Abstract
The endothelium-dependent vascular injury, a primary pathological feature of angiotensin II (Ang II)-induced hypertension. This study aimed to explore the role and underlying mechanisms of G protein-coupled receptor 39 (GPR39) in the pathogenesis of Ang II-induced hypertension. For in vivo studies, GPR39 knockout (KO) mice (C57BL/6 J, male) were generated and administered Ang II for 4 weeks. GPR39 expression was upregulated in the aorta of hypertensive patients and mice. The ablation of GPR39 mitigated vascular fibrosis, augmented endothelium-dependent vasodilation, and inhibited endothelial inflammation, oxidative stress, and apoptosis in mice. Additionally, GPR39 KO decreased NOD-like receptor protein 3 (Nlrp3) gene expression in Ang II-stimulated endothelial cells. Notably, Nlrp3 activation counteracted the therapeutic benefits of GPR39 KO. We identified the potential ligand of GPR39 using structure-based high throughput virtual screening (HTVS) and validated its antihypertensive function in vitro and in vivo. The small molecule ligand Z1780628919 of GPR39 can also reduce Ang II-induced hypertension and improve vascular function. GPR39 KO and the small molecule ligand Z1780628919 potentially downregulates Nlrp3, thereby mitigating vascular fibrosis, endothelial inflammation, oxidative stress, and apoptosis. This effect contributes to the alleviation of Ang II-induced hypertension and the rectification of vascular dysfunctions. These findings suggest new avenues for therapeutic intervention.
Similar content being viewed by others
Introduction
Hypertension is a leading and preventable cause of cardiovascular mortality1, affects over 1 billion individuals globally as of 2019, a doubling since 1990. This trend signifies a substantial global health challenge2. Several factors were significantly associated with the prevalence of hypertension, including lipid metabolism3, salt intake4, alcohol consumption5, tobacco6, smoking7 and obesity8 .
Angiotensin II (Ang II) and the Ang II type-1 receptor (AT1R) are pivotal in cardiovascular pathologies, including hypertension and atherosclerosis9,10. Ang II, a vasoactive peptide of the renin-angiotensin system (RAS), is a key molecular signal associated with hypertension11. Foulquier et al. found that mice treated with long-term Ang II were consistent with the pathophysiological mechanism of microvascular changes in human hypertension12. Independent of TGF-β, Ang II can activate the Smad signaling pathway13. Ang II-induced hypertension is known to precipitate the medial hypertrophy and perivascular fibrosis14.Ang II induces the overproduction of reactive oxygen species (ROS), upregulation of endothelial nitric oxide synthase (eNOS), and subsequent endothelial apoptosis15,16,17. Prior studies have identified eNOS as an indicator of endothelial dysfunction in the early-stage hypertension18. NOD-like receptor protein 3 (Nlrp3), a multi-protein cellular complex, is implicated in inflammatory responses. It contains the apoptosis-related dot-like protein19, closely linked to apoptosis20. Furthermore, evidence suggests the expression of the Nlrp3 inflammasome in endothelial cells, a mechanism associated with endothelial dysfunction21.
G protein-coupled receptor 39 (GPR39), also recognized as the Zn2+ sensing receptor (ZnR), can be activated by extracellular Zn2+at non-physiological concentrations22. While associations of GPR39 with cardiac hypertrophy23 and aortic valve calcification24 have been hypothesized, its involvement in blood pressure regulation remains uncertain.
In summary, this study aims to elucidate the role of GPR39 in Ang II-induced hypertension and aortic endothelial dysfunction. In addition, a small molecule ligand of GPR39 will be explored to improve hypertension via structure-based high throughput virtual screening (HTVS).
Results
GPR39 expression
Our investigation revealed markedly elevated protein and mRNA levels of GPR39 in the aortas of HTN patients (Fig. 1a, b), as well as in the aortas of mice with Ang II-induced hypertension, as compared to the control group (Fig. 1c, d). This elevation was further corroborated by GPR39 immunofluorescence staining in mouse aortic tissues (Fig. 1e, f). In a similar vein, HUVECs stimulated with Ang II exhibited significantly increased GPR39 levels relative to controls (Fig. 1g, h).
a, b Quantitative analysis of GPR39 mRNA (n = 5) and protein (n = 3) expression levels in the aorta of hypertensive patients (HTN) compared to normotensive patients. c, d Quantitative analysis of GPR39 mRNA and protein expression levels in the aorta of Ang II-infused mice compared to control mice (n = 6). e, f An immunofluorescence image depicting aortic cells marked with GPR39 (red) and DAPI (blue) at 40× magnification. Scale bar represents 50 μm. White arrows highlight endothelial cells with elevated GPR39 expression. Quantitative analysis of GPR39 expression is presented. g, h Analysis of GPR39 mRNA and protein expression levels in HUVECs post 48-h Ang II induction versus the PBS control group (n = 6). Data are expressed as mean ± SEM. Statistical significance is denoted as follows: ns (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
GPR39 knockout ameliorates hpertension and endothelium-dependent vascular dysfunction
To ascertain if GPR39 knockout could mitigate hypertension, GPR39 KO mice were developed (Supplemental Fig. 1a). We also validated the GPR39 antibody (Supplemental Fig. 1b). These mice, along with their control counterparts, were subjected to Ang II pump implantation to induce hypertension. Results indicated that blood pressure in the Ang II group was significantly higher than in the control group. Notably, GPR39 knockout markedly attenuated the Ang II-induced elevations in systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) (Fig. 2a–c).
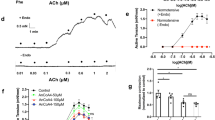
a–c Graphs displaying systolic pressure, diastolic pressure, and mean arterial pressure (tail artery measurements, n = 6) across saline, Ang II, GPR39 KO, and Ang II + GPR39 KO mouse groups. d, e Blood flow speckle analyses in the mesenteric artery of live mice, with statistical interpretation of mean blood flow (intensity of red indicates blood flow density, n = 6). f, g Examination of p-eNOS and eNOS protein levels in the aorta across the four groups (n = 6). h–k Analysis of isolated aortic vessel contraction and relaxation in the four mouse groups (n = 3). Pgf-2α and Ach assessed endothelium-dependent vascular responses, while Norepinephrine (NA) and sodium nitroprusside (SNP) were used for endothelium-independent systolic and diastolic function evaluation Data are expressed as the mean ± SEM. Statistical significance is indicated as ns (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
The analysis using the blood flow speckle test revealed that Ang II diminished blood flow in the arterioles of mice, particularly in the mesenteric arteries. Interestingly, GPR39 knockout appeared to mitigate this effect, resulting in improved blood flow (Fig. 2d, e). A key factor in vasodilation is endothelial Nitric Oxide Synthase (eNOS)-derived Nitric Oxide (NO). Ang II significantly lowered the protein levels of phosphorylated eNOS (p-eNOS) in the aorta, a trend that was substantially reversed in GPR39 KO mice (Fig. 2f, g). Endothelial dependent diastolic dysfunction is an important link in the pathogenesis of hypertension. Endothelial-dependent vasodilation (contraction) function refers to the vasodilation or contraction of blood vessels caused by certain vasoactive substances, which is closely related to the integrity of endothelial structure and function. The endothelial-independent relaxation (contraction) function refers to the vascular response caused by some drugs or vasoactive substances directly acting on vascular smooth muscle, which is not dependent on the endothelium. To further elucidate the role of GPR39 in modulating Ang II-induced hypertension, we performed contraction and relaxation experiments on isolated aortic rings from four groups of mice. The assays indicated no significant variance in endothelium-dependent systolic function, as induced by PGF-2α (Fig. 2k), or in endothelium-independent diastolic function, as induced by sodium nitroprusside, across the groups (Fig. 2i). However, both endothelium-dependent relaxation (acetylcholine-induced, Fig. 2h) and endothelium-independent contraction (NA-induced, Fig. 2j) were markedly diminished in mice with Ang II-induced hypertension. These impairments were significantly alleviated following GPR39 knockout.
