity value closer to 1 indicates a higher proportion of mitochondria with a circular shape. Violin plots display median and quartiles (dashed black line). L/L: 238 mitochondria, L/L, Cre: 161 mitochondria. Kruskal-Wallis test: *p < 0.05.
To further examine the intracellular organelle morphology of MII oocytes from 6-month-old females, transmission electron microscopy (TEM) was employed. To maintain the integrity of MII oocytes during sample preparation, we fixed the ampulla of the fallopian tubes, filled with ovulated MII oocytes from superovulated mice (Fig. 2d). Interestingly, a significant increase in lipid droplets was observed in L/L, Cre MII oocytes compared to the control oocytes (Fig. 2e). This observation was further confirmed by BODIPY staining, which showed that the fluorescence intensity was 45.9% higher in L/L, Cre oocytes than in L/L oocytes (Fig. 2f, g). Regarding mitochondrial morphology, a slight reduction in the mitochondrial area was observed in L/L, Cre oocytes compared to the controls (0.20 µm2 versus 0.23 µm2) (Fig. 2h). This reduction is consistent with the smaller major and minor axes in L/L, Cre oocytes (0.70 µm and 0.37 µm, respectively) as compared to L/L oocytes (0.74 µm and 0.42 µm, respectively) (Fig. 2i). Additionally, the mitochondrial shape in L/L, Cre oocytes is less circular than the controls, as indicated by the circularity calculation (Fig. 2j). Furthermore, we observed many multivesicular body (MVB)-like structures in L/L, Cre female oocytes (Fig. S2), which were rarely observed in the control group.
To investigate molecular changes in L/L, Cre and control MII oocytes from 6-month-old females, we utilized the Smart-seq2 method for oocyte transcriptome sequencing. We observed changes in a total of 388 transcripts in L/L, Cre MII oocytes compared to controls, with 239 showing downregulated expression and 149 showing upregulated expression (Fig. 3a, b). Based on previous observations of aberrant spindle structure, accumulated lipid droplets, and significantly decreased ATP levels, we manually categorized the differentially expressed genes into several groups, including mitochondrial OXPHOS subunits, DNA repair, RNA splicing, mRNA processing, translation, spindle organization, and lipid metabolism. Our analysis revealed that the majority of genes in these phenotype-related pathways were downregulated in L/L, Cre MII oocytes (Fig. 3c). Particularly noteworthy is the consistent downregulation of ten out of thirteen mitochondrial mRNAs, including mt-nd1, mt-nd2, mt-nd3, mt-nd4, mt-nd4l, mt-nd5, mt-nd6, mt-ctyb, mt-cox3, and mt-atp6, indicating there could be either downregulation of mtDNA transcription or the less stable of these transcripts under the conditions of mitochondrial ROS stress (Fig. 3b, c). To further validate these findings, we conducted unbiased pathway analyses on both the downregulated and upregulated gene lists (Fig. 3d, e). Consistent with our manual analysis, the downregulated genes primarily involved chromosome segregation and organization, nucleic acid metabolism, and OXPHOS (Fig. 3d). These results indicated that a decline in oocyte quality may be strongly associated with mitochondrial dysfunction and abnormalities in its gene expression, as illustrated in Fig. 3b. Furthermore, the upregulated genes in L/L, Cre MII oocytes were mainly related to negative regulation of cell cycles, proteasome-mediated ubiquitin-dependent protein catabolism, and the establishment of organelle localization through catabolic processes, suggesting that ROS affect cell cycle regulation and promote oocyte catabolism (Fig. 3e). In conclusion, the accumulation of ROS in oocytes leads to a deterioration in oocyte quality, potentially resulting from abnormal OXPHOS and mitochondrial function, consequently leading to irregular chromosome segregation and lipid accumulation.
a The Venn diagrams illustrating the numbers of transcripts that are up or down-regulated in MII oocytes of 6-month-old L/L, Cre female mice. b Volcano plot showing differentially expressed genes (DEGs; downregulated, green; upregulated, red) in L/L, Cre oocytes compared with L/L from 6-month-old mice oocytes, with significant DEGs highlighted. c Heatmap illustrating the manual-sorted pathways derived from DEGs. d Gene Ontology (GO) enrichment analysis of downregulated DEGs in L/L, Cre oocytes relative to L/L oocytes from 6-month-old mice. e Gene Ontology (GO) enrichment analysis of upregulated DEGs in L/L, Cre oocytes relative to L/L oocytes from 6-month-old mice.
Sod2 depletion in oocytes leads to reduced mitochondrial activity
To comprehend the intricate mechanism of decreased oocyte quality caused by accumulated mitochondrial matrix ROS, we assessed oocyte mitochondrial function. Specifically, we evaluated the MMP using tetramethylrhodamine methyl ester (TMRM) staining because of its direct relation to mitochondrial quality and activity. We observed a decreased MMP in L/L, Cre MII oocytes of 3-month-old females (82.9% of control levels). This decline became more pronounced as females aged, reaching 27.3% of control levels at 9 months old (Fig. 4a, b). Evaluation of OXPHOS protein levels using western blot in MII oocytes of different ages revealed a downward trend in complex II subunit level starting from 3-week-old L/L, Cre oocytes compared to control oocytes (p = 0.168). This declining trend worsened with increasing age, resulting in levels of 39% and 29% of control oocyte levels at 3 months and 6 months of age, respectively (Fig. 4c, d). However, other subunits of OXPHOS, such as complex I, III, IV, and V, as well as the total mitochondrial mass (indicated by either TOMM20, VDAC, or HSP60) didn’t show significant changes in L/L, Cre oocytes compared with controls (Fig. S3a, b). Furthermore, we used succinate dehydrogenase (SDH) activity staining to measure complex II activity in situ on frozen ovary sections from 3-month-old mice; in this assay, the blue formazan precipitate’s intensity directly positively correlates to complex II’s enzymatic function39. We observed an obvious decrease in CII activity in the L/L, Cre oocytes compared to the controls (Fig. 4e). Conclusively, our findings suggest that accumulated mitochondrial matrix ROS affects mitochondrial activity and disturbs oocyte complex II proteins.
a Representative image of TMRM staining of mature (MII) oocytes from control (L/L) and Sod2 knockout (L/L, Cre) mice, displaying mitochondrial membrane potential across different ages. b Quantification of TMRM signals from (a). Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, ***p < 0.001, ****p < 0.0001. c Representative image of western blots for SDHB protein in oocytes from L/L and L/L, Cre mice at various ages, with ACTIN as a loading control. N = 50 MII oocytes per lane, with 3-4 independent experiments. d Densitometric analysis of SDHB levels normalized to ACTIN from (c). Data were mean ± SEM and were analyzed using two-tailed unpaired t-test with Welch’ s correction, *p < 0.05, **p < 0.01. e Representative image of complex II activity in ovarian sections from 3-month-old L/L and L/L, Cre mice, with oocyte boundaries marked. f TEM images of early oocytes in primordial follicles from 2-week-old (upper panel) and 6-month-old (lower panel) L/L and L/L, Cre mice. g Mitochondrial area quantification of (f), presented in violin plots with median and quartiles (dashed black line). Mitochondria counts: L/L_2W = 153, L/L, Cre_2W = 177, L/L_6M = 64, L/L, Cre_6M = 94. Kruskal-Wallis test: *p < 0.05, ***p < 0.001. h Mitochondrial circularity calculation of (f), with values closer to 1 indicating greater circularity. Data presented in violin plots with median and quartiles (dashed black line). Mitochondria counts as in (g). Statistical analysis by Kruskal-Wallis test. i Major axis (longer diameter) measurements of (f), presented as violin plots with medians and quartiles (dashed black line). Mitochondria counts as in (g). Kruskal-Wallis test: ****p < 0.0001. j Minor axis (shorter diameter) measurements of (f), resented as violin plots with medians and quartiles (dashed black line). Mitochondria counts as in (g). Kruskal-Wallis test: *p < 0.05, ***p < 0.001, ****p < 0.0001.
