Abstract
The tumor microenvironment (TME) and the cellular interactions within it can be critical to tumor progression and treatment response. Although technologies to generate multiplex images of the TME are advancing, the many ways in which TME imaging data can be mined to elucidate cellular interactions are only beginning to be realized. Here, we present a novel approach for multipronged computational immune synapse analysis (CISA) that reveals T-cell synaptic interactions from multiplex images. CISA enables automated discovery and quantification of immune synapse interactions based on the localization of proteins on cell membranes. We first demonstrate the ability of CISA to detect T-cell:APC (antigen presenting cell) synaptic interactions in two independent human melanoma imaging mass cytometry (IMC) tissue microarray datasets. We then verify CISA’s applicability across data modalities with melanoma histocytometry whole slide images, revealing that T-cell:macrophage synapse formation correlates with T-cell proliferation. We next show the generality of CISA by extending it to breast cancer IMC images, finding that CISA quantifications of T-cell:B-cell synapses are predictive of improved patient survival. Our work demonstrates the biological and clinical significance of spatially resolving cell-cell synaptic interactions in the TME and provides a robust method to do so across imaging modalities and cancer types.
Similar content being viewed by others
Introduction
The immune system is a major component of the tumor microenvironment (TME) and plays a central role in the modern approach to oncology. The TME has been the target of recent therapeutics that increase the activity of the immune system1, improving survival for patients with tumors including metastatic melanoma2. Pre-existing anti-tumor immunity has long been a prognostic factor in patient survival3 and has been associated with response to immune checkpoint blockade4. Conversely, absent or dysfunctional anti-tumor immunity generally leads to poor survival and response to treatment5,6. Better parsing of the TME factors and mechanisms mediating anti-tumor immunity is therefore critical to the improvement of immunotherapy.
Cellular interactions within the TME modulate the immune response, often suppressing anti-tumor immunity and worsening patient survival7. T-cells are of particular interest owing to their ability to eliminate cancer cells8 and as key mediators in the immune checkpoint blockade pathway9,10. Immunosuppressive interactions targeting T-cells can prevent their infiltration into the TME, dampen anti-tumor functions, and lead to worse outcomes5, and tumor-resident cells such as dendritic cells may also play a role in T-cell-mediated anti-tumor immunity11. Further study to understand T-cell-TME interactions and their clinical implications is an important need.
A central aspect of T-cell interaction and function is the immune synapse (IS). The IS forms following recognition of antigens on antigen-presenting cells (APCs), triggering a molecular cascade where the T-cell receptor (TCR) and other signaling molecules aggregate at the point of contact with the APC12. This process is initially required for T-cell activation by professional APCs where T-cells are primed against non-self antigen13. Once primed, IS formation is necessary for and precedes acquired immunity functions such as delivering a cytotoxic payload to target (e.g., cancer) cells or triggering the release of cytokines14,15. Thus, IS formation is indicative of T-cell antigen recognition and induction of its functional capabilities. Recent studies have described important new T-cell behaviors by curating examples from image data, e.g., by using lattice light sheet microscopy to show macrophages with exhausted CD8 T-cells16 or by using 3D CyCIF to show CD8 T-cells with melanoma cells17. However, such approaches are limited by manual selection, and automated quantification would be beneficial.
Advances in tumor imaging provide new opportunities to understand immune synapses, but new analysis methods are needed. Proteomic imaging platforms can simultaneously measure dozens of epitopes at subcellular resolution within tumor samples18, and a number of groups have harnessed multiplex imaging methods to associate spatial distribution patterns of immune cells with patient survival or therapy response19,20,21,22,23,24,25, typically realized through analysis of cell-cell colocalization or cellular feature intensities. However, approaches based on subcellular information, such as the formation of immune synapses, remain little explored. Previously, a workflow has been proposed to analyze synaptic behaviors in blood samples using Imaging Flow Cytometry26, but it lacks the context of cell-cell spatial localization and cannot be applied to any solid tumor. New methods to automatically evaluate IS formation thus could be valuable to improve T-cell mechanistic quantification and discovery of TME-related biomarkers.
In this work we introduce a computational framework to investigate immune synapses in the TME, which we refer to as Computational Immune Synapse Analysis (CISA). We first assess its performance in capturing relevant T-cell IS biology and then demonstrate its effectiveness in identifying functionally relevant cell interactions from high-resolution multiplex TME imaging data across multiple imaging modalities and datasets. Our findings demonstrate the capability and value of interrogating computationally-defined immune synapse formation from imaging data to evaluate biologically and translationally relevant interactions in the in situ TME.
Results
Image-based computational immune synapse analysis
We hypothesized that immune synapses between cells can be detected based on the preferential localization of proteins at interfaces where cells contact one another. While this concept is intuitive, it has been unknown whether current spatial proteomic profiling technologies, such as imaging mass cytometry, have sufficient resolution and precision to reveal such synapses. It also remains poorly understood what fraction of cell-cell contacts will have active synapses in a given tissue sample, as this quantity will depend on the cell types of interest, cell densities, the strength and persistence of synapses, and the tissue type. In addition, synapse quantification is affected by the accuracy of cell segmentation algorithms in identifying cell-cell interfaces in 2D spatial proteomic images. Given these uncertainties, a statistical approach that not only evaluates individual cell-cell contacts, but also integrates across an image, would be valuable to quantifying synaptic activity.
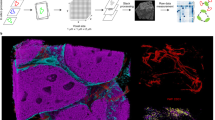
To interrogate cell-cell interactions in the in situ immunological context, we defined an image-based T-cell immune synapse metric. T-cells are known to interact with APCs, which motivated us to focus on the behavior of TCR proteins at interfaces between T-cells and APCs; however, the approach we describe here is generalizable to any cell types and synapse markers. CISA defines the strength of an immune synapse by calculating the degree of TCR localization towards the cell-cell interface: A T-cell’s synapse strength σ is defined as the logarithm of the mean CD3 signal intensity (a proxy for TCR signal) at the membrane region in contact with an APC divided by the mean signal in the noncontact region (Fig. 1a, Methods). A positive synapse strength indicates T-cell synapse formation and potential functional interaction with another cell in the TME, modeling how the TCR aggregates at the synapse with an APC upon recognition of the presented antigen12. Because individual contacts are affected by high variability in measurement noise, we more generally consider phenotypic evaluations at the whole image level based on average synapse strength, which reduces the effect of measurement noise. The average synapse strength \(\bar{\sigma }\) can be considered for each tissue sample, by averaging the values of σ for all contacts between T-cells and APCs (or specified cell type) in the sample (Fig. 1b, c). We also implemented a null synapse model based on random sets of contiguous T-cell membrane pixels to account for baseline CD3 aggregation (see Methods). This CISA provides a way to investigate the functional importance of cell-cell contacts from images. CISA requires single channel protein images, cell segmentation masks, and cell classification as input, all of which are fundamental and essential in this image-based analysis workflow (Fig. 1c), as well as common in typical image analysis workflows.
a Individual synapse strength \(\sigma\) is the logarithm of the mean membrane signal intensity of CD3 at the T-cell-APC contact interface relative to the intensity outside the contact interface. b Image level synapse strength \(\bar{{\rm{\sigma }}}\) is the average over all neighboring cell-cell pairs in the image. Statistically, \(\bar{{\rm{\sigma }}}\) < 0 indicates a lack of interaction; \(\bar{{\rm{\sigma }}}\) > 0 indicates an active interaction. Showing an example diagram of \(\bar{{\rm{\sigma }}}\) > 0. c The general niche of CISA in an image-based analysis workflow. CISA takes three required inputs, namely single channel images, a raster cell segmentation mask image, and the cell classification. CISA outputs a table of \({\rm{\sigma }}\) at each T-neighbor interface, which can be then aggregated for sample level \(\bar{{\rm{\sigma }}}\) for statistical characterization.
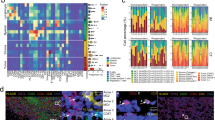
To test whether CISA is capable of quantifying immune synapses in high-resolution multiplexed images, we first applied it to a published melanoma IMC dataset from ref. 24 This dataset contains 30 pre-treatment patient tissue microarray samples from melanoma patients that received immune checkpoint inhibition therapy. We segmented cells in each image and mapped to the cell annotations from ref. 24. (see Methods). We then used CISA to compute σCD3(T-CD8 + , APC) for each T-cell:APC contact, i.e., the relative localization of CD3 to the contact region between a CD8 + T-cell and an adjacent APC. We also generalized CISA to compute σCD8(T-CD8 + , APC), the localization of CD8 toward the adjacent APC. We observed strong correlation (Fig. 2a) in the directional enrichment of CD3 and CD8 toward adjacent APCs, with r = 0.69 between σCD3(T-CD8 + , APC) and σCD8(T-CD8 + , APC), as would be expected from active T-cell: APC interactions. CD8 + T-cells can have a minor CD4 signal not expected to co-localize with CD3 in CD8+ cells, providing a control. Consistent with these expectations, the correlation of σCD3 and σCD4 (r = 0.17) in CD8+ cells is less than that between σCD3 and σCD8 (Fig. 2a). Likewise, for CD4 + T-cells, we observed a strong correlation of CD3 and CD4 toward adjacent APCs (σCD3(T-CD4 + , APC) and σCD4 (T-CD4 + , APC), r = 0.56), consistent with detection of TCR-mediated synapses (Fig. 2b). As expected, the correlation of σCD3(T-CD4 + , APC) and σCD8(T-CD4 + , APC) is lower (r = 0.26) than the CD3:CD4 correlation (Fig. 2b).
a Density plots showing correlation of σ(CD4) with σ(CD3) in CD8+ cells, and correlation of σ(CD8a) with σ(CD3) in CD8+ cells; (b) correlation of σ(CD4) with σ(CD3) in CD4+ cells, and correlation of σ(CD8a) with σ(CD3) in CD4+ cells. c Comparison of CD8/CD3 correlation in CD8+ cells at the pixel level to CISA. d Comparison of CD4/CD3 correlation in CD4+ cells to CISA. e–h Plots are analogous to (a–d) but for melanoma IMC data from (Hoch et al. 2022). Examples of positive (i) and negative (j) localization of CD3 and CD8a around the central T cell (dashed cell boundary) toward neighboring macrophages (solid white cell boundary). The “Combined” column shows the four channels listed as separate columns with corresponding colors, plus blue showing DNA. In the “Cell type” column, green marks macrophages and red marks T cells. In the CD3 and CD8a columns, numbers indicate synapse strengths of the shown marker between the central T cell and the indicated macrophage. Gray outlines: non-contacting or non-APC cells. Scale bar: 10 µm. k Correlations of σ(CD4), σ(CD8a) σ(CD7) and σ(ICOS) with σ(CD3) in CD4 + T cells. Each point is one TMA core. l Correlations of σ(CD4), σ(CD8a) σ(CD7) and σ(ICOS) with σ(CD3) in CD8 + T cells. m Average pixel intensity of markers within CD8 + T cells. ICOS (CD278), is significantly lower than CD4. Each point is the average in a TMA core. a–d, i, j are based on melanoma IMC spots from the (Moldoveanu et al. 2022)24 IMC dataset. e–h, k–m data are from the (Hoch et al. 2022)23 IMC data. Tc: CD8 + T cells; Th: CD4 + T cells. σ: interface-level synapse strength.