GPR39 knockout attenuates Ang II-Induced vascular fibrosis
Investigating the mechanistic basis of altered vasoconstriction and relaxation, we utilized Masson’s trichrome staining to examine vascular tissue (Fig. 3a). Comparative analysis revealed a pronounced increase in vascular fibrosis in the Ang II treated group, compared to the saline control. Notably, GPR39 knockout significantly mitigated this fibrotic response (Fig. 3a). Quantitative assessments via RT-PCR (Fig. 3b) and Western blotting (Fig. 3c) demonstrated comparable levels of fibrosis markers COL-1, α-SMA, and TGF-β, at both protein and mRNA levels in vascular tissues. These results suggest that GPR39 knockout may play a critical role in modulating non-endothelium-dependent systolic dysfunction.
a Quantification of fibrosis in mouse aortic vessels (n = 6) using Masson staining. The fibrotic area was meticulously calculated. b, c Analysis of fibrosis markers in aortic vessels at the protein (α-SMA, TGF-β and COL-1, n = 6) and mRNA levels (α-SMA, TGF-β and COL-1, n = 6). GAPDH served as the loading control. Data are presented as mean ± SEM, with statistical significance indicated as NS (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
GPR39 knockout mitigates vascular apoptosis, oxidative stress and inflammation
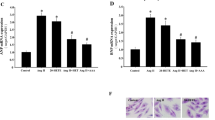
Protein (Fig. 4a) and mRNA levels (Supplemental Fig. 2a, b) of Bax, Bcl-2, and Caspase-3 were examined to assess the influence of Angiotensin II (Ang II) on vascular endothelial apoptosis in hypertensive mice. Results indicated a marked increase in apoptosis due to Ang II, which was substantially mitigated by GPR39 knockout. To visually ascertain apoptosis levels, TUNEL staining was employed on aortic sections from four groups (Fig. 4b). The findings echoed molecular observations, revealing complete endothelial cell apoptosis in the Ang II group, whereas GPR39 knockout significantly attenuated this effect. 8-hydroxy-2′,3′-deoxyguanosine (8-OHdG), an oxidative stress biomarker, was analyzed using immunofluorescence staining of vascular tissues. Elevated levels of 8-OHdG in the Ang II group were markedly reduced post-GPR39 knockout (Fig. 4c). ROS staining corroborated that Ang II augmented oxidative stress in the mouse aorta, akin to 8-OHdG findings, but this increase was less pronounced in GPR39 KO mice (Fig. 4d). Ang II is known to provoke vascular cell inflammation. The ablation of GPR39 attenuated Ang II-induced inflammation levels. Nlrp3, an essential inflammatory mediator was proposed to be modulated by GPR39, thereby curtailing apoptosis, oxidative stress, eNOS expression, and inflammation, ultimately diminishing Ang II-induced hypertension (Fig. 4e). mRNA expressions of IL-1, IL-6, TNF- α, Nlrp3, and other inflammatory markers further substantiated this hypothesis (Fig. 4f–i).
a Assessment of apoptosis marker proteins (Bax, Bcl-2, Caspase-3 and Cleaved caspase-3, n = 6) in aortic vessels. GAPDH was used as a loading control. b–d TUNEL, 8-OHdG, and DHE staining to visualize apoptosis and oxidative stress in the aortic vessels of mice from four groups (TUNEL, 8-OHdG, DHE: green; CD31: red; DAPI: blue; n = 6). White arrows highlight endothelial cells with elevated TUNEL, 8-OHdG, and DHE expression. Scale bar is 50 μm. e Nlrp3 protein levels in the aorta of the four groups (n = 6) were analyzed, with gray scales on WB images quantified using Image J. f–i mRNA expression levels of inflammatory markers (IL-1, IL-6, TNF-α and Nlrp3, n = 6) in the aortic vessels. Results are expressed as the mean ± SEM. Statistical significance is denoted as NS (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
GPR39 knockout mitigates Ang II-induced endothelial cell injury
This study extended its investigation to HUVECs. We engineered three small interfering RNAs targeting GPR39 and identified the most effective variant for GPR39 knockdown in these cells (Supplemental Fig. 3a–c). Subsequent analysis focused on the p-eNOS protein levels and apoptosis rates post-treatment across four groups. Notably, GPR39 knockdown markedly diminished both eNOS expression and HUVECs apoptosis induced by Ang II (Fig. 5a–e). The CCK8 assay further illustrated a significant decrease in HUVEC activity following Ang II exposure, which was effectively reversed by si-GPR39 treatment (Fig. 5g).
a–e Evaluation of apoptosis-related protein levels (P-eNOS, eNOS, Bax, Bcl-2, Caspase-3 and Cleaved caspase-3, n = 6) in HUVECs across PBS, Ang II, si-GPR39, and Ang II + si-GPR39 groups. GAPDH was employed as the loading control. f TUNEL staining to visualize apoptosis in the four HUVEC groups (TUNEL, green; DAPI, blue; n = 6; Scale, 50 μm). White arrows highlight endothelial cells with elevated TUNEL expression. g Cellular activity in the four groups was assessed using a CCK8 kit, reflecting enzymatic activity (n = 6). h ROS staining to detect oxidative stress in HUVECs of the four groups (ROS, green; DAPI, blue; n = 6; Scale, 50 μm). White arrows highlight endothelial cells with elevated ROS expression. i–l Quantitative analysis of mRNA levels of inflammatory markers (IL-1, IL-6, TNF-α and Nlrp3, n = 6) in HUVECs. Data are expressed as mean ± SEM. Statistical significance is denoted as NS (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Moreover, we quantified apoptosis and inflammation-related mRNA levels in the four cell groups. The findings consistently indicated that GPR39 knockdown inhibited both apoptosis and the expression of inflammatory markers in HUVECs induced by Ang II (Fig. 5i–l; Supplemental Fig. 2b, c). To visually corroborate these molecular alterations, we employed TUNEL (Fig. 5f) and ROS (Fig. 5h) staining across the groups. to observe their apoptosis and oxidative stress more intuitively. These assays demonstrated that GPR39 knockdown significantly alleviated both apoptosis and oxidative stress in HUVECs following Ang II treatment.