Given the unique features of oocyte development, including ongoing follicular recruitment from the same pool of primordial follicles40, it is reasonable to propose that early oocytes in these follicles may accumulate damage over time after depletion of the Sod2 gene. This could lead to older females relying on these early oocytes with mitochondria of diminished quality for robust mito-biogenesis during the maturation phase. Consequently, it could be a contributing factor to the declined oocyte quality observed in aged L/L, Cre mice. To investigate whether this hypothesis was true, we employed TEM to examine mitochondrial structure in the early oocytes from primordial follicles from mice aged 2 weeks and 6 months. Upon observation, mitochondria in the L/L, Cre early oocytes of 6-month-old mice exhibited reduced electron density with fewer cristae compared to those in L/L, Cre mice at 2 weeks and the controls at the same age (Fig. 4f). With measurements, we found mitochondria from L/L, Cre oocytes appeared to be larger by their total area, major and minor axis compared to both the oocytes at 2-week-old or the age-matched control oocytes without changes in the circularity (Fig. 4g–j). This suggests that the accumulated mitochondrial ROS could cause reduced mitochondrial quality in the early oocytes during aging. This results in an inferior initial state, ultimately contributing to the declined quality of aged mature oocytes.
Sod2 depletion in oocytes results in changes in mtDNA copy number without affecting mtDNA mutation load
Since the central premise of the free radical theory suggests that increased ROS levels trigger a higher occurrence of mtDNA mutations and mitochondrial stress, consequently leading to further ROS production, such theory hasn’t been examined in female germline. Here we determined oocyte mtDNA mutations under accumulated ROS at 3 weeks and 9 months. Using the rolling circle amplification technique, we successfully amplified adequate mtDNA content from individual oocytes of various females and prepared a PCR-free library (to minimize PCR bias) for subsequent next-generation sequencing (Fig. S4a).
To delve into the potential mutation mechanisms of the mitochondrial mutations, mutational signatures were extracted from all the de novo mutations and shared variations (see Methods). We identified that both de novo mutations and shared variations are randomly distributed throughout the mtDNA (Fig. S4b). The predominant mutation pattern identified in our research, G:C > A:T (Fig. S4c), is likely due to the replication error and spontaneous deamination of deoxycytidine41,42,43. Our results are also consistent with previously reported mutation patterns from multiple aging cases44,45.
To further investigate a possible link between the conditional knockout of Sod2 and characteristics of mutations, we analyzed both the de novo mutations and shared variants in L/L and L/L, Cre oocytes. Our findings suggest that the conditional knockout of Sod2 does not impact the quantity of mtDNA mutations or the variant allele frequencies (VAFs) in the oocytes of mice aged either 3 weeks or 9 months (as shown in Fig. 5a, b and Fig. S4d, e). In addition, the base substitution patterns of mutations observed in the L/L, Cre oocytes was in general consistent with that found in the control group. (Fig. 5c). Ultimately, the sequencing data indicated that increased oxidative stress in the oocyte mitochondrial matrix did not lead to a notable increase in mtDNA mutations.
a Mutation load, including de novo and shared variations, per oocyte in L/L and L/L, Cre mice at 3 weeks and 9 months of age. b Variant allele frequencies of de novo mutations and shared variations in L/L and L/L, Cre oocytes from mice aged 3 weeks and 9 months. c Mutation spectra comparison for de novo mutations and shared variations in L/L and L/L, Cre oocytes across the two age groups. d mtDNA copy number quantification in single MII oocytes from L/L and L/L, Cre mice at different ages; data were mean ± SEM, analyzed by unpaired two-tailed Student’s t-test (*p < 0.05, ****p < 0.0001). e Representative fluorescence images of oocytes from 6-month-old L/L and L/L, Cre mice, labeled with anti-dsDNA (green) and anti-TOMM20 (red) antibodies. f mtDNA copy number semi-quantification with the ratio of yellow to red fluorescence intensity in L/L normal (n = 9), L/L, Cre_normal (n = 5), and L/L, Cre_aberrant (n = 9) oocytes from (e). Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, **p < 0.01, ***p < 0.001.
We then tested the potential impact of accumulated mitochondrial matrix ROS on the mtDNA quantity in MII oocytes. We performed quantitative PCR (qPCR) to assess the mtDNA copy number in oocytes of varying ages. Our results showed a significant increase in mtDNA quantity in L/L, Cre oocytes at 6 months (56.6% higher than controls), followed by a slight decrease at 9 months (17.2% lower than controls). However, no changes in mtDNA quantity were observed at 3 weeks and 3 months when compared to the control group (Fig. 5d). Furthermore, we utilized immunofluorescence to separate the L/L, Cre oocytes into two categories based on the presence of normal or aberrant spindle structures (Fig. 5e). We then semi-quantified the relative mtDNA copy number by calculating the ratio of the fluorescent intensity of mtDNA (dsDNA signal co-localized with the mitochondria signal) to the total signals of mitochondria (Fig. 5e, f). Interestingly, despite a significant increase in bulk mtDNA copy number in L/L, Cre oocytes (Fig. 5d), this enhancement appeared to be limited to oocytes with normal spindle morphology.
In summary, we observed fluctuation in mtDNA copy number, with higher levels in 6-month-old oocytes and lower levels in 9-month-old oocytes from L/L, Cre females, compared to age-matched controls. This suggests that the impact of accumulated ROS on mtDNA quantity may depend on the different stages of oxidative stress. Upregulation could be a compensatory mechanism. However, when mitochondrial damage becomes more pronounced, such as in 9-month-old L/L, Cre female oocytes, this compensatory mechanism may no longer be initiated.
MtSSB heterozygous knockout accelerates the reproductive aging of Sod2 conditional knockouts
Next, we investigated the potential function of mtDNA copy number on oocyte quality and female fertility under accumulated ROS condition. To achieve this, we modulated the mtSSB level to manipulate the oocyte mtDNA copy number given its critical role in mtDNA replication31 and produced Sod2L/L, mtSSB+/L; Gdf9-Cre mice (hereafter referred to as L/L, M; Cre, Fig. 6a). To test the singular effect of mtSSB heterozygosity on oocyte quality, we firstly evaluated oocyte quantity and quality in mtSSB heterozygous mice (with the no-obvious phenotype reported before31) at 6 months of age. MtSSB heterozygous mice exhibited normal number of superovulated oocytes (Fig. S5a) without increasing oocytes with aberrant spindle structure (Fig. S5b). We utilized qPCR as well as the immunofluorescence semi-quantitative method and proved that mtSSB heterozygous oocytes presented decreased mtDNA copy number to 36% (qPCR, Fig. S5c) and 68% (immunofluorescence, Fig. S5d, e) of relative controls. Although the oocyte quality seems to have no change in mtSSB heterozygosity condition, we indeed observed a slight decrease (25.3% of control) in oocyte MMP, indicated by TMRM staining, compared to the controls (Fig. S5f, g). Our data demonstrated that reduced mtDNA copy number under physiological conditions could mildly reduce the mitochondrial activity without affecting the oocyte quality.
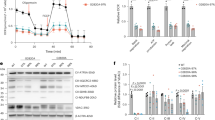
a Generation strategy for oocyte-specific Sod2 knockout and mtSSB heterozygous mice, using Gdf9-Cre-driven recombination in Sod2L/L males (L/L, Cre) crossed with Sod2L/L; mtSSBL/L females to generate Sod2L/L, mtSSB+/L; Gdf9-Cre (L/L, M; Cre) female mice. Created with BioRender.com. b Total pup number of each female during the 6-month fertility test, data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, **p < 0.01. c Number of total litters. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, ***p < 0.001. d Average litter sizes from each group. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test. e Statistical analysis of superovulated oocyte number in females aged 3 weeks and 3 months. Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, **p < 0.01, ****p < 0.0001. Sample sizes: control (n = 16, for the age of 3w and 3 m), L/L, Cre (n = 13 for 3w; n = 16 for 3 m), L/L, M; Cre (n = 11 for 3w; n = 16 for 3 m). f Representative image of the spindle morphology (red, tubulin; blue, chromosome), including oocytes with normal spindle (L/L) and oocytes with aberrant spindles (L/L, M; Cre). g Proportion of oocytes with aberrant spindle from (f), across three independent experiments, 3-4 mice per group. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, **p < 0.01. h Representative immunofluorescence images of MII oocytes from 3-month-old mice, stained for dsDNA (green) and TOMM20 (red). i mtDNA copy number semi-quantification with the ratio of yellow to red fluorescence intensity in L/L, M (n = 4), L/L, M; Cre_normal (n = 5), and L/L, M; Cre_aberrant (n = 6) oocytes from (j). Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05. j Quantitative PCR analysis of single oocyte mtDNA copy number from females of different ages. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, **p < 0.01, ****p < 0.0001. k Representative image of TMRM staining (red) of MII oocytes from control; L/L, Cre and L/L, M; Cre mice at 3-week-old and 3-month-old. l Quantification of TMRM signals in (k). Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, **p < 0.01, ****p < 0.0001. m ATP level of single oocytes from L/L, M and L/L, M; Cre mice at 3-month-old. Data were mean ± SEM and were analyzed using unpaired two-tailed Student’s t-test, *p < 0.05. n Mutation spectrum of de novo mutations and shared variations in control (L/L; L/L, M) and L/L, M; Cre 3-week-old and 6-month-old mice, respectively.