While our results show that colocalization of TCR proteins toward APCs can be detected by CISA, some protein colocalization might occur regardless of an APC contact. To evaluate the contact-independent effect, we calculated pixel-wise correlations between CD3 and CD4 (or CD8) intensity within the complete T-cell areas in each spot. CISA scores exhibited stronger correlations than the contact-naïve pixel-wise methods (Fig. 2c, d). This was observed for correlations of CD3 and CD8 in CD8 + T cells (t-test, p = 5.8e-9), as well as for correlations of CD3 and CD4 in CD4 + T cells (t-test, p = 3.9e-7). These results indicate that CISA captures information on contact-dependent synapse activity.
To verify the robustness of these results, we analyzed a second IMC dataset from ref. 23. It consists of 167 tissue microarray (TMA) spot images from melanoma patients. This dataset showed CISA behaviors consistent with the Moldoveanu et al. dataset24. CD3 and CD8 CISA correlations in CD8 + T cells were strong (r = 0.57, Fig. 2e) and higher than CD3-CD4 CISA correlations in the same cells (r = 0.09). CD3 and CD4 CISA correlations in CD4 + T cells were also strong (r = 0.43, Fig. 2f), and higher than CD3-CD8 CISA correlations in those cells (r = 0.14). As in the Moldoveanu et al. dataset, CISA correlations were stronger than pixel-wise correlations (Fig. 2g, h), both for CD3:CD8 in Tc cells (p = 1.8e-12) and for CD3:CD4 in Th cells (p = 3.8e-12). We also analyzed the correlations of T-cell:APC CISA scores for all pairs of proteins in the IMC data (Fig. S1). These showed a rich clustering structure reflecting behaviors such as TCR co-occurrence, T-cell specificity, and APC-specificity.
To clarify the observed behaviors, example images of T-cell:APC contacts with positive or negative CISA scores are shown in Fig. 2i, j. Figure 2i shows a CD8 + T cell that has positive σCD3 and σCD8a with its neighboring macrophages, suggesting potential synaptic interactions between them; Fig. 2j, on the other hand, depicts a CD8 + T cell that has negative σCD3 and σCD8a with the neighboring macrophages, suggesting lack of synaptic interactions between them. These images show the variability in signal along membrane boundaries, supporting the importance of statistical approaches that integrate information along over entire cells and tissue images to provide robustness. For example, in addition to CD3, CD4 and CD8a, we were also able to evaluate correlations in CISA scores between CD3 and other T cell surface proteins, including ICOS (CD278) and CD7 (Fig. 2k, l). It is worth noting that ICOS has a significantly (p = 3e-9) lower expression level than CD4 in CD8 + T cells (Fig. 2m), but the correlation between σCD3(T-CD8 + , APC) and σCD278(T-CD8 + , APC) (Fig. 2l) is stronger than between CD3 and CD4 in those cells. CISA’s ability to detect colocalization of CD3 and CD278 despite the low expression level of the latter suggests CISA can detect polarization of cell membrane proteins even at a low expression level. Together, these results support CISA as a robust method to quantify immune synapses between T cells and neighboring APCs.
Whole-slide images enable regional analysis of tumor microenvironmental interactions
Having demonstrated that CISA is able to quantify immune synapses between T cells and APCs in IMC data, we were interested in whether it would be applicable to other types of imaging data. Different types of imaging techniques have bespoke sample acquisition protocols that affect the scale of regions assayed and the cell-cell contacts within them, which could impact CISA characterization statistics. To further verify the applicability of CISA to other types of image data, we generated and analyzed histocytometry27 whole slide images (WSI) of metastatic melanoma28. These WSIs are much larger than TMA spots and are based on fluorescence, providing substantial differences from IMC. We analyzed a cohort of 21 human metastatic melanoma samples from 20 patients (see Methods). A 6-marker panel, consisting of nuclei, melanoma antigens, Ki67, CD45, CD3, CD14, and CD19/138, was employed to characterize cell phenotypes across whole-slide sections, providing extensive data for identification of TME features and interactions. Because CD14 is a broad marker that can be expressed by a range of cells including macrophages, monocytes, and monocytic-DCs, the individual CD14+ cells from the 9 of the patients were harvested with laser capture microdissection for in situ transcriptomic profiling. The RNA data showed expression of CD14, CD68, CD163, and CD206, confirming a typical monocyte/macrophage phenotype for the CD14 protein positive cells (see also Martinek et al.28). The images in our cohort averaged 67 mm2 of tissue imaged at a resolution of 663 nm per pixel.
We first analyzed regional cell type prevalence and spatial co-occurrence in these datasets, as cellular composition29 and APC expression28 could vary between the tumor stroma and tumor nests. The large size of WSI images makes them better than TMA spots for investigating potential region-specific T-cell interactions. After segmenting images into intratumor and stromal regions (see Methods, Fig. 3a, b), we observed that T-cells and CD14+ cells are both abundant in the stroma, but CD14+ cells make up the bulk of the intratumor immune infiltrate. Within the tumor, the cohort median density of T-cells is 86.3 cells per mm2, while the density of CD14+ cells is 252.6 cells per mm2 (CD14+ cell > T-cell: p = 2.4 × 10−4, Fig. 3c). CD14+ cells also exhibit differential melanoma antigen loading between the intratumoral and stromal regions, where loading is defined by the presence of melanoma antigen in the cytoplasm (Fig. 3d, see Methods). In the intratumoral region, the majority of CD14+ cells are loaded with unprocessed melanoma antigen (Fig. 3e, cohort median = 72.3%). In the stroma, a significantly lower fraction of CD14+ cells is loaded (cohort median = 7.7%, p = 9.5 × 10−7).
a An example whole-slide histocytometry image with select markers shown. White borders denote tumor-stroma interface. Yellow box denotes the zoomed in region for (b). b Zoomed in view of tumor/stroma segmentation. c Comparison of immune infiltrate in tumor and stroma. Within the tumor, CD14+ cells are denser than T-cells, but the difference is not statistically significant in the stroma. * denotes p < 0.05. d An example of a melanoma antigen-loaded CD14+ cell (white bound cell) within the tumor. Note green fluorescence (melanoma antigen stain) within the cytoplasm. e Melanoma antigen-loaded CD14+ cells make up the bulk of intratumor CD14+ cell infiltrate (fraction loaded, median = 72.3%), but a significantly smaller fraction in the stroma (median = 7.7%, p = 9.5 × 10–7). CD3: T-cell. CD45: Leukocytes.
T-cells colocalize with CD14+ cells in a region-dependent manner in melanoma
Because of the differential antigen-loading of CD14+ cells between the intratumoral and stromal regions, we hypothesized that cells of the TME interact in a region-specific manner. We first considered regional differences in cell-cell colocalization behavior, as these can impact CISA statistics. CISA statistics will be different in histocytometry compared to tissue microarray spots because the larger size of histocytometry images captures major variations in behavior across different types of tumor microenvironmental regions. We investigated this using a radial distribution function (RDF) analysis to identify spatial relationships between cell types in the TME (Fig. S2A, B). We applied RDF analysis to quantify the distance-dependent density of TME cells relative to T-cells (see Methods). We additionally devised a metric to quantify T-cell/TME cell colocalization relative to null expectations, ΔCDF, defined from the RDF curve as the excess of colocalization relative to a label-permuting null model—an approach that controls for variations in local cell density (Fig. S2C).
The use of RDF analysis to reveal cell-cell spatial associations is illustrated in Fig. 4 for sample Mel-512, with T-cells as the reference cell. In the intratumoral region, a peak in the RDF for loaded CD14+ cells at approximately 12 μm indicates that T-cells and loaded CD14+ cells are often in close proximity (Fig. 4a, solid cyan line). The observed distribution is above the cell-permuted null expectation (Fig. 4a, dashed cyan line) indicating excess colocalization. T-cell colocalization with loaded CD14+ cells is significant when calculated over the full histocytometry cohort (\(\overline{\Delta {\rm{CDF}}}\) = 4.184, 1-sample t-test p = 9.6 × 10−10, Fig. 4b). Unloaded CD14+ cell colocalization with intratumoral T-cells is also observed (Fig. 4a), though the effect is weaker than for loaded CD14+ cells. Such colocalization is moderately significant across the cohort (\(\overline{\Delta {\rm{CDF}}}\) = 0.908, 1-sample t-test p = 0.026, Fig. 4b). Tumor cells have high absolute densities near intratumoral T-cells, but do not significantly colocalize (\(\overline{\Delta {\rm{CDF}}}\) = −1.311, 1-sample t-test p = 1.0, Fig. 4a, b). Intratumor T-cell colocalization with loaded CD14+ cells is significantly greater than with either unloaded CD14+ cells or tumor cells (Fig. 4b, p = 1.2 × 10−4 and 5.9 × 10−5 respectively).
a RDFs for sample Mel-512 with T-cells as the reference cell demonstrate that intratumor T-cells colocalize with both loaded CD14+ cells (cyan curve) and unloaded CD14+ cells (blue) above the expected distribution (dashed lines). Conversely, intratumor T-cells contact melanoma cells below null expectations (green). b The cohort distributions of ΔCDF demonstrate significant intratumor T-cell colocalization with loaded CD14+ cells, unloaded CD14+ cells, and other T-cells but not tumor cells. ΔCDF is significantly greater for loaded CD14+ cells compared to both unloaded CD14+ cells and melanoma cells. c In the stroma, T-cells colocalize with unloaded CD14+ cells (blue, right) but no colocalization with loaded CD14+ cells. d Cohort wide ΔCDF demonstrates significant intratumor T-cell colocalization with unloaded CD14+ cells and other T-cells but not loaded CD14+ cells. ΔCDF for stromal T-cells is significantly greater for unloaded CD14+ cells compared to loaded CD14+ cells. T-cells similarly show strongly positive ΔCDF. #: 1-sample t-test p < 0.05. *Wilcoxon rank-sum test p < 0.05.