GPR39 knockdown mitigates Ang II-Induced HUVEC injury by inhibiting Nlrp3
This study hypothesized that GPR39 modulates endothelium-dependent injury both in vivo and in vitro via Nlrp3 regulation. To test this, HUVECs were treated with Nigericin, an Nlrp3 agonist. It was observed that GPR39 knockdown ameliorated the Ang II-induced impairment of HUVEC activity, an effect that Nigericin (Nlrp3 agonist, potassium ionophore, at the same time, it can inhibit the increase of intracellular Ca2+)25 counteracted (Fig. 6g). This suggests that Nlrp3 may serve as a critical intermediary gene. Subsequently, the study assessed the protein level of p-eNOS and apoptosis, along with mRNA expression, across six different cell groups. Findings revealed that Nlrp3 activation significantly negated the beneficial impact of GPR39 knockdown on the downregulation of p-eNOS levels and apoptosis in endothelial cells induced by Ang II (Fig. 6a–e and Supplemental Fig. 4a, b). Parallel TUNEL assays further corroborated these observations (Fig. 6f). Additionally, ROS staining indicated that GPR39 knockdown lessened the oxidative stress in HUVECs induced by Ang II, an effect reversed by Nlrp3 (Fig. 6h). Analysis of mRNA levels of inflammatory markers across the six cell groups demonstrated that Nlrp3 upregulation could negate the therapeutic effect of GPR39 knockdown on Ang II-induced inflammation (Fig. 6i–l). Notably, the inflammatory indicators levels in cells treated with both Nigericin and Ang II were comparable to those in cells treated with either agent alone, suggesting that Nigericin does not exacerbate Ang II’s effect on endothelial cells. Similar levels between the si-GPR39 + Nigericin + Ang II group and the Nigericin + Ang II group indicate that GPR39 knockdown mitigates Ang II’s effects via Nlrp3 inhibition. Ca2+ or other CASR agonists activate NLRP3 inflammasome in the absence of exogenous ATP26. Furthermore, Fluo4 assays conducted to assess Ca2+ signaling intensity in endothelial cells following GPR39 knockdown or activation (using TC-G-1008, a GPR39 agonist, at 10 μM)27 revealed that GPR39 activation enhances, while its absence reduces, intracellular Ca2+ signaling (Supplemental Fig. 5). In summary, GPR39 knockdown appears to alleviate endothelium-dependent injury induced by Ang II through the downregulation of Nlrp3 expression.
a–e Analysis of protein levels (P-eNOS, eNOS, Bax, Bcl-2, Caspase-3 and Cleaved caspase-3, n = 6) in HUVECs across. 6 groups. GAPDH served as the loading control. f TUNEL staining to assess apoptosis in the 6 groups of HUVECs (TUNEL, green; DAPI, blue; n = 6; Scale, 50 μm). White arrows highlight endothelial cells with elevated TUNEL expression. g Evaluation of cellular activity in the PBS, Ang II, Ang II + si-GPR39, Nigericin, Nigericin + Ang II and Nigericin + Ang II + si-GPR39 groups using a CCK8 kit (n = 6). h ROS staining depicting oxidative stress in HUVECs of the 6 groups (ROS, green; DAPI, blue; n = 6; Scale, 50 μm). White arrows highlight endothelial cells with elevated ROS expression.0.1. i–l Measurement of mRNA levels of inflammation markers (IL-1, IL-6, TNF-α and Nlrp3, n = 6) in HUVECs. Results are expressed as the mean ± SEM, with statistical significance indicated as NS, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Potential therapeutic effects of small molecule ligands for GRP39 on Ang II-induced hypertension and vascular damage
Utilizing high-throughput virtual screening (HTVS), our team identified potential small molecule ligands for GPR39, selecting five candidates (ID numbers: Z1780628919; Z2437696239; Z295870648; Z119834076; Z279582396; Supplemental Table 2) for validation on cells for cellular validation. Among these, Z1780628919 ((S)-N-(6-(difluoromethoxy)-1,2,3,4-tetrahydronaphthalen-1-yl)-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridine-1-carboxamide; Fig. 7a) demonstrated the most potent efficacy in ameliorating TC-G-1008-induced inflammatory effects in HUVECs (Supplemental Fig. 6a–c). The 3D molecular structure of Z1780628919 and its interaction with the GPR39 protein are depicted in Fig. 7b–e, highlighting key features such as the protein C skeleton in green, nitrogen atoms in blue, oxygen atoms in bright red, hydrogen atoms in white, and Z1780628919 in rose red. The hydrogen bond length is displayed as a red dashed line, and the longer the bond length, the weaker the hydrogen bond interaction. Hydrogen bonds and hydrophobic interactions between Z1780628919 and the Human GPR39 protein, including bonds with TRP314 and PRO179, are elucidated, emphasizing the relevance of bond length to interaction strength. The hexacyclic amide bond NH serves as a hydrogen bond donor and forms one hydrogen bond with TRP314 at 2.6 Å; Pyrazole, as a hydrogen bond donor, forms two hydrogen bonds with PRO179 at distances of 1.9 Å and 1.8 Å, respectively; In addition, Z1780628919 can form hydrophobic interactions with amino acid residues such as PRO98, VAL181, PHE319, etc. (Fig. 7b–e). In vivo, Z1780628919 exhibited no adverse effects on liver function in mice (Supplemental Fig. 7a) and was well-tolerated when administered via tail vein injection. At the cellular level, small molecule drugs did not affect cell activity (Supplemental Fig. 7b). In addition, to demonstrate the relationship between Z123 and GPR39, we detected the second messenger calcium ion signal of GPR39 in HEK293T cells and found that Z123 can block the calcium signal activation of HEK293T + GPR39 plasmid. And HEK293 itself does not express calcium signals (Supplemental Fig. 7c). Z1780628919 enhanced eNOS levels, reduced apoptosis, and mitigated inflammation in HUVECs exposed to Ang II (Fig. 7f–m; Supplemental Figs. 8a and 8b).
a Chemical structure of Z1780628919. b, c Two-dimensional and three-dimensional illustrations of the binding interaction between GPR39 and Z1780628919. GPR39 is shown as a green cartoon, and Z1780628919 as a rose-red stick. 2D and 3D representations were generated using Schrödinger Maestro 11.4 and PyMOL software, respectively. d, e Cartoon and surface diagrams depicting the interaction mode of GPR39 and Z1780628919. GPR39 is illustrated as a green cartoon, and Z1780628919 as a rose-red stick. Images produced with PyMOL software. f–j Analysis of apoptosis marker proteins (P-eNOS, eNOS, Bax, Bcl-2, Caspase-3, and Cleaved caspase-3, n = 6) in HUVECs treated with PBS, Ang II, Z1780628919, and Ang II + Z1780628919. GAPDH was used as a loading control. k–m mRNA expression levels of inflammatory markers (IL-1, IL-6, and TNF-α, n = 6) in HUVECs. Data are expressed as mean ± SEM. Statistical significance is indicated as NS (not significant), *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Consistent with these findings, Z1780628919 significantly attenuated Ang II-induced hypertension in mice (Fig. 8a). Additionally, Masson staining revealed that Z1780628919 reduced Ang II-induced aortic fibrosis, and analyses via Western blotting and qPCR (Supplemental Figure 9) confirmed similar expressions of fibrosis markers COL-1, α- SMA and TGF- β at the protein level in blood vessels (Fig. 8c; Supplemental Figure 9).