Next, we conducted a systematic evaluation of fertility and oocyte quantity and quality in L/L, M; Cre and control females. The 6-month breeding test revealed that L/L, M; Cre females produced an average of approximately 10.3 ± 2.0 total pups and 1.5 ± 0.4 litters per female, markedly less than the L/L, M controls (Fig. 6b, c and Fig. S6a), while the pup number from each litter did not show significant differences (Fig. 6d). Their breeding performance was inferior to that of L/L, Cre females producing 13.4 ± 2.3 total pups and 2.1 ± 0.3 litters per female on average (Fig. 1f, g). Additionally, when counting for the increased pup number with the mother’s age, we observed that the cessation of breeding occurred earlier in these individuals compared to L/L, Cre females (see Fig. S6b). The number of superovulated MII oocytes was reduced in L/L, M; Cre females at 3 weeks (averaging 32.3 in L/L, M; Cre versus 63.7 in controls) to 3 months of age (averaging 26.9 in L/L, M; Cre versus 33.8 in controls) (Fig. 6e). There was a significant increase in the ratio of oocytes with aberrant spindle structure in 3-month-old L/L, M; Cre mice, reaching as high as 47.6%, compared to only 9.1% in the control group (Fig. 6f, g). These data demonstrated that reduced mtSSB under the ROS stress exacerbates the reproductive aging phenotype in L/L, Cre females, leading to an accelerated decline in oocyte function.
We further detected the mtDNA quantity in these oocytes and found that there was a significant reduction in mtDNA content indicated by fluorescence in all L/L, M; Cre oocytes from 3-month-old mice, regardless of spindle structure (Fig. 6h, i). This was further confirmed by qPCR quantitative analysis of mtDNA levels in individual oocytes at all examined age points (Fig. 6j). The copy number decreased from 3 weeks of age (83.3% of control levels) and became more significant than that of the controls at 3 and 6 months of age (37.4% and 43.9% of controls, respectively). Additionally, we assessed the oocyte mitochondrial activity of L/L, M; Cre mice and observed a notable decline in MMP in the oocytes of both 3-week-old and 3-month-old female mice. The MMP levels in L/L, M; Cre oocytes were found to be significantly lower (41.5% of the controls) compared to those in L/L, Cre oocytes (which showed MMP levels at 74.9% of controls) at the same age of 3-month-old (Fig. 6k, l). Furthermore, we observed a significant decrease in ATP levels in MII oocytes from 3-month-old females (51.5% of L/L, M levels) (Fig. 6m).
Since we observed in the L/L, Cre oocytes at 6 months present lots of lipid droplets under TEM (Fig. 2e), we further detected whether such abnormal lipid droplets were significantly increased in L/L, M; Cre oocytes at 3-month-old. Our data showed that significantly increased lipid droplets under TEM already appeared in these L/L, M; Cre oocytes at 3 months of age, which is consistent with the further BODIPY staining, with 24.4% higher than that of the control group, while they did not show up in L/L, Cre oocytes at the same age (Fig. S6c, d, e). Besides this, we found mitochondria from L/L, M; Cre oocytes appeared to be smaller than the controls by their total area (0.18µm2 verse 0.22µm2), while there is no significant difference in the minor and major axis, as well as the circularity between L/L, M; Cre and L/L, M oocytes (Fig. S6f–h).
Due to the high sensitivity of complex II to mitochondrial ROS, a comparison was conducted to evaluate the expression of OXPHOS subunits using western blot analysis in oocytes obtained from mice at three weeks old, specifically L/L, M; Cre, L/L, Cre, and control mice. The quantification data exhibited a decreasing trend in SDHB, while the other complex subunits, as well as the total mitochondrial mass indicated by TOMM20 protein levels, were unaffected (Fig. S6i, j). Notably, the reduction in mtSSB did not lead to further decrease in complex II levels under ROS conditions (Fig. S6j). These findings support the fact that mtDNA-encoded genes do not contribute to complex II subunits. Consequently, we speculated that the reduced levels of mtDNA copy number make the oocyte mitochondria less active, similar to the observations made in mtSSB+/d oocytes (Fig. S5f, g). This further decline in mitochondrial activity increases the oocyte’s susceptibility to ROS accumulation, ultimately resulting in poorer oocyte function in L/L, M; Cre females.
Given the importance of mtSSB during mtDNA replication, which could protect the single-stranded heavy strand during delayed replication, it was essential to test whether the severe reproductive aging phenotype observed in L/L, M; Cre mice could also be due to potential increases in mtDNA mutations caused by insufficient mtSSB protection. We sequenced the mtDNA in oocytes from 3-weeks-old and 6-months-old mice and found that even in the reduced protection by mtSSB during replication under mitochondrial ROS conditions, we did not observe significantly elevated mutation load in mtDNA (Fig. S7a). A slight increase in the VAF of both de novo mutations and shared variations were observed in the L/L, M; Cre oocytes at the 3-week-old and 6-month-old stages (Fig. S7b), likely driven by mutations/variations in the individual mice of high VAF mutations (Fig. S7c, d). The L/L, M; Cre mice did not show a preference for the G:C > T:A mutation in either de novo mutations or shared variations (Fig. 6n). Based on these findings, we can largely exclude the possibility that accelerated reproductive aging caused by reduced mtSSB contribute to mutations in mtDNA.
NMN improves L/L, M; Cre oocyte quality through increasing mitochondrial activity
The lower mitochondrial activity in L/L, M; Cre oocytes in 3-month-old mice is consistent with previous findings in naturally aged oocytes with lower mtDNA copy number8 and reduced antioxidant capacity46,47. We aim to investigate whether improving the mitochondrial activity in these oocytes can mitigate the reproductive aging phenotype.
Nicotinamide mononucleotide (NMN), a precursor to NAD+, can rapidly convert to NAD+ upon entering cells, thereby increasing intracellular NAD+ levels. NAD+ serves as a coenzyme for many essential enzymes and is involved in various vital metabolic processes, including glycolysis, the TCA cycle, and OXPHOS, tightly linked to mitochondrial activity48. Previous research has demonstrated that NMN treatment in aged mice can reverse several dysfunctions associated with organ aging, including reproductive aging49,50,51,52. Therefore, we aimed to determine whether NMN could improve oocyte quality in 3-month-old L/L, M; Cre mice. We followed the methodology described by Miao et al.52 and recorded changes in mouse weight during the NMN treatment period (Fig. 7a). Our results indicated that NMN treatment did not significantly change the body weight, or the number of superovulated oocytes compared to the group treated with PBS (Fig. 7b, c). However, the mitochondrial activity, as indicated by MMP in oocytes, significantly increased in the NMN treatment group compared to PBS groups (Fig. 7d, e). Specifically, compared to the PBS-treated group, the NMN-treated control oocytes exhibited a 1.53-fold increase, while the NMN-treated L/L, M; Cre oocytes displayed a 1.67-fold increase. There was an increasing trend in ATP levels in NMN-treated L/L, M; Cre oocytes compared to their PBS group, while NMN-treatment did not increase ATP levels in control oocytes compared to their PBS group (Fig. 7f). Finally, we analyzed meiotic spindle assembly in MII oocytes from different groups using immunofluorescence staining (Fig. 7g, h). The results showed that the proportion of aberrant spindles in oocytes from NMN-treated L/L, M; Cre mice were reduced to 16.7%, reaching levels comparable to those of the control group (18.8%).
a Schematic representation of NMN or PBS administration and hormone-induced superovulation in 3-month-old L/L, M and L/L, M; Cre female mice. Created with BioRender.com. b Body weight monitoring in mice subjected to NMN or PBS treatment (n = 3 per group). c Oocyte number after ovulation of mice with NMN or PBS treatment, n = 3 for each group. d Representative image of TMRM staining (red) of MII oocytes from PBS- and NMN-treated mice. e Quantification of TMRM signals in MII oocytes from (d). Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05, ****p < 0.0001. f ATP levels in single oocytes of PBS- and NMN-treated female mice. Data were mean ± SEM and were analyzed using one-way ANOVA with Tukey’s multiple comparison test, *p < 0.05. g Representative images of spindle morphology and chromosome alignment in MII oocytes from PBS- and NMN-treated mice. Red, tubulin; blue, chromosome. h Quantitative analysis of the percentage of oocytes with aberrant spindles from (g). Each group consisted of oocytes obtained from 3 mice, and Fisher’s exact test was used to analyze the statistical differences between L/L, M and L/L, M; Cre treated with PBS or MNM, respectively.