In the stromal regions, cell co-localization relationships differ from those within the tumor. For example, in the stroma of sample Mel-512, unloaded CD14+ cells have a small RDF peak with respect to T-cells at a distance of approximately 10 μm (Fig. 4c, blue). This colocalization exceeds the null expectation by a statistically significant but small margin (\(\overline{\Delta {\rm{CDF}}}\) = 2.642, 1-sample t-test p = 1.5 × 10−6, Fig. 4d). On the other hand, loaded CD14+ cells have lower colocalization with stromal T-cells than expected (Fig. 4c). Negative colocalization between these cell types is observed across the cohort (\(\overline{\Delta {\rm{CDF}}}\) = −3.567, 1-sample t-test p = 1.0, Fig. 4d). The unloaded CD14+ cell ΔCDF is significantly greater than for loaded CD14+ cells (p = 5.9 × 10−5). Meanwhile, T-cells exhibit strong colocalization to other T-cells both in the stroma and within the tumor (\(\overline{\Delta {\rm{CDF}}}\) = 4.616 and 4.713, 1-sample t-test p = 1.8 × 10−6 and 4.7 × 10−7, stroma and intratumor respectively Fig. 4b, d; S2B).
CISA reveals region-specific T-cell/CD14+ cell synapses in whole slide images
Given the distinct cell co-localization behaviors in the intratumor and stromal regions, we hypothesized that CISA analysis would detect regional differences in immune synapse formation within whole slide histocytometry images. Indeed, CISA analysis of intratumoral regions showed that intratumor T-cells tend to form synapses to loaded CD14+ cells (\(\bar{\sigma }\) = 0.098, 1-sample t-test p = 3.9 × 10−3, Fig. 5a), consistent with the IMC results. However, this relationship was not observed in the stroma, and stromal T-cells instead tend to form synapses to unloaded CD14+ cells (\(\bar{\sigma }\) = 0.059, 1-sample t-test p = 5.0 × 10−3). Both synapse strengths are significantly greater than the null model (p = 2.4 × 10−5 and 2.9 × 10−6 respectively). T-cell synapses to loaded CD14+ cells are significantly stronger within the tumor than in the stroma, while T-cell synapses to unloaded CD14+ cells are significantly stronger in the stroma than in the tumor (Fig. 5b, p = 6.9 × 10−5 for both respectively). Within the tumor, T-cell synapse strength to loaded CD14+ cells is significantly stronger than to unloaded CD14+ cells (Fig. 5b, p = 3.7 × 10−4). In the stroma, T-cells have significantly stronger synapses to unloaded CD14+ cells than to loaded CD14+ cells (p = 1.3 × 10−3). In the tumor, T-cells showed no tendency to form synapses to melanoma cells (\(\bar{\sigma }\) = −0.101, 1-sample t-test p = 0.98), with synapse strength not significantly greater than the null model (p = 0.41, Fig. 5a). The greater T-cell synapse strength to loaded CD14+ cells than to melanoma cells is also statistically significant (p = 6.9 × 10−5).
a Intratumor T-cell synapses with loaded CD14+ cells are significantly stronger than those with unloaded CD14+ cells and melanoma (p = 3.7 × 10–4 and p = 6.9 × 10–5 respectively) and are also stronger than expected from the intratumor null synapse model (denoted by *p = 2.4 × 10–5). Stromal T-cell synapses with unloaded CD14+ cells are significantly stronger than those with loaded CD14+ cells (p = 1.3 × 10–3) and the stromal null model (denoted by *p = 2.9 × 10–6). b Intratumor T-cell synapses with loaded CD14+ cells are stronger than stromal T-cell synapses to loaded CD14+ cells (p = 6.9 × 10–5). In contrast, T-cell synapses with unloaded CD14+ cells are stronger in the stroma than in the tumor (p = 1.3 × 10–3). c Super-resolution imaging of two stromal T-cells in contact with CD14+ cells in the TME. The T-cell on the right is in contact with the neighboring CD14+ cell’s dendrite, and CD3 is aggregating in the contact region. Scale bar = 4 μm. d Volumetric rendering of the right T-cell in C with ICAM-1 shown. The CD14+ cell’s ICAM-1 can be seen aggregating in the dendrite. Scale bar = 2 μm. APC antigen-presenting cell. \(\bar{{\rm{\sigma }}}\): sample-level mean synapse strength. Rendering was performed using the Imaris software.
We employed synapse-focused super-resolution imaging to further verify T-cell synapses in the TME. Figure 5c shows two T-cell-CD14+ cell contacts with different CD3 aggregation behavior via stimulated emission depletion microscopy (Fig. 5c). The left T-cell exhibits relatively homogenous CD3 along its membrane without aggregation towards its neighboring CD14+ cell. Volumetric rendering suggests a CD3-containing T-cell protrusion towards the CD14+ cell which may signify early synapse formation or antigen sampling (Fig. S3A), though there is not a reciprocal concentration of ICAM-1 in the CD14+ cell that would demonstrate mature synapse formation (Fig. S3B). The right T-cell shows CD3 concentration towards the dendrite protrusion on the target CD14+ cell (Fig. 5C, yellow box). Volumetric rendering shows not only contact between the T-cell CD3 and the CD14+ cell dendrite but also reciprocal CD14+ cell ICAM-1 in the contact area, as expected from mature synapse formation and functional interaction (Fig. 5d, Fig. S3B).
T-cell-CD14+ cell interactions are associated with T-cell proliferation in metastatic melanoma
T-cells recognizing their cognate antigen in conjunction with pro-inflammatory signals undergo clonal expansion30 typically within the lymph node, but in vivo mouse data suggest this may occur in the tumor as well11. We therefore hypothesized that proliferating T-cells in the TME might have stronger synapse strengths than other T-cells. We extended CISA to address this by classifying T-cells as proliferating (Ki-67 + ) or non-proliferating (Ki-67 − ) from KI-67 imaging data. We found that intratumor Ki-67 + T-cells have significantly stronger synapses to loaded CD14+ cells than Ki-67 − T-cells (Fig. 6a, p = 4.2 × 10−4) and significantly greater than the Ki-67 + T-cell null model (p = 9.5 × 10−7). There was no significant difference in synapse strength between Ki-67+ and Ki-67− stromal T-cells, unlike in the intratumor region (Fig. 6a). Ki-67 + T-cell synapse strength to melanoma cells is also stronger than for Ki-67 − T-cells (p = 0.021), although not significantly greater than the null model. To further address the synapse-proliferation association, we compared the fraction of T-cells that are Ki-67+ in the synapse positive and non-positive groups. For intratumoral T-cells in contact with loaded CD14+ cells, we observed a significantly greater fraction of Ki-67 positivity in synapse-positive T-cells compared to non-positive synapse T-cells (p = 2.8 × 10−4, Fig. 6b). We observed a similar association between KI-67 and T-cell/melanoma synapses, albeit with lower statistical significance (p = 0.014).
a Proliferative (Ki67+, orange boxes) T-cell synapses are significantly stronger than non-proliferative (Ki67-, green boxes) T-cell synapses, for both loaded CD14+ cells and melanoma cells (p = 4.2 × 10–4 and p = 0.021 respectively). Proliferative T-cell synapses with loaded CD14+ cells are stronger than those with unloaded CD14+ cells (#p = 6.0 × 10–5) and the null model (@, p = 9.5 × 10–7). In the stroma, there are no significant differences in synapses between proliferative and non-proliferative T-cells with either loaded or unloaded CD14+ cells. b Paired difference plot comparing the fraction of intratumor Ki67+ T-cells vs. synapse strength. The fraction of T-cells that are Ki67+ is significantly greater for T-cells with positive synapses compared to non-positive synapses, both for synapses to loaded CD14+ cells (p = 2.8 × 10–4) and to melanoma cells (p = 0.014). Black lines indicate a positive difference while red lines indicate a negative difference.\(\bar{{\rm{\sigma }}}\): sample-level mean synapse strength.
On the other hand, T-cell synapse formation with unloaded CD14+ cells was not associated with a proliferative response. We observed no significant difference in average synapse strength between Ki-67+ and Ki-67 − T-cells in contact with unloaded CD14+ cells, either within the tumor or in the stroma (Fig. 6a). Additionally, intratumoral Ki-67 + T-cells have significantly higher average synapse strengths with loaded CD14+ cells than with unloaded CD14+ cells (Fig. 6a, p = 6.0 × 10−5). In the stroma, T-cell Ki-67 status is not associated with synapse strength to an adjacent CD14+ cell, regardless of whether the CD14+ cell has an antigen load.
Synapse analysis of breast cancer imaging mass cytometry data reveal T-cell/B-cell interactions
To test the applicability of the RDF and CISA approaches to other types of tumors, we applied these methods to a cohort of breast cancer IMC images of primary tumors from 281 patients20, of which 275 had associated survival data and clinical subtyping. Because these IMC images were derived from tissue microarrays, individual IMC spot images had far fewer cells than histocytometry images, resulting in noisy and uninformative sample-level T-cell RDF curves. Consequently, we aggregated all breast cancer IMC images to generate a single cohort RDF for each class of cell-cell co-localizations. We did not distinguish antigen-loaded from -unloaded macrophages in this analysis because loaded macrophages make up only 0.3% of macrophages in the stroma and only 11% of the total macrophage content.
RDF analysis showed no distinguishable colocalization of T-cells with tumor cells within breast tumors (ΔCDF = − 0.465, Fig. S4), similar to T-cells within melanomas. This behavior was consistent across clinical subtypes, with HER2+ tumors showing the largest (negative) deviation from the null (ΔCDF = − 2.260, Fig. S5A–C). Intratumor T-cell/macrophage associations were close to null expectations (ΔCDF = 0.509). T-cell/B-cell interactions were below null expectations, though noisy. This is related to the fact that only 2.6% of B-cells reside within tumor nests, which hinders statistical assessment of colocalization. In the stromal region, T-cells show a co-localization with both macrophages and B-cells, and these effects are of comparable magnitude (ΔCDF = 1.261 and 1.567 respectively). The T-cell co-localization with B-cells is apparent despite the presence of B-cells in only 130 of 275 images, compared to 272 patient images containing macrophages. Stromal T-cell colocalization with B-cells and macrophages is consistent across clinical subtypes with some variation in effect size (Fig. S5A–C).