a Blood pressure measurements (systolic pressure, diastolic pressure and mean arterial pressure; tail artery measurements, n = 6) in saline, Ang II, Z1780628919, and Ang II + Z1780628919 treated mice. b Assessment of fibrosis in mouse aortic vessels (n = 6) using Masson’s trichrome staining and quantification of fibrotic areas. c Protein levels of fibrosis markers (α-SMA, TGF-β and COL-1, n = 6) in the aortic vessels. GAPDH served as the loading control. d Analysis of apoptosis marker proteins (eNOS, p-eNOS, Bax, Bcl-2, Caspase-3 and Cleaved caspase-3, n = 6) in the aortic vessels. GAPDH used as a loading control. e, f TUNEL staining for apoptosis visualization in aortic vessels of mice across the four groups (TUNEL, green; CD31, red; DAPI, blue; n = 6; Scale, 50 μm). g, h 8-OHdG staining to detect oxidative stress in aortic vessels of the four groups (8-OHdG, green; CD31, red; DAPI, blue; n = 6; Scale, 50 μm). i, j DHE staining for oxidative stress assessment in aortic vessels of the four groups (DHE, green; CD31, red; DAPI, blue; n = 6; Scale, 50 μm). k–m mRNA levels of inflammation markers (IL-1, IL-6 and TNF-α, n = 6) in the aortic vessels. GAPDH as the loading control. Data are expressed as the mean ± SEM. Statistical significance is denoted as NS, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001.
The levels of Bax, Bcl-2 and Caspase-3 further illustrated that Z1780628919 countered Ang II-induced apoptosis in vascular cells (Fig. 8d, Supplemental Fig. 8c, d), a finding corroborated by TUNEL staining of mouse aorta (Fig. 8e, f).
Moreover, 8-OHdG immunofluorescence staining, and ROS assays revealed a decrease in oxidative stress in the aortas of mice treated with Z1780628919, as compared to those exposed to Ang II alone (Fig. 8g–j). Importantly, Z1780628919 also alleviated the vascular inflammatory response induced by Ang II (Fig. 8k–m).
In summary, the GPR39 small molecule ligand Z1780628919, identified through this research, appears to be a promising therapeutic candidate for hypertension, exhibiting significant efficacy without observed side effects.
Discussion
The global incidence and mortality rates associated with hypertension-related complications, notably cardiovascular diseases, are projected to rise28. Angiotensin II, a pivotal ligand in the renin-angiotensin system (RAS), plays a significant role in the pathogenesis of hypertension29. The findings of this study suggest that downregulation of GPR39 suppresses Nlrp3, thereby mitigating vascular dysfunction induced by Ang II and consequently reducing Ang II-induced hypertension.
GPR39, belonging to the ghrelin family of G protein-coupled receptors, exhibits zinc sensitivity30. The role of GPR39 in physiological processes remains a subject of debate. Certain studies have indicated that zinc, mediated by the ERK pathway involving GPR39, can attenuate arterial calcification31, and GPR39 activation may reduce inflammation and apoptosis in macrophages32. However, recent studies posited a potentially harmful role of GPR39 in the cardiovascular system. For instance, Liao H et al. reported that GPR39 exacerbates cardiac hypertrophy via the AMPK-mTOR pathway and influences protein synthesis23. Similarly, H. Kaur et al. demonstrated that GPR39 contributes to the dedifferentiation of smooth muscle cells, as revealed through single-cell sequencing33. GPR39 is hypothesized to act as a homeostatic regulator in diverse biological processes, including neuronal excitability, vascular tone, and immune responses34. The present study highlights the beneficial impact of GPR39 knockdown in vascular tissues on Ang II-induced hypertension.
Historical and contemporary studies have underscored the critical role of endothelial cells in regulating vascular tone, with endothelial dysfunction being a prevalent pathophysiological factor in hypertension35. Our research indicates that GPR39 knockout ameliorates endothelium-dependent relaxation in mouse vessels subjected to Ang II-induced stress, and a heightened expression of GPR39 was observed in Ang II-stimulated endothelial cells. This implies that GPR39 ablation in endothelial cells may ameliorate endothelial dysfunction triggered by Ang II, thereby contributing to blood pressure reduction.
Contrasting evidence exists regarding the relationship between hypertension and vascular smooth muscle function. While some studies associate hypertension with enhanced vascular smooth muscle contraction36,37, others report that Ang II infusion diminishes aortic smooth muscle contractility in mice38. Our findings indicate that GPR39 knockout in mice increases endothelium-independent vasoconstrictor capacity, while simultaneously reducing blood pressure in the Ang II-treated group. This could be attributed to Ang II-induced fibrosis in smooth muscle, which impairs vascular contractility. GPR39 knockout seemingly alleviates this fibrosis. Using the Langendorff method, one study observed that Ang II does not affect coronary perfusion pressure (CPP), but SNP significantly attenuates CPP in GPR39-deleted mice39. This is an acute experiment in vitro. All drugs (Ang II, etc.) were administered by cardiac perfusion. The researchers selected mice aged 3–6 months and injected ketamine into the mice before separating the heart. Therefore, there are some differences between this study and our results, which may be due to the different functions of GPR39 in different parts of smooth muscle cells of mice treated with different treatments, which needs to be further explored.
Hypertension, along with vasoconstriction, diastolic dysfunction, fibrosis of vascular smooth muscle cells, and inflammatory response, is intimately linked to endothelial cell dysfunction and endothelial cell apoptosis40. ROS play crucial roles in physiological cell functions such as host defense, post-translational proteins processing, cell signaling, gene expression regulation, and cell differentiation41. However, excessive ROS production may induce endothelial dysfunction and endothelial cell apoptosis42. Ang II has demonstrated to augment local ROS production via interaction with AT1R43. This ROS-induced endothelial dysfunction is believed to be a key early step in the development of hypertension44. Moreover, ROS exert significant pro-inflammatory effects. Elevations in ROS, cytokines, and adhesion molecules triggered by Ang II can activate inflammatory genes in vascular cells19,45. Nlrp3 is closely associated with inflammation and apoptosis20. Vascular fibrosis, characterized by the accumulation of extracellular matrix proteins46, particularly collagen synthesis, is a notable consequence. The ROS induced by Ang II can inhibit eNOS activity and provoke inflammation, thereby promoting vascular fibrosis and contributing to the damage and apoptosis of vascular cells47,48.