Overall, NMN treatment appears to have a beneficial effect through increasing mitochondrial activity in L/L, M; Cre oocytes.
Discussion
The interplay between mitochondrial ROS and the maternal mtDNA, as well as oocyte quality, remains an enigmatic area of study9,53. Our subject holds significant relevance in light of the rising trend of delayed childbearing and the crucial role of maternal mtDNA for next generation. Utilizing the Sod2 conditional knockout mouse model, we showed that mitochondrial matrix ROS could precipitate female reproductive aging, possibly through ROS-mediated disruption of complex II, and perturb the steady-state of mtDNA-encoded transcripts, without inducing mutations to maternal mtDNA. Intriguingly, the increased ROS could shift the mtDNA quantity in different ages, indicating it’s susceptible to accumulated mitochondrial matrix ROS. Reducing the basal quantity level of mtDNA on the Sod2 knockout background amplified the reproductive aging phenotype.
The reproductive aging phenotype in our Sod2 knockout model offers a contrast to prior experiments involving Sod2-null ovary transplantation26. Previous studies involving the transplantation of ovaries from Sod2 knockout juvenile mice into wild-type mice resulted in successful follicular development, ovulation, and fertility, implying that SOD2 is not indispensable for ovarian function. However, our data, which involved knocking out the Sod2 gene from the postnatal early oocytes, yielded divergent results. The discrepancies could be ascribed to two factors. First, the transplantation experiments employed a Sod2 heterozygous ovary as a control, and our fertility tests revealed that Sod2 heterozygotes exhibit a certain degree of reduced fertility compared to controls. Second, the broad range of pups born from transplanted females might not provide a sensitive enough measure for long-term observation of female fertility. Despite different conclusions on reproduction, both studies found that the overall impact of mitochondrial matrix ROS on postnatal oocyte maturation is limited, since even in our Sod2 knockout models, the reproductive aging phenotype appears after 2 to 3 litters, approximately 6 months of age. The major impact of mitochondrial matrix ROS on female fertility is through reducing the quality of the oocytes. The aberrant spindle structure observed in our mouse models aligns with the phenomenon observed in aged human oocytes38,54 and is consistent with what has been observed in Drosophila models55 with Sod1 or Sod2 knockdown experiments, emphasizing the importance of antioxidant capacity during reproductive aging.
We initiated Sod2 knockout in the early oocytes from the primordial follicle pool and evaluated the mutation load in 9-month-old mature oocytes. Intriguingly, such long-term exposure did not induce an increase in mtDNA mutations in our Sod2 knockouts. This finding aligns with the mtDNA sequence from Sod2-specific knockout hearts25, although the phenotype of Sod2 conditional knockout oocytes and heart are affected to different degrees: much more severe in the heart compared with oocytes, while neither organ showed a change in mtDNA mutation level under such oxidative stress. Our results also consistent with deep sequencing data from macaque45, human56, and mouse oocytes44, suggesting that aged oocytes do not have oxidative damage-caused mutations: G:C > T:A, but rather replication error-caused mutations. Thus, our study provides evidence that the accumulated mitochondrial matrix ROS do not trigger an increase in mtDNA mutations, indicating that the core tenet of the free radical theory of aging may need to be revised in the context of female germlines. Additionally, a recent study demonstrated that mitochondrial-released H2O2 is unable to impair nuclear genomic DNA mutations57. In line with our findings, we suggest that mitochondrial ROS plays a limited role in initiating both nuclear and mtDNA mutations.
Unexpectedly, we observed an intriguing rise in mtDNA levels at 6 months of age under ROS condition. The effect could be direct, resulting from increased mtDNA replication stimulated by ROS such as H2O2 or singlet oxygen, which has been previously observed both in vivo25 and in vitro58. Alternatively, the effect could be the consequence of cellular responses aimed at enhancing mitochondrial biogenesis in response to cellular damage59,60,61. However, this compensatory mechanism may not sufficiently rescue other defects, such as reduced complex II and decreased levels of mtDNA-encoded mRNA in 6-month-old females. Consequently, our data underscore the need for alternative strategies, enhancing mtDNA gene expression and complex II activity, which can be more directly compared with increasing mtDNA copy number at this stage. The latter approach may sometimes exacerbate the condition, as it can restrict mtDNA transcription further62. Furthermore, the observed increase in mtDNA was transient, as it declined back to lower levels in 9-month-old females. Such observation may help elucidate inconsistent findings regarding mtDNA levels in clinical patient oocytes, which may arise from different stages of accumulated oxidative stress63. Although increasing mtDNA levels after the occurrence of ROS damage might provide limited assistance to oocytes, our data obtained from Sod2 knockout mice with mtSSB heterozygosity supports the notion that maintaining the baseline level of mtDNA is crucial for oocytes to sustain normal function when confronted with ROS stress. This may also be a contributing factor in how certain chemotherapy drugs, such as cyclophosphamide (CPM)64 and 14-hydroxydaunomicyn (DOX)65,66 can affect female fertility. The mechanism behind this could involve the inhibition of oocyte mtDNA replication, ultimately leading to a reduction in the baseline mtDNA levels within the oocytes.
The preservation of mtDNA copy number appears to support oocyte function under ROS stress not due to any inherent antioxidative characteristics of mtDNA but through potentially maintaining OXPHOS efficiency, thereby enhancing mitochondrial function. While directly assessing OXPHOS efficiency in limited samples like oocytes is difficult, the noted reduction in MMP in mtSSB heterozygotes is consistent with this hypothesis. A decrease in mtSSB compromises mitochondrial activity, increasing oocyte vulnerability to ROS-induced damage. This interpretation is further supported by the positive response to NMN treatment, which increases NAD+ level, a critical component in mitochondrial-related metabolic pathways, indicating a relationship between improved mitochondrial performance and increased oocyte resistance to oxidative stress.
In summary, our study significantly contributes to the understanding of the various states of maternal mtDNA under ROS stress within the female germline. The positive effects observed with NMN treatments present a promising option for increasing mitochondrial activity, which, in turn, could enhance oocyte function during the early stages of ROS accumulation. Additionally, future treatment directions could also focus on up-regulated mtDNA gene transcription or increasing complex II activity once oxidative damage occurs to improve declined oocyte quality during reproductive aging.
Materials and Methods
Mouse models and husbandry
All mice were housed under specific pathogen-free environmental conditions with free access to water and food, a temperature of 20–22 °C, 50–70% humidity, and a 12 hour light/dark cycle at the Animal Core Facility of Westlake University. All procedures involving animals were conducted strictly according to the guidelines for the Care and Use of Laboratory Animals of Westlake University and approved by the Institutional Animal Care and Use Committee at Westlake University. We have complied with all relevant ethical regulations for animal use.
To obtain SodL/L; Gdf9-Cre females (abbreviated as L/L, Cre) for our study, we initially mated SodL/L females with Gdf9-Cre males, resulting in Sod2+/L; Gdf9-Cre males. These males were subsequently bred with Sod2L/L females to generate the desired Sod2L/L; Gdf9-Cre mice (L/L, Cre). To improve breeding efficiency, L/L females were directly mated with L/L, Cre males, as shown in Fig. 1a. The offspring from these matings, composed of L/L, Cre females (abbreviated as L/L), served as the control group for the L/L, Cre females.