We then applied CISA to the breast cancer IMC images to investigate T-cell synapses with macrophages and B-cells. Because some samples have very limited number of B cells, only samples with at least 5 contacts between T cells and the neighboring cell type are included in the following analysis to increase statistical stability. In the intratumoral region (Fig. 7a), T-cells do not have significant synapse formation to macrophages (mean \(\bar{\sigma }\) = 0.094, 1-sample t-test p = 0.21) or tumor cells (mean \(\bar{\sigma }\) = −0.108, 1-sample t test p = 1.0), consistent with the lack of T-cell/cancer synapses in melanoma. A noteworthy effect in the breast IMC images is that intratumor T-cells form strong synapses with B-cells (mean \(\bar{\sigma }\) = 0.837, 1-sample t-test p = 7.5 × 10−5). These effect sizes are larger than those for the T-cell/loaded macrophage interactions in melanoma (Figs. 5a, b, 6a). Remarkably, this synapse effect is highly significant despite the lack of frequent colocalization between B-cells and T-cells -- intratumor T/B-cell contacts occur in only 45 of 275 (16%) images. In the stroma, T-cell synapse behaviors are consistent with the tendency of both macrophages and B-cells to co-localize with T-cells. Stromal T-cells have significant synapse formation with macrophages (mean \(\bar{\sigma }\,\)= 0.106, 1-sample t test p = 3.5 × 10−5, Fig. 7a), on par with the effect size seen in melanoma (Fig. 5a). Moreover, stromal T-cells form even stronger immune synapses with B-cells (mean \(\bar{\sigma }\,\)= 0.565, 1-sample t-test p = 2.6 × 10-21). Curiously, due to the paucity of B-cells, stromal T-/B-cell contacts are present in only 75 of 275 (27%) patients, compared to macrophage contacts being present in 182 patients. Synapse behaviors do not significantly differ across breast cancer subtypes (1-way ANOVA p > 0.05 for each target cell, Fig. S5D–F).
a Immune synapses of T-cells to B-cells are significantly >0 in tumor nests (denoted by *, mean = 0.837, N = 45, 1-sample t-test p = 7.5 × 10–5), and in the stroma (mean = 0.565, N = 75, 1-sample t-test p = 2.6 × 10–21). There is a significant but low effect size for T-cells to macrophages in the stroma (mean \(\bar{\sigma }\,\)= 0.106, N = 182, 1-sample t-test p = 3.5 × 10–5). b HR + HER2− patients with stromal T-cell synapses to B-cells have significantly improved disease-free survival (DFS) in Kaplan-Meier (KM) survival analysis (p = 0.016). c Patients with increased immune infiltration do not have significantly improved DFS (p = 0.073). d Cox proportional hazards (PH) shows that stromal synapses to B-cells have a significantly negative log hazard ratio (HR) in HR + HER2− patients when compared to other infiltrative and clinical factors (log HR = −1.45, p < 0.005) in determining DFS. e Triple-negative breast cancer patients with stromal T-cell synapses to B-cells have significantly improved DFS in Kaplan–Meier (KM) survival (p = 0.018). f Patients with increased immune infiltration do not have significantly improved DFS (p = 0.377). g Cox proportional hazards (PH) show the stromal synapses to B-cells have a significantly negative log HR in triple-negative patients when compared to other infiltrative and clinical factors (log HR = −2.45, p = 0.02). \(\bar{{\rm{\sigma }}}\): sample-level mean synapse strength. N: number of samples that have at least 5 cell-cell contact between T cells and the indicated neighbor cell type.
Stromal T-cell synapses with B-cells are associated with improved breast cancer survival
B-cells can hinder or help tumor growth depending on the context, and prior studies have suggested T-cells may be necessary for the beneficial B-cell effects31. We therefore tested whether the T-cell synapses to B-cells were associated with clinical outcomes in breast cancer. We first analyzed the 173 hormone-receptor positive HER2 negative (HR + HER2 − ) patients. We divided these patients into two groups based on synapse formation to B-cells: (1) those with a stromal T-cell:B-cell σ > 0 (representing contact and synapse formation), vs. (2) those with either σ <= 0 (representing contact with no synapse formation) or no contact (and therefore no synapse formation) between T- and B-cells. We observed a significant difference in disease-free survival (DFS) between these groups by Kaplan-Meier (KM) estimation, with improved survival for group 1 (p = 0.016, Fig. 7B). This DFS benefit is specific to the stroma, as splitting by the intratumor σ does not result in a significant survival difference (p = 0.88, Fig. S6A), nor does splitting by the combined intratumor and stromal T-cell σ (p = 0.106, Fig. S6B). DFS differences cannot be explained simply by contact between stromal T- and B-cells: grouping patients by the presence or absence of T-cells in contact with B-cells regardless of synapse strength resulted in a non-significant DFS difference (p = 0.131, Fig. S6C). Additionally, we analyzed the association of T-cell/B-cell infiltration on survival. In some images there could be sparse lymphocytes which would not be expected to influence outcome. So to determine an appropriate threshold for separating patients into high and low infiltration groups, we fit a two-component Gaussian Mixture Model, which yielded a separating threshold of lymphocyte infiltration of 5.4% (Fig. S6D). T- plus B-cell infiltration, while qualitatively associated with improved outcome, was not sufficient to distinguish patient DFS (p = 0.073, Fig. 7C). We next integrated these factors into a multivariable survival regression model using Cox Proportional Hazards, regressing the aforementioned factors and patient metadata against DFS. Stromal T-cell synapse formation with B-cells exhibited a negative and significant hazard ratio (log HR = − 1.45, CI [ − 2.45, −0.45], p < 0.005, Fig. 7d) in distinction from other factors, including increased immune cell infiltration.
We next investigated the association of DFS with T-cell/B-cell interactions in other breast cancer molecular subtypes. The Jackson et al. cohort20 has 48 TNBC (HR − HER2 − ) and 52 HER2+ (including 29 HR+ and 23 HR − ) patients. Splitting patients again by stromal T-cell σ to B-cells, the survival benefit in TNBC is significant by KM survival estimation (p = 0.018, Fig. 7e). No samples had a non-positive sample-average synapse strength in the TNBC cohort, either when considering only stromal or both stromal and intratumor synapses. As such, we could not test for additional survival benefits of T-cell-B-cell contact. Survival was not significantly different when splitting patients by quantity of T- and B-cell infiltration (p = 0.377, Fig. 7f). Multivariable survival regression demonstrated T-cell synapse formation to B-cells has a strong effect on DFS after controlling for infiltration factors (log HR = − 2.45, CI [ − 4.52, −0.38], p = 0.02, Fig. 7g). In the HER2+ cohort, KM survival estimation shows no DFS benefit for stromal T-cell synapse formation to B-cells (p = 0.727, Fig. S6E) or increased T- and B-cell infiltration (p = 0.515, Fig. S6F). Rather, the presence of hormone receptors estrogen receptor (ER) and progesterone receptor (PR) are the factors influencing survival in the Cox Proportional Hazards model with opposite effects (ER: log HR = 1.56, CI [0.59, 2.72], p = 0.01, PR: log HR = − 1.72, CI [−3.02, −0.41], p = 0.01, Fig. S6G). Thus, T-cell synapses to B-cells have strong survival benefits in TNBC but not HER2+ patients in this cohort.
Different cell segmentation approaches can identify different sets of pixels as the cell boundary areas, which are involved in the calculation of CISA. We therefore investigated the sensitivity of CISA with respect to the segmentation method used, by comparing CISA results after applying two different cell segmentation methods to the same Jackson et al. breast cancer dataset20. This dataset was originally segmented using nuclear expansion as implemented in Ilastik32 and CellProfiler33. We additionally applied MESMER34 cell segmentation to the raw images and labeled the segmented cells according to the annotation of matched cells in the original segmentation. A visual inspection shows that cells segmented by MESMER tend to be larger and rounder, but fewer in number (Fig. S7A, B). Nevertheless, a comparison of CD3-based synapse strength shows that the strong synaptic signal between T cells and B cells in stroma regions which was observed with original segmentation (mean = 0.565, N = 75, 1-sample t-test p = 2.6 × 10−21), is also observed with MESMER segmentation (mean = 0.485, N = 55, 1-sample t-test p = 7.5 × 10−13) (Fig. S7C, D). Similarly, for the intratumoral T cell-B cell synapse observed in intratumoral regions using the original segmentation (mean = 0.837, N = 45, 1-sample t-test p = 7.5 × 10−5), comparable synapses were observed with MESMER segmentation (mean = 0.714, N = 30, 1-sample t-test p = 0.0012) (Fig. S7C, D), though the statistical significance is lower for the MESMER segmentation, likely because there are fewer neighboring T-B cell pairs due to decreased numbers of segmented cells.
To further test the TNBC findings, we performed a similar CISA analysis on a TNBC IMC35 dataset generated by our team (referred to as JAX TNBC IMC, see Methods. Additional manuscript in preparation). As expected, in the CD8 + T cells, we observed strong correlation between σCD3(T-CD8 + , APC) and σCD8(T-CD8 + , APC), but weak correlation between σCD3(T-CD8 + , APC) and σCD4(T-CD8 + , APC) (Fig. S8A). Also as expected, in the CD4 + T cells, we observed strong correlation between σCD3(T-CD4 + , APC) and σCD4(T-CD4 + , APC), but weak correlation between σCD3(T-CD4 + , APC) and σCD8(T-CD4 + , APC) (Fig. S8B). The correlations indicate that CISA effectively characterizes the distribution of TCR molecules along the T cell membrane in this dataset.
Interestingly, unlike the other breast cancer dataset, we do not detect a positive average synapse between T cells and neighboring macrophages, B cells, or tumor cells, either in the intratumoral or stroma regions, in the new dataset (Fig. S8C, D). We performed survival analysis on T-B synapse strength (Fig. S8E). Positive synapse patients do exhibit superior survival, but the effect is not statistically significant. However, notably none of the patients with a positive T-B synapse strength in the dataset were yet deceased. Using the same threshold of 5%, patients with increased T cell and B cell infiltration also had better survival but the effect was not statistically significantly (log ranked p = 0.158) (Fig. S8F). Thus, the two IMC datasets show qualitatively similar survival associations between each of synapse strength and lymphocyte infiltration, but with different statistical significance.
Image preprocessing can enhance CISA performance
Because CISA is based on the logarithm of intensity ratios, it is innately robust to linear noise. Still, denoising has the potential to enhance image data quality. To test the robustness of CISA to image denoising, we applied several alternative preprocessing workflows to the ref. 20 TNBC IMC dataset and the ref. 24 dataset and then reperformed CISA analysis.