Previous research has established that GPR39 suppresses AMPK activation, thereby stimulating the mammalian target of rapamycin (mTOR) and ribosomal protein S6 kinase β-1 (S6K1), which in turn promotes de novo protein synthesis23. Additionally, the AMPK/mTOR pathway has been identified as an inhibitor of NLRP3 inflammasomes in cellular models49. The NLRP3 inflammasome, a prominent multimeric protein complex, initiates the cleavage and secretion of mature forms of pro-interleukin (IL)-1β and pro-IL-18, mediated by caspase-1. This activation plays a pivotal role in propagating the inflammatory process and oxidative stress21. Therefore, we speculate that GPR39 inhibits the AMPK/mTOR signaling pathway in endothelial cells, thereby reducing the inhibition of NLRP3 and causing inflammation and even apoptosis in endothelial cells.
In this study, we observed that nigericin, an NLRP3 inflammasome activator, reversed the anti-apoptotic effects of GPR39 downregulation in HUVECs exposed to Ang II. This finding suggests a novel mechanism by which Ang II induces vascular dysfunction, wherein GPR39 downregulation mitigates such dysfunction and consequent hypertension through Nlrp3 downregulation. The advancements in genomic technology have significantly enhanced the diagnostic precision for cardiovascular diseases50, and future studies will focus on multi-omic analyses of GPR39.
Additionally, HTVS identified the small molecule Z1780628919 as a potent GPR39 ligand, previously unstudied. Subsequent therapeutic validation in animals’ models and HUVECs revealed no significant side effects of Z1780628919. Importantly, Z1780628919 demonstrated efficacy in mitigating Ang II-induced hypertension and endothelial cell damage, suggesting its potential as a promising hypertension treatment.
In summary, the overexpression of Nlrp3 in endothelial cells may contribute to endothelial dysfunction. GPR39 knockout reduces Nlrp3 expression, thereby inhibiting inflammation, oxidative stress, vascular fibrosis, as well as apoptosis, ultimately alleviating Ang II-induced hypertension. Therefore, GPR39 emerges as a novel therapeutic target for Ang II-induced hypertension, with its small molecule inhibitor, Z1780628919, showing promise as a potential treatment option.
Limitations
There are some limitations to this article. Firstly, the gold standard for blood pressure measurement in rodents is radio telemetry of conscious, unconstrained animals. Although the tail cuff data is convincing, this is still a limitation as these mice are constrained during blood pressure measurement. Secondly, this study was only conducted on male mice. While Ang II led to hypertension and worsening vascular reactivity in both sexes, aortic remodeling and stiffening occurred only in males51. In DOCA-salt model, the increase of blood pressure in female mice was significantly lower than that in male mice52. Therefore, in C57/B6 mice, female mice have certain blood pressure resistance. Compared with male mice, female mice are not susceptible to a variety of models to induce long-term stable hypertension and corresponding vascular injury. Therefore, we chose male mice as the research object. However studies have shown that, male but not female GPR39 KO mice exhibited larger infarcts and lower capillary RBC flux than WT controls after stroke53. This indicates that GPR39 knockout has sex differences, which is another limitation of this study and needs further study.
Methods
Artery tissue samples of patients
Collect human aortic tissue samples from subjects ( > 18 years old) who require aortic dissection surgery. The study focuses on primary hypertension patients (4 males and 1 female) and control group patients (5 males) admitted to the Cardiovascular Surgery Department of the First Affiliated Hospital of Nanjing Medical University from June 2022 to June 2023. According to international standards54, hypertension is defined as >130/80 mmHg. This study was approved by the Institutional Review Committee at the First Affiliated Hospital with Nanjing Medical University (2019-SR-067).
Animal ethics statement
All animal experiments were conducted in strict compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The Institutional Animal Care and Use Committee of Nanjing Medical University (Nanjing, China) approved the animal experimental protocols. The study design ensured the use of the minimum number of animals and aimed to minimize their discomfort.
Animals and model establishment
We employed CRISPR/Cas9 technology for targeted modification of the GPR39 gene. Specifically, we selected exon 1 of the GPR39-201 (ENSMUST00000027581.6) transcript as the knockout region, which includes the start codon ATG. This knockout disrupts protein function. Genes influenced by GPR39 predominantly affect various physiological systems, including adipose tissue; neurological behavior; cardiovascular, digestive/alimentary system; endocrine/exocrine glands; growth and body size, homeostasis/metabolism; and the renal/urinary system This information was corroborated by data from the MGI database (Mouse Genome Informatics).
We generated GPR39-deficient mice (GPR39 KO, C57BL/6JGpt, male, 8-10 weeks) and compared them with their wild-type (WT) counterparts. Both groups were housed under a standard 12-hour light/dark cycle and had ad libitum access to food (AIN-93M, Research Diet, <0.3% NaCl) and water. For experimental purposes, the mice were randomly assigned to receive either saline or Angiotensin II (Ang II, 1.44 mg/kg/day, Sigma, Shanghai, China) administered via Alzet osmotic minipumps (Model 2004, CA, USA) for a duration of four weeks. This resulted in four distinct experimental groups: Saline, Ang II, GPR39 KO, and GPR39 KO + Ang II. Additionally, we investigated the effects of (S)-N-(6-(difluoromethoxy)-1,2,3,4-tetrahydronaphthalen-1-yl)-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-c]pyridine-1-carboxamide (Z1780628919; 10 μg/kg/day, Topscience, Shanghai, China). This compound was intravenously injected into the mice via the tail vein once weekly. Similar to the previous setup, another cohort of mice was divided into four groups to assess the responses to Saline, Ang II, Z1780628919, and Ang II + Z1780628919 treatments.
Tissue sample collection
The tissue sample collection started at about 9 a.m. that day. Mice were anesthetized using an intraperitoneal injection of 2% pentobarbital sodium and subsequently euthanized. To facilitate blood clearance, a minor incision was made in the right atrial appendage, followed by perfusion of the left ventricle with phosphate-buffered saline (PBS). This process ensured the effective removal of blood from the organs. Subsequently, the aortic vascular tissues were meticulously dissected and immediately fixed in formalin for preservation. These tissues were then carefully transferred into enzyme-free Eppendorf tubes and stored at -80°C until further analysis.
Masson’s trichome staining
For histological analysis, aorta tissues underwent a meticulous dehydration process using a series of ethanol solutions with ascending concentrations: 75%, 80%, 90%, 90%, and twice in 100%, each for a duration of 1.5 h. Subsequently, the tissues were cleared with xylene twice and embedded in paraffin. Utilizing flakers (RM2016, Leica, Shanghai, China), we sectioned the tissues into slices measuring 5–7 μm in thickness. These sections were then subjected to baking at 65 °C to enhance adherence to the slides. Next, the tissue sections were dewaxed in xylene (two sessions, 5 min each), followed by a graded series of ethanol (100%, 95%, 85%, and 75%, 5 min each) to effectively remove any residual xylene and rehydrate the tissues. The tissue sections were dewaxed and rehydrated before staining with Masson’s Trichome (ServiceBio, Wuhan, China). For imaging and analysis, we utilized a fluorescence upright microscope (Carl Zeiss, Jena, Germany) to capture whole-section images. Quantitative analysis of the extent of fibrosis in the aortic tissues was conducted using Image-Pro software (version 6.0; Media Cybernetics, MD, USA), specifically focusing on the percentage of fibrotic areas within the tissues.