To generate Sod2L/L; mtSSB+/L; Cre females (abbreviated as L/L, M; Cre), we crossed Sod2L/L mice with mtSSBL/L mice, resulting in Sod2+/L; mtSSB+/L mice. These mice were then used for mating to obtain Sod2L/L; mtSSBL/L females. These females were directly mated with Sod2L/L; Gdf9-Cre males to generate the desired L/L, M; Cre females, as depicted in Fig. 6a. All of these mice are maintained in a C57BL/6 genetic background. The efficiency of depletion of Sod2 in the L/L, Cre line was detected via immunohistochemistry of mouse ovaries and western blot of oocytes.
For fertility tests, 8-week-old L/L, Cre, L/L, M; Cre and control females were mated with C57BL/6 wild-type males for 6 months. Fertility was assessed by the total pup number, total litter number, and litter size per female.
Oocyte collection
To obtain mature (MII) oocytes, the ages of female mice were determined based on experimental demands. Initially, mice were intraperitoneally injected with 10 IU of pregnant mare serum gonadotropin (PMSG, Ningbo Second Hormone Factory). After 48 h, the mice received the same dose of human chorionic gonadotropin (hCG, Ningbo Second Hormone Factory) for superovulation. Subsequently, cervical dislocation was performed to euthanize the mice, and cumulus-oocyte complexes (COCs) were extracted from the ampulla of the mouse oviduct 12–14 h after hCG injection. MII oocytes were then washed in M2 medium (Sigma, M7167) with 1 mg/mL hyaluronidase (Sigma, H4272) and collected using a mouth pipette under the visual field of a dissecting microscope (Nikon SMZ25) for further experimentation.
NMN treatment
We employed the method described in Miao et al.52. Briefly, female mice were administered NMN (Sigma, N3501, 200 mg/kg body weight) or an equivalent volume of PBS by intraperitoneal injection on a daily basis for 10 consecutive days. Their weights were recorded daily. To evaluate the impact of NMN on MII oocyte quality, superovulation was conducted from day 8 using the same procedure as mentioned above. Oocyte collection was carried out on day 11.
Immunohistochemical analysis (IHC)
To assess the efficiency of Sod2 knockout in oocytes, mouse ovaries were harvested and fixed in 4% PFA (Leagene, DF0135) overnight at 4 °C and then dehydrated through a series of graded ethanol solutions and xylene before paraffin embedding. IHC analysis was performed on 7 μm-thick ovary sections according to the manufacturer’s instructions. In brief, the slides were incubated in 3% H2O2 in methanol at 37 °C for 10 min to block endogenous peroxidase, 0.1 M citrate buffer (Beyotime, P0083) was used for antigen retrieval of deparaffinized paraffin sections, and the sections were then incubated with permeabilization buffer containing 3% BSA (Gibco, 10099141 C) and 0.35% Triton X-100 (Sigma, SLCD3237) in PBS (Meilunbio, MA0015). After that, the tissue sections were covered with 5% goat serum in PBS-T (0.05% Tween-20 in PBS) for 30 min at room temperature to block any nonspecific binding. The slides were incubated with primary antibodies at 4 °C overnight. After three washes with PBS, the slides were incubated with secondary antibodies (Zhongshan Golden Bridge Biotechnology, Beijing, PV6001,) for 2 h at room temperature. After three washes with PBS, DAB was added to each slide until the sections developed, and the slides were stained with hematoxylin before dehydrating and mounting the slides. It should be noted that the control, L/L, Cre ovary sections were mounted on the same slide. IHC was performed on the same slides to facilitate comparison.
Oocyte mitochondrial function assay
To evaluate the quality of oocyte mitochondria, oocyte mitochondrial function was evaluated by mitochondrial ROS level, mitochondrial membrane potential (ΔΨm), lipids content, and ATP content.
For mitochondrial matrix ROS detection, we utilized HKSOX-2m67 probe (an improved version of HKSOX-1m probe68). In brief, oocytes were stained with 10 mM HKSOX-2m for 30 min at 37 °C in M2 medium (Sigma, M7167) and washed with fresh M2 medium before imaging.
For mitochondrial membrane potential detection. Oocytes were incubated with 25 nM TMRM (Invitrogen, I34361) at 37 °C for 30 min and immediately viewed under the confocal microscope (Nikon C2Si) without washing.
For oocyte lipid content staining, Oocytes were incubated with 1 μM BODIPY (Invitrogen, D3823) at 37 °C for 30 min, then washed with fresh M2 medium before imaging.
For the detection of ATP content from single oocytes, the assay was performed using the CellTiter-Glo® Luminescent Cell Viability Assay Kit (Promega, G7570) in accordance with the manufacturer’s instructions. The luminescence measurement was carried out using a microplate reader (Varioskan LUX, Thermo). The ATP content in single oocytes was calculated by utilizing a standard curve derived from a standard solution, which was prepared following the provided guidelines.
Immunofluorescence assay of oocytes
The morphological assessment of spindles, mitochondrial distribution, and mtDNA quantification in MII oocytes was conducted using an immunofluorescence assay. MII oocytes were gently harvested and transferred in small groups (no more than three) to a Tyrode’s solution (Sigma, T1788). The dissolution of the zona pellucida was monitored microscopically. Upon complete dissolution, the oocytes were promptly moved to PBS to remove any residual Tyrode’s solution. Subsequently, the oocytes were fixed with 4% PFA for 30 minutes at room temperature.
After fixation, the oocytes were rinsed in Cell Wash buffer (10 μL FBS in 10 mL PBS) for 15 minutes, followed by a 15 min incubation in an ammonium chloride solution (0.03 g NH4Cl with 10 μL FBS in 10 mL PBS). The oocytes were then blocked using a cell blocking buffer (10 μL Triton X-100 and 1 mL FBS in 9 mL PBS) for 2 h at room temperature.
Subsequent to blocking, the oocytes were incubated with the primary antibody at 4 °C overnight. The following day involved three washes with staining wash buffer (1 mL FBS in 10 mL PBS) prior to a 2 h incubation at room temperature with a fluorescence-labeled secondary antibody targeting α-tubulin. Between the second and third washes, Hoechst 33342 was added to the staining wash buffer at a 1:5000 dilution for nuclear visualization.
Finally, the oocytes were carefully placed in a confocal dish (NEST, 801001) and embedded using Aqua Poly/Mount (Polysciences, 18606-5). Imaging was performed utilizing a Zeiss LSM 900 confocal microscope to capture the detailed internal structures of the oocytes.Oocyte immunofluorescence assay.
Western blot
To detect the expression of target proteins in oocytes, 50 MII oocytes were collected with 3 µl of PBS and pooled as a single sample. Then, 5 µl of RIPA (Beyotime, P0013B) was added to each sample and lysed on ice for 10 min. After that, 2 μL of 5X SDS-loading buffer (CWBIO, CW0027S) was added, resulting in a final volume of 10 µl per sample. The samples were then heated for 15 min at 37 °C. Oocyte protein expression levels were measured with standard western blotting. Briefly, each sample was separated by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane (Sigma, IEVH00005). The PVDF membranes were blocked with 5% skim milk (2.5 g of milk powder diluted in 50 mL of TBS with 0.1% Tween 20) for 1 h at room temperature. After blocking, the membranes were incubated with primary antibody diluted in 5% skim milk overnight at 4 °C. The membranes were then incubated with the secondary antibody diluted, followed by three washes with TBST (Tris-buffered saline with 0.1% Tween 20). Finally, the membrane signals were visualized using Thermo Scientific SuperSignal West Pico PLUS (Thermo Fisher, 4580). Images were captured using the GEL Imaging System (AI680RGB, GE). The band intensity was assessed with software (GE) and normalized to that of the reference protein.
Antibodies
For Western blot, primary antibodies against OXPHOS (a premixed cocktail of antibodies against CI-NDUFB8, CII-SDHB, CIII-UQCRC2, CIV-MTCO1, and CV-ATP5A, 1:500, ab110413), HSP60 (1:1000, ab190828), and TOMM20 (1:1000, ab186735) were purchased from Abcam. VDAC1 (1;1000; MABN504) was purchased from Merck. GAPDH (1:1000, #2118) was purchased from Cell Signaling Technology. ACTIN (1:10000, A3854) was purchased from Sigma, and SOD2 antibody (13141S) for both Western Blot (1:1000) and immunohistochemistry (1:250) was purchased from Cell Signaling Technology.