First, we compared CISA performance on the breast cancer IMC dataset using: raw images, IMC-Denoise36, and a simple thresholding-based denoising. Stronger T-B synapse strengths are observed with both denoising approaches, with IMC-denoise showing reduced variance and strengthened significance (Fig. S9A, B). Using thresholding denoising, in intratumoral regions immune synapses of T-cells to B are significantly > 0 (mean = 0.849, n = 45, one sample t test p = 0.00012); and in stroma regions immune synapses of T-cells to B are significantly > 0 (mean = 0.493, n = 75, one sample t-test p = 4e-16). With IMC-Denoise, in intratumoral regions immune synapses of T-cells to B are significantly > 0 (mean = 0.750, n = 45, one sample t test p = 4.5e-13); and in stroma regions immune synapses of T-cells to B are significantly > 0 (mean = 0.607, n = 75, one sample t test p = 5.2e-40).
Because the breast cancer dataset lacks CD4 and CD8a in its marker panel, we used the ref. 24 dataset for correlation analysis. With IMC-denoising, the expected correlation pattern is still observed: CD3 distribution is strongly correlated with CD8 distribution but not CD4 distribution in CD8 + T cells; and CD3 distribution is strongly correlated with CD4 distribution but not CD8 distribution in CD4 + T cells (Fig. S9C, D). This implies that CISA can successfully detect membrane distribution of TCR markers in images processed with IMC-denoising.
Discussion
Image analysis of in situ human tumor samples can deepen our understanding of the functional and compositional heterogeneity within the TME. Here we have presented CISA, a novel algorithm to quantify T-cell immune synapses by analyzing the distribution of proteins at cell-cell interfaces in multiplexed images of tumor tissues. We have verified the effectiveness of CISA across multiple imaging mass cytometry datasets in melanoma and breast cancer and further validated it with whole slide histocytometry images and super resolution emission depletion microscopy images generated by our team. Using CISA, we were able to elucidate many aspects of T-cell synaptic interactions, including the quantification of T-cell:macrophage interactions in melanomas, the increased proliferation of T-cells forming synapses with macrophages, and the differential behavior of T-cell:macrophage interactions between tumor and stromal regions. To our knowledge, CISA is the first computational method able to mine high resolution imaging data to quantify immune synapses. While we have focused on TCR proteins, CISA can handle any synapse-associated molecules. For example, CISA could be used to study other clinically important synapse-mediated mechanisms such as reciprocal PD-1/PD-L1 engagement at immune synapses37,38 and their downstream effects on TCR engagement strength39.
CISA’s methodology is distinctive compared to prior approaches to study cell synapses. For example, Danielson et al. studied neuronal puncta by: (1) using CellProfiler to segment all neuronal puncta within a sample, then (2) classifying each puncta as excitatory or inhibitory based on marker expression, with a threshold determined by comparison to a control sample40. Such an approach is sensitive to batch effects that might systematically impact marker detection throughout a sample. In contrast, CISA is based on the relative localization of synaptic proteins along a cell’s surface. Thus, each cell is internally calibrated, reducing the impact of batch effects. This internal calibration approach is also more realistic, as cells naturally vary in their gross protein expression.
Prior studies have investigated how protein-localization-based measures of synapses reflect cell state, e.g., Purbhoo et al. described cases of CD8 T cells requiring few peptide-MHC complexes to form immune synapses, while also noting that TCR clustering is observed in matured immune synapses41. Other studies, such as Wiedemann et al. described TCR clustering that is maintained at the cell-cell interface for a prolonged time42. Such studies show that dynamics affect how synaptic protein localization relates to cell activity. A limitation of CISA is that it is based on static images, from which it is challenging to infer dynamics for individual cells. However, CISA is able to extract information from static images by using a simplified concept of synaptic state and by averaging over many cells, both of which improve signal detection.
Our analysis of a large breast cancer cohort highlights the flexibility and potential clinical utility of CISA. We discovered that CISA quantification of T-cell: B-cell synapses is predictive of improved survival. Remarkably, this predicted benefit is beyond what would be expected simply from lymphocyte infiltration3, particularly in HR + HER2− and triple-negative breast cancer tumors. These findings are notable given the small sizes of TMA spots, the rarity of B-cells, and the small number of immune markers in the IMC data, suggesting that the scale of data considered here will be sufficient for further synapse biology discoveries. When applied to two TNBC IMC datasets, CISA revealed qualitatively similar survival associations between each of synapse strength and lymphocyte infiltration, but with different statistical significance. We speculate that such differences could be due to sample collection factors such as patient demographics, tumor staging, or processes for spot acquisition, though further study is needed.
The T-cell synaptic associations we have observed are relevant to a number of prior studies. For example, enhancement of macrophage tumor cell phagocytosis with anti-CD47 antibodies has been shown to yield tumor regression43,44 in a T-cell-dependent manner45. Our observation that T-cells form synapses with melanoma-loaded macrophages suggests that macrophage phagocytosis of tumor cells impacts priming and proliferation of T-cells to maintain anti-tumor response. B-cells are known to exert and promote pro- or anti-tumor immunity in a context-dependent fashion31,46. T-cells may be necessary for the beneficial B-cell effects31 and vice versa47, including within the context of immunotherapy48,49,50. Our results support that these behaviors stem from the enhanced activation of anti-tumor T cell responses via synapses with antigen-presenting B cells51. Differences in B-cell infiltration may explain the lack of observed effects in melanoma compared to primary breast tumors52,53.
One curious finding in the melanoma studies was that intratumoral CD8 + T cell-antigen loaded macrophage interactions were associated with stronger T cell synapses and increased ki67, but synapse strengths for CD8 + T cell-melanoma interactions were low. We speculate that the lack of T-cell/cancer-cell interactions may be because some T cells in the TME may not actually be cancer-specific. Previous studies have suggested T-cells acting against viruses54,55. In addition, half of the samples in this cohort are LN metastases, which increases the possibility that T cells are not tumor specific. T-cell / macrophage interactions may also have a stronger signal than T-cell/melanoma interactions because T cells have prolonged interactions with TAMs16. The lack of T-cell/tumor interactions might also be related to the fact that the metastasized tumors are already treatment-resistant28. However, the low level of CD3 clustering detected at T-cell/cancer-cell interfaces does not eliminate the possibility of other form of interactions between T cells and cancer cells.
We expect that CISA analyses will improve as multiplexed tumor imaging with broader marker panels increase, as these will enable finer interrogation of synaptic protein co-localization patterns. Multiplexed imaging approaches are limited by the number of concurrent assayable markers27 or capture duration56, and current panel sizes necessitate tradeoffs among tumor and immune proteins. This makes it difficult to resolve drivers of spatial effects such as the proliferation observed in the melanoma histocytometry data. Larger marker panels would also refine understanding of how specific T-cell populations such as Tregs57 contribute to prognosis. 3-dimensional imaging58 is also likely to improve the quantification of immune synapses and their impact on tumor cell killing in time and space. It would also be exciting to combine CISA with complementary methods such as Immunoscore59 for patient outcome prediction, though this will require deeper investigation of CISA robustness when applied to clinically-practical protein localization measurements.
A caveat of CISA is that it depends on the quality of cell segmentation to accurately identify membrane pixels. We showed that melanoma datasets imaged and segmented by distinct approaches yielded similar synaptic behaviors when analyzed with CISA, but there remain uncertainties due to variations in data characteristics and associated segmentation approaches. For example, the cell segmentations from our histocytometry cohort were based on Imaris’ proprietary algorithms. In the breast cancer cohort, segmentations were generated by the combination of Ilastik32 and CellProfiler33, a common protocol but potentially dependent on manual tuning. Machine-learning segmentation techniques such as StarDist60, Cellpose61, and MESMER34 offer high-throughput options that should improve as more publicly-available datasets emerge. A related consideration is that cell classification methods also vary, e.g., cell classification schemes designed specifically for single-cell mass cytometry applications such as X-shift62 may be important to optimal analysis of IMC data. Nevertheless, the reason that CISA is able to yield robust results in spite of such uncertainties is likely because it integrates over the many cell-cell interactions within a TMA spot or WSI, clarifying the signal. Another caveat of using CISA is that synapse formation does not guarantee T-cell activation. Classic studies showing accumulation of TCR at the site of T-APC synapses in immunofluorescence data suggest that TCR protein localization is associated with T-cell triggering63, but such conclusions should be justified by data. CISA enables such data-driven quantifications of the synapse.
In conclusion, CISA is a powerful approach capable of capturing region-specific T-cell synaptic interactions in the TME associated with biological and clinical outcomes. Moreover, it can in principle be applied to any highly-multiplexed epitope imaging modalities. We expect that CISA analysis will be valuable for understanding the functional and clinical correlates of specific T-cell interactions to guide future therapies.
Methods
Immune synapse model
To study immune synapse formation in imaging, we formulated the T-cell synapse strength as a means to quantify TCR localization. A T-cell’s synapse strength with an APC is calculated as the average signal intensity of CD3 at the membrane in contact with an APC divided by the average signal in the noncontact area followed by a log2 transformation (Fig. 1a):
Where \({{\rm{T}}}_{{\rm{i}}}\) is the i-th T cell, \({{\rm{N}}}_{{\rm{j}}}\) is the j-th neighboring cell for \({{\rm{T}}}_{{\rm{i}}}\), and \({{\rm{I}}}_{{\rm{CD}}3,{\rm{C}}\left({{\rm{T}}}_{{\rm{i}}},{{\rm{N}}}_{{\rm{j}}}\right)}\) and \({{\rm{I}}}_{{\rm{CD}}3,{\rm{NC}}\left({{\rm{T}}}_{{\rm{i}}},{{\rm{N}}}_{{\rm{j}}}\right)}\) are the mean intensities of CD3 in the contacting and noncontact areas of \({{\rm{T}}}_{{\rm{i}}}\) with respect to \({{\rm{N}}}_{{\rm{j}}}\), respectively.
To decide the contacting and noncontact areas, for a given T-cell, we identify the two pixel-wide internal boundary of the cell mask as the cell membrane. A one pixel-wide internal boundary was used for IMC synapse analysis due to the lower resolution. We use a 2-pixel diamond element for morphological dilation on each single cell mask of cells near T-cells to determine which are in contact. The dilated pixels of the neighboring cell intersecting with the cell of interest’s membrane pixels are considered the contact interface (Fig. 1a). The remaining membrane pixels are part of the non-contact interface. In cases where a T-cell is in contact with multiple cells of the same cell type, the non-contact interface is modified to contain only pixels not considered in contact with that cell type. This model is a simple quantitative improvement to a previous in situ approach which requires multiple molecule colocalization to identify qualitative synapses64.