Immunofluorescence analysis
Paraffin-embedded sections were rehydrated using the above standard protocol. Antigen retrieval was achieved by microwaving the sections, followed by cooling at room temperature in tap water. Sections were then sealed with 10% goat serum (C-0005, Haoran Biotechnology Co. Shanghai, China) for 120 min. Incubation proceeded with primary antibodies: rabbit anti‐GPR39 (1:50; AGR-045; Alomone, Jerusalem, Israel), 8-OHdG (1:50; sc-393871; Santa Cruz Co. Ltd., TX, USA), CD31(1:50; sc-376764; Santa Cruz Co. Ltd., TX, USA) and Beclin-1 (1:50; sc-48341; Santa Cruz Co., Ltd., TX, USA) at 4 °C overnight. The sections were washed thrice with PBS (0.1 mol/L, PH = 7.4) for 5 min each, which was followed by a 2-h room temperature incubation with rabbit IgG antibodies (Cy3) and DAPI for nuclear staining. Fluorescence imaging was conducted using an inverted microscope equipped with Zeiss optics. Quantitative analysis of images was performed using ImageJ software.
Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were cultured in DMEM medium (GIBCO, USA) supplemented with glutamine, 10% fetal bovine serum (FBS) (GIBCO, USA), 100 U0/ml penicillin, and 100 U/ml streptomycin at 37 °C in 5% CO2 atmosphere. For experimental treatments, cells were exposed to Angiotensin II (Ang II, 1 μM, Bachem, CA, USA) for 48 hours. si-GPR39 (RiboBio, GGGCTTTGCCACAAGGAAA, CCAAGCCCAACATGATCAT, GGTGGAATGTGTTATGAAA) and GPR39 plasmid (purchased from Shanghai Jikai GeneChemical Technology Co., Ltd) transfection was performed using Lipofectamine according to the manufacturer’s instructions. Nigericin (50 μM) treatment duration and concentration were determined based on prior studies, with a 1-hour exposure being optimal55. Additional compounds including Z1780628919, 36, 48, 76, and 96 (10 μM; TsBiochem, Shanghai, China) were administered to the cells for 24 hours. According to the official ingredient statement, no high concentration of Zn was detected in any of the reagents, and no additional Zn ions were added.
Intracellular calcium assay
Endothelial cell medium was replaced with a buffer containing 5 μg/ml Fluo4-AM (Beyotime, Shanghai, China). Cells were incubated at 37°C for one hour followed by three washes with PBS. Subsequently, cells were examined using a confocal laser scanning microscope. Wnt5a (500 ng/ml) was introduced to elicit calcium responses, monitored by LSM510 Meta. An inverted microscope with Zeiss fluorescence optics facilitated section analysis. Quantitative image analysis was conducted using ImageJ software.
qPCR analysis
Total RNA was isolated from samples using TRIzol reagent (Invitrogen, Shanghai, China). Subsequently, mRNA was reverse transcribed into cDNA utilizing a PrimeScript™ RT Master Mix kit (TaKaRa Biomedical Technology, Beijing, China) within a 10 μL reaction system. Quantitative PCR (qPCR) was performed in triplicate for all samples using 384-well plates. Specific primers (Genscript, Nanjing, China), listed in Supplemental Table 1, facilitated gene quantification. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was employed as the normalization control. Relative gene expression levels were calculated using the 2 − ΔΔ CT method.
Western blotting analysis
Mouse aorta tissues and cultured cells, preserved at −80 °C, were subjected to ultrasonic disruption followed by homogenization in Radio Immunoprecipitation Assay (RIPA) lysis buffer. The homogenate was centrifuged at 14000 °C × G to remove cellular debris, and the supernatant was collected 20 minutes. This protein lysate was mixed with sample buffer (Invitrogen) and heated for 5 minutes to denature proteins. Subsequent to electrophoresis and membrane transfer, the proteins were probed with primary antibodies specific to GPR39 (1:200; AGR-045; Alomone, Jerusalem, Israel), Collagen I (1:1000; No.14695-1-AP; Proteintech Co., Wuhan, China), α-SMA (1:1000; No.14395-1-AP; Proteintech), TGF-β (1:1000; No.21898-1-AP; Proteintech), Cleaved caspase-3 (1:1000, ab-32042, Abcam, Shanghai, China), Caspase-3 (1:1000; No.19677-1-AP; Proteintech), p-eNOS (1:1000, No. 28939-1-AP, Proteintech), eNOS (1:1000, No. 27120-1-AP, Proteintech), Bcl-2 (1:1000; No.26593-1-AP; Proteintech), Nlrp3 (1:1000, No. 19771-1-AP, Proteintech) and Bax (1:1000; No.50599-2-lg; Proteintech). GAPDH (1:1000, AF0006; Beyotime Biotechnology Co., Shanghai, China) served as an internal control. Following overnight incubation, membranes were treated with appropriate secondary antibodies for 2 h, washed thrice in TBST on a shaker (5 min each). Blots were stripped and re-probed as required. Although no imprint of molecular weight ladder can be seen on some uncut/unedited images, the marker is visible at the stage of electrophoresis. We manually mark the matched molecular weight in advance to find the corresponding band at the subsequent exposure. The bands were visualized, and images were quantitatively analyzed using ImageJ software.
Assessment of blood flow using laser speckle contrast imaging
Blood flow with the mesenteric artery was quantified utilizing a laser perfusion imaging system. Mice underwent anesthesia with isoflurane (1.5–2.5%) and were maintained at physiological temperature using thermostatic heating pads. Initially, the mouse was positioned laterally and their abdominal fur was removed with an electric razor, and a midline-adjacent incision was executed. To facilitate mesenteric artery visualization, a portion of the gastrointestinal tract was gently retracted and covered with gauze moistened in sterile normal saline at 37 °C. Care was taken to maintain arterial integrity by applying 37 °C normal saline. The imaging laser was fixed 20 cm above the renal area of the mouse, and adjustments to zoom and focus were made to capture high-resolution images. Ambient room illumination was standardized to ensure image consistency.
TUNEL analysis
TUNEL test kit (Beyotime, Shanghai, China) was used to determine TUNEL following the manufacturer’s guidelines. Briefly, sectioned samples underwent permeabilization with 0.3% Triton X-100 for 15 minutes. This step was followed by the fixation of adherent cells using 4% paraformaldehyde for 30 min. After a single wash with PBS, the cells were again treated with 0.3% Triton X-100 for 5 min to ensure adequate permeabilization. The TUNEL reaction was then initiated by applying 50 µL of the reaction mixture, composed of 5 µL of TdT enzyme solution and 45 µL of labeling solution, to the samples. The samples were incubated for 1 hour at 37 °C in a dark, humidified chamber to facilitate the labeling process. Post-incubation, the cells were co-cultured with DAPI for nuclear visualization and examined under an Olympus FV3000 confocal microscope to assess apoptotic activity.