For immunohistochemistry, antibodies against dsDNA (1:1000, ab27156) and goat anti-rabbit IgG H&L Cy3 preabsorbed (1:1000, ab6939) were purchased from Abcam. Goat anti-mouse IgG (H + L) Alexa Fluor® 488 (1:1000, A11029) was purchased from Invitrogen. Anti-α-Tubulin antibody clone DM1A, Alexa Fluor® 647 conjugate (1:250, 05-829-AF647) was purchased from Merck.
MtDNA copy number analysis
To observe the amount of mtDNA in every single oocyte, quantitative real-time PCR (qPCR) was performed. First, a single oocyte was lysed for 16 hours in 5 µl of lysis buffer (50 mM Tris-HCL, with 0.5% Tween 20 and 100 µg/mL proteinase K, pH 8.5) at 55 °C, followed by heat inactivation at 95 °C for 10 min. Quantitative real-time PCR (qPCR) was performed on a Qtower384G System (Jena). Mitochondrial DNA copy number was calculated by absolute quantification using a singleplex TaqMan assay targeting the mitochondrial mt-nd5 gene (Thermo Fisher, 4331348). Standard curves using PCR-generated templates were used for absolute quantification.
In vitro fertilization and embryo culture
To evaluate the fertilization capacity of oocytes and its potential for early embryonic development, L/L, Cre and L/L female mice were superovulated with 10 IU PMSG and 10 IU hCG as described before. The COCs were transferred to Human tubal fluid (HTF, provided by the Animal Research Committee of Westlake University). For IVF, 12 ~ 14-week-old wild-type C57BL/6 male mice were euthanized by cervical dislocation, the epididymides were collected, a small transverse incision was made with scissors at the end of the epididymides, and the sperm were allowed to spill out. Then, the epididymides were obtained with forceps and placed in Krebs-Ringer’s bicarbonate solution (TYH, provided by the Animal Research Committee of Westlake University), 500 µl TYH per epididymis, for sperm capacitation (approximately 50 min). COCs were co-incubated with spermatozoa for 5 h. All the above incubations were carried out at 37 °C with 5% CO2. After 4–5 h of fertilization, the superfluous sperms were washed off from the surface of the oocytes using M16 medium (Sigma, M7292) and then transferred to fresh M16. The fertilization rate was calculated by observing the 2-cell embryos on the next day. The 2-cell embryos were transferred to potassium simplex optimization medium (KSOM medium, Merck Millipore, MR-121-D) for early embryo culture, and the blastocyst rate was calculated at E3.5 (while the 2-cell stage was considered at E1.5). It should be noted that the incubation was conducted at 37 °C under a humidified atmosphere of either 1% O2 (1% O2/ 5% CO 2/ 94% N2) or 5% O2 (5% O2/ 5% CO2/ 90% N2) after fertilization.
Transmission electron microscopy (TEM)
Fresh mouse fallopian tubes or 1/4 fresh mouse ovarian tissues were fixed in fixation fluid immediately (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylic acid, pH=7.2) overnight at 4 °C. Then, the samples were washed three times with 0.1 M PB/cacodylate and fixed with 1% osmic acid in 0.1 M PB/cacodylate for 90 min on ice. The samples were washed with 0.1 M PB/cacodylate three times again and then washed with double-distilled water three times. Next, the samples were stained with 1% uranyl acetate in double distilled water for 1 h at room temperature or overnight at 4 °C and washed with double distilled water three times on ice. After dehydration in 30%, 50%, 70% and 100% ethanol (30 min/wash), the samples were washed twice with 100% acetone at room temperature. Then, the samples were infiltrated in 2:1 mixture of acetone and Epon 812 and 1:2 mixture of acetone and Epon 812 for 1 h at room temperature. Then, the samples were infiltrated into Epon 812 overnight at room temperature. The epon was changed every 3 h for three times on the second day. Finally, the tissue was embedded in Epon fixative. Fixed tissue was cut into 70 nm sections on an ultramicrotome (UC7, Leica) on a grid and contrasted with 1.5% uranyl acetate aqueous solution for 15 min at 37 °C. Cuts were washed five times in water, incubated for 4 min in lead citrate, washed five times with distilled water and then dried on filter paper. Images were acquired with a transmission electron microscope (Talos L120C G2) and a OneView 4 K camera (Ceta) with Digital Micrograph software at 120 KV at room temperature.
The assessment of mitochondrial size and the calculation of their circularity were conducted with Fiji. Circularity was determined using the formula: four times pi (π) multiplied by the ratio of the object’s area to the square of its perimeter. This calculation facilitates the quantification of how closely the shape of mitochondria approximates a perfect circle.
Succinate dehydrogenase (SDH) activity assay
Mouse ovaries were quickly frozen in liquid nitrogen and stored at −80 °C until use. Frozen tissues were cut into 7 μm slides with CM1950 Cryostat (Leica) and mounted onto adhesion microscope slides. Sections were preincubated in 0.1 M PBS (mixing 0.04 M sodium dihydrogen orthophosphate monohydrate with 0.06 M disodium hydrogen phosphate anhydrous, pH 7.0) for 10 min at room temperature. Then, the PBS was removed, and the sections were covered with 1 mL of NBTx reaction buffer [0.13 M sodium succinate (Sigma, S2378), 0.2 mM phenazine methosulfate (Sigma, P9625), 2 mM nitrotetrazolium blue chloride (NBT, Sigma, N6876) and 1 mM sodium azide] for 30 min at 20 °C, then washed with 0.1 M PBS for three times, and dehydrated through increasing concentrations of ethanol (5 min in 50%, 75%, 96%, and 100%, followed by 10 min in the final 100% ethanol). Finally, sections proceeded with 100% xylenes (5 min) two times before applying a few drops of a mounting medium on the tissue section and covering it with a coverslip.
Oocyte RNA sequencing analysis
After superovulation, three oocytes were collected from each female mouse (four females from the L/L group and four females from L/L, Cre group, all at 6 months of age). The oocytes collected from the same female were merged and treated as an individual sample to minimize individual oocyte variations. Subsequently, the merged oocytes were placed in 5 µl of cell lysis buffer for immediate transfer. Smart-seq2 was employed to analyze the oocytes, following the method described previously69. In brief, after the completion of the reverse transcription reactions, the amplified cDNA products of each sample were quantified using the Agilent Bioanalyzer 2100 (MGI). Subsequently, the products were purified using the MGIEASY DNA Purification Magnetic Bead Kit (MGI) to facilitate library construction. The libraries were prepared using the TruePrep DNA Library Prep Kit (VAZYME). Finally, all the samples were sequenced on the MGISEQ-2000 (MGI) sequencer, with 100-bp paired-end reads.
The quality control of raw data was applied by FASTQC70. Then, Kallisto71 was used to pseudoalign and quantify reads at the transcript level. Differential expression analysis was performed with the sleuth R package72, comparing isoform expression levels between the control and knockout groups at 6 months. The isoforms with p-value ≤ 0.05 and |FC | > 1 was defined as differentially expressed. The volcano plot was generated with the ScatterD3 package73 and the heatmap was generated with the ComplexHeatmap package74,75. Finally, the isoforms were annotated by the biomaRt package76,77 and enriched using the clusterProfiler package78.
MtDNA mutation load analysis
Sample and library preparation
To obtain sufficient DNA samples for downstream sequencing analysis, single oocyte lysate was prepared as previously described to release DNA, and then selective amplification of mitochondrial DNA from total DNA samples was performed using the REPLI-g Mitochondrial DNA Kit (QIAGEN, 151023) according to the manufacturer’s instructions. This amplification method relied on the Phi29 DNA polymerase, which has an error rate of 1 × 10−6–10−7 nucleotides both in its intrinsic enzymatic activity and during the amplification reaction. After the REPLI-g reaction, the REPLI-g amplified DNA was equilibrated to room temperature, and the purification of amplified DNA was performed immediately. In brief, 150 µl ethanol (96–100%) was added. Mix carefully by flicking the tube, centrifuge at maximum speed for 1–2 min and discard the supernatant. Then, the pellet was washed twice with 70% ethanol. Finally, REPLI-g amplified DNA was dissolved in TE buffer (pH 8.0) for downstream DNA sequencing library preparation. The amount of purified mtDNA of each sample was quantified by Qubit™ dsDNA HS Assay Kits (Thermo Fisher, Q32851) according to the manufacturer’s instructions. See Table S1 for detailed sample information.