We made adjustments to the synapse strength calculation for IMC images due to high signal-to-noise ratio compared to histocytometry for which our synapse analysis was originally designed. The mean CD3 signal in either the contact and/or non-contact interface was 0 for a non-trivial number of T-cells. Rather than discarding these T-cells from the analysis, we added a noise term to provide a fill-in value in these situations. We estimated this noise term for each sample as the background cell CD3 level, calculated as the average of the CD3 signal intensity in cells not labeled as a T-cell. In cases where the mean signal at the contact interface was 0 but the mean signal in the non-contact interface was less than the estimated noise term, we set the synapse strength to 0.
We devised a null synapse model for our histocytometry cohort to account for potential cell segmentation biases and serve as a control comparison in our synapse analysis. The null synapse model was derived by generating randomly-selected contiguous pixel regions on T-cell membranes. We used a seed-and-grow approach to create contiguous regions from membrane pixels to act as the null contact pixels in our synapse model, with the remaining membrane pixels acting as the non-contact pixels for synapse strength calculations. In the seed-and-grow approach, a pixel was randomly chosen from the membrane as a seed. Neighboring pixels were iteratively added until the region consisted of at least 15 pixels. A cell’s null synapse value was calculated from the average of five randomly seeded null synapses. This process was repeated for each T-cell in contact with another cell, with the null model synapse value for an iteration calculated from the sample average of these cell’s null synapse values. The mean of 100 iterations of sample averages were calculated to generate the final null model synapse value. In our initial histocytometry synapse analysis (Fig. 5), T-cells were split into intratumor and stroma to generate separate null models for each region. We further split these populations by Ki67 positivity for the proliferation analysis (Fig. 6).
CISA computation
The CISA code requires the following data as input:
-
1.
A cell boundary mask, where each pixel has an integer value corresponding to the cell ID it belongs to. A value of 0 indicates background or the area outside the object of interest. Because CISA characterizes the subcellular behaviors on cell membrane, any pixels in nuclear regions should also be 0;
-
2.
A cell classification table that contains the cell phenotype of each cell;
-
3.
Images of the proteins of interest, in the same resolution as the cell boundary mask, one grayscale image per protein.
First, CISA will calculate the contacting and non-contacting membrane regions between each T cell and their neighboring cells. This is done by dilating the cell boundary mask of neighboring cells with a diamond element of certain width, 2 pixels by default. Any pixels on the T cell membrane that overlap with the dilated neighbor membrane are defined as contacting regions, and the non-overlapping pixels are treated as non-contacting regions. In cases where a T-cell is in contact with multiple cells of the same cell type, the non-contact interface is modified to contain only pixels not considered in contact with that cell type.
Next, a T-cell’s synapse strength σ of a given TCR molecule of interest with a neighboring cell is calculated as the average signal intensity of the molecule at the membrane in contact with the neighboring cell divided by the average signal in the noncontact area followed by a log2 transformation. In the case where the average signal in either the contact or noncontact area is 0, CISA estimates a background noise value using the average intensity in all non-T cells, and uses the noise value as a fill-in value. In cases where the mean signal at the contact interface was 0 but the mean signal in the non-contact interface was less than the estimated noise term, we set the synapse strength to 0.
CISA returns a pandas data frame table containing all calculated σ values between all T-neighbor interfaces, along with the provided cell identity for neighbor cells. Since CISA is intended to be used as a sample-level statistical method, a sample mean \(\bar{\sigma }\) can be easily calculated using built-in data frame aggregation with customizable criteria.
Melanoma imaging mass cytometry data analysis
For the IMC data of (Moldoveanu et al.)24, the published data include cell center coordinates and cell classification, but no cell segmentation. We performed cell segmentation with MESMER34, then mapped the cell centers to the cell classification of the nearest cells, within a maximum center-to-center distance of 15 pixels. For the IMC data of (Hoch et al.)23, cell segmentations were available, but cell classifications were not provided. We calculated the average intensity of markers in each cell, and rescaled the mean intensity to a [0,1] range where values equal or greater to the 99th percentile of all cells were mapped to 1. Then we applied hierarchical clustering with Ward’s method and annotated the top 20 clusters based on known cell type specific marker patterns in the cluster mean intensity. This process yielded cell classifications for major immune and tumor cell types. In Fig. 2g, expression was calculated by averaging pixel intensity within each cell, then averaging the behavior of cells within each TMA.
Data from the breast cancer IMC cohort20 were obtained from https://doi.org/10.5281/zenodo.3518284. Data were minimally processed, as the necessary elements for our RDF and synapse analysis were provided from the publication. This includes spillover-corrected images, tumor/stroma masks, single-cell masks, and cell labels. We used the provided cell labels in our analysis, combining the metaclusters considered epithelial cells into a single tumor cell label for our general analysis. In addition, we combined the two T-cell only metaclusters into a single cluster and the two macrophage metaclusters into a single cluster. We excluded metacluster 2 for B- and T-cell labeling due to the ambiguity.
To mitigate the bias of random single strong pixels in IMC data, all raw images were log-transformed before analyzed by CISA. To avoid inducing additional bias, low intensity pixels were not removed as noise. Such noise is addressed implicitly as CISA compares the contacting region with the non-contacting region.
CISA correlation analyses
For the correlations in CISA scores (Fig. 1b, d), σ values were computed for all contacts between cells of the two specified types. The figures provide density plots of the σ values. The overall correlation r was evaluated across these σ values. For the pixel-wise correlation analyses (left panels of Fig. 1c, e), for each sample all pixels within the area of any segmented T-cells were identified. We then computed the correlation of the intensities for the specified proteins (CD3 vs. CD8; or CD3 vs CD4) over all pixels within the specified T-cell type (Tc or Th). Thus each dot in Fig. 1c, e corresponds to a sample. The CISA correlation values in Fig. 1c, e were computed analogously to the correlations in Fig. 1b, d, but on a sample-specific basis.
Metastatic melanoma samples for histocytometry
De-identified metµastatic melanoma surgical samples were obtained from the Cooperative human tissue network (CHTN) or from the Baylor Research Institute Tissue Bank. The use of these samples was determined to be non-human subjects research by the Jackson Laboratory Institutional Review Board. Resection location was provided but other clinical data such as past treatment was incomplete. No patient outcomes were available as no follow-up was allowed per the agreement with CHTN. Additional information provided in ref. 28.
Tissue immunofluorescence staining
Optimal cutting temperature compound (OCT) embedded samples were cryo-sectioned (8 µm) and consecutively, fixed with acetone, washed with 1x PBS, treated with Fc Receptor Block (Innovex bioscience) for 40 min + Background Buster (Innovex bioscience) for an additional 30 min. The sections were then stained with primary antibodies, diluted in PBS + 5% BSA 0.1% Saponin for 1 h at room temperature, washed, and stained with the secondary antibodies at room temperature for 30 min, secondary antibodies were saturated using mouse normal serum (Jackson ImmunoResearch) for 15 min at room temperature. Tissues were stained with directly conjugated antibody mix for 1 h at room temperature and washed. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (1 µg/mL) or SytoxBlue 1/1000 for 2 min. Tissues were mounted in Fluoromount-G mounting media.
Primary anti human antibodies: CD3 (HIT3a, Biolegend); CD14 (UCHM1, Genetex); MART-1 (M2-7C10 + M2-9E3 Novus Biologicals); PMEL (NIKI/beteb LSBio).
Conjugated anti human antibodies: CD3-BV421 (UCHT1, Biolegend); CD45-AF488 (HI30, Biolegend) CD19-AF700 (HIB19, Biolegend); CD138-AF700 (MI15, Biolegend); Ki67-AF555 (MKI67, BD Pharmingen); ICAM-1-APC (HA58, Biolegend); Melanoma-JF549 (M2-7C10 + M2-9E3 + T311 + HMB45, Novus Biologicals).
Secondary antibodies: depending on primary antibody combinations, species and isotype specific secondary antibody conjugated with either Alexa Fluor 488, Alexa Fluor 568 and Alexa Fluor 647 (Molecular Probes) were used in appropriated mixtures.
Whole section scan
Whole tissue scans were acquired on the Leica SP8 confocal microscope (Leica Microsystems) equipped with an automated motorized stage. Sequential acquisition was performed with a 20X objective. For each tile, focal plane was defined by autofocus function based on nuclear staining. Tiles were max projected and stitched using Leica LAS X software.
Super resolution microscopy (3D STED)
Tissue sections were stained following a modified immunofluorescence staining protocol with 2-fold increase in antibody concentration and one additional wash at each washing step. Vector Shield mounting media was left to cure for 72 h. Super resolution acquisition was performed on an inverted Leica SP8 confocal microscope equipped with STED modules, with 3 depletions lasers and an HC PL APO 100x GLYC objective (Leica Microsystems). Z-stacks were acquired with the 3D STED function using the 775 nm depletion laser. Images were analyzed and surface rendering was performed using the Imaris software (Bitplane).
Histocytometry
In situ quantitative analysis of melanoma tissue was based on published methodology27,65,66. Briefly, whole tissue scans were acquired using a Leica SP8 confocal microscope (Leica Microsystems, Germany). Each scan was then analyzed using image analysis software Imaris 8.4 (Bitplane). Using the “spot” function in Imaris, the images were segmented into individual cells, defined based on having a nucleus diameter equal or larger than 5 µm, used as a seeding point for each cell’s spot. The accuracy of the segmentation was visually assessed and adjusted if needed for each sample. Finally, for each generated spot, x;y coordinates and the mean intensity values for all channels were exported into an fcs file to be visualized and quantified using Flowjo software (version 10, Flowjo LLC).
Histocytometry image processing and analysis strategy
Single-cell masks from cell segmentation were exported from Imaris, and processed using the Python package scikit-image67. Small holes in the masks were filled and per marker single-cell average fluorescence intensities recalculated from the modified masks. The one pixel-wide internal mask boundary was considered as the membrane and the remaining pixels as the cytoplasm for additional signal quantification.
We used a K-means-based adaptive thresholding scheme for both tumor/stroma segmentation and cell label assignment in the histocytometry cohort to accommodate for inter-sample signal variation. Tumor/stroma segmentation for each image was conditioned on the signal intensity of the melanoma and CD45 channels respectively. The two image channels were passed through a gaussian filter (σ = 1) and log2(x + 1) transformed, where x represents the pixel value, to prevent negative values. We used Multi-Otsu thresholding, which is equivalent to the K-means clustering of the intensity histograms68, to determine thresholding levels. We considered four clusters which approximate to non-tissue background, tissue background, low intensity foreground, and high intensity foreground. The final signal threshold value was chosen as the mean signal in the low intensity foreground cluster to help limit undersegmentation of the tumor. Intensities greater than the threshold were considered part of the individual mask. Individual masks were furthered processed with a morphological closing (10-pixel square) followed by removing small holes (fewer than 2500 pixels) to eliminate small gaps. Small objects (fewer than 2500 pixels) were also removed to ignore isolated cell debris. We considered the tumor segmentation as the processed melanoma channel mask; the stroma segmentation was the processed CD45 channel mask excluding intersection with the melanoma channel mask. This allows for immune cells which reside in tumor nests to be differentiated from those in the stromal regions of the tumor.