Measurement of intracellular reactive oxygen species
Measurement of ROS generation in aortas was quantified via dihydroethidium fluorescence intensity, following established protocols. Fresh-frozen RPE-choroid sections were thawed, washed in Tris-buffered saline, and incubated with 10 μmol/L DHE under dark conditions at 37 °C for 15 min. Following incubation, sections were washed and counterstained with DAPI. Fluorescence imaging was conducted using an Olympus confocal microscope. Analysis involved quantifying the area of lesion and neovascular tissue in laser-treated sections, alongside integrated DHE fluorescence intensity, utilizing ImageJ software. Intracellular ROS levels were quantitatively assessed using the Reactive Oxygen Species Assay Kit (Beyotime Biotechnology, Shanghai, China). The assay utilizes 2′,7′-dichlorofluorescein diacetate (DCFH-DA), a compound readily oxidized to fluorescent dichlorofluorescein (DCF) in the presence of ROS. HUVECs seeded in 96-well plates were treated with DCFH-DA at 37 °C for 20 min. Post-incubation, fluorescence images were acquired and analyzed, as previously mentioned.
Non-Invasive Tail-Cuff Blood Pressure Measurement
Blood pressure was assessed on days 0 (baseline), 7, 14 and 21 (endpoint) days of the experiment using a computerized, non-invasive tail-cuff system (Kent Science, USA). To mitigate stress-related variations in tail artery blood pressure, daily measurements were conducted for at least 7 days prior to the commencement of the experiments. Tail cuff blood pressure measurement started at 8-10 am. Prior to each measurement, rats underwent a warming period of 10 to 20 min at a temperature of 28 °C. This protocol was essential for ensuring the reliable detection of tail arterial pulsations and establishing a consistent pulse rate.
Measurement of vasoconstriction and relaxation
The aorta of the mouse swiftly excised and meticulously freed from fat and connective tissues in an ice-cold, carbon dioxide -supplemented Tyrode solution. Subsequently, a 2-mm segment of the artery was mounted in a wire myograph (610 M and 620 M models, Danish Myo Technology A/S, Aarhus, Denmark). To determine the effective transmural pressure, each artery segment was normalized. The arterial tension was adjusted to equate to the tension observed at a 100 mm Hg pressure, corresponding to 90% of the artery’s diameter. Following a 45-min equilibration period, the arterial segments were exposed to a high potassium chloride (KCl) solution to confirm their viability. The endothelium-dependent contractile response was evaluated through the cumulative addition of Prostaglandin F2α (Pgf-2α; ranging from 10-9M to 10-4M). To ascertain non-endothelium-dependent contractile function, norepinephrine was added in a cumulative manner (from 10-9M to 10-4M). The assessment of endothelium-dependent relaxation involved the gradual addition of acetylcholine (Ach; from 10-9M to 10-4M) to the pre-contracted arteries using a high KCl solution. Additionally, endothelium-independent relaxation was gauged through the cumulative introduction of sodium nitroprusside (from 10-9M to 10-4M).
Cell viability assessment
To determine the viability of HUVECs, we utilized the CCK-8 cell viability assay kit (CK04; Dojindo Laboratories, Kumamoto, Japan). Briefly, 10 µl of CCK-8 solution was introduced into each well of the cell culture. The absorbance value was then measured at 450 nm with an enzyme marker. Cell viability was calculated with the formula: cell viability (%) = (A of experimental well / A of control well) × 100%.
High-throughput virtual screening (HTVS)
For structure-based HTVS, Schrödinger Maestro (version 11.4) was employed to identify potential ligands of GRP39. In the absence of a published 3D structure for human GPR39, its configuration was predicted using AlphaFold2 (AlphaFold2 ID: AF-O43194-F1), with the prediction yielding a high reliability score. The binding site for GPR39 was delineated using the Sitemap module in Schrödinger Maestro, forming the basis for subsequent HTVS procedures.
Enamine’s HTS Compound Library, which comprises 2,163,000 compounds (available at https://enamine.net/compound-collections/screening-collection), was prepared for virtual screening. This preparation involved energy optimization via the LigPrep module in Schrödinger, followed by molecular docking using the Virtual Screening Workflow module. Initially, compounds were screened using the High Throughput Virtual Screening (HTVS) mode, with the top 5% scoring compounds advancing to the Standard Precision mode. Subsequently, the top 10% compounds in the SP mode were further assessed using the Glide Extra Precision (XP) mode. Small molecules exhibiting the highest docking scores were earmarked as potential hits for further experimental verification. The visualizations and superpositions of protein-ligand interactions were generated using PyMol.
Statistics and reproducibility
For this study, all data were presented as the mean ± standard error of the mean (SEM). To compare differences between two groups, we employed the Student’s t-test. For analyses involving more than two groups, one- or two-way analysis of variance was utilized. Subsequent to ANOVA, post-hoc comparisons were conducted using the Bonferroni correction method. Both the statistical analyses and the creation of statistical graphs were executed using GraphPad Prism version 8.0.
References
Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2, 634–647 https://doi.org/10.1016/s2213-8587(14)70102-0 (2014).
Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398, 957–980 https://doi.org/10.1016/s0140-6736(21)01330-1 (2021).
Niu, Z. et al. Plasma lipidomic subclasses and risk of hypertension in middle-aged and elderly chinese. Phenomics 2, 283–294 (2022).
He, F. J., Tan, M., Ma, Y. & MacGregor, G. A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 75, 632–647 (2020).
Ikehara, S. & Iso, H. Alcohol consumption and risks of hypertension and cardiovascular disease in Japanese men and women. Hypertens. Res. 43, 477–481 (2020).
Pipe, A. Tobacco addiction and hypertension. J. Hum. Hypertens. 10, S13–S16 (1996).
Virdis, A., Giannarelli, C., Neves, M. F., Taddei, S. & Ghiadoni, L. Cigarette smoking and hypertension. Curr. Pharm. Des. 16, 2518–2525 (2010).
Seravalle, G. & Grassi, G. Obesity and hypertension. Pharmacol Res 122, 1–7 https://doi.org/10.1016/j.phrs.2017.05.013 (2017).
Daugherty, A. & Cassis, L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc. Med. 14, 117–120 (2004).
Crowley, S. D., Gurley, S. B. & Coffman, T. M. AT(1) receptors and control of blood pressure: the kidney and more. Trends Cardiovasc. Med. 17, 30–34 (2007).
Giani, J. F. et al. Renal generation of angiotensin II and the pathogenesis of hypertension. Curr. Hypertens. Rep. 16, 477 (2014).
Foulquier, S. et al. Hypertension-induced cognitive impairment: insights from prolonged angiotensin II infusion in mice. Hypertens. Res. 41, 817–827 (2018).
Ruiz-Ortega, M., Rodríguez-Vita, J., Sanchez-Lopez, E., Carvajal, G. & Egido, J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 74, 196–206 (2007).