Reads pre-processing and quality control
Raw reads were preprocessed and released by Novogene Co., Ltd. First, the clean reads were evaluated by FastQC v0.11.970. To improve the base precision and reduce artifactual deletions, the overlapped ends of paired reads were interleaved by Seqtk v1.3-r117 (https://github.com/lh3/seqtk) and merged by pre-meta (https://github.com/lh3/pre-pe). Afterward, processed reads from each sample were mapped to the mitochondrial mm10 genome using BWA v0.7.17-r118879. Subsequently, the derived sequence alignment map (SAM) files were transformed into the binary version (BAM) and sorted by SAMtools v1.1380. Duplicates were marked by MarkDuplicates in Picard v2.25.1 (http://broadinstitute.github.io/picard/). Insert size distributions were investigated by CollectInsertSizeMetrics in Picard v2.25.1 (http://broadinstitute.github.io/picard/). Statistics from BAM files were collected by SAMtools v1.1379. The above quality control across different samples was summarized and visualized using MultiQC v1.1281, based on which certain samples failed quality control and were omitted in the following analysis.
Reads mapping and mpileup
The expected age-related accumulation mutation rate per mitochondrion would be approximately 10−6~10−7/bp, as previously reported44, and a major proportion of neutral spontaneous mutations arising during this process would have a very low allele frequency (AF). To distinguish the very low-AF de novo mutations from artifacts (e.g., artifacts due to read-mapping issues or sequencing errors), we hypothesized that it is highly unlikely to observe multiple de novo mutations at the same position; as a result, the read abundance of different alleles could be used to estimate the position-specific signal/noise threshold. For this purpose, base mpileup information at each position was extracted using SAMtools v1.1380 (samtools mpileup -s -B -Q 0 -q 0 -d 100000), and low-quality bases (baseQ<20) and low-quality reads (mapQ<20) were further filtered. Meanwhile, repeat masked regions in the reference genome were ruled out in the following analysis.
Filtration of candidate variants
Based on all the base information at each position in each oocyte, read counts of the top two non-reference alleles (alternative 1 and alternative 2) were compared using Fisher’s exact test. The AF threshold of high-confidence alleles was set to ≥ 0.1%, and the p-value threshold was set to 10−15 to filter out most of the technical artifacts, with the passed oocytes genotyped as 1 and the failed ones genotyped as 0 (in other words, the alternative allele supported by the highest allele read count and the alternative allele supported by the second highest allele read count could not be well distinguished). Afterward, sites with variants occurring in only one mouse and in at least two mice were considered as candidate de novo mutations and shared variations, respectively. To further filter out potential artifacts, we extracted mutant AFs across all samples and compared the VAF of mutant samples (i.e., genotyped as 1) and un-mutant samples (i.e., genotyped as 0) using Student’s t-test with p values adjusted using the Bonferroni correction. Finally, only candidate variants with an adjusted p-value < 0.001 were retained. Additionally, de novo mutations in mito-mRNA regions were annotated using the UCSC mm10 ncbiRefSeq gene model (https://hgdownload.soe.ucsc.edu/goldenPath/mm10/bigZips/genes/mm10.ncbiRefSeq.gtf.gz), and mutational signatures were deconstructed using deconstructSigs82 according to COSMIC11 v3.3.
Statistics and reproducibility
Statistical parameters and significance are indicated in the text or figure legends. Statistical analyses of differences between two groups were conducted using Student’s t-test or Fisher’s exact test, and comparisons among several groups were performed using one-way ANOVA or the Kruskal‒Wallis test with GraphPad Prism 9 software (GraphPad Software). The number of experimental and technical replicates are specified in the figures or figure legends. Data are presented as the mean ± SEM. Significance is indicated by asterisks: ns, no significance: p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All source data are supplied with this paper. RNA-seq data were deposited in the GEO database under accession GSE251734. All DNA-seq data were deposited in NCBI databases under accession PRJNA1057590. The uncropped and unedited gel images supporting Figs. 1b, 4c, Figs. S3a and S6i are available in Supplementary Fig. S8–S12. Numerical source data for all graphs is provided in Supplementary Data.
References
Wai, T., Teoli, D. & Shoubridge, E. A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet 40, 1484–1488 (2008).
Cree, L. M. et al. A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat. Genet 40, 249–254 (2008).
Wai, T. et al. The Role of Mitochondrial DNA Copy Number in Mammalian Fertility. Biol. Reprod. 83, 52–62 (2010).
Larsson, N.-G. et al. Mitochondrial transcription factor A is necessary for mtDNA maintance and embryogenesis in mice. Nat. Genet 18, 231–236 (1998).
Broekmans, F. J., Soules, M. R. & Fauser, B. C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 30, 465–493 (2009).
Kushnir, V. A. et al. Reproductive aging is associated with decreased mitochondrial abundance and altered structure in murine oocytes. J. Assist Reprod. Gen. 29, 637–642 (2012).
May-Panloup, P. et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update 22, 725–743 (2016).
Babayev, E. et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas 93, 121–130 (2016).
Mihalas, B. P., Redgrove, K. A., McLaughlin, E. A. & Nixon, B. Molecular Mechanisms Responsible for Increased Vulnerability of the Ageing Oocyte to Oxidative Damage. Oxid Med Cell Longev 2017, 1–22 (2017).
Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 11, 298–300 (1956).
Hekimi, S., Lapointe, J. & Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 21, 569–576 (2011).
Murphy, M. P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Brand, M. D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Bio Med 100, 14–31 (2016).
Lebovitz, R. M. et al. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl Acad. Sci. 93, 9782–9787 (1996).
Reaume, A. G. et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat. Genet. 13, 43–47 (1996).
Wang, Y., Branicky, R., Noë, A. & Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217, 1915–1928 (2018).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 (1979).
Sies, H. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 23, 499–515 (2022).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Bio 21, 363–383 (2020).
Lakshmanan, L. N. et al. Clonal expansion of mitochondrial DNA deletions is a private mechanism of aging in long‐lived animals. Aging Cell 17, e12814 (2018).
Kennedy, S. R., Salk, J. J., Schmitt, M. W. & Loeb, L. A. Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage. PLoS Genet. 9, e1003794 (2013).
Greaves, L. C. et al. Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing. Plos Genet 10, e1004620 (2014).
Ziada, A. S. et al. Mitochondrial DNA somatic mutation burden and heteroplasmy are associated with chronological age, smoking, and HIV infection. Aging Cell 18, e13018 (2019).
Itsara, L. S. et al. Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations. Plos Genet 10, e1003974 (2014).
Kauppila, J. H. K. et al. Base-excision repair deficiency alone or combined with increased oxidative stress does not increase mtDNA point mutations in mice. Nucleic Acids Res 46, gky456 (2018).
Matzuk, M. M., Dionne, L., Guo, Q., Kumar, T. R. & Lebovitz, R. M. Ovarian Function in Superoxide Dismutase 1 and 2 Knockout Mice. Endocrinology 139, 4008–4011 (1998).
Rodríguez-Nuevo, A. et al. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 607, 1–6 (2022).
Cao, L. et al. The mitochondrial bottleneck occurs without reduction of mtDNA content in female mouse germ cells. Nat. Genet. 39, 386–390 (2007).
Adhikari, D. & Liu, K. Molecular Mechanisms Underlying the Activation of Mammalian Primordial Follicles. Endocr. Rev. 30, 438–464 (2009).
Ruhanen, H. et al. Mitochondrial single-stranded DNA binding protein is required for maintenance of mitochondrial DNA and 7S DNA but is not required for mitochondrial nucleoid organisation. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1803, 931–939 (2010).
Jiang, M. et al. The mitochondrial single-stranded DNA binding protein is essential for initiation of mtDNA replication. Sci. Adv. 7, eabf8631 (2021).
Strassburger, M. et al. Heterozygous deficiency of manganese superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium in vivo. Free Radic. Biol. Med. 38, 1458–1470 (2005).
Lan, Z.-J., Xu, X. & Cooney, A. J. Differential Oocyte-Specific Expression of Cre Recombinase Activity in GDF-9-iCre, Zp3cre, and Msx2Cre Transgenic Mice. Biol. Reprod. 71, 1469–1474 (2004).
Fischer, B. & Bavister, B. D. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Reproduction 99, 673–679 (1993).
Lister, L. M. et al. Age-Related Meiotic Segregation Errors in Mammalian Oocytes Are Preceded by Depletion of Cohesin and Sgo2. Curr. Biol. 20, 1511–1521 (2010).