Our K-means clustering approach for cell label assignment considered two clusters, approximating non-membership and membership. We clustered the log2-transformed single cell average fluorescence intensities (again biased by +1) for each channel within a sample separately. To reduce the potential for false positives, we chose the mean of the membership cluster as our threshold to label cells. We modified the quantification of single cell signal intensities to call antigen-loaded macrophages in our histocytometry cohort. Instead of using the entire macrophage area to determine melanoma signal to call antigen-loaded or unloaded, we took the upper-quartile average of the melanoma signal in the cytoplasm mask and determined loaded macrophage membership using the melanoma channel threshold. Our gating strategy allows for membership in multiple classes, such as cells labeled as both CD3+ (T-cell) and CD14+ (macrophage) which should not be expressed in a single immune cell together. However, we observed that these cases were due to undersegmentation of cells such that T-cells in contact with macrophages were pressed up closely enough such that nuclei were indistinguishable for segmentation. This did not affect our spatial analyses significantly but is an artifact to consider that may cause our immune synapse analysis to underestimate the strength of T-cell synapses with macrophages.
Cell densities and the radial distribution function
Immune cell densities in histocytometry were calculated using the tumor/stroma masks and cell labels. For sample-level densities (Fig. 3c), we took the label count divided by the area of the respective tumor and stroma mask. B-cells were excluded from analysis due to antibody cross-reactivity with tumors cells, limiting our ability to reliably detect B-cells. The RDF (Fig. 4) was calculated using the nearest neighbors algorithm from the Python package scikit-learn69. RDFs describe the density of target particles for a given distance from a reference particle70 (Fig. S2A). RDFs have been used previously to calculate cell-cell separation distances in cell cultures71, but have not been applied to quantify whole-slide TME spatial relationships to our knowledge. We adapted this concept using T-cells as the reference particle, with the other TME cell types assayed as target particles. RDFs quantify the average relationships between cell types, integrating the full information from selected regions. An advantage of RDFs over nearest neighbor statistics is that the RDF can be interpreted as a function of cell-cell distance.
After calculating all neighbors within 100 µm for each T-cell, we collected the frequency of the cell types of interest at distances binned into 1 µm increments. 100 µm is the range at which most RDF curves reached an asymptotic density; further distances did not yield additional information. We calculated histograms for both stromal and intratumor T-cells, then normalized to the number of T-cells in each region. Each bin of the histograms was further normalized by the area of the annulus representing each binned distance (Fig. S2A, yellow). This formulation of the RDF differs from the textbook definition in the final normalization step, where a traditional RDF definition further normalizes to the overall particle density70. We chose to omit this step to reflect the true density near T-cells as an additional value to compare spatial behaviors. We modified the final normalization step for the IMC analysis, as the smaller imaging area led to edge effects. For each T-cell, we adjusted the counts in each distance bin by the area of the annulus residing in the image divided by the original area of the annulus. Expected RDF curves were generated using label permutation. As we iterated through each T-cell to aggregate distance histograms for the observed RDF curve, we randomly permuted cell labels within 100 µm 100 times while keeping distances constant. We repeated the original RDF procedure for each permutation and averaged all the permutations together to generate the expected RDF curves for each cell type of interest.
The T-cell RDF can be thought of as a probability distribution function (PDF) of finding a target cell a given distance away from the reference cell. We used this concept in our ΔCDF calculations to determine T-cell spatial associations. RDFs were divided by the sum of RDF distance bins out to 100 µm to generate a pseudo-PDF which were then converted to discrete cumulative distribution functions (CDF). ΔCDF was calculated by summing the differences between the observed and expected CDFs at each of the 100 1-μm bins.
Jackson et al. 20 Breast cancer IMC survival analysis
Survival analysis was performed on 275 patients with DFS values and clinical subtyping information in the breast cancer IMC cohort. Two patients with available clinical data were excluded due to their lone B-cell residing on the edge of the image, raising the possibility of misclassification and ambiguity of infiltrate. In the case of patients with multiple images, the first was selected if no T-cell-B-cell contact occurred. If T-cell-B-cell contact occurred in one of the images, that image was chosen. There were no instances where one image had a negative synapse and the other image had a positive synapse. The remaining 273 patients were split into three clinical subtypes based on the clinical metadata provided by the authors: HR + HER2 − , HER2+ (both HR+ and HR − ), and Triple Negative (HR − HER2 − ).
Patients were further subdivided based on parameters of interest. T-cell-B-cell contact was determined based on our synapse analysis. A σ greater than 0 implies contact between T-cells and B-cells and a tendency to form synapses, whereas a σ less than or equal to 0 implies contact but no tendency to form synapses. Samples with no σ do not have contact between T- and B-cells; contact is thus determined as any value of σ. For Fig. 7e, h, patients were split by the percentage of T- and B-cells compared to total cellular content. We used the sum of cells labeled as T- or B-cells, including the ambiguous label previously excluded from synapse analysis. A 5% threshold was set to differentiate tumors with increased lymphocyte infiltrate. This was chosen as the frequency of samples as the level of infiltrate dropped substantially below this cutoff point and is approximately the median lymphocyte infiltration. Survival analysis was performed using the Python package lifelines (version 0.23.0) implementation of Kaplan-Meier survival estimation and the Cox Proportional Hazards model using default parameters. Tumor stage in the Cox Proportional Hazards analysis is based on the column “PTNM_T” using only the numeric stage.
MESMER segmentation and cell classification for Jackson et al. 20 Breast cancer IMC
Histone H3 channel is used as the nuclear channel. A max projection of pan cytokeratin, E/P-cadherin, CD44, and CD45 is used as the membrane channel. The nuclear and membrane channels are log-transformed before being inputted into MESMER34. The segmented cells are annotated by mapping the cell centers to the nearest cells in the original segmentation, with a maximum center-to-center distance of 15 pixels. The cell boundary masks are calculated from cell masks using binary pixel erosion by 1 pixel.
Considering the number of B cells in this dataset are further reduced with MESMER segmentation, a threshold of at least 5 T-/B- contact was used to reduce the effect of randomness. Samples below this threshold were not included in synapse strength comparison.
JAX TNBC IMC TMA tissue source
The YTMA-341 TNBC TMA slide at Yale Pathology Tissue Services was ordered. The TMA was built under Yale IRB# 0003011706, which allows construction and annotation of TMAs with waiver of patient consent. The Jackson Laboratory IRB has determined the use of this TNBC TMA in this project as not human subject research under IRB reference number 2024-NHSR-044.
JAX TNBC IMC processing and analysis
We log-normalized raw IMC channels, removed pixels with low intensity (intensity < T), and removed connected components (8-connectivity) with less than K connected pixels. We used T = 0.75 and K = 5 except for the following markers: for CD11b and CD14 T = 1.5 and K = 5, for vimentin, CD20, CD11c, CD74, CD68, and HLA-ABC T = 1 and K = 5, and for Epcam-Ecadh T = 0.75 and K = 4.
We used MESMER34 for cell segmentation, and associated each cell with the average expression of preprocessed IMC markers on cell area. We used a hierarchical approach for phenotyping. We first separated tumor, stroma, and immune cells, where for a cell X we assumed:
where \(C\in \left\{{tumor},{stroma},{immune}\right\}\) denotes a class, \({M}_{{tumor}}=\{{pan}- {cytokeratin},\,B7-H4,{epcam}-{ecadherin}\}\), \({M}_{{stroma}}=\{{collagen},{aSMA}, {vimentin}\}\), and \({M}_{{immune}}=\left\{{CD}45,{CD}45 {RA},{CD}45 {RO},{CD}33,{CD}3, {CD}4,{CD}8a,{CD}20, {CD}11b, {CD}14,{CD}74,{CD}68,{HLA}-{DR}\right\}.\) \({w}_{i}\)’s are weights that are optimized to maximize separation between classes. We then separated lymphocytes, myeloid, and NK cells using \({M}_{{lym}}= \{{CD}3,{CD}8a,{CD}20\}\), \({M}_{{myl}}=\{{CD}11b,{CD}11c,{CD}68,{CD}33,{CD}15\}\), and \({M}_{{NK}}=\{{CD}56\}\). We separated T-cells and B-cells using \({M}_{T-{cell}}=\{{CD}3,{CD}4,{CD}8a,{CD}15\}\) and \({M}_{B-{cell}}=\{{CD}20\}\). We separated myeloids to neutrophils and macrophages-monocytes using \({M}_{{neut}.}=\{{CD}15\}\) and \({M}_{{mac}.{mono}.}=\{{CD}11b,{CD}16,{CD}68,{CD}11c\}\). We separated CD8+ and CD4 + T-cells using expressions od CD8a and CD4 markers, respectively, and separated activated and naïve T-cells using CD45RA and CD45RO markers, respectively.
Denoising using simple thresholding or IMC-Denoise
The thresholding method uses the lowest peak on the pixel intensity histogram as the threshold for noise level. Any pixels below this threshold are set to 0.
The small DeepSNif model of IMC-Denoise36 is trained with default parameters for 200 epochs. The trained model is used together with the DIMR module from IMC-Denoise to remove both hot pixels and pervasive noises. The existing cell segmentation and classification results were reused. In terms of CISA computation, the only difference is how the inputted protein images were denoised.
Statistics and reproducibility
Wilcoxon signed-rank test p values are presented for comparisons between cell types and regions unless otherwise specified. When 1-sample t-tests are noted, the one-sided greater-than alternative hypothesis was used with the population mean considered to be 0. Presented p-values are unadjusted. Log-rank test was used to determine Kaplan–Meier survival estimation p values. Additional statistics including sample sizes and replicates are described throughout the manuscript for each analysis.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Hoch et al. melanoma IMC, the Moldoveanu et al. melanoma IMC, and the Jackson et al. Breast cancer IMC datasets are downloaded from the corresponding Zenodo repositories published in the original studies (https://zenodo.org/records/6004986, https://zenodo.org/records/5903190, and https://zenodo.org/records/3518284)72,73,74. The melanoma histocitometry images are available at https://zenodo.org/records/1327149575. The Jax TNBC IMC dataset is available at https://zenodo.org/records/13271783 with access provided upon request35. It will be made publicly accessible once another manuscript, currently in preparation, is published. All source data for generating main figures are provided in Supplementary Data 1.