Takayanagi, T. et al. Vascular ADAM17 as a novel therapeutic target in mediating cardiovascular hypertrophy and perivascular fibrosis induced by angiotensin II. Hypertension 68, 949–955 (2016).
Mendell, J. T. & Olson, E. N. MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 (2012).
Paravicini, T. M. & Touyz, R. M. Redox signaling in hypertension. Cardiovasc Res 71, 247–258 (2006).
Wang, J. et al. Vitamin E renders protection to PC12 cells against oxidative damage and apoptosis induced by single-walled carbon nanotubes. Toxicol. Vitr. 26, 32–41 (2012).
Sessa, W. C. eNOS at a glance. J. Cell Sci. 117, 2427–2429 (2004).
Bruder-Nascimento, T. et al. Angiotensin II induces Fat1 expression/activation and vascular smooth muscle cell migration via Nox1-dependent reactive oxygen species generation. J. Mol. Cell Cardiol. 66, 18–26 (2014).
Swanson, K. V., Deng, M. & Ting, J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Bai, B. et al. NLRP3 inflammasome in endothelial dysfunction. Cell Death Dis. 11, 776 (2020).
Hershfinkel, M., Moran, A., Grossman, N. & Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl Acad. Sci. USA 98, 11749–11754 (2001).
Liao, H. et al. GPR39 promotes cardiac hypertrophy by regulating the AMPK-mTOR pathway and protein synthesis. Cell Biol. Int 45, 1211–1219 (2021).
Chen, Z. et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signalling pathway. Cardiovasc Res 117, 820–835 (2021).
Zeng, S., Guo, Z. G. & Chen, H. Mobilization of intracellular Ca2+ modulates activation of Na+/H+ exchange in thrombin-stimulated platelets. Zhongguo Yao Li Xue Bao 19, 151–154 (1998).
Lee, G. S. et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492, 123–127 (2012).
Muneoka, S. et al. G Protein-Coupled Receptor 39 Agonist Improves Concanavalin A-Induced Hepatitis in Mice. Biol. Pharm. Bull. 42, 1415–1418 (2019).
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260 (2012).
Eguchi, S., Kawai, T., Scalia, R. & Rizzo, V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension 71, 804–810 (2018).
Laitakari, A., Liu, L., Frimurer, T. M. & Holst, B. The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22083872 (2021).
Satianrapapong, W., Pongkorpsakol, P. & Muanprasat, C. A G-protein coupled receptor 39 agonist stimulates proliferation of keratinocytes via an ERK-dependent pathway. Biomed. Pharmacother. 127, 110160 (2020).
Muneoka, S. et al. G protein-coupled receptor 39 plays an anti-inflammatory role by enhancing IL-10 production from macrophages under inflammatory conditions. Eur. J. Pharm. 834, 240–245 (2018).
Kaur, H. et al. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat. Commun. 8, 15700 (2017).
Xu, Y., Barnes, A. P. & Alkayed, N. J. Role of GPR39 in neurovascular homeostasis and disease. Int. J. Mol. Sci. 22 https://doi.org/10.3390/ijms22158200 (2021).
Xu, S. et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharm. Rev. 73, 924–967 (2021).
Qu, H. & Khalil, R. A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 319, H661–h681 (2020).
Li, Y. et al. Vascular smooth muscle cell-specific miRNA-214 knockout inhibits angiotensin II-induced hypertension through upregulation of Smad7. Faseb j. 35, e21947 (2021).
Song, T. et al. SLC44A2 regulates vascular smooth muscle cell phenotypic switching and aortic aneurysm. J. Clin. Invest. 134 https://doi.org/10.1172/jci173690 (2024).
Alkayed, N. J. et al. Control of coronary vascular resistance by eicosanoids via a novel GPCR. Am. J. Physiol. Cell Physiol. 322, C1011–C1021 (2022).
Zheng, D. et al. ROS-triggered endothelial cell death mechanisms: Focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 13, 1039241 (2022).
Bedard, K. & Krause, K. H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313 (2007).
Gimbrone, M. A. Jr. & García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res 118, 620–636 (2016).
Adams, V. et al. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111, 555–562 (2005).
Costantino, S. et al. Interplay among H3K9-editing enzymes SUV39H1, JMJD2C and SRC-1 drives p66Shc transcription and vascular oxidative stress in obesity. Eur. Heart J. 40, 383–391 (2019).
Viel, E. C., Lemarié, C. A., Benkirane, K., Paradis, P. & Schiffrin, E. L. Immune regulation and vascular inflammation in genetic hypertension. Am. J. Physiol. Heart Circ. Physiol. 298, H938–H944 (2010).
Tayebjee, M. H., MacFadyen, R. J. & Lip, G. Y. Extracellular matrix biology: a new frontier in linking the pathology and therapy of hypertension? J. Hypertens. 21, 2211–2218 (2003).
Satoh, C. et al. Role of endogenous angiotensin II in the increased expression of growth factors in vascular smooth muscle cells from spontaneously hypertensive rats. J. Cardiovasc Pharm. 37, 108–118 (2001).
Montezano, A. C., Nguyen Dinh Cat, A., Rios, F. J. & Touyz, R. M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 16, 431 (2014).
Yang, F. et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int J. Biol. Sci. 15, 1010–1019 (2019).
Franks, P. W. et al. Precision medicine for cardiometabolic disease: a framework for clinical translation. Lancet Diab. Endocrinol. 11, 822–835 (2023).
Wang, H. et al. Sex differences and role of lysyl oxidase-like 2 in angiotensin II-induced hypertension in mice. Am. J. Physiol. Heart Circ. Physiol. 327, H642–h659 (2024).
Karatas, A. et al. Deoxycorticosterone acetate-salt mice exhibit blood pressure-independent sexual dimorphism. Hypertension 51, 1177–1183 (2008).
Xu, Y. et al. GPR39 knockout worsens microcirculatory response to experimental stroke in a sex-dependent manner. Transl. Stroke Res 14, 766–775 (2023).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324 (2018).
Sun, W. et al. NEDD4 ameliorates myocardial reperfusion injury by preventing macrophages pyroptosis. Cell Commun. Signal 21, 29 (2023).
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (82070438 and 82373892), the Academy Talent Special Fund of The First Affiliated Hospital of Nanjing Medical University (YNRCQN0312), and The Gusu School of Nanjing Medical University (GSRCKY20210203 to Y.S.).
Author information
Authors and Affiliations
Contributions
D.H. and W.H.: Conceptualization, Investigation, and Writing – original draft. Q.X. and W.X.: Methodology. L.T., M.L., and X.W.: Formal analysis and Data curation. Q.Z. and X.C.: Software. Y.S. and P.L.: Conceptualization, Formal analysis, Writing – review & editing, Supervision, Funding and acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hua, D., Huang, W., Xie, Q. et al. Targeting GPR39 in structure-based drug discovery reduces Ang II-induced hypertension. Commun Biol 7, 1441 (2024). https://doi.org/10.1038/s42003-024-07132-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s42003-024-07132-2