Chiang, T., Duncan, F. E., Schindler, K., Schultz, R. M. & Lampson, M. A. Evidence that Weakened Centromere Cohesion Is a Leading Cause of Age-Related Aneuploidy in Oocytes. Curr. Biol. 20, 1522–1528 (2010).
Tatone, C. et al. Evidence that carbonyl stress by methylglyoxal exposure induces DNA damage and spindle aberrations, affects mitochondrial integrity in mammalian oocytes and contributes to oocyte ageing. Hum. Reprod. 26, 1843–1859 (2011).
Charalambous, C., Webster, A. & Schuh, M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat. Rev. Mol. Cell Bio. 1–18 (2022)
Vivarelli, E., Siracusa, G. & Mangia, F. A histochemical study of succinate dehydrogenase in mouse oocytes and early embryos. Reproduction 47, 149–NP (1976).
McGee, E. A. & Hsueh, A. J. W. Initial and Cyclic Recruitment of Ovarian Follicles. Endocr. Rev. 21, 200–214 (2000).
Sanchez-Contreras, M. et al. A replication-linked mutational gradient drives somatic mutation accumulation and influences germline polymorphisms and genome composition in mitochondrial DNA. Nucleic Acids Res. 49, 11103–11118 (2021).
Árnadóttir, E. R. et al. The rate and nature of mitochondrial DNA mutations in human pedigrees. Cell 187, 3904–3918.e8 (2024).
Weng, C. et al. Deciphering cell states and genealogies of human haematopoiesis. Nature 627, 389–398 (2024).
Arbeithuber, B. et al. Age-related accumulation of de novo mitochondrial mutations in mammalian oocytes and somatic tissues. Plos Biol. 18, e3000745 (2020).
Arbeithuber, B. et al. Advanced age increases frequencies of de novo mitochondrial mutations in macaque oocytes and somatic tissues. Proc. Natl Acad. Sci. 119, e2118740119 (2022).
Hamatani, T. et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 13, 2263–2278 (2004).
Ben-Meir, A. et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 14, 887–895 (2015).
Covarrubias, A. J., Perrone, R., Grozio, A. & Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 22, 119–141 (2021).
Gomes, A. P. et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 155, 1624–1638 (2013).
Mills, K. F. et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 24, 795–806 (2016).
Bertoldo, M. J. et al. NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 30, 1670–1681.e7 (2020).
Miao, Y., Cui, Z., Gao, Q., Rui, R. & Xiong, B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 32, 107987 (2020).
Wang, L. et al. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 236, 7966–7983 (2021).
Nagaoka, S. I., Hassold, T. J. & Hunt, P. A. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13, 493–504 (2012).
Perkins, A. T., Das, T. M., Panzera, L. C. & Bickel, S. E. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc. Natl. Acad. Sci. 113, E6823–E6830 (2016).
Boucret, L. et al. Deep sequencing shows that oocytes are not prone to accumulate mtDNA heteroplasmic mutations during ovarian ageing. Hum. Reprod. 32, 2101–2109 (2017).
Soest, D. M. Kvan et al. Mitochondrial H2O2 release does not directly cause damage to chromosomal DNA. Nat. Commun. 15, 2725 (2024).
Hori, A., Yoshida, M., Shibata, T. & Ling, F. Reactive oxygen species regulate DNA copy number in isolated yeast mitochondria by triggering recombination-mediated replication. Nucleic Acids Res. 37, 749–761 (2009).
Peng, K. et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxid. Med. Cell. Longev. 2016, 6705621 (2016).
Peng, K. et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1α Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol. Neurobiol. 54, 3783–3797 (2017).
Gureev, A. P., Shaforostova, E. A. & Popov, V. N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 10, 435 (2019).
Bonekamp, N. A. et al. High levels of TFAM repress mammalian mitochondrial DNA transcription in vivo. Life Sci. Alliance 4, e202101034 (2021).
Pasquariello, R. et al. Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humans†. Biol. Reprod. 100, 971–981 (2019).
Emidio, G. D. et al. The Natural Carotenoid Crocetin and the Synthetic Tellurium Compound AS101 Protect the Ovary against Cyclophosphamide by Modulating SIRT1 and Mitochondrial Markers. Oxid. Med. Cell. Longev. 2017, 8928604 (2017).
Bar-Joseph, H. et al. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod. Toxicol. 30, 566–572 (2010).
Zhang, T., He, W. H., Feng, L. L. & Huang, H. G. Effect of doxorubicin-induced ovarian toxicity on mouse ovarian granulosa cells. Regul. Toxicol. Pharmacol. 86, 1–10 (2017).
DAN, Y., NAI-KEI, W. & JUN, H. Bistrifilate-based fluorogenic probes for detection of superoxide anion radical. US010877051B2.
Hu, J. J. et al. Fluorescent Probe HKSOX-1 for Imaging and Detection of Endogenous Superoxide in Live Cells and In Vivo. J. Am. Chem. Soc. 137, 6837–6843 (2015).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
S, A. FastQC: a quality control tool for high throughput sequence data. http://www.BioinformaticsBabrahamAcUk/Projects/ Fastqc/2010; http://www.bioinformatics.babraham.ac.uk/projects/.
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Pimentel, H., Bray, N. L., Puente, S., Melsted, P. & Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14, 687–690 (2017).
J, Barnier, et al. scatterD3: D3, JavaScript Scatterplot from R. R package version 1.0.1. https://CRAN.R-project.org/package=scatterD (2021).
Gu, Z. Complex heatmap visualization. iMeta 1, e43 (2022).
Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 32, 2847–2849 (2016).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Durinck, S. et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440 (2005).
Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innov 2, 100141 (2021).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Rosenthal, R., McGranahan, N., Herrero, J., Taylor, B. S. & Swanton, C. deconstructSigs: delineating mutational processes in single tumors distinguishes DNA repair deficiencies and patterns of carcinoma evolution. Genome Biol. 17, 31 (2016).
Acknowledgements
We express our gratitude to Prof. Nils-Göran Larsson from Karolinska Institutet for kindly sharing the mtSSB loxp mouse, and to Prof. Ran Huo from Nanjing Medical University for providing the Gdf9 Cre mouse as a gift. We thank Prof. Dan Yang (Westlake University) for generously sharing the sensitive probe used for superoxide detection. We also extend our thanks to the Westlake Laboratory Animal Resources Center for their efforts in mouse husbandry, as well as to the Westlake Biomedical Research Core Facilities (BRCF) for their invaluable technical support. This project was supported by the Key R&D Program of Zhejiang (2024SSYS0033 and 2024SSYS0034), Zhejiang Provincial Key Laboratory Construction Project, Westlake Education Foundation, the German Research Foundation within the Collaborative Research Center CRC1506 (Project-ID 450627322) “Aging at Interfaces” and the Graduate Training Group GRK 1789 “Cellular and Molecular Mechanisms in Ageing (CEMMA)”.
Author information
Authors and Affiliations
Contributions
M.J. and Y.D. directed and supervised the project. S.L. conducted the majority of the animal-related work and molecular characterization. Y.Z. and X.D. performed all bioinformatics analyses on high-throughput sequencing data. J.G. provided guidance for experiments related to oocytes. K.S.K. and Z.X. supported the animal-related work. The manuscript was written by M.J., Y.D., S.L., and Y.Z.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Andrew Pomiankowski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Christopher Hine and Joao Valente.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Long, S., Zheng, Y., Deng, X. et al. Maintaining mitochondrial DNA copy number mitigates ROS-induced oocyte decline and female reproductive aging. Commun Biol 7, 1229 (2024). https://doi.org/10.1038/s42003-024-06888-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-06888-x
This article is cited by
-
Targeting MFF succinylation: a novel therapeutic strategy for premature ovarian insufficiency by restoring mitochondrial dynamics in granulosa cells
Journal of Ovarian Research (2026)
-
The impact of mitochondrial dysfunction on ovarian aging
Journal of Translational Medicine (2025)
-
Oxidoreductase delivery by cell therapies in cyclophosphamide-induced premature ovarian insufficiency: a mechanistic insight into ovarian rejuvenation
Journal of Ovarian Research (2025)
-
Mitochondrial dysfunction in oocytes: implications for fertility and ageing
Journal of Ovarian Research (2025)
-
Mitochondria-driven inflammation: a new frontier in ovarian ageing
Journal of Translational Medicine (2025)