Code availability
Software for CISA analysis has been made available through Github at https://github.com/SamLiu1218/CISA, and through Zenodo at https://zenodo.org/records/1334111776.
References
Bejarano, L., Jordāo, M. J. C. & Joyce, J. A. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 11, 933–959 (2021).
Kahlon, N. et al. Melanoma treatments and mortality rate trends in the US, 1975 to 2019. JAMA Netw. Open 5, e2245269 (2022).
Fridman, W. H., Pagès, F., Sautès-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Wang, S., He, Z., Wang, X., Li, H. & Liu, X.-S. Antigen presentation and tumor immunogenicity in cancer immunotherapy response prediction. eLife 8, e49020 (2019).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 541, 321–330 (2017).
Palucka, A. K. & Coussens, L. M. The basis of oncoimmunology. Cell 164, 1233–1247 (2016).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Kurtulus, S. et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1−CD8+ tumor-infiltrating T Cells. Immunity 50, 181–194.e6 (2019).
Alspach, E. et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Huppa, J. B. & Davis, M. M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Dustin, M. L. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 221, 77–89 (2008).
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Kabanova, A., Zurli, V. & Baldari, C. T. Signals controlling Lytic Granule polarization at the Cytotoxic immune synapse. Front. Immunol. 9, 307 (2018).
Kersten, K. et al. Spatiotemporal co-dependency between macrophages and exhausted CD8+ T cells in cancer. Cancer Cell 40, 624–638 (2022).
Nirmal, A. J. et al. The spatial landscape of progression and immunoediting in primary melanoma at single cell resolution. Cancer Discov. 12, 1518 (2022).
Lewis, S. M. et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 18, 997–1012 (2021).
Keren, L. et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387.e19 (2018).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Schürch, C. M. et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182, 1341–1359.e19 (2020).
Schapiro, D. et al. histoCAT: analysis of cell phenotypes and interactions in multiplex image cytometry data. Nat. Methods 14, 873–876 (2017).
Hoch, T. et al. Multiplexed imaging mass cytometry of the chemokine milieus in melanoma characterizes features of the response to immunotherapy. Sci. Immunol. 7, eabk1692 (2022).
Moldoveanu, D. et al. Spatially mapping the immune landscape of melanoma using imaging mass cytometry. Sci. Immunol. 7, eabi5072 (2022).
Martinez-Morilla, S. et al. Biomarker discovery in patients with immunotherapy-treated melanoma with imaging mass cytometry. Clin. Cancer Res. 27, 1987–1996 (2021).
Wabnitz, G., Kirchgessner, H. & Samstag, Y. Qualitative and quantitative analysis of the immune synapse in the human system using imaging flow cytometry. J. Vis. Exp. 143, e55345 (2019).
Gerner, M. Y., Kastenmuller, W., Ifrim, I., Kabat, J. & Germain, R. N. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376 (2012).
Martinek, J. et al. Transcriptional profiling of macrophages in situ in metastatic melanoma reveals localization-dependent phenotypes and function. Cell Rep. Med. 3, 100621 (2022).
Li, H., Fan, X. & Houghton, J. Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell. Biochem. 101, 805–815 (2007).
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Sarvaria, A., Madrigal, J. A. & Saudemont, A. B cell regulation in cancer and anti-tumor immunity. Cell. Mol. Immunol. 14, 662–674 (2017).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Greenwald, N. F. et al. Whole-cell segmentation of tissue images with human-level performance using large-scale data annotation and deep learning. Nat. Biotechnol. 40, 555–565 (2022).
Sivajothi, S., Martinek, J. & Palucka, K. Triple Negative Breast Cancer TMA IMC. Zenodo https://doi.org/10.5281/zenodo.13271783 (2024).
Lu, P. et al. IMC-Denoise: a content aware denoising pipeline to enhance imaging mass cytometry. Nat. Commun. 14, 1601 (2023).
Ambler, R. et al. PD-1 suppresses the maintenance of cell couples between cytotoxic T cells and target tumor cells within the tumor. Sci. Signal. 13, eaau4518 (2020).
Yokosuka, T. et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209, 1201–1217 (2012).
Elliot, T. A. E. et al. Antigen and checkpoint receptor engagement recalibrates T cell receptor signal strength. Immunity 54, 2481–2496.e6 (2021).
Danielson, E. et al. Molecular diversity of glutamatergic and GABAergic synapses from multiplexed fluorescence imaging. eneuro 8, ENEURO.0286–20.2020 (2021).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Wiedemann, A., Depoil, D., Faroudi, M. & Valitutti, S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc. Natl Acad. Sci. USA 103, 10985–10990 (2006).
Gholamin, S. et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 9, eaaf2968 (2017).
Sikic, B. I. et al. First-in-human, first-in-class phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 37, 946–953 (2019).
Liu, X. et al. CD47 blockade triggers T cell–mediated destruction of immunogenic tumors. Nat. Med. 21, 1209–1215 (2015).
Fremd, C., Schuetz, F., Sohn, C., Beckhove, P. & Domschke, C. B cell-regulated immune responses in tumor models and cancer patients. OncoImmunology 2, e25443 (2013).
DiLillo, D. J., Yanaba, K. & Tedder, T. F. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J. Immunol. 184, 4006–4016 (2010).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Hua, Z. & Hou, B. The role of B cell antigen presentation in the initiation of CD4+ T cell response. Immunol. Rev. 296, 24–35 (2020).
Ladányi, A. et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol. Immunother. 60, 1729–1738 (2011).
Mahmoud, S. M. A. et al. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 132, 545–553 (2012).
Erkes, D. A. et al. Virus-Specific CD8(+) T cells infiltrate melanoma lesions and retain function independently of PD-1 expression. J. Immunol. 198, 2979–2988 (2017).
Rosato, P. C. et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10, 567 (2019).
Lin, J.-R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 7, e31657 (2018).
Saleh, R. & Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 490, 174–185 (2020).
Kuett, L. et al. Three-dimensional imaging mass cytometry for highly multiplexed molecular and cellular mapping of tissues and the tumor microenvironment. Nat. Cancer 3, 122–133 (2021).
Angell, H. & Galon, J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 25, 261–267 (2013).
Schmidt, U., Weigert, M., Broaddus, C. & Myers, G. (2018). Cell detection with star-convex polygons. In Conference of the Medical Image Computing and Computer Assisted Intervention – MICCAI 2018 (eds Frangi, Ú., Schnabel, J., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) 265-273 (Springer, 2018).
Stringer, C., Wang, T., Michaelos, M. & Pachitariu, M. Cellpose: a generalist algorithm for cellular segmentation. Nat. Methods 18, 100–106 (2021).
Samusik, N., Good, Z., Spitzer, M. H., Davis, K. L. & Nolan, G. P. Automated mapping of phenotype space with single-cell data. Nat. Methods 13, 493–496 (2016).
Das, V. et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse: involvement of SNARE complexes. Immunity 20, 577–588 (2004).
Barcia, C. et al. Infiltrating CTLs in human glioblastoma establish immunological synapses with tumorigenic cells. Am. J. Pathol. 175, 786–798 (2009).
Wu, T.-C. et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 78, 5243–5258 (2018).
Wang, M. et al. Humanized mice in studying efficacy and mechanisms of PD-1-targeted cancer immunotherapy. FASEB J. 32, 1537–1549 (2018).
Walt, Svander et al. scikit-image: image processing in Python. PeerJ 2, e453 (2014).
Liu, D. & Yu, J. Otsu Method and K-means. 2009 Ninth Int. Conf. Hybrid. Intell. Syst. 1, 344–349 (2009).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
McQuarrie, D. A. Statistical Mechanics (Harper & Row, 1976).
Jubran, M. R. et al. Dissecting the role of crosstalk between glioblastoma subpopulations in tumor cell spreading. Oncogenesis 9, 1–15 (2020).
Hoch, T. et al. Multiplexed imaging mass cytometry of chemokine milieus in metastatic melanoma - Raw Data https://doi.org/10.5281/zenodo.6004986 (2022).
Jackson, H. W. et al. The Single-Cell Pathology Landscape of Breast Cancer. Zenodo https://doi.org/10.5281/ZENODO.3518284 (2019).
Moldoveanu, D. et al. Raw CyTOF images associated with Moldoveanu et al. 2022, Science Immunology. Zenodo https://doi.org/10.5281/zenodo.5903190 (2022).
Martinek, J., Boruchov, H., Glutali, A., Seeniraj, S. & Palucka, K. Histocytometry profiling of immune infiltrate in metastatic melanoma. Zenodo https://doi.org/10.5281/zenodo.13271495 (2022).
Liu, Z., Wang, V. & Chuang, J. Code for Computational Immune Synapse Analysis. Zenodo https://doi.org/10.5281/ZENODO.13341116 (2024).
Acknowledgements
We would like to thank Ananya Gulati for her assistance with performing the single-cell segmentation on the histocytometry images in the early stages of this work. We would like to thank David Rimm, Katherine Bates, and Yale Pathology Tissue Services for sharing the tissues for the Jax TNBC IMC data. We would also like to acknowledge Javad Noorbakhsh for initially suggesting the radial distribution function as a way to measure local density. All authors acknowledge support from NIH grants P30CA034196 and R01CA219880. V.G.W., Z.L., A.F.P., J.Z., and J.H.C. acknowledge support from R21CA191848, R01CA230031. J.M. and K.P. acknowledge support from R01CA204115 and R01CA195712.
Author information
Authors and Affiliations
Contributions
Conceptualization, V.G.W., J.M., K.P., and J.H.C.; Methodology, V.G.W. and J.M.; Software, V.G.W., Z.L., J.Z.; Validation, J.M.; Investigation, V.G.W., Z.L., J.M., A.F., J.Z., H.B., and K.R.; Writing – Original Draft, V.G.W., Z.L., J.M., K.P., and J.H.C.; Visualization, V.G.W., Z.L., and J.M.; Supervision, K.P. and J.H.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Laura Rodríguez Pérez. A peer review file is available. This manuscript has been previously reviewed at another Nature Portfolio journal. This document only contains reviewer comments and rebuttal letters for versions considered at Communications Biology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, V.G., Liu, Z., Martinek, J. et al. Computational immune synapse analysis reveals T-cell interactions in distinct tumor microenvironments. Commun Biol 7, 1201 (2024). https://doi.org/10.1038/s42003-024-06902-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-024-06902-2
This article is cited by
-
Immunological synapse: structures, molecular mechanisms and therapeutic implications in disease
Signal Transduction and Targeted Therapy (2025)